A New X-ray Medical-Image-Enhancement Method Based on Multiscale Shannon–Cosine Wavelet
Abstract
1. Introduction
2. Wavelet Multiscale Transform Fundamentals
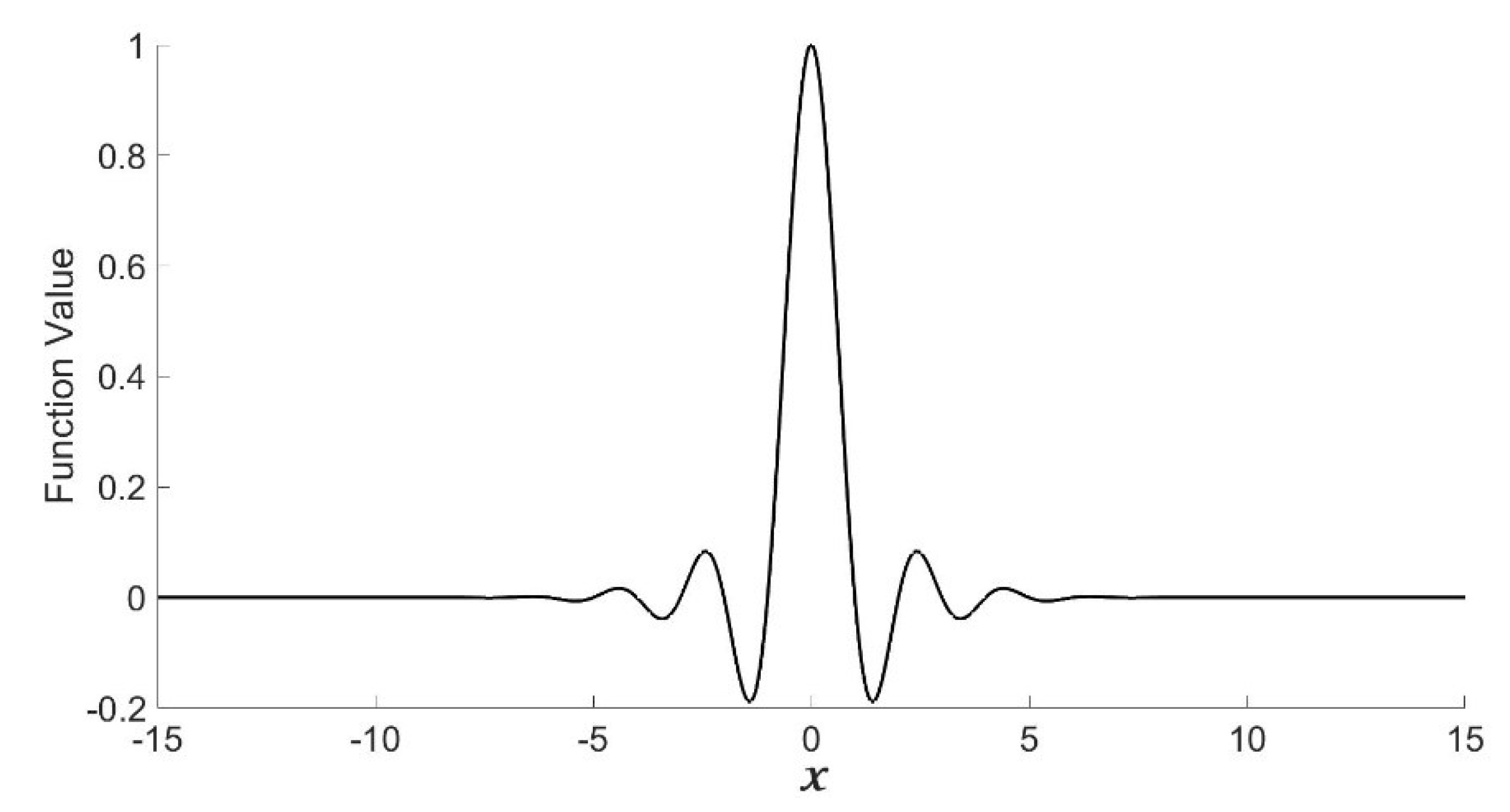
2.1. Shannon–Cosine Wavelet and Their Properties
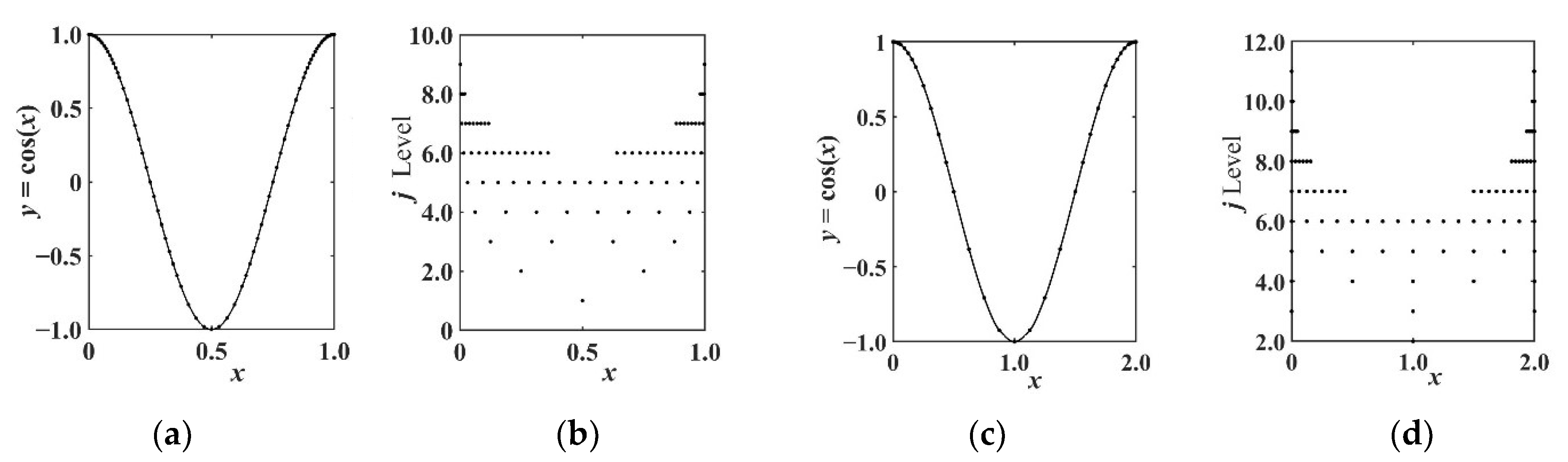
2.2. Multiscale Interpolation Wavelet Operator
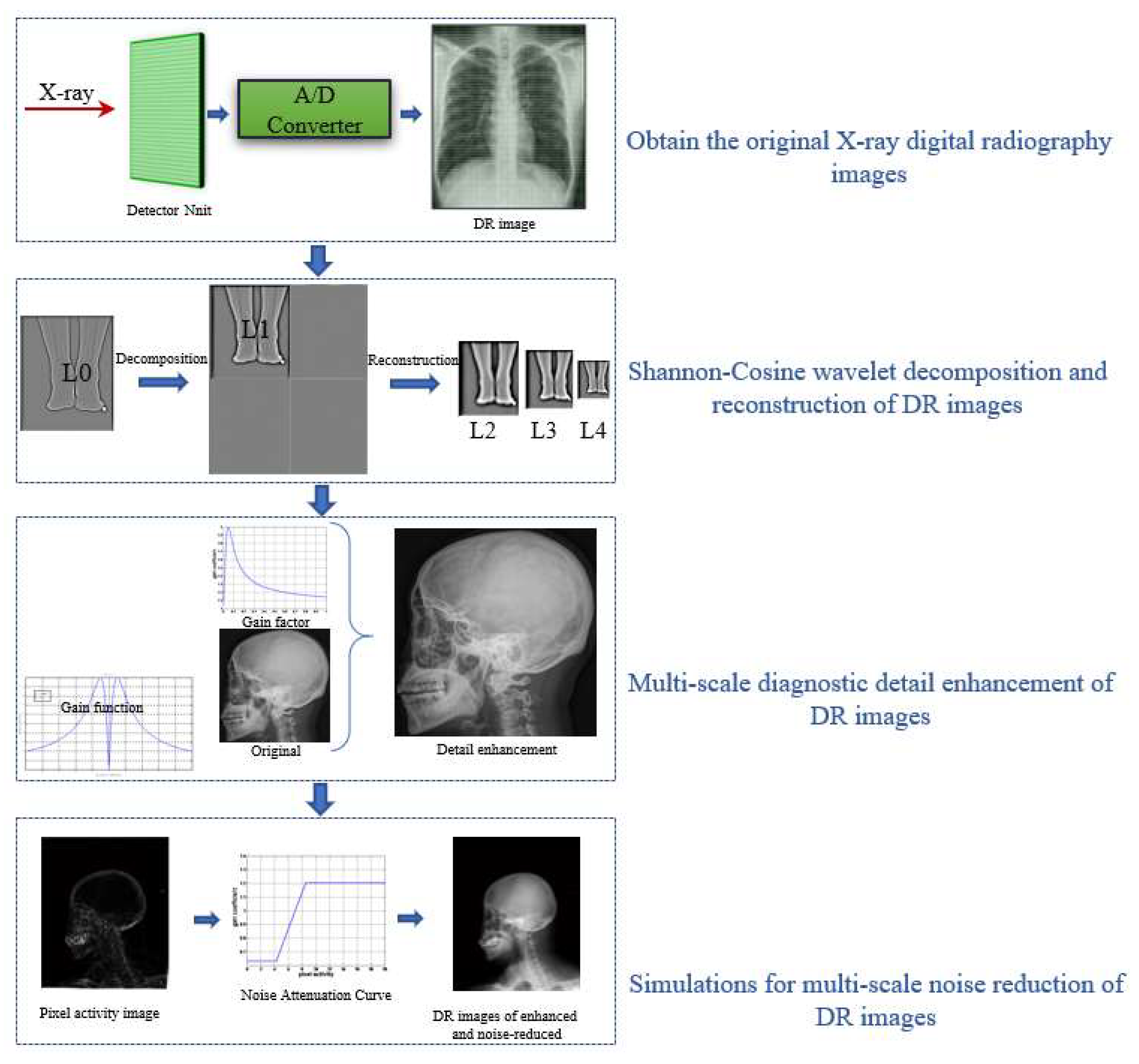
3. Multiscale Digital X-ray Image-Enhancement and Noise-Reduction Algorithm
- 1.
- X-rays are passed through the analog-to-digital (A/D) converter to obtain the original X-ray digital photography image, .
- 2.
- The Shannon–Cosine wavelet transform is applied to the original image by using the multiscale properties of the Shannon–Cosine wavelet. A set of high-frequency images, , , ,… , and a low-frequency image, , are obtained after multilayer decomposition.
- 3.
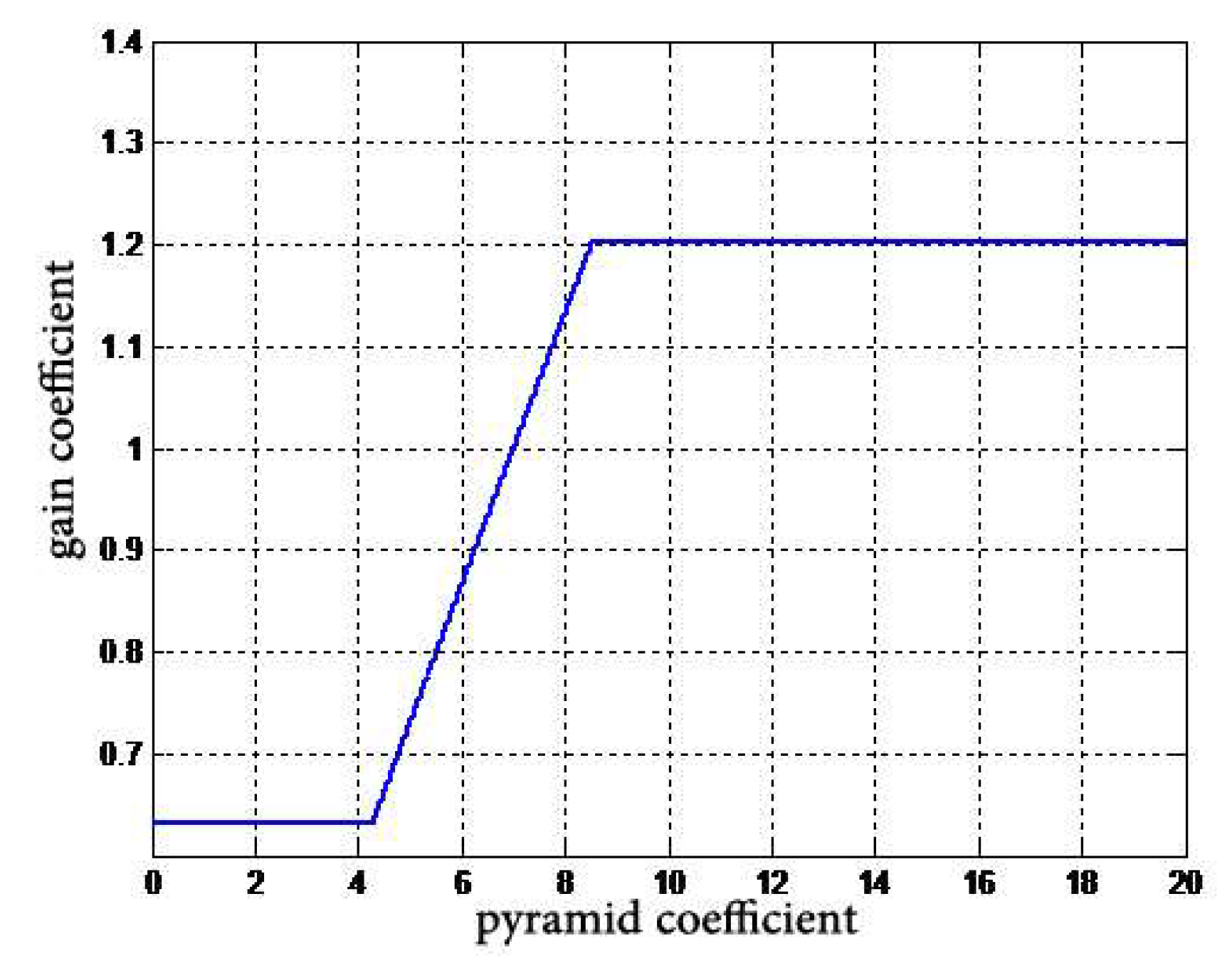
- A nonlinear gain function,is designed to control the degree of enhancement for the large dynamic range of digital X-ray images. The gain coefficient is multiplied by the gray value of each pixel at different scales to change the detail image, and then the image is reconstructed to achieve the enhancement effect.
- 4.
- The degree of pixel activity is defined by calculating the standard deviation within the local neighborhood of the central pixel. The pixel activity level is then bilinearly interpolated to correspond to high-frequency images at different scales, . Finally, the pixel gray-scale values are attenuated and enhanced according to the strength of each pixel’s activity level to achieve noise reduction.
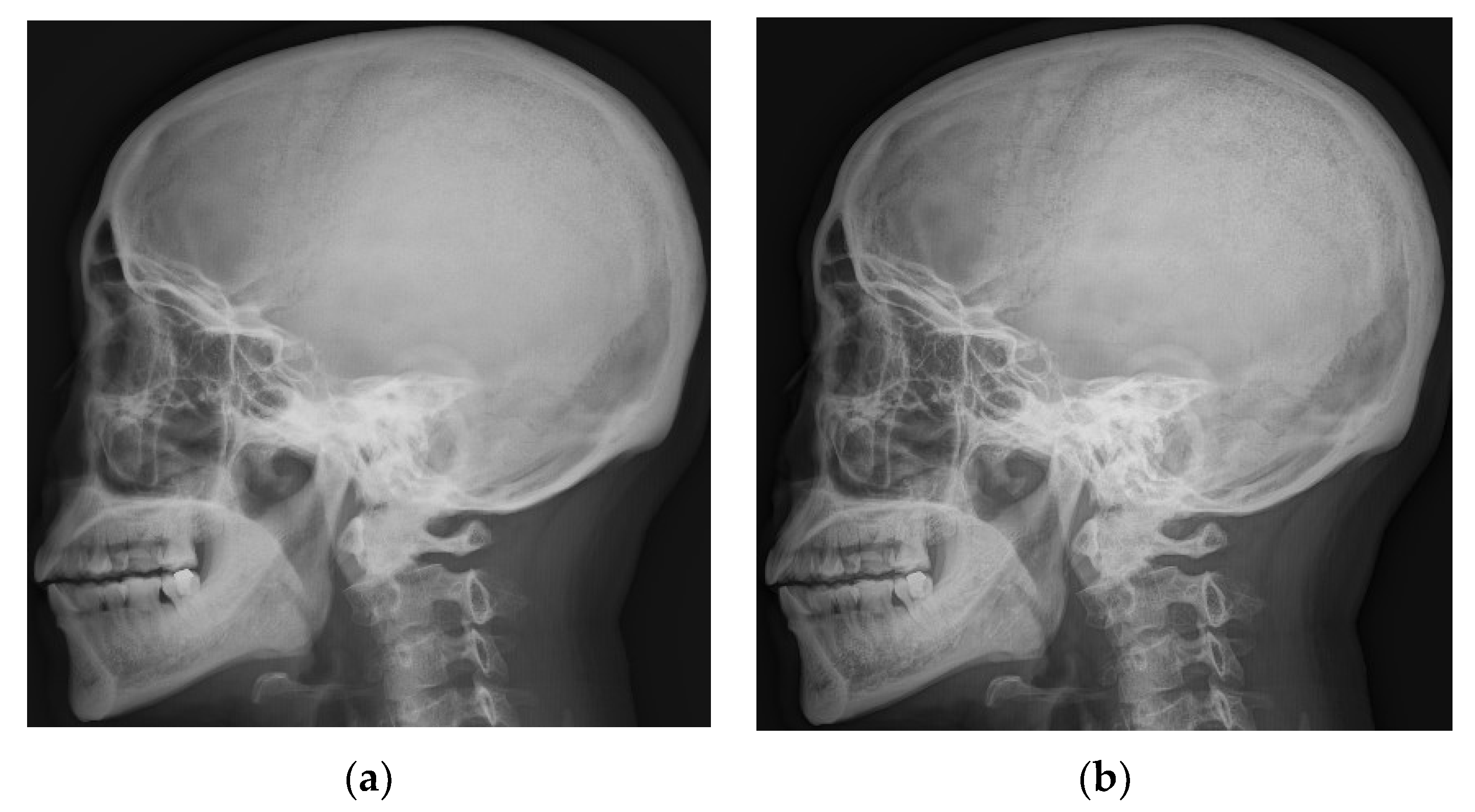
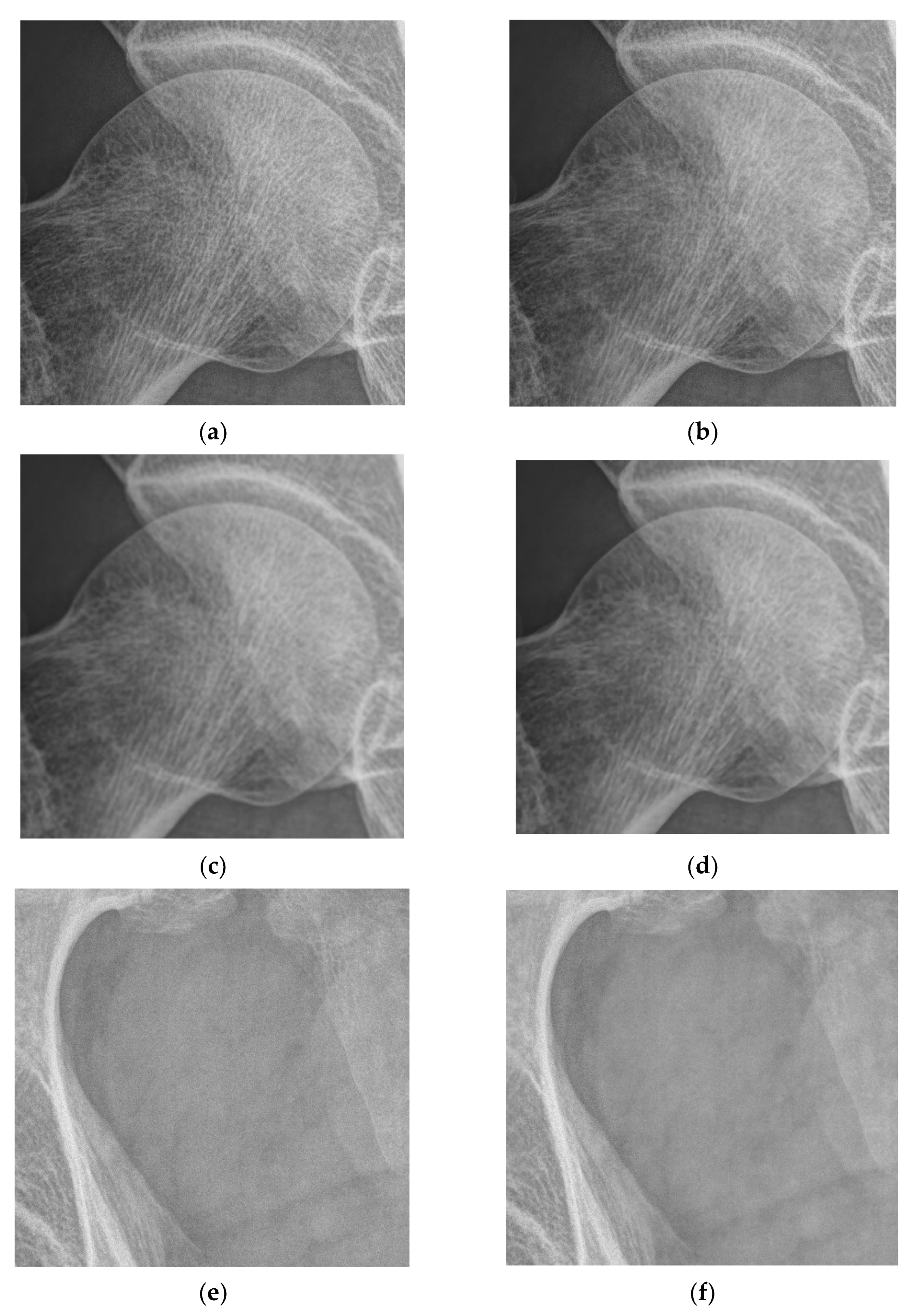
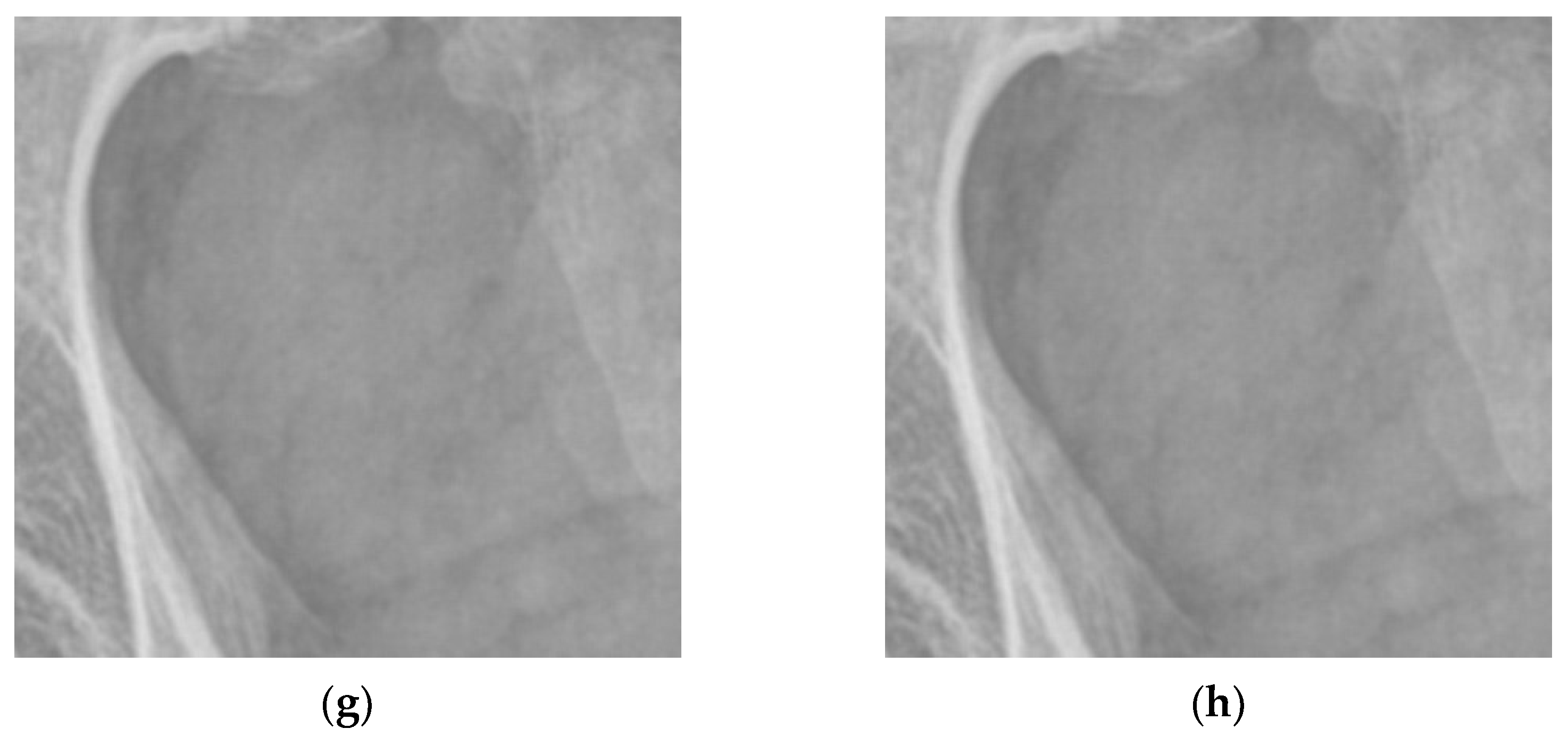
3.1. Multiscale Diagnostic Detail Enhancement on DR Images
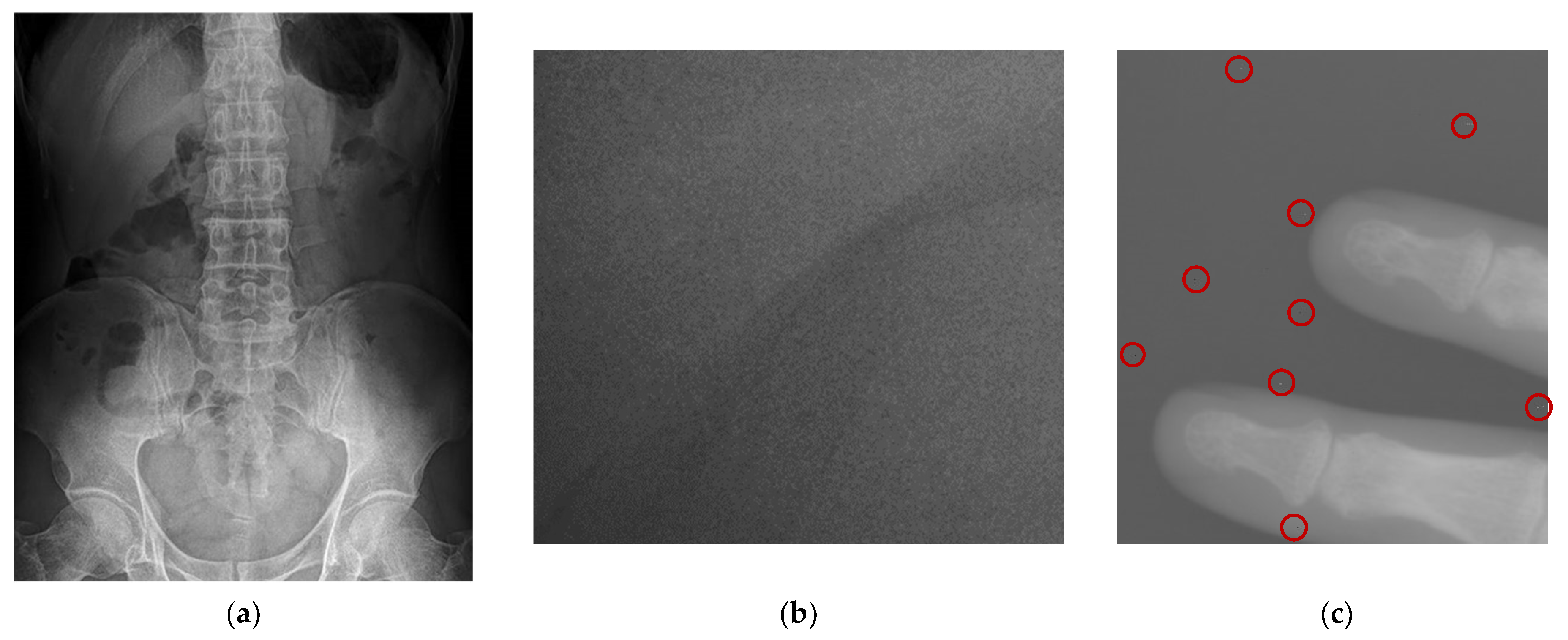
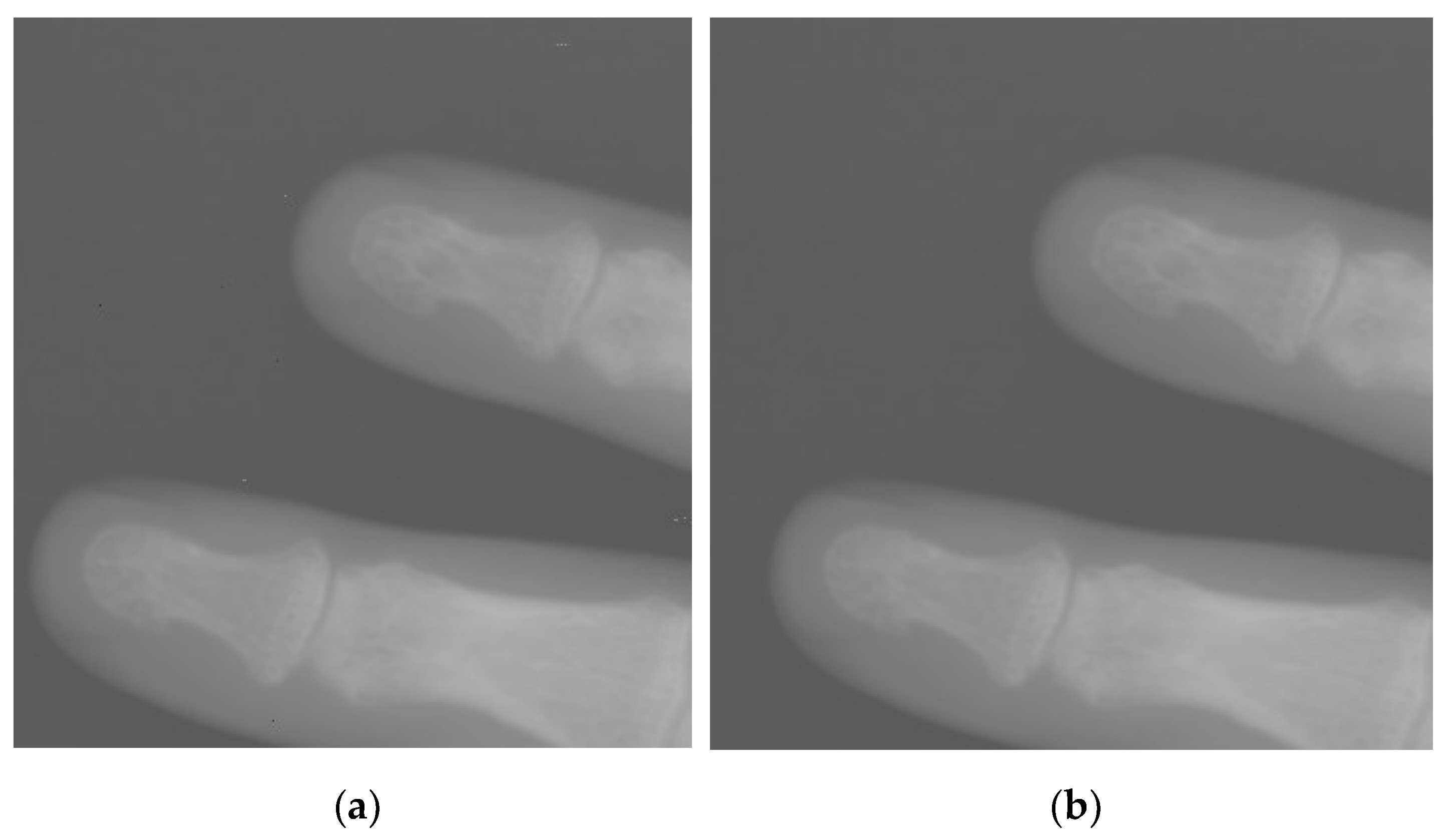
3.2. Adaptive Multiscale Noise Reduction in DR Images
4. Multiscale Medical-Image-Enhancement and Noise-Reduction Simulation
4.1. Simulations for Multicale Diagnostic Detail Enhancement on DR Images
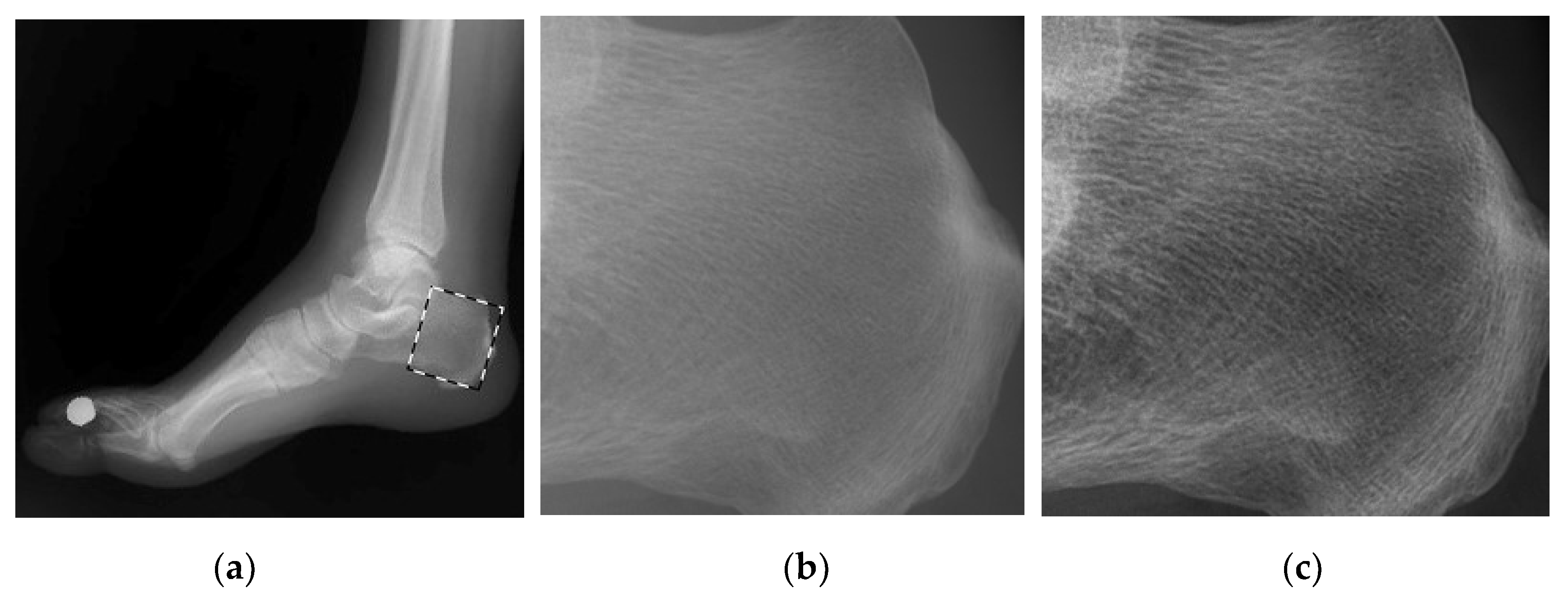
4.2. Simulations for Multiscale Noise Reduction in DR Images
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Reutershan, T.; Effarah, H.H.; Lagzda, A.; Barty, C.P.J. Numerical evaluation of high-energy, laser-Compton X-ray sources for contrast enhancement and dose reduction in clinical imaging via gadolinium-based K-edge subtraction. Appl. Opt. 2022, 61, C162–C178. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xue, W.; Wang, T.; Ni, D. Cardiac Motion Scoring Based on CNN with Attention Mechanism. In Proceedings of the Third International Symposium on Image Computing and Digital Medicine, Xi’an, China, 24–26 August 2019; pp. 128–132. [Google Scholar]
- Zhao, Q.; Yang, W.; Liao, Q. Adasan: Adaptive Cosine Similarity Self-Attention Network for Gastrointestinal Endoscopy Image Classification. In Proceedings of the 2021 IEEE 18th International Symposium on Biomedical Imaging (ISBI), Nice, France, 13–16 April 2021; pp. 1855–1859. [Google Scholar]
- Cai, Y.; Yu, J.G.; Chen, Y.; Liu, C.; Xiao, L.; Grais, E.M.; Zheng, Y. Investigating the use of a two-stage attention-aware convolutional neural network for the automated diagnosis of otitis media from tympanic membrane images: A prediction model development and validation study. BMJ Open 2021, 11, e041139. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.; Sivaraman, J.; Periyasamy, R.; Simi, V.R. An objective method to identify optimum clip-limit and histogram specification of contrast limited adaptive histogram equalization for MR images. Biocybern. Biomed. Eng. 2017, 37, 489–497. [Google Scholar] [CrossRef]
- Ismail, W.Z.W.; Sim, K.S. Contrast enhancement dynamic histogram equalization for medical image processing application. Int. J. Imaging Syst. Technol. 2011, 21, 280–289. [Google Scholar] [CrossRef]
- Isa, I.S.; Sulaiman, S.N.; Mustapha, M.; Karim, N.K.A. Automatic contrast enhancement of brain MR images using Average Intensity Replacement based on Adaptive Histogram Equalization (AIR-AHE). Biocybern. Biomed. Eng. 2017, 37, 24–34. [Google Scholar] [CrossRef]
- Subramani, B.; Veluchamy, M. MRI brain image enhancement using brightness preserving adaptive fuzzy histogram equalization. Int. J. Imaging Syst. Technol. 2018, 28, 217–222. [Google Scholar] [CrossRef]
- Nigam, M.; Bhateja, V.; Arya, A.; Bhadauria, A.S. An evaluation of contrast enhancement of brain MR images using morphological filters. In Embedded Systems and Artificial Intelligence; Springer: Singapore, 2020; pp. 571–577. [Google Scholar]
- Dinh, P.H.; Giang, N.L. A new medical image enhancement algorithm using adaptive parameters. Int. J. Imaging Syst. Technol. 2022, 32, 2198–2218. [Google Scholar] [CrossRef]
- Xu, C.; Xu, L.; Gao, Z.; Zhao, S.; Zhang, H.; Zhang, Y.; Li, S. Direct delineation of myocardial infarction without contrast agents using a joint motion feature learning architecture. Med. Image Anal. 2018, 50, 82–94. [Google Scholar] [CrossRef]
- Tian, X.; Wang, J.; Du, D.; Li, S.; Han, C.; Zhu, G.; Tan, Y.; Ma, S.; Chen, H.; Lei, M. Medical imaging and diagnosis of subpatellar vertebrae based on improved Laplacian image enhancement algorithm. Comput. Methods Programs Biomed. 2020, 187, 105082. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Mei, S. Laplace Multiscale Image Enhancement Denoising Algorithm. Agric. Netw. Inf. 2010, 3, 142–144. [Google Scholar]
- Ruyi, X.; Meng, L.; Meng, K.; Mei, S. Coupling Technique of Haar Wavelet Transform and Variational Iteration Method for nonlinear Option Pricing Model. Mathematics 2021, 9, 1642. [Google Scholar] [CrossRef]
- Mei, S.; Liu, M.; Kudreyko, A.; Cattani, P.; Baikov, D.; Villecco, F. Bendlet Transform Based Adaptive Denoising Method for Microsection Images. Entropy 2022, 24, 869. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Kexin, M.; Ruyi, X.; Mei, S.; Cattani, C. Haar wavelet transform and variational iteration method for fractional option pricing models. Math. Methods Appl. Sci. 2022, 1–10. [Google Scholar] [CrossRef]
- Kexin, M.; Meng, L.; Shujun, G.; Shuli, M. Hermite–Shannon–Cosine Interval Wavelet and Its Application in Adaptive Distribute Interpolation on Curves. J. Comput. -Aided Des. Comput. Graph. 2021, 33, 1573–1581. [Google Scholar]
- Xing, R.; Li, Y.; Wang, Q.; Wu, Y.; Mei, S. Point-Symmetric Extension-Based Interval Shannon–Cosine Spectral Method for Fractional PDEs. Discret. Dyn. Nat. Soc. 2020, 2020, 4565036. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; Liu, L.; Zhao, M.; Mei, S. Coupling technology of OpenSURF and Shannon–Cosine wavelet interpolation for locust slice images inpainting. Comput. Electron. Agric. 2022, 198, 107110. [Google Scholar] [CrossRef]
- Wang, A.; Li, L.; Mei, S.; Meng, K. Hermite Interpolation Based Interval Shannon–Cosine Wavelet and Its Application in Sparse Representa-tion of Curve. Mathematics 2021, 9, 1. [Google Scholar] [CrossRef]
- Vuylsteke, P.; Schoeters, E. Multiscale image contrast amplification (MUSICA). Proc. SPIE 1994, 2167, 551–560. [Google Scholar]
- Stahl, M.; Aach, T.; Dippel, S. Digital radiography enhancement by nonlinear multiscale processing. Med. Phys. 2000, 27, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Xiangdan, H.; Mengjing, Z.; Hongpu, L.; Li, B. Medical Image Enhancement Algorithm Based on Shearlet Domain and Improve Pal-King Algorithm. Laser Optoelectron. Prog. 2019, 56, 031006. [Google Scholar] [CrossRef]
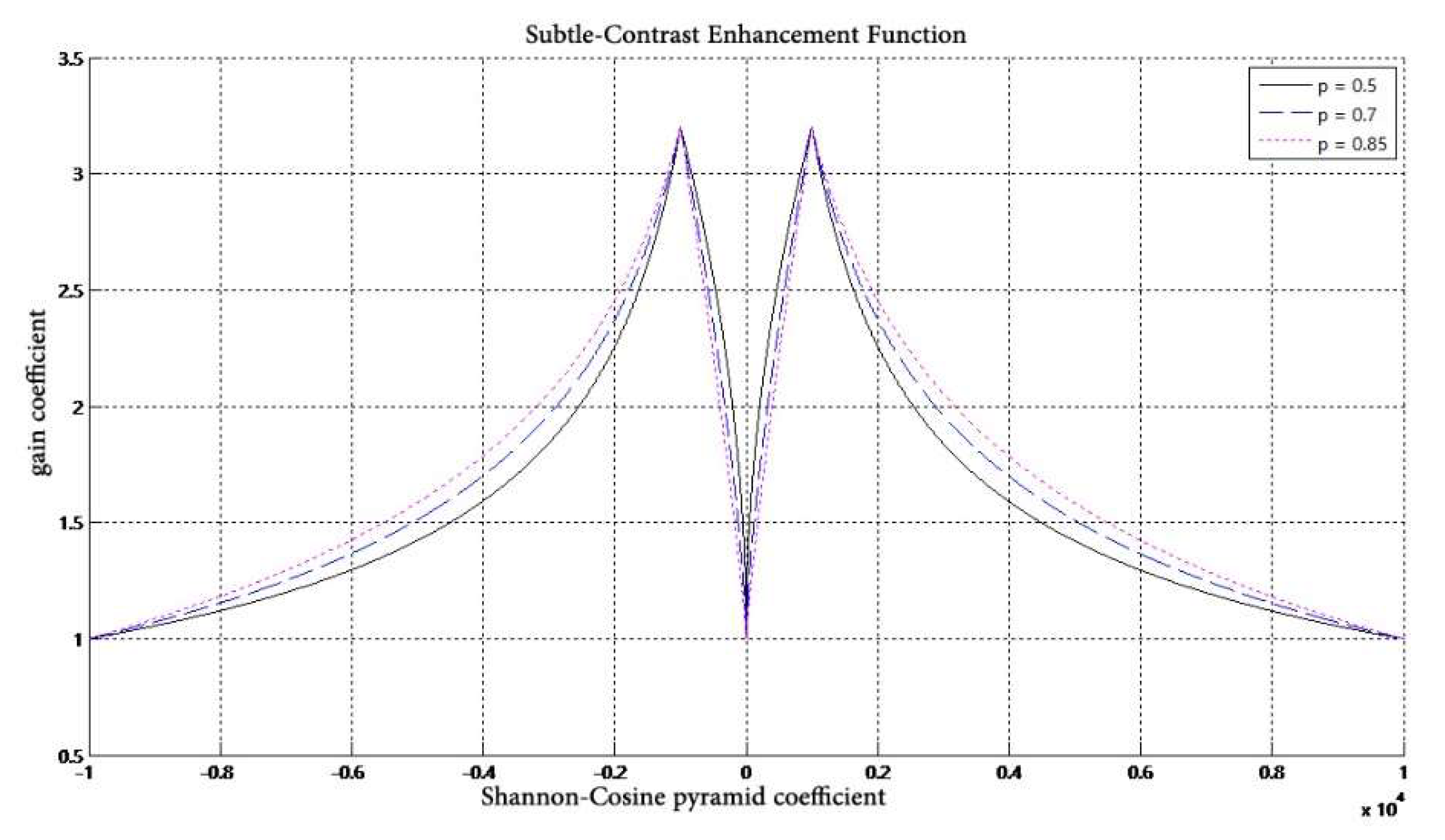
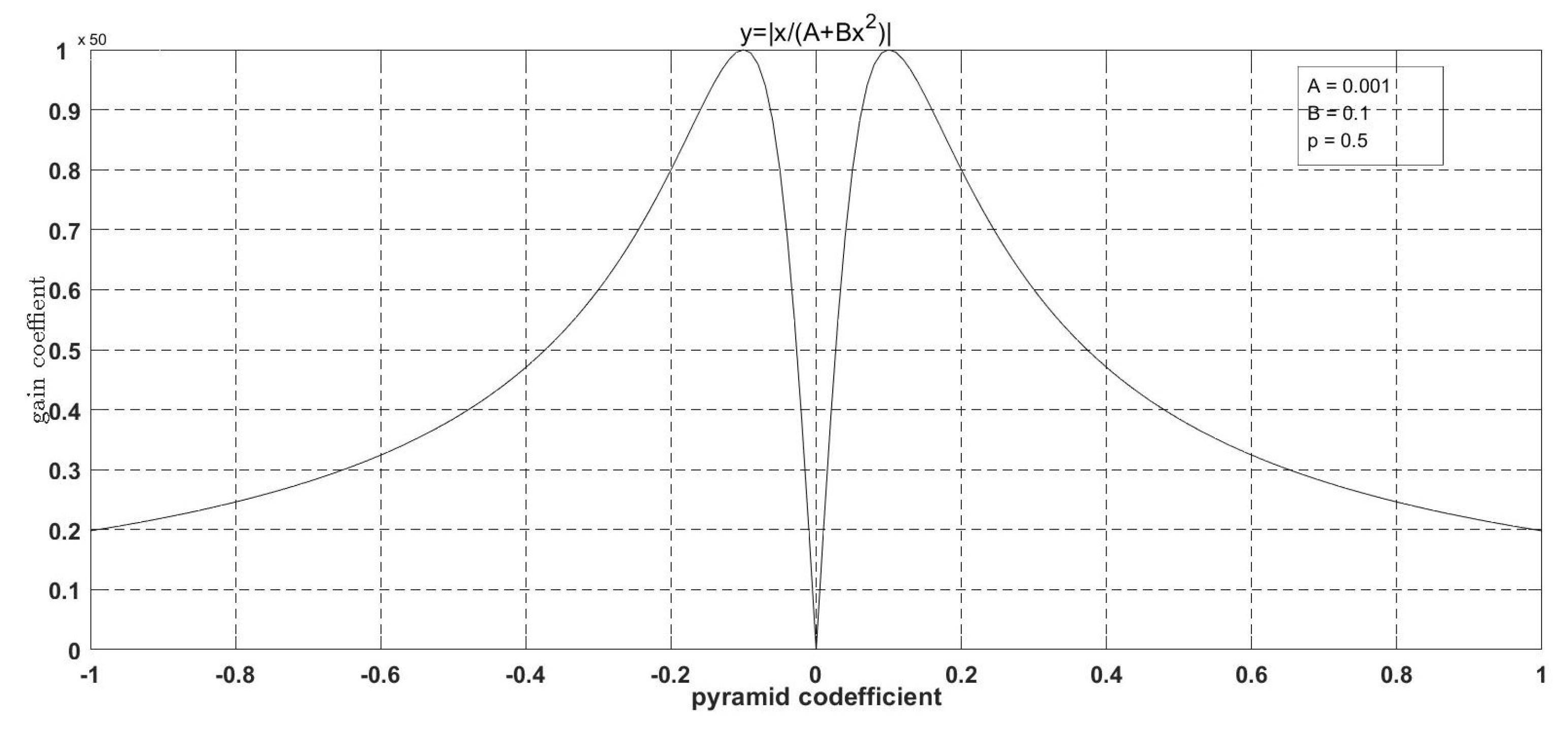
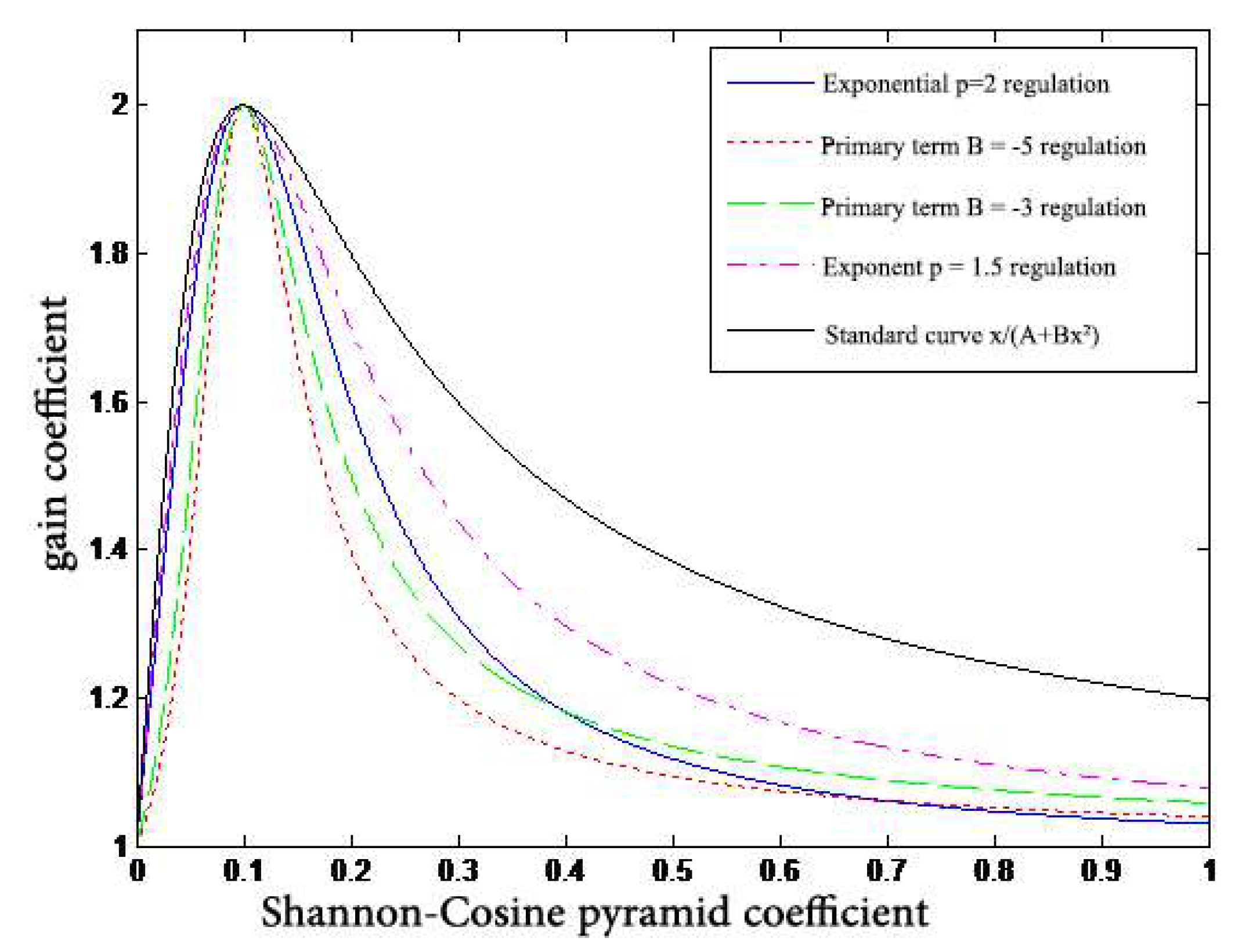
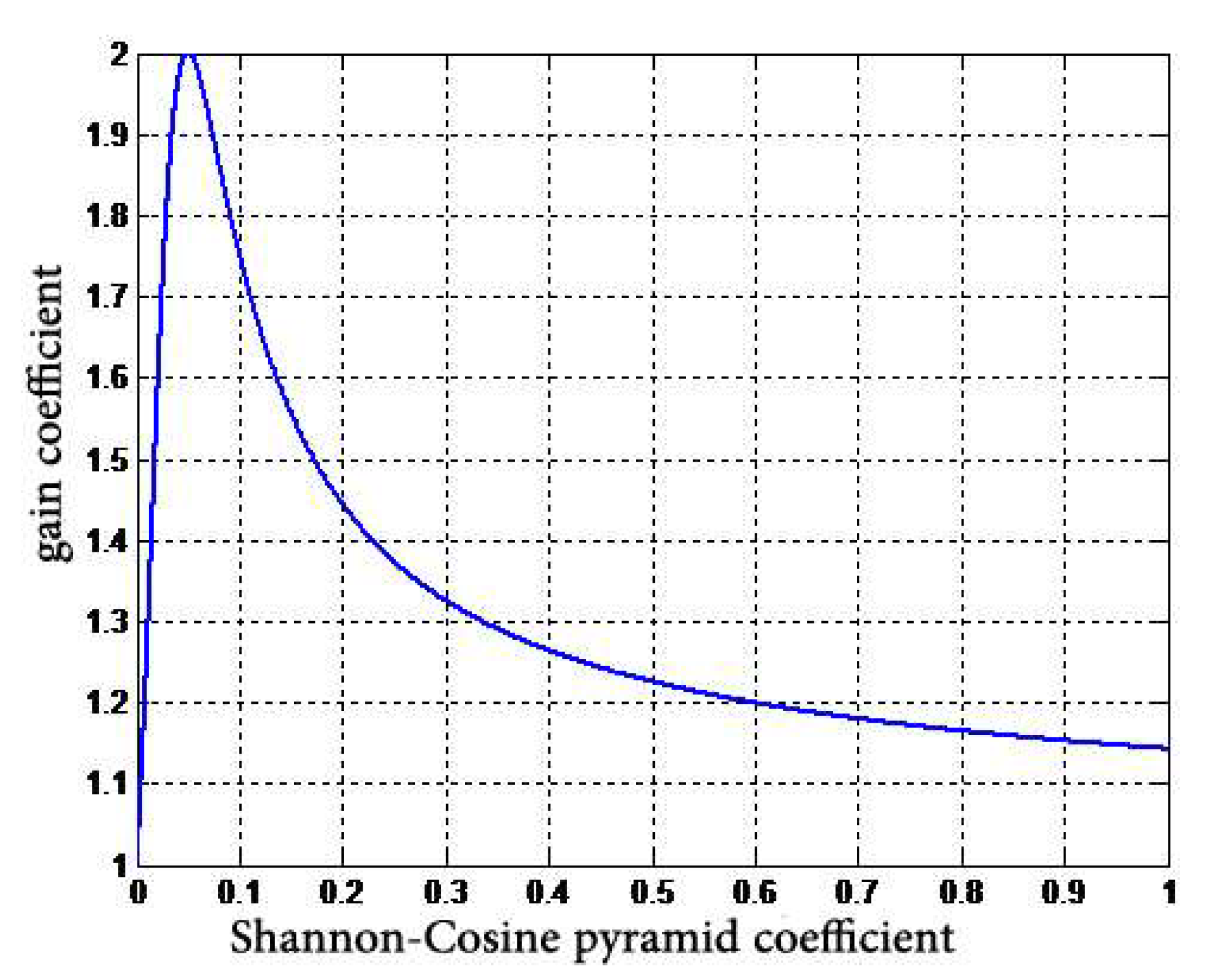


| Algorithms | PSNR | SSIM |
|---|---|---|
| HE | 28.8677 | 0.6946 |
| CLAHE | 32.2087 | 0.8426 |
| Wavelets | 30.4563 | 0.5261 |
| This study | 36.9548 | 0.8297 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Mei, S.; Liu, P.; Gasimov, Y.; Cattani, C. A New X-ray Medical-Image-Enhancement Method Based on Multiscale Shannon–Cosine Wavelet. Entropy 2022, 24, 1754. https://doi.org/10.3390/e24121754
Liu M, Mei S, Liu P, Gasimov Y, Cattani C. A New X-ray Medical-Image-Enhancement Method Based on Multiscale Shannon–Cosine Wavelet. Entropy. 2022; 24(12):1754. https://doi.org/10.3390/e24121754
Chicago/Turabian StyleLiu, Meng, Shuli Mei, Pengfei Liu, Yusif Gasimov, and Carlo Cattani. 2022. "A New X-ray Medical-Image-Enhancement Method Based on Multiscale Shannon–Cosine Wavelet" Entropy 24, no. 12: 1754. https://doi.org/10.3390/e24121754
APA StyleLiu, M., Mei, S., Liu, P., Gasimov, Y., & Cattani, C. (2022). A New X-ray Medical-Image-Enhancement Method Based on Multiscale Shannon–Cosine Wavelet. Entropy, 24(12), 1754. https://doi.org/10.3390/e24121754







