A New Model of Hemoglobin Oxygenation
Abstract
:1. Introduction
2. Materials and Methods
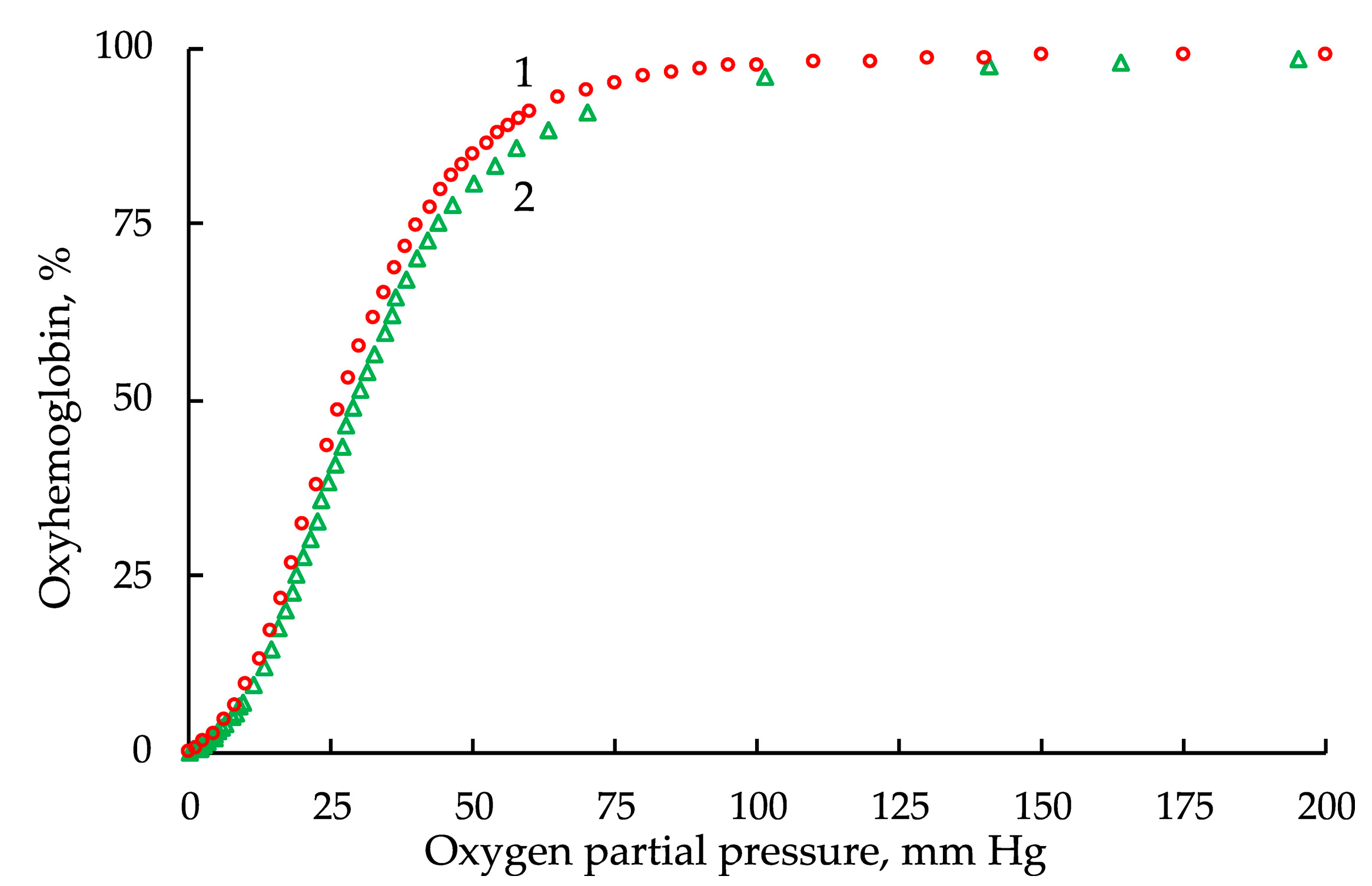
3. Results
3.1. The Hill Equation and Its Limitations
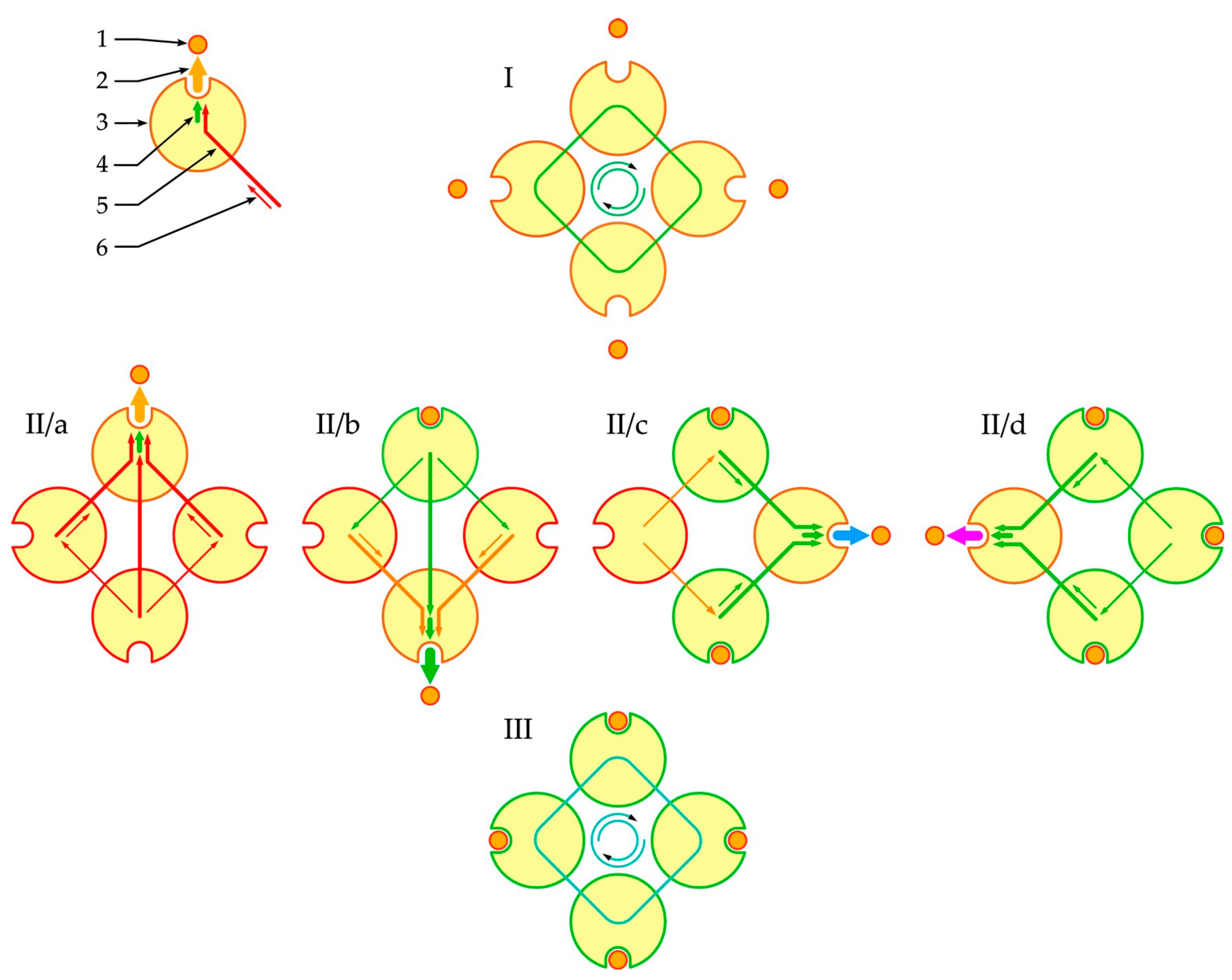
3.2. Phenomenological Model of Oligomer Liganding
- Each subunit of the oligomer could be either vacant for the ligand or liganded and also in different conformational states;
- In these conformational states, the oligomer subunits experienced coordinated perturbation by neighboring subunits;
- The current superposition of the conformational states of the subunit states could change the affinity of a subunit for an attachable or already attached ligand;
- Ligand binding reconfigured the interaction between the subunits and created a new self-consistent level of cooperativity between them, which determined the nature and strength of the interaction of the attachable or already attached ligand according to a feedback mechanism.
3.3. The Hill Coefficient as a Function of Oxygen Partial Pressure
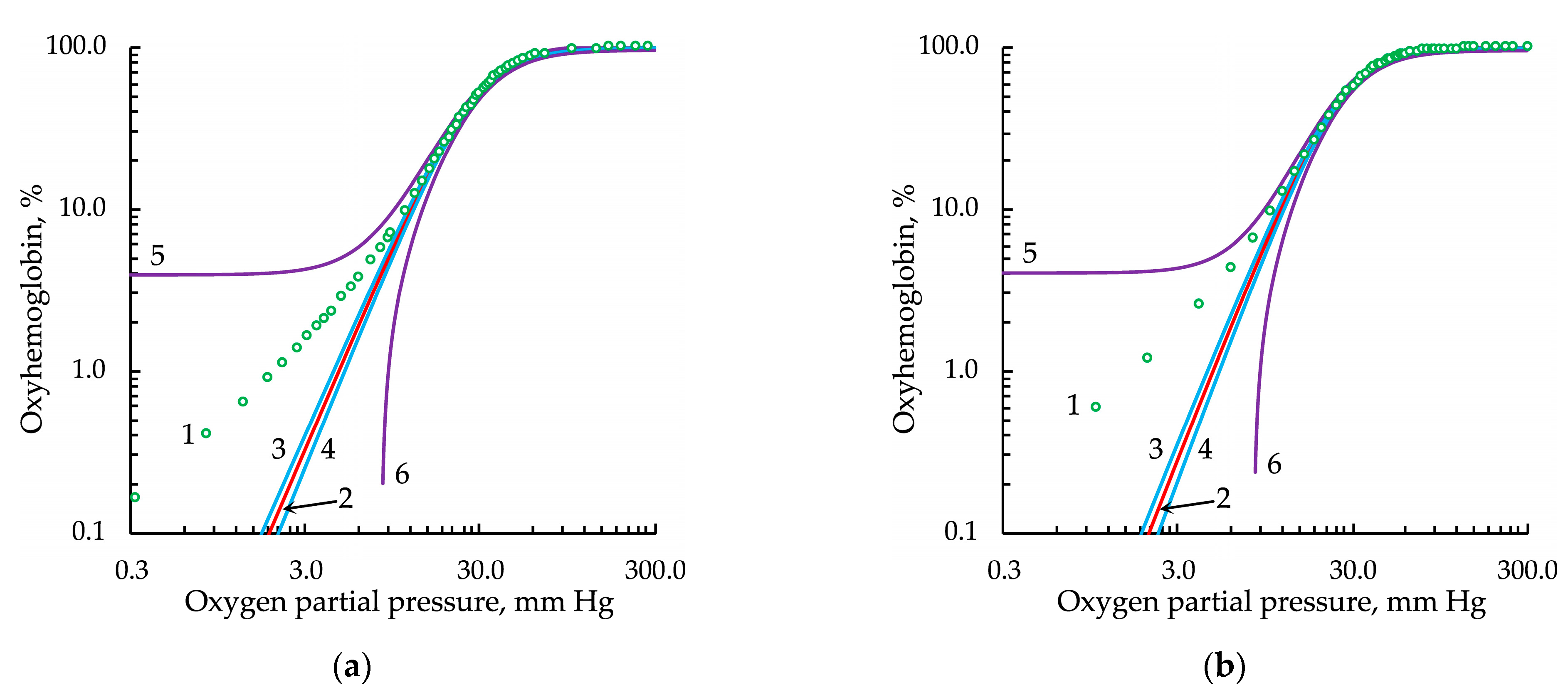
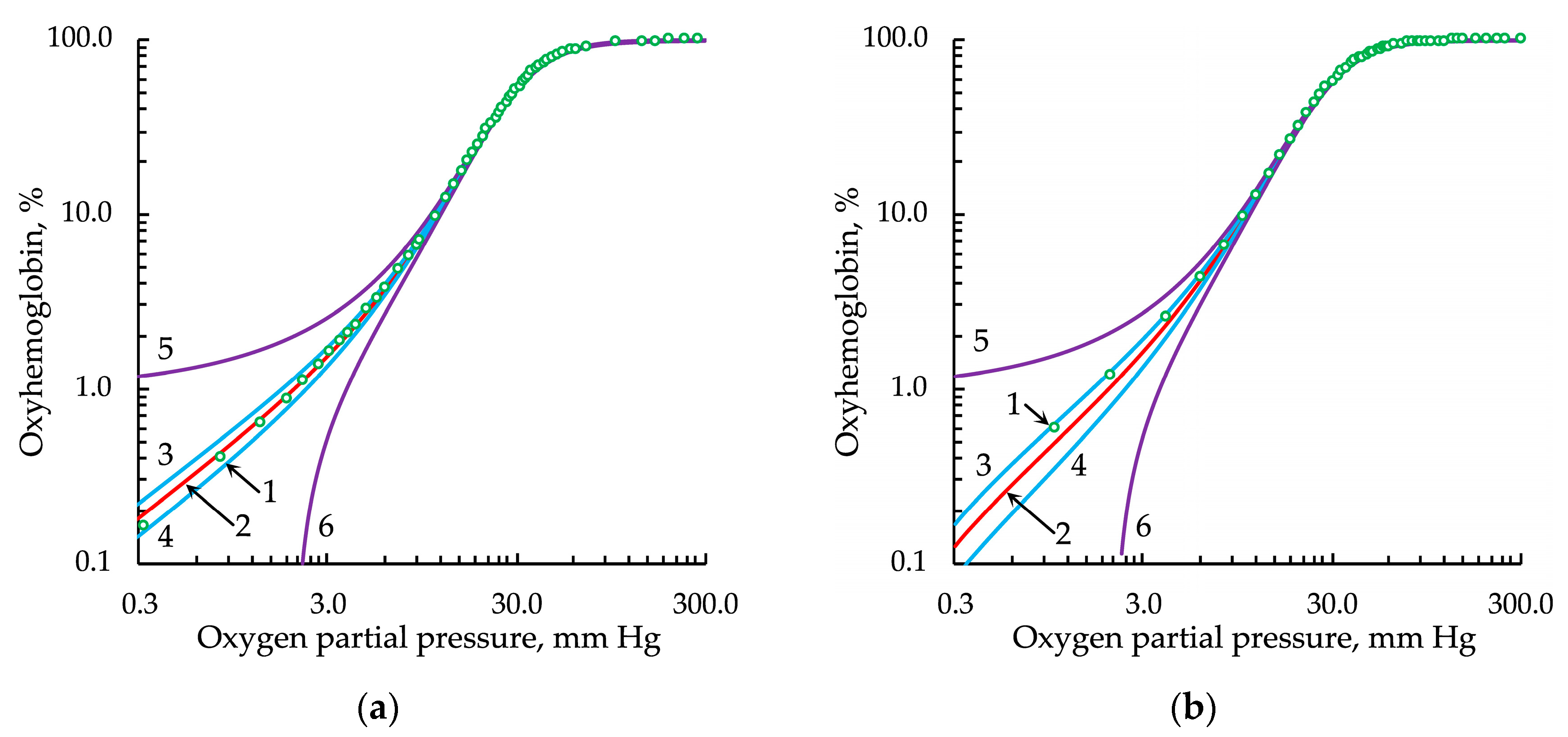
3.4. Evaluation of the Effectiveness of Approximation of Experimental Data by Modified Hill Equations
3.5. Fitting and Derived Parameters in the Modified Hill Equations
3.6. Calculation of Fitting and Derived Parameters in the Modified Hill Equations
4. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cui, Q.; Karplus, M. Allostery and cooperativity revisited. Protein Sci. 2008, 17, 1295–1307. [Google Scholar] [CrossRef]
- Lisi, G.P.; Loria, J.P. Solution NMR Spectroscopy for the Study of Enzyme Allostery. Chem. Rev. 2016, 116, 6323–6369. [Google Scholar] [CrossRef]
- Biddle, J.W.; Martinez-Corral, R.; Wong, F.; Gunawardena, J. Allosteric conformational ensembles have unlimited capacity for integrating information. eLife 2021, 10, e65498. [Google Scholar] [CrossRef]
- Aristov, V.V.; Buchelnikov, A.S.; Nechipurenko, Y.D. The Use of the Statistical Entropy in Some New Approaches for the Description of Biosystems. Entropy 2022, 24, 172. [Google Scholar] [CrossRef]
- Nikitin, E.S.; Malyshev, A.Y.; Balaban, P.M.; Volgushev, M.A. Physiological Aspects of the Use of the Hodgkin–Huxley Model of Action Potential Generation for Neurons in Invertebrates and Vertebrates. Neurosci. Behav. Physiol. 2017, 47, 751–757. [Google Scholar] [CrossRef]
- Agnati, L.F.; Guidolin, D.; Leo, G.; Fuxe, K. A boolean network modelling of receptor mosaics relevance of topology and cooperativity. J. Neural Transm. 2007, 114, 77–92. [Google Scholar] [CrossRef]
- Gruber, R.; Horovitz, A. Unpicking allosteric mechanisms of homo-oligomeric proteins by determining their successive ligand binding constants. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 20170176. [Google Scholar] [CrossRef]
- Pabis, A.; Risso, V.A.; Sanchez-Ruiz, J.M.; Kamerlin, S.C. Cooperativity and flexibility in enzyme evolution. Curr. Opin. Struct. Biol. 2018, 48, 83–92. [Google Scholar] [CrossRef]
- Bellelli, A. Hemoglobin and cooperativity: Experiments and theories. Curr. Protein Pept. Sci. 2010, 11, 2–36. [Google Scholar] [CrossRef]
- Brunori, M. Hemoglobin is an honorary enzyme. Trends Biochem. Sci. 1999, 24, 158–161. [Google Scholar] [CrossRef]
- Gell, D.A. Structure and function of haemoglobins. Blood Cells Mol. Dis. 2018, 70, 13–42. [Google Scholar] [CrossRef]
- Premont, R.T.; Singel, D.J.; Stamler, J.S. The enzymatic function of the honorary enzyme: S-nitrosylation of hemoglobin in physiology and medicine. Mol. Asp. Med. 2022, 84, 101056. [Google Scholar] [CrossRef]
- Perutz, M.F.; Rossmann, M.G.; Cullis, A.F.; Muirhead, H.; Will, G.; North, A.C. Structure of haemoglobin: A three-dimensional Fourier synthesis at 5.5-A. resolution, obtained by X-ray analysis. Nature 1960, 185, 416–422. [Google Scholar] [CrossRef]
- Weiss, J.N. The Hill equation revisited: Uses and misuses. FASEB J. 1997, 11, 835–841. [Google Scholar] [CrossRef]
- Prinz, H. Hill coefficients, dose–response curves and allosteric mechanisms. J. Chem. Biol. 2010, 3, 37–44. [Google Scholar] [CrossRef]
- Bounias, M. Algebraic potential of the Hill equation as an alternative tool for plotting dose (or time)/effects relationships in toxicology: A theoretical study. Fundam. Clin. Pharmacol. 1989, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gesztelyi, R.; Zsuga, J.; Kemeny-Beke, A.; Varga, B.; Juhasz, B.; Tosaki, A. The Hill equation and the origin of quantitative pharmacology. Arch. Hist. Exact Sci. 2012, 66, 427–438. [Google Scholar] [CrossRef]
- Santillán, M. On the Use of the Hill Functions in Mathematical Models of Gene Regulatory Networks. Math. Model. Nat. Phenom. 2008, 3, 85–97. [Google Scholar] [CrossRef]
- Shi, X. A Hill type equation can predict target gene expression driven by p53 pulsing. FEBS Open Bio. 2021, 11, 1799–1808. [Google Scholar] [CrossRef]
- Zimmerman, A.; Baylor, D. Cyclic GMP-sensitive conductance of retinal rods consists of aqueous pores. Nature 1986, 321, 70–72. [Google Scholar] [CrossRef]
- Ruiz, M.; Brown, R.L.; He, Y.; Haley, T.L.; Karpen, J.W. The Single-Channel Dose−Response Relation Is Consistently Steep for Rod Cyclic Nucleotide-Gated Channels: Implications for the Interpretation of Macroscopic Dose−Response Relations. Biochemistry 1999, 38, 10642–10648. [Google Scholar] [CrossRef] [PubMed]
- Guldberg, C.M.; Waage, P. Studies concerning affinity. CM Forh. Vidensk. Selsk. Christ. 1864, 35, 1864. [Google Scholar]
- van’t Hoff, J.H. Die grenzebene, ein beitrag zur kenntniss der esterbildung. Ber. Dtsch. Chem. Ges. 1877, 10, 669–678. [Google Scholar]
- McLean, F.C. Application of the law of chemical equilibrium (law of mass action) to biological problems. Physiol. Rev. 1938, 18, 495–523. [Google Scholar]
- Lavrinenko, I.A.; Vashanov, G.A.; Sulin, V.Y.; Nechipurenko, Y.D. An Analysis of Models of Cooperative Oxygen Binding by Hemoglobin. Biophysics 2021, 66, 905–912. [Google Scholar] [CrossRef]
- Lavrinenko, I.A.; Vashanov, G.A.; Nechipurenko, Y.D. New Mathematical Model to Describe Hemoglobin Oxygenation. Biophysics 2022, 67, 347–352. [Google Scholar] [CrossRef]
- Winslow, R.M.; Swenberg, M.L.; Berger, R.L.; Shrager, R.I.; Luzzana, M.; Samaja, M.; Rossi-Bernardi, L. Oxygen equilibrium curve of normal human blood and its evaluation by Adair’s equation. J. Biol. Chem. 1977, 252, 2331–2337. [Google Scholar] [CrossRef]
- Severinghaus, J.W. Simple, accurate equations for human blood O2 dissociation computations. J. Appl. Physiol. 1979, 46, 599–602. [Google Scholar] [CrossRef]
- Facó, J.L.D. A Generalized Reduced Gradient Algorithm for Solving Large-Scale Discrete-Time Nonlinear Optimal Control Problems. IFAC Proc. Vol. 1989, 22, 45–50. [Google Scholar] [CrossRef]
- Dekking, M.; Kraaikamp, C.; Lopuhaä, H.P.; Meester, L.E. A Modern Introduction to Probability and Statistics: Understanding Why and How; Springer: London, UK, 2005; p. xv. 486p. [Google Scholar]
- Katz, M.H. Multivariable Analysis: A Practical Guide for Clinicians and Public Health Researchers, 3rd ed.; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Härdle, W.; Müller, M.; Sperlich, S.; Werwatz, A. Nonparametric and Semiparametric Models; Springer: Berlin/Heidelberg, Germany, 2004; Volume 1. [Google Scholar]
- Lavrinenko, I.A.; Vashanov, G.A.; Buchelnikov, A.S.; Nechipurenko, Y.D. Cooperative Oxygen Binding with Hemoglobin as a General Model in Molecular Biophysics. Biophysics 2022, 67, 327–337. [Google Scholar] [CrossRef]
- Coval, M.L. Analysis of Hill interaction coefficients and the invalidity of the Kwon and Brown equation. J. Biol. Chem. 1970, 245, 6335–6336. [Google Scholar]
- Hofmeyr, J.-H.S.; Cornish-Bowden, H. The reversible Hill equation: How to incorporate cooperative enzymes into metabolic models. Bioinformatics 1997, 13, 377–385. [Google Scholar]
- Goutelle, S.; Maurin, M.; Rougier, F.; Barbaut, X.; Bourguignon, L.; Ducher, M.; Maire, P. The Hill equation: A review of its capabilities in pharmacological modelling. Fundam. Clin. Pharmacol. 2008, 22, 633–648. [Google Scholar] [CrossRef] [PubMed]
- Cantor, C.R.; Schimmel, P.R. Biophysical Chemistry: Part III: The Behavior of Biological Macromolecules; WH Freeman: New York, NY, USA, 1980. [Google Scholar]
- Tyuma, I.; Imai, K.; Shimizu, K. Analysis of oxygen equilibrium of hemoglobin and control mechanism of organic phosphates. Biochemistry 1973, 12, 1491–1498. [Google Scholar] [CrossRef] [PubMed]
- Tanford, C. Physical Chemistry of Macromolecules; Wiley: Hoboken, NJ, USA, 1961. [Google Scholar]
- Podrabinek, P.A.; Kamenskii, I.I. Relation between the oxygenation of hemoglobin and its molecular changes. Biofizika 1967, 12, 983–988. [Google Scholar] [PubMed]
- Nechipurenko, Y.D. Analysis of binding of ligands to nucleic acids. Biophysics 2014, 59, 6–27. [Google Scholar] [CrossRef]
- Konkoli, Z. Safe uses of Hill’s model: An exact comparison with the Adair-Klotz model. Theor. Biol. Med. Model. 2011, 8, 10. [Google Scholar] [CrossRef]
- Purich, D.L.; Allison, R.D. Handbook of Biochemical Kinetics: A Guide to Dynamic Processes in the Molecular Life Sciences; Elsevier: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Ingalls, B.P. Mathematical Modeling in Systems Biology: An Introduction; MIT press: Cambridge, MA, USA, 2013. [Google Scholar]
- Chang, J.B.; Quinnies, K.M.; Realubit, R.; Karan, C.; Rand, J.H.; Tatonetti, N.P. A novel, rapid method to compare the therapeutic windows of oral anticoagulants using the Hill coefficient. Sci. Rep. 2016, 6, 29387. [Google Scholar] [CrossRef]
- Hüfner, G. Über die Bedeutung der in der vorigen Abhandlung vorgethangenen Lehre für die Spectroskopie und Photometrie des Blutes. Arch. Physiol 1890, 31, 28–30. [Google Scholar]
- Hill, A.V. The possible effects of the aggregation of the molecules of hæmoglobin on its dissociation curves. J. Physiol. 1910, 40, i–vii. [Google Scholar]
- Michaelis, L.; Menten, M.L.; Johnson, K.A.; Goody, R.S. The original Michaelis constant: Translation of the 1913 Michaelis-Menten paper. Biochemistry 2011, 50, 8264–8269. [Google Scholar] [CrossRef] [PubMed]
- Sebaugh, J.L. Guidelines for accurate EC50/IC50 estimation. Pharm. Stat. 2011, 10, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Lavrinenko, I.A.; Vashanov, G.A.; Nechipurenko, Y.D. New Interpretation of the Hill Coefficient. Biophysics 2022, 67, 171–174. [Google Scholar] [CrossRef]
- Ilgenfritz, G.; Schuster, T.M. Kinetics of Oxygen Binding to Human Hemoglobin: Temperature Jump Relaxation Studies. J. Biol. Chem. 1974, 249, 2959–2973. [Google Scholar] [CrossRef] [PubMed]
- Galanello, R.; Cao, A. Alpha-thalassemia. Genet. Med. 2011, 13, 83–88. [Google Scholar] [CrossRef]
- Bordbar, A.K.; Saadati, Z.; Sohrabi, N. Analysis of ligand binding process using binding capacity concept. Acta Biochim. Pol. 2004, 51, 963–970. [Google Scholar] [CrossRef]
- Li, J.; Zhu, X.; Byrnes, M.; Nelson, J.W.; Chang, S.H. Site-directed mutagenesis of rabbit muscle phosphofructokinase cDNA. Mutations at glutamine 200 affect the allosteric properties of the enzyme. J. Biol. Chem. 1993, 268, 24599–24606. [Google Scholar]
- Aramaki, H.; Kabata, H.; Takeda, S.; Itou, H.; Nakayama, H.; Shimamoto, N. Formation of repressor-inducer-operator ternary complex: Negative cooperativity of d-camphor binding to CamR. Genes Cells 2011, 16, 1200–1207. [Google Scholar] [CrossRef]
- Ding, S.; Sachs, F. Single channel properties of P2X2 purinoceptors. J. Gen. Physiol. 1999, 113, 695–720. [Google Scholar] [CrossRef]
- Srinivasan, S.; Waghu, F.H.; Idicula-Thomas, S.; Venkatesh, K.V. A steady-state modeling approach for simulation of antimicrobial peptide-cell membrane interaction. Biochim. Biophys. Acta (BBA)-Biomembr. 2020, 1862, 183242. [Google Scholar] [CrossRef]
- Monod, J.; Wyman, J.; Changeux, J.-P. On the nature of allosteric transitions: A plausible model. J. Mol. Biol. 1965, 12, 88–118. [Google Scholar] [CrossRef]
- Koshland, D.E., Jr.; Némethy, G.; Filmer, D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry 1966, 5, 365–385. [Google Scholar]
- Eaton, W.A.; Henry, E.R.; Hofrichter, J.; Bettati, S.; Viappiani, C.; Mozzarelli, A. Evolution of allosteric models for hemoglobin. IUBMB Life 2007, 59, 586–599. [Google Scholar] [CrossRef]
- Henry, E.R.; Mozzarelli, A.; Viappiani, C.; Abbruzzetti, S.; Bettati, S.; Ronda, L.; Bruno, S.; Eaton, W.A. Experiments on Hemoglobin in Single Crystals and Silica Gels Distinguish among Allosteric Models. Biophys. J. 2015, 109, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Nagatomo, S.; Nagai, Y.; Aki, Y.; Sakurai, H.; Imai, K.; Mizusawa, N.; Ogura, T.; Kitagawa, T.; Nagai, M. An Origin of Cooperative Oxygen Binding of Human Adult Hemoglobin: Different Roles of the α and β Subunits in the α2β2 Tetramer. PLoS ONE 2015, 10, e0135080. [Google Scholar] [CrossRef]
- Henry, E.R.; Harper, J.; Glass, K.E.; Metaferia, B.; Louis, J.M.; Eaton, W.A. MWC allosteric model explains unusual hemoglobin-oxygen binding curves from sickle cell drug binding. Biophys. J. 2021, 120, 2543–2551. [Google Scholar] [CrossRef]
- Horovitz, A.; Mondal, T. Discriminating between Concerted and Sequential Allosteric Mechanisms by Comparing Equilibrium and Kinetic Hill Coefficients. J. Phys. Chem. B 2021, 125, 70–73. [Google Scholar] [CrossRef]


| Equation | r2 |
|---|---|
| Hill/L | 0.999962 |
| Adair | 0.999953 |
| Hill/G | 0.999944 |
| Hill classic | 0.999603 |
| Equation | r2 |
|---|---|
| Hill/L | 0.999951 |
| Hill/G | 0.999943 |
| Adair | 0.999907 |
| Hill classic | 0.999446 |
| No. | Equation Parameters | Hill/G Equation | Hill/L Equation |
|---|---|---|---|
| 1 | p50, mm Hg | 29.08 | 29.11 |
| 2 | 2.30 | 2.30 | |
| 3 | hmax(−1) | 1.66 | 1.68 |
| 4 | hmax (hmax(−1) + 1) | 2.66 | 2.68 |
| 5 | ∆h (hmax − ) | 0.36 | 0.38 |
| 6 | pmax, mm Hg | 52.92 | 47.82 |
| 7 | sg | 3.33 | — |
| 8 | sl | — | 1.80 |
| 9 | pO2low (for ω = 0.99), mm Hg | 37.92 | 36.36 |
| 10 | pO2high (for ω = 0.99), mm Hg | 73.87 | 62.89 |
| 11 | ∆pO2 (pO2high − pO2low), mm Hg | 35.95 | 26.53 |
| 12 | HbO2, % (for pmax) | 83.12 | 79.09 |
| No. | Equation Parameters | Hill/G Equation | Hill/L Equation |
|---|---|---|---|
| 1 | p50, mm Hg | 26.82 | 26.86 |
| 2 | 2.62 | 2.60 | |
| 3 | hmax(−1) | 1.82 | 1.82 |
| 4 | hmax (hmax(−1) + 1) | 2.82 | 2.82 |
| 5 | ∆h (hmax − ) | 0.20 | 0.22 |
| 6 | pmax, mm Hg | 80.85 | 67.28 |
| 7 | sg | 3.59 | — |
| 8 | sl | — | 1.91 |
| 9 | pO2low (for ω = 0.99), mm Hg | 56.42 | 50.25 |
| 10 | pO2high (for ω = 0.99), mm Hg | 115.85 | 90.08 |
| 11 | ∆pO2 (pO2high − pO2low), mm Hg | 59.43 | 39.83 |
| 12 | HbO2, % (for pmax) | 95.74 | 93.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lavrinenko, I.A.; Vashanov, G.A.; Hernández Cáceres, J.L.; Buchelnikov, A.S.; Nechipurenko, Y.D. A New Model of Hemoglobin Oxygenation. Entropy 2022, 24, 1214. https://doi.org/10.3390/e24091214
Lavrinenko IA, Vashanov GA, Hernández Cáceres JL, Buchelnikov AS, Nechipurenko YD. A New Model of Hemoglobin Oxygenation. Entropy. 2022; 24(9):1214. https://doi.org/10.3390/e24091214
Chicago/Turabian StyleLavrinenko, Igor A., Gennady A. Vashanov, José L. Hernández Cáceres, Anatoly S. Buchelnikov, and Yury D. Nechipurenko. 2022. "A New Model of Hemoglobin Oxygenation" Entropy 24, no. 9: 1214. https://doi.org/10.3390/e24091214
APA StyleLavrinenko, I. A., Vashanov, G. A., Hernández Cáceres, J. L., Buchelnikov, A. S., & Nechipurenko, Y. D. (2022). A New Model of Hemoglobin Oxygenation. Entropy, 24(9), 1214. https://doi.org/10.3390/e24091214








