Epileptic Seizure Detection Using Geometric Features Extracted from SODP Shape of EEG Signals and AsyLnCPSO-GA
Abstract
1. Introduction
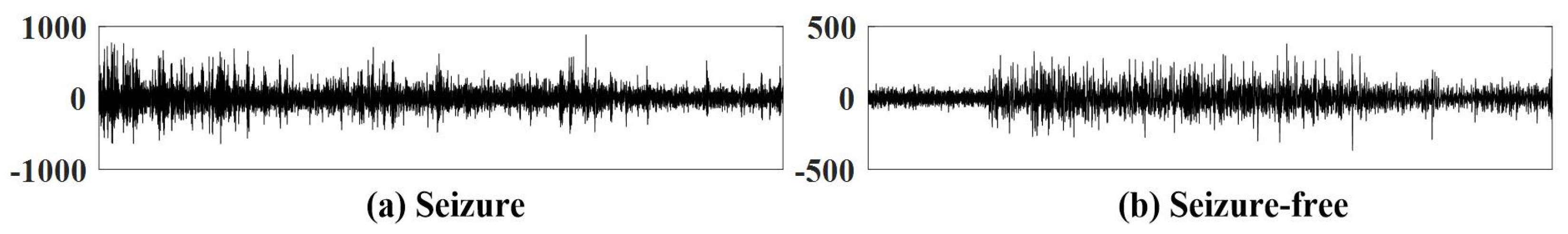
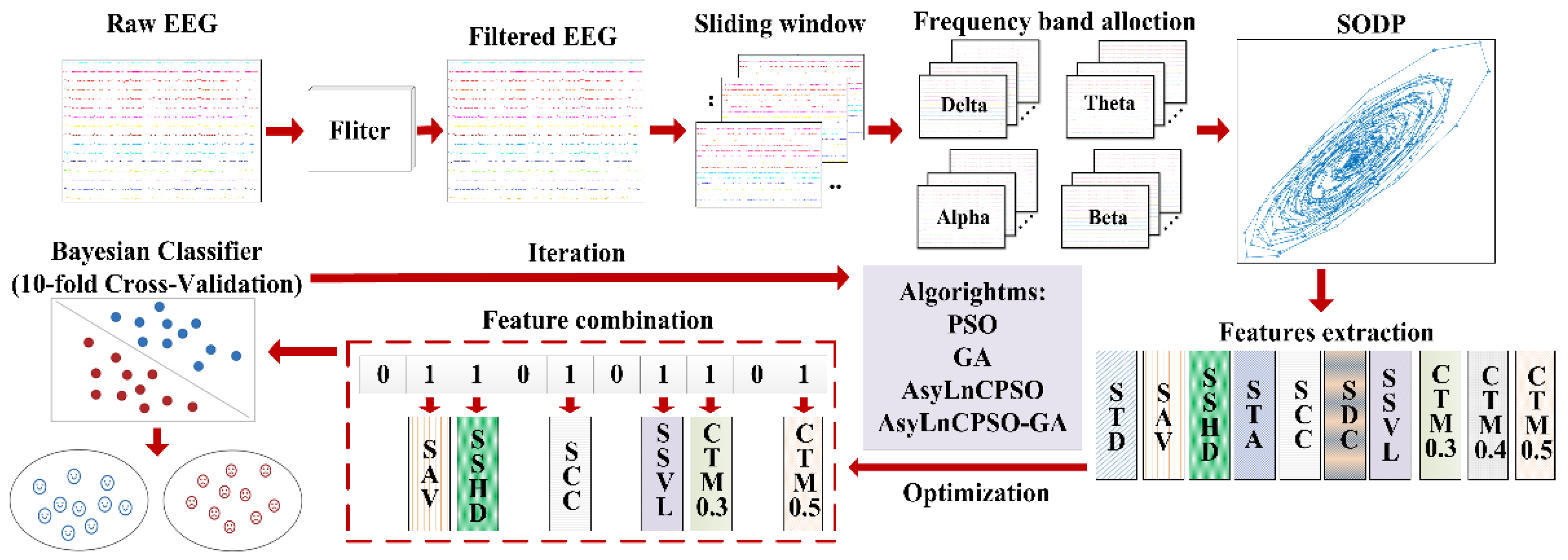
2. Data Description and Preprocessing
3. Method
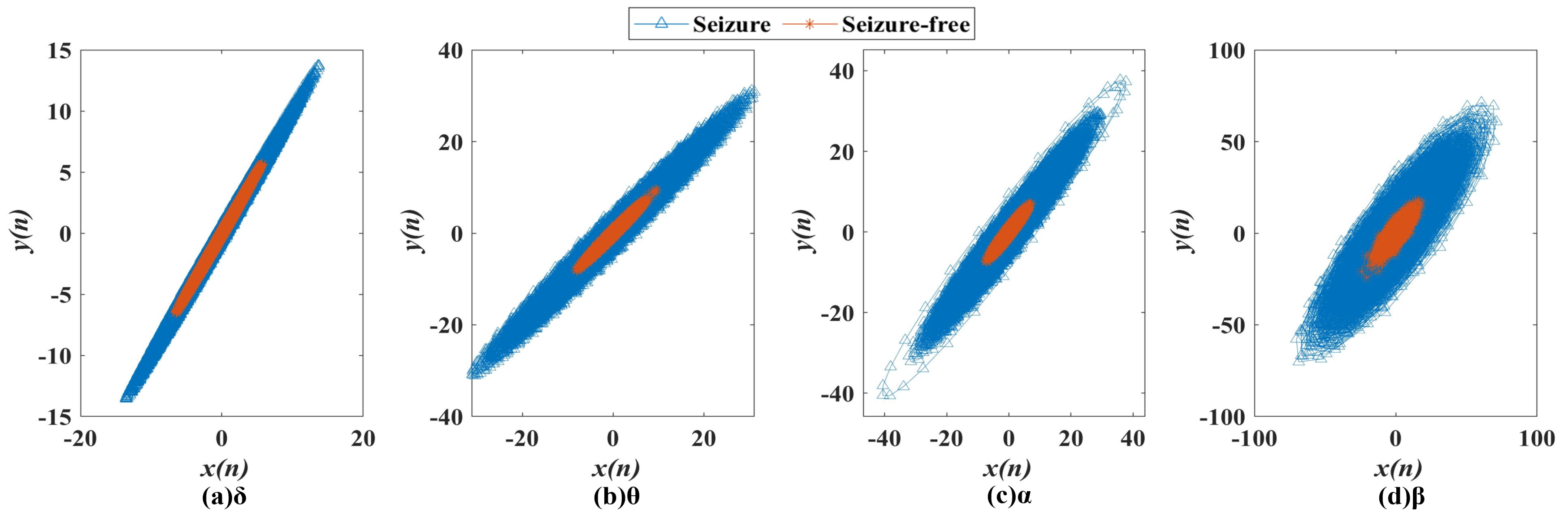
3.1. Second-Order Differential Plot (SODP)
3.2. Feature Extraction
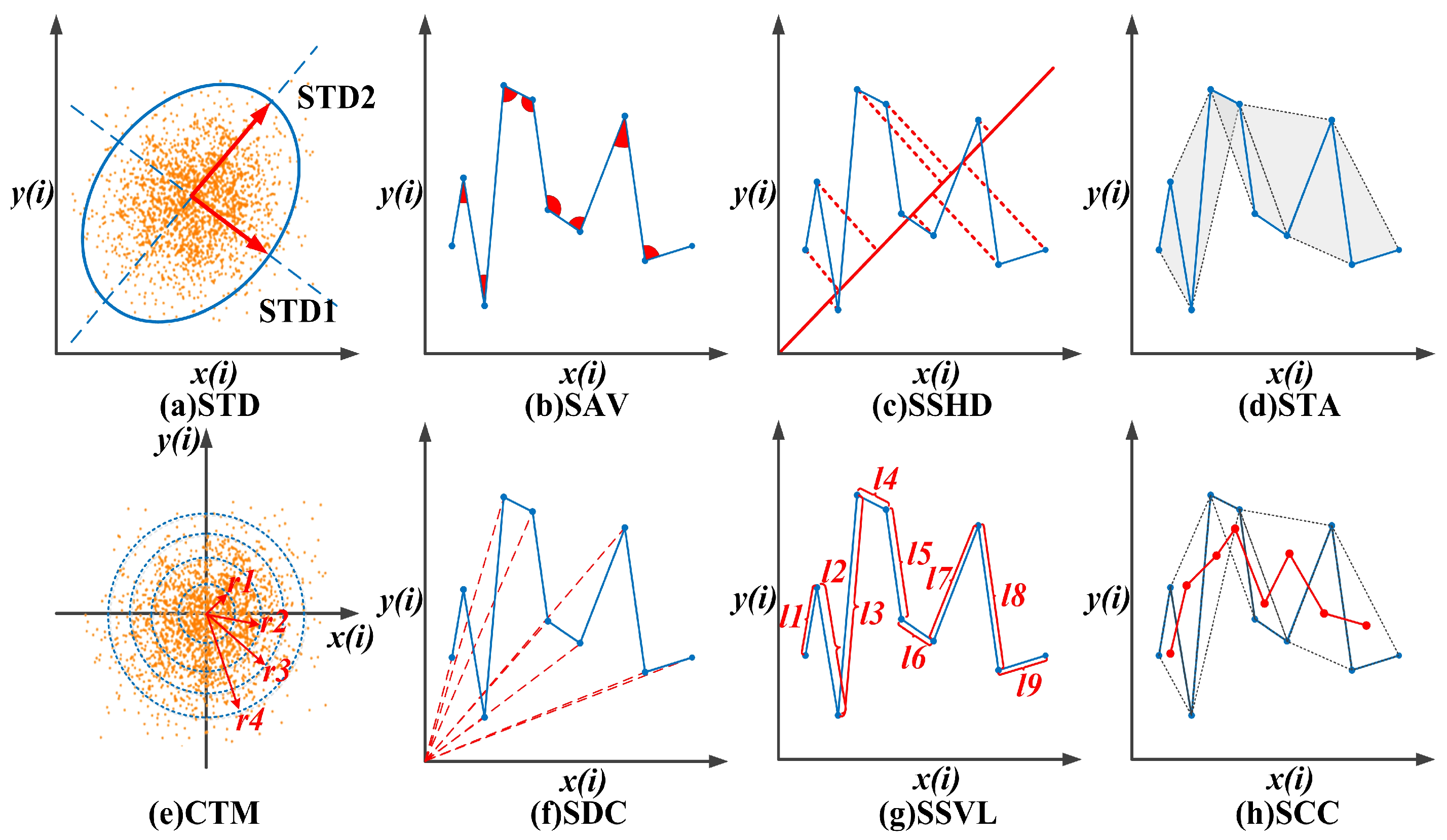
3.2.1. Standard Descriptors (STDs)
3.2.2. Sum of the Angles between Consecutive Vectors (SAV)
3.2.3. Sum of the Shortest Distance of Each Point from the 45-Degree Line (SSHD)
3.2.4. Sum of the Triangle Area Using Consecutive Vectors (STA)
3.2.5. Central Tendency Measure (CTM)
3.2.6. Sum of Distances to Coordinate (SDC)
3.2.7. Sum Successive of Vectors Length (SSVL)
3.2.8. Sum of the Centroid-to-Centroid Distance of Successive Triangles (SCC)
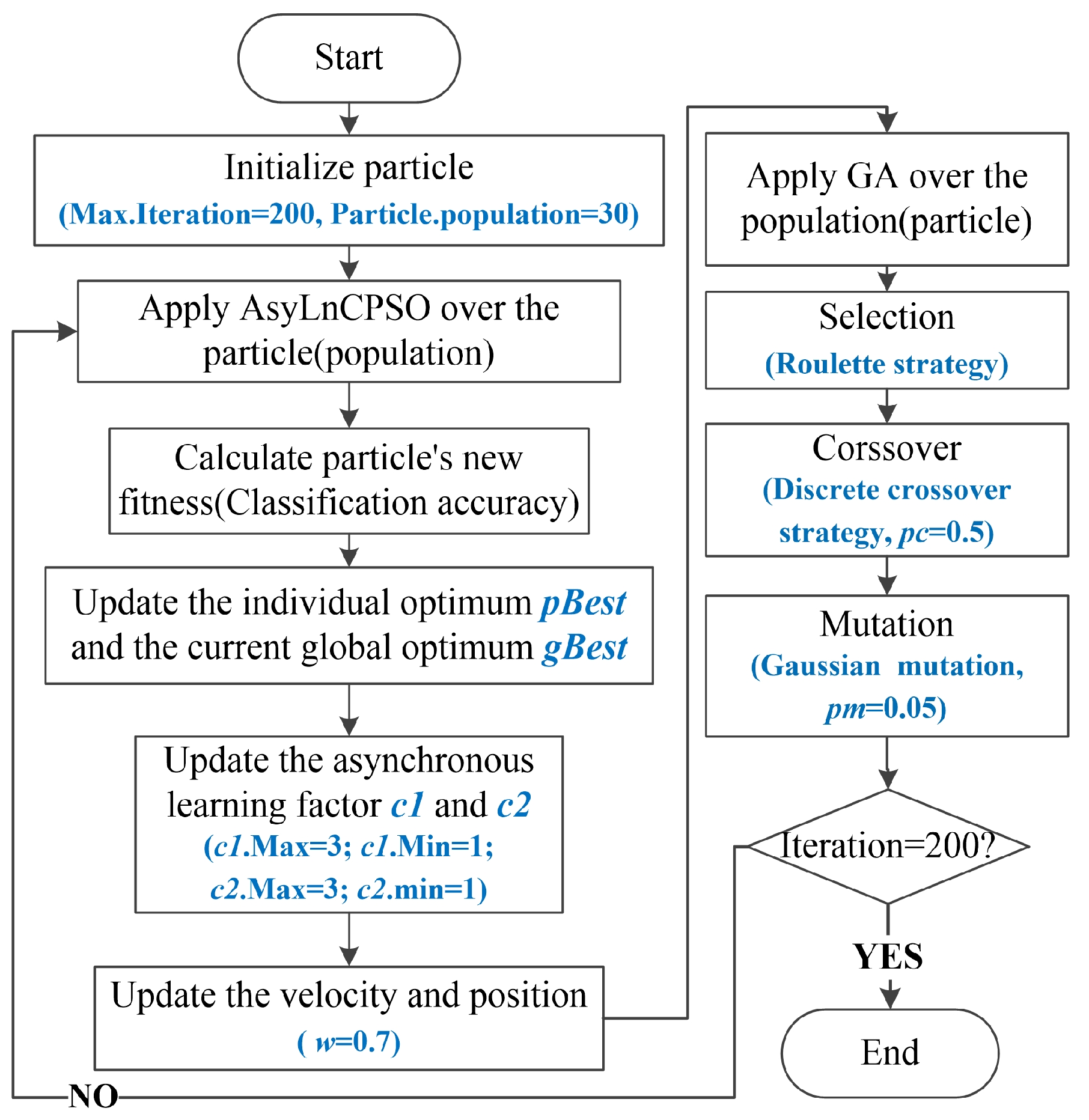
3.3. AsyLnCPSO-GA
3.4. Application of AsyLnCPSO-GA in Feature Selection
4. Results
4.1. Analysis of SODP
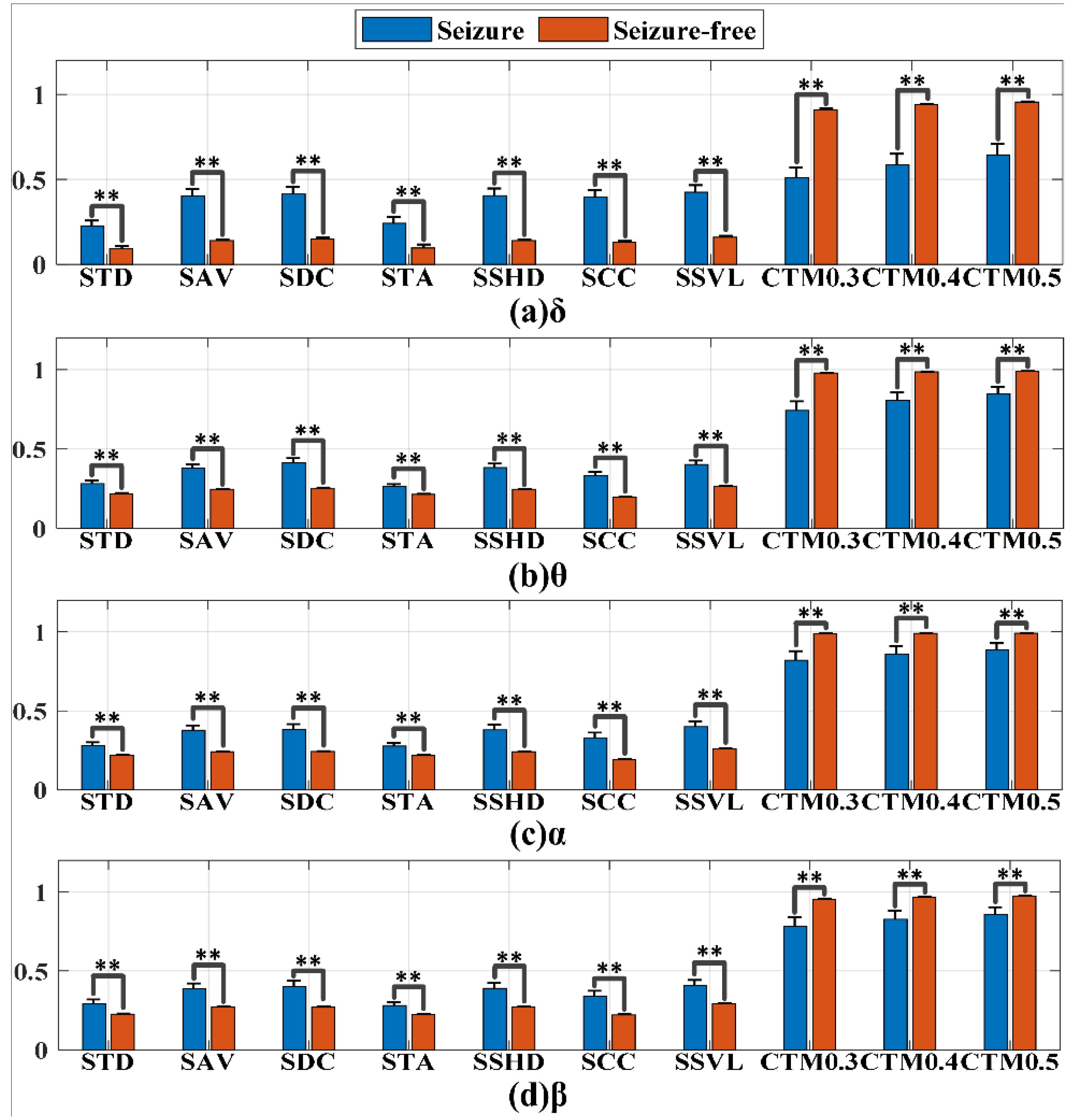
4.2. Statistical Analysis of Features
4.3. Classification Analysis of Features
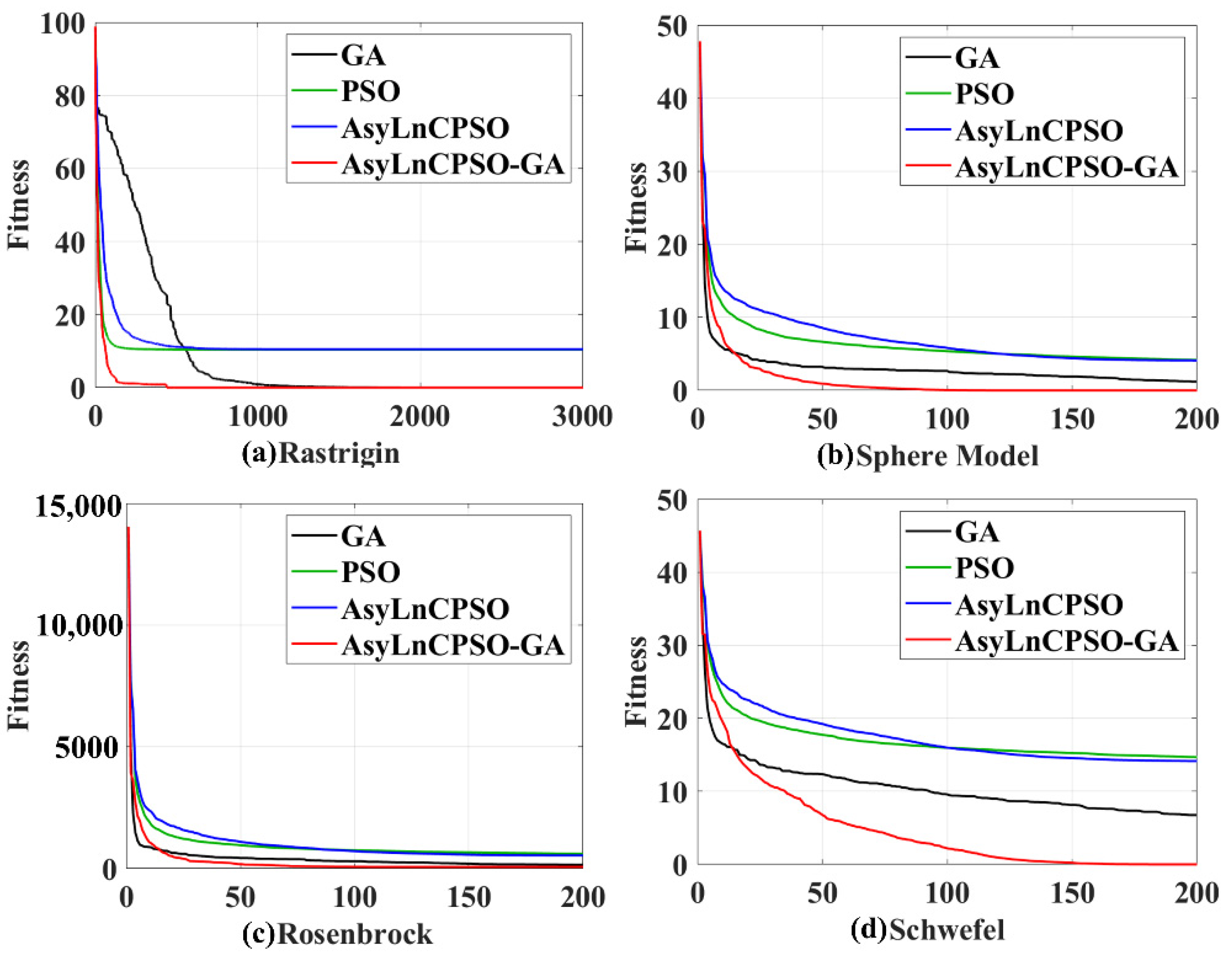
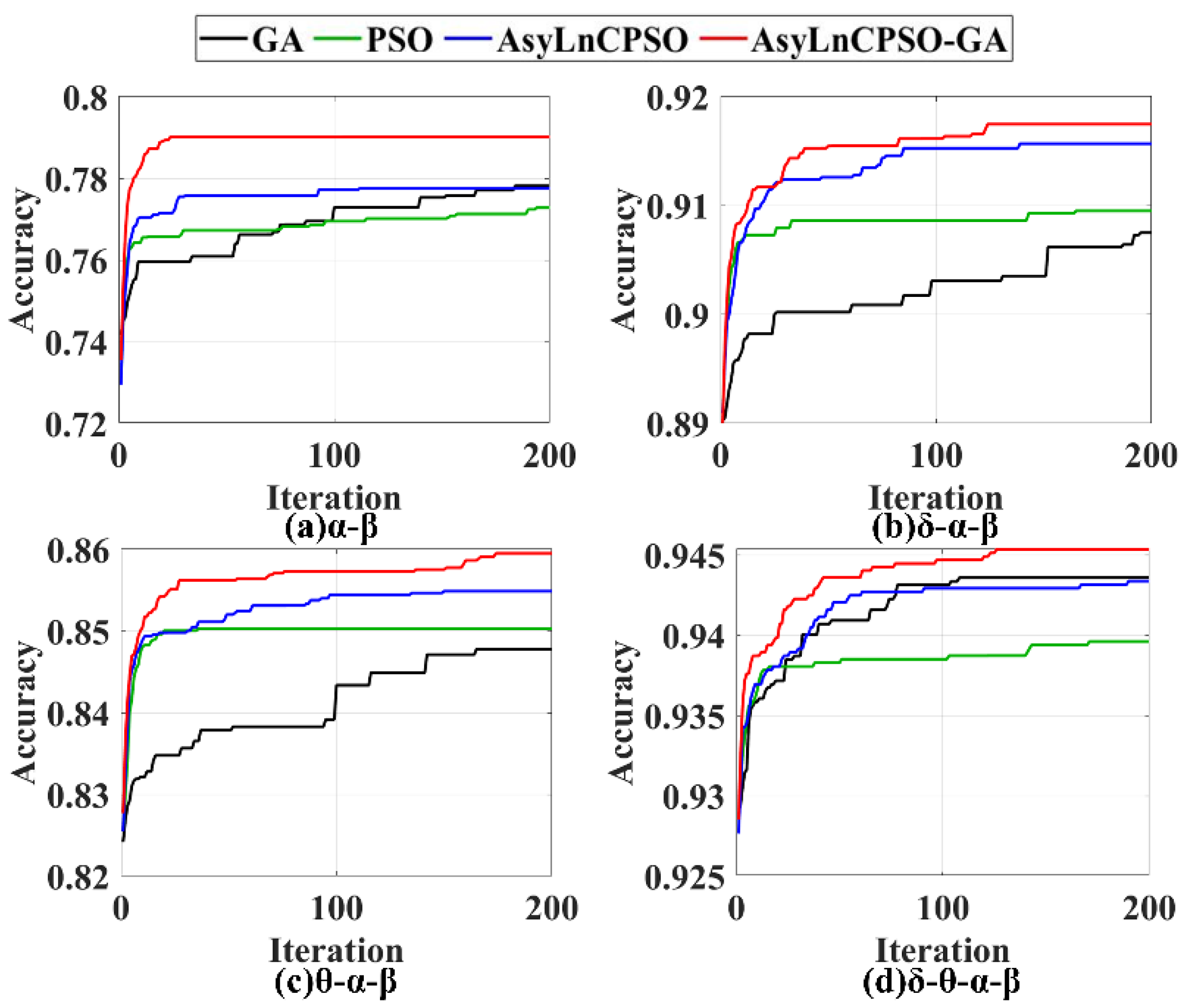
4.4. Application Results of GA, PSO, AsyLnCPSO and AsyLnCPSO-GA
4.4.1. Simulation Test
4.4.2. Application Analysis of Seizure Detection
4.4.3. Analysis of Key Features
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations of Terms
| Abbreviation | Description |
| EEG | Electroencephalography |
| SODP | Second-order differential plot |
| GA | Genetic algorithm |
| PSO | Particle swarm optimization |
| AsyLnCPSO | PSO with asynchronous learning factor |
| STD | Standard descriptor |
| SAV | Sum of the angles between consecutive vectors |
| SSHD | Sum of the shortest distance of each point from the 45-degree line |
| STA | Sum of the triangle area using consecutive vectors |
| CTM | Central tendency measure |
| SDC | Sum of distances to coordinate |
| SSVL | Sum of successive vectors length |
| SCC | Sum of the centroid-to-centroid distance of successive triangles |
Appendix A. Description of the GA, PSO and AsyLnCPSO Algorithms
Appendix A.1. Genetic Algorithm (GA)
Appendix A.2. Particle Swarm Optimization (PSO)
Appendix A.3. Asynchronous Learning Factor Changes of PSO (AsyLnCPSO)
Appendix B. Extra Tables
| δ Frequency Band | θ Frequency Band | |||||||
|---|---|---|---|---|---|---|---|---|
| Feature | Seizure | Seizure-Free | F-Value | p-Value | Seizure | Seizure-Free | F-Value | p-Value |
| STD | 10.68 ± 10.66 | 2.626 ± 7.62 | 85.472 | 9.454 × 10−19 | 91.66 ± 172.09 | 19.98 ± 79.76 | 37.062 | 2.4 × 10−8 |
| SAV | 727.74 ± 385.36 | 227.05 ± 167.5 | 320.896 | 1.454 × 10−54 | 3106.43 ± 2827.25 | 908.09 ± 815.07 | 159.752 | 5.67 × 10−26 |
| SDC | 20115.8 ± 10368.1 | 6682.9 ± 4630.7 | 316.268 | 5.663 × 10−54 | 26917.5 ± 24837.9 | 908.09 ± 815.07 | 172.344 | 1.24 × 10−25 |
| STA | 6.3249 ± 6.502 | 1.548 ± 4.698 | 80.123 | 9.296 × 10−18 | 1249.8 ± 2318.1 | 269.47 ± 1077.14 | 33.044 | 1.512 × 10−8 |
| SSHD | 734.79 ± 391.5 | 228.44 ± 168.35 | 319.049 | 2.5 × 10−54 | 3264.8 ± 3015.5 | 941.19 ± 844.78 | 154.255 | 1.122 × 10−25 |
| SCC | 1252.1 ± 667.25 | 389.1 ± 286.8 | 318.951 | 2.573 × 10−54 | 5591.3 ± 5164.8 | 1611.7 ± 1446.9 | 154.138 | 1.126 × 10−25 |
| SSVL | 1474.24 ± 785.4 | 458.3 ± 337.7 | 319.061 | 2.491 × 10−54 | 6635.6 ± 6128.6 | 1912.9 ± 1717.1 | 154.149 | 1.117 × 10−25 |
| CTM-0.3 | 0.619 ± 0.19 | 0.928 ± 0.069 | 525.113 | 1.393 × 10−77 | 0.86 ± 0.186 | 0.991 ± 0.029 | 211.460 | 5.377 × 10−23 |
| CTM-0.4 | 0.708 ± 0.178 | 0.957 ± 0.048 | 408.887 | 3.736 × 10−65 | 0.903 ± 0.153 | 0.993 ± 0.026 | 137.766 | 5.638 × 10−17 |
| CTM-0.5 | 0.773 ± 0.161 | 0.971 ± 0.037 | 323.897 | 6.052 × 10−55 | 0.93 ± 0.125 | 0.994 ± 0.024 | 96.527 | 2.511 × 10−13 |
| α Frequency Band | β Frequency Band | |||||||
| Feature | Seizure | Seizure-Free | F-Value | p-Value | Seizure | Seizure-Free | F-Value | p-Value |
| STD | 56.996 ± 97.713 | 12.81 ± 48.52 | 32.280 | 2.453 × 10−9 | 1156.2 ± 2083.7 | 327.1 ± 835.6 | 30.832 | 4.818 × 10−8 |
| SAV | 2071.1 ± 1631.3 | 629.57 ± 527.9 | 126.153 | 1.502 × 10−31 | 13547.4 ± 12057.4 | 5610.7 ± 4671.2 | 85.142 | 1.088 × 10−18 |
| SDC | 28810.4 ± 22047.6 | 8580.12 ± 7112.2 | 124.175 | 1.471 × 10−33 | 64792.5 ± 59132.5 | 25392.3 ± 20699.6 | 89.382 | 1.806 × 10−19 |
| STA | 215.2 ± 395.9 | 48.92 ± 179.9 | 33.244 | 1.664 × 10−8 | 170741 ± 307823 | 52644 ± 123649 | 28.643 | 1.389 × 10−7 |
| SSHD | 2142.5 ± 1736.4 | 641.1 ± 536.59 | 124.421 | 1.167 × 10−30 | 16434.1 ± 14936.7 | 6777.1 ± 5677.7 | 82.541 | 3.298 × 10−18 |
| SCC | 3661.6 ± 2968.9 | 1095.3 ± 917.2 | 124.411 | 1.219 × 10−30 | 27275 ± 24803 | 11195.7 ± 9358.4 | 83.143 | 2.55 × 10−18 |
| SSVL | 4321.1 ± 3502.5 | 1293.5 ± 1082.1 | 124.431 | 1.214 × 10−30 | 34545.9 ± 31394.8 | 14276.9 ± 11977.1 | 82.233 | 3.762 × 10−18 |
| CTM-0.3 | 0.793 ± 0.192 | 0.982 ± 0.033 | 109.004 | 1.514 × 10−39 | 0.811 ± 0.21 | 0.959 ± 0.063 | 102.430 | 7.895 × 10−22 |
| CTM-0.4 | 0.858 ± 0.165 | 0.989 ± 0.029 | 75.944 | 6.15 × 10−28 | 0.864 ± 0.180 | 0.973 ± 0.048 | 77.163 | 3.327 × 10−17 |
| CTM-0.5 | 0.898 ± 0.141 | 0.992 ± 0.026 | 56.941 | 9.065 × 10−21 | 0.898 ± 0.154 | 0.981 ± 0.038 | 61.583 | 3.124 × 10−14 |
| Time-Domain Feature (δ Frequency Band) | |||||
|---|---|---|---|---|---|
| Feature | Seizure | Seizure-Free | F-Value | p-Value | ACC |
| Root Mean Square | 55.057 ± 27.982 | 20.794 ± 13.312 | 273.3 | 1.011 × 10−48 | 0.8031 |
| Peak–Peak Value | 545.311 ± 234.13 | 265.673 ± 113.991 | 260.61 | 1.4 × 10−46 | 0.7972 |
| Skewness | 75139.13 ± 725886.695 | 31472.762 ± 63317.906 | 0.8116 | 0.368 | 0.5984 |
| Kurtosis | 4.306 ± 1.588 | 6.373 ± 2.863 | 90.005 | 1.389 × 10−19 | 0.6902 |
| Shape Indicator | 1.32 ± 0.079 | 1.413 ± 0.116 | 98.715 | 3.656 × 10−21 | 0.6817 |
| Crest Indicator | 3.245 ± 0.408 | 3.817 ± 0.585 | 144.977 | 3.881 × 10−29 | 0.7025 |
| Impulse Indicator | 4.346 ± 0.84519 | 5.574 ± 1.413 | 125.735 | 6.68 × 10−26 | 0.6989 |
| Clearance Indicator | 5.343 ± 1.279 | 7.199 ± 2.289 | 113.119 | 1.017 × 10−23 | 0.6874 |
References
- Yang, C.; Liu, Z.; Wang, Q.; Wang, Q.; Luan, G. Epilepsy as a dynamical disorder orchestrated by epileptogenic zone: A review. Nonlinear Dyn. 2021, 104, 1901–1916. [Google Scholar] [CrossRef]
- Milligan, T.A. Epilepsy: A clinical overview. Am. J. Med. 2021, 134, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Yuvaraj, R.; Thomas, J.; Kluge, T.; Dauwels, J. A deep learning scheme for automatic seizure detection from long-term scalp EEG. In Proceedings of the 2018 52nd Asilomar Conference on Signals, Systems, and Computers, Pacific Grove, CA, USA, 28–31 October 2018; pp. 368–372. [Google Scholar]
- Yu, H.; Zhu, L.; Cai, L.; Wang, J.; Liu, C.; Shi, N.; Liu, J. Variation of functional brain connectivity in epileptic seizures: An EEG analysis with cross-frequency phase synchronization. Cognit. Neurodyn. 2020, 14, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Thangavel, P.; Peh, W.Y.; Jing, J.; Yuvaraj, R.; Cash, S.S.; Chaudhari, R.; Karia, S.; Rathakrishnan, R.; Saini, V.; et al. Automated adult epilepsy diagnostic tool based on interictal scalp electroencephalogram characteristics: A six-center study. Int. J. Neural Syst. 2021, 31, 2050074. [Google Scholar] [CrossRef]
- Thomas, J.; Jin, J.; Thangavel, P.; Bagheri, E.; Yuvaraj, R.; Dauwels, J.; Rathakrishnan, R.; Halford, J.J.; Cash, S.S.; Westover, J. Automated detection of interictal epileptiform discharges from scalp electroencephalograms by convolutional neural networks. Int. J. Neural Syst. 2020, 30, 2050030. [Google Scholar] [CrossRef]
- Boylan, G.B.; Kharoshankaya, L.; Mathieson, S.R. Diagnosis of seizures and encephalopathy using conventional EEG and amplitude integrated EEG. Handb. Clin. Neurol. 2019, 162, 363–400. [Google Scholar]
- Akbari, H.; Ghofrani, S.; Zakalvand, P.; Sadiq, M.T. Schizophrenia recognition based on the phase space dynamic of EEG signals and graphical features. Biomed. Signal Process. Control 2021, 69, 102917. [Google Scholar] [CrossRef]
- Rincón, A.Q.; Flugelman, M.; Prendes, J.; d’Giano, C. Study on epileptic seizure detection in EEG signals using largest Lyapunov exponents and logistic regression. Rev. Argent. Bioing. 2019, 23, 17–24. [Google Scholar]
- Lehnertz, K.; Elger, C.E. Spatio-temporal dynamics of the primary epileptogenic area in temporal lobe epilepsy characterized by neuronal complexity loss. Electroencephalogr. Clin. Neurophysiol. 1995, 95, 108–117. [Google Scholar] [CrossRef]
- Namazi, H.; Aghasian, E.; Ala, T.S. Complexity-based classification of EEG signal in normal subjects and patients with epilepsy. Technol. Health Care 2020, 28, 57–66. [Google Scholar] [CrossRef]
- Li, P.; Karmakar, C.; Yearwood, J.; Venkatesh, S.; Palaniswami, M.; Liu, C. Detection of epileptic seizure based on entropy analysis of short-term EEG. PLoS ONE 2018, 13, e0193691. [Google Scholar] [CrossRef]
- Accardo, A.; Affinito, M.; Carrozzi, M.; Bouquet, F. Use of the fractal dimension for the analysis of electroencephalographic time series. Biol. Cybern. 1997, 77, 339–350. [Google Scholar] [PubMed]
- Fei, S.W.; Chu, Y.B. A novel classification strategy of motor imagery EEG signals utilizing WT-PSR-SVD-based MTSVM. Expert Syst. Appl. 2022, 199, 116901. [Google Scholar] [CrossRef]
- Anuragi, A.; Sisodia, D.S.; Pachori, R.B. Epileptic-seizure classification using phase-space representation of FBSE-EWT based EEG sub-band signals and ensemble learners. Biomed. Signal Process. Control 2022, 71, 103138. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, J.; Huang, S.; Lu, J.; Ye, M.; Wang, M. Detection and classification of epileptic EEG signals by the methods of nonlinear dynamics. Chaos Solitons Fract. 2021, 151, 111032. [Google Scholar] [CrossRef]
- Wang, X.Y.; Meng, J.; Tan, G.L.; Zou, L.X. Research on the Relation of EEG Signal Chaos Characteristics with High-Level Intelligence Activity of Human Brain. Models Appl. Chaos Theory Mod. Sci. 2010, 4, 2. [Google Scholar] [CrossRef]
- Pachori, R.B.; Patidar, S. Epileptic seizure classification in EEG signals using second-order difference plot of intrinsic mode functions. Comput. Methods Programs Biomed. 2014, 113, 494–502. [Google Scholar] [CrossRef]
- Abdulhay, E.; Alafeef, M.; Alzghoul, L.; Al Momani, M.; Al Abdi, R.; Arunkumar, N.; Munoz, R.; de Albuquerque, V.H.C. Computer-aided autism diagnosis via second-order difference plot area applied to EEG empirical mode decomposition. Neural Comput. Appl. 2020, 32, 10947–10956. [Google Scholar] [CrossRef]
- Akbari, H.; Ghofrani, S.; Ghofrani, S. Fast and accurate classification F and NF EEG by using SODP and EWT. Int. J. Image Gr. Signal Process. 2019, 11, 29–35. [Google Scholar] [CrossRef]
- Ullal, A.; Pachori, R.B. EEG signal classification using variational mode decomposition. arXiv 2020, arXiv:2003.12690. [Google Scholar]
- Wang, R.; Wang, J.; Li, S.; Yu, H.; Deng, B.; Wei, X. Multiple feature extraction and classification of electroencephalo-graph signal for Alzheimers’ with spectrum and bispectrum. Chaos Interdiscip. J. Nonlinear Sci. 2015, 25, 013110. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, J.; Yu, H.; Wei, X.; Yang, C.; Deng, B. Power spectral density and coherence analysis of Alzheimer’s EEG. Cognit. Neurodyn. 2015, 9, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, H.; Yang, Z.; Gui, Y.; Yin, Y.; Wang, W. Recognition of Alzheimer’s Brain Network Using Hybrid PSO-SVM Frame. In Proceedings of the 2021 40th Chinese Control Conference (CCC), Shanghai, China, 26–28 July 2021; pp. 3155–3160. [Google Scholar]
- Ghosh, M.; Guha, R.; Alam, I.; Lohariwal, P.; Jalan, D.; Sarkar, R. Binary genetic swarm optimization: A combination of GA and PSO for feature selection. J. Intell. Syst. 2020, 29, 1598–1610. [Google Scholar] [CrossRef]
- Khaire, U.M.; Dhanalakshmi, R. Stability of feature selection algorithm: A review. J. King Saud Univ.-Comput. Inf. Sci. 2022, 34, 1060–1073. [Google Scholar] [CrossRef]
- Kennedy, J.; Eberhart, R. Particle Swarm Optimization. In Proceedings of the ICNN’95-International Conference on Neural Networks, Perth, WA, Australia, 27 November–1 December 1995; Volume 4, pp. 1942–1948. [Google Scholar]
- Deng, W.; Zhao, H.; Yang, X.; Xiong, J.; Sun, M.; Li, B. Study on an improved adaptive PSO algorithm for solving multi-objective gate assignment. Appl. Soft Comput. 2017, 59, 288–302. [Google Scholar] [CrossRef]
- Jiang, W.; Zhang, Y.; Wang, R. Comparative Study on Several PSO Algorithms. In Proceedings of the 26th Chinese Control and Decision Conference (2014 CCDC), Changsha, China, 31 May–2 June 2014; pp. 1117–1119. [Google Scholar]
- Holland, J.H. Genetic algorithms. Sci. Am. 1992, 267, 66–73. [Google Scholar] [CrossRef]
- Shon, D.; Im, K.; Park, J.H.; Lim, D.S.; Jang, B.; Kim, J.M. Emotional stress state detection using genetic algorithm-based feature selection on EEG signals. Int. J. Environ. Res. Public Health 2018, 15, 2461. [Google Scholar] [CrossRef]
- Lokman, M.; Dabag, A.; Ozkurt, N.; Miqdad, S.; Najeeb, M. Feature Selection and Classification of EEG Finger Movement Based on Genetic Algorithm. In Proceedings of the 2018 Innovations in Intelligent Systems and Applications Conference (ASYU), Adana, Turkey, 4–6 October 2018; pp. 1–5. [Google Scholar]
- Huang, C.L.; Wang, C.J. A GA-based feature selection and parameters optimizationfor support vector machines. Expert Syst. Appl. 2006, 31, 231–240. [Google Scholar] [CrossRef]
- Hadoush, H.; Alafeef, M.; Abdulhay, E. Automated identification for autism severity level: EEG analysis using empirical mode decomposition and second order difference plot. Behav. Brain Res. 2019, 362, 240–248. [Google Scholar] [CrossRef]
- Salankar, N.; Mishra, P.; Garg, L. Emotion recognition from EEG signals using empirical mode decomposition and second-order difference plot. Biomed. Signal Process. Control 2021, 65, 102389. [Google Scholar] [CrossRef]
- Brennan, M.; Palaniswami, M.; Kamen, P. Do existing measures of Poincare plot geometry reflect nonlinear features of heart rate variability? IEEE Trans. Biomed. Eng. 2001, 48, 1342–1347. [Google Scholar] [CrossRef] [PubMed]
- Karimi Moridani, M.; Setarehdan, S.K.; Motie Nasrabadi, A.; Hajinasrollah, E. Non-linear feature extraction from HRV signal for mortality prediction of ICU cardiovascular patient. J. Med. Eng. Technol. 2016, 40, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Azizi, A.; Moridani, M.K.; Saeedi, A. A Novel Geometrical Method for Depression Diagnosis Based on EEG Signals. In Proceedings of the 2019 IEEE 4th Conference on Technology in Electrical and Computer Engineering, Tehran, Iran, 4–25 April 2019. [Google Scholar]
- Sadiq, M.T.; Akbari, H.; Siuly, S.; Yousaf, A.; Rehman, A.U. A novel computer-aided diagnosis framework for EEG-based identification of neural diseases. Comput. Biol. Med. 2021, 138, 104922. [Google Scholar] [CrossRef] [PubMed]
- Sawada, T.; Matsumoto, N.; Yoshida, N. Phase Transition Model between Swarm Behavior and Territorial Behavior. J. Comput. Model. 2022, 12, 1–15. [Google Scholar] [CrossRef]
- Tinuper, P.; Bisulli, F. From nocturnal frontal lobe epilepsy to sleep-related hypermotor epilepsy: A 35-year diagnostic challenge. Seizure 2017, 44, 87–92. [Google Scholar] [CrossRef]
- Ray, G.C. An algorithm to separate nonstationary part of a signal using mid-prediction filter. IEEE Trans. Signal Process. 1994, 42, 2276–2279. [Google Scholar] [CrossRef]
- Vanrumste, B.; Jones, R.D.; Bones, P.J.; Carroll, G.J. Slow-wave activity arising from the same area as epileptiform activity in the EEG of paediatric patients with focal epilepsy. Clin. Neurophysiol. 2005, 116, 9–17. [Google Scholar] [CrossRef]
- Tao, J.X.; Chen, X.J.; Baldwin, M. Interictal regional delta slowing is an EEG marker of epileptic network in temporal lobe epilepsy. Epilepsia 2011, 52, 467–476. [Google Scholar] [CrossRef]
- Schönherr, M.; Stefan, H.; Hamer, H.M. The delta between postoperative seizure freedom and persistence: Automatically detected focal slow waves after epilepsy surgery. NeuroImage Clin. 2017, 13, 256–263. [Google Scholar] [CrossRef]
- Settles, M.; Soule, T. Breeding Swarms: A GA/PSO Hybrid. In Proceedings of the 7th Annual Conference on Genetic and Evolutionary Computation, Washington, DC, USA, 25–29 June 2005; pp. 161–168. [Google Scholar]
- Gupta, I.K.; Shakil, S.; Shakil, S. A Hybrid GA-PSO Algorithm to Solve Traveling Salesman Problem. In Computational Intelligence: Theories, Applications and Future Directions; Springer: Singapore, 2019; Volume 1, pp. 453–462. [Google Scholar]
- Liu, Y.; Dai, J.; Zhao, S. Optimization of five-parameter BRDF model based on hybrid GA-PSO algorithm. Optik 2020, 219, 164978. [Google Scholar] [CrossRef]
- Howbert, J.J.; Patterson, E.E.; Stead, S.M.; Brinkmann, B.; Vasoli, V.; Crepeau, D.; Vite, C.H.; Sturges, B.; Ruedebusch, V.; Mavoori, J.; et al. Forecasting seizures in dogs with naturally occurring epilepsy. PLoS ONE 2014, 9, e81920. [Google Scholar] [CrossRef] [PubMed]
- Andrzejak, R.G.; Lehnertz, K.; Mormann, F.; Rieke, C.; David, P.; Elger, C.E. Indications of nonlinear deterministic and finite-dimensional structures in time series of brain electrical activity: Dependence on recording region and brain state. Phys. Rev. E 2001, 64, 061907. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, T.K.; Chakraborty, P.; Roy, G.G.; Panigrahi, B.K. Discrete harmony search based expert model for epileptic seizure detection in electroencephalography. Expert Syst. Appl. 2012, 39, 4055–4062. [Google Scholar] [CrossRef]
- Panichev, O.; Popov, A.; Kharytonov, V. Patient-specific epileptic seizure prediction using correlation features. In Proceedings of the 2015 Signal Processing Symposium (SPSympo), Debe, Poland, 10–12 June 2015; pp. 1–5. [Google Scholar]
- Truong, N.D.; Kuhlmann, L.; Bonyadi, M.R.; Yang, J.W.; Faulks, A.; Kavehei, O. Supervised learning in automatic channel selection for epileptic seizure detection. Expert Syst. Appl. 2017, 86, 199–207. [Google Scholar] [CrossRef]
- Usman, S.M.; Khalid, S.; Bashir, S. A deep learning based ensemble learning method for epileptic seizure prediction. Comput. Biol. Med. 2021, 136, 104710. [Google Scholar] [CrossRef]
- Assi, E.B.; Sawan, M.; Nguyen, D.K.; Rihana, S. A Hybrid mRMR-Genetic Based Selection Method for the Prediction of Epileptic Seizures. In Proceedings of the 2015 IEEE Biomedical Circuits and Systems Conference (BioCAS), Atlanta, GA, USA, 22–24 October 2015; pp. 1–4. [Google Scholar]
- Peng, H.; Lei, C.; Zheng, S.; Zhao, C.; Wu, C.; Sun, J.; Hu, B. Automatic epileptic seizure detection via Stein kernel-based sparse representation. Comput. Biol. Med. 2021, 132, 104338. [Google Scholar] [CrossRef]
- Sharma, M.; Bhurane, A.A.; Acharya, U.R. MMSFL-OWFB: A novel class of orthogonal wavelet filters for epileptic seizure detection. Knowl.-Based Syst. 2018, 160, 265–277. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, Y.; Jia, Z.; Ma, Y.; Wang, J. Representation based on ordinal patterns for seizure detection in EEG signals. Comput. Biol. Med. 2020, 126, 104033. [Google Scholar] [CrossRef]
- Djemili, R.; Bourouba, H.; Korba, M.C.A. Application of empirical mode decomposition and artificial neural network for the classification of normal and epileptic EEG signals. Biocybern. Biomed. Eng. 2016, 36, 285–291. [Google Scholar] [CrossRef]
- Tajmirriahi, M.; Amini, Z. Modeling of seizure and seizure-free EEG signals based on stochastic differential equations. Chaos Solitons Fract. 2021, 150, 111104. [Google Scholar] [CrossRef]
- Darjani, N.; Omranpour, H. Phase space elliptic density feature for epileptic EEG signals classification using metaheuristic optimization method. Knowl.-Based Syst. 2020, 205, 106276. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Pachori, R.B. A Multivariate Approach for Patient-Specific EEG Seizure Detection Using Empirical Wavelet Transform. IEEE Trans. Biomed. Eng. 2017, 64, 2003–2015. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Xu, P.; Xie, L.; Choi, K.S.; Wang, S. Transductive Joint-Knowledge-Transfer TSK FS for Recognition of Epileptic EEG Signals. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 1481–1494. [Google Scholar] [CrossRef] [PubMed]
- Raghu, S.; Sriraam, N.; Temel, Y.; Rao, S.V.; Hegde, A.S.; Kubben, P.K. Performance evaluation of DWT based sigmoid entropy in time and frequency domains for automated detection of epileptic seizures using SVM classifier. Comput. Biol. Med. 2019, 110, 127–143. [Google Scholar] [CrossRef]
- Raghum, S.; Sriraam, N.; Gommer, E.D.; Hilkman, D.M.W.; Temel, Y.; Rao, S.V.; Hegde, A.S.; Kubben, P.L. Cross-database evaluation of EEG based epileptic seizures detection driven by adaptive median feature baseline correction. Clin. Neurophysiol. 2020, 131, 1567–1578. [Google Scholar] [CrossRef]
- Shylo, V.P.; Glybovets, M.M.; Gulayeva, N.M.; Nikishchikhina, K.V. Genetic Algorithm of Tournament Crowding Based on Gaussian Mutation. Cybern. Syst. Anal. 2020, 56, 231–242. [Google Scholar] [CrossRef]
- Houssein, E.H.; Gad, A.G.; Hussain, K.; Suganthan, P.N. Major advances in particle swarm optimization: Theory, analysis, and application. Swarm Evol. Comput. 2021, 63, 100868. [Google Scholar] [CrossRef]



| Band | STD | SAV | SDC | STA | SSHD | SCC | SSVL | CTM-0.3 | CTM-0.4 | CTM-0.5 |
|---|---|---|---|---|---|---|---|---|---|---|
| δ | 0.6734 | 0.8284 | 0.8228 | 0.6491 | 0.8347 | 0.8252 | 0.8042 | 0.8356 | 0.8198 | 0.8011 |
| θ | 0.5758 | 0.7692 | 0.7701 | 0.5796 | 0.7557 | 0.7581 | 0.7582 | 0.7822 | 0.7509 | 0.6927 |
| α | 0.5712 | 0.7158 | 0.7087 | 0.5711 | 0.7062 | 0.7011 | 0.6985 | 0.7108 | 0.6523 | 0.6252 |
| β | 0.5693 | 0.6272 | 0.6238 | 0.5642 | 0.6102 | 0.6124 | 0.6107 | 0.6317 | 0.6093 | 0.5953 |
| Algorithm | Test Function | |||||||
|---|---|---|---|---|---|---|---|---|
| Rastrigin | Sphere Model | Rosenbrock | Schwefel | |||||
| Time Consumption | Fitness Value | Time Consumption | Fitness Value | Time Consumption | Fitness Value | Time Consumption | Fitness Value | |
| GA | 0.313 s | 1.92 × 10−4 | 0.016 s | 1.201 | 0.013 s | 154.149 | 0.045 s | 6.762 |
| PSO | 0.392 s | 10.494 | 0.021 s | 4.173 | 0.02 s | 609.415 | 0.065 s | 14.687 |
| AsyLnCPSO | 0.425 s | 10.417 | 0.021 s | 4.105 | 0.021 s | 541.367 | 0.066 s | 14.143 |
| AsyLnCPSO-GA | 0.671 s | 0 | 0.035 s | 2.03 × 10−7 | 0.03 s | 68.287 | 0.094 s | 1.93× 10−3 |
| GA | PSO | AsyLnCPSO | AsyLnCPSO-GA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Avg | Min | Max | Avg | Min | Max | Avg | Min | Max | Avg | |
| δ | 0.8606 | 0.8719 | 0.8660 | 0.8651 | 0.8719 | 0.8677 | 0.8651 | 0.8719 | 0.8675 | 0.8651 | 0.8719 | 0.8682 |
| θ | 0.8121 | 0.8251 | 0.8181 | 0.8186 | 0.8251 | 0.8212 | 0.8187 | 0.8251 | 0.8215 | 0.8206 | 0.8251 | 0.8217 |
| α | 0.7698 | 0.7856 | 0.7772 | 0.7719 | 0.7878 | 0.7786 | 0.7701 | 0.7878 | 0.7786 | 0.7763 | 0.7878 | 0.7790 |
| β | 0.6547 | 0.6792 | 0.6689 | 0.6592 | 0.6768 | 0.6707 | 0.6638 | 0.6792 | 0.6716 | 0.6681 | 0.6792 | 0.6729 |
| GA | PSO | AsyLnCPSO | AsyLnCPSO-GA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Avg | Min | Max | Avg | Min | Max | Avg | Min | Max | Avg | |
| δ-α | 0.8627 | 0.8784 | 0.8710 | 0.8671 | 0.8739 | 0.8708 | 0.8627 | 0.8783 | 0.8719 | 0.8671 | 0.8783 | 0.8726 |
| θ-α | 0.7940 | 0.8293 | 0.8110 | 0.8052 | 0.8229 | 0.8150 | 0.8054 | 0.8343 | 0.8219 | 0.8187 | 0.8316 | 0.8245 |
| δ-θ-α | 0.9070 | 0.9292 | 0.9183 | 0.9114 | 0.9291 | 0.9208 | 0.9203 | 0.9271 | 0.9239 | 0.9204 | 0.9292 | 0.9252 |
| δ-β | 0.8716 | 0.8916 | 0.8801 | 0.8760 | 0.8959 | 0.8845 | 0.8760 | 0.8959 | 0.8865 | 0.8783 | 0.8959 | 0.8880 |
| θ-β | 0.7963 | 0.8320 | 0.8186 | 0.8166 | 0.8320 | 0.8255 | 0.8142 | 0.8321 | 0.8235 | 0.8185 | 0.8363 | 0.8281 |
| δ-θ-β | 0.9003 | 0.9271 | 0.9144 | 0.9048 | 0.9249 | 0.9150 | 0.9070 | 0.9226 | 0.9161 | 0.9138 | 0.9315 | 0.9210 |
| GA | PSO | AsyLnCPSO | AsyLnCPSO-GA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Avg | Min | Max | Avg | Min | Max | Avg | Min | Max | Avg | |
| α-β | 0.7593 | 0.7876 | 0.7782 | 0.7628 | 0.7900 | 0.7728 | 0.7480 | 0.7946 | 0.7775 | 0.7767 | 0.7969 | 0.7901 |
| δ-α-β | 0.8936 | 0.9250 | 0.9075 | 0.8963 | 0.9203 | 0.9095 | 0.9003 | 0.9270 | 0.9157 | 0.9026 | 0.9269 | 0.9175 |
| θ-α-β | 0.8294 | 0.8673 | 0.8478 | 0.8364 | 0.8630 | 0.8503 | 0.8517 | 0.8606 | 0.8549 | 0.8494 | 0.8718 | 0.8595 |
| δ-θ-α-β | 0.9314 | 0.9535 | 0.9436 | 0.9315 | 0.9425 | 0.9396 | 0.9315 | 0.9535 | 0.9434 | 0.9381 | 0.9535 | 0.9454 |
| Algorithm (Naïve Bayesian) | Single-Band | Dual-Band | Three-Band | Four-Band | ||||
|---|---|---|---|---|---|---|---|---|
| Time Consumption | Feature Dimension | Time Consumption | Feature Dimension | Time Consumption | Feature Dimension | Time Consumption | Feature Dimension | |
| GA | 78.14 s | 6.5 | 91.82 s | 12.4 | 103.21 s | 16.5 | 125.36 s | 19.2 |
| PSO | 117.28 s | 4.8 | 144.57 s | 9.9 | 173.5 s | 13.3 | 198.69 s | 15.3 |
| AsyLnCPSO | 121.39 s | 4.9 | 152.23 s | 10.4 | 187.31 s | 15.1 | 201.54 s | 18.1 |
| AsyLnCPSO-GA | 174.25 s | 5.2 | 213.84 s | 11.3 | 259.64 | 16.7 | 288.13 s | 21.6 |
| Band | The Proportion of Features in gBest |
|---|---|
| δ | δ: CTM-0.3 (75%), CTM-0.4 (95%), SAV (60%%), SSHD (50%). |
| θ | θ: CTM-0.4 (100%), SSHD (75%), SAV (70%), CTM-0.5 (60%). |
| α | α: SSHD (100%), SAV (80%), CTM-0.3 (60%), STD (50%). |
| β | β: CTM-0.4 (95%), SAV (80%), CTM-0.3 (60%), SDC (30%). |
| δ-θ | δ: CTM-0.3 (100%), SSHD (85%), SAV (85%), CTM-0.4 (50%); θ: SAV (85%), CTM-0.3 (75%), STD (60%), STA (30%). |
| δ-α | δ: CTM-0.3 (95%), SAV (95%), SSHD (90%), SCC (40%); α: SSHD (55%), SAV (50%), CTM-0.3 (40%), STA (15%). |
| δ-β | δ: SSHD (95%), CTM-0.3 (90%), STD (90%), CTM-0.5 (45%); β: SSHD (90%), SAV (70%), CTM-0.3 (35%), SCC (20%). |
| θ-α | θ: SAV (95%), CTM-0.3 (90%), SSHD (85%), SSVL (30%); α: SSHD (60%), CTM-0.3 (20%), SDC (10%), SAV (10%). |
| θ-β | θ: CTM-0.3 (100%), SAV (95%), SSHD (65%), SDC (30%); β: SAV (65%), SSHD (25%), STD (5%), SCC (5%). |
| α-β | α: SAV (100%), SSHD (80%), CTM-0.3 (30%), SSVL (25%); β: SAV (25%), SSHD (25%), STD (10%), CTM-0.3 (10%). |
| δ-θ-α | δ: CTM-0.3 (80%), CTM-0.5 (40%), SAV (35%), CTM-0.4 (30%); θ: CTM-0.5 (100%), SAV (100%), SSHD (65%), STD (55%); α: SAV (60%), CTM-0.5 (55%), SSHD (50%), SSVL (25%). |
| δ-θ-β | δ: CTM-0.3 (70%), SAV (65%), STD (40%), CTM-0.5 (40%); θ: CTM-0.4 (100%), SAV.5 (80%), CTM-0.3 (80%), SSHD (60%); β: CTM-0.5 (65%), SAV (40%), SSHD (30%), CTM-0.3 (25%). |
| δ-α-β | δ: SSHD (75%), CTM-0.5 (70%), SAV (70%), STD (60%); α: SAV (100%), SSVL (85%), SSHD (80%), CTM-0.3 (65%); β: SSVL (35%), CTM-0.3 (35%), STD (35%), CTM-0.5 (35%). |
| θ-α-β | θ: STA (75%), CTM-0.4 (70%), CTM-0.5 (70%), SAV (65%); α: SSHD (90%), SAV (65%), CTM-0.3 (60%), CTM-0.4 (60%); β: CTM-0.4 (55%), STD (40%), SSVL (35%), SSHD (30%). |
| δ-θ-α-β | δ: CTM-0.4 (75%), CTM-0.5 (75%), CTM-0.3 (70%), STD (65%); θ: SAV (65%), STD (60%), SSHD (55%), SSVL (55%); α: SSHD (75%), STA (65%), SSVL (65%), SCC (50%); β: SAV (60%), SSVL (55%), CTM-0.4 (50%), STD (50%). |
| Dataset | The Proportion of Features in gBest | Classification Accuracy | ||
|---|---|---|---|---|
| δ-θ-α-β | Min | Max | Avg | |
| Kaggle (Dog) | δ: CTM-0.5 (90%), CTM-0.4 (85%), SAV (55%), CTM-0.3 (55%); θ: SSVL (85%), SCC (85%), STD (70%), SAV (65%); α: CTM-0.5 (65%), SSVL (55%), STD (55%), SSHD (45%); β: CTM-0.5 (75%), CTM-0.3 (75%), SSVL (75%), STD (65%); | 0.9584 | 0.9714 | 0.9612 |
| Kaggle (Human) | δ: CTM-0.4 (90%), CTM-0.5 (80%), SSHD (55%), SCC (55%); θ: STD (70%), CTM-0.3 (65%), CTM-0.5 (65%), SSVL (55%); α: SDC (80%), SAV (55%), STD (50%), CTM-0.4 (50%); β: STA (90%), STD (80%), SAV (70%), SSVL (70%); | 0.9273 | 0.9455 | 0.9371 |
| U-Bonn | δ: SSHD (95%), STA (75%), SDC (65%), SCC (65%); θ: CTM-0.3 (80%), SSHD (75%), SSVL (70%), SCC (55%); α: STD (75%), SDC (70%), SSVL (70%), SSVL (65%); β: STD (70%), CTM-0.5 (70%), SCC (65%), SSVL (60%); | 0.9839 | 0.9936 | 0.9877 |
| NSC-ND | δ: SSHD (85%), STA (85%), SSHD (60%), SDC (55%); θ: SSHD (60%), SSVL (60%), STA (50%), SAV (50%); α: CTM-0.4 (80%), STA (75%), CTM-0.3 (60%), SSHD (55%); β: STD (60%), CTM-0.3 (60%), CTM-0.4 (60%), SSVL (55%); | 0.9921 | 0.9987 | 0.9954 |
| Dataset | Method | Features | Subjects | Classifier | Cross Validation | Performance |
|---|---|---|---|---|---|---|
| Kaggle | STW [52] | Correlation coefficient | 5 dogs, 2 patients | SVM | 10 | Dog: 0.9432 (AUC) Human: 0.9349 (AUC) |
| ACS [53] | Spectral power, correlation between channels | 4 dogs, 8 patients | Random forest | Leave-one-out | Dog: 0.9651 (ACC) Human: 0.9172 (ACC) | |
| EMD [54] | Statistical and spectral moments | 5 dogs, 2 patients | MAML | 10 | Dog: 0.9563 (ACC) Human: 0.9528 (ACC) | |
| mRMR-GA [55] | Decorrelation time, Hjorth parameters, etc. | 5 dogs, 2 patients | SVM | 5 | Dog: 0.9028 (SEN) Human: 0.8853 (SEN) | |
| Proposed method | Geometric features | 4 dogs, 2 patients | Bayesian | 10 | Dog: 0.9714 (ACC) Human: 0.9455 (ACC) | |
| U-Bonn | RKHS [56] | Covariance descriptors | Set A–E | SR | 10 | Set D, E: 0.9888 (ACC) |
| MMSFL-OWFB [57] | Kraskov entropy | Set A–E | SVM | 10 | 0.992 (ACC) | |
| Niobium [58] | Euclidean distance, cosine distance, etc. | Set A–E | SVM | 5 | Set D, E: 0.96 (ACC) | |
| EMD [59] | IMFs | Set A–E | MLPNN | 10 | 0.952(ACC) | |
| Proposed method | Geometric features | Set D, E | Bayesian | 10 | Set D, E: 0.9936 (ACC) | |
| NSC-ND | RKHS [56] | Covariance descriptors | 10 patients | SR | 10 | 0.9848 (ACC) |
| SDE [60] | Parameters of fitted histograms | 10 patients | SVM | 10 | 0.991 (ACC) | |
| MMSFL-OWFB [57] | Kraskov entropy | 10 patients | SVM | 10 | 0.98 (ACC) | |
| ED [61] | Signal data density | 10 patients | 1-NN | 10 | 0.9897 (ACC) | |
| Proposed method | Geometric features | 10 patients | Bayesian | 10 | 0.9987 (ACC) | |
| CHB-MIT | EMD [62] | Mean of joint instantaneous amplitude, etc. | 24 patients | RF, Bayesian | 10 | 0.9941 (RF, ACC); 0.9516 (Bayesian, ACC) |
| WPD [63] | Wavelet coefficients, etc. | 24 patients | ANFIS | 10 | 0.9404 (ACC) | |
| DWT [64] | Sigmoid entropy, etc. | 23 patients | SVM | 10 | 0.9421 (SEN) | |
| AM-FBC [65] | Matrix determinant, etc. | 23 patients | SVM | Leave-one-out | 0.99 (SEN) 0.89 (SPE) | |
| Proposed method | Geometric features | 23 patients | Bayesian | 10 | 0.9535 (ACC) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Wang, H.; Shi, L.; Han, C.; Che, Y. Epileptic Seizure Detection Using Geometric Features Extracted from SODP Shape of EEG Signals and AsyLnCPSO-GA. Entropy 2022, 24, 1540. https://doi.org/10.3390/e24111540
Wang R, Wang H, Shi L, Han C, Che Y. Epileptic Seizure Detection Using Geometric Features Extracted from SODP Shape of EEG Signals and AsyLnCPSO-GA. Entropy. 2022; 24(11):1540. https://doi.org/10.3390/e24111540
Chicago/Turabian StyleWang, Ruofan, Haodong Wang, Lianshuan Shi, Chunxiao Han, and Yanqiu Che. 2022. "Epileptic Seizure Detection Using Geometric Features Extracted from SODP Shape of EEG Signals and AsyLnCPSO-GA" Entropy 24, no. 11: 1540. https://doi.org/10.3390/e24111540
APA StyleWang, R., Wang, H., Shi, L., Han, C., & Che, Y. (2022). Epileptic Seizure Detection Using Geometric Features Extracted from SODP Shape of EEG Signals and AsyLnCPSO-GA. Entropy, 24(11), 1540. https://doi.org/10.3390/e24111540






