Pressure Injury Link to Entropy of Abdominal Temperature
Abstract
1. Introduction
2. Methods
2.1. Study Design and Data Collection
2.2. Primary Measures
2.3. Entropy Measures
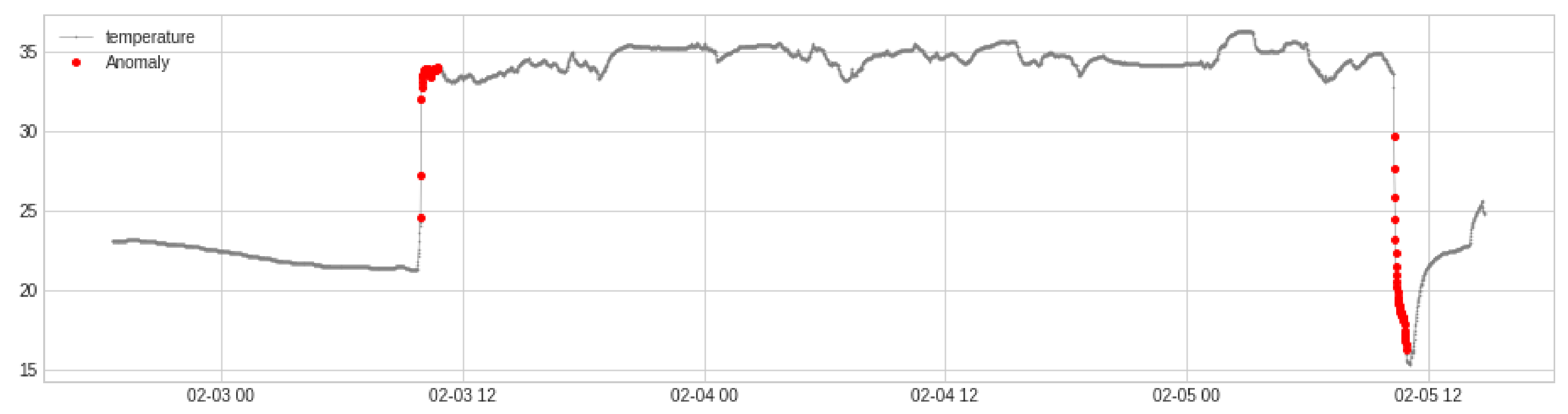
2.4. Temperature Time Series
2.5. Data Analysis and Models
2.5.1. Summary Measures of Multiscale Entropy
2.5.2. Simple Bivariate Models
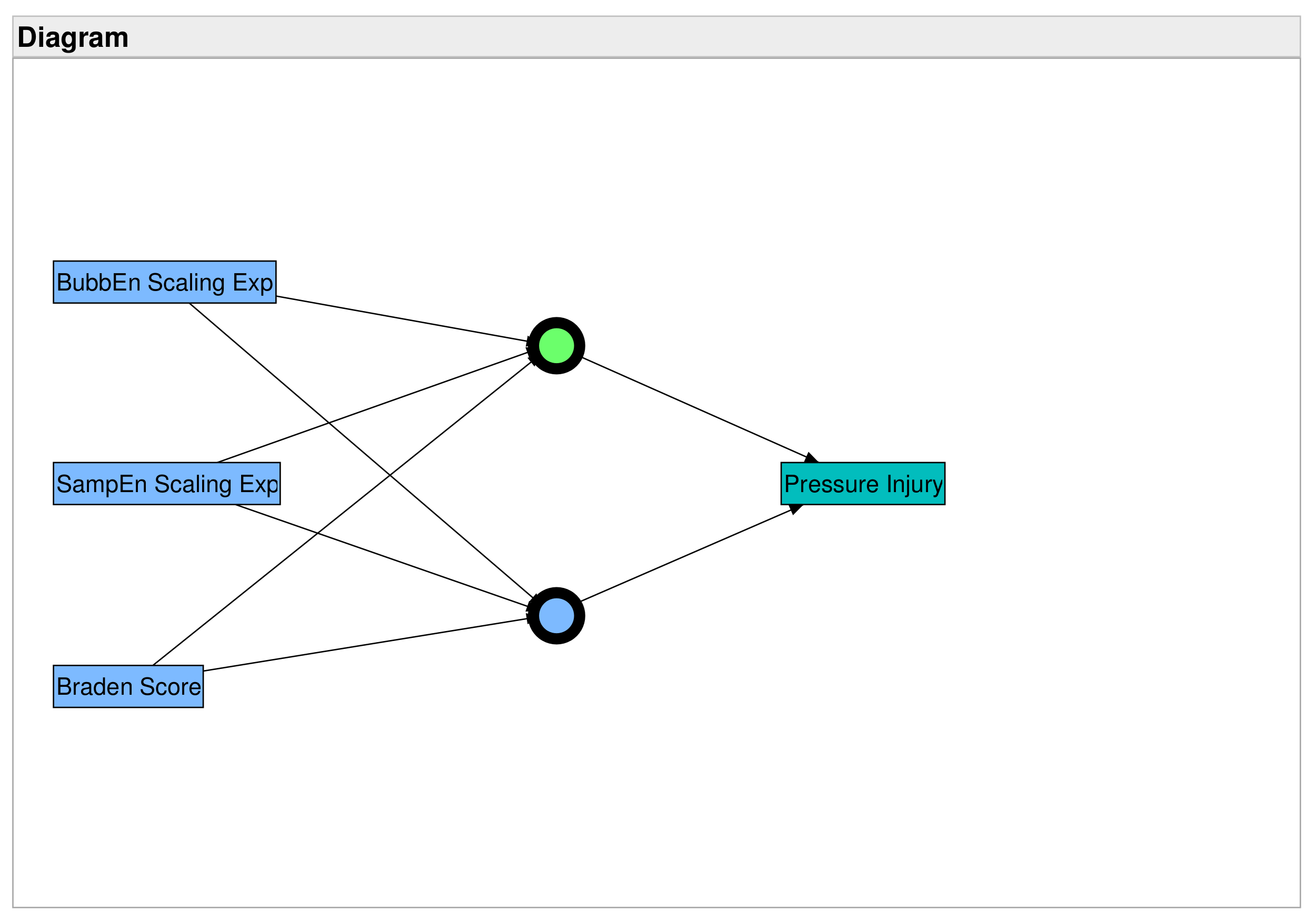
2.5.3. Predictive Models
3. Results
3.1. Sample Description
3.2. Multiscale Entropy and Pressure Injuries
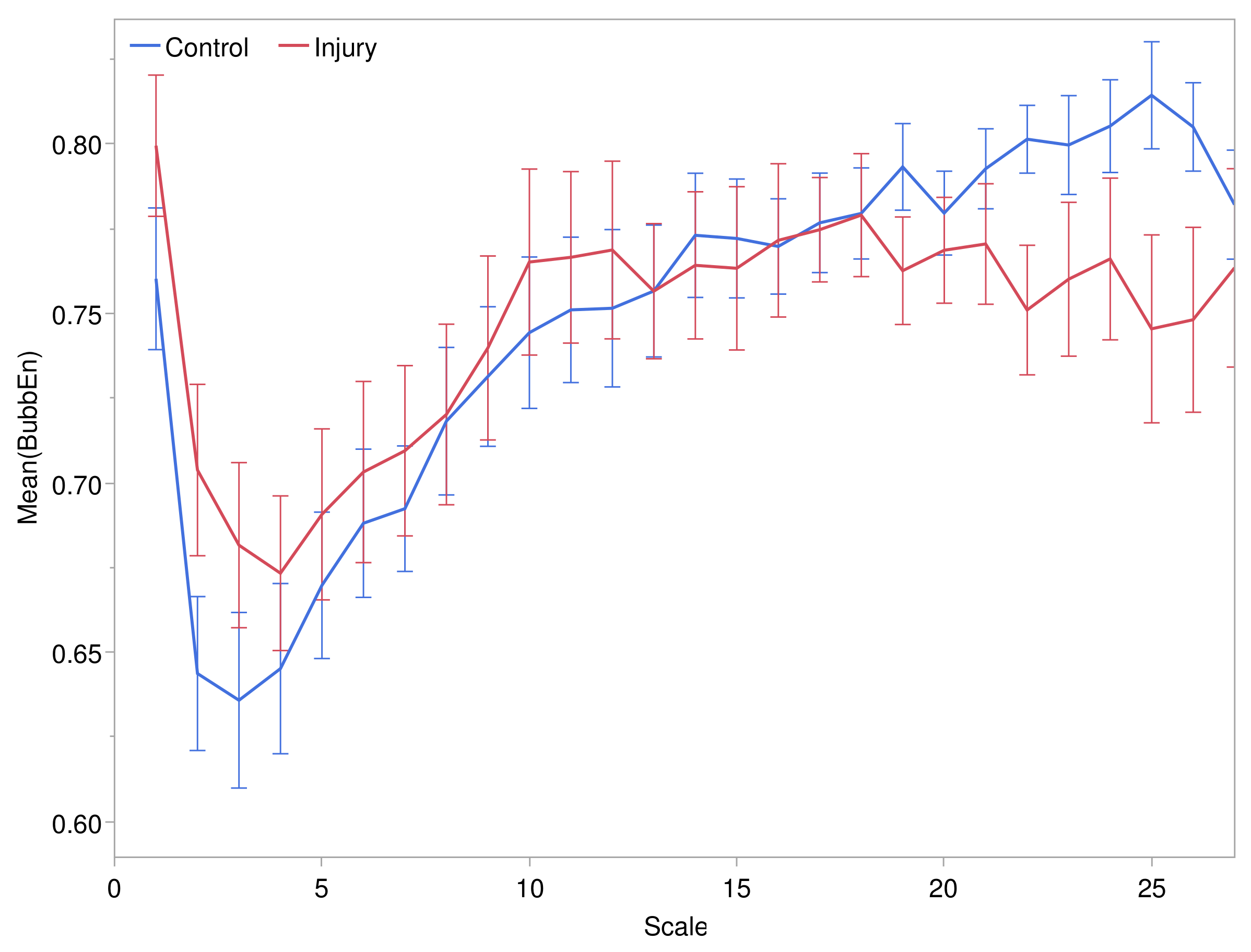
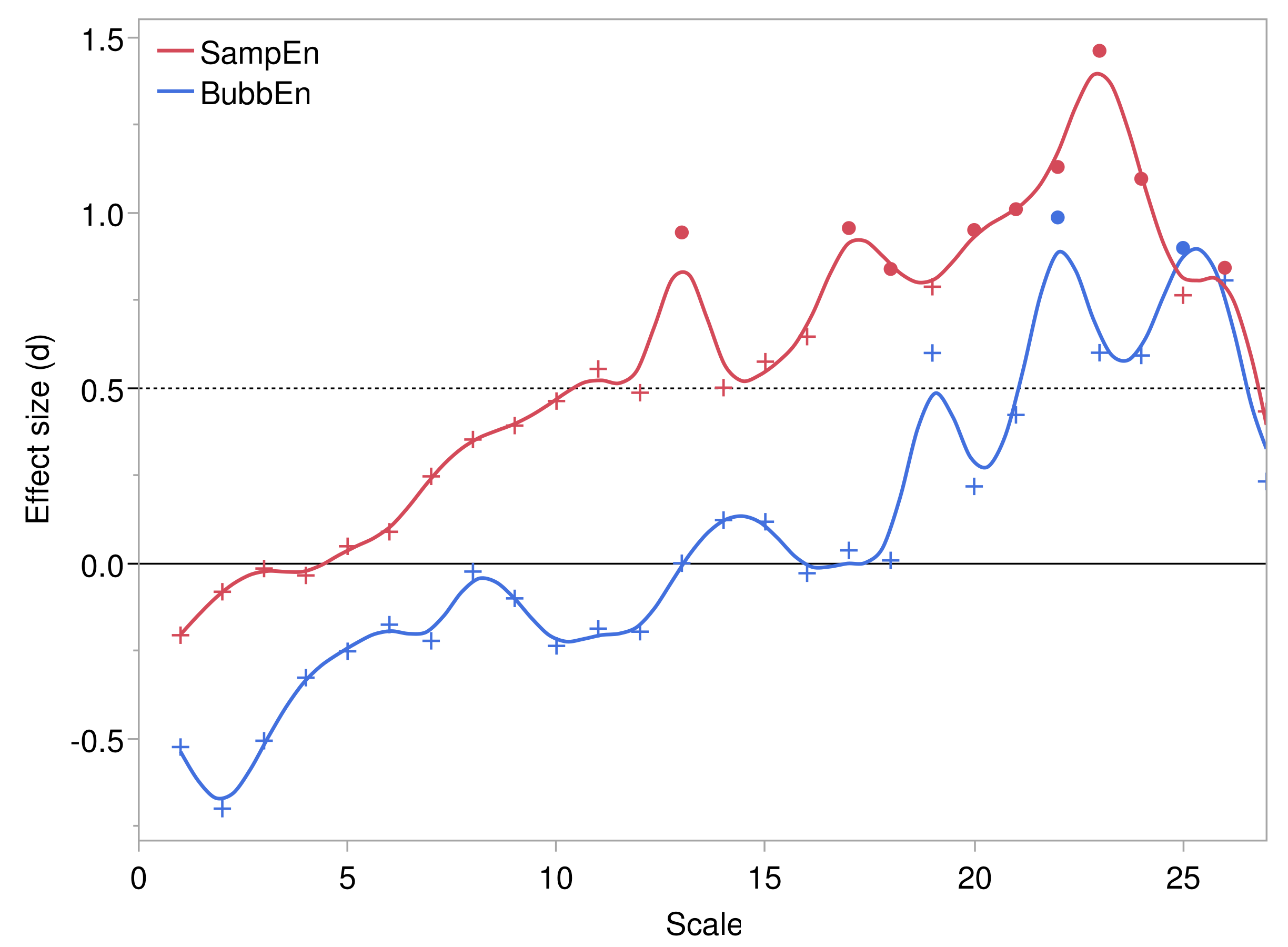
3.2.1. Scale Structure of Entropies
3.2.2. Comparison of Entropies by Pressure Injury Group
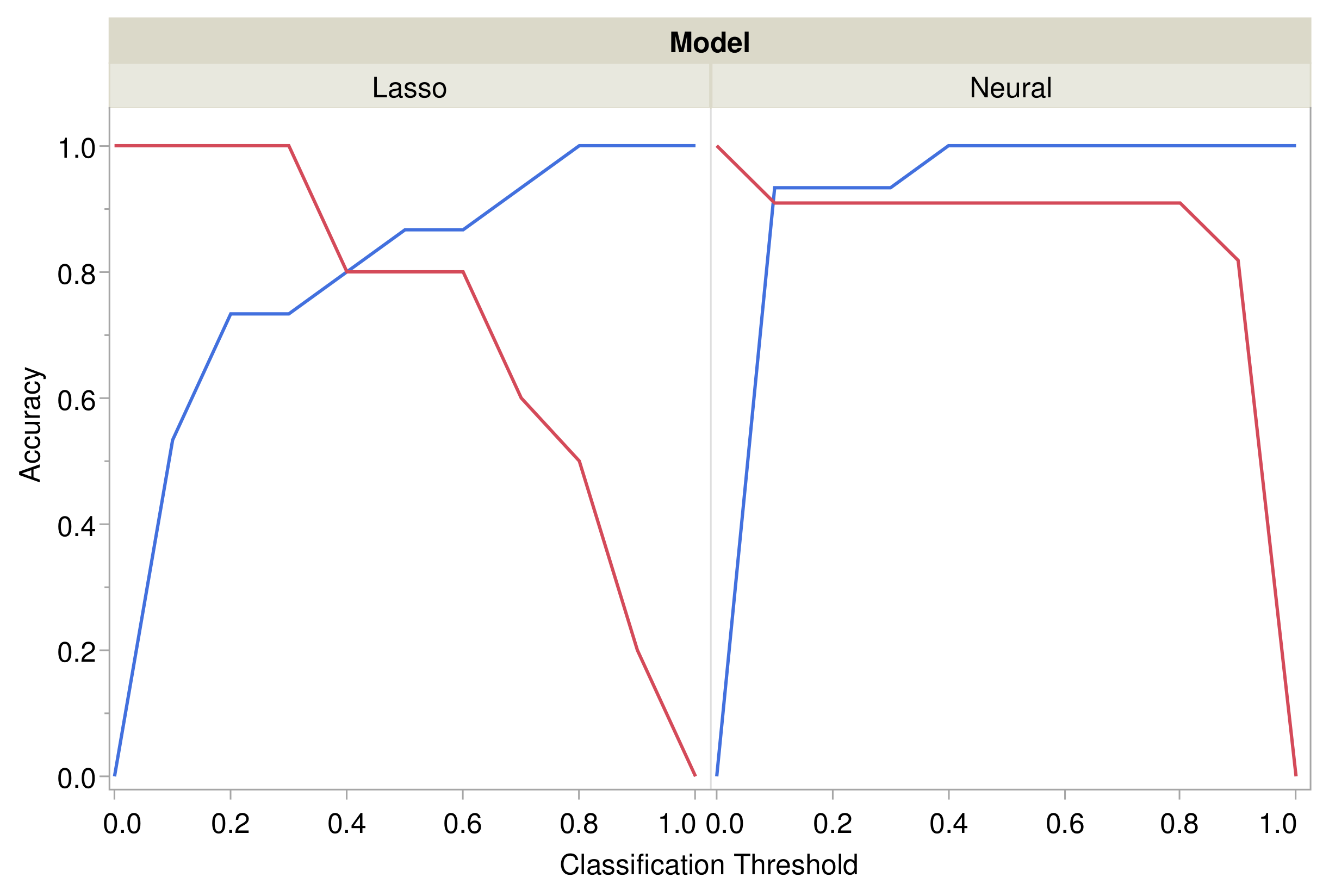
3.3. Prediction of Pressure Injuries
3.3.1. Generalized Regression Models
3.3.2. Neural Models
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SampEn | Sample Entropy |
| BubbEn | Bubble Entropy |
| AUC | Area Under Curve |
| ROC | Receiver Operating Characteristic (Curve) |
| AUROC | Area Under ROC Curve |
| IQR | Interquartile range |
Appendix A
References
- Kosiak, M. Etiology and pathology of ischemic ulcers. Arch. Phys. Med. Rehabil. 1959, 40, 62–69. [Google Scholar] [PubMed]
- Braden, B.; Bergstrom, N. A conceptual schema for the study of the etiology of pressure sores. Rehabil. Nurs. 1987, 12, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Grey, J.E.; Harding, K.G.; Enoch, S. Pressure ulcers. BMJ 2006, 332, 472–475. [Google Scholar] [CrossRef] [PubMed]
- Gefen, A.; Brienza, D.M.; Cuddigan, J.; Haesler, E.; Kottner, J. Our contemporary understanding of the aetiology of pressure ulcers/pressure injuries. Int. Wound J. 2021, 19, 692–704. [Google Scholar] [CrossRef]
- Blair, M.J.; Jones, J.D.; Woessner, A.E.; Quinn, K.P. Skin Structure–Function Relationships and the Wound Healing Response to Intrinsic Aging. Adv. Wound Care 2020, 9, 127. [Google Scholar] [CrossRef]
- Kottner, J.; Black, J.; Call, E.; Gefen, A.; Santamaria, N. Microclimate: A critical review in the context of pressure ulcer prevention. Clin. Biomech. 2018, 59, 62–70. [Google Scholar] [CrossRef]
- Sun, P.C.; Jao, S.H.E.; Cheng, C.K. Assessing foot temperature using infrared thermography. Foot Ankle Int. 2005, 26, 847–853. [Google Scholar] [CrossRef]
- Kelechi, T.J.; Michel, Y. A Descriptive Study of Skin Temperature, Tissue Perfusion, and Tissue Oxygen in Patients With Chronic Venous Disease. Biol. Res. Nurs. 2007, 9, 70–80. [Google Scholar] [CrossRef]
- Sayre, E.; Kelechi, T.; Neal, D. Sudden increase in skin temperature predicts venous ulcers: A case study. J. Vasc. Nurs. Off. Publ. Soc. Peripher. Vasc. Nurs. 2007, 25, 46–50. [Google Scholar] [CrossRef]
- Stern, M.D.; Lappe, D.L.; Bowen, P.D.; Chimosky, J.E.; Holloway, G., Jr.; Keiser, H.; Bowman, R. Continuous measurement of tissue blood flow by laser-Doppler spectroscopy. Am. J. Physiol.-Heart Circ. Physiol. 1977, 232, H441–H448. [Google Scholar] [CrossRef]
- Ratheesh, K.M.; Seah, L.K.; Murukeshan, V.M. Spectral phase-based automatic calibration scheme for swept source-based optical coherence tomography systems. Phys. Med. Biol. 2016, 61, 7652–7663. [Google Scholar] [CrossRef] [PubMed]
- Meleppat, R.K.; Miller, E.B.; Manna, S.K.; Zhang, P.; Pugh, E.N., Jr.; Zawadzki, R.J. Multiscale Hessian filtering for enhancement of OCT angiography images. In Proceedings of the Ophthalmic Technologies XXIX, San Francisco, CA, USA, 2–3 February 2019; SPIE: Bellingham, WA, USA, 2019; Volume 10858, pp. 64–70. [Google Scholar] [CrossRef]
- Ioannou, S.; Gallese, V.; Merla, A. Thermal infrared imaging in psychophysiology: Potentialities and limits. Psychophysiology 2014, 51, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Ring, E.F.J.; Ammer, K. Infrared thermal imaging in medicine. Physiol. Meas. 2012, 33, R33–R46. [Google Scholar] [CrossRef] [PubMed]
- Grayson, J. Responses of the microcirculation to hot and cold environments. Pharmacol. Ther. 1988, 38, 201–214. [Google Scholar] [CrossRef]
- Lipsitz, L.A. Dynamics of stability: The physiologic basis of functional health and frailty. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, B115–B125. [Google Scholar] [CrossRef] [PubMed]
- Goldberger, A.L.; Peng, C.K.; Lipsitz, L.A. What is physiologic complexity and how does it change with aging and disease? Neurobiol. Aging 2002, 23, 23–26. [Google Scholar] [CrossRef]
- Goldberger, A.L.; Amaral, L.A.N.; Hausdorff, J.M.; Ivanov, P.C.; Peng, C.K.; Stanley, H.E. Fractal dynamics in physiology: Alterations with disease and aging. Proc. Natl. Acad. Sci. USA 2002, 99, 2466–2472. [Google Scholar] [CrossRef]
- Langemo, D.K.; Brown, G. Skin fails too: Acute, chronic, and end-stage skin failure. Adv. Skin Wound Care 2006, 19, 206–211. [Google Scholar] [CrossRef]
- Meijer, J.H.; Schut, G.L.; Ribbe, M.W.; Goovaerts, H.G.; Nieuwenhuys, R.; Reulen, J.P.; Schneider, H. Method for the measurement of susceptibility to decubitus ulcer formation. Med. Biol. Eng. Comput. 1989, 27, 502–506. [Google Scholar] [CrossRef]
- Van Marum, R.J.; Meijer, J.H.; Ribbe, M.W. The relationship between pressure ulcers and skin blood flow response after a local cold provocation. Arch. Phys. Med. Rehabil. 2002, 83, 40–43. [Google Scholar] [CrossRef]
- Liao, F.; Yang, T.D.; Wu, F.L.; Cao, C.; Mohamed, A.; Jan, Y.K. Using Multiscale Entropy to Assess the Efficacy of Local Cooling on Reactive Hyperemia in People with a Spinal Cord Injury. Entropy 2019, 21, 90. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Goldberger, A.L.; Peng, C.K. Multiscale entropy analysis of complex physiologic time series. Phys. Rev. Lett. 2002, 89, 068102. [Google Scholar] [CrossRef] [PubMed]
- Porta, A.; Bari, V.; Ranuzzi, G.; De Maria, B.; Baselli, G. Assessing multiscale complexity of short heart rate variability series through a model-based linear approach. Chaos 2017, 27, 093901. [Google Scholar] [CrossRef] [PubMed]
- Rapp, M.P.; Bergstrom, N.; Padhye, N.S. Contribution of skin temperature regularity to the risk of developing pressure ulcers in nursing facility residents. Adv. Skin Wound Care 2009, 22, 506–513. [Google Scholar] [CrossRef]
- Bergstrom, N.; Horn, S.D.; Rapp, M.P.; Stern, A.; Barrett, R.; Watkiss, M. Turning for Ulcer ReductioN: A Multisite Randomized Clinical Trial in Nursing Homes. J. Am. Geriatr. Soc. 2013, 61, 1705–1713. [Google Scholar] [CrossRef]
- Padhye, N.S.; Bergstrom, N.; Rapp, M.P.; Etcher, L.; Redeker, N.S. Pressure ulcer risk detection from complexity of activity. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju, Korea, 11–15 July 2017; Volume 2017, pp. 2304–2307. [Google Scholar] [CrossRef]
- Da Rosa Silva, C.F.; Santana, R.F.; de Oliveira, B.G.R.B.; do Carmo, T.G. High prevalence of skin and wound care of hospitalized elderly in Brazil: A prospective observational study. BMC Res. Notes 2017, 10, 81. [Google Scholar] [CrossRef][Green Version]
- Bauer, K.; Rock, K.; Nazzal, M.; Jones, O.; Qu, W. Pressure Ulcers in the United States’ Inpatient Population From 2008 to 2012: Results of a Retrospective Nationwide Study. Ostomy Wound Manag. 2016, 62, 30–38. [Google Scholar]
- Demarré, L.; Verhaeghe, S.; Annemans, L.; Van Hecke, A.; Grypdonck, M.; Beeckman, D. The cost of pressure ulcer prevention and treatment in hospitals and nursing homes in Flanders: A cost-of-illness study. Int. J. Nurs. Stud. 2015, 52, 1166–1179. [Google Scholar] [CrossRef]
- Paulden, M.; Bergstrom, N.; Horn, S.; Rapp, M.; Stern, A.; Barrett, R.S.; Watkiss, M.; Krahn, M. Turning for Ulcer Reduction (TURN) Study: An Economic Analysis. Ont. Health Technol. Assess. Ser. 2014, 14, 1. [Google Scholar]
- Bergstrom, N.; Braden, B.J.; Laguzza, A.; Holman, V. The Braden Scale for Predicting Pressure Sore Risk. Nurs. Res. 1987, 36, 205–210. [Google Scholar] [CrossRef]
- Valencia, J.F.; Porta, A.; Vallverdu, M.; Claria, F.; Baranowski, R.; Orlowska-Baranowska, E.; Caminal, P. Refined Multiscale Entropy: Application to 24-h Holter Recordings of Heart Period Variability in Healthy and Aortic Stenosis Subjects. IEEE Trans. Biomed. Eng. 2009, 56, 2202–2213. [Google Scholar] [CrossRef]
- Manis, G.; Aktaruzzaman, M.; Sassi, R. Bubble Entropy: An Entropy Almost Free of Parameters. IEEE Trans. Biomed. Eng. 2017, 64, 2711–2718. [Google Scholar] [CrossRef]
- Richman, J.S.; Moorman, J.R. Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H2039–H2049. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Kring, D.L. Reliability and validity of the Braden Scale for predicting pressure ulcer risk. J. Wound Ostomy Cont. Nurs. 2007, 34, 399–406. [Google Scholar] [CrossRef]
- NPUAP Pressure Injury Stages|The National Pressure Ulcer Advisory Panel—NPUAP. Available online: https://npiap.com/page/PressureInjuryStages (accessed on 31 May 2017).
- Flood, M.W.; Grimm, B. EntropyHub: An open-source toolkit for entropic time series analysis. PLoS ONE 2021, 16, e0259448. [Google Scholar] [CrossRef]
- Ribeiro, M.; Henriques, T.; Castro, L.; Souto, A.; Antunes, L.; Costa-Santos, C.; Teixeira, A. The Entropy Universe. Entropy 2021, 23, 222. [Google Scholar] [CrossRef]
- Bandt, C.; Pompe, B. Permutation entropy: A natural complexity measure for time series. Phys. Rev. Lett. 2002, 88, 174102. [Google Scholar] [CrossRef]
- Cuesta-Frau, D.; Miró-Martínez, P.; Oltra-Crespo, S.; Jordán-Núñez, J.; Vargas, B.; González, P.; Varela-Entrecanales, M. Model Selection for Body Temperature Signal Classification Using Both Amplitude and Ordinality-Based Entropy Measures. Entropy 2018, 20, 853. [Google Scholar] [CrossRef]
- Arundo Analytics. ADTK (version 0.6.2). 2020. Available online: https://github.com/arundo/adtk (accessed on 1 December 2021).
- Magagnin, V.; Bassani, T.; Bari, V.; Turiel, M.; Maestri, R.; Pinna, G.D.; Porta, A. Non-stationarities significantly distort short-term spectral, symbolic and entropy heart rate variability indices. Physiol. Meas. 2011, 32, 1775–1786. [Google Scholar] [CrossRef]
- MacKinnon, J.G. Critical Values for Cointegration Tests; Technical Report 1227, Publication Title: Working Paper; Economics Department, Queen’s University: Kingston, ON, Canada, 2010. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio, PBC: Boston, MA, USA, 2022. [Google Scholar]
- JMP 15 Fittting Linear Models; SAS Institute Inc.: Cary, NC, USA, 2020.
- Tibshirani, R. Regression Shrinkage and Selection Via the Lasso. J. R. Stat. Soc. Ser. B Methodol. 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Zou, H. The Adaptive Lasso and Its Oracle Properties. J. Am. Stat. Assoc. 2012, 101, 1418–1429. [Google Scholar] [CrossRef]
- JMP 15 Predictive and Specialized Modeling; SAS Institute Inc.: Cary, NC, USA, 2020.
- Porta, A.; Bari, V.; Marchi, A.; Maria, B.D.; Castiglioni, P.; Rienzo, M.d.; Guzzetti, S.; Cividjian, A.; Quintin, L. Limits of permutation-based entropies in assessing complexity of short heart period variability. Physiol. Meas. 2015, 36, 755–765. [Google Scholar] [CrossRef]
- Stefanovska, A.; Bracic, M.; Kvernmo, H. Wavelet analysis of oscillations in the peripheral blood circulation measured by laser Doppler technique. IEEE Trans. Biomed. Eng. 1999, 46, 1230–1239. [Google Scholar] [CrossRef]
- Porta, A.; Bari, V.; Maria, B.D.; Cairo, B.; Vaini, E.; Malacarne, M.; Pagani, M.; Lucini, D. Peripheral Resistance Baroreflex During Incremental Bicycle Ergometer Exercise: Characterization and Correlation With Cardiac Baroreflex. Front. Physiol. 2018, 9, 688. [Google Scholar] [CrossRef]
- Pagani, M.; Montano, N.; Porta, A.; Malliani, A.; Abboud, F.M.; Birkett, C.; Somers, V.K. Relationship Between Spectral Components of Cardiovascular Variabilities and Direct Measures of Muscle Sympathetic Nerve Activity in Humans. Circulation 1997, 95, 1441–1448. [Google Scholar] [CrossRef]
- Hodges, G.J.; Mallette, M.M.; Tew, G.A.; Saxton, J.M.; Moss, J.; Ruddock, A.D.; Klonizakis, M. Effect of age on cutaneous vasomotor responses during local skin heating. Microvasc. Res. 2017, 112, 47–52. [Google Scholar] [CrossRef]
- Mufti, A.; Maliyar, K.; Syed, M.; Pagnoux, C.; Alavi, A. Approaches to Microthrombotic Wounds: A Review of Pathogenesis and Clinical Features. Adv. Skin Wound Care 2020, 33, 68–75. [Google Scholar] [CrossRef]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental Algorithms for Scientific Computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef]
- Kanazawa, T.; Kitamura, A.; Nakagami, G.; Goto, T.; Miyagaki, T.; Hayashi, A.; Sasaki, S.; Mugita, Y.; Iizaka, S.; Sanada, H. Lower temperature at the wound edge detected by thermography predicts undermining development in pressure ulcers: A pilot study. Int. Wound J. 2016, 13, 454–460. [Google Scholar] [CrossRef]
- Bennett, S.L.; Goubran, R.; Knoefel, F. Long term monitoring of a pressure ulcer risk patient using thermal images. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju, Korea, 11–15 July 2017. [Google Scholar]
- Jiang, X.; Hou, X.; Dong, N.; Deng, H.; Wang, Y.; Ling, X.; Guo, H.; Zhang, L.; Cai, F. Skin temperature and vascular attributes as early warning signs of pressure injury. J. Tissue Viability 2020, 29, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, X.; Yu, K.; Shi, F.; Qin, L.; Zhou, H.; Cai, F. Infrared Thermal Images Classification for Pressure Injury Prevention Incorporating the Convolutional Neural Networks. IEEE Access 2021, 9, 15181–15190. [Google Scholar] [CrossRef]
- Polak, A.; Kucio, C.; Kloth, L.C.; Paczula, M.; Hordynska, E.; Ickowicz, T.; Blaszczak, E.; Kucio, E.; Oleszczyk, K.; Ficek, K.; et al. A Randomized, Controlled Clinical Study to Assess the Effect of Anodal and Cathodal Electrical Stimulation on Periwound Skin Blood Flow and Pressure Ulcer Size Reduction in Persons with Neurological Injuries. Ostomy Wound Manag. 2018, 64, 10–29. [Google Scholar] [CrossRef]

| Characteristic | Control 1 | Pressure Injury 1 | Total 1 |
|---|---|---|---|
| () | () | () | |
| Age (y) | 78 (66, 83) | 74 (68, 82) | 75 (67, 82) |
| Sex: | |||
| Male | 7 (44%) | 8 (67%) | 15 (54%) |
| Female | 9 (56%) | 4 (33%) | 13 (46%) |
| Race: | |||
| Black | 8 (50%) | 7 (58%) | 15 (54%) |
| White | 8 (50%) | 5 (42%) | 13 (46%) |
| Num. Comorbidities | 8 (5, 10) | 8 (6, 9) | 8 (6, 9) |
| Unknown | 0 | 2 | 2 |
| Dementia | 3 (19%) | 1 (8%) | 4 (14%) |
| Vascular Disease | 12 (75%) | 10 (83%) | 22 (79%) |
| Treated Vasc. Dis. | 12 (75%) | 9 (75%) | 21 (75%) |
| Heart Rate (bpm) | 81 (68, 90) | 69 (63, 81) | 77 (66, 89) |
| Blood Pressure (mm-Hg) | |||
| Diastole | 75 (65, 81) | 72 (60, 78) | 73 (64, 80) |
| Systole | 137 (124, 154) | 132 (119, 141) | 136 (122, 144) |
| Temperature (F) | 98.2 (97.9, 98.6) | 97.6 (97.1, 98.1) | 98.0 (97.5, 98.4) |
| BMI (kg/m) | 28.2 (25.1, 37.6) | 25.7 (21.3, 32.1) | 27.2 (24.5, 33.6) |
| Weight (lb) | 184 (145, 225) | 168 (141, 220) | 169 (144, 222) |
| Braden Scale Score | 15.5 (15, 16) | 14 (13, 15.8) | 15 (14, 16) |
| Time Series Summary | |||
| Median (C) | 35.1 (34.4, 35.9) | 35.1 (34.2, 35.9) | 35.1 (34.3, 35.9) |
| Interquartile Range (C) | 1.1 (0.8, 1.3) | 0.9 (0.8, 1.3) | 1.0 (0.8, 1.3) |
| Trimmed Range (C) | 3.1 (2.3, 3.6) | 3.0 (2.4, 3.9) | 3.0 (2.4, 3.6) |
| Unknown | 1 | 1 | 2 |
| Characteristic | Eff. Size | Difference | 95% Conf. Int. | p | |
|---|---|---|---|---|---|
| d | Lower | Upper | |||
| SampEn | |||||
| Single scale () | 1.46 | 0.310 | 0.134 | 0.486 | 0.001 |
| Scaling exponent | 1.53 | 1.113 | 0.522 | 1.704 | <0.001 |
| Requisite AUC | 1.38 | 0.628 | 0.248 | 1.009 | 0.003 |
| BubbEn | |||||
| Single scale () | 0.99 | 0.050 | 0.005 | 0.096 | 0.033 |
| Scaling exponent | 1.04 | 3.079 | 0.624 | 5.534 | 0.016 |
| Requisite AUC | 0.82 | 0.081 | 0.008 | 0.170 | 0.035 |
| Model | AUROC a | Term | OR b | 95% Conf. Int. (OR) | p | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| B1 | 0.727 | BubbEn req. AUC | 6.3 × 10 −4 | 2.9 × 10 −7 | 1.4 | 0.061 |
| B2 | 0.740 | Braden Score | 0.47 | 0.26 | 0.84 | 0.012 |
| B3 | 0.782 | BubbEn scaling exp. | 0.70 | 0.55 | 0.88 | 0.003 |
| B4 | 0.807 | SampEn req. AUC | 3.9 × 10 −2 | 8.3 × 10 −3 | 0.18 | <0.001 |
| B5 | 0.861 | SampEn scaling exp. | 0.15 | 0.05 | 0.42 | <0.001 |
| Model | AUROC a | Term | OR b | 95% Conf. Int. (OR) | p | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| M1 | 0.940 | BubbEn scaling exp. | 0.58 | 0.36 | 0.93 | 0.025 |
| SampEn scaling exp. | 0.21 | 0.05 | 0.88 | 0.033 | ||
| SampEn req. AUC | 0.19 | 3.7 × 10 −2 | 1.02 | 0.053 | ||
| BubbEn req. AUC | 1.6 × 10 −9 | 1.2 × 10 −18 | 2.05 | 0.058 | ||
| M2 | 0.967 | Braden Score | 0.25 | 0.12 | 0.54 | <0.001 |
| BubbEn scaling exp. | 0.68 | 0.49 | 0.95 | 0.024 | ||
| SampEn scaling exp. | 0.25 | 7.5 × 10 −2 | 0.85 | 0.026 | ||
| BubbEn req. AUC | 6.8 × 10 −2 | 1.7 × 10 −7 | 2.6 × 10 | 0.682 | ||
| SampEn req. AUC | 0.88 | 0.20 | 3.9 | 0.867 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padhye, N.; Rios, D.; Fay, V.; Hanneman, S.K. Pressure Injury Link to Entropy of Abdominal Temperature. Entropy 2022, 24, 1127. https://doi.org/10.3390/e24081127
Padhye N, Rios D, Fay V, Hanneman SK. Pressure Injury Link to Entropy of Abdominal Temperature. Entropy. 2022; 24(8):1127. https://doi.org/10.3390/e24081127
Chicago/Turabian StylePadhye, Nikhil, Denise Rios, Vaunette Fay, and Sandra K. Hanneman. 2022. "Pressure Injury Link to Entropy of Abdominal Temperature" Entropy 24, no. 8: 1127. https://doi.org/10.3390/e24081127
APA StylePadhye, N., Rios, D., Fay, V., & Hanneman, S. K. (2022). Pressure Injury Link to Entropy of Abdominal Temperature. Entropy, 24(8), 1127. https://doi.org/10.3390/e24081127






