Study on Hydrate Production Behaviors by Depressurization Combined with Brine Injection in the Excess-Water Hydrate Reservoir
Abstract
:1. Introduction
2. Experimental Sections
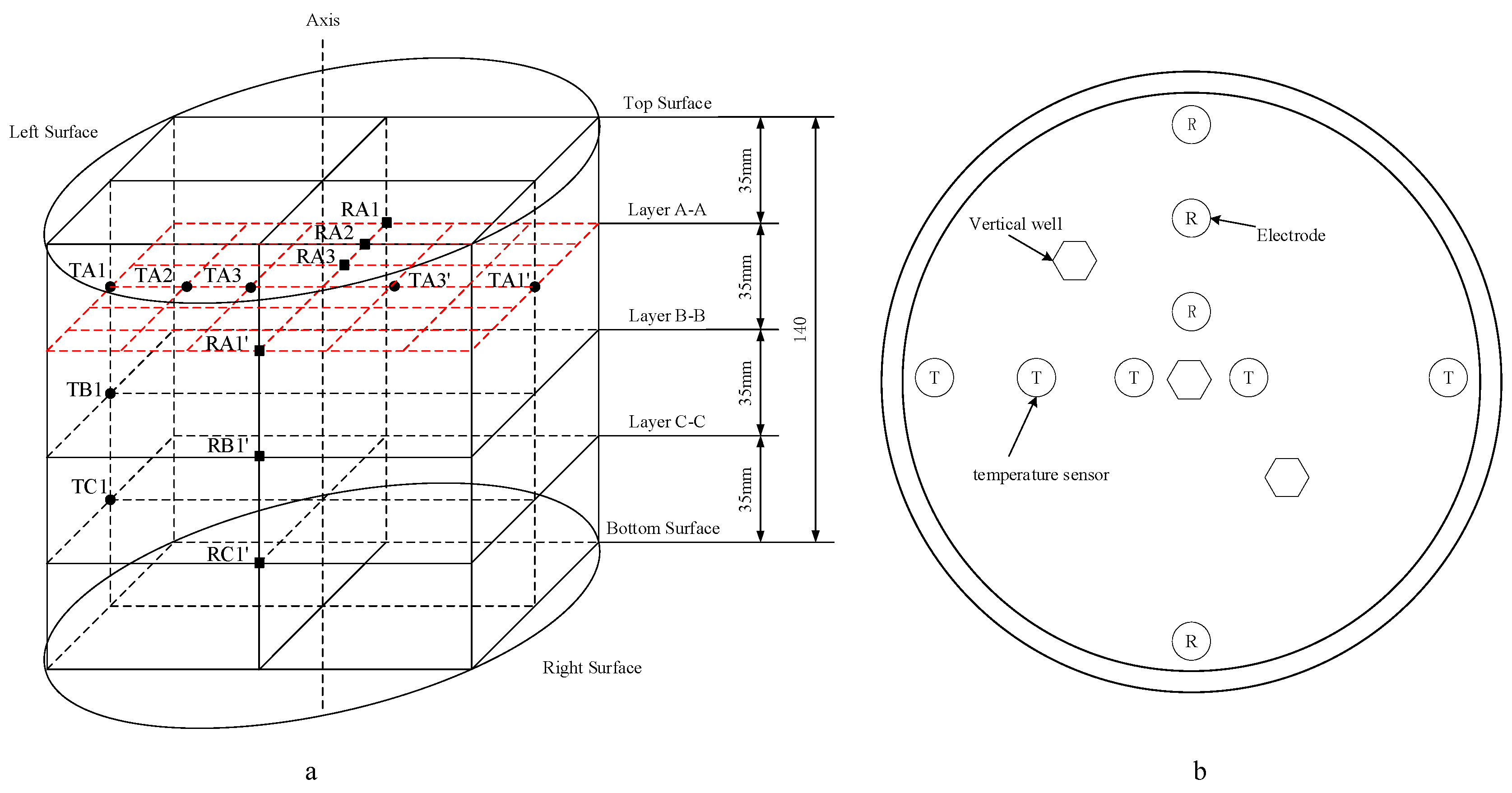
2.1. Experimental Apparatus
2.2. Materials
2.3. Experimental Procedure
2.3.1. Hydrate Formation
2.3.2. Hydrate Decomposition
3. Results and Discussion
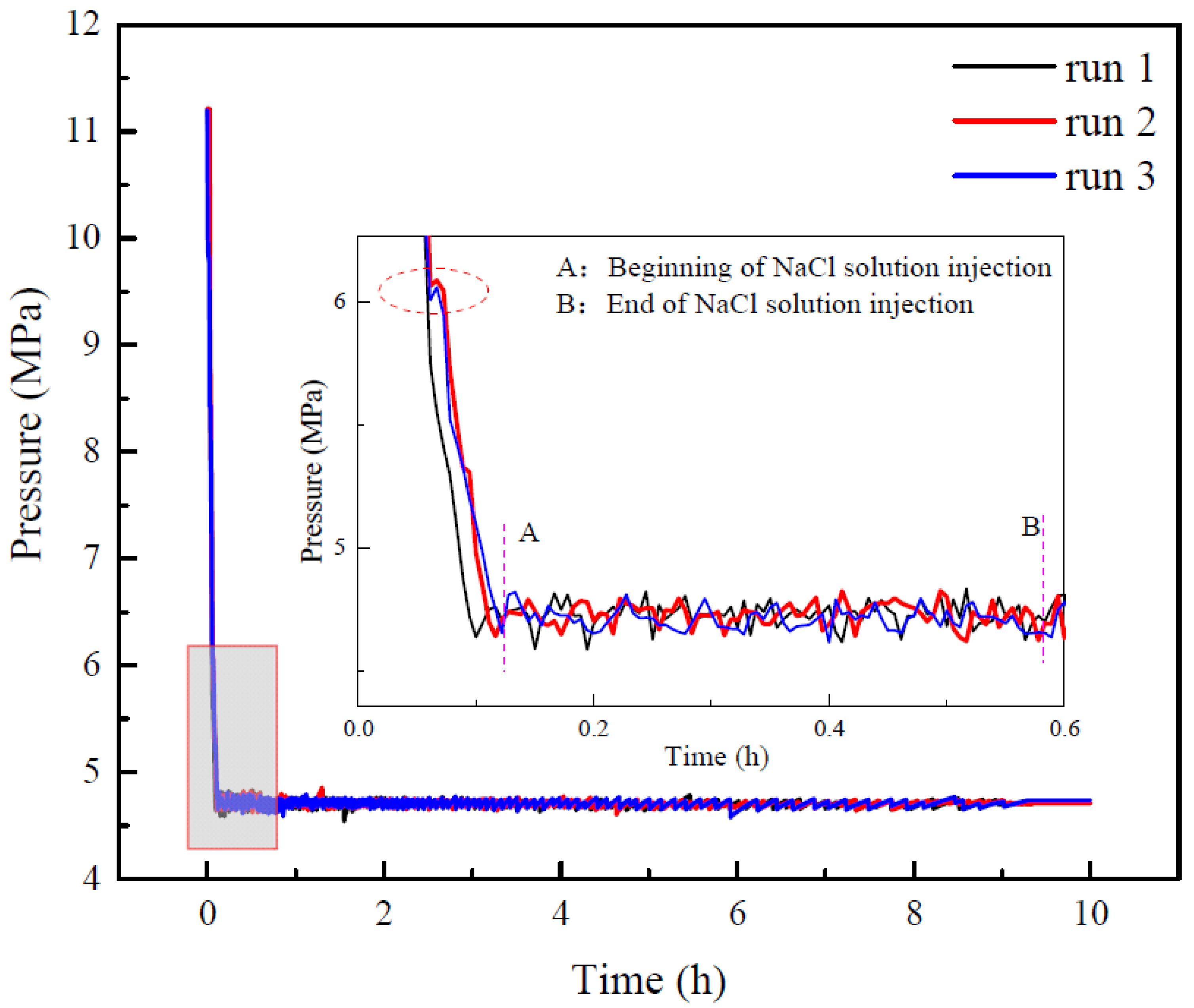
3.1. Evolution of Pressure during Production
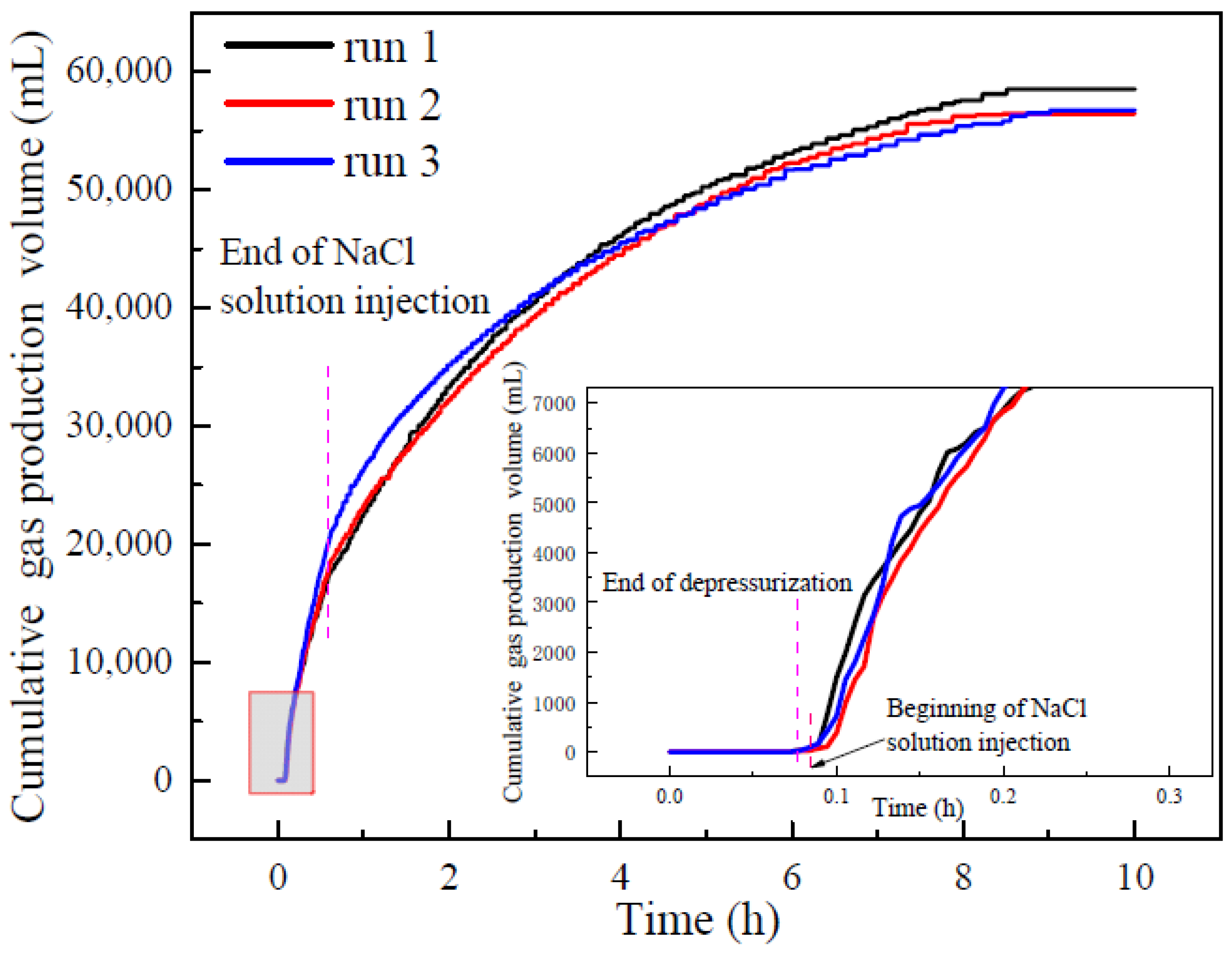
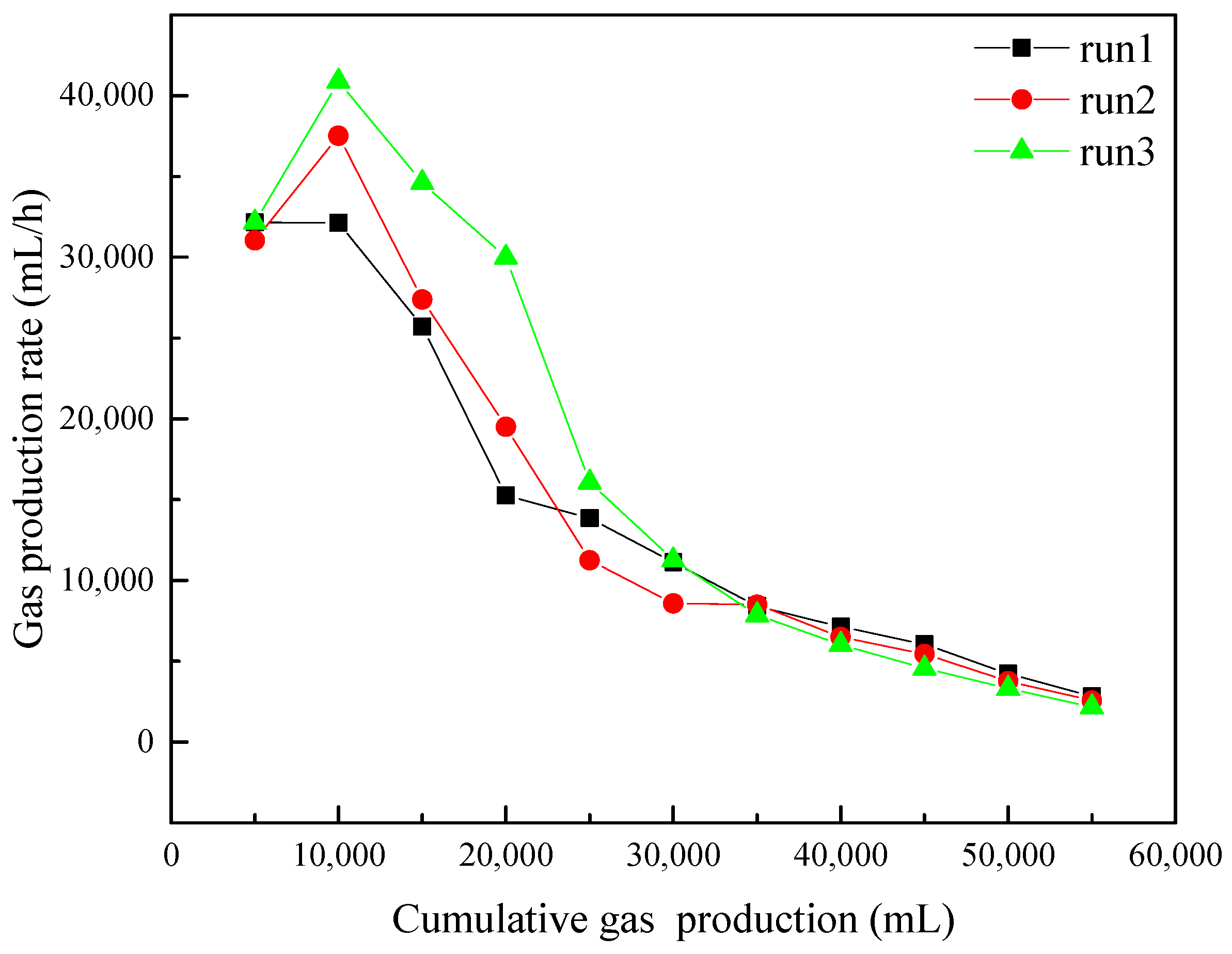
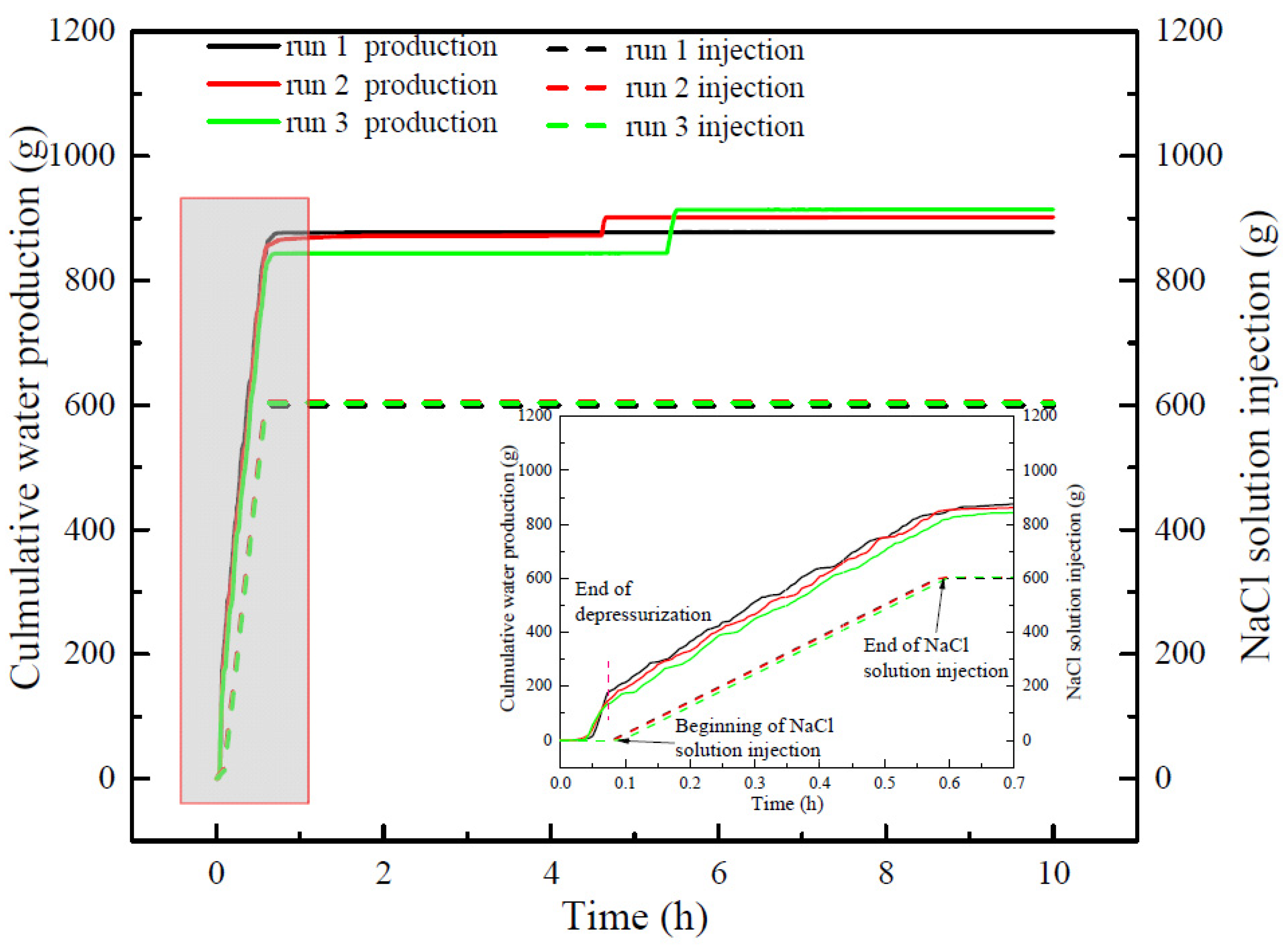
3.2. Gas Production and Water Production
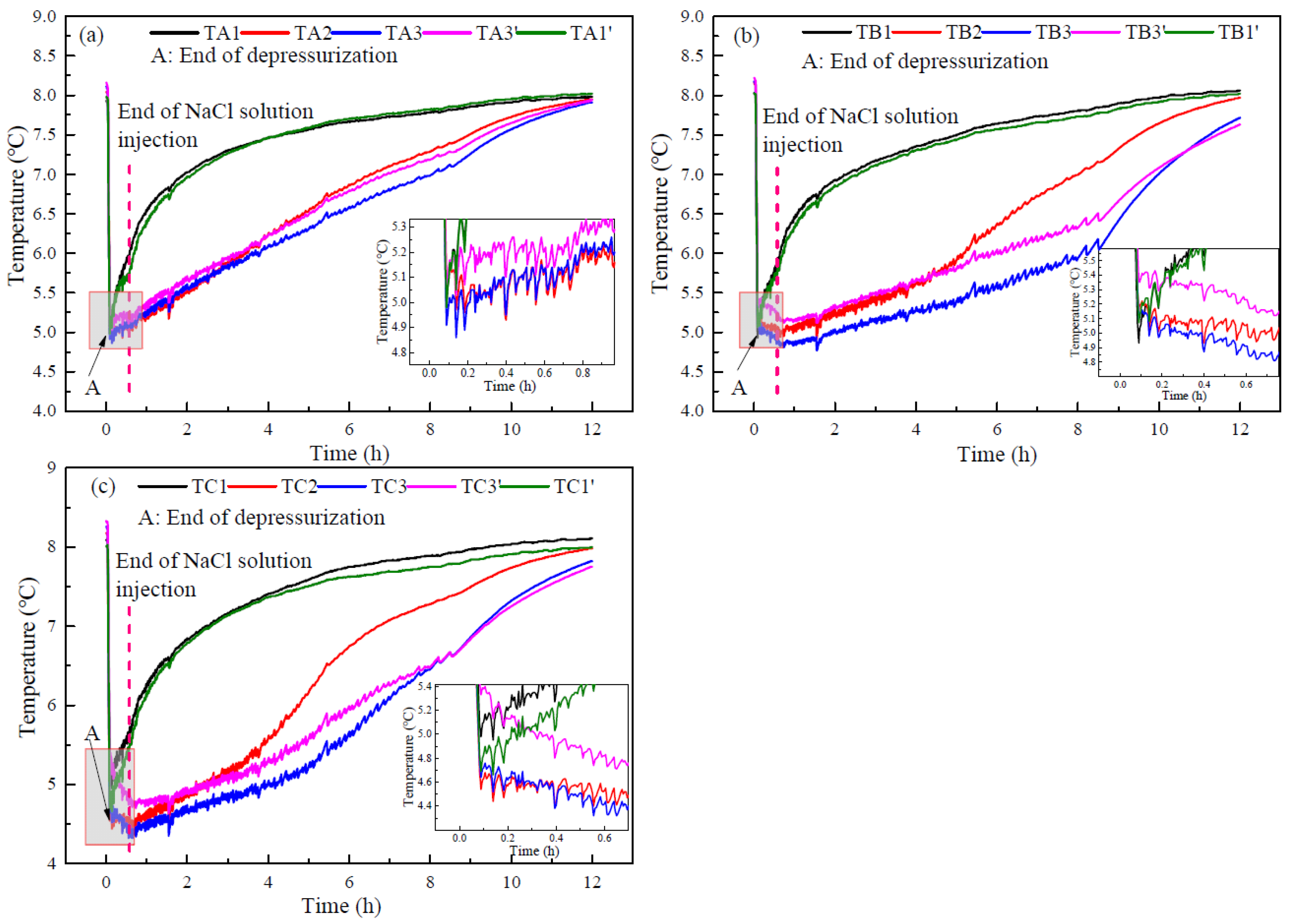
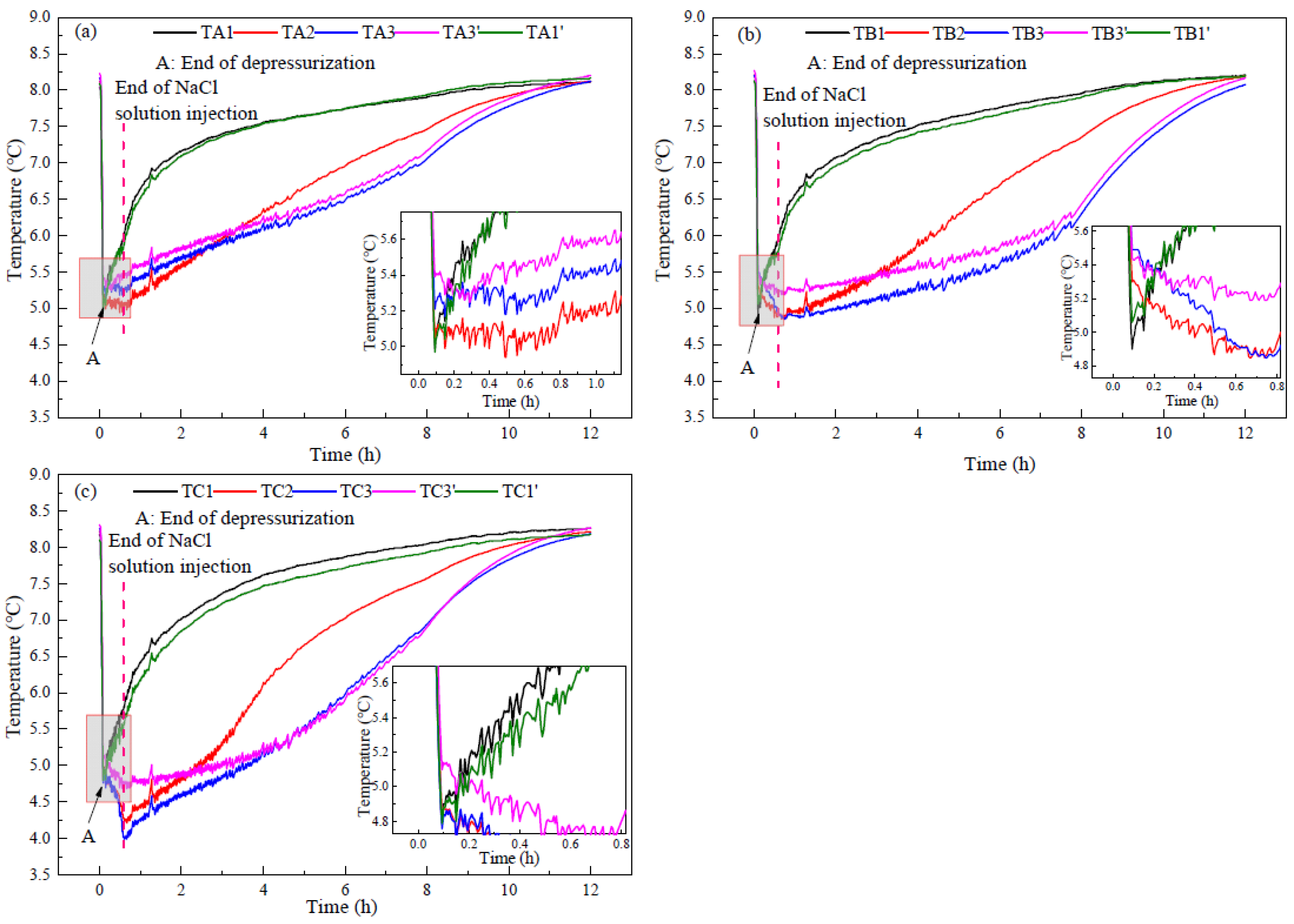
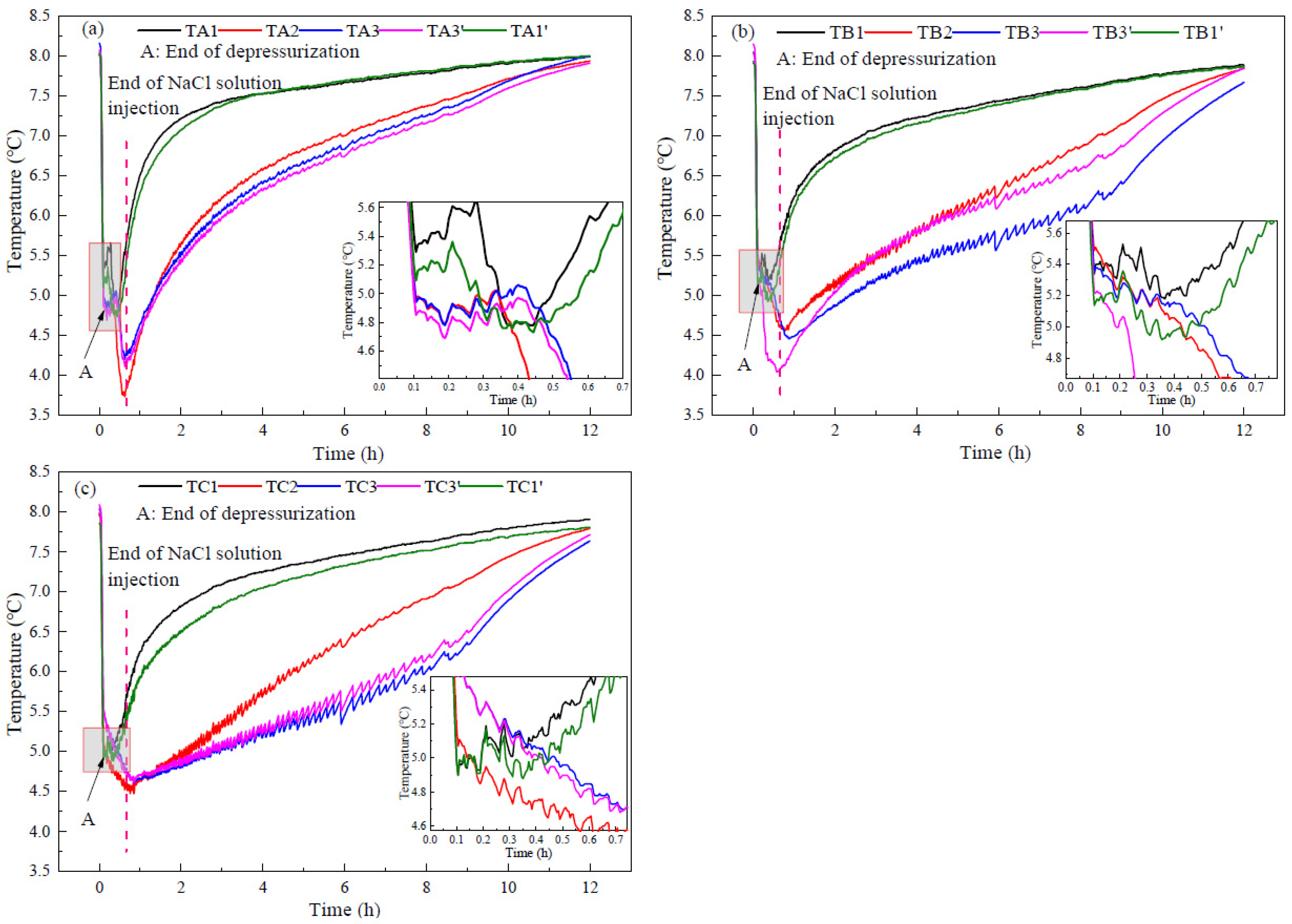
3.3. Heat Transfer Characteristics
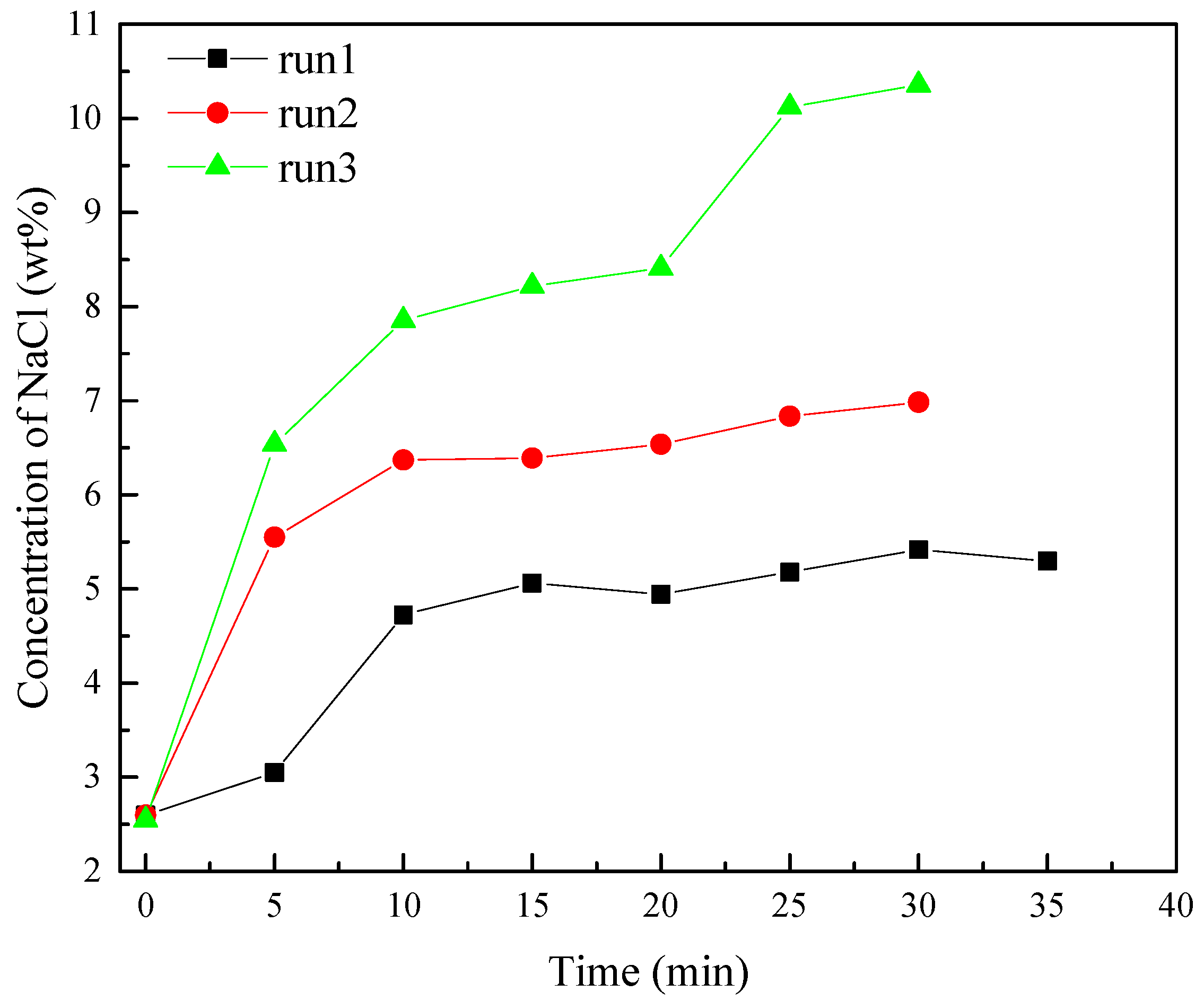
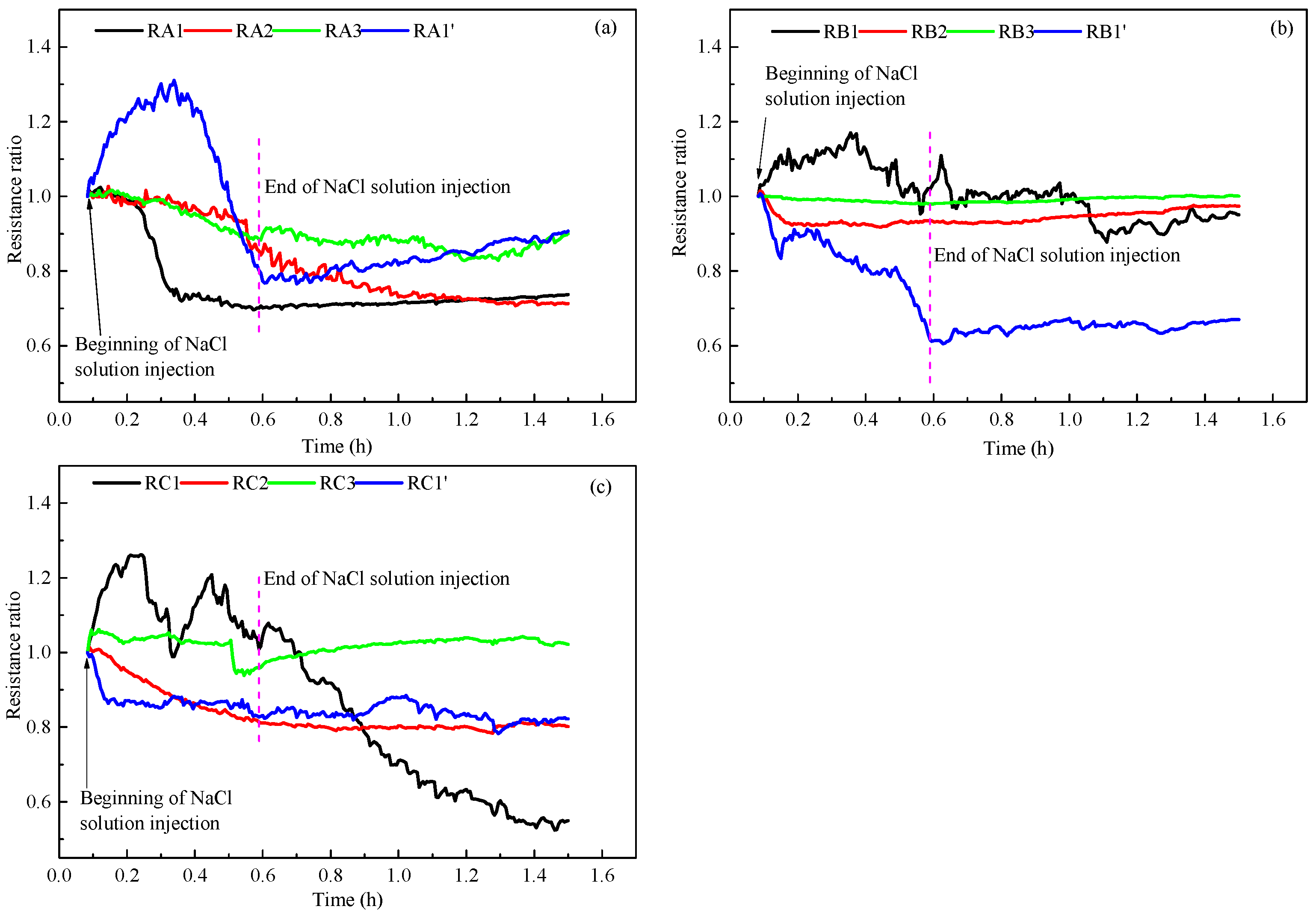
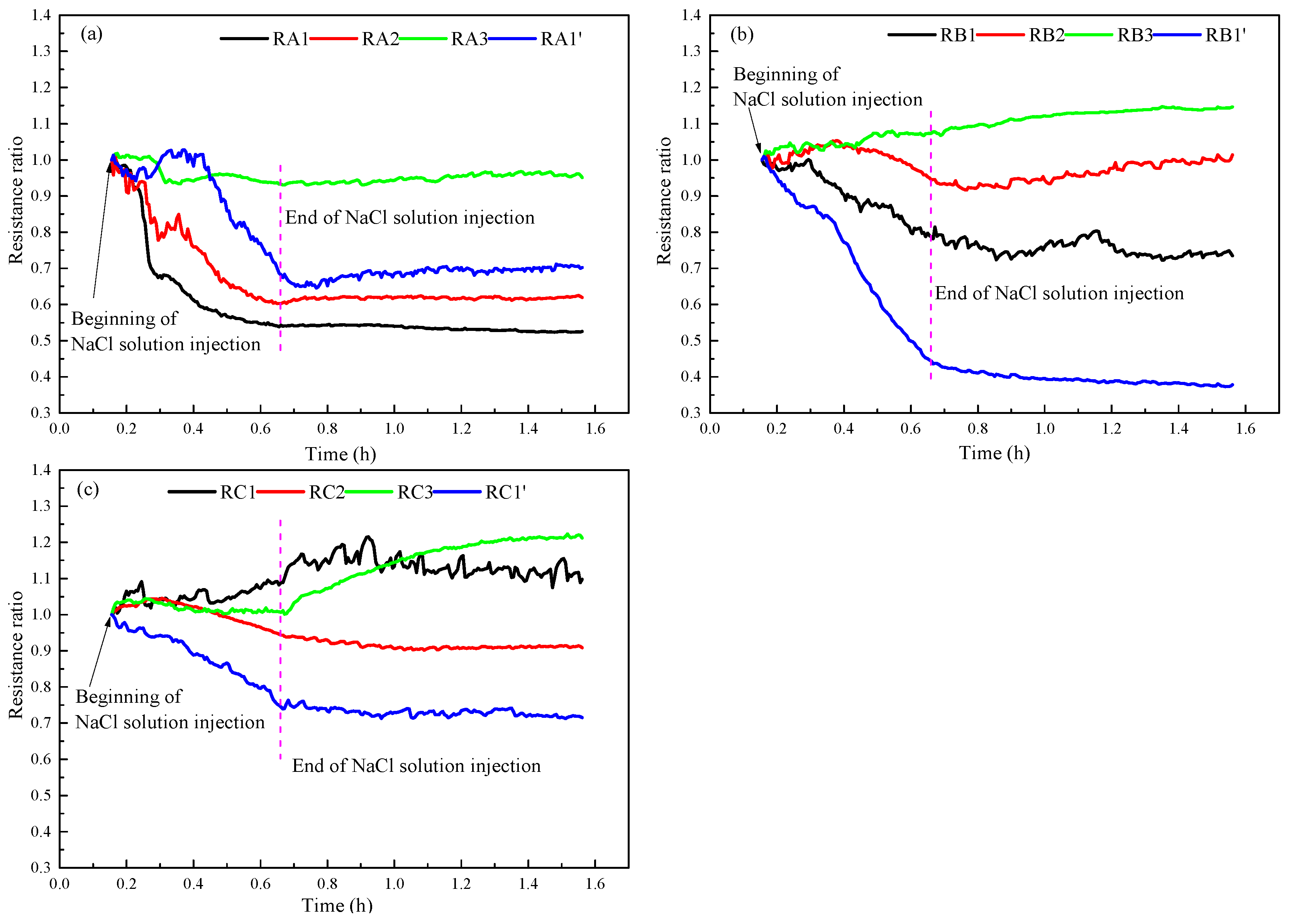
3.4. NaCl Solution Distribution
4. Conclusions
- In an excess-water hydrate reservoir, there is little gas production during depressurization and the gas dissociated from hydrate is trapped in pores of sediments. The high-water production reduces the final gas recovery, which was lower than 70% in the experiments, resulting in the weak influence of NaCl concentration on the final gas production.
- The increasing NaCl concentration effectively promotes gas production rate in the early stage. However, after a period of production, the gas production rate becomes similar in the experiments with different NaCl concentrations. The final cumulative gas production and average gas production rate had little difference in different experiments.
- The NaCl concentration of the produced water is significantly higher than that in contact with hydrate in the reactor due to the water produced by hydrate decomposition existing on the surface of undissociated hydrate. The high concentration of NaCl of produced water significantly reduces the promoting effect and efficiency of NaCl solution on hydrate decomposition. It is important to ensure that the injected salt solution can be more widely distributed in the hydrate reservoir and reduce the loss of salt solution caused by water production.
- The injection of NaCl solution decreases the lowest temperature during hydrate production and increases the sensible heat and heat transfer from environment for hydrate decomposition. The changes of temperature and resistance reflect the distribution of the injected NaCl solution in the hydrate reservoir.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boswell, R. Is Gas Hydrate Energy Within Reach? Science 2009, 325, 957–958. [Google Scholar] [CrossRef] [PubMed]
- Moridis, G.J.; Collett, T.S.; Boswell, R.; Kurihara, M.; Reagan, M.T.; Koh, C.; Sloan, E.D. Toward Production From Gas Hydrates: Current Status, Assessment of Resources, and Simulation-Based Evaluation of Technology and Potential. SPE Reserv. Eval. Eng. 2009, 12, 745–771. [Google Scholar] [CrossRef] [Green Version]
- Boswell, R.; Collett, T.S. Current perspectives on gas hydrate resources. Energy Environ. Sci. 2011, 4, 1206–1215. [Google Scholar] [CrossRef]
- Milkov, A.V. Global estimates of hydrate-bound gas in marine sediments: How much is really out there? Earth Sci. Rev. 2004, 66, 183–197. [Google Scholar] [CrossRef]
- Kurihara, M.; Sato, A.; Funatsu, K.; Ouchi, H.; Ashford, D. Analysis of Production Data for 2007/2008 Mallik Gas Hydrate Production Tests in Canada. In Proceedings of the International Oil and Gas Conference and Exhibition in China, Beijing, China, 8–10 June 2010. [Google Scholar]
- Hunter, R.B.; Collett, T.S.; Boswell, R.; Anderson, B.J.; Digert, S.A.; Pospisil, G.; Baker, R.; Weeks, M. Mount Elbert Gas Hydrate Stratigraphic Test Well, Alaska North Slope: Overview of scientific and technical program. Mar. Pet. Geol. 2011, 28, 295–310. [Google Scholar] [CrossRef]
- Konno, Y.; Fujii, T.; Sato, A.; Akamine, K.; Naiki, M.; Masuda, Y.; Yamamoto, K.; Nagao, J. Key Findings of the World’s First Offshore Methane Hydrate Production Test off the Coast of Japan: Toward Future Commercial Production. Energy Fuels 2017, 31, 2607–2616. [Google Scholar] [CrossRef]
- Ye, J.; Qin, X.; Xie, W.; Lu, H.; Ma, B.; Qiu, H.; Liang, J.; Lu, J.a.; Kuang, Z.; Lu, C.; et al. Main progress of the second gas hydrate trial production in the South China Sea. Geol. China 2020, 47, 557–568. [Google Scholar]
- Li, J.-F.; Ye, J.-L.; Qin, X.-W.; Qiu, H.-J.; Wu, N.-Y.; Lu, H.-L.; Xie, W.-W.; Lu, J.-A.; Peng, F.; Xu, Z.-Q.; et al. The first offshore natural gas hydrate production test in South China Sea. China Geol. 2018, 1, 5–16. [Google Scholar] [CrossRef]
- Chong, Z.R.; Yang, S.; Babu, P.; Linga, P.; Li, X.S. Review of natural gas hydrates as an energy resource: Prospects and challenges. Appl. Energy 2016, 162, 1633–1652. [Google Scholar] [CrossRef]
- Konno, Y.; Masuda, Y.; Hariguchi, Y.; Kurihara, M.; Ouchi, H. Key Factors for Depressurization-Induced Gas Production from Oceanic Methane Hydrates. Energy Fuels 2010, 24, 1736–1744. [Google Scholar] [CrossRef]
- Fitzgerald, G.C.; Castaldi, M.J.; Zhou, Y. Large scale reactor details and results for the formation and decomposition of methane hydrates via thermal stimulation dissociation. J. Pet. Sci. Eng. 2012, 94–95, 19–27. [Google Scholar] [CrossRef]
- Wang, Y.; Lang, X.; Fan, S.; Wang, S.; Li, G. Review on Enhanced Technology of Natural Gas Hydrate Recovery by Carbon Dioxide Replacement. Energy Fuels 2021, 35, 3659–3674. [Google Scholar] [CrossRef]
- Selim, M.S.; Sloan, E.D. Heat and mass transfer during the dissociation of hydrates in porous media. AIChE J. 2010, 35, 1049–1052. [Google Scholar] [CrossRef]
- Yang, M.; Zheng, J.N.; Gao, Y.; Ma, Z.; Song, Y. Dissociation characteristics of methane hydrates in South China Sea sediments by depressurization. Appl. Energy 2019, 243, 266–273. [Google Scholar] [CrossRef]
- Li, X.-S.; Xu, C.-G.; Zhang, Y.; Ruan, X.-K.; Li, G.; Wang, Y. Investigation into gas production from natural gas hydrate: A review. Appl. Energy 2016, 172, 286–322. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Cheng, C.; Zhao, J.; Zhu, Z.; Liu, W.; Yang, M.; Xue, K. Evaluation of gas production from methane hydrates using depressurization, thermal stimulation and combined methods. Appl. Energy 2015, 145, 265–277. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, J.C.; Li, X.S.; Zhang, Y.; Li, G. Large scale experimental evaluation to methane hydrate dissociation below quadruple point in sandy sediment. Appl. Energy 2016, 162, 372–381. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, J.C.; Li, X.S. Pilot-scale experimental test on gas production from methane hydrate decomposition using depressurization assisted with heat stimulation below quadruple point. Int. J. Heat Mass Transfer 2019, 131, 965–972. [Google Scholar] [CrossRef]
- Feng, J.-C.; Wang, Y.; Li, X.-S. Hydrate dissociation induced by depressurization in conjunction with warm brine stimulation in cubic hydrate simulator with silica sand. Appl. Energy 2016, 174, 181–191. [Google Scholar] [CrossRef]
- Bai, D.; Wu, Z.; Lin, C.; Zhou, D. The effect of aqueous NaCl solution on methane hydrate nucleation and growth. Fluid Phase Equilib. 2019, 487, 76–82. [Google Scholar] [CrossRef]
- Chong, Z.R.; Koh, J.W.; Linga, P. Effect of KCl and MgCl2 on the kinetics of methane hydrate formation and dissociation in sandy sediments. Energy 2017, 137, 518–529. [Google Scholar] [CrossRef]
- Feng, J.-C.; Wang, Y.; Li, X.-S.; Zhang, Y. Influence of Hydrate Saturation on Methane Hydrate Dissociation by Depressurization in Conjunction with Warm Water Stimulation in the Silica Sand Reservoir. Energy Fuels 2015, 29, 7875–7884. [Google Scholar] [CrossRef]
- Chen, Z.; Feng, J.; Li, X.; Zhang, Y.; Li, B.; Lv, Q. Preparation of Warm Brine in Situ Seafloor Based on the Hydrate Process for Marine Gas Hydrate Thermal Stimulation. Ind. Eng. Chem. Res. 2014, 53, 14142–14157. [Google Scholar] [CrossRef]
- Li, X.S.; Wan, L.H.; Li, G.; Li, Q.P.; Chen, Z.Y.; Yan, K.F. Experimental Investigation into the Production Behavior of Methane Hydrate in Porous Sediment with Hot Brine Stimulation. Ind. Eng. Chem. Res. 2008, 47, 9–11. [Google Scholar] [CrossRef]
- Kamath, V.A.; Mutalik, P.N.; Sira, J.H.; Patil, S.L. Experimental Study of Brine Injection Depressurization of Gas Hydrates Dissociation of Gas Hydrates. SPE Form. Eval. 1991, 6, 477–484. [Google Scholar] [CrossRef]
- Wang, J.; Han, F.; Li, S.; Ge, K.; Zheng, Z. Investigation of gas hydrate production with salinity via depressurization and thermal stimulation methods. J. Pet. Sci. Eng. 2020, 194, 107465. [Google Scholar] [CrossRef]
- Lee, J. Experimental Study on the Dissociation Behavior and Productivity of Gas Hydrate by Brine Injection Scheme in Porous Rock. Energy Fuels 2010, 24, 456–463. [Google Scholar] [CrossRef]
- Jin, Y.; Li, S.; Yang, D.; Jiang, X. Determination of dissociation front and operational optimization for hydrate development by combining depressurization and hot brine stimulation. J. Nat. Gas. Sci. Eng. 2018, 50, 215–230. [Google Scholar] [CrossRef]
- Feng, J.-C.; Li, G.; Li, X.-S.; Li, B.; Chen, Z.-Y. Evolution of Hydrate Dissociation by Warm Brine Stimulation Combined Depressurization in the South China Sea. Energies 2013, 6, 5402–5425. [Google Scholar] [CrossRef]
- Phillips, S.C.; Flemings, P.B.; You, K.; Meyer, D.W.; Dong, T. Investigation of in situ salinity and methane hydrate decomposition in coarse-grained sediments by slow, stepwise depressurization. Mar. Pet. Geol. 2019, 109, 128–144. [Google Scholar] [CrossRef]
- Yin, Z.; Huang, L.; Linga, P. Effect of wellbore design on the production behaviour of methane hydrate-bearing sediments induced by depressurization. Appl. Energy 2019, 254, 113635. [Google Scholar] [CrossRef]
- Li, S.; Wang, Z.; Xu, X.; Zheng, R.; Hou, J. Experimental study on decomposition of hydrate reservoirs with different saturations by hot brine injection. J. Nat. Gas. Sci. Eng. 2017, 46, 555–562. [Google Scholar] [CrossRef]

| Run 1 | Run 2 | Run 3 | |
|---|---|---|---|
| Initial pressure (MPa) | 17.93 | 17.9 | 18.02 |
| Formation end pressure (MPa) | 10.56 | 10.43 | 10.58 |
| Hydrate saturation (%) | 27.45 | 27.27 | 27.91 |
| Methane saturation before replacement (%) | 50.77 | 50.68 | 50.8 |
| Water saturation before replacement (%) | 21.78 | 22.05 | 21.29 |
| Net injection volume of NaCl solution (g) | 478.21 | 480.32 | 463.97 |
| Gas production during replacement (mL) | 34,825 | 35,187 | 36,087 |
| Methane saturation after replacement (%) | 4.83 | 3.49 | 3.29 |
| Water saturation after replacement (%) | 57.39 | 59.19 | 59.21 |
| Hydrate saturation after replacement (%) | 37.78 | 37.32 | 37.5 |
| Run 1 | Run 2 | Run 3 | |
|---|---|---|---|
| Amount of NaCl solution injected (g) | 600 | 600 | 600 |
| Injected NaCl solution concentration (wt%) | 7 | 10 | 14 |
| Production pressure (MPa) | 4.7 | 4.7 | 4.7 |
| Average gas production rate (mL/h) | 7799 | 8093 | 7903 |
| Cumulative water production (g) | 877.61 | 901.62 | 914.06 |
| Cumulative gas production (mL) | 58,512 | 56,253 | 56,740 |
| Total gas volume before production (mL) | 83,818 | 81,801 | 81,106 |
| Gas recovery ratio (%) | 69.81 | 68.77 | 69.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, H.; Zhang, Y.; Zhang, L.; Chen, Z.; Li, X. Study on Hydrate Production Behaviors by Depressurization Combined with Brine Injection in the Excess-Water Hydrate Reservoir. Entropy 2022, 24, 765. https://doi.org/10.3390/e24060765
Zeng H, Zhang Y, Zhang L, Chen Z, Li X. Study on Hydrate Production Behaviors by Depressurization Combined with Brine Injection in the Excess-Water Hydrate Reservoir. Entropy. 2022; 24(6):765. https://doi.org/10.3390/e24060765
Chicago/Turabian StyleZeng, Haopeng, Yu Zhang, Lei Zhang, Zhaoyang Chen, and Xiaosen Li. 2022. "Study on Hydrate Production Behaviors by Depressurization Combined with Brine Injection in the Excess-Water Hydrate Reservoir" Entropy 24, no. 6: 765. https://doi.org/10.3390/e24060765
APA StyleZeng, H., Zhang, Y., Zhang, L., Chen, Z., & Li, X. (2022). Study on Hydrate Production Behaviors by Depressurization Combined with Brine Injection in the Excess-Water Hydrate Reservoir. Entropy, 24(6), 765. https://doi.org/10.3390/e24060765







