Thermodynamic Properties of the First-Generation Hybrid Dendrimer with “Carbosilane Core/Phenylene Shell” Structure
Abstract
:1. Introduction
2. Experimental
2.1. Sample
- Found (%): C, 83.18; H, 7.51; Si, 8.41.
- Calculated (%): C, 83.92; H, 7.69; Si, 8.39.
2.2. Apparatus and Measurement Procedure
2.2.1. Adiabatic Vacuum Calorimetry
2.2.2. Differential Scanning Calorimetry
3. Results and Discussion
3.1. Heat Capacity
3.2. Standard Thermodynamic Characteristics of the Glass Transition and the Glassy State
3.3. Standard Thermodynamic Functions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fréchet, J.M.J.; Tomalia, D.A. Dendrimers and Other Dendritic Polymers; John Wiley & Sons: Chichester, UK, 2001. [Google Scholar] [CrossRef]
- Newkome, G.R.; Moorefield, C.N.; Vögtle, F. Dendrimers and Dendrons: Concepts, Syntheses, Applications; Wiley-VCH: Weinheim, Germany, 2001. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Christensen, J.B.; Boas, U. Dendrimers, Dendrons, and Dendritic Polymers: Discovery, Applications, and the Future; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar] [CrossRef]
- Inoue, K. Functional dendrimers, hyperbranched and star polymers. Prog. Polym. Sci. 2000, 25, 453–571. [Google Scholar] [CrossRef]
- Grayson, S.M.; Fréchet, J.M.J. Convergent dendrons and dendrimers: From synthesis to applications. Chem. Rev. 2001, 101, 3819–3867. [Google Scholar] [CrossRef]
- van Heerbeek, R.; Kamer, P.C.J.; van Leeuwen, P.W.N.M.; Reek, J.N.H. Dendrimers as support for recoverable catalysts and reagents. Chem. Rev. 2002, 102, 3717–3756. [Google Scholar] [CrossRef] [PubMed]
- Boas, U.; Heegaard, P.M.H. Dendrimers in drug research. Chem. Soc. Rev. 2004, 33, 43–63. [Google Scholar] [CrossRef]
- Gade, L.H. Dendrimer Catalysis; Springer: Heidelberg, Germany, 2006. [Google Scholar] [CrossRef]
- Astruc, D.; Boisselier, E.; Ornelas, C. Dendrimers Designed for Functions: From Physical, Photophysical, and Supramolecular Properties to Applications in Sensing, Catalysis, Molecular Electronics, Photonics, and Nanomedicine. Chem. Rev. 2010, 110, 1857–1959. [Google Scholar] [CrossRef] [PubMed]
- Svenson, S.; Tomalia, D.A. Dendrimers in biomedical applications—Reflections on the field. Adv. Drug Deliv. Rev. 2012, 64, 102–115. [Google Scholar] [CrossRef]
- Sebestik, J.; Reinis, M.; Jezek, J. Biomedical Applications of Peptide-, Glyco- and Glycopeptide Dendrimers, and Analogous Dendrimeric Structures; Springer: Vienna, Austria, 2012. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Q.; Chang, H.; Cheng, Y. Surface-Engineered Dendrimers in Gene Delivery. Chem. Rev. 2015, 115, 5274–5300. [Google Scholar] [CrossRef] [PubMed]
- Carlmark, A.; Hawker, C.; Hult, A.; Malkoch, M. New methodologies in the construction of dendritic materials. Chem. Soc. Rev. 2009, 38, 352–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majoral, J.-P.; Caminade, A.-M. Dendrimers Containing Heteroatoms (Si, P, B, Ge, or Bi). Chem. Rev. 1999, 99, 845–880. [Google Scholar] [CrossRef]
- Frey, H.; Schlenk, C. Silicon-based dendrimers. Top. Curr. Chem. 2000, 210, 69–129. [Google Scholar] [CrossRef]
- Rebrov, E.A.; Leshchiner, I.D.; Muzafarov, A.M. Synthesis of carbosilane dendrimers with variable distance between branching nodes. Macromolecules 2012, 45, 8796–8804. [Google Scholar] [CrossRef]
- Dvornic, P.R.; Owen, M.J. Silicon-Containing Dendritic Polymers; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar] [CrossRef]
- Miller, T.M.; Neenan, T.X. Convergent synthesis of monodisperse dendrimers based upon 1,3,5-trisubstituted benzenes. Chem. Mater. 1990, 2, 346–349. [Google Scholar] [CrossRef]
- Morgenroth, F.; Reuther, E.; Müllen, K. Polyphenylene dendrimers: From three-dimensional to two-dimensional structures. Angew. Chem. Int. Ed. 1997, 36, 631–634. [Google Scholar] [CrossRef]
- Morgenroth, F.; Kübel, C.; Müllen, K. Nanosized polyphenylene dendrimers based upon pentaphenylbenzene units. J. Mater. Chem. 1997, 7, 1207–1211. [Google Scholar] [CrossRef]
- Morgenroth, F.; Müllen, K. Dendritic and hyperbranched polyphenylenes via a simple Diels–Alder route. Tetrahedron 1997, 53, 15349–15366. [Google Scholar] [CrossRef]
- Hammer, B.A.G.; Müllen, K. Dimensional evolution of polyphenylenes: Expanding in all directions. Chem. Rev. 2016, 116, 2103–2140. [Google Scholar] [CrossRef] [PubMed]
- Hammer, B.A.G.; Wu, Y.; Fischer, S.; Liu, W.; Weil, T.; Müllen, K. Controlling cellular uptake and toxicity of polyphenylene dendrimers by chemical functionalization. ChemBioChem 2017, 18, 960–964. [Google Scholar] [CrossRef] [PubMed]
- Shifrina, Z.B.; Rajadurai, M.S.; Firsova, N.V.; Bronstein, L.M.; Huang, X.; Rusanov, A.L.; Muellen, K. Poly(phenylene-pyridyl) dendrimers: Synthesis and templating of metal nanoparticles. Macromolecules 2005, 38, 9920–9932. [Google Scholar] [CrossRef]
- Rajadurai, M.S.; Shifrina, Z.B.; Kuchkina, N.V.; Rusanov, A.L.; Müllen, K. Rigid aromatic dendrimers. Russ. Chem. Rev. 2007, 76, 767–783. [Google Scholar] [CrossRef]
- Bronstein, L.M.; Shifrina, Z.B. Dendrimers as encapsulating, stabilizing, or directing agents for inorganic nanoparticles. Chem. Rev. 2011, 111, 5301–5344. [Google Scholar] [CrossRef]
- Yuzik-Klimova, E.Y.; Kuchkina, N.V.; Sorokina, S.A.; Morgan, D.G.; Boris, B.; Nikoshvili, L.Z.; Lyubimova, N.A.; Matveeva, V.G.; Sulman, E.M.; Stein, B.D.; et al. Bronstein, Magnetically recoverable catalysts based on polyphenylenepyridyl dendrons and dendrimers. RSC Adv. 2014, 4, 23271–23280. [Google Scholar] [CrossRef]
- Kuchkina, N.V.; Morgan, D.G.; Kostopoulou, A.; Lappas, A.; Brintakis, K.; Boris, B.S.; Yuzik-Klimova, E.Y.; Stein, B.D.; Svergun, D.I.; Spilotros, A.; et al. Hydrophobic periphery tails of polyphenylenepyridyl dendrons control nanoparticle formation and catalytic properties. Chem. Mater. 2014, 26, 5654–5663. [Google Scholar] [CrossRef] [Green Version]
- Serenko, O.; Strashnov, P.; Kapustin, G.; Kalinin, M.; Kuchkina, N.; Serkova, E.; Shifrina, Z.; Muzafarov, A. Adsorption properties of pyridylphenylene dendrimers. RSC Adv. 2017, 7, 7870–7875. [Google Scholar] [CrossRef] [Green Version]
- Sorokina, S.A.; Kuchkina, N.V.; Lawson, B.P.; Krasnova, I.Y.; Nemygina, N.A.; Nikoshvili, L.Z.; Talanova, V.N.; Stein, B.D.; Pink, M.; Morgan, D.G.; et al. Pyridylphenylene dendrons immobilized on the surface of chemically modified magnetic silica as efficient stabilizing molecules of Pd species. Appl. Surf. Sci. 2019, 488, 865–873. [Google Scholar] [CrossRef]
- Schmaltz, B.; Weil, T.; Müllen, K. Polyphenylene-based materials: Control of the electronic function by molecular and supramolecular complexity. Adv. Mater. 2009, 21, 1067–1078. [Google Scholar] [CrossRef]
- Türp, D.; Nguyen, T.T.T.; Baumgarten, M.; Müllen, K. Uniquely versatile: Nano-site defined materials based on polyphenylene dendrimers. New J. Chem. 2012, 36, 282–298. [Google Scholar] [CrossRef]
- Stangenberg, R.; Wu, Y.; Hedrich, J.; Kurzbach, D.; Wehner, D.; Weidinger, G.; Kuan, S.L.; Jansen, M.I.; Jelezko, F.; Luhmann, H.J.; et al. A polyphenylene dendrimer drug transporter with precisely positioned amphiphilic surface patches. Adv. Healthc. Mater. 2015, 4, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.; Dillenburger, M.; Simon, J.; Oberländer, J.; Landfester, K.; Mailänder, V.; Ng, D.Y.W.; Müllen, K.; Weil, T. Amphiphilic dendrimers control protein binding and corona formation on liposome nanocarriers. Chem. Commun. 2020, 56, 8663–8666. [Google Scholar] [CrossRef]
- Milenin, S.A.; Cherkaev, G.V.; Demchenko, N.V.; Serkova, E.S.; Krasnova, I.Y.; Selezneva, E.V.; Buzin, M.I.; Bakirov, A.V.; Vasil’ev, V.G.; Shifrina, Z.B.; et al. Influence of the growing flexible shell on the molecular behavior of hybrid dendrimers. Macromolecules 2020, 53, 9706–9716. [Google Scholar] [CrossRef]
- Lebedev, B.V.; Ryabkov, M.V.; Tatarinova, E.A.; Rebrov, E.A.; Muzafarov, A.M. Thermodynamic properties of the first to fifth generations of carbosilane dendrimers with allyl terminal groups. Russ. Chem. Bull. 2003, 52, 545–551. [Google Scholar] [CrossRef]
- Smirnova, N.N.; Sologubov, S.S.; Sarmini, Y.A.; Markin, A.V.; Novozhilova, N.A.; Tatarinova, E.A.; Muzafarov, A.M. Thermodynamic properties of first- and third-generation carbosilane dendrimers with terminal phenyldioxolane groups. Russ. J. Phys. Chem. A 2017, 91, 2317–2325. [Google Scholar] [CrossRef]
- Sologubov, S.S.; Markin, A.V.; Smirnova, N.N.; Novozhilova, N.A.; Tatarinova, E.A.; Muzafarov, A.M. Thermodynamic properties of a first-generation carbosilane dendrimer with terminal phenylethyl groups. Russ. J. Phys. Chem. A 2018, 92, 235–243. [Google Scholar] [CrossRef]
- Markin, A.V.; Sarmini, Y.A.; Sologubov, S.S.; Smirnova, N.N.; Boldyrev, K.L.; Tatarinova, E.A.; Meshkov, I.B.; Muzafarov, A.M. Thermodynamic properties of a first-generation siloxane dendrimer with terminal trimethylsilyl groups. Russ. J. Phys. Chem. A 2020, 94, 240–248. [Google Scholar] [CrossRef]
- Sologubov, S.S.; Markin, A.V.; Sarmini, Y.A.; Samosudova, Y.S.; Smirnova, N.N.; Boldyrev, K.L.; Tatarinova, E.A.; Meshkov, I.B.; Muzafarov, A.M. Calorimetric study of siloxane dendrimer of the third generation with trimethylsilyl terminal groups. J. Therm. Anal. Calorim. 2019, 138, 3301–3310. [Google Scholar] [CrossRef]
- Sologubov, S.S.; Markin, A.V.; Sarmini, Y.A.; Smirnova, N.N.; Boldyrev, K.L.; Tatarinova, E.A.; Meshkov, I.B.; Muzafarov, A.M. Thermodynamic investigation of G2 and G4 siloxane dendrimers with trimethylsilyl terminal groups. J. Chem. Thermodyn. 2021, 153, 106318. [Google Scholar] [CrossRef]
- Smirnova, N.N.; Markin, A.V.; Zakharova, Y.A.; Kuchkina, N.V.; Rusanov, A.L.; Shifrina, Z.B. Thermodynamic properties of pyridine-containing polyphenylene dendrimers of the first–fourth generations. Russ. Chem. Bull. 2011, 60, 132–138. [Google Scholar] [CrossRef]
- NSmirnova, N.N.; Zakharova, Y.A.; Markin, A.V.; Kuchkina, N.V.; Yuzik-Klimova, E.Y.; Shifrina, Z.B. Thermodynamics of hard poly(phenylene-pyridyl) dendrimers. Russ. Chem. Bull. 2013, 62, 2258–2262. [Google Scholar] [CrossRef]
- Serkova, E.S.; Krasnova, I.Y.; Milenin, S.; Selezneva, E.V.; Tatarinova, E.A.; Boldyrev, K.L.; Korlyukov, A.A.; Zubavichus, Y.V.; Buzin, M.I.; Serenko, O.A.; et al. Core/shell hybrid dendrimers: Controllable rigidity determines molecular behaviour. Polymer 2018, 138, 83–91. [Google Scholar] [CrossRef]
- Meija, J.; Coplen, T.B.; Berglund, M.; Brand, W.A.; De Bièvre, P.; Gröning, M.; Holden, N.E.; Irrgeher, J.; Loss, R.D.; Walczyk, T.; et al. Atomic weights of the elements 2013 (IUPAC Technical Report). Pure Appl. Chem. 2016, 88, 265–291. [Google Scholar] [CrossRef]
- Varushchenko, R.M.; Druzhinina, A.I.; Sorkin, E.L. Low-temperature heat capacity of 1-bromoperfluorooctane. J. Chem. Thermodyn. 1997, 29, 623–637. [Google Scholar] [CrossRef]
- Sabbah, R.; Xu-Wu, A.; Chickos, J.S.; Leitão, M.L.P.; Roux, M.V.; Torres, L.A. Reference materials for calorimetry and differential thermal analysis. Thermochim. Acta 1999, 331, 93–204. [Google Scholar] [CrossRef]
- Höhne, G.W.H.; Hemminger, W.F.; Flammersheim, H.-J. Differential Scanning Calorimetry, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar] [CrossRef]
- della Gatta, G.; Richardson, M.J.; Sarge, S.M.; Stølen, S. Standards, calibration, and guidelines in microcalorimetry. Part 2. Calibration standards for differential scanning calorimetry (IUPAC Technical Report). Pure Appl. Chem. 2006, 78, 1455–1476. [Google Scholar] [CrossRef] [Green Version]
- Kaisersberger, E.; Janoschek, J.; Wassmer, E. A heat flux DSC for enthalpy and specific heat determinations to 1700 K. Thermochim. Acta 1989, 148, 499–505. [Google Scholar] [CrossRef]
- Alford, S.; Dole, M. Specific heat of synthetic high polymers. VI. A study of the glass transition in polyvinyl chloride. J. Am. Chem. Soc. 1955, 77, 4774–4777. [Google Scholar] [CrossRef]
- Adam, G.; Gibbs, J.H. On the Temperature Dependence of Cooperative Relaxation Properties in Glass-Forming Liquids. J. Chem. Phys. 1965, 43, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Kauzmann, W. The Nature of the Glassy State and the Behavior of Liquids at Low Temperatures. Chem. Rev. 1948, 43, 219–256. [Google Scholar] [CrossRef]
- Bestul, A.B.; Chang, S.S. Excess Entropy at Glass Transformation. J. Chem. Phys. 1964, 40, 3731–3733. [Google Scholar] [CrossRef]
- Debye, P. Zur Theorie der spezifischen Wärmen. Ann. Phys. 1912, 344, 789–839. [Google Scholar] [CrossRef] [Green Version]
- JMcCullough, P.; Scott, D.W. Experimental Thermodynamics. Calorimetry of Non-Reacting Systems; Butterworth & Co. Ltd.: London, UK, 1968; Volume I, Available online: https://sciencedirect.com/science/book/9781483213279 (accessed on 22 November 2021).
- Lebedev, B. Application of precise calorimetry in study of polymers and polymerization processes. Thermochim. Acta 1997, 297, 143–149. [Google Scholar] [CrossRef]
- Chase, M.W. NIST-JANAF Thermochemical Tables, 4th ed.; Monograph No. 9; National Institute of Standards and Technology: Gaithersburg, MD, USA, 1998; pp. 1–1951. [CrossRef]
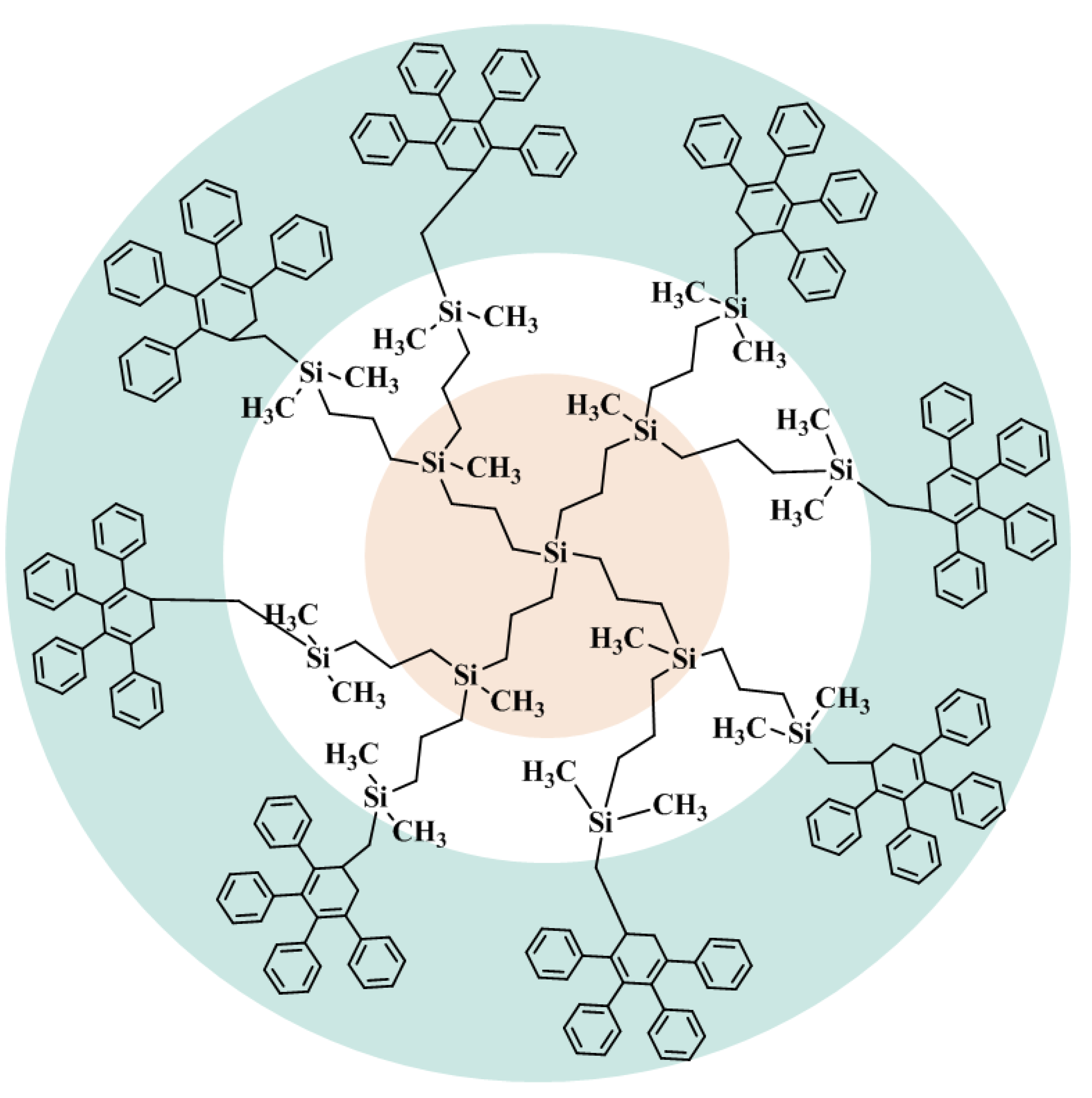



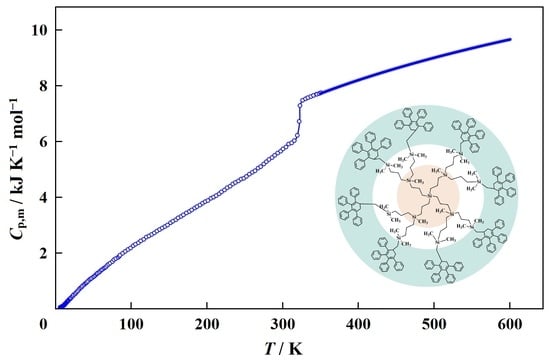
| Designation of Dendrimer | Brutto Formula | Source | Purification Method | Final Mass Fraction Purity | Analysis Method |
|---|---|---|---|---|---|
| G1{Si}13[Ar]32 | C304H332Si13 | Synthesis [44] | Gel permeation chromatography | 0.98 | Elemental analysis, NMR spectroscopy, MALDI-TOF mass spectrometry |
| Type of Dendrimer | Designation of Dendrimer | ΔT (K) | Source | |||
|---|---|---|---|---|---|---|
| Hybrid | G1{Si}13[Ar]32 | 300–350 | 323 | 1312 ± 16 | 334 ± 5 | This work |
| Carbosilane | G1{Si}5[CH2CH=CH2]8 | 150–160 | 154 | 406 ± 5 | 103 ± 2 | [36] |
| G1{Si}13[(C6H4)C3H5O2]8 | 200–260 | 231 | 1180 ± 11 | 301 ± 3 | [37] | |
| G1{Si}13[CH2CH2C6H5]8 | 176–215 | 198 | 960 ± 10 | 245 ± 3 | [38] | |
| Siloxane | G1[OSi(CH3)3]6 | 137–153 | 147 | 245 ± 3 | 63 ± 1 | [39] |
| Pyridylphenylene | G1[C5H4N]12 | 290–350 | 323 | 225 ± 3 | 57 ± 1 | [43] |
| T (K) | (H°(T) − H°(0)) (kJ·mol−1) | (S°(T) − S°(0)) (kJ·K−1·mol−1) | −(G°(T) − H°(0)) (kJ·mol−1) | |
|---|---|---|---|---|
| Amorphous (glassy) state | ||||
| 5 | 0.0196 | 0.0249 | 0.00667 | 0.00838 |
| 10 | 0.0948 | 0.298 | 0.04161 | 0.118 |
| 15 | 0.224 | 1.08 | 0.103 | 0.468 |
| 20 | 0.3689 | 2.569 | 0.1879 | 1.189 |
| 30 | 0.6452 | 7.638 | 0.3897 | 4.053 |
| 40 | 0.9112 | 15.46 | 0.6129 | 9.056 |
| 50 | 1.142 | 25.73 | 0.8412 | 16.33 |
| 60 | 1.359 | 38.23 | 1.068 | 25.88 |
| 70 | 1.572 | 52.87 | 1.294 | 37.69 |
| 80 | 1.798 | 69.74 | 1.519 | 51.75 |
| 90 | 2.005 | 88.69 | 1.741 | 68.04 |
| 100 | 2.196 | 109.7 | 1.963 | 86.56 |
| 150 | 3.034 | 240.9 | 3.015 | 211.4 |
| 200 | 3.862 | 413.8 | 4.004 | 387.1 |
| 250 | 4.692 | 628.1 | 4.957 | 611.1 |
| 298.15 | 5.675 | 877.0 | 5.865 | 871.6 |
| 300 | 5.706 | 887.6 | 5.900 | 882.5 |
| 320 | 6.070 | 1006 | 6.282 | 1004 |
| 323 | 6.100 | 1022 | 6.331 | 1020 |
| Amorphous (devitrified) state | ||||
| 323 | 7.412 | 1022 | 6.331 | 1020 |
| 330 | 7.534 | 1076 | 6.499 | 1068 |
| 350 | 7.736 | 1229 | 6.949 | 1203 |
| 400 | 8.216 | 1628 | 8.014 | 1577 |
| 450 | 8.638 | 2050 | 9.006 | 2003 |
| 500 | 9.016 | 2491 | 9.936 | 2477 |
| 550 | 9.358 | 2951 | 10.81 | 2996 |
| 600 | 9.671 | 3427 | 11.64 | 3557 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sologubov, S.S.; Markin, A.V.; Smirnova, N.N.; Chamkina, E.S.; Krasnova, I.Y.; Milenin, S.A.; Serenko, O.A.; Shifrina, Z.B.; Muzafarov, A.M. Thermodynamic Properties of the First-Generation Hybrid Dendrimer with “Carbosilane Core/Phenylene Shell” Structure. Entropy 2021, 23, 1557. https://doi.org/10.3390/e23121557
Sologubov SS, Markin AV, Smirnova NN, Chamkina ES, Krasnova IY, Milenin SA, Serenko OA, Shifrina ZB, Muzafarov AM. Thermodynamic Properties of the First-Generation Hybrid Dendrimer with “Carbosilane Core/Phenylene Shell” Structure. Entropy. 2021; 23(12):1557. https://doi.org/10.3390/e23121557
Chicago/Turabian StyleSologubov, Semen S., Alexey V. Markin, Natalia N. Smirnova, Elena S. Chamkina, Irina Yu. Krasnova, Sergey A. Milenin, Olga A. Serenko, Zinaida B. Shifrina, and Aziz M. Muzafarov. 2021. "Thermodynamic Properties of the First-Generation Hybrid Dendrimer with “Carbosilane Core/Phenylene Shell” Structure" Entropy 23, no. 12: 1557. https://doi.org/10.3390/e23121557
APA StyleSologubov, S. S., Markin, A. V., Smirnova, N. N., Chamkina, E. S., Krasnova, I. Y., Milenin, S. A., Serenko, O. A., Shifrina, Z. B., & Muzafarov, A. M. (2021). Thermodynamic Properties of the First-Generation Hybrid Dendrimer with “Carbosilane Core/Phenylene Shell” Structure. Entropy, 23(12), 1557. https://doi.org/10.3390/e23121557