Every level is autonomous and differs from other levels in the amount of details (that are taken into account in both experimental observations and the mathematical formulation) and in the range of applicability. However self-contained are the levels, their mathematical formulation is closely related to their relationship to other levels. From investigating relations to upper levels (i.e., to levels involving more details) comes a structure that we shall call reduced structure and from investigating relations to lower levels (i.e., to levels involving fewer details) comes the reducing structure. Both structures equip the state space with a geometry and a vector field. The geometry is a mathematical formulation of thermodynamics. Every level has thus reduced and reducing thermodynamics and reduced and reducing vector fields. Below, we limit ourselves only to the reducing thermodynamics on the upper level and the reduced thermodynamics on the lower level (which is in this section the equilibrium level).
We emphasize that the term “reduction” has in this paper the same meaning as “emergence”. Some details on the upper level are lost in the reduction from an upper level to a lower level but at the same time an emerging overall pattern is gained. The process of reduction, as well as processes conductive to an emergence of overall features (pattern-recognition processes), involve both a loss and a gain. The terms “upper” and “lower” levels that we use in this paper have a different meaning than they have in, say, social sciences. The lower level is inferior from the upper level in the amount of details but superior in the ability to see overall patterns.
2.1. 2-Level Equilibrium Thermodynamics
Among many questions about the origin of both reduced and reducing structures and about their relations, we shall discuss only the one that is directly relevant to the Landau theory. We shall investigate the passage from the reducing thermodynamics on the upper level to the reduced thermodynamics on the equilibrium level. A few comments about the placement of the investigation of this passage in the larger context of multiscale thermodynamics are discussed at the end of this section.
The upper reducing thermodynamic relation
is one of several possible forms of the mathematical formulation of the upper reducing thermodynamics. The quantities introduced in (
1) are the upper energy per unit volume
, the upper number of moles per unit volume
, and the upper reducing entropy per unit volume
and are assumed to be sufficiently regular.
The reduced equilibrium thermodynamic relation
is obtained from (
1) by the following reducing Legendre transformation. We make this transformation in four steps.
Step 1: We introduce upper reducing thermodynamic potential (Note that
x can be any state variable, e.g., distribution function, hydrodynamic fields, electromagnetic fields, see [
5])
where
are conjugate equilibrium state variables. In the standard equilibrium thermodynamic notation
, where
T is the equilibrium temperature, and
, where
is the equilibrium chemical potential.
Step 2: We solve the equation
We use hereafter the notation:
, where
is an appropriate functional derivative if
x is a function (i.e., an element of an infinite dimensional space). Let
be the solution to (
4).
Step 3: We introduce
called a reduced conjugate entropy.
Step 4: Finally, we pass from to by the Legendre transformation (i.e., we introduce the equilibrium thermodynamic potential , solve , , and arrive at , where are solutions to , .
The reducing Legendre transformation (
1) → (
2) can also be seen as maximization of the upper reducing entropy
subjected to constraints
[
5,
6]. The conjugate equilibrium state variables
play the role of Lagrange multipliers. This viewpoint then gives the passage (
1) → (
2) the name Maximum Entropy principle (MaxEnt principle)
Summing up, the MaxEnt passage from the upper level to the equilibrium level, via the upper reducing thermodynamic relation (
1), is the following sequence of two mappings
The second mapping in (
6) is the standard Legendre transformation. The first mapping is the upper reducing Legendre transformation expressing the MaxEnt principle.
In the particular case when
and
, there is no reduction in (
6) and both arrows in (
6) are (one-to-one) standard Legendre transformations:
To conclude this section we turn to questions like where the potentials
come from and why the upper entropy
is maximized subjected to constraints
and
. These questions are answered simply by the existence of the autonomous upper level and the existence of the autonomous equilibrium level. The autonomous existence implies that there exists a way to prepare macroscopic systems for their investigations on the equilibrium level and that the time evolution describing the preparation process can be formulated on the upper level as a reducing time evolution. It is in this reducing time evolution where the potentials
make their first appearance. The entropy
generates the reducing time evolution. Maximization of
reflects the property of its solutions expressing mathematically the approach to the equilibrium level. We note that in the context of the classical formulation of equilibrium thermodynamics the existence of the preparation process for the equilibrium level (the existence of equilibrium states) is a subject of the zero axiom (see [
2]). The 2-level formulation of equilibrium thermodynamics can be thus seen as a way to bring the zero axiom to an active participation in the formulation of equilibrium thermodynamics.
2.3. Van der Waals Theory
We now illustrate the Landau theory on the van der Waals theory of a gas composed of particles interacting via long range attractive and short range repulsive forces. The macroscopic physical system investigated in this illustration is a gas composed of particles interacting via long range attractive forces and short range repulsive forces (van der Waals gas). The latter forces are treated as constraints and their influence enters the entropy rather than energy. The mathematical model of this system on the equilibrium level is the well known classical van der Waals model, on the level of kinetic theory the van Kampen model [
7] (see also [
8]) and its dynamical extension ([
9]).
The upper level is the level of kinetic theory with the one particle distribution function
serving as a single state variable,
r is the position coordinate and
v the momentum of one particle. In this example we specify explicitly the upper reducing thermodynamic relation
by using arguments developed mainly in the Gibbs equilibrium statistical mechanics. Having
, we identify the critical point and subsequently restrict
to the critical region. The resulting potentials take the form of the Landau critical thermodynamic potentials. This illustration has already been presented in [
8], we can therefore omit details.
Following van Kampen [
7], the upper reducing thermodynamic relation (
1) representing on the level of kinetic theory the van der Waals gas is
where we put the volume of the region in which the van der Waals gas is confined equal to one, the mass of one particle is also equal to one,
is the potential energy,
and where
is a small (proportional to the volume of one particle) parameter. Note that
is the local particle density. In
, the first term is the kinetic energy, the second the potential energy. In
, the first term is the Boltzmann entropy, the second term is the contribution to the entropy due to the excluded volume constraint.
By making the transformations in (
6), we arrive at the classical well known van der Waals thermodynamic relation (see details in [
5] (pp. 43–44) and [
8]).
Now we proceed to investigate the critical region. First note that van Kampen [
7] showed that the critical points corresponding to the end points of the critical curve in van der Waals gas are spatially homogeneous. This knowledge can be translated within this multiscale framework formulation into a restriction of the MaxEnt distribution function and the reduction to the level with state variable
n (see the
Appendix A and [
8] for details). In short, we begin by looking for solutions of (
4) only among
that are independent of
r. With this restriction, we arrive at
where we use the shorthand notation
,
and
.
The critical point is
A MaxEnt value: an extremum of reducing thermodynamic potential that governs the evolution and hence this extremum corresponds to an equilibrium state;
A point where loss of convexity occurs: the extremum (equilibrium point) is ambiguous, multiple or continuum of extrema are plausible;
Critical point of the whole system (including the inverse temperature ), i.e., the lowest temperature (T) and chemical potential () for which a critical point given by the above two points still exists: an extremal point, i.e., the third derivative with respect to n has to vanish as follows from Taylor expansion and the fact that the two above requirements can be translated into vanishing first two derivatives.
In short, the critical point is a point
where the potential has a stationary point, just loses convexity and has a minimum. By taking the Taylor expansion about the critical point,
the requirements on the critical point lead to equations
Hence the critical point of van der Waals gas is given by
We now make an explicit choice of the order parameter
where
is the critical value of
n, and define
With the explicit knowledge of the reducing fundamental thermodynamic potential (
11) we know that in a neighborhood of the critical point we arrive at
where
as the coefficient of
in
is linear in
n while the coefficient of
is quadratic in
n. Note that the cubic term is missing in the expansion as its coefficient is independent of
and hence
due to (
13). This form of the thermodynamic relation in the critical region
can be put in a more general framework due to Landau [
10] (Chapter XIV). Expressions for
involve the parameters
, serving as the material parameters in the van der Waals theory.
From (
16) we obtain
where
is a solution to
The lower reduced entropy (
18) provides complete information about the behavior (the behavior seen in equilibrium-thermodynamic observations) of the van der Waals gas in a small neighborhood of the critical point. In particular, we obtain the critical exponents arising in the dependence of
on
and
.
A simple way to see that
is a generalized homogeneous function and thus to identify the critical exponents is to use (
16) with
now being the variables instead of
. We replace
in (
16) with
. We obtain
and consequently, noting
is unaffected by the rescaling,
Finally, one could invert
from (
17) to get a (generalized) scaling for
.
Still another view of this relation can serve as an introduction to the renormalization-group theory of critical phenomena discussed below in
Section 4. We start again with (
16) and write it in the form
, where
is given in (
17). Our aim is to introduce a
renormalization time evolution (i.e., renormalization group of transformations generated by a vector field) of
and of
such that:
with the initial conditions
and the constraint
The renormalization time is denoted by the symbol
. We emphasize that the renormalization time
has nothing to do with the real time
t. The renormalization time evolution will become the basis for a new definition of critical points discussed in
Section 4. From the physical point of view, the constraint expresses the requirement that the material parameter
B entering the repulsive short range forces in (
10) remains unchanged in the renormalization process.
We begin with
with
being at this point an unspecified parameter and with the initial condition given by the second line in (
21). It can be easily verified that [
8]
with the initial condition given by the first line in (
21) and
We see now that with
we satisfy both (
21) and the constraint (
23).
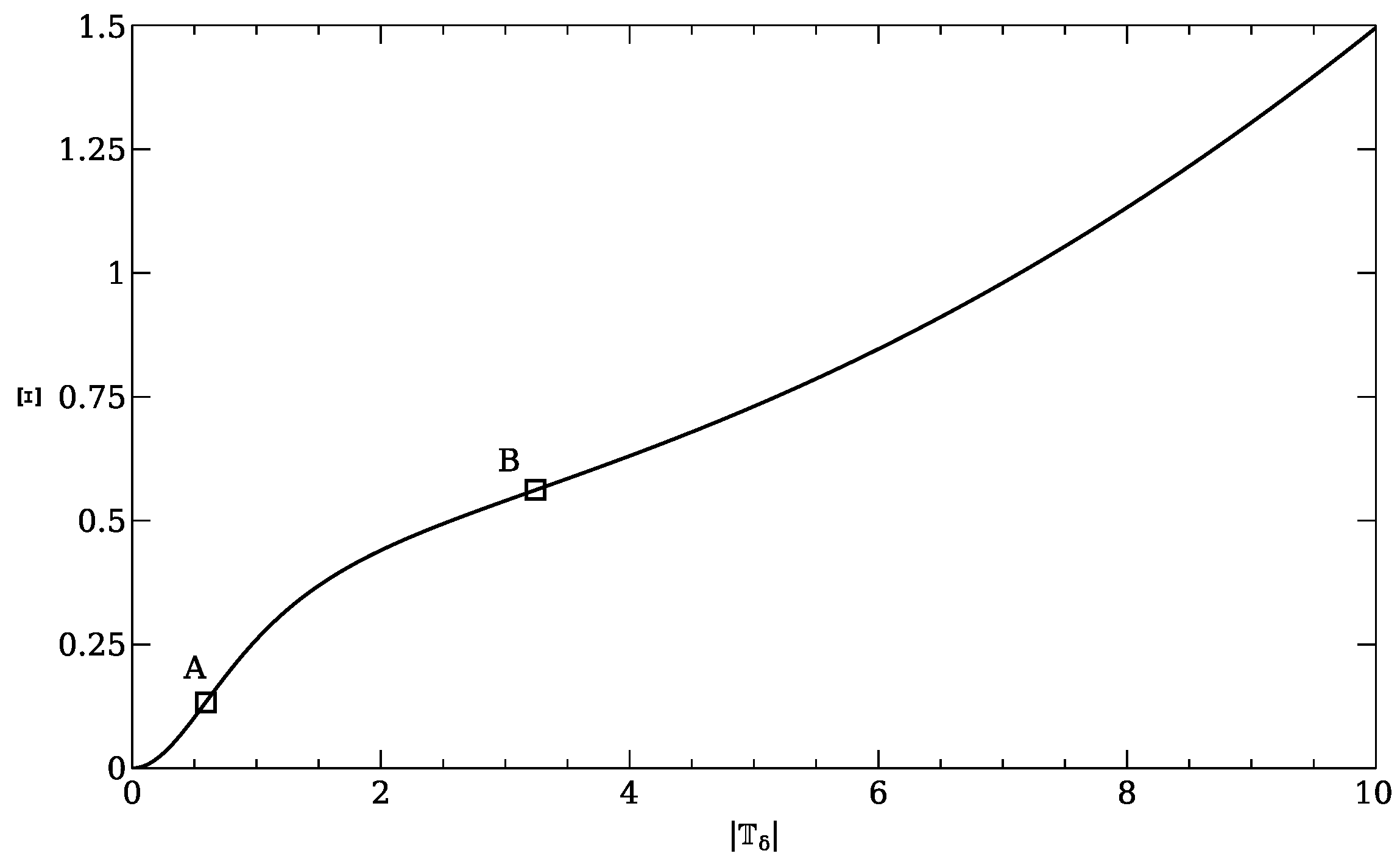
The fixed point of the renormalization time evolution is the critical point and the eigenvalues of the vector field linearized about the fixed point are the critical exponents.
This statement, which has arisen as a simple observation in the particular context discussed above, is in fact a definition of the critical points and the critical exponents in the renormalization-group theory of critical phenomena (see
Section 4). In the case of (
25) the linearization is, of course, unnecessary since the vector field is already linear.
Finally we compare the classical analysis of the van der Waals gas with the analysis based on the Landau theory. The starting point of the classical analysis is the physical insight that led us to the upper reducing thermodynamic relation (
9). By restricting it to the critical region we have arrived at the Landau expression (
16). The starting point of the Landau theory is the expression (
16). The quantity
, called in the Landau theory an order parameter, does not need to have a specific physical interpretation, nor the coefficients
are specified in the Landau theory.
The extra information about the critical phenomena that the classical van der Waals theory provides (but only for the van der Waals gas) is thus: (i) the location of the critical point in the state space
, (ii) physical interpretation of the order parameter, (iii) a detailed knowledge of the critical behavior beyond a small neighborhood of the critical point. On the other hand, the advantage of the Landau theory is its universal applicability. In
Section 4 we make a comment about the renormalization group theory, the objective of which is to bring the critical exponents implied by the van der Waals theory (and thus also the Landau theory) closer to those seen in experiments.
Before leaving the van der Waals theory, we mention that the static version of the theory recalled above has been upgraded to the dynamical theory in [
9]. The kinetic equation of which solutions make the maximization of the entropy
subjected to constraints
(see (
9)) is the Enskog Vlasov kinetic equation.