Joint Angle Variability Is Altered in Patients with Peripheral Artery Disease after Six Months of Exercise Intervention
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Supervised Exercise Training Protocol
2.3. Experimental Data Collection
2.4. Data Analysis
2.5. Statistics
3. Results
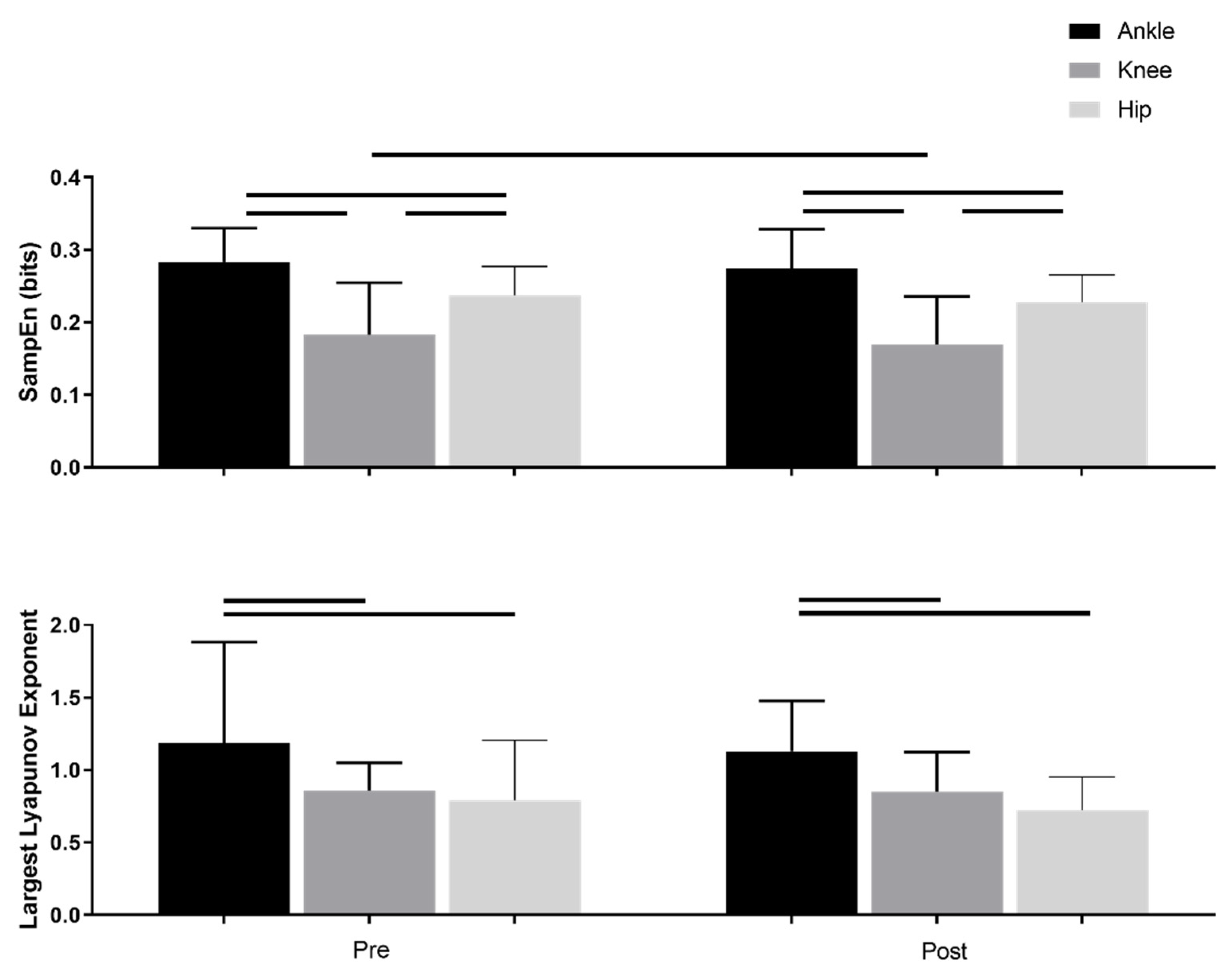
3.1. Nonlinear Measures
3.2. Linear Measures
3.3. Bayesian Statistics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Signorelli, S.S.; Marino, E.; Scuto, S.; Di Raimondo, D. Pathophysiology of Peripheral Arterial Disease (PAD): A Review on Oxidative Disorders. Int. J. Mol. Sci. 2020, 21, 4393. [Google Scholar] [CrossRef] [PubMed]
- Fuglestad, M.A.; Hernandez, H.; Gao, Y.; Ybay, H.; Schieber, M.N.; Brunette, K.E.; Myers, S.A.; Casale, G.P.; Pipinos, I.I. A low-cost, wireless near-infrared spectroscopy device detects the presence of lower extremity atherosclerosis as measured by computed tomographic angiography and characterizes walking impairment in peripheral artery disease. J. Vasc. Surg. 2020, 71, 946–957. [Google Scholar] [CrossRef] [PubMed]
- Rahman, H.; Pipinos, I.I.; Johanning, J.M.; Myers, S.A. Gait variability is affected more by peripheral artery disease than by vascular occlusion. PLoS ONE 2021, 16, e0241727. [Google Scholar] [CrossRef]
- Webber, M.S.C.; Porter, M.M.; Menec, V.H. Mobility in older adults: A comprehensive framework. Gerontologist 2010, 50, 443–450. [Google Scholar] [CrossRef]
- Koutakis, P.; Pipinos, I.I.; Myers, S.A.; Stergiou, N.; Lynch, T.G.; Johanning, J.M. Joint torques and powers are reduced during ambulation for both limbs in patients with unilateral claudication. J. Vasc. Surg. 2010, 51, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Koutakis, P.; Miserlis, D.; Myers, S.A.; Kim, J.K.-S.; Zhu, Z.; Papoutsi, E.; Swanson, S.A.; Haynatzki, G.; Ha, D.M.; Carpenter, L.A.; et al. Abnormal Accumulation of Desmin in Gastrocnemius Myofibers of Patients with Peripheral Artery Disease: Associations with Altered Myofiber Morphology and Density, Mitochondrial Dysfunction and Impaired Limb Function. J. Histochem. Cytochem. 2015, 63, 256–269. [Google Scholar] [CrossRef]
- Gerhard-Herman, M.D.; Gornik, H.L.; Barrett, C.; Barshes, N.R.; Corriere, M.A.; Drachman, D.E.; Fleisher, L.A.; Fowkes, F.G.R.; Hamburg, N.; Kinlay, S.; et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: Executive Summary: A report of the American college of cardiology/American Heart Association task force on clinical practice guidelines. Circulation 2017, 135, e686–e725. [Google Scholar] [CrossRef]
- Peripheral Arterial Disease (PAD)|cdc.gov. Available online: https://www.cdc.gov/heartdisease/pad.htm (accessed on 29 June 2022).
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2021 Update A Report from the American Heart Association. Circulation 2021, 143, E254–E743. [Google Scholar] [CrossRef]
- Myers, S.A.; Johanning, J.M.; Stergiou, N.; Celis, R.I.; Robinson, L.; Pipinos, I.I. Gait variability is altered in patients with peripheral arterial disease. J. Vasc. Surg. 2009, 49, 924–931.e1. [Google Scholar] [CrossRef]
- Schieber, M.N.; Pipinos, I.I.; Johanning, J.M.; Casale, G.P.; Williams, M.A.; DeSpiegelaere, H.K.; Senderling, B.; Myers, S.A. Supervised walking exercise therapy improves gait biomechanics in patients with peripheral artery disease. J. Vasc. Surg. 2020, 71, 575–583. [Google Scholar] [CrossRef]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G.R. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J. Vasc. Surg. 2007, 45 (Suppl. 1), S5–S67. [Google Scholar] [CrossRef] [PubMed]
- McCamley, J.D.; Pisciotta, E.J.; Yentes, J.M.; Wurdeman, S.R.; Rennard, S.I.; Pipinos, I.I.; Johanning, J.M.; Myers, S.A. Gait deficiencies associated with peripheral artery disease are different than chronic obstructive pulmonary disease. Gait Posture 2017, 57, 258–264. [Google Scholar] [CrossRef]
- Myers, S.A.; Johanning, J.M.; Pipinos, I.I.; Schmid, K.K.; Stergiou, N. Vascular occlusion affects gait variability patterns of healthy younger and older individuals. Ann. Biomed. Eng. 2013, 41, 1692–1702. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, A.; Schmitz, R.W.; Erlacher, D. Near-infrared spectroscopy-derived muscle oxygen saturation on a 0% to 100% scale: Reliability and validity of the Moxy Monitor. J. Biomed. Opt. 2019, 24, 115001. [Google Scholar] [CrossRef] [PubMed]
- Paldán, K.; Steinmetz, M.; Simanovski, J.; Rammos, C.; Ullrich, G.; Jánosi, R.A.; Moebus, S.; Rassaf, T.; Lortz, J. Supervised Exercise Therapy Using Mobile Health Technology in Patients With Peripheral Arterial Disease: Pilot Randomized Controlled Trial. JMIR Mhealth Uhealth 2021, 9, e24214. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Myers, S.A.; Thompson, J.; Kim, J.; Koutakis, P.; Williams, M.; Zhu, Z.; Schieber, M.; Lackner, T.; Willcockson, G.; et al. Different Outcomes after Revascularization or Standard Supervised Exercise Treadmill Training of Claudicating Patients with Peripheral Artery Disease. JVS Vasc. Sci. 2020, 1, 255. [Google Scholar] [CrossRef]
- Mitchell, A.; Martin, A.E. Quantifying the effect of sagittal plane joint angle variability on bipedal fall risk. PLoS ONE 2022, 17, e0262749. [Google Scholar] [CrossRef]
- Myers, S.A.; Pipinos, I.I.; Johanning, J.M.; Stergiou, N. Gait variability of patients with intermittent claudication is similar before and after the onset of claudication pain. Clin. Biomech. 2011, 26, 729–734. [Google Scholar] [CrossRef]
- Dounskaia, N. The internal model and the leading joint hypothesis: Implications for control of multi-joint movements. Exp. Brain Res. 2005, 166, 1–16. [Google Scholar] [CrossRef]
- McDonald, P.V.; Van Emmerik, R.E.A.; Newell, K.M. The effects of practice on limb kinematics in a throwing task. J. Mot. Behav. 1989, 21, 245–264. [Google Scholar] [CrossRef]
- Kang, H.G.; Dingwell, J. Dynamic stability of superior vs. inferior segments during walking in young and older adults. Gait Posture 2009, 30, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Decker, L.M.; Cignetti, F.; Stergiou, N. Wearing a safety harness during treadmill walking influences lower extremity kinematics mainly through changes in ankle regularity and local stability. J. Neuroeng. Rehabil. 2012, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Raffalt, P.C.; Vallabhajosula, S.; Renz, J.J.; Mukherjee, M.; Stergiou, N. Lower limb joint angle variability and dimensionality are different in stairmill climbing and treadmill walking. R. Soc. Open Sci. 2018, 5, 180996. [Google Scholar] [CrossRef] [PubMed]
- Haas, T.L.; Lloyd, P.G.; Yang, H.; Terjung, R.L. Exercise training and peripheral arterial disease. Compr. Physiol. 2012, 2, 2933–3017. [Google Scholar] [CrossRef]
- Bulmer, A.C.; Coombes, J.S. Optimising exercise training in peripheral arterial disease. Sport. Med. 2004, 34, 983–1003. [Google Scholar] [CrossRef]
- Treat-Jacobson, D.; McDermott, M.M.; Bronas, U.G.; Campia, U.; Collins, T.C.; Criqui, M.H.; Gardner, A.W.; Hiatt, W.R.; Regensteiner, J.G.; Rich, K.; et al. Optimal Exercise Programs for Patients with Peripheral Artery Disease: A Scientific Statement from the American Heart Association. Circulation 2019, 139, E10–E33. [Google Scholar] [CrossRef]
- Nigg, B.M.; Cole, G.K.; Nachbauer, W. Effects of arch height of the foot on angular motion of the lower extremities in running. J. Biomech. 1993, 26, 909–916. [Google Scholar] [CrossRef]
- Vaughan, C.L.; Davis, B.L.; O’connor, J.C. Dynamics of Human Gait, 2nd ed.; Kiboho Publishers: Cape Town, South Africa, 1999; Available online: http://www.kiboho.co.za/GaitCD (accessed on 25 July 2022).
- Lee, M.; Kim, J.; Son, J.; Kim, Y. Kinematic and kinetic analysis during forward and backward walking. Gait Posture 2013, 38, 674–678. [Google Scholar] [CrossRef]
- Gonabadi, A.M.; Cesar, G.M.; Buster, T.W.; Burnfield, J.M. Effect of gap-filling technique and gap location on linear and nonlinear calculations of motion during locomotor activities. Gait Posture 2022, 94, 85–92. [Google Scholar] [CrossRef]
- Stergiou, N.; Decker, L.M. Human movement variability, nonlinear dynamics, and pathology: Is there a connection? Hum. Mov. Sci. 2011, 30, 869–888. [Google Scholar] [CrossRef]
- Ahmadi, S.; Sepehri, N.; Wu, C.; Szturm, T. Sample Entropy of Human Gait Center of Pressure Displacement: A Systematic Methodological Analysis. Entropy 2018, 20, 579. [Google Scholar] [CrossRef] [PubMed]
- Richman, J.S.; Moorman, J.R. Physiological time-series analysis using approximate and sample entropy. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, 2039–2049. [Google Scholar] [CrossRef] [PubMed]
- Fallahtafti, F.; Wurdeman, S.R.; Yentes, J.M. Sampling rate influences the regularity analysis of temporal domain measures of walking more than spatial domain measures. Gait Posture 2021, 88, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Yentes, J.M.; Hunt, N.; Schmid, K.K.; Kaipust, J.P.; McGrath, D.; Stergiou, N. The appropriate use of approximate entropy and sample entropy with short data sets. Ann. Biomed. Eng. 2013, 41, 349–365. [Google Scholar] [CrossRef]
- Delgado-Bonal, A.; Marshak, A. Approximate entropy and sample entropy: A comprehensive tutorial. Entropy 2019, 21, 541. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.; Swift, J.B.; Swinney, H.L.; Vastano, J.A. Determining Lyapunov Exponents From A Time Series. Phys. D Nonlinear Phenom. 1985, 16, 285–317. [Google Scholar] [CrossRef]
- Rouder, J.N.; Speckman, P.L.; Sun, D.; Morey, R.; Iverson, G. Bayesian t tests for accepting and rejecting the null hypothesis. Psychon. Bull. Rev. 2009, 16, 225–237. [Google Scholar] [CrossRef]
- Jeffreys, H. The Theory of Probability; OUP Oxford: Oxford, UK, 1998; 247p, Available online: https://books.google.com/books?hl=en&lr=&id=vh9Act9rtzQC&oi=fnd&pg=PA1&dq=Jeffreys,+H.+(1961).+Theory+of+probability,+(3rd+ed.)+Oxford,+UK:+Oxford+University+Press.&ots=ffVuDUY2jT&sig=lwq4Wm9HURu-OSp247pSSj4dGo4#v=onepage&q&f=false (accessed on 18 August 2022).
- Wagenmakers, E.-J.; Love, J.; Marsman, M.; Jamil, T.; Ly, A.; Verhagen, J.; Selker, R.; Gronau, Q.F.; Dropmann, D.; Boutin, B.; et al. Bayesian inference for psychology. Part II: Example applications with JASP. Psychon. Bull. Rev. 2018, 25, 58–76. [Google Scholar] [CrossRef]
- Crowther, R.G.; Spinks, W.L.; Leicht, A.S.; Sangla, K.; Quigley, F.; Golledge, J. The influence of a long term exercise program on lower limb movement variability and walking performance in patients with peripheral arterial disease. Hum. Mov. Sci. 2009, 28, 494–503. [Google Scholar] [CrossRef]
- Myers, S.A.; Stergiou, N.; Pipinos, I.I.; Johanning, J.M. Gait Variability Patterns are Altered in Healthy Young Individuals During the Acute Reperfusion Phase of Ischemia-Reperfusion. J. Surg. Res. 2010, 164, 6–12. [Google Scholar] [CrossRef][Green Version]
- Hausdorff, J.M. Gait variability: Methods, modeling and meaning. J. Neuroeng. Rehabil. 2005, 2, 19. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, H.; Sawada, S.; Osawa, T.; Natsume, T.; Yoshihara, T.; Deng, P.; Machida, S.; Naito, H. Muscle Size and Strength of the Lower Body in Supervised and in Combined Supervised and Unsupervised Low-Load Resistance Training. J. Sports Sci. Med. 2020, 19, 721–726. [Google Scholar] [PubMed]
- McDermott, M.M. Lower extremity manifestations of peripheral artery disease: The pathophysiologic and functional implications of leg ischemia. Circ. Res. 2015, 116, 1540–1550. [Google Scholar] [CrossRef]
- Hussain, V.S.; Spano, M.L.; Lockhart, T.E. Effect of data length on time delay and embedding dimension for calculating the Lyapunov exponent in walking. J. R. Soc. Interface. 2020, 17, 20200311. [Google Scholar] [CrossRef] [PubMed]
- Barrett, R.; Noordegraaf, M.V.; Morrison, S. Gender differences in the variability of lower extremity kinematics during treadmill locomotion. J. Mot. Behav. 2008, 40, 62–70. [Google Scholar] [CrossRef]
- Koutakis, P.; Johanning, J.M.; Haynatzki, G.R.; Myers, S.A.; Stergiou, N.; Longo, G.M.; Pipinos, I.I. Abnormal Joint Powers Before And After The Onset Of Claudication Symptoms. J. Vasc. Surg. 2010, 52, 340–347. [Google Scholar] [CrossRef] [PubMed]
- McDermott, M.M.; Tian, L.; Ferrucci, L.; Liu, K.; Guralnik, J.M.; Liao, Y.; Pearce, W.H.; Criqui, M.H. Associations between lower extremity ischemia, upper and lower extremity strength, and functional impairment with peripheral arterial disease. J. Am. Geriatr. Soc. 2008, 56, 724–729. [Google Scholar] [CrossRef] [PubMed]
- McDermott, M.M.; Ferrucci, L.; Gonzales-Freire, M.; Kosmac, K.; Leeuwenburgh, C.; Peterson, C.A.; Saini, S.; Sufit, R. Skeletal Muscle Pathology in Peripheral Artery Disease: A Brief Review. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2577–2585. [Google Scholar] [CrossRef]
- Rao, S.; Saltzman, C.; Yack, H.J. Segmental foot mobility in individuals with and without diabetes and neuropathy. Clin. Biomech. 2007, 22, 464–471. [Google Scholar] [CrossRef]
- Li, S.; Lackner, T.; Willcockson, G.; Shields, C.; Brunette, K.; Zhu, Z.; Kim, J.; Myers, S.; Williams, M.; Despiegelaere, H.; et al. Abstract 631: Histopathological Study of Calf Muscle in Claudicating Patients with Peripheral Artery Disease, After Supervised Exercise Therapy. Arterioscler. Thromb. Vasc. Biol. 2019, 39, A631. Available online: https://www.ahajournals.org/doi/10.1161/atvb.39.suppl_1.631 (accessed on 13 August 2022).


| Age (Years) | Body Mass (kg) | Height (cm) | BMI (kg/m2) |
|---|---|---|---|
| 63.79 (6.21) | 91.53 (18.6) | 1.76 (0.07) | 29.49 (5.7) |
| Ankle (Pre) | Ankle (Post) | Knee (Pre) | Knee (Post) | Hip (Pre) | Hip (Post) | p-Value-Joint | p-Value-Intervention | p-Value-Interaction | |
|---|---|---|---|---|---|---|---|---|---|
| SampEn | 0.28(0.04) | 0.27(0.05) | 0.18(0.7) | 0.17(0.06) | 0.23(0.03) | 0.22(0.03) | p < 0.001 | p = 0.008 | p = 0.93 |
| Largest Lyapunov Exponent | 1.18(0.69) | 1.13(0.34) | 0.85(0.19) | 0.85(0.27) | 0.79(0.41) | 0.72(0.22) | p < 0.001 | p = 0.43 | p = 0.79 |
| ROM_Average (degree) | 22.93(4.3) | 24.84(3.83) | 47.79(6.24) | 47.92(5.98) | 36.80(5.41) | 36.80(4.61) | p < 0.001 | p = 0.11 | p = 0.08 |
| ROM_STD (degree) | 4.80(2.07) | 4.38(1.92) | 13.56(3.34) | 15.55(3.59) | 3.75(3.20) | 3.38(3.21) | p < 0.001 | p = 0.29 | p < 0.001 |
| p < 0.05 denotes significant differences between factors | |||||||||
| Measure 1 | Measure 2 | BF01 | Error% |
|---|---|---|---|
| LyE_Ankle_Pre | LyE_Ankle_Post | 5.27 | 7.03 × 10−6 |
| LyE_Knee_Pre | LyE_Knee_Post | 5.72 | 7.96 × 10−6 |
| LyE_Hip_Pre | LyE_Hip_Post | 3.68 | 4.73 × 10−6 |
| N | Mean | SD | SE | 95% Confidence Interval | ||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| LyE_Ankle_Pre | 39 | 1.185 | 0.698 | 0.112 | 0.959 | 1.411 |
| LyE_Ankle_Post | 39 | 1.132 | 0.345 | 0.055 | 1.020 | 1.243 |
| LyE_Knee_Pre | 39 | 0.859 | 0.192 | 0.031 | 0.797 | 0.921 |
| LyE_Knee_Post | 39 | 0.850 | 0.273 | 0.044 | 0.762 | 0.939 |
| LyE_Hip_Pre | 39 | 0.791 | 0.414 | 0.066 | 0.656 | 0.925 |
| LyE_Hip_Post | 39 | 0.726 | 0.226 | 0.036 | 0.653 | 0.799 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fallahtafti, F.; Salamifar, Z.; Hassan, M.; Rahman, H.; Pipinos, I.; Myers, S.A. Joint Angle Variability Is Altered in Patients with Peripheral Artery Disease after Six Months of Exercise Intervention. Entropy 2022, 24, 1422. https://doi.org/10.3390/e24101422
Fallahtafti F, Salamifar Z, Hassan M, Rahman H, Pipinos I, Myers SA. Joint Angle Variability Is Altered in Patients with Peripheral Artery Disease after Six Months of Exercise Intervention. Entropy. 2022; 24(10):1422. https://doi.org/10.3390/e24101422
Chicago/Turabian StyleFallahtafti, Farahnaz, Zahra Salamifar, Mahdi Hassan, Hafizur Rahman, Iraklis Pipinos, and Sara A. Myers. 2022. "Joint Angle Variability Is Altered in Patients with Peripheral Artery Disease after Six Months of Exercise Intervention" Entropy 24, no. 10: 1422. https://doi.org/10.3390/e24101422
APA StyleFallahtafti, F., Salamifar, Z., Hassan, M., Rahman, H., Pipinos, I., & Myers, S. A. (2022). Joint Angle Variability Is Altered in Patients with Peripheral Artery Disease after Six Months of Exercise Intervention. Entropy, 24(10), 1422. https://doi.org/10.3390/e24101422






