Numerical Investigation of Exergy Loss of Ammonia Addition in Hydrocarbon Diffusion Flames
Abstract
:1. Introduction
2. Methodology
2.1. Chemical Kinetic Modeling
2.2. Energy Generation Rate
2.3. Exergy Loss Rate
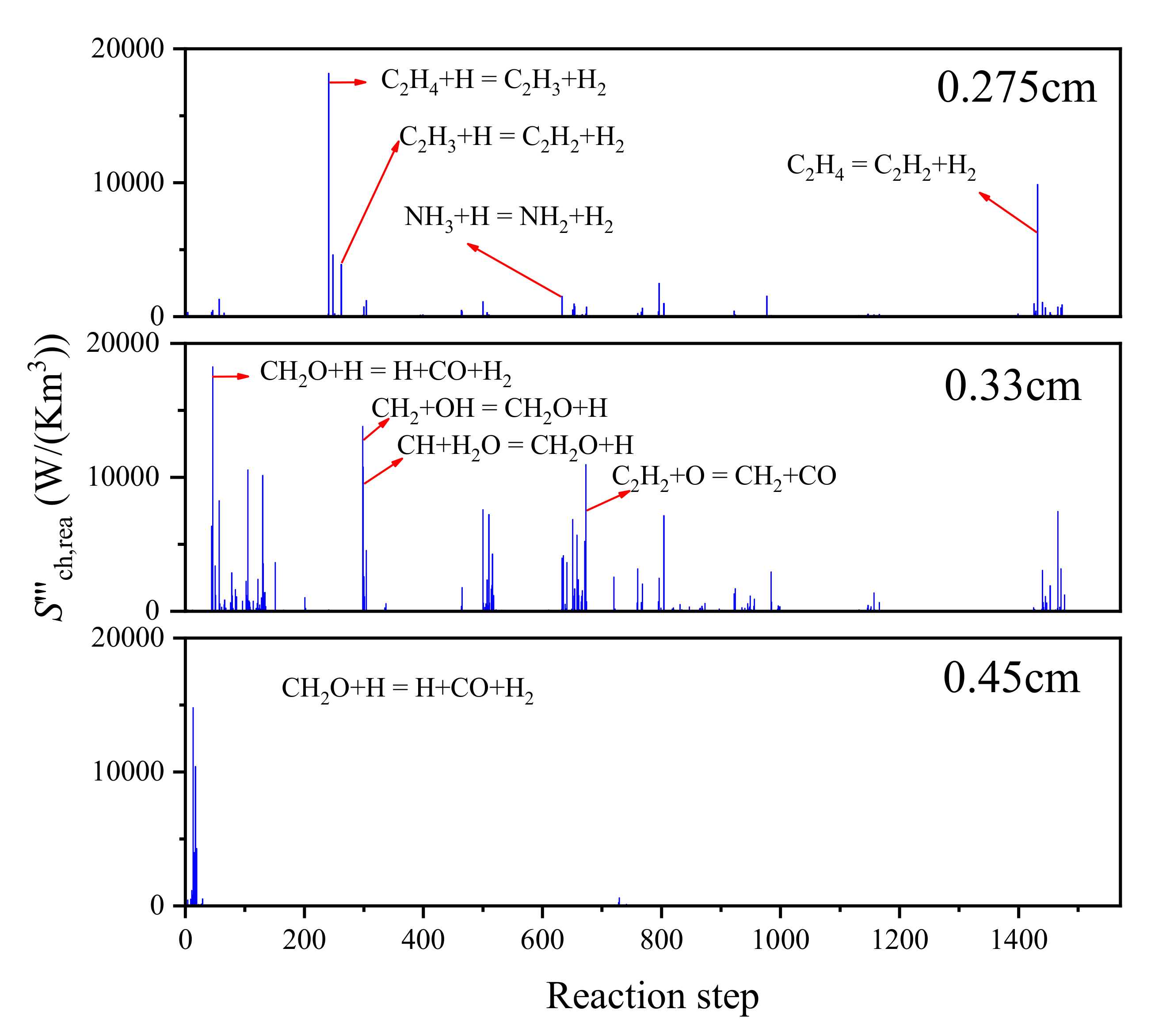
3. Results
3.1. Exergy Loss Ratio Due to Heat Transfer, Mass Diffusion and Chemical Reaction
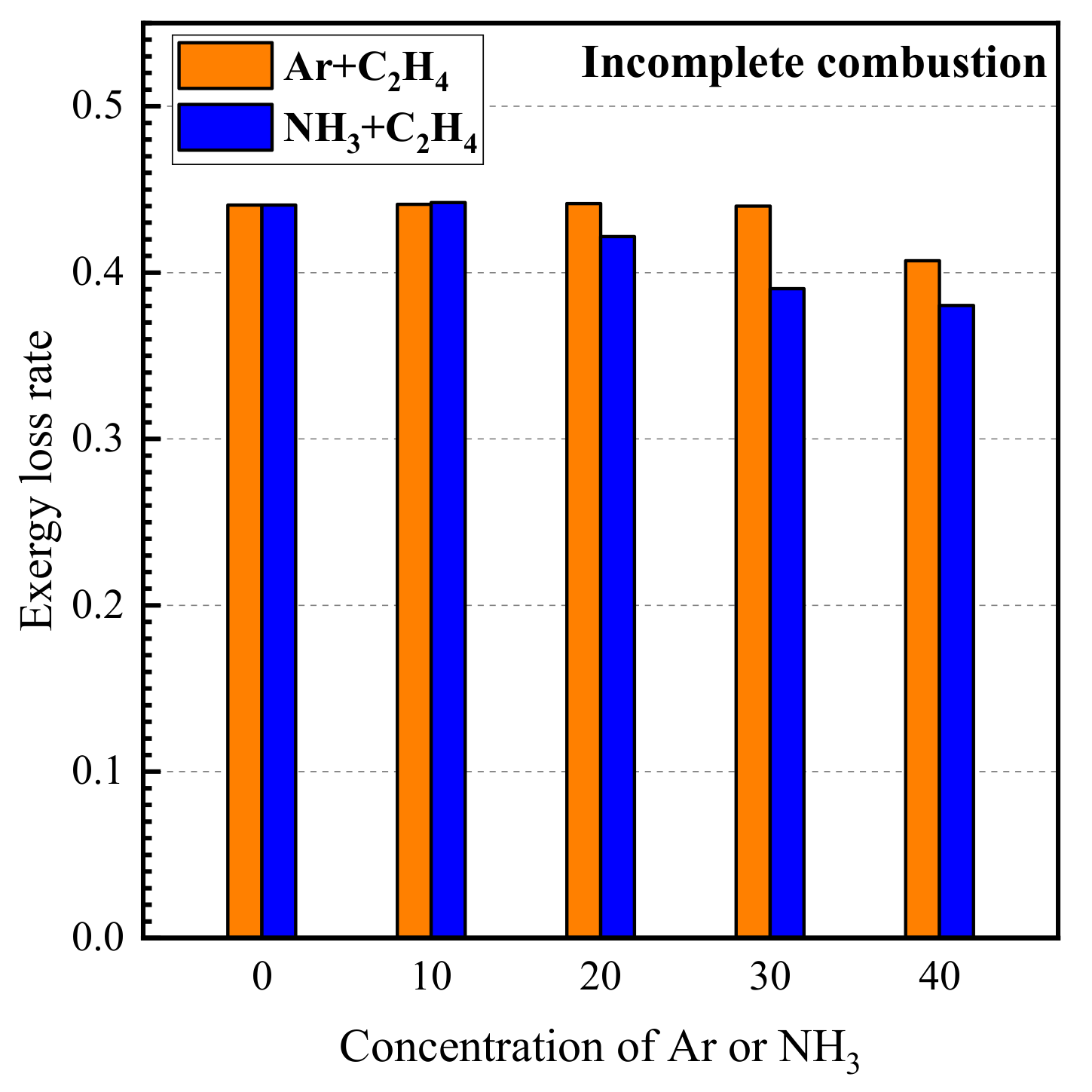
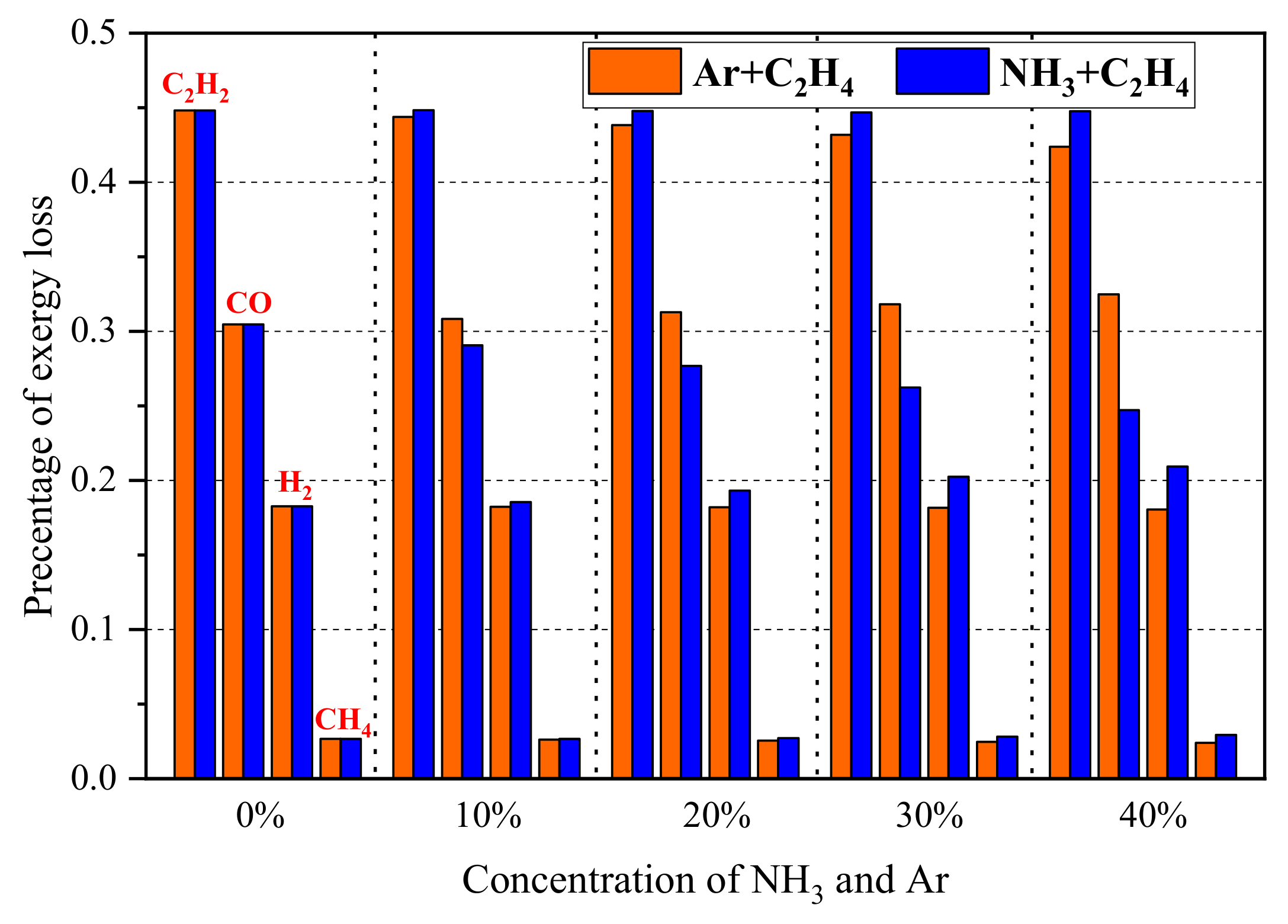
3.2. Exergy Loss Rate Due to Incomplete Combustion
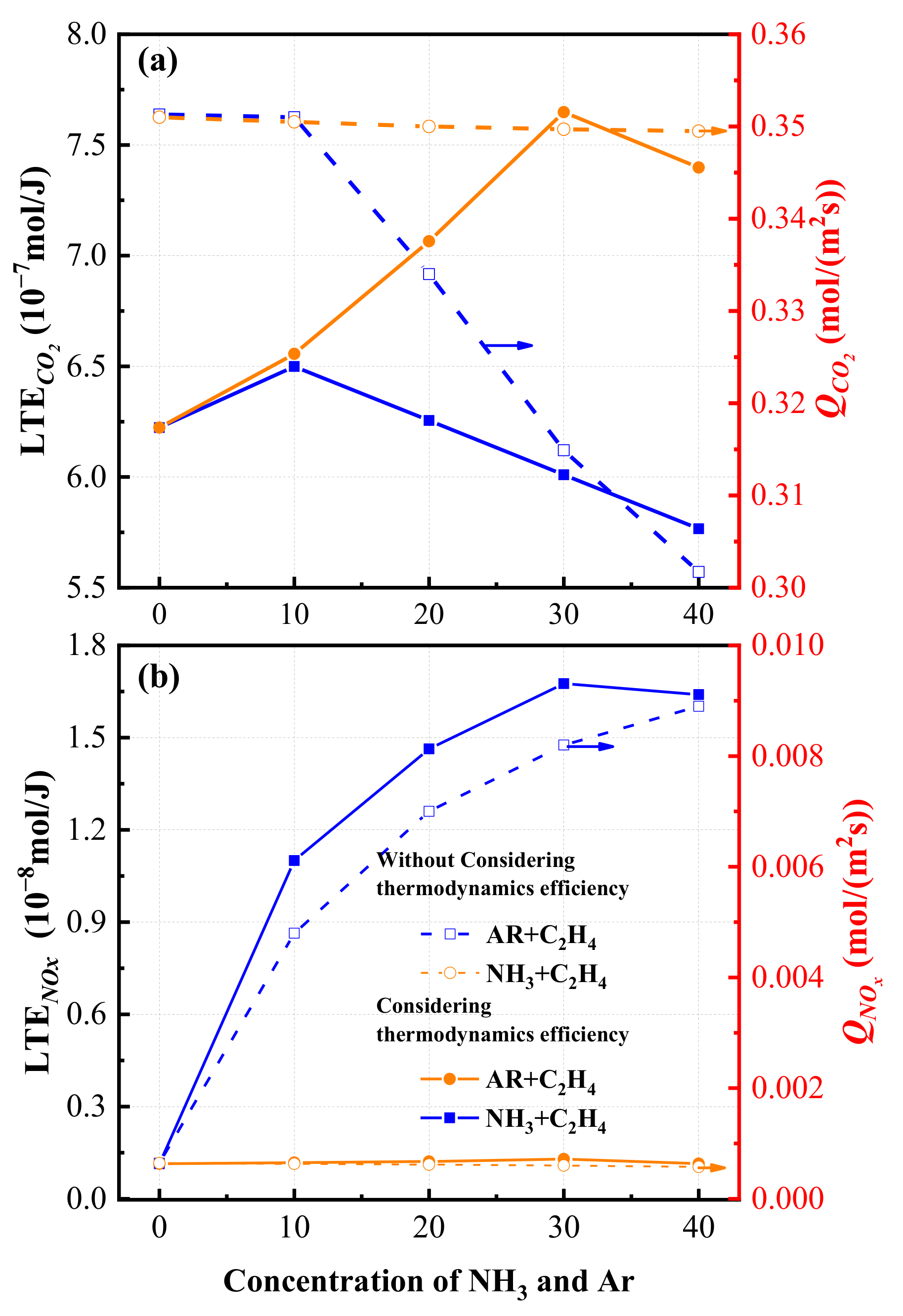
3.3. Total Exergy Loss Rate and the Lowest Emission of Pollutants
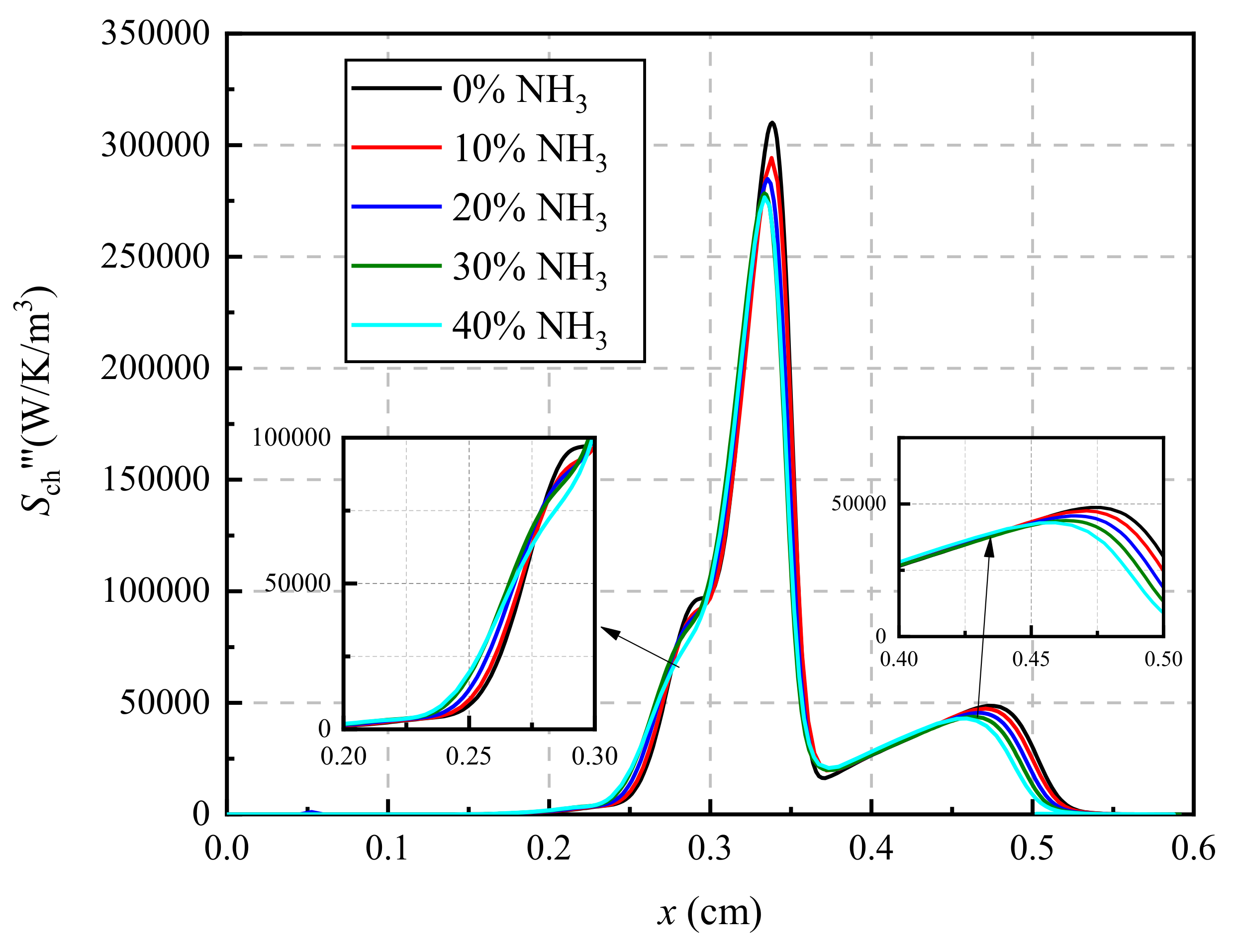
3.4. Chemical Reaction Paths Analysis Based on Thermodynamics Analysis
4. Conclusions
- (1)
- The exergy loss rate due to heat conduction and exergy loss rate due to chemical reaction increase with the increase in ammonia concentration. The exergy loss rate due to the mass diffusion process has a trend of first increasing and then decreasing with the increase in ammonia concentration. The ammonia addition has a significant enhancement on heat transfer and chemical reaction in the flames, but it has no obvious effect on the mass diffusion.
- (2)
- Incomplete combustion is an important cause for the production of exergy loss and C2H2, CO, and H2 are the main species for exergy loss due to incomplete combustion. The exergy loss rate due to incomplete combustion decreases with the increase in the concentration of argon and ammonia in the fuel.
- (3)
- Total exergy loss rate has a tendency of first increasing slightly and then decreasing with an increase in the concentration of ammonia. When ammonia concentration is high, ammonia addition decreases the total exergy loss rate and enhances the thermodynamics effectivity of combustion obviously. A parameter, the lowest emission of pollutants, is proposed based on thermodynamic analysis. The results indicate that ammonia addition can effectively reduce the emission of CO2. When ammonia concentration increases, the increasing tendency of LEPNOx weakens gradually and it reaches the maximum when ammonia concentration is 30%.
- (4)
- The chemical reactions in the ammonia/ethylene flame can be divided into three parts. These are the pyrolysis process of fuel, chemical reactions between pyrolysis products, as well as the oxidation of elementary substances. The results reveal that CH2O is recognized as an important substance in the chemical reactions.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Glarborg, P.; Miller, J.A.; Ruscic, B.; Klippenstein, S.J. Modeling nitrogen chemistry in combustion. Prog. Energ. Combust. 2018, 67, 31–68. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Cheng, X.; Li, Y.; Qiu, L.; Wang, X.; Xu, Y. Effects of ammonia addition on soot formation in ethylene laminar diffusion flames. Fuel 2021, 292, 120416. [Google Scholar] [CrossRef]
- Bennett, A.M.; Liu, P.; Li, Z.; Kharbatia, N.M.; Boyette, W.; Masri, A.R.; Roberts, W.L. Soot formation in laminar flames of ethylene/ammonia. Combust. Flame 2020, 220, 210–218. [Google Scholar] [CrossRef]
- Zhou, M.; Yan, F.; Ma, L.; Jiang, P.; Wang, Y.; Chung, S.H. Chemical speciation and soot measurements in laminar counterflow diffusion flames of ethylene and ammonia mixtures. Fuel 2022, 308, 122003. [Google Scholar] [CrossRef]
- Steinmetz, S.A.; Ahmed, H.A.; Boyette, W.R.; Dunn, M.J.; Roberts, W.L.; Masri, A.R. Effects of ammonia and hydrogen on the sooting characteristics of laminar coflow flames of ethylene and methane. Fuel 2022, 307, 121914. [Google Scholar] [CrossRef]
- Lou, C.; Chen, C.; Sun, Y.; Zhou, H. Review of soot measurement in hydrocarbon-air flames. Sci. China Technol. Sci. 2022, 53, 2129–2141. [Google Scholar] [CrossRef]
- Guo, Z.; Lou, C.; Liu, Z. The impact of combustion characteristics and flame structure on soot formation in oxy-enhanced and oxy-fuel diffusion flames. Sci. China Technol. Sci. 2013, 56, 1618–1628. [Google Scholar] [CrossRef]
- Lou, C.; Zhang, L.; Pu, Y. Research advances in passive techniques for combustion diagnostics based on analysis of spontaneous emission radiation. J. Exp. Fluid Mech. 2021, 35, 1–17. [Google Scholar]
- Bowen, P.J. Ammonia for power. Prog. Energy Combust. Sci. 2018, 69, 63–102. [Google Scholar]
- Liu, S.; Zou, C.; Song, Y.; Cheng, S.; Lin, Q. Experimental and numerical study of laminar flame speeds of CH4/NH3 mixtures under oxy-fuel combustion. Energy 2019, 175, 250–258. [Google Scholar] [CrossRef]
- Moran, M.J.; Shapiro, H.N.; Boettner, D.D.; Bailey, M.B. Fundamentals of Engineering Thermodynamics; Wiley: New York, NY, USA, 2018. [Google Scholar]
- Som, S.K.; Datta, A. Thermodynamic irreversibilities and exergy balance in combustion processes. Prog. Energy Combust. Sci. 2008, 34, 351–376. [Google Scholar] [CrossRef]
- Dunbar, W.R.; Lior, N. Sources of combustion irreversibility. Combust. Sci. Technol. 1994, 103, 41–61. [Google Scholar] [CrossRef]
- Lior, N.; Sarmiento-Darkin, W. The exergy fields in transport processes: Their calculation and use. Energy 2006, 31, 553578. [Google Scholar] [CrossRef]
- Zhang, J.; Zhong, A.; Huang, Z.; Han, D. Second-law thermodynamic analysis in premixed flames of ammonia and hydrogen binary fuels. J. Eng. Gas Turbines Power 2019, 141, 071007. [Google Scholar] [CrossRef]
- Nishida, K.; Takagi, T.; Kinoshita, S. Analysis of entropy generation and exergy loss during combustion. Proc. Combust. Inst. 2002, 29, 869–874. [Google Scholar] [CrossRef]
- Datta, A. Entropy generation in a confined laminar diffusion flame. Combust. Sci. Technol. 2000, 159, 39–56. [Google Scholar] [CrossRef]
- Datta, A. Effects of gravity on structure and entropy generation of confined laminar diffusion flames. Int. J. Therm. Sci. 2005, 44, 429–440. [Google Scholar] [CrossRef]
- Zhang, Z.; Lou, C.; Long, Y.; Kumfer, B.M. Thermodynamics second-law analysis of hydrocarbon diffusion flames: Effects of soot and temperature. Combust. Flame 2021, 234, 111618. [Google Scholar] [CrossRef]
- Lou, C.; Zhang, Z. Experimental and numerical analysis of radiative entropy generation in industrial and boiler furnaces. J. Quant. Spectros. Radiat. Transf. 2019, 232, 27–34. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Z.; Lou, C. Numerical analysis of radiative entropy generation in a parallel plate system with non-uniform temperature distribution participation medium. J. Quant. Spectros. Radiat. Transf. 2019, 225, 319–326. [Google Scholar] [CrossRef]
- Zhang, Z.; Lou, C.; Li, Z.; Long, Y. Evaluation of radiative entropy generation in a high temperature system including H2O, CO2 and soot with non-gray wall. J. Quant. Spectros. Radiat. Transf. 2020, 253, 107175. [Google Scholar] [CrossRef]
- Yan, H.; Tang, G.; Wang, C.; Li, L.; Zhou, Y.; Zhang, Z.; Lou, C. Thermodynamics Irreversibilities Analysis of Oxy-Fuel Diffusion Flames: The Effect of Oxygen Concentration. Entropy 2022, 24, 205. [Google Scholar] [CrossRef] [PubMed]
- Jejurkar, S.Y.; Mishra, D.P. Effects of wall thermal conductivity on entropy generation and exergy losses in a H2-air premixed flame microcombustor. Int. J. Hydrogen Energy 2011, 36, 15851–15859. [Google Scholar] [CrossRef]
- Yang, W.; Jiang, D.; Yong, C.K. Combustion process and entropy generation in a novel microcombustor with a block insert. Chem. Eng. J. 2015, 275, 231–237. [Google Scholar]
- Safer, K.; Ouadha, A.; Tabet, F. Entropy generation in turbulent sygas counter-flow diffusion flames. Int. J. Hydrogen Energy 2017, 42, 29532–29544. [Google Scholar] [CrossRef]
- Acampora, L.; Marra, F.S. Second law thermodynamic analysis of syngas premixed flames. Int. J. Hydrogen Energy 2020, 45, 12185–12202. [Google Scholar] [CrossRef]
- Zhang, J.; Luong, M.B.; Pérez, F.H.; Han, D. Exergy loss characteristics of DME/air and ethanol/air mixtures with temperature and concentration fluctuations under HCCI/SCCI conditions: A DNS study. Combust. Flame 2021, 226, 334–346. [Google Scholar] [CrossRef]
- Zhang, J.; Han, D.; Huang, Z. Second-law thermodynamic analysis for premixed hydrogen flames with diluents of argon/nitrogen/carbon dioxide. Int. J. Hydrogen Energy 2019, 44, 5020–5029. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Ju, D.; Huang, Z.; Han, D. Analysis of exergy losses in laminar premixed flames of methane/hydrogen blends. Int. J. Hydrogen Energy 2019, 44, 24043–24053. [Google Scholar] [CrossRef]
- Sciacovelli, A.; Verda, V.; Sciubba, E. Entropy generation analysis as a design tool—A review. Renew. Sust. Energ. Rev. 2015, 43, 1167–1181. [Google Scholar] [CrossRef]


| Ar/C2H4 Flame | NH3/C2H4 Flame | ||
|---|---|---|---|
| XC2H4 | XAR | XC2H4 | XNH3 |
| 1 | 0 | 1 | 0 |
| 0.9 | 0.1 | 0.9 | 0.1 |
| 0.8 | 0.2 | 0.8 | 0.2 |
| 0.7 | 0.3 | 0.7 | 0.3 |
| 0.6 | 0.4 | 0.6 | 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.; Zhang, Z.; Sun, H.; Yao, B.; Lou, C. Numerical Investigation of Exergy Loss of Ammonia Addition in Hydrocarbon Diffusion Flames. Entropy 2022, 24, 922. https://doi.org/10.3390/e24070922
Sun H, Zhang Z, Sun H, Yao B, Lou C. Numerical Investigation of Exergy Loss of Ammonia Addition in Hydrocarbon Diffusion Flames. Entropy. 2022; 24(7):922. https://doi.org/10.3390/e24070922
Chicago/Turabian StyleSun, Haifeng, Zhongnong Zhang, Hanxiao Sun, Bin Yao, and Chun Lou. 2022. "Numerical Investigation of Exergy Loss of Ammonia Addition in Hydrocarbon Diffusion Flames" Entropy 24, no. 7: 922. https://doi.org/10.3390/e24070922
APA StyleSun, H., Zhang, Z., Sun, H., Yao, B., & Lou, C. (2022). Numerical Investigation of Exergy Loss of Ammonia Addition in Hydrocarbon Diffusion Flames. Entropy, 24(7), 922. https://doi.org/10.3390/e24070922







