Recent Advances in Conservation–Dissipation Formalism for Irreversible Processes
Abstract
:1. Introduction
2. Physical Motivation and Mathematical Foundation
2.1. Symmetry, Scale Separation and Conservation Laws
2.2. Entropy, Free Energy and Onsager’s Relation
2.3. The Conservation-Dissipation Formalism
2.4. Structural Conditions for the Existence of Global Smooth Solutions
- (a)
- There is a strictly concave smooth entropy function defined in a convex compact neighborhood G of , such that is symmetric and under consideration;
- (b)
- There is a dissipation matrix such that ;
- (c)
- The kernel of contains no eigenvector of the matrix , (the unit sphere in );
2.5. Gradient Flows in the Absence of Source Terms
2.6. A Typical Example: The Generalized Newton–Stokes-Fourier’s Law
- Compatibility Assumption: at local equilibrium, i.e., and , we haveHere denotes the dissipation matrix at local equilibrium, such that
- Causality Assumption: let , then
3. Classical Models in Mathematical Physics
- Routine 1 (stochastic models): Master equations → Fokker–Planck (F-P) equations → Chemical mass-action euqations;
- Routine 2 (hydrodynamic systems): Moment hierarchies of Boltzmann equation → Euler equations → Navier–Stokes-Fourier (NSF) equations → Non-NSF equations;
- Routine 3 (optics, radiation and etc.): Quasi-linear Maxwell’s equations for nonlinear optics → Radiation hydrodynamics → Chemically reactive flows.
3.1. Stochastic Models
3.2. Hydrodynamic Systems
3.3. Optics, Radiation, etc.
4. Novel Applications
4.1. Non-Fourier Heat Conduction
4.2. Waves Transportation in Neuroscience
- ;
- ;
- is irreducibility;
- There exit i and j such that .
4.3. Soft Matter Physics
4.3.1. Polymer Diffusion
4.3.2. Phase Separation
4.3.3. Isothermal Flows of Liquid Crystals
4.3.4. Non-Isothermal Flows of Liquid Crystals
4.4. Boundary Control of Linear Hyperbolic Balance Laws
- (A1) There exists a symmetric positive-definite matrix such that is symmetric and is block diagonal, with ;
- (A2) is positive definite.
- (A3) The matrix has only positive eigenvalues.
5. Validation of CDF
5.1. Global Existence for Viscoelastic Fluids with Finite Strain
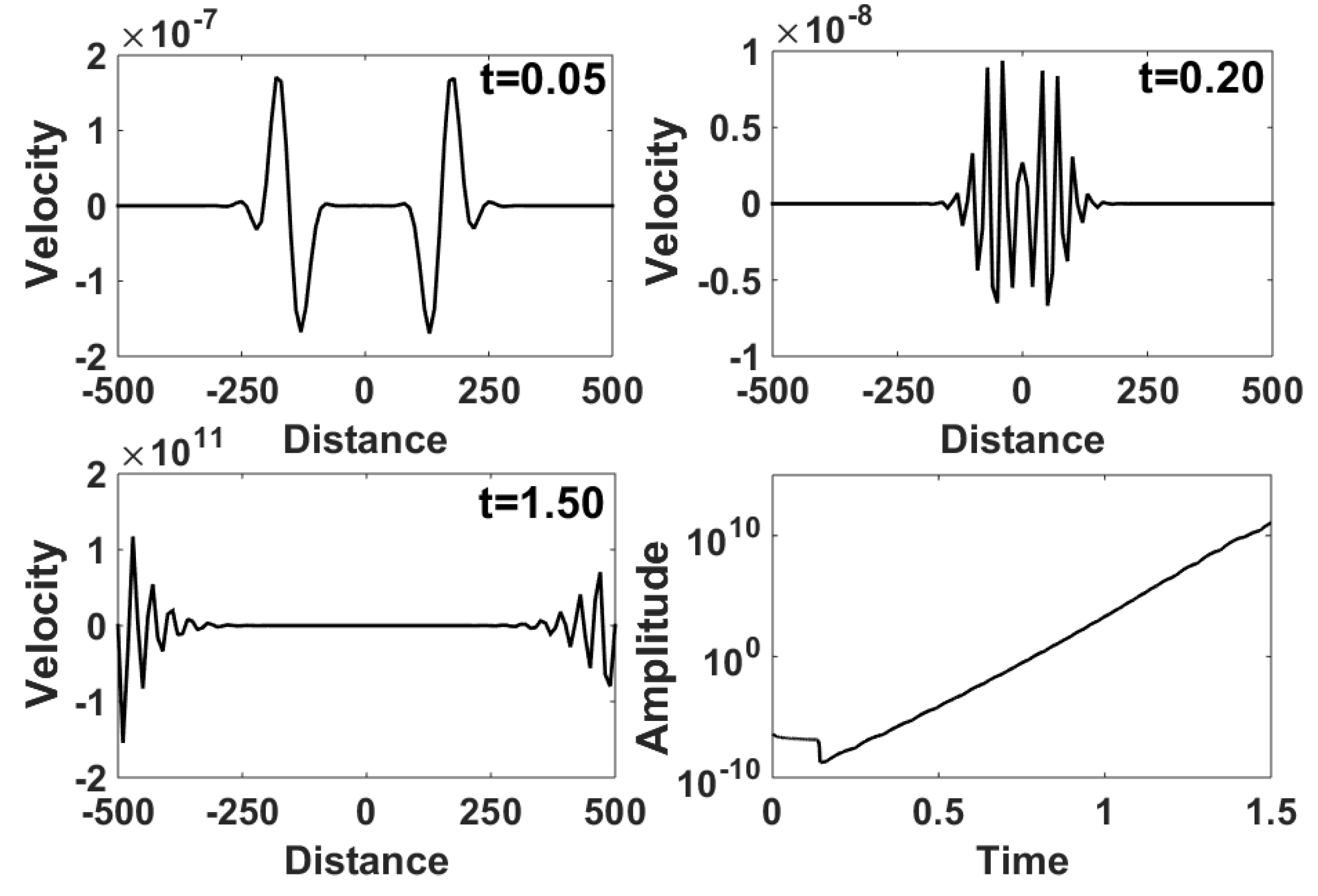
5.2. Unstable Modes of BISQ Model in Geophysics
5.3. Vibrations of Bipyramidal Particles in Viscoelastic Fluids
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Groot, S.R.; Mazur, P. Non-Equilibrium Thermodynamics; North-Holland Publishing Company: Amsterdam, The Netherlands, 1962. [Google Scholar]
- Coleman, B.D. Thermodynamics of materials with memory. Arch. Ration. Mech. Anal. 1964, 17, 1–46. [Google Scholar] [CrossRef]
- Truesdell, C. Rational Thermodynamics; Springer: New York, NY, USA, 1984. [Google Scholar]
- Noll, W. The Foundations of Mechanics and Thermodynamics; Springer: Berlin/Heidelberg, Germany, 1974. [Google Scholar]
- Jou, D.; Casas-Vázquez, J.; Lebon, G. Extended irreversible thermodynamics revisited (1988–98). Rep. Prog. Phys. 1999, 62, 1035. [Google Scholar] [CrossRef]
- Müller, I.; Ruggeri, T. Extended Thermodynamics; Springer Science and Business Media: Berlin, Germany, 2013; Volume 37. [Google Scholar]
- Jou, D.; Casas-Vázquez, J.; Lebon, G. Extended Irreversible Thermodynamics; Springer: New York, NY, USA, 2010. [Google Scholar]
- Grmela, M. Hamiltonian mechanics of complex fluids. J. Phys. Math. Gen. 1989, 22, 4375. [Google Scholar] [CrossRef]
- Grmela, M.; Öttinger, H.C. Dynamics and thermodynamics of complex fluids. I. Development of a general formalism. Phys. Rev. E 1997, 56, 6620–6632. [Google Scholar] [CrossRef]
- Grmela, M.; Öttinger, H.C. Dynamics and thermodynamics of complex fluids. II. Illustrations of a general formalism. Phys. Rev. E 1997, 56, 6633–6655. [Google Scholar] [CrossRef]
- Lebon, G.; Jou, D.; Grmela, M. Extended reversible and irreversible thermodynamics: A Hamiltonian approach with application to heat waves. J. -Non Thermodyn. 2017, 42, 153–168. [Google Scholar] [CrossRef]
- Doi, M. Soft Matter Physics; Oxford University Press: Oxford, UK, 2013. [Google Scholar]
- Doi, M. Onsager principle in polymer dynamics. Prog. Polym. Sci. 2021, 112, 101339. [Google Scholar] [CrossRef]
- Wang, H.; Qian, T.; Xu, X. Onsager’s variational principle in active soft matter. Soft Matter 2021, 17, 3634–3653. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Doi, M. Dynamics of viscoelastic filaments based on Onsager principle. Phys. Rev. Fluids 2018, 3, 084004. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.; Zhao, Q.; Qian, T.; Srolovitz, D.J.; Bao, W. Application of the Onsager’s variational principle to the dynamics of a solid toroidal island on a substrate. Acta Mater. 2019, 163, 154–160. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.; Wang, Y.; Man, X.; Doi, M. Deposition Patterns of Two Neighbouring Droplets: Onsager Variational Principle Studies. Langmuir 2017, 33, 5965–5972. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wu, H. An energetic variational approach for the Cahn–Hilliard equation with dynamic boundary condition: Model derivation and mathematical analysis. Arch. Ration. Mech. Anal. 2019, 233, 167–247. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Sun, H. On energetic variational approaches in modeling the nematic liquid crystal flows. Discret. Contin. Dyn. Syst. 2009, 23, 455–475. [Google Scholar]
- Hyon, Y.; Kwak, D.Y.; Liu, C. Energetic variational approach in complex fluids: Maximum dissipation principle. Discret. Contin. Dyn. Syst. 2017, 26, 1291–1304. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, T.F.; Liu, C. A two species micro–macro model of wormlike micellar solutions and its maximum entropy closure approximations: An energetic variational approach. J. -Non Fluid Mech. 2021, 293, 104559. [Google Scholar] [CrossRef]
- Yong, W.A. An interesting class of partial differential equations. J. Math. Phys. 2008, 49, 033503. [Google Scholar] [CrossRef] [Green Version]
- Peng, L.; Zhu, Y.; Hong, L. Generalized Onsager’s reciprocal relations for the master and Fokker-Planck equations. Phys. Rev. E 2018, 97, 062123. [Google Scholar] [CrossRef]
- Godunov, S.K. An interesting class of quasilinear systems. Dokl. Akad. Nauk SSSR 1961, 139, 520–523. [Google Scholar]
- Friedrichs, K.O.; Lax, P.D. Systems of conservation equations with a convex extension. Proc. Natl. Acad. Sci. USA 1971, 68, 1686–1688. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Hong, L.; Yang, Z.; Yong, W.A. Conservation-dissipation formalism of irreversible thermodynamics. J. Non-Equilib. Thermodyn. 2015, 40, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Huo, X. Modeling and Analysis of Viscoelastic Fluids. Ph.D. Thesis, Tsinghua University, Beijing, China, 2017. [Google Scholar]
- Józsa, V.; Kovács, R. General Aspects of Thermodynamical Modeling. In Solving Problems in Thermal Engineering; Springer: Berlin/Heidelberg, Germany, 2020; pp. 15–42. [Google Scholar]
- Huo, X.; Yong, W.A. Structural stability of a 1D compressible viscoelastic fluid model. J. Differ. Equ. 2016, 261, 1264–1284. [Google Scholar] [CrossRef]
- Yang, Z.; Yong, W.A.; Zhu, Y. A Generalized hydrodynamics and its classical hydrodynamic limit. arXiv 2018, arXiv:1809.01611. [Google Scholar]
- Liu, J.; Yong, W.A. Stability Analysis of the Biot/squirt and Double-porosity Models for Wave Propagation in Saturated Porous Media. In Proceedings of the 78th EAGE Conference and Exhibition 2016, Vienna, Austria, 30 May–2 June 2016. [Google Scholar]
- Liu, J.; Yong, W.A. Stability analysis of the Biot/squirt models for wave propagation in saturated porous media. Geophys. J. Int. 2016, 204, 535–543. [Google Scholar] [CrossRef]
- Yan, H.; Yong, W.A. Weak entropy solutions of nonlinear reaction-hyperbolic systems for axonal transport. Math. Model. Methods Appl. Sci. 2011, 21, 2135–2154. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.; Yong, W.A. Stability of steady solutions to reaction-hyperbolic systems for axonal transport. J. Hyperbolic Differ. Equ. 2012, 9, 325–337. [Google Scholar] [CrossRef] [Green Version]
- Hong, L.; Yang, Z.; Zhu, Y.; Yong, W.A. A novel construction of thermodynamically compatible models and its correspondence with Boltzmann-equation-based moment-closure hierarchies. J. Non-Equilib. Thermodyn. 2015, 40, 247–256. [Google Scholar] [CrossRef]
- Sun, M.; Jou, D.; Zhang, J. A nonlinear viscoelastic model and non-equilibrium entropies. J. Non-Newton. Fluid Mech. 2016, 229, 96–100. [Google Scholar] [CrossRef] [Green Version]
- Qian, H.; Kjelstrup, S.; Kolomeisky, A.B.; Bedeaux, D. Entropy production in mesoscopic stochastic thermodynamics: Nonequilibrium kinetic cycles driven by chemical potentials, temperatures, and mechanical forces. J. Phys. Condens. Matter 2016, 28, 153004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, T.; Mallick, K.; Zia, R. Non-equilibrium statistical mechanics: From a paradigmatic model to biological transport. Rep. Prog. Phys. 2011, 74, 116601. [Google Scholar] [CrossRef]
- Dahlsten, O.C. Non-equilibrium statistical mechanics inspired by modern information theory. Entropy 2013, 15, 5346–5361. [Google Scholar] [CrossRef]
- Grmela, M. Geometry of multiscale nonequilibrium thermodynamics. Entropy 2015, 17, 5938–5964. [Google Scholar] [CrossRef] [Green Version]
- Pavelka, M.; Klika, V.; Grmela, M. Multiscale Thermo-Dynamics: Introduction to GENERIC; De Gruyter: Berlin, Germany, 2018. [Google Scholar] [CrossRef]
- Hong, L.; Qian, H. Stochastic Dynamics, Large Deviations Principle, and Non-equilibrium Thermodynamics. Phys. Rev. E 2021, 104, 044113. [Google Scholar] [CrossRef]
- Hong, L.; Qian, H. The statistical foundation of entropy in extended irreversible thermodynamics. J. Phys. A Math. Theor. 2020, 53, 425202. [Google Scholar] [CrossRef]
- Dafermos, C.M. Hyperbolic Conservation Laws in Continuum Physics; Springer: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- Yong, W.A. Entropy and global existence for hyperbolic balance laws. Arch. Ration. Mech. Anal. 2004, 172, 247–266. [Google Scholar] [CrossRef]
- Shizuta, Y.; Kawashima, S. Systems of equations of hyperbolic-parabolic type with applications to the discrete Boltzmann equation. Hokkaido Math. J. 1985, 14, 249–275. [Google Scholar] [CrossRef]
- Zeng, Y. Gas Dynamics in Thermal Nonequilibrium and General Hyperbolic Systems with Relaxation. Arch. Ration. Mech. Anal. 1999, 150, 225–279. [Google Scholar] [CrossRef]
- Peshkov, I.; Pavelka, M.; Romenski, E.; Grmela, M. Continuum mechanics and thermodynamics in the Hamilton and the Godunov-type formulations. Contin. Mech. Thermodyn. 2018, 30, 1343–1378. [Google Scholar] [CrossRef] [Green Version]
- Villani, C. Topics in Optimal Transportation (Graduate Studies in Mathematics 58); American Mathematical Society: Providence, RI, USA, 2003. [Google Scholar]
- Villani, C. Optimal Transport; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Santambrogio, F. Optimal transport for applied mathematicians. Birkäuser N. Y. 2015, 55, 94. [Google Scholar]
- Muschik, W. Empirical foundation and axiomatic treatment of non-equilibrium temperature. Arch. Ration. Mech. Anal. 1977, 66, 379–401. [Google Scholar] [CrossRef]
- Casas-Vázquez, J.; Jou, D. Temperature in non-equilibrium states: A review of open problems and current proposals. Rep. Prog. Phys. 2003, 66, 1937–2023. [Google Scholar] [CrossRef]
- Hewgill, A.; De Chiara, G.; Imparato, A. Quantum thermodynamically consistent local master equations. Phys. Rev. Res. 2021, 3, 013165. [Google Scholar] [CrossRef]
- Ge, H.; Qian, H. Mesoscopic kinetic basis of macroscopic chemical thermodynamics: A mathematical theory. Phys. Rev. E 2016, 94, 052150. [Google Scholar] [CrossRef] [Green Version]
- Rao, R.; Esposito, M. Nonequilibrium Thermodynamics of Chemical Reaction Networks: Wisdom from Stochastic Thermodynamics. Phys. Rev. X 2016, 6, 041064. [Google Scholar] [CrossRef] [Green Version]
- Van Kampen, N.G. Stochastic Processes in Physics and Chemistry; Elsevier: New York, NY, USA, 1983. [Google Scholar]
- Schaller, G.; Ablamayer, J. Thermodynamics of the Coarse-Graining Master Equation. Entropy 2020, 22, 525. [Google Scholar] [CrossRef]
- Kwon, S.; Park, J.M. Derivation of evolutionary entropy in the steady-state thermodynamics of evolutionary dynamics. Phys. Rev. E 2021, 103, 062405. [Google Scholar] [CrossRef] [PubMed]
- Risken, H. Fokker-Planck Equation: Methods of Solutions and Applications; Springer: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Dong, X. Conservation-dissipation structure of linear stochastic systems. In Proceedings of the 2016 IEEE 55th Conference on Decision and Control (CDC), Las Vegas, NV, USA, 12–14 December 2016; pp. 5980–5985. [Google Scholar]
- Kurtz, T.G. The relationship between stochastic and deterministic models for chemical reactions. J. Chem. Phys. 1972, 57, 2976–2978. [Google Scholar] [CrossRef]
- Yong, W.A. Conservation-dissipation structure of chemical reaction systems. Phys. Rev. E 2012, 86, 067101. [Google Scholar] [CrossRef]
- Peng, L.; Zhu, Y.; Hong, L. The Markov process admits a consistent steady-state thermodynamic formalism. J. Math. Phys. 2018, 59, 013302. [Google Scholar] [CrossRef] [Green Version]
- Ge, H.; Qian, H. Nonequilibrium thermodynamic formalism of nonlinear chemical reaction systems with Waage-Guldberg’s law of mass action. Chem. Phys. 2016, 472, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Levermore, C.D. Moment closure hierarchies for kinetic theories. J. Stat. Phys. 1996, 83, 1021–1065. [Google Scholar] [CrossRef]
- Krivtsov, A.; Sokolov, A.; Müller, W.; Freidin, A. One-dimensional heat conduction and entropy production. In Advances in Mechanics of Microstructured Media and Structures; Springer: Berlin/Heidelberg, Germany, 2018; pp. 197–213. [Google Scholar]
- Yuan, D.; Cao Bing-Yang, G.Z.Y. Generalized heat conduction laws based on thermomass theory and phonon hydrodynamics. J. Appl. Phys. 2011, 110, 063504. [Google Scholar] [CrossRef] [Green Version]
- Guyer, R.A.; Krumhansl, J.A. Solution of the Linearized Phonon Boltzmann Equation. Phys. Rev. 1966, 148, 766–778. [Google Scholar] [CrossRef]
- Chen, G. Ballistic-Diffusive Heat-Conduction Equations. Phys. Rev. Lett. 2001, 86, 2297–2300. [Google Scholar] [CrossRef] [PubMed]
- Carr, D.D. Global existence of solutions to reaction-hyperbolic systems in one space dimension. SIAM J. Math. Anal. 1995, 26, 399–414. [Google Scholar] [CrossRef]
- Reed, M.C.; Venakides, S.; Blum, J.J. Approximate Traveling Waves in Linear Reaction-Hyperbolic Equations. SIAM J. Appl. Math. 1990, 50, 167–180. [Google Scholar] [CrossRef]
- Peng, L.; Hu, Y.; Hong, L. Conservation-Dissipation Formalism for soft matter physics: I. Augmentation to Doi’s variational approach. Eur. Phys. J. E 2019, 42, 73. [Google Scholar] [CrossRef]
- Yong, W.A. Diffusive relaxation limit of multidimensional isentropic hydrodynamical models for semiconductors. SIAM J. Appl. Math. 2004, 64, 1737–1748. [Google Scholar] [CrossRef]
- Doi, M. Onsager’s variational principle in soft matter. J. Phys. Condens. Matter 2011, 23, 284118. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, P.; Weinan, E. Modified models of polymer phase separation. Phys. Rev. E 2006, 73, 061801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, F.H.; Liu, C. Existence of Solutions for the Ericksen-Leslie System. Arch. Ration. Mech. Anal. 2000, 154, 135–156. [Google Scholar] [CrossRef]
- Lin, F.H.; Liu, C. Nonparabolic dissipative systems modeling the flow of liquid crystals. Commun. Pure Appl. Math. 1995, 48, 501–537. [Google Scholar] [CrossRef]
- Leslie, F.M. Theory of Flow Phenomena in Liquid Crystals. Adv. Liq. Cryst. 1979, 4, 1–81. [Google Scholar]
- Stewart, I.W. The Static and Dynamic Continuum Theory of Liquid Crystals: A Mathematical Introduction; Taylor and Francis: London, UK; New York, NY, USA, 2004. [Google Scholar]
- Flory, P.J. Molecular theory of liquid crystals. Adv. Polym. Sci. 1984, 59, 1–36. [Google Scholar]
- Lekkerkerker, H.N.W.; Coulon, P.; Haegen, R.V.D.; Deblieck, R. On the isotropic-liquid crystal phase separation in a solution of rodlike particles of different lengths. J. Chem. Phys. 1984, 80, 3427–3433. [Google Scholar] [CrossRef]
- He, H.; Sevick, E.M.; Williams, D.R.M. Isotropic and nematic liquid crystalline phases of adaptive rotaxanes. J. Chem. Phys. 2016, 144, 2186–2199. [Google Scholar] [CrossRef]
- Frank, F.C.I. Liquid crystals. On the theory of liquid crystals. Discuss Faraday Soc. 1958, 25, 19–28. [Google Scholar] [CrossRef]
- Qian, T.; Sheng, P. Generalized hydrodynamic equations for nematic liquid crystals. Phys. Rev. E 1998, 58, 7475–7485. [Google Scholar] [CrossRef] [Green Version]
- Bastin, G.; Coron, J.M. Stability and Boundary Stabilization of 1-d Hyperbolic Systems; Springer: Berlin/Heidelberg, Germany, 2016; Volume 88. [Google Scholar]
- Herty, M.; Yong, W.A. Feedback boundary control of linear hyperbolic systems with relaxation. Automatica 2016, 69, 12–17. [Google Scholar] [CrossRef]
- Yong, W.A. Boundary stabilization of hyperbolic balance laws with characteristic boundaries. Automatica 2019, 101, 252–257. [Google Scholar] [CrossRef]
- Diagne, A.; Bastin, G.; Coron, J.M. Lyapunov exponential stability of 1-D linear hyperbolic systems of balance laws. Automatica 2012, 48, 109–114. [Google Scholar] [CrossRef]
- Herty, M.; Yu, H. Feedback boundary control of linear hyperbolic equations with stiff source term. Int. J. Control 2018, 91, 230–240. [Google Scholar] [CrossRef]
- Maxwell, J.C. The Scientific Papers of James Clerk Maxwell; Courier Corporation: Washington, DC, USA; Springer: Tokyo, Japan, 2013. [Google Scholar]
- De Gennes, P.G.; Prost, J. The Physics of Liquid Crystals; Oxford University Press: Oxford, UK, 1995. [Google Scholar]
- Sarma, R.; Nath, A.J.; Konwar, T.; Mondal, P.K.; Wongwises, S. Thermo-hydrodynamics of a viscoelastic fluid under asymmetrical heating. Int. J. Heat Mass Transf. 2018, 125, 515–524. [Google Scholar] [CrossRef]
- Chakrabarti, N. Velocity shear effect on Jeans instability in a viscoelastic fluid. Phys. Lett. A 2021, 389, 127084. [Google Scholar] [CrossRef]
- Coleman, B.D.; Noll, W. Foundations of Linear Viscoelasticity. Rev. Mod. Phys. 1961, 33, 239. [Google Scholar] [CrossRef]
- Lin, F.H.; Liu, C.; Zhang, P. On hydrodynamics of viscoelastic fluids. Commun. Pure Appl. Math. 2005, 58, 1437–1471. [Google Scholar] [CrossRef]
- Huo, X.; Yong, W.A. Global existence for viscoelastic fluids with infinite Weissenberg number. Commun. Math. Sci. 2017, 15, 1129–1140. [Google Scholar] [CrossRef]
- Dvorkin, J.; Nur, A. Dynamic poroelasticity; a unified model with the squirt and the Biot mechanisms. Geophysics 1993, 58, 524–533. [Google Scholar] [CrossRef]
- Yong, W.A. Singular perturbations of first-order hyperbolic systems with stiff source terms. J. Differ. Equ. 1999, 155, 89–132. [Google Scholar] [CrossRef] [Green Version]
- Yong, W.A. Basic aspects of hyperbolic relaxation systems. In Advances in the Theory of Shock Waves; Springer: Berlin/Heidelberg, Germany, 2001; pp. 259–305. [Google Scholar]
- Liu, J. Stability Analysis of Wave Propagation Theories in Rock Physics. Ph.D. Thesis, Tsinghua University, Beijing, China, 2017. [Google Scholar]
- Chakraborty, D.; Sader, J.E. Constitutive models for linear compressible viscoelastic flows of simple liquids at nanometer length scales. Phys. Fluids 2015, 27, 052002. [Google Scholar] [CrossRef]
- Landau, L.; Lifshitz, E. Fluid Mechanics; Pergamon: Oxford, UK, 1987. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, L.; Hong, L. Recent Advances in Conservation–Dissipation Formalism for Irreversible Processes. Entropy 2021, 23, 1447. https://doi.org/10.3390/e23111447
Peng L, Hong L. Recent Advances in Conservation–Dissipation Formalism for Irreversible Processes. Entropy. 2021; 23(11):1447. https://doi.org/10.3390/e23111447
Chicago/Turabian StylePeng, Liangrong, and Liu Hong. 2021. "Recent Advances in Conservation–Dissipation Formalism for Irreversible Processes" Entropy 23, no. 11: 1447. https://doi.org/10.3390/e23111447
APA StylePeng, L., & Hong, L. (2021). Recent Advances in Conservation–Dissipation Formalism for Irreversible Processes. Entropy, 23(11), 1447. https://doi.org/10.3390/e23111447





