Are Gait and Balance Problems in Neurological Patients Interdependent? Enhanced Analysis Using Gait Indices, Cyclograms, Balance Parameters and Entropy
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Methods
2.2.1. Balance
Balance on Kistler Force Plate
Balance on Biodex Balance System SD
- -
- On a stable platform with eyes open;
- -
- On an unstable platform with eyes open (level of stability 4);
- -
- On a slightly unstable platform with eyes open (level of stability 8);
- -
- On a platform with changing level of stability with eyes open (from level 12 to 4);
- -
- Limits of Stability Test (LOS) with moderate difficulty level [12] on stable platform;
- -
- Modified Clinical Test of Sensory Integration in Balance (m-CTSIB). This test comprised four different conditions: standing on firm surface with eyes open, standing on firm surface with eyes closed, standing on foam with eyes open, and standing on foam with eyes closed.
2.2.2. Gait Analysis
2.2.3. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crowther, R.C.; Pohlman, J.M. Gait retraining for balance improvement. In Handbook of Human Motion; Müller, B., Wolf, S.I., Eds.; Springer: Cham, Switzerland, 2018; pp. 277–285. [Google Scholar]
- Guffey, K.; Regier, M.; Mancinelli, C.; Pergami, P. Gait parameters associated in balance in healthy 2- to 4-years old children. Gait Posture 2017, 43, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, J.; An, R. Effectiveness of backward walking training on balance performance: A systematic review and meta-analysis. Gait Posture 2019, 68, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Mudge, S.; Rochester, L.; Recordon, A. The effect of treadmill training on gait, balance, and trunk control in a hemiplegic subject: A single system design. Disabil. Rehabil. 2003, 25, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Langhammer, B.; Lindmark, B.; Stanghelle, J.K. The relation between gait velocity and static and dynamic balance in the early rehabilitation of patients with acute stroke. Adv. Physiother. 2006, 8, 60–65. [Google Scholar] [CrossRef]
- Bland, D.C.; Zampieri, C.; Damiano, D.L. Effectiveness of physical therapy for improving gait and balance in individuals with traumatic brain injury: A systematic review. Brain Injury 2011, 25, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lee, M.; Song, C. Balance training improves postural balance, gait and functional strength in adolescents with intellectual disabilities: Single-blinded, randomized clinical trial. Disabil. Health J. 2016, 9, 416–422. [Google Scholar] [CrossRef]
- Domagalska-Szopa, M.; Szopa, A.; Czamara, A. Dependence of gait deviation on weight-bearing asymmetry and postural instability in children with unilateral cerebral palsy. PLoS ONE 2016, 11, e0165583. [Google Scholar] [CrossRef] [PubMed]
- Syczewska, M.; Szczerbik, E.; Kalinowska, M.; Święcicka, A.; Graff, G.; Graff, K.; Pawlak, P.; Rostek, T.; Łukowicz, M. Results of balance tests and gait parameters are not dependent on each other in patients with balance problems. Gait Posture 2019, 73 (Suppl. 1), 93–94. [Google Scholar]
- Syczewska, M.; Szczerbik, E.; Kalinowska, M.; Święcicka, A. Connection between Gait and Balance Functions in Pediatric Patients with Either Neurological or Sensory Integration Problems. In Lecture Notes in Computational Vision and Biomechanics Vol. 36, Computer Methods, Imaging and Visualization in Biomechanics and Biomedical Engineering; Ateshian, G.A., Myers, K.M., Tavares, J.M.R.S., Eds.; Springer: Cham, Switzerland, 2020; pp. 335–338. [Google Scholar]
- Szczerbik, E.; Iwanicka-Pronicka, K.; Syczewska, M.; Kalinowska, M.; Grasff, K. Balance control of children and adolescents suffering from vertigo symptoms: In what way posturography is helpful in clinical evaluation of vestibular system pathology? Acta Bioeng. Biomech. 2019, 21, 73–78. [Google Scholar] [PubMed]
- Biodex Balance System, S.D. Operation and Service Manual 950-441; Biodex Medical System Inc.: New York, NY, USA, 2010. [Google Scholar]
- Baker, R.; McGinley, J.L.; Schwartz, M.H.; Beynon, S.; Rozumalski, A.; Kerr Graham, K.; Tirosh, O. The gait profile score and movement analysis profile. Gait Posture 2019, 30, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Goswami, A. A new gait parametrization technique by means of cyclogram moments: An application to human slope walking. Gait Posture 1998, 8, 15–36. [Google Scholar] [CrossRef]
- Assainate, C.; Amblard, B. An ontogenetic model for the sensorimotor organization of balance control in humans. Hum. Mov. Sci. 1995, 14, 13–43. [Google Scholar] [CrossRef]
- Assaiante, C. Development of locomotor balance control in healthy children. Neurosci. Biobehav. Rev. 1998, 22, 527–532. [Google Scholar] [CrossRef]
- Guertin, P.A. Central pattern generator for locomotion: Anatomical, physiological, and pathophysiological considerations. Front. Neurol. 2012, 3, 183. [Google Scholar] [CrossRef] [PubMed]
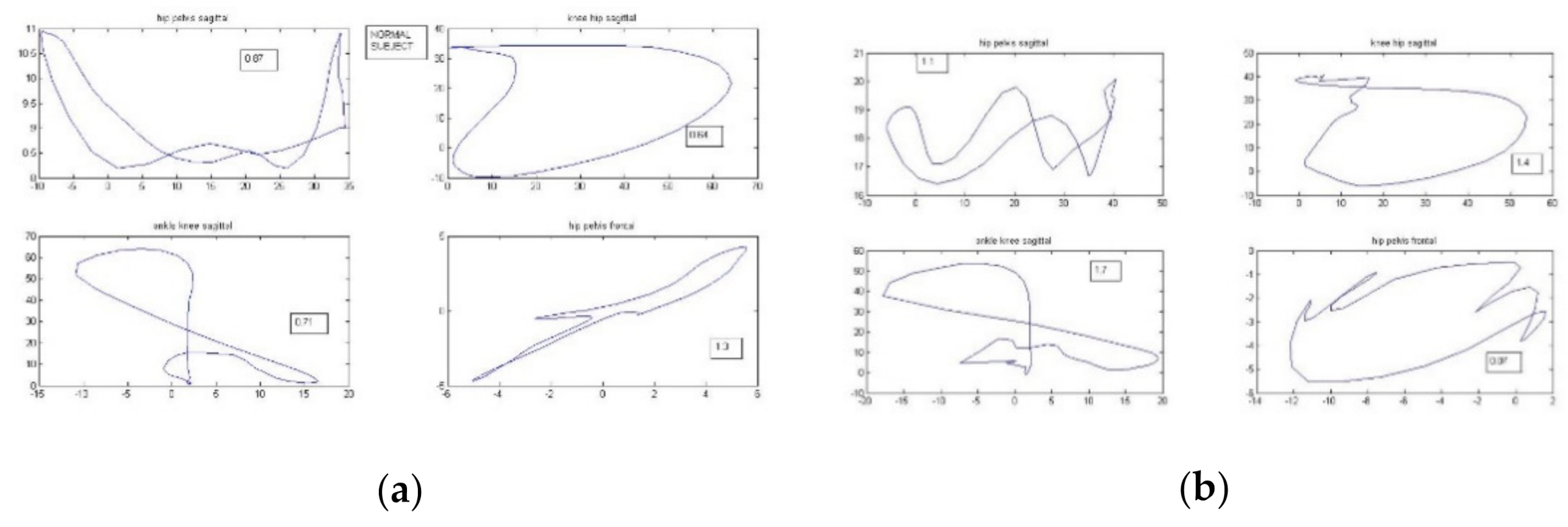
| Dependent Variable | F Test | Statistically Significant Independent Variables |
|---|---|---|
| Entropy eyes open (Kistler) | F = 3.509, p = 0.007 | Ankle-knee cyclogram sagittal plane, GVS hip frontal plane, GDI, knee-hip cyclogram sagittal plane |
| Entropy eyes closed (Kistler) | F = 5.054, p < 0.001 | GVS hip transversal plane, GVS pelvis transversal plane, GVS hip sagittal plane, GVS knee sagittal plane |
| Stability index on stable platform (Biodex) | F = 8.217, p < 0.001 | GDI, cyclogram index |
| Stability index on mildly unstable platform (Biodex) | F = 3.755, p = 0.005 | Knee-hip cyclogram sagittal plane, GVS hip transversal plane |
| Stability index on unstable platform (Biodex) | F = 4.148, p = 0.004 | Knee-hip cyclogram sagittal plane, cyclogram index, GVS hip transversal plane |
| Limits of Stability index (Biodex) | F = 7.180, p < 0.001 | GDI, GVS knee sagittal plane |
| mCTSIB | F = 4.202, p = 0.009 | GDI |
| GPS | F = 8.110, p < 0.001 | Stability index on stable platform (Biodex), total length path eyes closed, maximal sway to the right eyes closed, |
| GDI | F = 7.693, p < 0.001 | Stability index on stable platform (Biodex) |
| F to Remove (1, 40) | p | |
|---|---|---|
| No of parameters exceeding normal values in eyes closed condition (Kistler) | 13.582 | <0.001 * |
| Entropy eyes open (Kistler) | 1.037 | 0.315 |
| Maximal sway to the right in eyes closed condition (Kistler) | 5.441 | 0.025 * |
| Hip-pelvis cyclogram in sagittal plane | 5.204 | 0.028 * |
| GVS ankle sagittal plane | 12.157 | 0.001 * |
| Maximal radius of sway in eyes closed condition (Kistler) | 7.377 | 0.010 * |
| Average radius of sway in eyes closed condition (Kistler) | 9.320 | 0.001 * |
| Entropy eyes closed (Kistler) | 11.953 | 0.001 * |
| Total path length in eyes open condition (Kistler) | 9.480 | 0.004 * |
| GVS knee sagittal plane | 7.824 | 0.008 * |
| GVS hip sagittal plane | 11.039 | 0.002 * |
| Entropy eyes closed (Kistler) | 9.251 | 0.004 * |
| Stability index on unstable platform (Biodex) | 5.654 | 0.022 * |
| Stability index on platform with changing stability (Biodex) | 8.620 | 0.005 * |
| GVS pelvis transversal plane | 0.062 | 0.805 |
| Stability index in mCSTIB test | 4.219 | 0.047 * |
| Hip-pelvis cyclogram in frontal plane | 8.479 | 0.006 * |
| GVS hip transversal plane | 7.702 | 0.008 * |
| Maximal sway to the left in eyes closed condition (Kistler) | 7.482 | 0.009 * |
| GDI | 4.617 | 0.038 * |
| GVS pelvis sagittal plane | 2.714 | 0.148 |
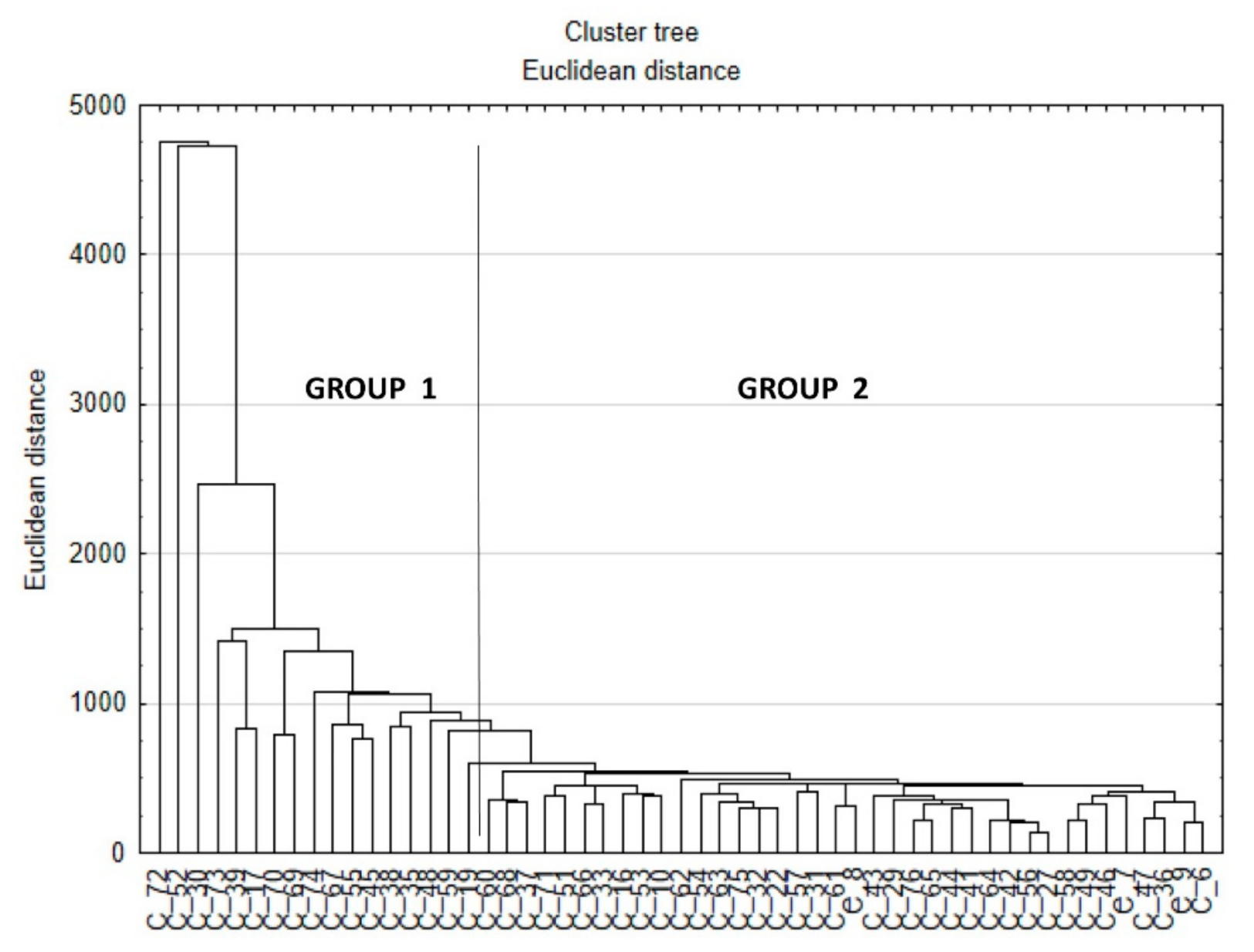
| Correctly Classified Cases [%] | Group 1 | Group 2 | |
|---|---|---|---|
| Group 1 | 100 | 17 | 0 |
| Group 2 | 100 | 0 | 44 |
| Total | 100 | 17 | 44 |
| Parameters | All Patients | Group 1 | Group 2 |
|---|---|---|---|
| Kistler Eyes | Open Condition | ||
| Maximal radius of sway | 18.65 <13.75–24.05> | 24.3 <20.2–35.8> | 18.1 <13.2–22.57> |
| Average radius of sway | 6.4 <4.75–9.25> | 8.5 <6.0–10.9> | 6.2 <4.5–8.2> |
| Total sway path | 513.0 <386.5–634.5> | 690.0 <562.0–853.0> | 481.0 <345.0–557.0> |
| Maximal sway to the left | 11.0 <8.35–15.95> | 15.6 <9.4–23.6> | 10.9 <8.1–14.2> |
| Maximal sway to the right | 11.4 <8.75–18.6> | 17.6 <11.5–24.4> | 10.6 <7.5–15.0> |
| Maximal forward sway | 13.3 <9.75–18.35> | 17.8 <13.8–23.5> | 12.3 <9.5–17.1 > |
| Maximal backward sway | 11.4 <10.7–19.7> | 19.0 <16.1–20.6> | 13.6 <10.3–18.0> |
| No of parameters exceeding normal values | 1 <0–1.5> | 2 <1–3> | 1 <0–1> |
| Entropy | 2892.8 <2159.5–3734.6> | 3932.9 <2894.4–4967.9> | 2832.7 <2058.6–3308.4> |
| Kistler Eyes | Closed Condition | ||
| Maximal radius of sway | 21.95 <18.05–30.5> | 34.5 <26.1–52.9> | 20.6 <15.6–26.6> |
| Average radius of sway | 7.8 <6.05–10.65> | 11.6 <8.4–15.7> | 7.2 <6.0–9.4> |
| Total sway path | 641.6 <469.0–977.5> | 1136.0 <880.0–1481.0> | 591.0 <439.0–765.0> |
| Maximal sway to the left | 15.4 <10.55–20.0> | 19.7 <18.0–31.0> | 13.4 <9.99–18.8> |
| Maximal sway to the right | 14.55 <9.7–21.45> | 17.0 <13.9–40.3> | 12.9 <9.0–19.4> |
| Maximal forward sway | 18.0 <11.75–26.3> | 31.4 <19.6–39.5> | 16.0 <11.0–21.8> |
| Maximal backward sway | 17.0 <13.15–23.05> | 28.9 <22.5–31.0> | 15.1 <11.7–20.5> |
| No of parameters exceeding normal values | 1 <0–2> | 3 <1–5> | 1 <0–1> |
| Entropy | 3287.05 <2570.05–4736.1> | 5112.4 <4538.6–6560.5> | 2962.2 <2362.3 -3753.9> |
| Biodex | |||
| Stability index on stable platform | 0.7 <0.5–1.1> | 1.1 <0.6–1.2> | 0.6 <0.4–1.1> |
| Stability index on slightly unstable platform | 0.8 <0.6–1.1> | 0.7 <0.6–1.0> | 0.8 <0.6–1.1> |
| Stability index on unstable platform | 0.95 <0.7–1.4> | 1.0 <0.7–1.4> | 0.9 <0.6–1.3> |
| Stability index on platform with changing instability | 0.95 <0.7–1.4> | 0.9 <0.6–1.4> | 0.9 <0.7–1.3> |
| Stability index of LOS test | 38.0 <27.0–55.0> | 27.0 <17.0–42> | 42.5 <31.0–57.5> |
| Stability index of mCSTIB test | 2.22 <1.58–2.83> | 2.94 <2.16–4.0> | 2.115 <1.53–2.58> |
| Gait | |||
| GDI | 82.1 <73.45–87.0> | 83.15 <73.45–87.9> | 81.95 <73.45–86.95> |
| GPS | 7.15 <6.23–8.14> | 6.78 <6.2–7.95> | 7.22 <6.26–8.26> |
| GVS pelvis sagittal plane | 4.02 <2.25–6.77> | 3.20 <2.02–7.41> | 4.08 <2.44–6.2> |
| GVS hip sagittal plane | 8.13 <6.24–10.85> | 8.8 <6.18–10.38> | 8.13 <6.51–11.07> |
| GVS knee sagittal plane | 11.94 <9.84–13.4> | 11.15 <9.88–12.61> | 12.29 <9.72–13.71> |
| GVS ankle sagittal plane | 6.64 <5.36–7.91> | 6.0 <4.83–7.91> | 6.67 <5.48–7.91> |
| GVS pelvis frontal plane | 2.56 <1.84–3.28> | 1.92 <1.55–3.73> | 2.61 <2.16–3.19> |
| GVS hip frontal plane | 3.5 <2.69–4.74> | 3.37 <2.69–4.8> | 3.62 <2.69–4.68> |
| GVS pelvis transversal plane | 4.09 <2.65–5.83> | 4.13 <2.62–4.84> | 4.05 <2.78–5.99> |
| GVS hip transversal plane | 11.65 <9.51–15.03> | 11.37 <10.09–14.54> | 11.79 <9.11–15.99> |
| GVS foot progression | 8.27 <5.84–10.69> | 8.8 <5.35–10.77> | 8.21 <5.96–10.61> |
| Hip-pelvis cyclogram sagittal | −0.011 <−0.105–0.099> | −0.006 <−0.119–0.107> | −0.024 <−0.097–0.09> |
| Knee-hip cyclogram sagittal | −0.121 <−0.20–−0.037> | −0.148 <−0.206–−0.017> | −0.12 <−0.2–−0.037> |
| Ankle-hip cyclogram sagittal | −0.053 <−0.152–0.053> | −0.084 <−0.192–0.051> | −0.035 <−0.148–0.054> |
| Hip-pelvis cyclogram frontal | 0.064 <−0.125–0.218> | 0.077 <−0.124–0.22> | 0.056 <−0.125–0.216> |
| Cyclogram index | −0.028 <−0.108–0.068> | −0.003 <−0.133–0.052> | −0.032 <−0.104–0.072> |
| All Patients | Group no 1 | Group no 2 | |
|---|---|---|---|
| Age (years) | 11.4 ± 3.5 | 10.8 ± 3.5 | 11.6 ± 3.5 |
| Height (cm) | 149.4 ± 20.3 | 146.3 ± 15.5 | 150.2 ± 21.6 |
| Weight (kg) | 48.8 ± 22.7 | 40.6 ± 14.4 | 51.2 ± 24.1 |
| BMI | 19.4 ± 5.1 | 18.4 ± 3.7 | 19.6 ± 5.4 |
| Females/Males | 35/40 | 7/10 | 28/30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Syczewska, M.; Szczerbik, E.; Kalinowska, M.; Swiecicka, A.; Graff, G. Are Gait and Balance Problems in Neurological Patients Interdependent? Enhanced Analysis Using Gait Indices, Cyclograms, Balance Parameters and Entropy. Entropy 2021, 23, 359. https://doi.org/10.3390/e23030359
Syczewska M, Szczerbik E, Kalinowska M, Swiecicka A, Graff G. Are Gait and Balance Problems in Neurological Patients Interdependent? Enhanced Analysis Using Gait Indices, Cyclograms, Balance Parameters and Entropy. Entropy. 2021; 23(3):359. https://doi.org/10.3390/e23030359
Chicago/Turabian StyleSyczewska, Malgorzata, Ewa Szczerbik, Malgorzata Kalinowska, Anna Swiecicka, and Grazyna Graff. 2021. "Are Gait and Balance Problems in Neurological Patients Interdependent? Enhanced Analysis Using Gait Indices, Cyclograms, Balance Parameters and Entropy" Entropy 23, no. 3: 359. https://doi.org/10.3390/e23030359
APA StyleSyczewska, M., Szczerbik, E., Kalinowska, M., Swiecicka, A., & Graff, G. (2021). Are Gait and Balance Problems in Neurological Patients Interdependent? Enhanced Analysis Using Gait Indices, Cyclograms, Balance Parameters and Entropy. Entropy, 23(3), 359. https://doi.org/10.3390/e23030359






