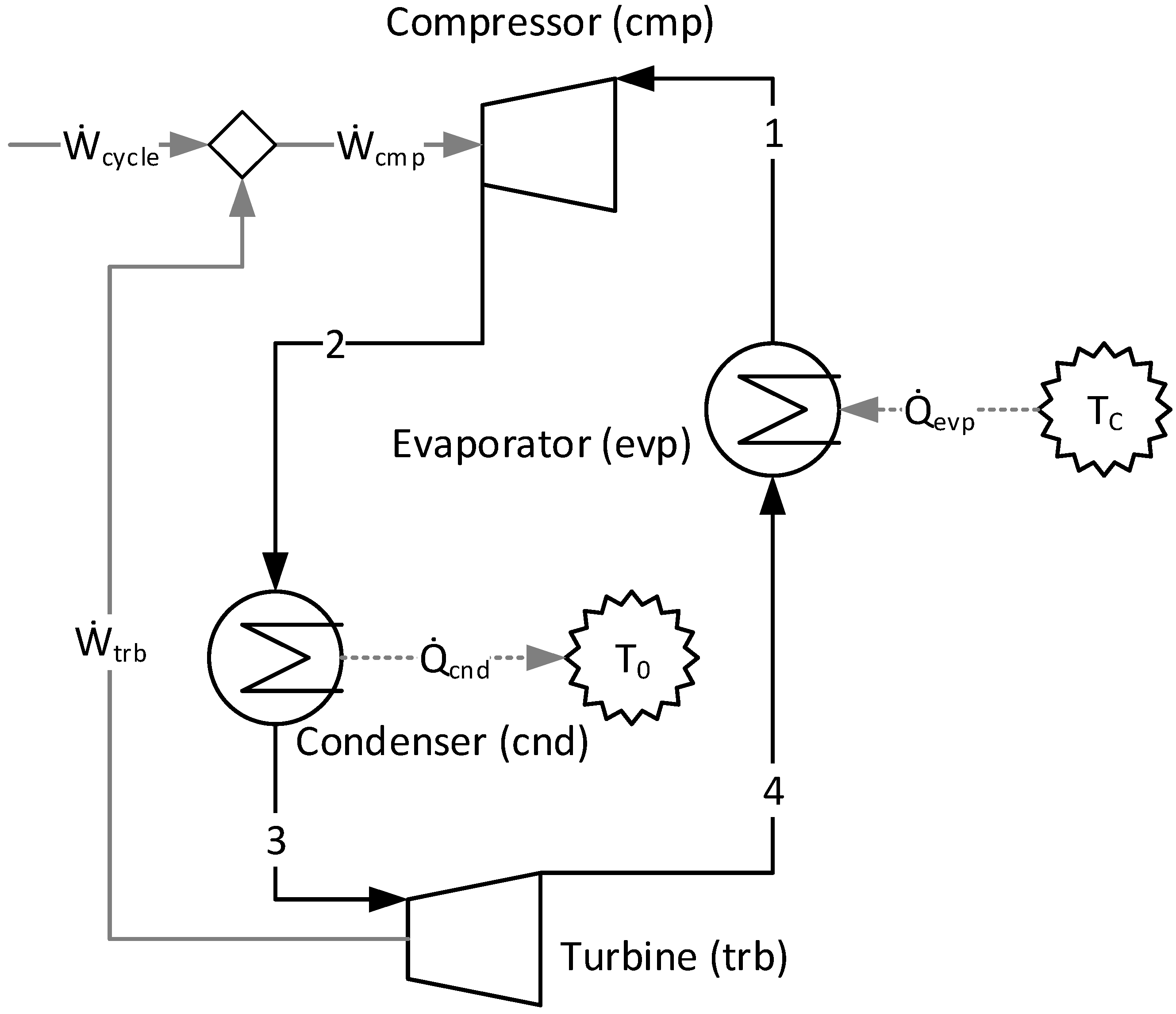
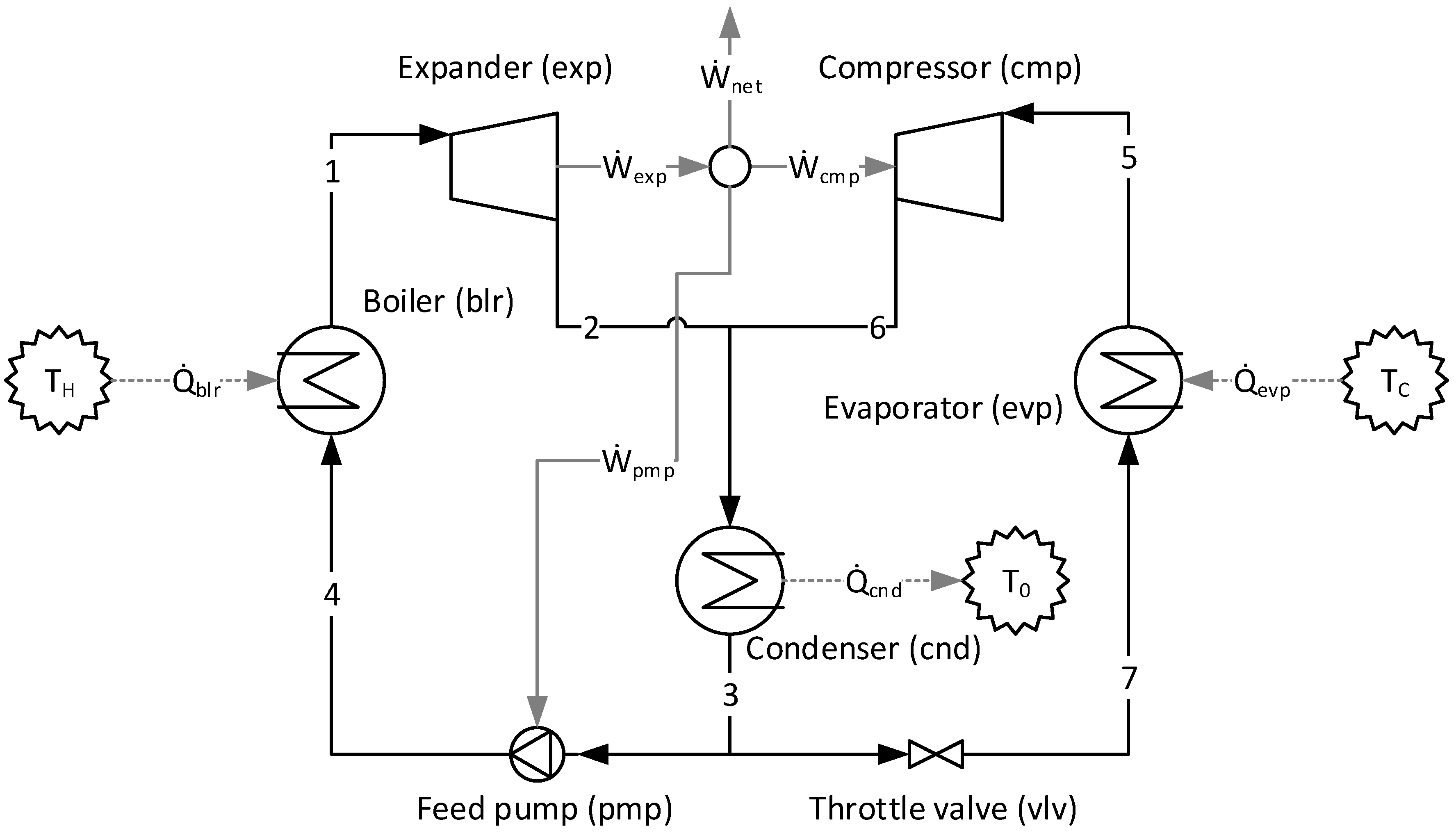
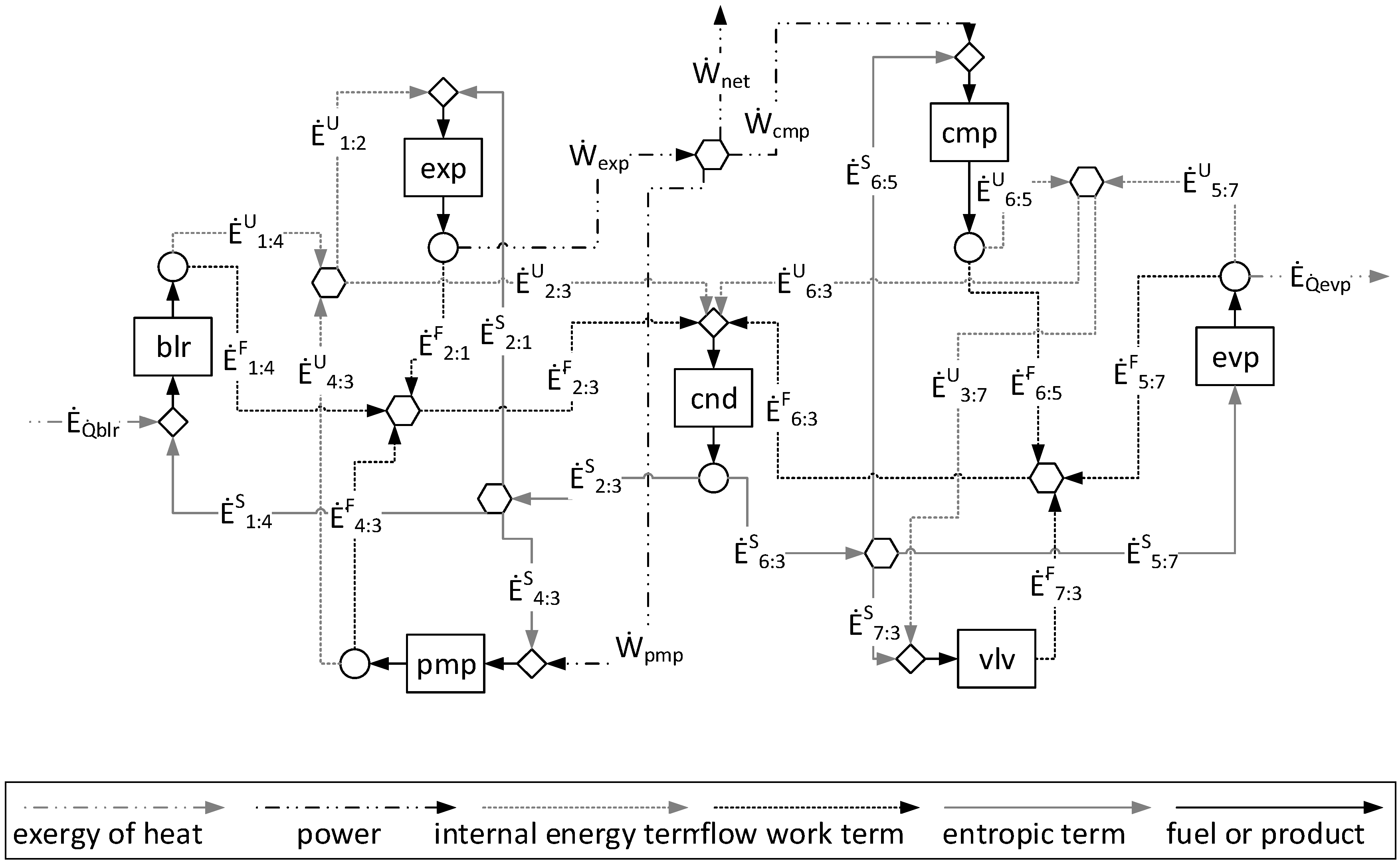
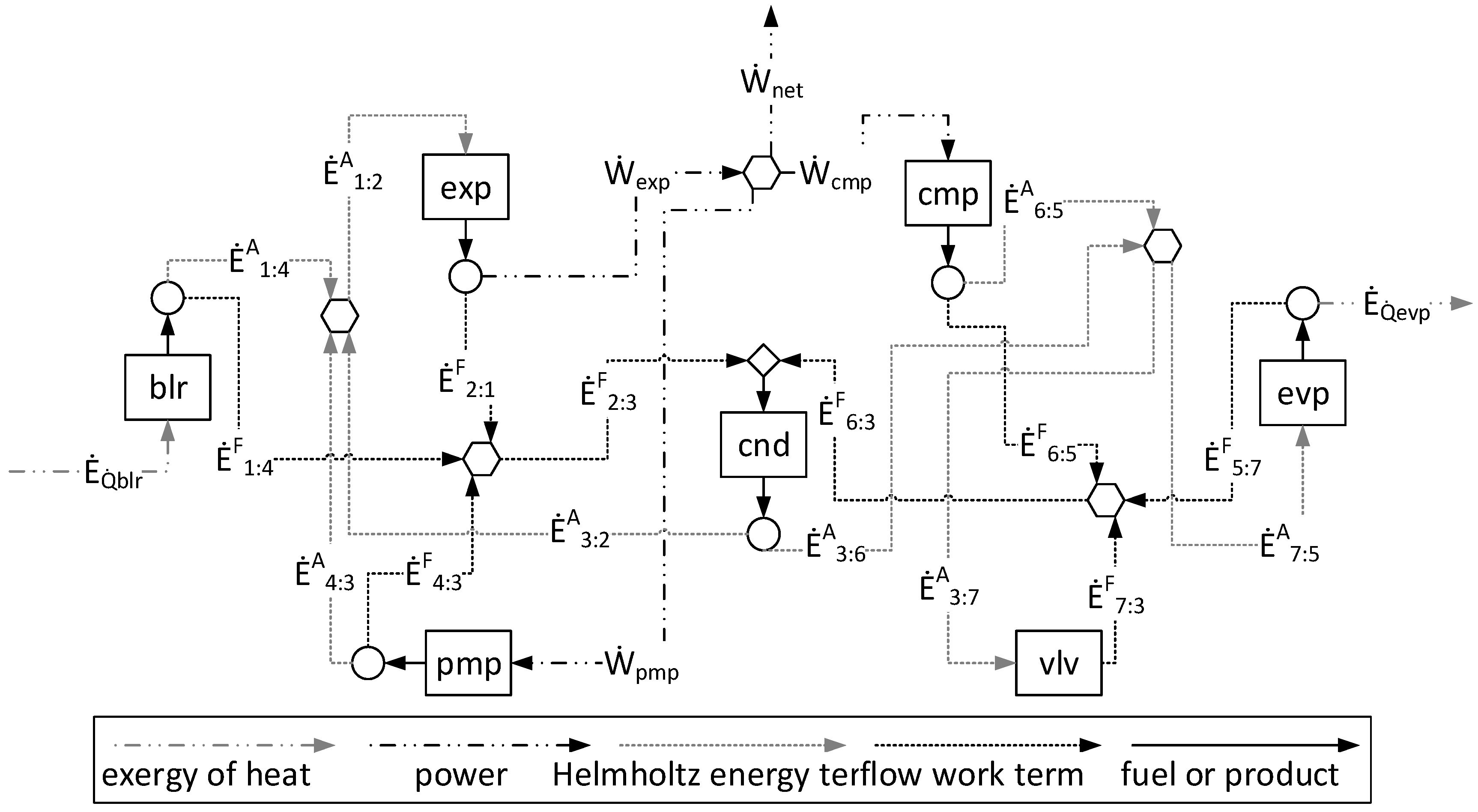
1. Introduction
For elaborate energy systems, the use of traditional energy analysis techniques—established on energy and mass balance (First Law of Thermodynamics)—to solve problems can be either hard, not enough, or inconclusive. Thus, thermoeconomics is a science that, by joining economics and thermodynamics, supplies knowledge to resolve these issues within energy cycles. For instance, logical cost estimation of cycle products is established on physical criteria [
1].
The way in which we define the productive structure (fuel and product) is a key point of thermoeconomic modeling [
2], and the most adequate thermodynamic magnitude to be associated with cost is exergy, according to several researchers [
3,
4]. Since it contains information from both the first and the second laws of thermodynamics, it qualifies the energy streams and identifies the irreversibilities of the subsystems.
Depending on the type of analysis, different levels of accuracy of the results are required, meaning that each thermoeconomic analysis requires a specific disaggregation level of the components and flows [
4]. For local optimization design and operational diagnosis, for instance, total disaggregation of the system components is generally required in order to isolate each of the subsystems.
In agreement with Lozano & Valero [
2], the deeper and more detailed the disaggregation is, the clearer the interpretation of the obtained costs will be and the wider the catalog of applications of theoretical and practical problems.
It is feasible to get more accurate results in thermoeconomic studies when the physical exergy is disaggregated. Nonetheless, there is a consequent increase in the modeling complexity [
5,
6].
The isolation of dissipative components and waste treatment represent two challenges in thermoeconomics. Waste treatment became more evident in the 1990s, during the CGAM problem evaluations [
7], and it directly affects the cost allocation, making it difficult for the discipline to deal with environmental costs. In this regard, several studies have proposed and applied different methods for waste cost allocation in recent years [
8,
9,
10]. The method introduced by [
10] was extended into alternative methodologies by [
8,
9]. Recently, these concepts were used in an application of thermoeconomic diagnostics to a gas turbine [
11].
The problem of dissipative component treatment (the isolation of condensers, valves, intercoolers, etc.) lies in the impossibility of defining their products and/or fuels using total exergy flows only. The total exergy flows cannot guarantee the explicit waste cost allocation (exhaust gases in gas cycle cogeneration plants and combined cycle systems). Thus, this thermoeconomic problem remains open [
8], since the dissipative equipment cannot be isolated in the productive diagrams.
A usual solution is to merge the dissipative with productive equipment, such as condensers with turbines in steam cycles, valves and evaporators in refrigeration cycles, or intercoolers with compressors in gas cycles. However, this solution can be arbitrary, and it limits the quality of thermoeconomic analysis.
Frangopoulos [
12] presented an elegant solution to isolate the condenser in the steam cycles, which used both total exergy and negentropy flows (E&S Model). Nonetheless, the negentropy is a fictitious flow [
8,
13]. Although this proposal allowed the fuel and product of the condensers to be defined, it has already been shown that this solution presents uncommon values of exergetic unit costs, i.e., lower than the unit [
8,
14]. Moreover, this solution does not allow us to define the fuel and product of the valves in order to isolate them in the productive diagrams.
In the study by d’Accadia & Rossi [
15], thermoeconomic modeling of a refrigeration system was carried out, disaggregating both the valve and the condenser, and combining the concept of negentropy with the exergy disaggregated into its thermal and mechanical terms. Nevertheless, it maintains the uncommon results associated with the use of negentropy as a fictitious flow; and this type of disaggregation of exergy into thermal and mechanical terms [
16] is only advisable, without arbitrariness, for fluids modeled as ideal gases [
6]. Additionally, it cannot isolate condensers.
In order to achieve consistent values of exergetic unit costs, Santos et al. [
17] approached the concept and magnitude of negentropy from a different perspective. The authors proposed it as an entropic term to be used in conjunction with enthalpy. In other words, the physical exergy was split into enthalpic (
H − H0) and entropic components (
T0S −
T0S0). This model already considers the dissipative component (condensers and intercoolers) treatment and residue cost allocation naturally through its productive diagram, without the uncommon values obtained when the E&S Model is performed for cost allocation.
The H&S Model (
H: enthalpic term,
S: entropic term) is considered a consistent approach, but it is not capable of defining a product for any of the types of expansion or bypass valves. For the expansion valves of refrigeration and heat pump cycles, a solution proposed for the valve is the use of thermal and mechanical exergy disaggregation by performing the calculation methodology presented by [
18,
19]. However, it is not a generalized approach capable of isolating any type of valves. The UFS Model [
20] (
U: internal energy term,
F: flow work term,
S: entropic term) was then proposed as an extension of the H&S Model to solve this issue by disaggregating the physical exergy into three terms: internal energy term (
U −
U0), flow work term (
pV −
p0V0), and the entropic term (
T0S −
T0S0), while keeping all the characteristics of the H&S Model. Lourenço et al. [
21] showed that internal energy, flow work, and entropic can be interpreted as physical exergy terms.
The UFS Model has been applied for cost allocation purposes [
22], diagnosis applications [
23], and combining cost allocation with environmental analyses [
22,
24].
At this point, it is worth emphasizing that exergy disaggregation for dissipative component isolation plays an important role, not only for cost allocation applications, but also for diagnosis and local design optimization through the Principle of Thermoeconomic Isolation [
25].
One can say that the UFS Model is an extension of the H&S Model, and the application of the first one could only be justified if there were a valve in the system, because of the increase in modeling complexity. To be more precise, the UFS Model presents one more term of exergy to determine the product and fuel of all equipment in the productive diagrams in comparison with the H&S Model.
After presenting a review of the state of the art, it can be observed that a comprehensive methodology combined with a lower degree of associated complexity is always a point of interest and study in thermoeconomics studies. Bearing this in mind, this study aims to present a new exergy disaggregation approach to reduce the complexity of thermoeconomic modeling and also its computational efforts. This new approach, herein called the A&F Model (A: Helmholtz energy term, F: flow work term), disaggregates the exergy into only two terms: Helmholtz energy and flow work.
The novelty of this study is to present this new physical exergy disaggregation approach, the A&F Model, which adequately deals with dissipative components (condensers, intercoolers, and valves) with less modeling complexity.
It is evaluated from a thermodynamic and thermoeconomic point of view through cost allocation in an ideal Carnot Cycle, and it is also applied and compared with the UFS Model in an organic Rankine cycle-powered vapor compression refrigeration system.
2. Physical Exergy Disaggregation and Definitions of Fuel and Product
According to [
13], physical exergy desegregation has been used in thermoeconomics since 1990 and was proposed by Tsatsaronis. In agreement with [
6], the split of exergy improves the accuracy of the results.
The concept of the A&F Model begins with the disaggregation proposed by the UFS Model (internal energy, flow work, and entropic terms). It is possible to combine internal energy and entropic terms, such as
U −
T0S. This rearrangement has a thermodynamic meaning called Helmholtz energy [
26]. Hence, the physical exergy disaggregation is proposed into Helmholtz energy combined with flow work (A&F Model) terms. Thus, the main advantage of this approach is the reduction of complexity associated with the productive diagram since the physical exergy disaggregation will be carried out in only two terms.
Moreover, there is no arbitrariness to disaggregating the physical exergy Equation (1) into its Helmholtz energy term Equation (5) and flow work Equation (6) terms when applying the A&F Model. Thus, for this disaggregation, the physical exergy of the i-th stream is given by Equation (1) when kinetic, potential, and other energy forms are neglected. From Equation (1), the terms of the H&S Model are obtained directly, without arbitrariness, according to the physical exergy of a flow.
Applying the definition of specific enthalpy (
h = u + Pv), Equation (2) is written.
Rearranging Equation (2), the three terms of UFS Model are obtained and given by Equation (3).
The first and third terms of Equation (3) are combined so that Equation (4) is written.
The specific Helmholtz energy of a closed system under a heat bath (reservoir at
T0) is given by
a = u −
T0s. This can be applied to both
i-th and dead states. Equations (5) and (6) show the Helmholtz energy term and flow work terms, respectively. In this way, the physical exergy could be written as in Equation (7), which represents a novel exergy disaggregation model.
In this study, the novel approach shall be called the A&F Model. It is worth mentioning that, for the thermoeconomic methodologies, one of the main points is to know the cost formation process and identify the causes that generate the cost in order to define a function or a productive purpose for each subsystem and plant [
13]. It is noteworthy that different productive structures generate different costs [
2]. According to [
4], irreversibility is the physical magnitude generating the cost. Thus, irreversibility is, qualitatively, the cause of cost generation. Efficiency is the proportion of the desired result for an event to the inlet required to achieve it [
27]. Moran et al. [
26] stated that efficiency gauges how effectively the input is converted to the product. Hence, the concepts of fuel (
Fu), product (
Pr), irreversibility (
Ir), losses (
Lo), and efficiency (
ƞ) are not independent in thermoeconomics, as evidenced in Equation (8) and (9). Equation (8) describes a general balance for each unit and the whole plant. The resources (fuel,
Fu) expended are not fully converted into the product (
Pr) or service due to the irreversibilities (
Ir) and losses (
Lo) associated with the production process. In other words, the efficiency defined by the product fuel ratio is, quantitatively, the parameter for the cost generation index.
It is worth mentioning that in cost accounting methodologies, the values of costs strongly depend on the
Pr/Fu definition of the devices and on the set of auxiliary equations. In other words, the values of costs change depending on the
Pr/Fu definition in the subsystems and also depending on how the different subsystems are interconnected in the productive structure [
4].
In the A&F Model, the physical exergy is disaggregated into Helmholtz energy and flow work terms. The products and the fuels of all subsystems, in terms of Helmholtz energy and flow work terms, are determined in accordance with the quantity of these magnitudes added to and removed from the working fluid, respectively. These two new terms have a positive contribution to the physical exergy (Equations (4) and (7)), and the consideration allows one to define the fuels and the products of the dissipative and productive component.
3. Thermoeconomic Modeling
The thermoeconomic model must be accomplished by utilizing Equations (10) and (11). The resolution of this cost equation set, Equation (10), is the monetary unit costs of all internal flow and final product. The monetary unit cost of a flow is the amount of external monetary units required to obtain one unit of this flow, meaning that the monetary unit costs of a flow are the measure of the economic efficiency of the production process when producing it [
4].
In Equation (10),
Z represents the external hourly cost of the subsystem due to the capital, operation, and maintenance costs of each subsystem (in
$/h);
is consumption (kW) and
is the monetary unit cost, both from the external fuel exergy. The unknown variables
cout and
cin are the monetary unit cost of the internal flows at the outlet and at the inlet of each subsystem (in
$/kWh), respectively; and
and
mean the generic thermodynamic magnitude of the internal flows at inlet and outlet of each subsystem. The solution of the set of equations results in the monetary unit costs of each internal flow and each final product [
28]. In this paper,
assumes the thermodynamic magnitudes, for power (
W), heat exergy (
Q), total exergy (
E), Helmholtz energy term (
EA), flow work term (
EF), internal energy (
EU), and entropic term (
ES).
In Equation (11), the unknown
and
are the exergetic unit costs of the internal flows (output/input) of every subsystem; and the hourly cost of the subsystem, due to the capital cost, operation, and maintenance, must be zero (
Z = 0). Since there is no information about the resource,
is assumed to be 1.00 kW/kW [
4]. The exergetic unit cost of a flow is the amount of external exergy unit required to obtain one unit of this flow, meaning that the exergetic unit cost of a flow is a measure of the thermodynamic efficiency of the production process when producing this flow [
4].
Each subsystem has a single cost equation, thus auxiliary equations are necessary. Thermoeconomic models based on the productive diagrams consider the equality criteria [
29,
30,
31], where productive flows exiting the same productive unit must have the same unit cost.
5. Results and Discussions
The thermoeconomic results of the case studies presented in
Section 4 are presented in
Section 5. The exergetic unit cost of the Carnot refrigeration cycle by the A&F Model in
Section 5.1 and a comparison discussion between the A&F Model and the UFS Model, considering both the design point analysis in
Section 5.2 and a parametric study analysis, is presented in
Section 5.3 for the ORC–VCR.
5.1. Carnot Refrigeration Cycle
It is possible to observe, when using the A&F Model in the productive diagram for the Carnot refrigeration cycle, the values of the productive flows and their respective exergetic unit costs in
Table 4.
When applying the cost allocation of a thermoeconomic model in a Carnot refrigeration cycle, it is expected (from a thermodynamic point of view) that the value of the products and the fuels of each subsystem are equal and, consequently, the exergetic unit cost of each productive flow and the efficiency are equal to one (there are no irreversibilities). Additionally, the four conversion processes of this cycle are isolated in the productive diagram. All of this information can be observed and comprised in
Table 4 and/or
Figure 2. It may, therefore, be affirmed that the A&F Model is a consistent approach.
5.2. Design Point (ORC-VCR)
Table 5 shows the values of fuel (
Fu), product (
Pr), irreversibility (
Ir), and efficiency (
η) of each component of the plant according to the approaches applied. Note that although they define different productive structures (different fuel and products in each production unit, as can be seen in
Figure 4 and
Figure 5), the irreversibility (the entity generating costs) of each component is the same. However, the methodologies determine different costs since the fuels and products of the productive units are different. It is extremely important to point out that different productive structures (fuel and product) generate different costs [
2] and, depending on the productive structure definition (fuel and product), different cost values can be obtained [
4]. By comparing the E Model with the A&F Model, three pieces of equipment have the same efficiency (boiler, feed pump, and compressor). It is plausible since the sum of Helmholtz energy and the flow work defines the total physical exergy, and the Helmholtz energy is a summation of internal energy and entropic terms.
It is worth mentioning that the A&F Model obtains efficiency values equal to or closer to the conventional efficiency based on total exergy flow (E Model) than the UFS Model. In addition to isolating the valve and condenser with a coherent product/fuel ratio, the A&F Model does not significantly differentiate the efficiency of other productive equipment concerning the E Model.
By using the A&F Model, the product-fuel ratios (efficiency) have results between zero (if the processes are totally irreversible) and one (if the processes are totally reversible) for each component present in the plant (even the dissipative ones, such as the condenser), as can be seen in the results of this study presented in
Table 5. The same conclusion can be extracted for the product-fuel ratio considering the UFS model.
Finally, note in
Table 5 that although the models define different productive structures (different fuel and products in each production unit), the irreversibility (
Ir) (the entity generating costs) of each component is the same. Thus, it can be stated that the models are coherent from a thermodynamic point of view. The cost allocation models had the challenge of determining the exergetic unit cost of net power and useful heat. Regardless of the allocation method, the results are a pair of exergetic unit costs for both final products along the defined straight line. Thus, for each model, the higher the exergetic unit cost of the useful heat exergy, the lower the exergetic unit cost of the net power. The straight-line solution is formed by two thermodynamic parameters: the overall exergetic efficiency and the ratio between the final products of the plant. Thus, independently of the kind of cogeneration plant, the straight line will be the same if there is no change at these two parameters.
Figure 6 illustrates the straight line with the results estimated by the A&F and UFS Models for the case study. The useful heat exergy and net power unit costs can be considered as coherent results when intercession of both (ordered pairs) are on the line with the solution, as can be observed at the two points in
Figure 6. The comparison between both methodologies, using the productive diagram, shows a distinction in the exergetic unit cost of net power of around 17%. Consequently, the useful heat unit cost differs by around 20% between both models.
Table 6 confirms that when comparing the A&F and UFS thermoeconomic models, a distinct behavior can be observed, meaning that the A&F model overcharges the useful heat to the detriment of net power. This is explained by the difference in the thermodynamic properties associated with the product and the fuel for each subsystem.
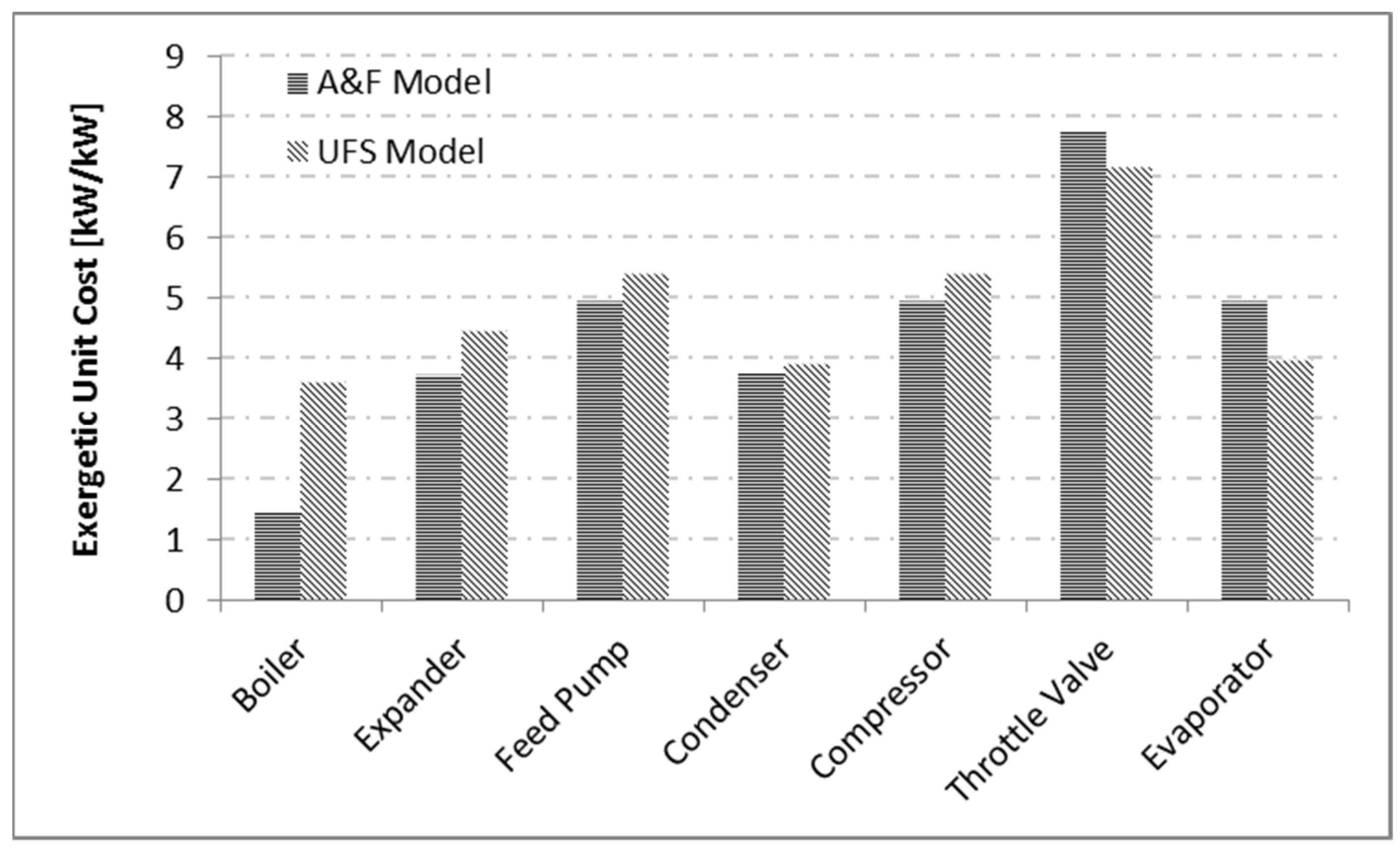
Figure 7 illustrates the results obtained for the subsystem product unit cost by the A&F and UFS models. Even when a device has more than one product, the exergetic unit costs of these products are the same. There is a similar tendency in the cost behavior of equipment products. For example, boiler products have the lowest exergetic unit cost; and this occurs because it is where the system cost formation process begins. The valve, on the other hand, has the highest exergetic unit cost for products among all equipment. It is noteworthy that all products in the system have a unit cost greater than one.
Although the exergetic efficiency of the boiler in the UFS model has a high value, the entropic term, that is, fuel for this equipment, has a high cost. Consequently, the cost of the products for this equipment by the UFS Model is also high. Regarding the A&F model, which has the same efficiency as the E model, despite not being highly efficient, its fuel is only associated with fuel cost, therefore the costs of its products are not high.
Table 7 shows all the productive flows, their internal energy, flow work, entropic, and Helmholtz terms values, and their respective exergetic unit costs. It is noteworthy that all the results are consistent since they are not less than one.
For all the models used in this study, the products of the equipment are less than its fuels. Consequently, all costs are greater than one.
Regarding the modeling complexity, by comparing the A&F and UFS models represented in the productive diagrams for the ORC–VCR system (
Figure 4 and
Figure 5), it is observed that the A&F model has reduced the quantity of flows by approximately 27%, the quantity of junctions–bifurcations by 33%, and the quantity of cost equations by 38%, in relation to the UFS Model. Thus, both models are able to isolate the dissipative components of the system, but the complexity associated with the A&F model is significantly smaller than that of the UFS model. This comparison of complexity considering the quantities of flows, junctions–bifurcations, and auxiliary equations is highlighted in
Table 8.
5.3. Parametric Study (ORC–VCR)
A parametric analysis is carried out to investigate the effects of several parameters, such as equipment efficiency and temperature variations, on the exergetic unit cost and product-fuel ratio (P/F), obtained by applying the E, A&F, and UFS models in the case study of the ORC–VCR system. The E model was applied for thermodynamic reference only, avoiding unnecessary arbitrariness involved in dissipative equipment.
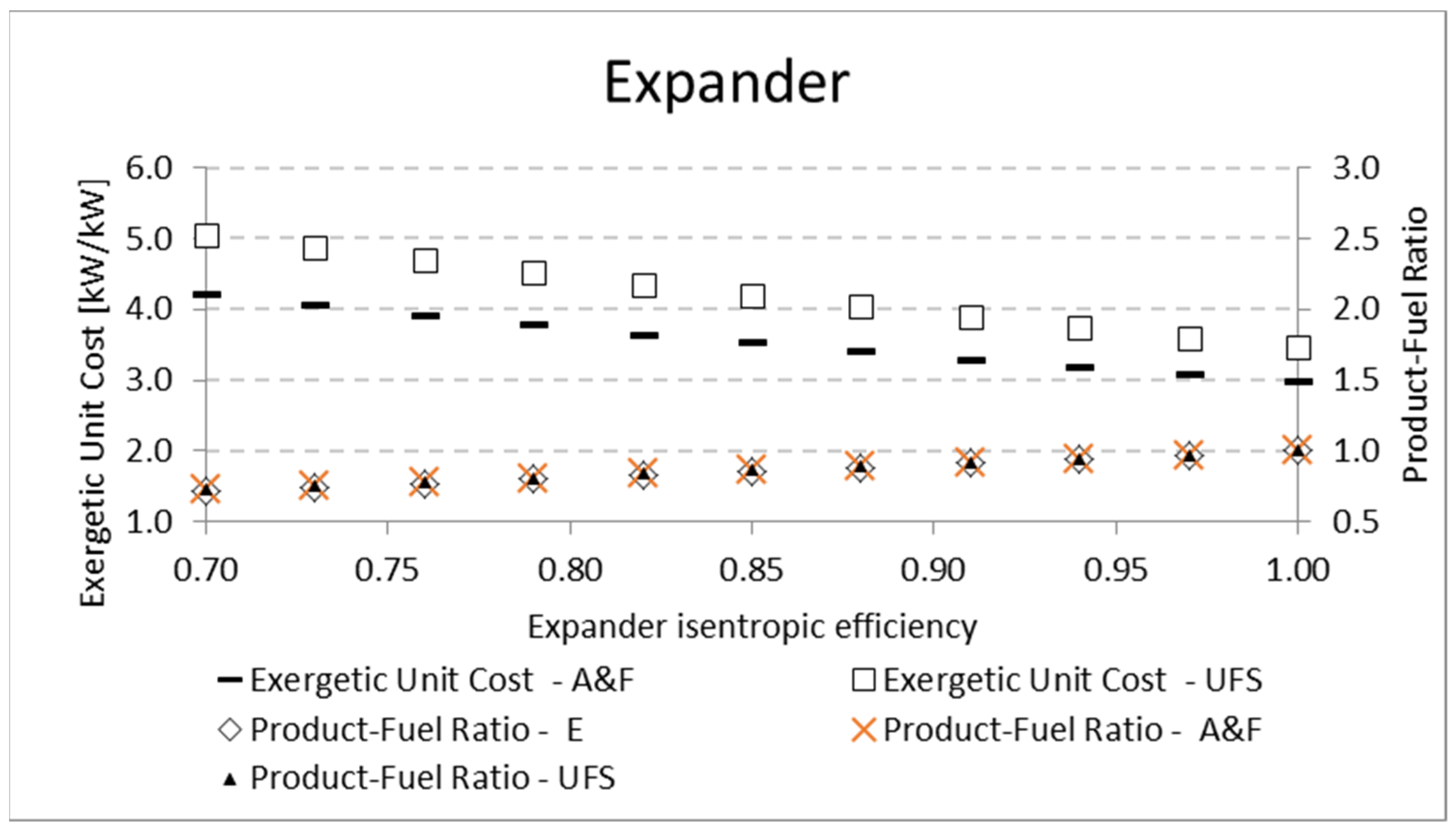
Table 9 shows the variation of expander isentropic efficiency and its influence on the product-fuel ratio according to the E, A&F, and UFS Models.
Figure 8 shows how the exergetic unit cost of the expander product and product-fuel ratio (
P/F) vary with an expander isentropic efficiency variation. The higher the isentropic efficiency, the higher the exergetic efficiency (
P/F) due to the lower irreversibility generation for both the A&F and UFS models. In addition, the
P/F, regardless of the thermoeconomic model, increases until it reaches 1 for a fully reversible and adiabatic process (efficiency of 100%). Consequently, the exergetic unit cost will be lower, as shown in
Figure 8. Although the
P/F ratio seems to be the same (overlapping) in
Figure 8, there is a small difference between the E model and the remaining ones (A&F and UFS), as shown in
Table 9. This small difference was expected due to the different manner that the E model defines the product and fuel of the expander compared to the A&F and UFS models (
Table 3).
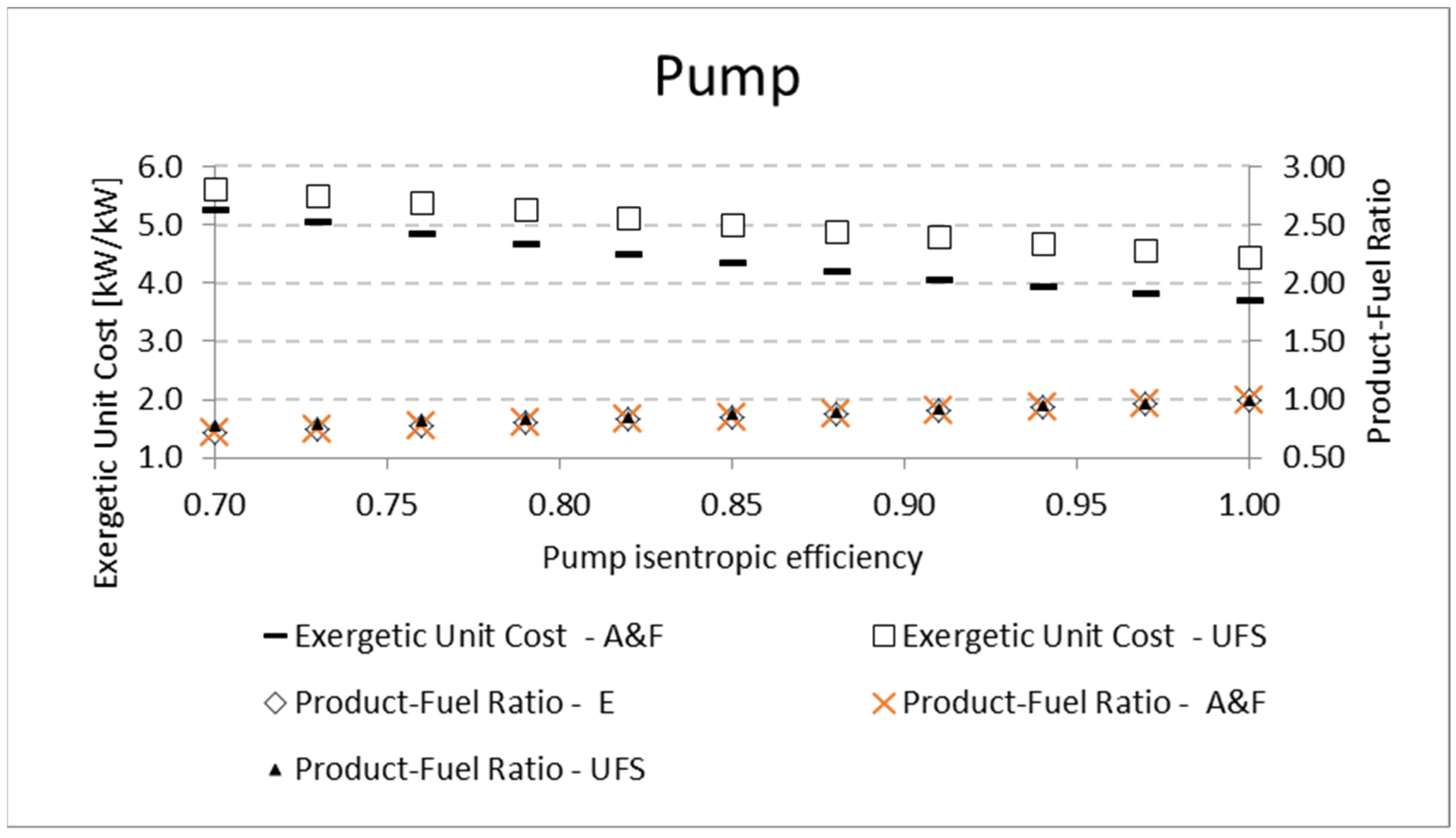
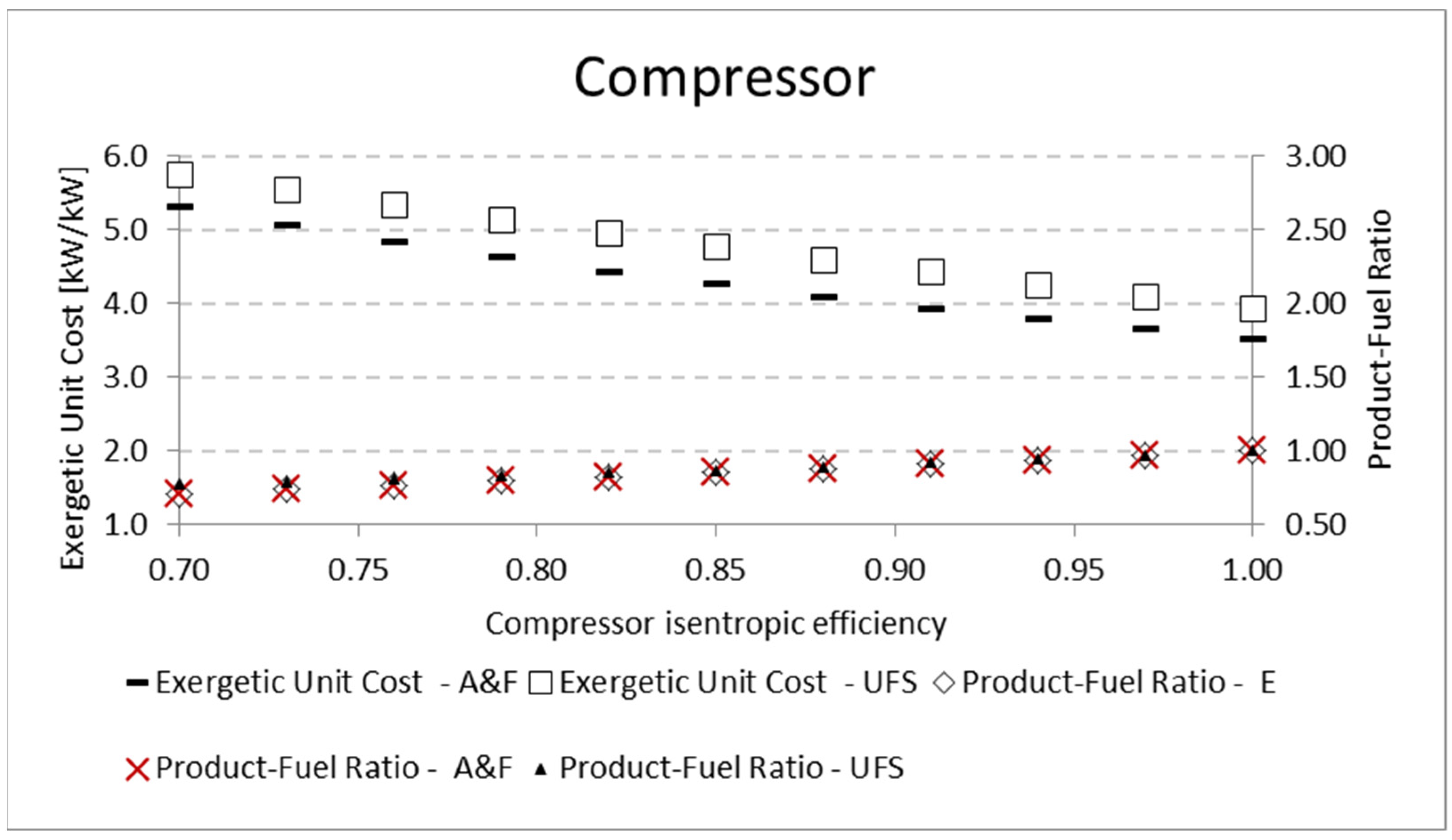
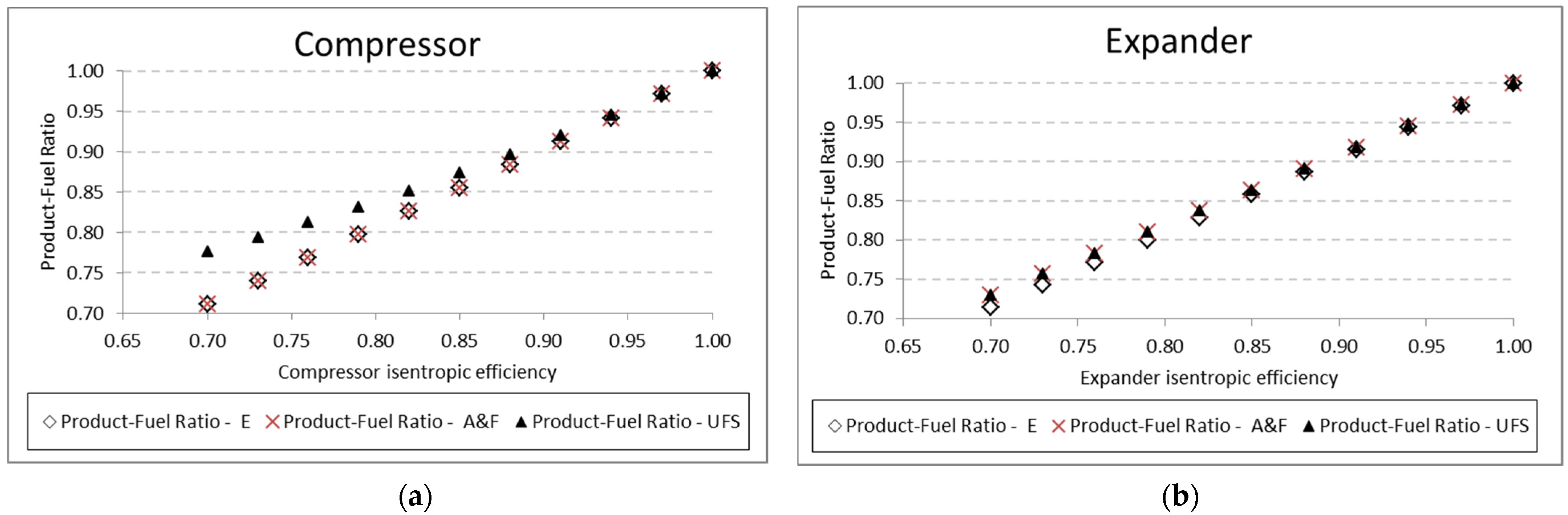
A similar analysis is performed for the pump and the compressor. The results for the product-fuel ratio and exergetic unit cost present the same tendency as those obtained in the analysis of the expander, increasing the isentropic efficiency and reducing the unit cost, due to the increase in the
P/F. The same kinds of results are obtained for the compressor. For the pump, the results are shown in
Table 10 and
Figure 9. For the compressor, similar results are presented in
Table 11 and
Figure 10. In these analyses, for the three mentioned components (expander, pump, and compressor), the variation in the exergetic unit cost of the equipment products, obtained by the A&F and UFS models, is in accordance with the definition of the fuel and product of each model in its productive diagram, as shown in
Table 3 and explained in
Section 4.2.
For the evaporator and boiler, the parametric analysis is carried out by increasing the temperature variation (Δ
T) between the working fluid and external reservoirs, T
COLD and T
HOT, respectively. Regarding the exergetic unit cost, both analyses for the evaporator and boiler present the same tendency: the increase in the temperature difference (Δ
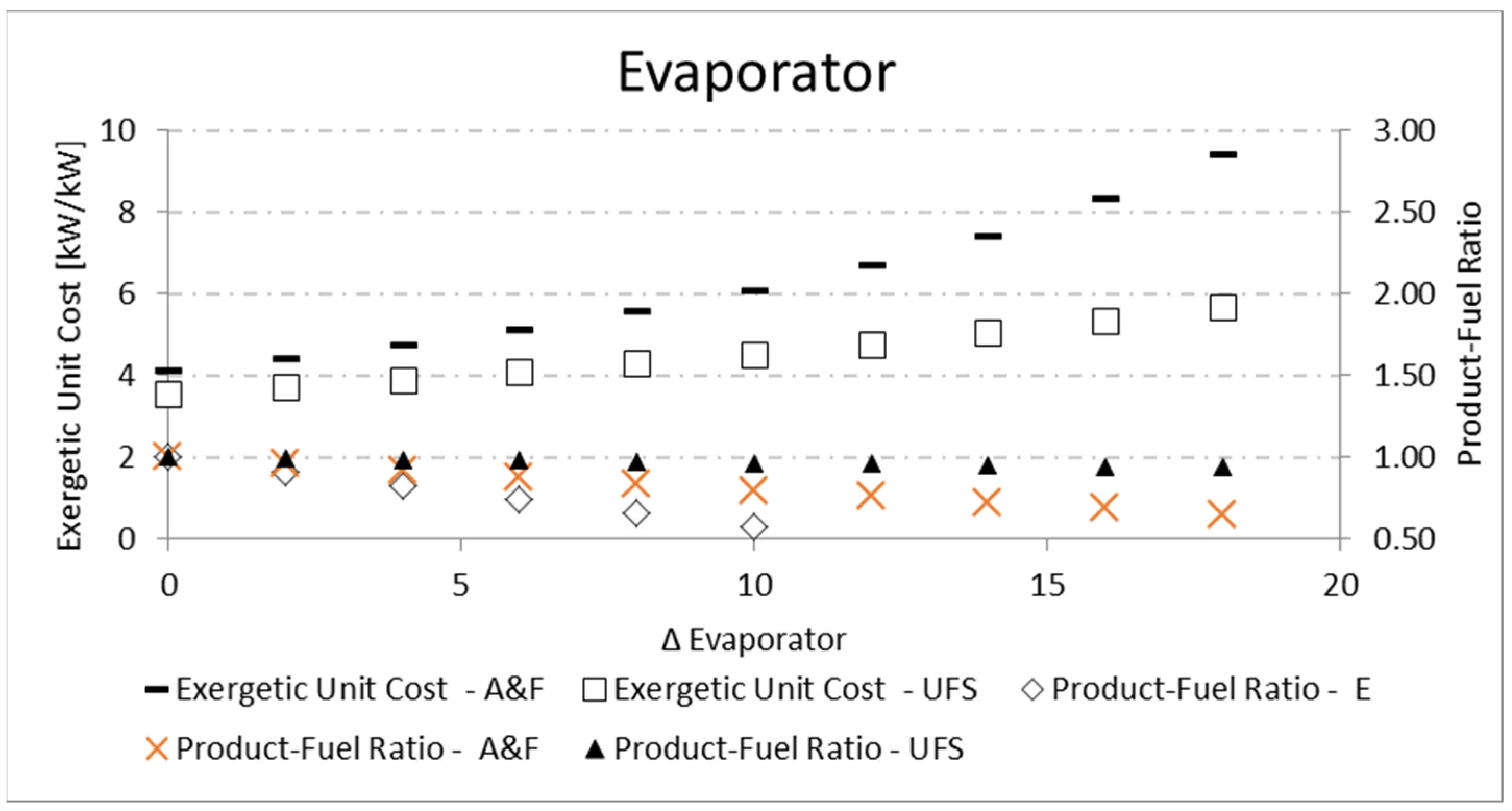
T) causes more irreversibilities associated with heat exchange, and therefore the unit cost increases. Nevertheless, the A&F model obtains unit costs higher than the UFS model for the evaporator (
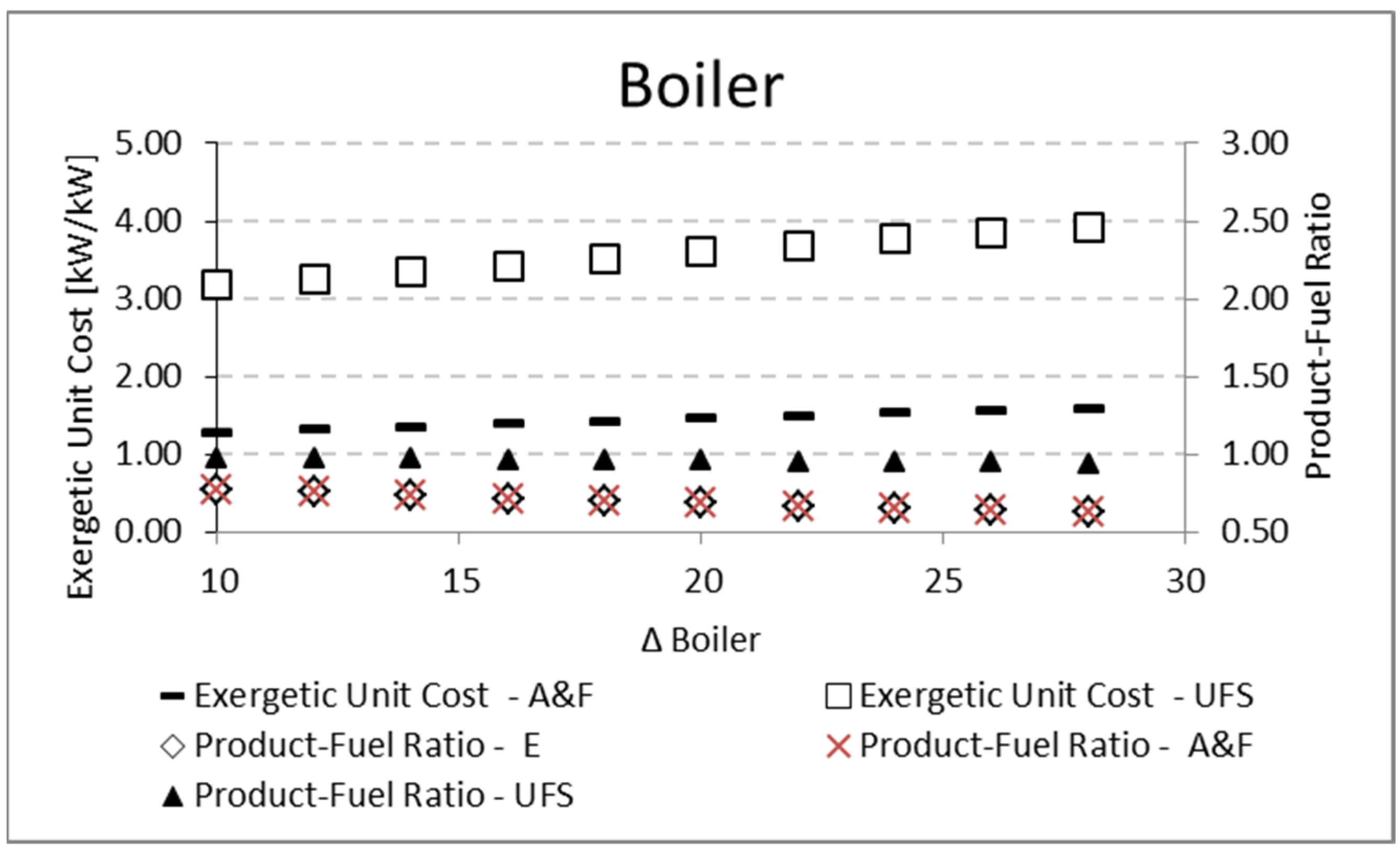
Figure 11), while the opposite happens for the boiler (
Figure 12). This is due to the different behavior in the
P/F definition (
Table 3) since the working fluid has a fixed temperature, the component with greater fuel will present greater irreversibility and, therefore, greater unit cost.
For both the evaporator and the boiler, the increase in the temperature difference (Δ
T) between the reservoirs and the working fluid reduces the
P/F, as shown in
Table 12 and
Figure 11 for the evaporator, and
Table 13 and
Figure 12 for the boiler. In both cases, there is an increase in the fuel for a constant product, so the product-fuel ratio decreases and, consequently, the unit cost increases.
From a thermodynamic point of view, the A&F model has advantages over the two other models applied in this work. In relation to the E model, it allows isolating dissipative equipment and still presents product-fuel ratio values that are very close or equal, which is different from the UFS model. For example, for the compressor and expander, respectively, in
Figure 13a,b, the efficiency values for the E and A&F models are practically the same, which does not happen in the UFS model. It is important to highlight that they are all consistent since for isentropic efficiency equal to 1, all have an efficiency value equal to 1.
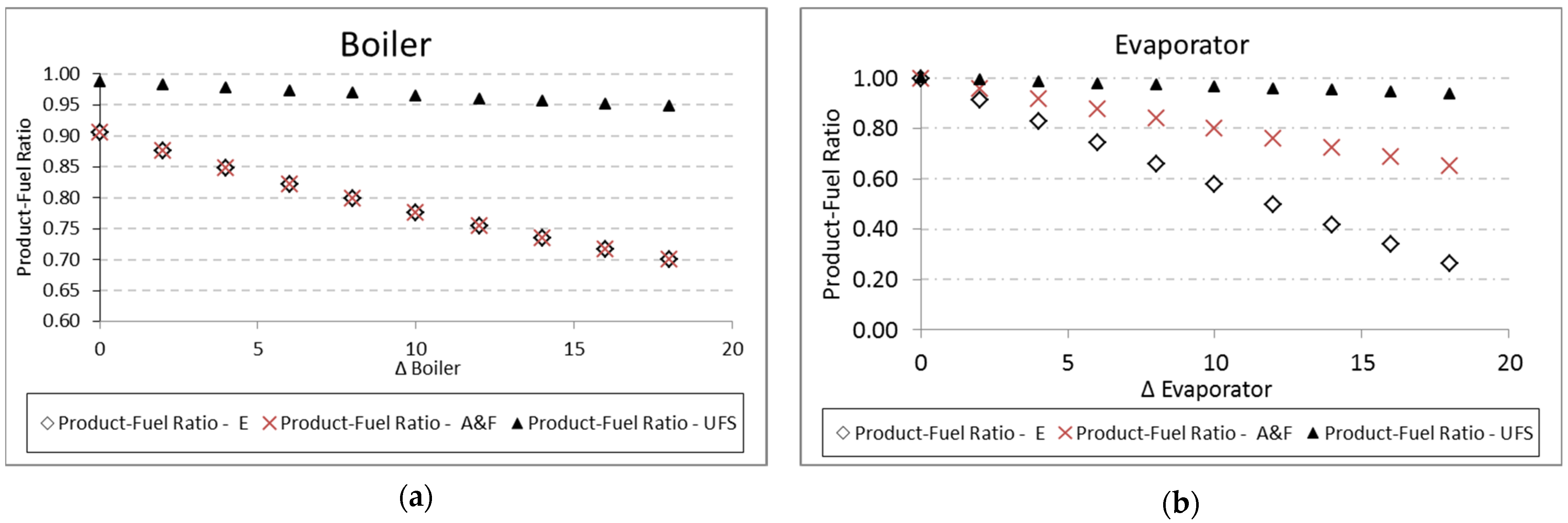
In the case of heat exchangers, the same tendency is observed for the boiler and evaporator, respectively, in
Figure 14a,b. The values of the product and fuel ratio between Models A&F and E are very close. It is necessary to emphasize that, in this case, the values are also coherent because, for a temperature variation equal to zero, the efficiency value of the evaporator models is equal to 100%. In relation to the boiler, this does not happen because the addition of heat does not happen at a constant temperature; therefore, it is not an internally reversible process.
All these analyses, considering the design point and the parametric study, allowed us to conform to the proposed objective of this study, allowing us to extract the conclusion below.
6. Conclusions
This work presented a new exergy disaggregation approach, herein called the A&F model, which is a solid option for total disaggregation of each subsystem in a thermodynamic cycle, especially when there are dissipative components under analysis. The physical exergy terms of this model have a thermodynamic meaning: Helmholtz energy and flow work. The results of application to a Carnot refrigeration cycle confirmed consistent values of exergetic unit costs and productive efficiencies were yielded, so that the new approach agrees with the Second Law of Thermodynamics. The product-fuel ratios must have their results between zero (when the processes are totally irreversible) and one (when the processes are totally reversible) for each component present in the plant, as can be seen in the results of this study.
The complexity in the A&F model can be considered much lower than in the UFS model due to the decrease in the number of exergy terms and, consequently, in the productive flows and junctions/bifurcations encompassed in the analysis.
Parametric analyses were carried out in order to investigate the effects of some parameters on the exergetic unit cost and on the product-fuel ratio. The results confirm the consistency of the A&F model, as they present the expected tendency. By increasing the temperature variation (∆T) between the working fluid and external reservoirs, the cost has to increase and the P/F ratio has to be reduced. On the other hand, the efficiency increase reduces the exergetic costs due to lower irreversibility generation; and, the P/F, regardless of the thermoeconomic model, increases until it reaches 1 for a fully reversible process and adiabatic (efficiency of 100%). It is exactly what happens with the A&F and UFS models.
It is worth mentioning that the A&F model obtains efficiency values equal to or closer to the conventional efficiency based on total exergy flow (E model) than the UFS model. In addition to isolating the valve and condenser with a coherent product-fuel ratio, the A&F model does not significantly differentiate the efficiency of other production equipment concerning the E model. Even though defining different productive structures (fuel and product), the magnitude of generating costs (irreversibilities) is the same in all components of the plant for all models. Therefore, the different thermoeconomic methods achieve different exergetic unit costs.
The A&F model is an alternative for physical exergy disaggregation, keeping the vast majority of characteristics as the UFS model. The work was proposed to present a new approach to physical exergy disaggregation as a solid and strong option to resolve hard questions in thermoeconomics associated with the treatment and isolation of dissipative equipment and to reduce the complexity in thermoeconomic modeling since the physical exergy disaggregation will be carried out in two terms only. By comparing the productive diagrams of both models, the A&F model has reduced the quantity of flows by approximately 27%, the quantity of junctions–bifurcations by 33%, and the quantity of cost equations by 38%, in relation to the UFS model. Thus, the complexity and computational effort reduction in the A&F model are significantly smaller than in the UFS model.
Furthermore, this novel approach can be easily applied in different refrigeration systems, especially when there are dissipative components in the cycle under analysis, because it is a strong option for disaggregation of each subsystem in thermodynamic plants. In future works, the A&F model can be applied to other thermodynamic systems, and based on the results so far, the expectations are positive.