Neurofeedback Training Based on Motor Imagery Strategies Increases EEG Complexity in Elderly Population
Abstract
:1. Introduction
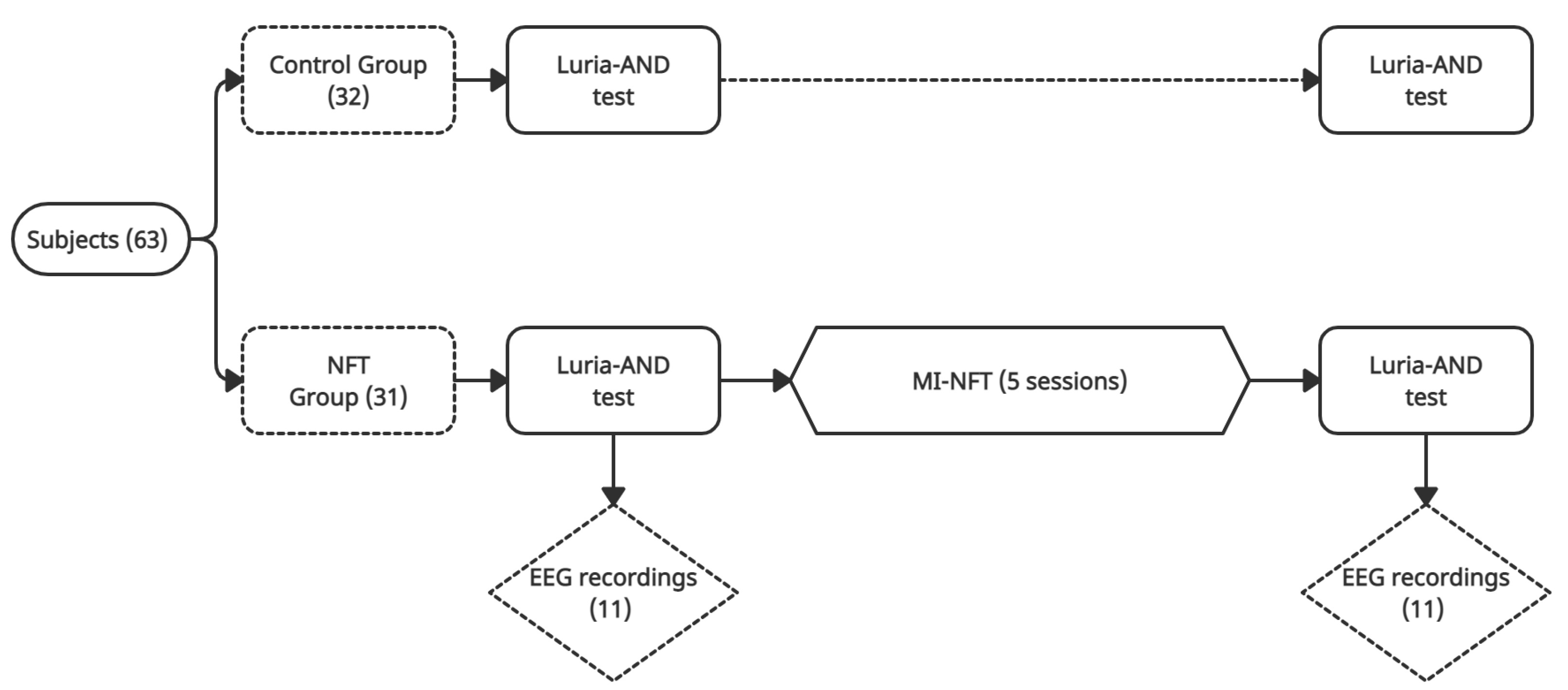
2. Materials and Methods
2.1. Experimental Protocol
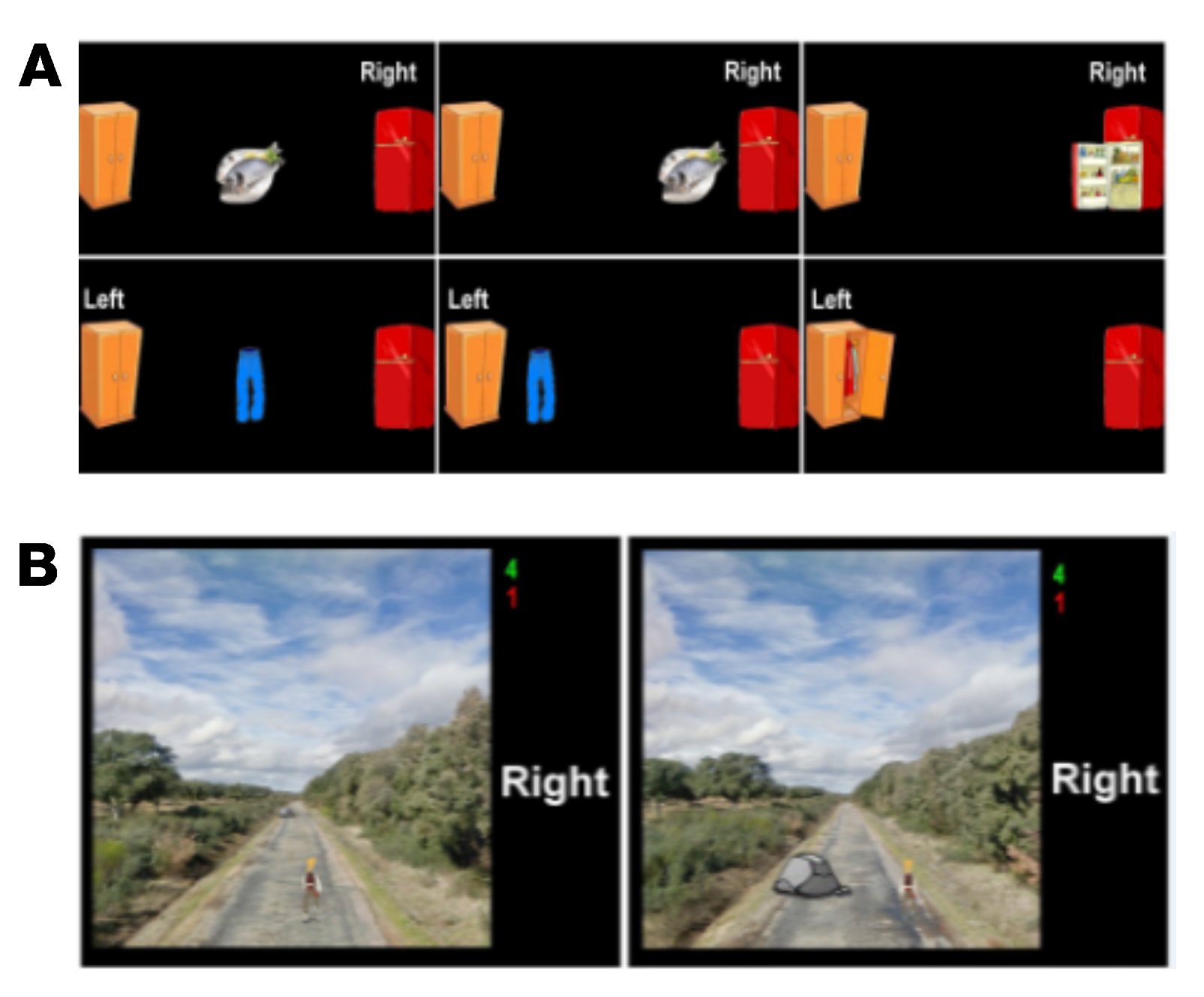
- Task 1: Subjects were required to imagine hand movements. Alternatively, a closed door or a closed window was displayed on the screen. Then, the paradigm indicated whether users had to perform right- or left-hand MI, respectively. If the user correctly performed the exercise, the displayed object was opened. This task aimed to help subjects learn to modulate their SMR.
- Task 2: A target randomly located on the right or the left of the screen was displayed 3 s prior to the start of the trial. In order to reach the target with a displayed cursor, subjects had to continuously perform MI for a maximum duration of 10 s. The target and the cursor were represented by different pairs of related pictures, such as fish/fridge or person/house.
- Task 3: Subjects had to control a cursor in order to reach one of the two displayed targets (one of them was a picture related to cursor’s picture, and the other was unrelated to it) by performing MI. Targets were displayed 3 s prior to the start of the trial, and subjects had to complete the task in a maximum time of 10 s. Therefore, this task not only involves MI but also the use of logic.
- Task 4: A person walking forward continuously on a path was displayed. Through the MI of their hands, subjects had to control the horizontal position in order to overcome the different obstacles that appeared on the path. This task required the use of the visuospatial function of the subjects. In each trial, the duration of the feedback period was 18 s.
- Task 5: Two pairs of images were shown sequentially for 3 s each, so that one image was common to both pairs. Subjects had to identify the image shown twice and move the cursor towards it by performing MI. They had a maximum of 12 s to do so. Thus, this activity also involved the use of memory.
2.2. Dataset
2.3. Luria Adult Neuropsychological Diagnosis
2.4. Signal Pre-Processing
2.5. Metrics for Assessing EEG Changes
2.5.1. Relative Power
2.5.2. Multiscale Entropy
- 1.
- Form N-m+1 template vectors of m consecutive data points , where each template vector is defined by = , i = . These vectors represent m consecutive values of X commencing with the i-th sample.
- 2.
- Define the distance between and , . In this context, Chebyshev is the most common metric used to compute the distance between vectors [59],
- 3.
- Define a tolerance criterion in terms of the standard deviation () of the time series, , where r is a parameter to set. Thus, two template vectors, and , are considered similar if their distance is less than the tolerance value:
- 4.
- For each , count the number of vectors , given i≠j, that fulfil the tolerance criterion (Equation (3)). This count is denoted as . Then, the frequency of patterns similar to a given one of window length m is calculated by
- 5.
- Define the probability that two sequences will match for m points as
- 6.
- Increase the embedding dimension to m+1, and repeat steps 1 to 5 in order to calculate as the probability that two vectors, and , given i ≠ j, match for m+1 points.
- 7.
- Then, we can estimate SampEn by computing:
2.6. Statistical Analysis
3. Results
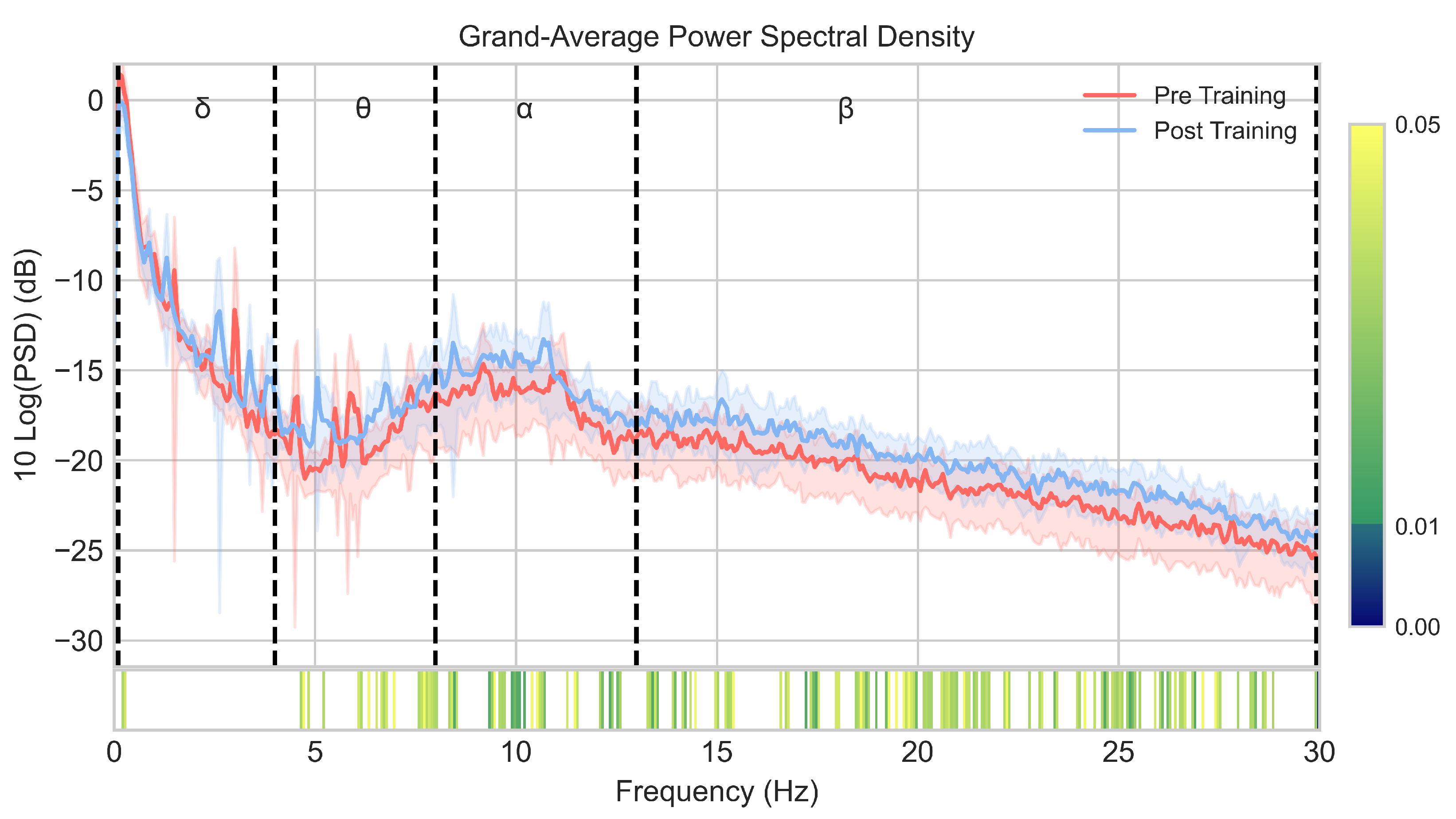
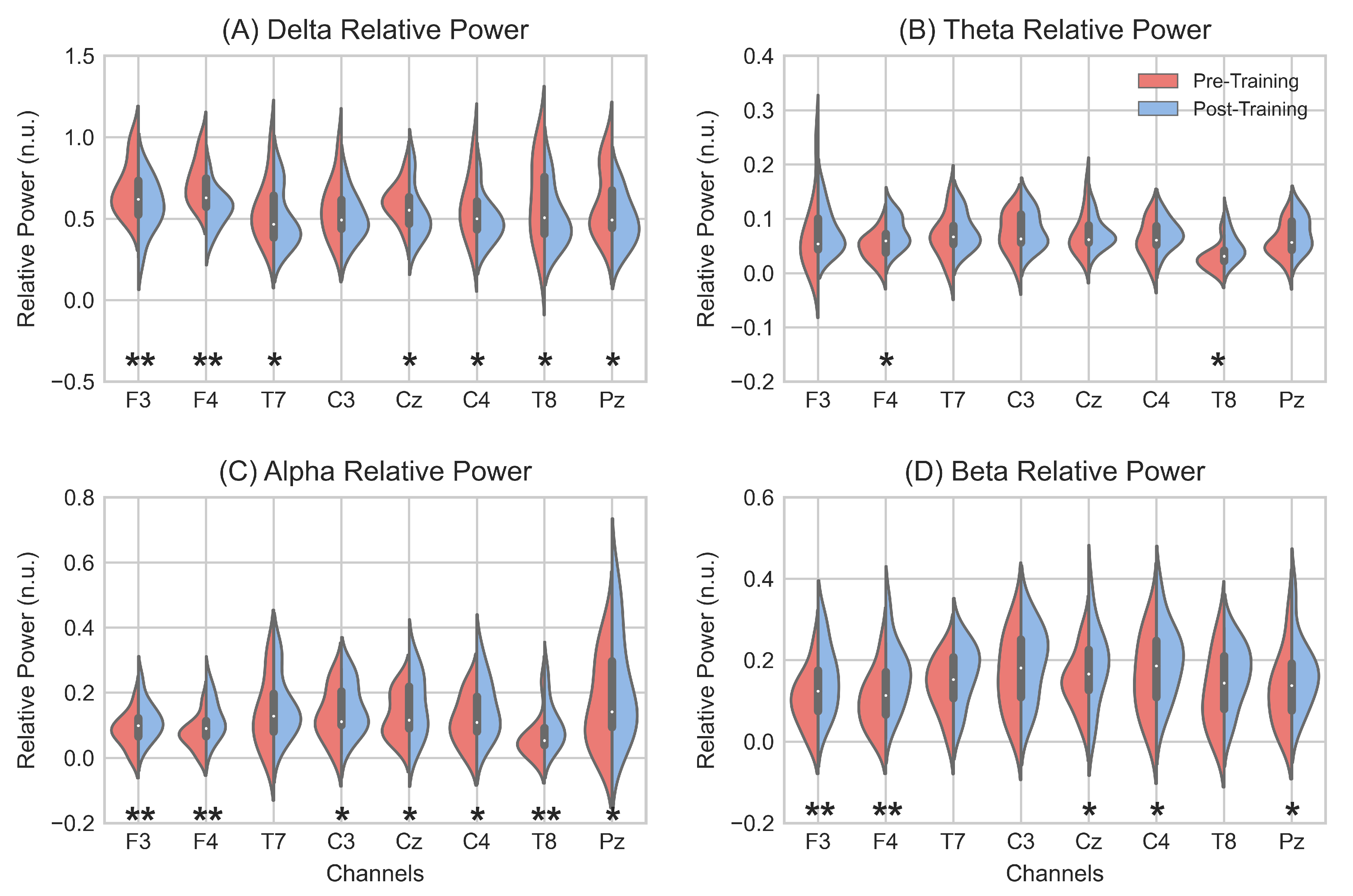
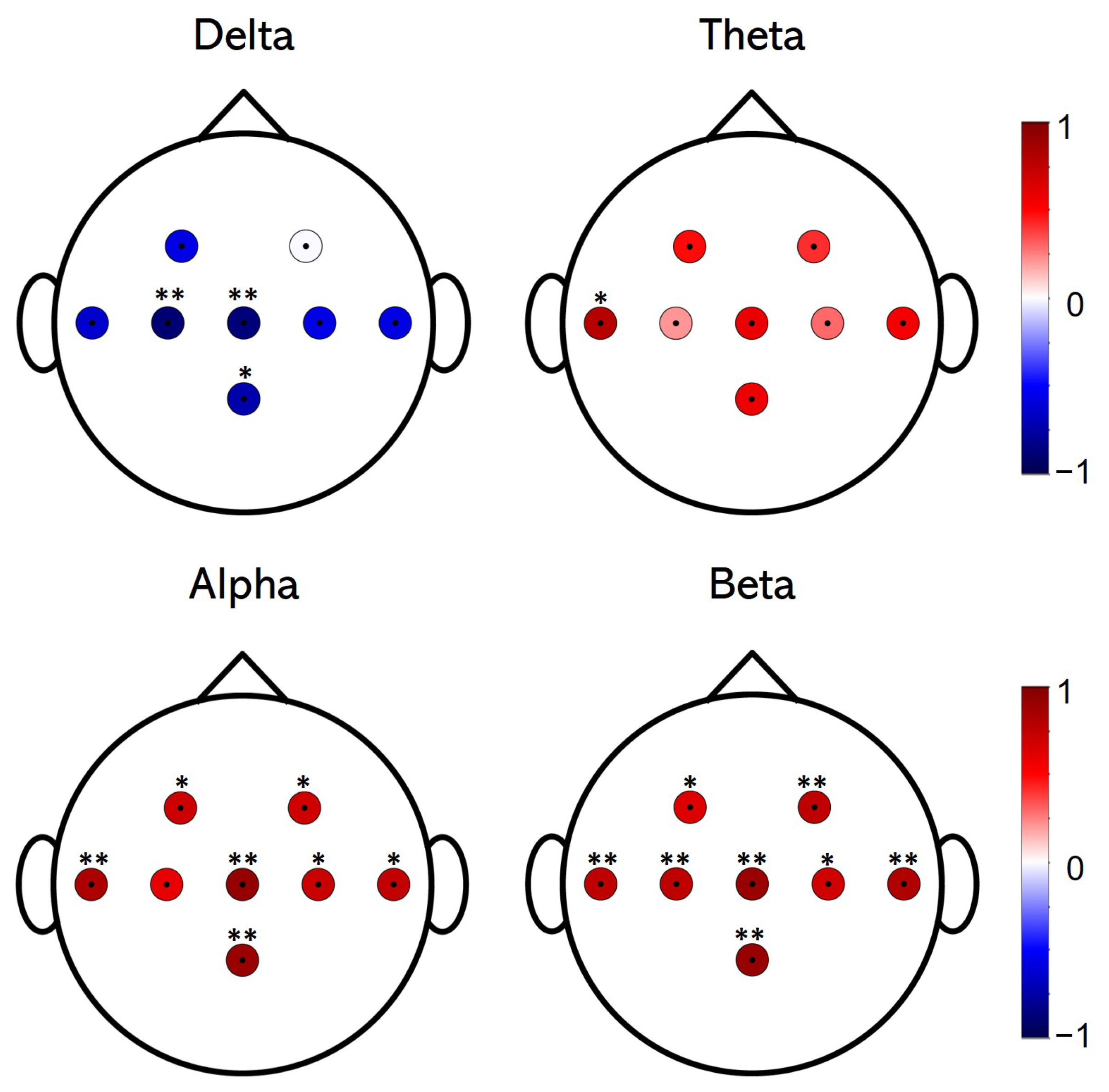
3.1. EEG Spectral Analysis
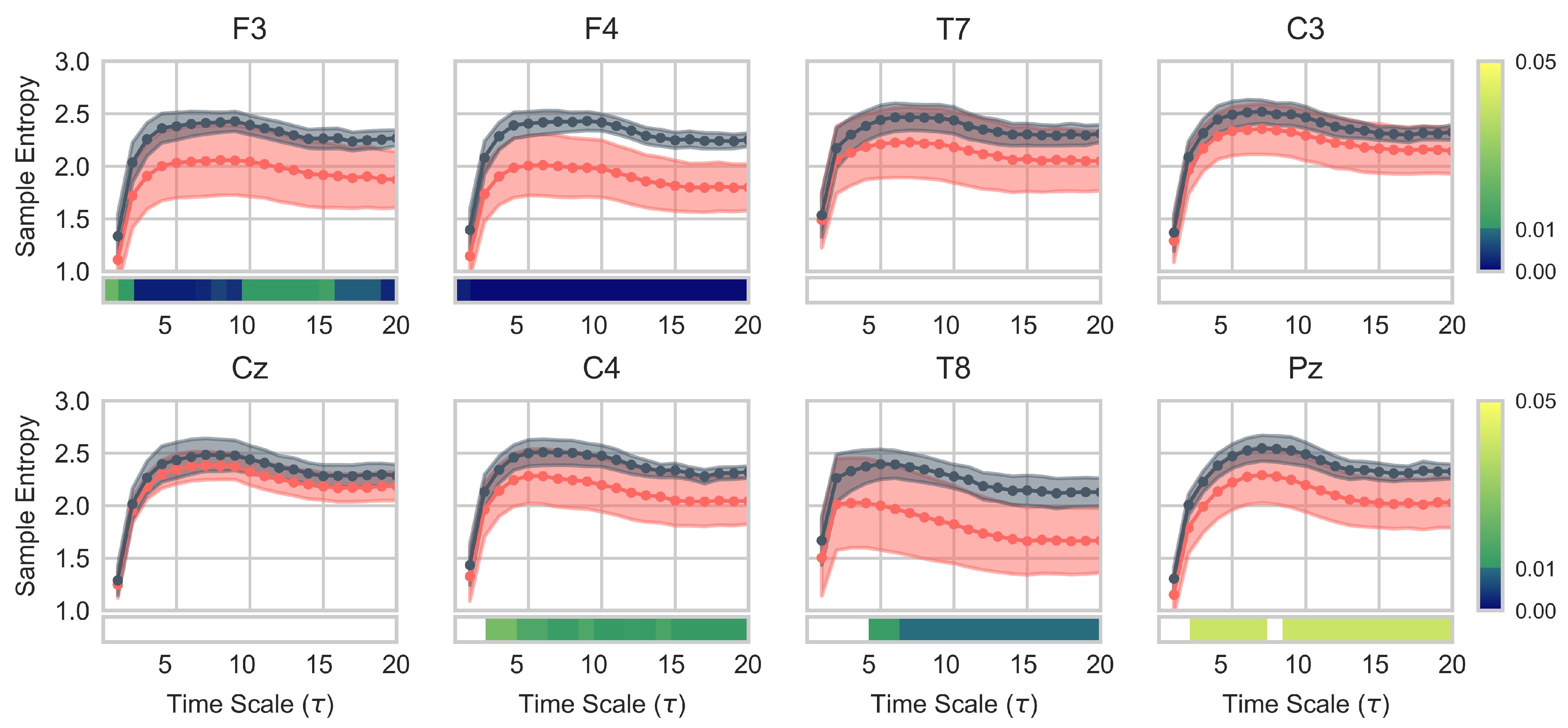
3.2. EEG Complexity Analysis
3.3. Correlation Analysis of Spectral and Complexity EEG Changes
3.4. Luria-AND Analysis
4. Discussion
4.1. Induced EEG Spectral Changes
4.2. Induced EEG Complexity Changes
4.3. Correlations between Spectral Power and EEG Complexity
4.4. Changes in Neuropsychological Functions
4.5. Limitations and Future Research Lines
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wolpaw, J.R.; Birbaumer, N.; McFarland, D.J.; Pfurtscheller, G.; Vaughan, T.M. Brain-computer interfaces for communication and control. Clin. Neurophysiol. 2002, 113, 767–791. [Google Scholar] [CrossRef]
- Wolpaw, J.; Wolpaw, E.W. Brain-Computer Interfaces: Principles and Practice; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Mane, R.; Chouhan, T.; Guan, C. BCI for stroke rehabilitation: Motor and beyond. J. Neural Eng. 2020, 17, 041001. [Google Scholar] [CrossRef]
- Sebastián-Romagosa, M.; Cho, W.; Ortner, R.; Murovec, N.; Von Oertzen, T.; Kamada, K.; Allison, B.Z.; Guger, C. Brain Computer Interface Treatment for Motor Rehabilitation of Upper Extremity of Stroke Patients—A Feasibility Study. Front. Neurosci. 2020, 14, 1056. [Google Scholar] [CrossRef]
- Enriquez-Geppert, S.; Huster, R.J.; Herrmann, C.S. EEG-neurofeedback as a tool to modulate cognition and behavior: A review tutorial. Front. Hum. Neurosci. 2017, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Sitaram, R.; Ros, T.; Stoeckel, L.; Haller, S.; Scharnowski, F.; Lewis-Peacock, J.; Weiskopf, N.; Blefari, M.L.; Rana, M.; Oblak, E.; et al. Closed-loop brain training: The science of neurofeedback. Nat. Rev. Neurosci. 2017, 18, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Ros, T.; Munneke, M.A.; Ruge, D.; Gruzelier, J.H.; Rothwell, J.C. Endogenous control of waking brain rhythms induces neuroplasticity in humans. Eur. J. Neurosci. 2010, 31, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Arns, M.; Heinrich, H.; Strehl, U. Evaluation of neurofeedback in ADHD: The long and winding road. Biol. Psychol. 2014, 95, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Arns, M.; Clark, C.R.; Trullinger, M.; DeBeus, R.; Mack, M.; Aniftos, M. Neurofeedback and Attention-Deficit/Hyperactivity-Disorder (ADHD) in Children: Rating the Evidence and Proposed Guidelines. Appl. Psychophysiol. Biofeedback 2020, 45, 39–48. [Google Scholar] [CrossRef]
- Tan, G.; Thornby, J.; Hammond, D.C.; Strehl, U.; Canady, B.; Arnemann, K.; Kaiser, D.A. Meta-analysis of EEG biofeedback in treating epilepsy. Clin. EEG Neurosci. 2009, 40, 173–179. [Google Scholar] [CrossRef]
- Prichep, L.S.; John, E.R.; Ferris, S.H.; Reisberg, B.; Almas, M.; Alper, K.; Cancro, R. Quantitative EEG correlates of cognitive deterioration in the elderly. Neurobiol. Aging 1994, 15, 85–90. [Google Scholar] [CrossRef]
- Jeong, J. EEG dynamics in patients with Alzheimer’s disease. Clin. Neurophysiol. 2004, 115, 1490–1505. [Google Scholar] [CrossRef] [PubMed]
- Czigler, B.; Csikós, D.; Hidasi, Z.; Anna Gaál, Z.; Csibri, É.; Kiss, É.; Salacz, P.; Molnár, M. Quantitative EEG in early Alzheimer’s disease patients—Power spectrum and complexity features. Int. J. Psychophysiol. 2008, 68, 75–80. [Google Scholar] [CrossRef]
- Benwell, C.S.; Davila-Pérez, P.; Fried, P.J.; Jones, R.N.; Travison, T.G.; Santarnecchi, E.; Pascual-Leone, A.; Shafi, M.M. EEG spectral power abnormalities and their relationship with cognitive dysfunction in patients with Alzheimer’s disease and type 2 diabetes. Neurobiol. Aging 2020, 85, 83–95. [Google Scholar] [CrossRef]
- Laborda-Sánchez, F.; Cansino, S. The Effects of Neurofeedback on Aging-Associated Cognitive Decline: A Systematic Review. Appl. Psychophysiol. Biofeedback 2021, 46, 1–10. [Google Scholar] [CrossRef] [PubMed]
- United Nations. World Population Ageing; Department of Economic and Social Affairs: New York, NY, USA, 2020; pp. 40–1307. [Google Scholar]
- Wang, J.R.; Hsieh, S. Neurofeedback training improves attention and working memory performance. Clin. Neurophysiol. 2013, 124, 2406–2420. [Google Scholar] [CrossRef]
- Enriquez-Geppert, S.; Huster, R.J.; Scharfenort, R.; Mokom, Z.N.; Zimmermann, J.; Herrmann, C.S. Modulation of frontal-midline theta by neurofeedback. Biol. Psychol. 2014, 95, 59–69. [Google Scholar] [CrossRef]
- Vernon, D.; Egner, T.; Cooper, N.; Compton, T.; Neilands, C.; Sheri, A.; Gruzelier, J. The effect of trainin distinct neurofeedback protocols on aspects of cognitive performance. Int. J. Psuchophysiol. 2003, 47, 75–85. [Google Scholar] [CrossRef]
- Zoefel, B.; Huster, R.J.; Herrmann, C.S. Neurofeedback training of the upper alpha frequency band in EEG improves cognitive performance. NeuroImage 2011, 54, 1427–1431. [Google Scholar] [CrossRef] [PubMed]
- Kober, S.E.; Witte, M.; Stangl, M.; Väljamäe, A.; Neuper, C.; Wood, G. Shutting down sensorimotor interference unblocks the networks for stimulus processing: An SMR neurofeedback training study. Clin. Neurophysiol. 2015, 126, 82–95. [Google Scholar] [CrossRef]
- Campos da Paz, V.K.; Garcia, A.; Campos da Paz Neto, A.; Tomaz, C. SMR neurofeedback training facilitates working memory performance in healthy older adults: A behavioral and EEG study. Front. Behav. Neurosci. 2018, 12, 321. [Google Scholar] [CrossRef]
- Marlats, F.; Bao, G.; Chevallier, S.; Boubaya, M.; Djabelkhir-Jemmi, L.; Wu, Y.H.; Lenoir, H.; Rigaud, A.S.; Azabou, E. SMR/Theta Neurofeedback Training Improves Cognitive Performance and EEG Activity in Elderly With Mild Cognitive Impairment: A Pilot Study. Front. Aging Neurosci. 2020, 12, 147. [Google Scholar] [CrossRef]
- Becerra, J.; Fernández, T.; Roca-Stappung, M.; Díaz-Comas, L.; Galán, L.; Bosch, J.; Espino, M.; Moreno, A.J.; Harmony, T. Neurofeedback in healthy elderly human subjects with electroencephalographic risk for cognitive disorder. J. Alzheimer’s Dis. 2012, 28, 357–367. [Google Scholar] [CrossRef]
- Staufenbiel, S.M.; Brouwer, A.M.; Keizer, A.W.; van Wouwe, N.C. Effect of beta and gamma neurofeedback on memory and intelligence in the elderly. Biol. Psychol. 2014, 95, 74–85. [Google Scholar] [CrossRef]
- Luijmes, R.E.; Pouwels, S.; Boonman, J. L’efficacité du neurofeedback sur le fonctionnement cognitif chez les patients atteints de la maladie d’Alzheimer: Résultats préliminaires. Neurophysiol. Clin. 2016, 46, 179–187. [Google Scholar] [CrossRef]
- Pfurtscheller, G. Event-related synchronization (ERS): An electrophysiological correlate of cortical areas at rest. Electroencephalogr. Clin. Neurophysiol. 1992, 83, 62–69. [Google Scholar] [CrossRef]
- Sterman, M.B. Physiological origins and functional correlates of EEG rhythmic activities: Implications for self-regulation. Biofeedback Self-Regul. 1996, 21, 3–33. [Google Scholar] [CrossRef]
- Neuper, C.; Scherer, R.; Wriessnegger, S.; Pfurtscheller, G. Motor imagery and action observation: Modulation of sensorimotor brain rhythms during mental control of a brain–computer interface. Clin. Neurophysiol. 2009, 120, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Pilar, J.; Corralejo, R.; Nicolas-Alonso, L.F.; Álvarez, D.; Hornero, R. Neurofeedback training with a motor imagery-based BCI: Neurocognitive improvements and EEG changes in the elderly. Med. Biol. Eng. Comput. 2016, 54, 1655–1666. [Google Scholar] [CrossRef]
- Mulder, T. Motor imagery and action observation: Cognitive tools for rehabilitation. J. Neural Transm. 2007, 114, 1265–1278. [Google Scholar] [CrossRef] [PubMed]
- Vourvopoulos, A.; Jorge, C.; Abreu, R.; Figueiredo, P.; Fernandes, J.C.; Bermúdez i Badia, S. Efficacy and brain imaging correlates of an immersive motor imagery BCI-driven VR system for upper limb motor rehabilitation: A clinical case report. Front. Hum. Neurosci. 2019, 13, 244. [Google Scholar] [CrossRef] [PubMed]
- Pichiorri, F.; Mattia, D. Brain-Computer Interfaces in Neurologic Rehabilitation Practice, 1st ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2020; Volume 168, pp. 101–116. [Google Scholar] [CrossRef]
- Yang, S.; Li, R.; Li, H.; Xu, K.; Shi, Y.; Wang, Q.; Yang, T.; Sun, X. Exploring the Use of Brain-Computer Interfaces in Stroke Neurorehabilitation. BioMed Res. Int. 2021, 2021, 9967348. [Google Scholar] [CrossRef] [PubMed]
- Baniqued, P.D.E.; Stanyer, E.C.; Awais, M.; Alazmani, A.; Jackson, A.E.; Mon-Williams, M.A.; Mushtaq, F.; Holt, R.J. Brain-computer interface robotics for hand rehabilitation after stroke: A systematic review. J. NeuroEng. Rehabil. 2021, 18, 15. [Google Scholar] [CrossRef]
- Cervera, M.A.; Soekadar, S.R.; Ushiba, J.; Millán, J.d.R.; Liu, M.; Birbaumer, N.; Garipelli, G. Brain-computer interfaces for post-stroke motor rehabilitation: A meta-analysis. Ann. Clin. Transl. Neurol. 2018, 5, 651–663. [Google Scholar] [CrossRef] [PubMed]
- De Castro-Cros, M.; Sebastian-Romagosa, M.; Rodríguez-Serrano, J.; Opisso, E.; Ochoa, M.; Ortner, R.; Guger, C.; Tost, D. Effects of Gamification in BCI Functional Rehabilitation. Front. Neurosci. 2020, 14, 882. [Google Scholar] [CrossRef]
- Klimesch, W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res. Rev. 1999, 29, 169–195. [Google Scholar] [CrossRef]
- Bastiaansen, M.; Hagoort, P. Chapter 12 Oscillatory neuronal dynamics during language comprehension. Prog. Brain Res. 2006, 159, 179–196. [Google Scholar] [CrossRef]
- Yılmaz, N.H.; Çalışoğlu, P.; Güntekin, B.; Hanoğlu, L. Correlation between alpha activity and neuropsychometric tests in Parkinson’s disease. Neurosci. Lett. 2020, 738. [Google Scholar] [CrossRef]
- Doppelmayr, M.; Klimesch, W.; Stadler, W.; Pöllhuber, D.; Heine, C. EEG alpha power and intelligence. Intelligence 2002, 30, 289–302. [Google Scholar] [CrossRef]
- Klimesch, W. Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn. Sci. 2012, 16, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, M.; Herman, P.; Warden, M.R.; Brincat, S.L.; Miller, E.K. Gamma and beta bursts during working memory readout suggest roles in its volitional control. Nat. Commun. 2018, 9, 394. [Google Scholar] [CrossRef]
- Schmidt, R.; Ruiz, M.H.; Kilavik, B.E.; Lundqvist, M.; Starr, P.A.; Aron, A.R. Beta oscillations in working memory, executive control of movement and thought, and sensorimotor function. J. Neurosci. 2019, 39, 8231–8238. [Google Scholar] [CrossRef]
- Weiss, S.; Mueller, H.M. “Too many betas do not spoil the broth”: The role of beta brain oscillations in language processing. Front. Psychol. 2012, 3, 201. [Google Scholar] [CrossRef] [PubMed]
- Egner, T.; Gruzelier, J.H. EEG Biofeedback of low beta band components: Frequency-specific effects on variables of attention and event-related brain potentials. Clin. Neurophysiol. 2004, 115, 131–139. [Google Scholar] [CrossRef]
- Costa, M.; Goldberger, A.L.; Peng, C.K. Multiscale Entropy Analysis of Complex Physiologic Time Series. Phys. Rev. Lett. 2002, 89, 6–9. [Google Scholar] [CrossRef]
- Escudero, J.; Abásolo, D.; Hornero, R.; Espino, P.; López, M. Analysis of electroencephalograms in Alzheimer’s disease patients with multiscale entropy. Physiol. Meas. 2006, 27, 1091. [Google Scholar] [CrossRef]
- Hornero, R.; Abásolo, D.; Escudero, J.; Gómez, C. Nonlinear analysis of electroencephalogram and magnetoencephalogram recordings in patients with Alzheimer ’ s disease Receive free email alerts when new articles cite this article-sign up. Philos. Trans. R. Soc. A 2009, 367, 317–336. [Google Scholar] [CrossRef]
- Takahashi, T.; Cho, R.Y.; Murata, T.; Mizuno, T.; Kikuchi, M.; Mizukami, K.; Kosaka, H.; Takahashi, K.; Wada, Y. Age-related variation in EEG complexity to photic stimulation: A multiscale entropy analysis. Clin. Neurophysiol. 2009, 120, 476–483. [Google Scholar] [CrossRef]
- Maturana-Candelas, A.; Gómez, C.; Poza, J.; Pinto, N.; Hornero, R. EEG characterization of the Alzheimer’s disease continuum by means of multiscale entropies. Entropy 2019, 21, 544. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Li, X.; Liu, Y.; Cui, W.; Xie, P.; Han, Y. Evaluation of the Brain Function State During Mild Cognitive Impairment Based on Weighted Multiple Multiscale Entropy. Front. Aging Neurosci. 2021, 13, 430. [Google Scholar] [CrossRef]
- Costa, M.; Goldberger, A.L.; Peng, C.K. Multiscale entropy analysis of biological signals. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2005, 71, 021906. [Google Scholar] [CrossRef] [PubMed]
- Christensen, A.L. A Practical Application of the Luria Methodology. J. Clin. Neuropsychol. 1979, 1, 241–247. [Google Scholar] [CrossRef]
- Klem, G.H.; Lüders, H.O.; Henri, H.; Elger, C. The ten±twenty electrode system of the International Federation. Electroencephalogr. Clin. Neurophysiol. 1958, 10, 371–375. [Google Scholar]
- Schalk, G.; Mcfarland, D.J.; Hinterberger, T.; Birbaumer, N.; Wolpaw, J.R.; Technology 753 ABcIBCI. BCI2000: A General-Purpose Brain-Computer Interface (BCI) 754 System. IEEE Trans. Biomed. Eng. 2004, 51, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Kane, N.; Acharya, J.; Benickzy, S.; Caboclo, L.; Finnigan, S.; Kaplan, P.W.; Shibasaki, H.; Pressler, R.; van Putten, M.J. A revised glossary of terms most commonly used by clinical electroencephalographers and updated proposal for the report format of the EEG findings. Revision 2017. Clin. Neurophysiol. Pract. 2017, 2, 170–185. [Google Scholar] [CrossRef]
- Welch, P.D. The Use of Fast Fourier Transform for the Estimation of Power Spectra. IEEE Trans. Audio Electroacoust. 1976, 15, 70–73. [Google Scholar] [CrossRef]
- Humeau-Heurtier, A. The multiscale entropy algorithm and its variants: A review. Entropy 2015, 17, 3110–3123. [Google Scholar] [CrossRef]
- Richman, J.S.; Moorman, J.R. Physiological time-series analysis using approximate and sample entropy. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H2039–H2049. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Cagigal, V.; Santamaría-Vázquez, E.; Hornero, R. Asynchronous control of P300-based brain–computer interfaces using sample entropy. Entropy 2019, 21, 230. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Amarantidis, L.C.; Abásolo, D. Interpretation of entropy algorithms in the context of biomedical signal analysis and their application to EEG analysis in epilepsy. Entropy 2019, 21, 840. [Google Scholar] [CrossRef]
- Duncan, J.; Emslie, H.; Williams, P.; Johnson, R.; Freer, C. Intelligence and the frontal lobe: The organization of goal-directed behavior. Cogn. Psychol. 1996, 30, 257–303. [Google Scholar] [CrossRef]
- Duncan, J.; Seitz, R.J.; Kolodny, J.; Bor, D.; Herzog, H.; Ahmed, A.; Newell, F.N.; Emslie, H. A neural basis for general intelligence. Science 2000, 289, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Fuster, J.M. Frontal lobe and cognitive development. J. Neurocytol. 2002, 31, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Elhalal, A.; Davelaar, E.J.; Usher, M. The role of the frontal cortex in memory: An investigation of the Von Restorff effect. Front. Hum. Neurosci. 2014, 8, 410. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, J.F.; Frank, M.J. Frontal theta as a mechanism for cognitive control. Trends Cogn. Sci. 2014, 18, 414–421. [Google Scholar] [CrossRef]
- Fernández, A.; Pinal, D.; Díaz, F.; Zurrón, M. Working memory load modulates oscillatory activity and the distribution of fast frequencies across frontal theta phase during working memory maintenance. Neurobiol. Learn. Mem. 2021, 183, 107476. [Google Scholar] [CrossRef]
- Jurewicz, K.; Paluch, K.; Kublik, E.; Rogala, J.; Mikicin, M.; Wróbel, A. EEG-neurofeedback training of beta band (12–22 Hz) affects alpha and beta frequencies—A controlled study of a healthy population. Neuropsychologia 2018, 108, 13–24. [Google Scholar] [CrossRef]
- De Munck, J.C.; Gonçalves, S.I.; Mammoliti, R.; Heethaar, R.M.; Lopes da Silva, F.H. Interactions between different EEG frequency bands and their effect on alpha-fMRI correlations. NeuroImage 2009, 47, 69–76. [Google Scholar] [CrossRef]
- Mizuno, T.; Takahashi, T.; Cho, R.Y.; Kikuchi, M.; Murata, T.; Takahashi, K.; Wada, Y. Assessment of EEG dynamical complexity in Alzheimer’s disease using multiscale entropy. Clin. Neurophysiol. 2010, 121, 1438–1446. [Google Scholar] [CrossRef]
- Goldberger, A.L.; Peng, C.K.; Lipsitz, L.A. What is physiologic complexity and how does it change with aging and disease? Neurobiol. Aging 2002, 23, 23–26. [Google Scholar] [CrossRef]
- Van Noordt, S.; Willoughby, T. Cortical maturation from childhood to adolescence is reflected in resting state EEG signal complexity. Dev. Cogn. Neurosci. 2021, 48, 100945. [Google Scholar] [CrossRef] [PubMed]
- Eroğlu, G.; Gürkan, M.; Teber, S.; Ertürk, K.; Kırmızı, M.; Ekici, B.; Arman, F.; Balcisoy, S.; Özgüz, V.; Çetin, M. Changes in EEG complexity with neurofeedback and multi-sensory learning in children with dyslexia: A multiscale entropy analysis. Appl. Neuropsychol. Child 2020, 9, 1–12. [Google Scholar] [CrossRef]
- Angelakis, E.; Stathopoulou, S.; Frymiare, J.L.; Green, D.L.; Lubar, J.F.; Kounios, J. EEG neurofeedback: A brief overview and an example of peak alpha frequency training for cognitive enhancement in the elderly. Clin. Neuropsychol. 2007, 21, 110–129. [Google Scholar] [CrossRef] [PubMed]
- Ros, T.; Enriquez-Geppert, S.; Zotev, V.; Young, K.D.; Wood, G.; Whitfield-Gabrieli, S.; Wan, F.; Vuilleumier, P.; Vialatte, F.; Van De Ville, D.; et al. Consensus on the reporting and experimental design of clinical and cognitive-behavioural neurofeedback studies (CRED-nf checklist). Brain 2020, 143, 1674–1685. [Google Scholar] [CrossRef] [PubMed]
| Socio-Demographic Data | ||
|---|---|---|
| Identifier | Sex | Age (Years) |
| U01 | Female | 70 |
| U02 | Female | 65 |
| U03 | Female | 65 |
| U04 | Male | 71 |
| U05 | Male | 68 |
| U06 | Female | 73 |
| U07 | Male | 81 |
| U08 | Male | 65 |
| U09 | Female | 75 |
| U10 | Female | 70 |
| U11 | Female | 60 |
| Delta | Theta | Alpha | Beta | |
|---|---|---|---|---|
| Pre-NFT RP (mean ± std) | 0.622 ± 0.154 | 0.060 ± 0.029 | 0.112 ± 0.068 | 0.132 ± 0.073 |
| Post-NFT RP (mean ± std) | 0.520 ± 0.131 | 0.073 ± 0.028 | 0.149 ± 0.077 | 0.175 ± 0.080 |
| p-value | 0.0013 | 0.0013 | 0.0013 | 0.0034 |
| Test | U01 | U02 | U3 | U4 | U5 | U6 | U7 | U8 | U9 | U10 | U11 | Mean ± Std | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Attentional control | 0 | 0 | 5 | −15 | 55 | 5 | 0 | 45 | −5 | 5 | 0 | 8.6 ± 21.3 | 0.1310 |
| Thematic draws | 5 | 0 | 0 | 5 | 0 | 25 | 10 | 15 | 0 | 0 | 15 | 6.8 ± 8.4 | 0.0403 |
| Conceptual activity | 10 | 5 | 5 | 0 | 0 | 10 | 5 | 5 | 0 | 10 | 0 | 4.5 ± 4.2 | 0.0403 |
| Immediate memory | −5 | 0 | 0 | 20 | 10 | 10 | 20 | 0 | 0 | −10 | 0 | 4.1 ± 9.7 | 0.1074 |
| Logical memory | −5 | 0 | 0 | 30 | 15 | 20 | 25 | −5 | 15 | 10 | 20 | 11.4 ± 12.3 | 0.0403 |
| Receptive speech | 5 | 0 | 5 | 15 | 0 | 5 | 0 | 10 | −10 | 5 | 10 | 4.1 ± 6.6 | 0.0655 |
| Expressive speech | 0 | 0 | 10 | 15 | 0 | 10 | −15 | 10 | 10 | 5 | 0 | 4.1 ± 8.3 | 0.1102 |
| Visual perception | 5 | 0 | 25 | 30 | 35 | 20 | −10 | 20 | −10 | 5 | 0 | 10.1 ± 15.8 | 0.0590 |
| Spatial orientation | 0 | −10 | 15 | 10 | 0 | −5 | 15 | 15 | 15 | −5 | 35 | 7.7 ± 13.1 | 0.0590 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcos-Martínez, D.; Martínez-Cagigal, V.; Santamaría-Vázquez, E.; Pérez-Velasco, S.; Hornero, R. Neurofeedback Training Based on Motor Imagery Strategies Increases EEG Complexity in Elderly Population. Entropy 2021, 23, 1574. https://doi.org/10.3390/e23121574
Marcos-Martínez D, Martínez-Cagigal V, Santamaría-Vázquez E, Pérez-Velasco S, Hornero R. Neurofeedback Training Based on Motor Imagery Strategies Increases EEG Complexity in Elderly Population. Entropy. 2021; 23(12):1574. https://doi.org/10.3390/e23121574
Chicago/Turabian StyleMarcos-Martínez, Diego, Víctor Martínez-Cagigal, Eduardo Santamaría-Vázquez, Sergio Pérez-Velasco, and Roberto Hornero. 2021. "Neurofeedback Training Based on Motor Imagery Strategies Increases EEG Complexity in Elderly Population" Entropy 23, no. 12: 1574. https://doi.org/10.3390/e23121574
APA StyleMarcos-Martínez, D., Martínez-Cagigal, V., Santamaría-Vázquez, E., Pérez-Velasco, S., & Hornero, R. (2021). Neurofeedback Training Based on Motor Imagery Strategies Increases EEG Complexity in Elderly Population. Entropy, 23(12), 1574. https://doi.org/10.3390/e23121574








