A Comprehensive, Three-Dimensional Analysis of a Large-Scale, Multi-Fuel, CFB Boiler Burning Coal and Syngas. Part 2. Numerical Simulations of Coal and Syngas Co-Combustion
Abstract
1. Introduction
2. Materials and Methods
- Variant K1 corresponds to the use of Nozzle No. 1, with a total 4.6 kg/s of syngas supplied through two side, start-up burners,
- Variant K2 considers the simultaneous use of Nozzles No. 1 and No. 2, with a total of 9.2 kg/s of syngas supplied to the combustion chamber through four side, start-up burners, and
- Variant K3 matches the simultaneous use of Nozzles No’s. 1, 2 and 3, with a total of 13.8 kg/s of syngas supplied to the combustion chamber through four side and two front, start-up burners.
3. Results
3.1. Validation
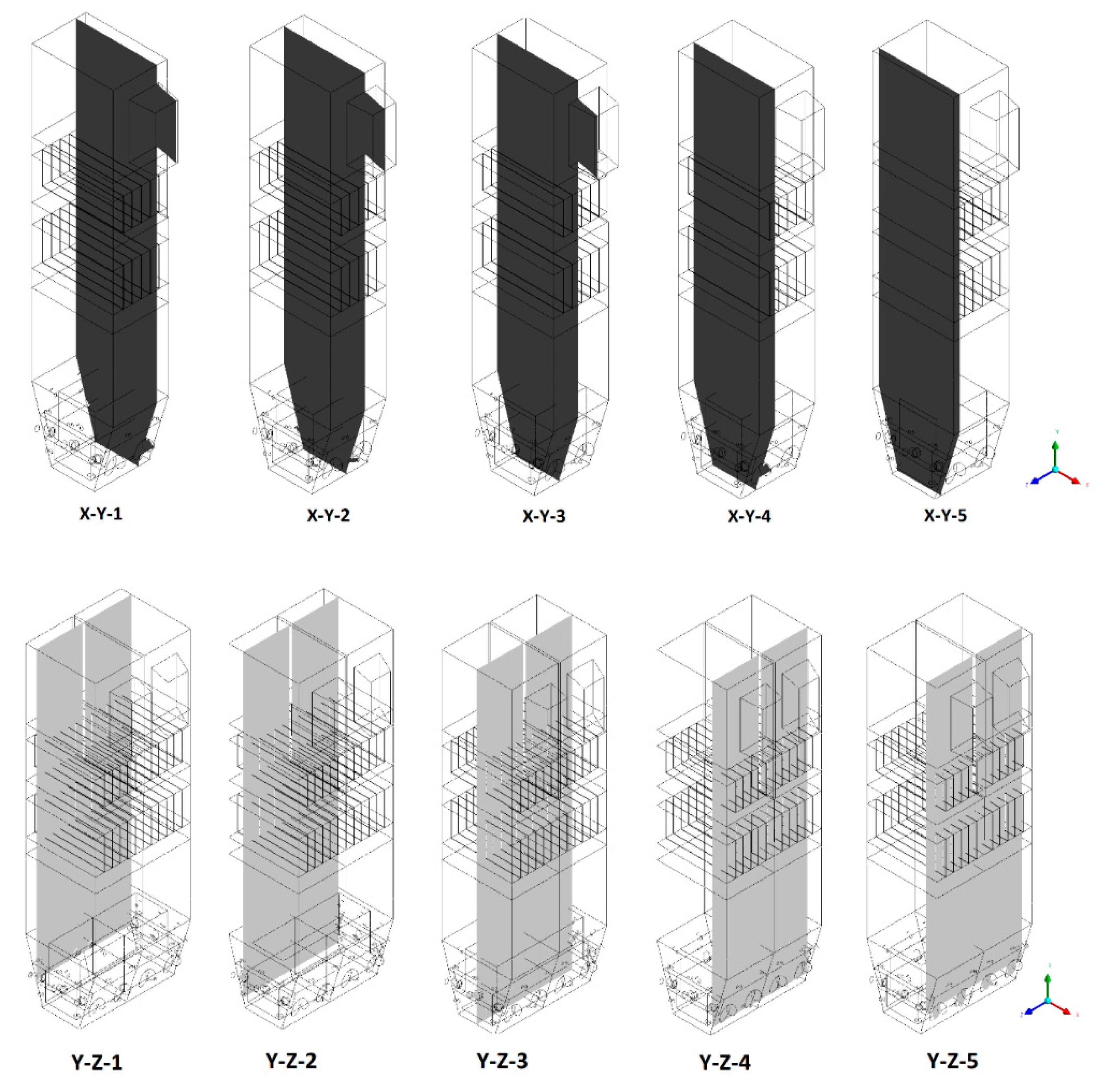
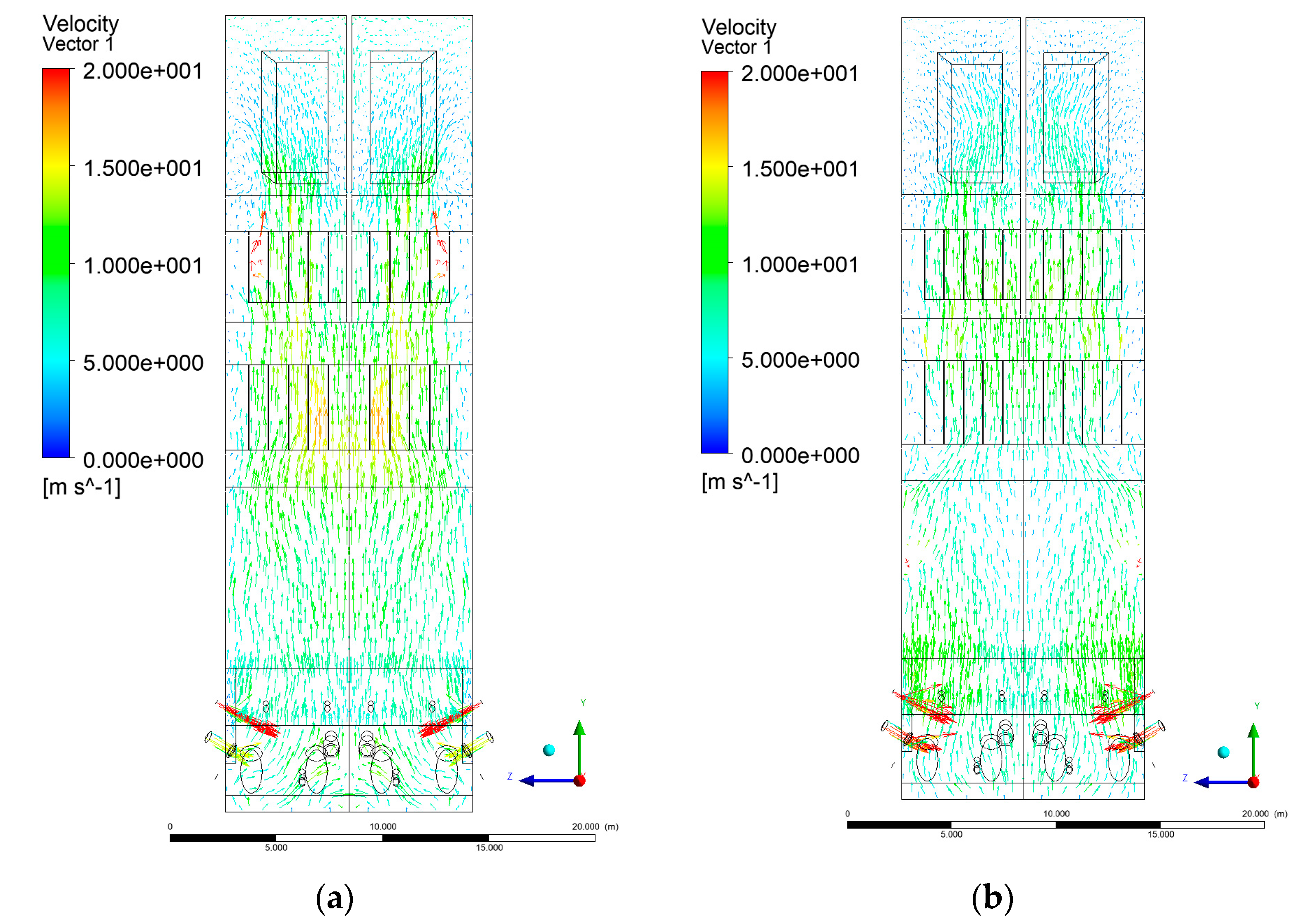
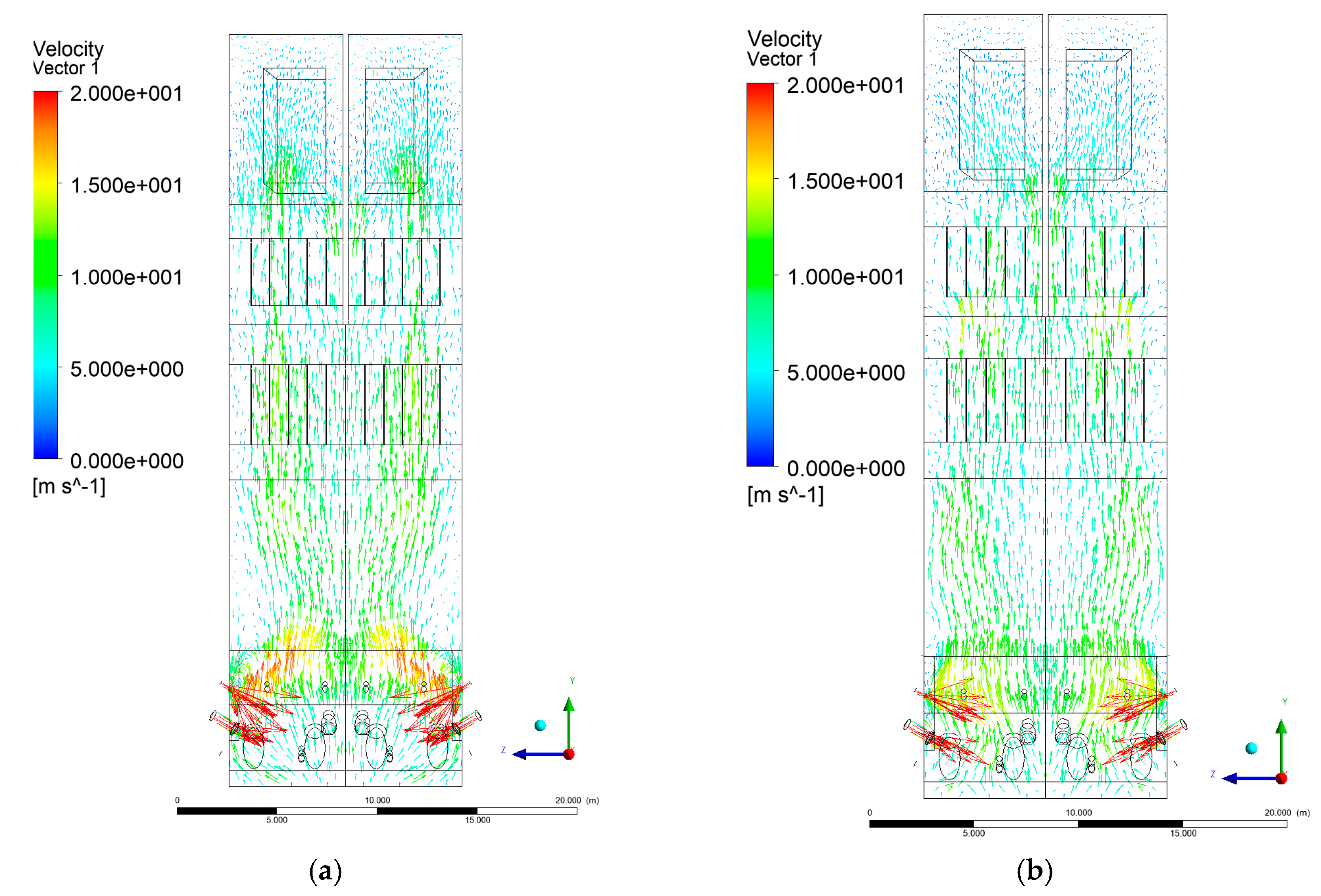
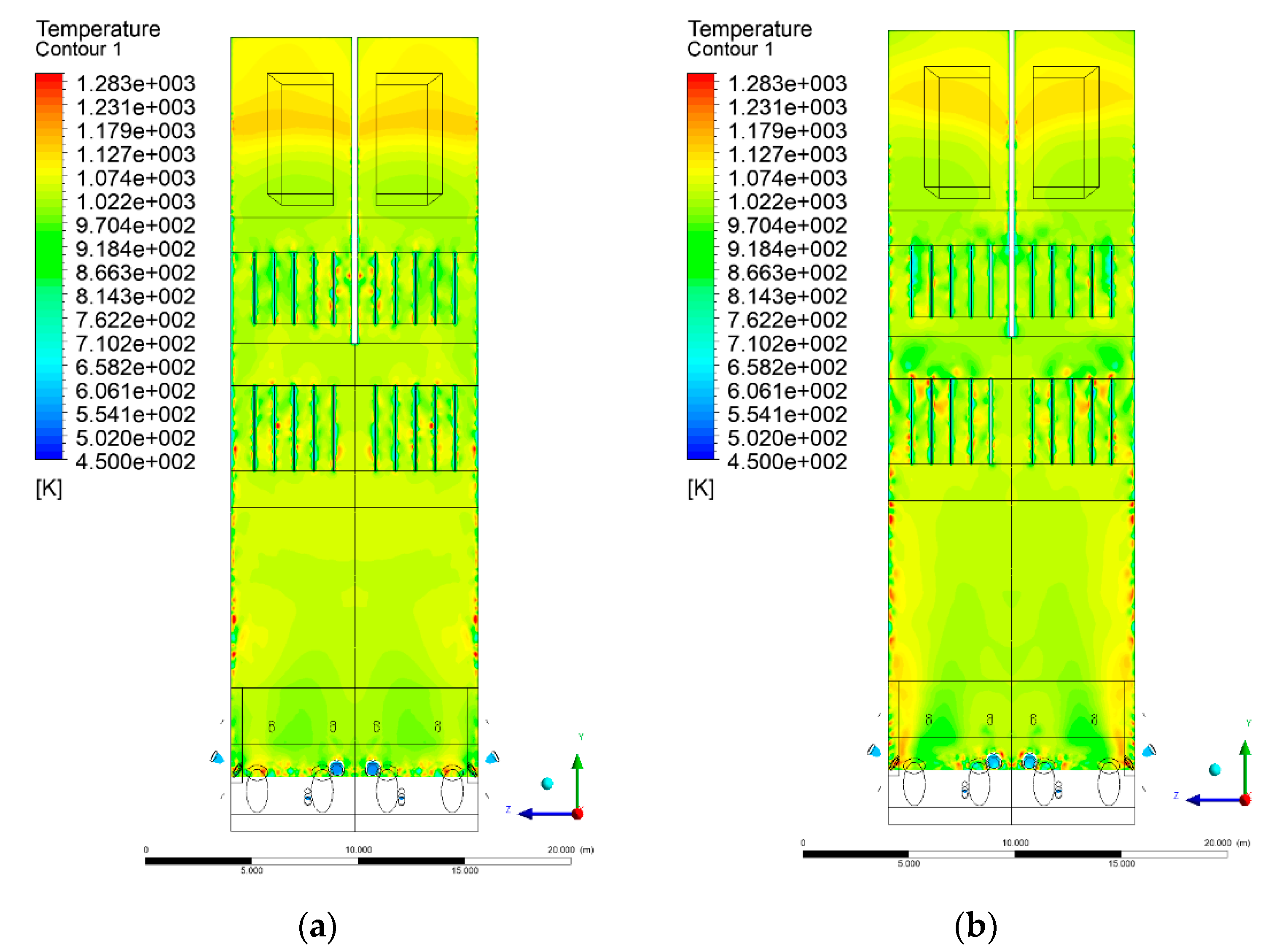
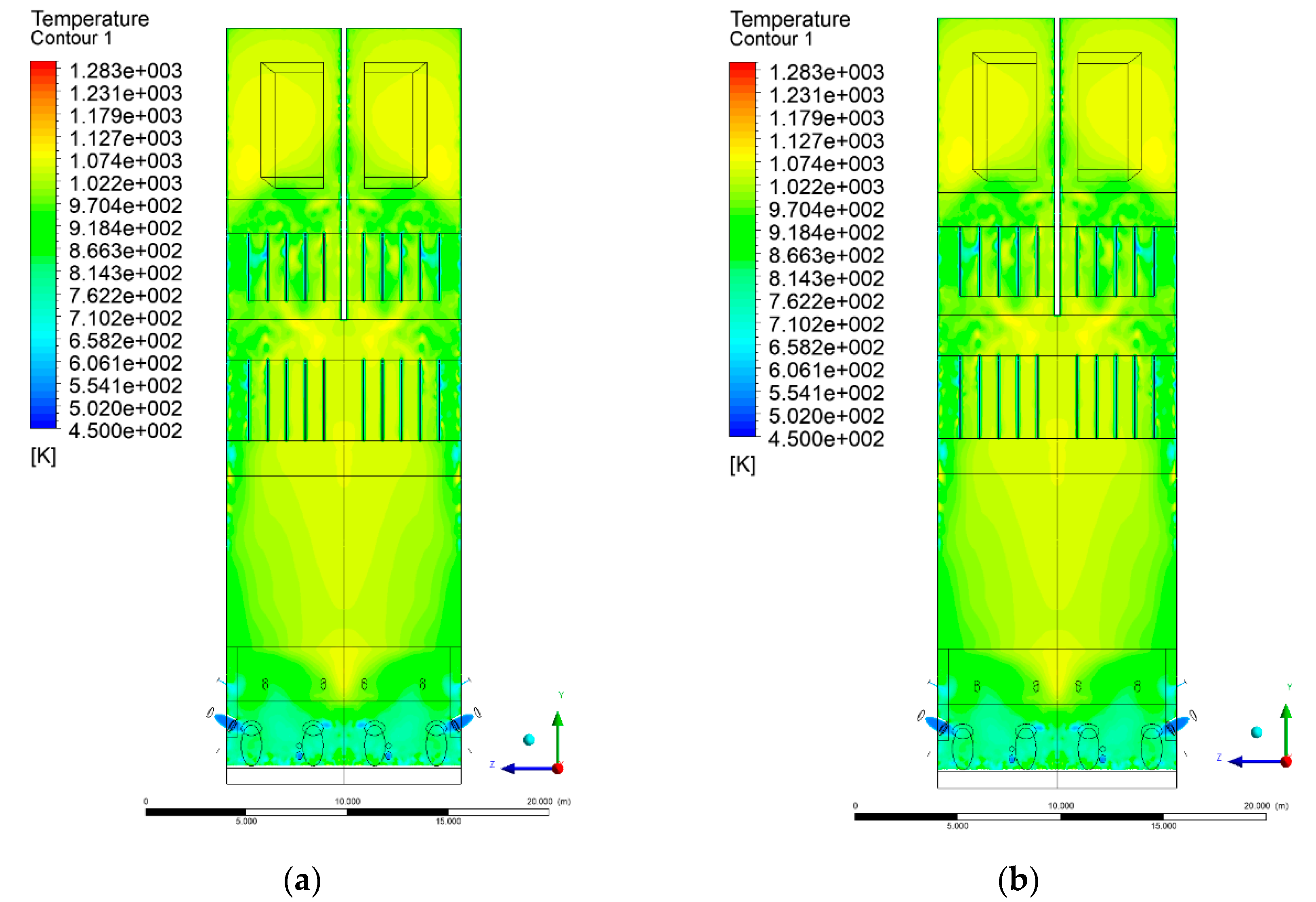
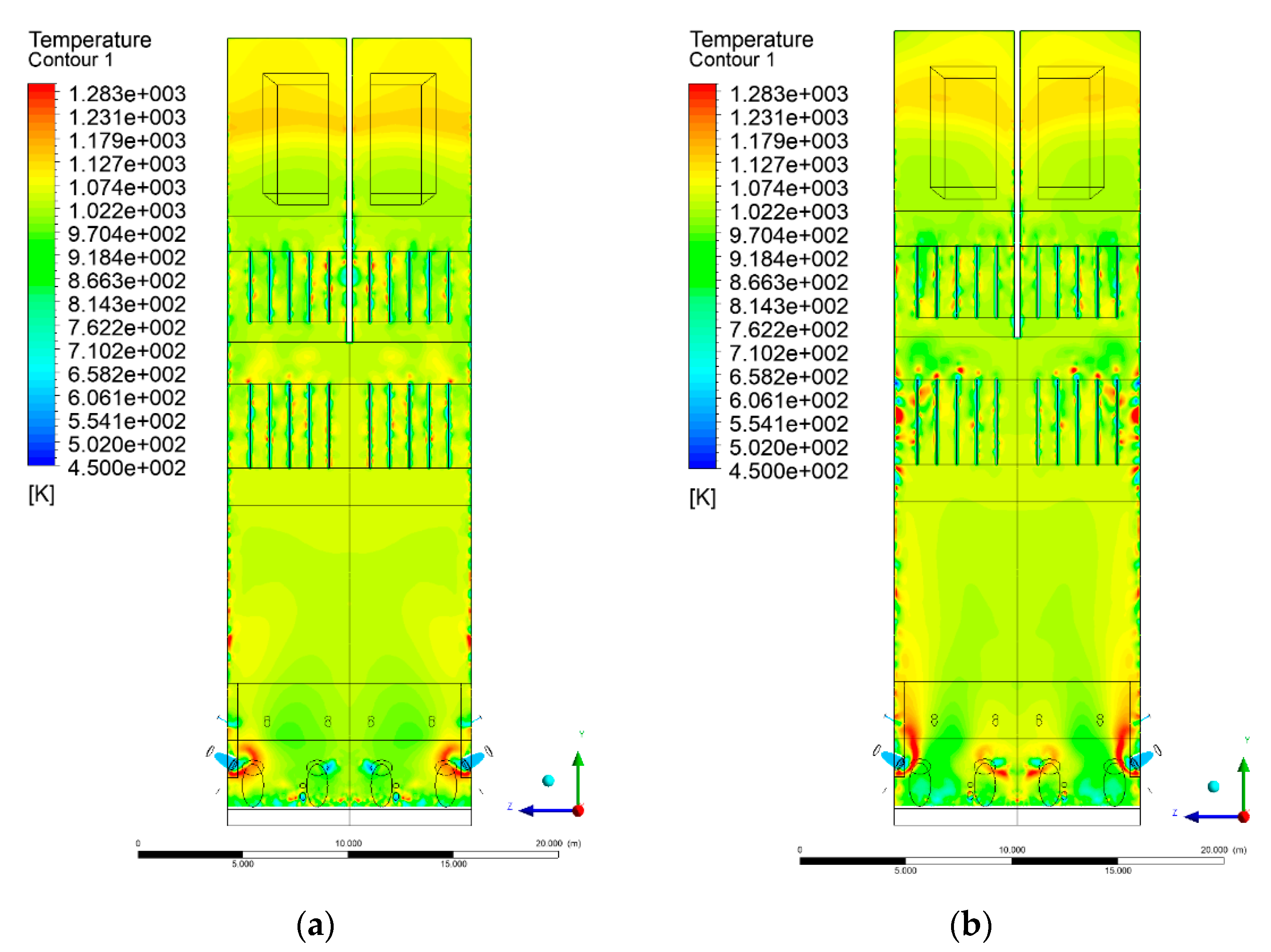
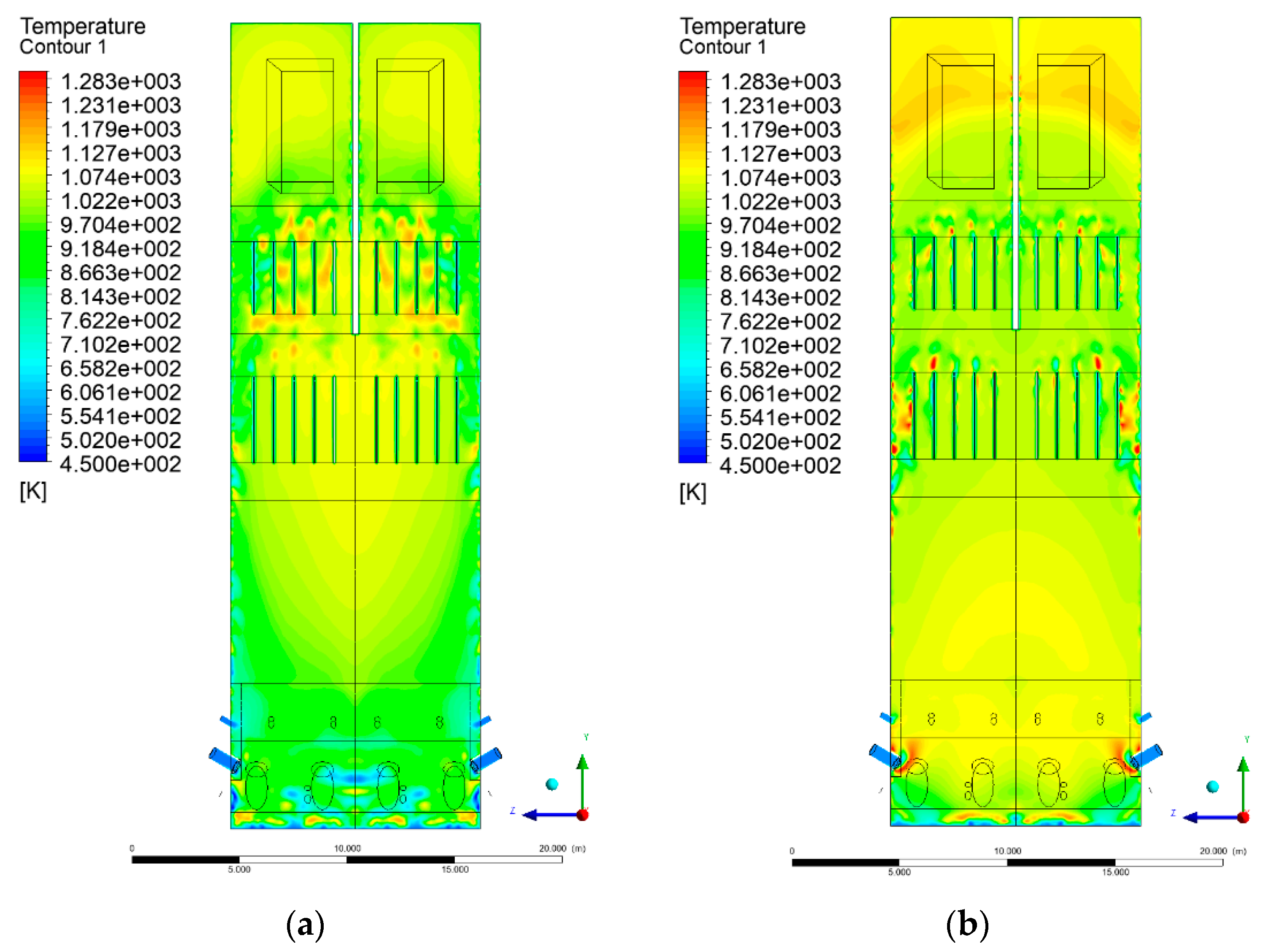
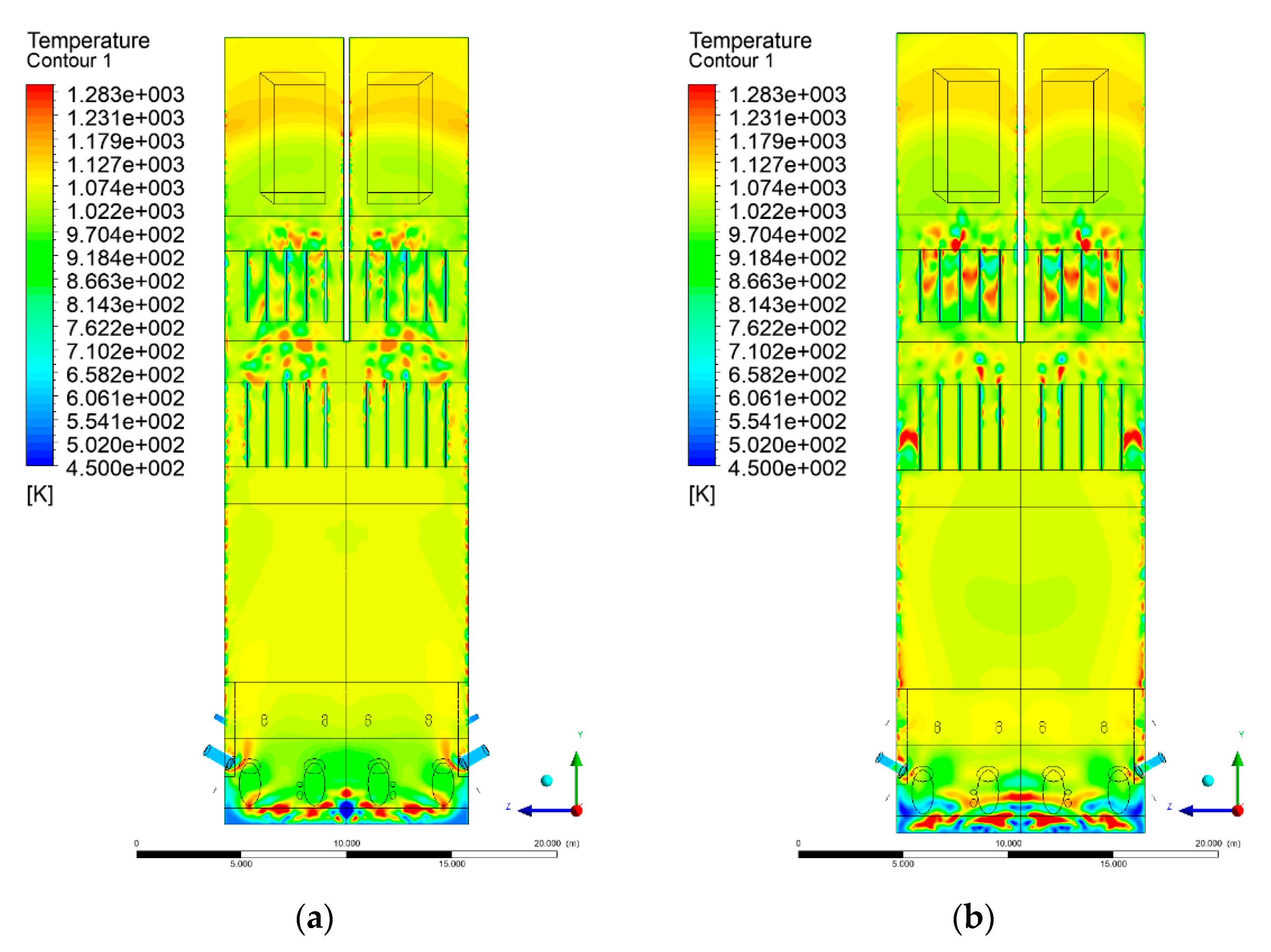
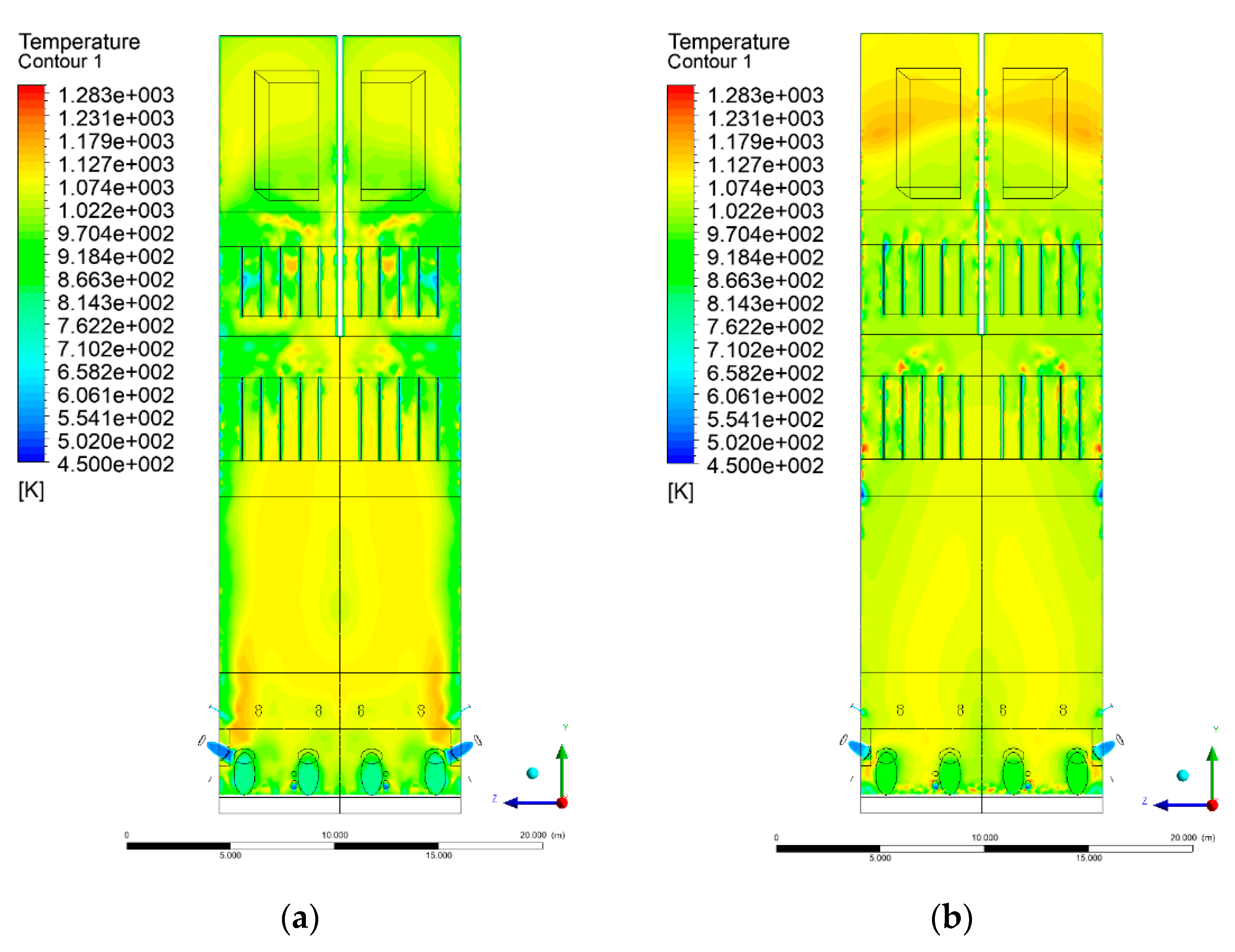
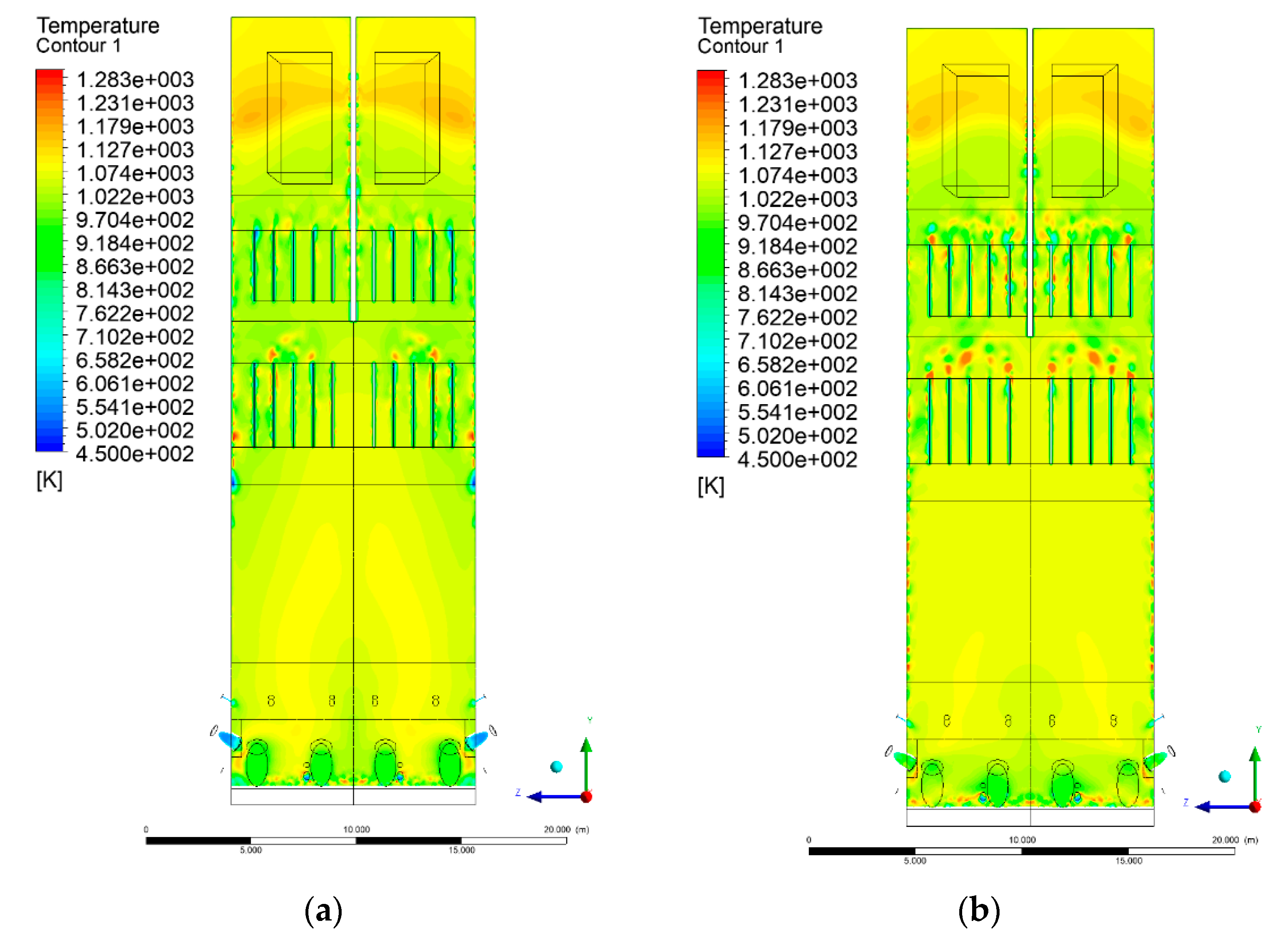
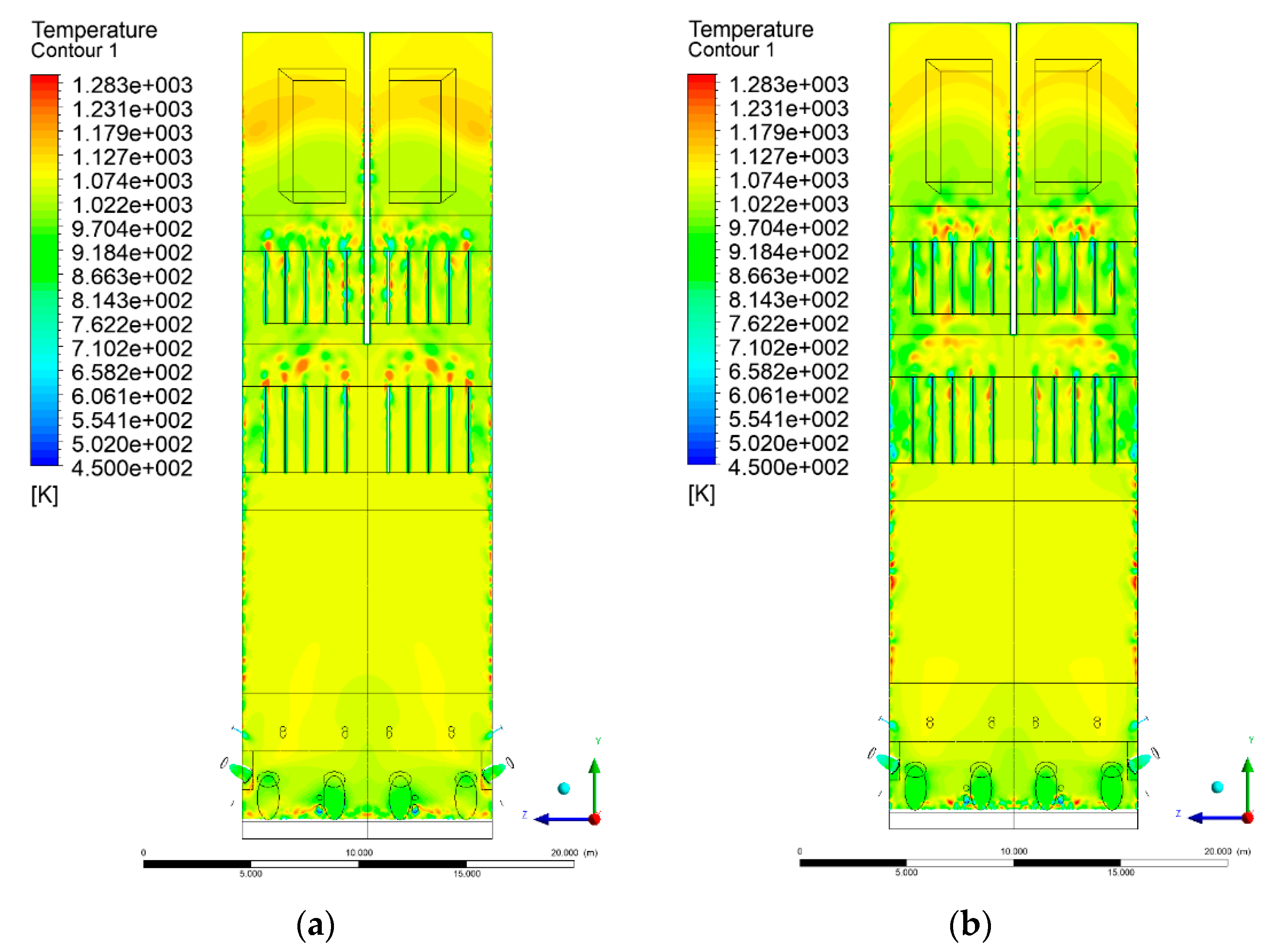
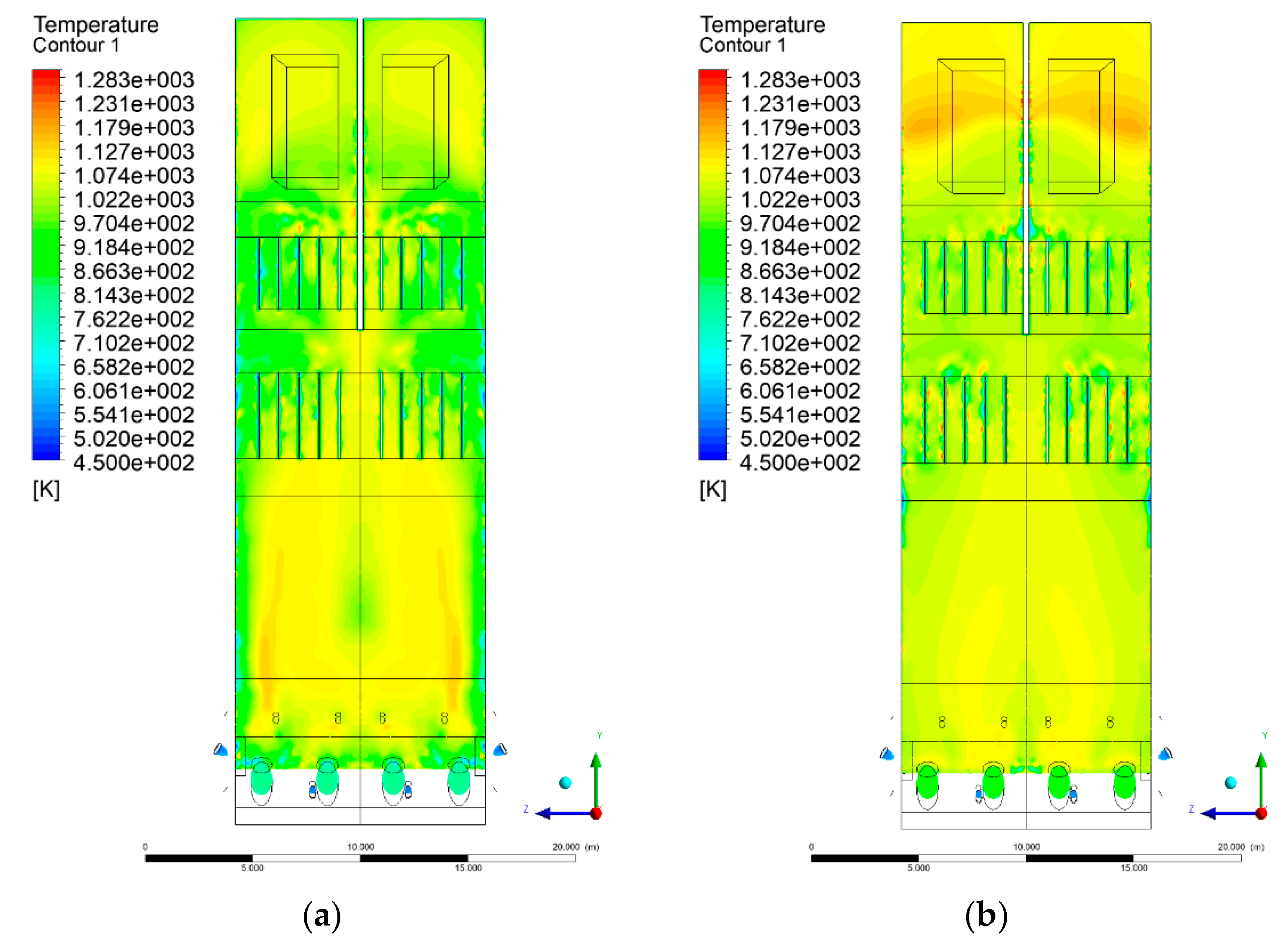
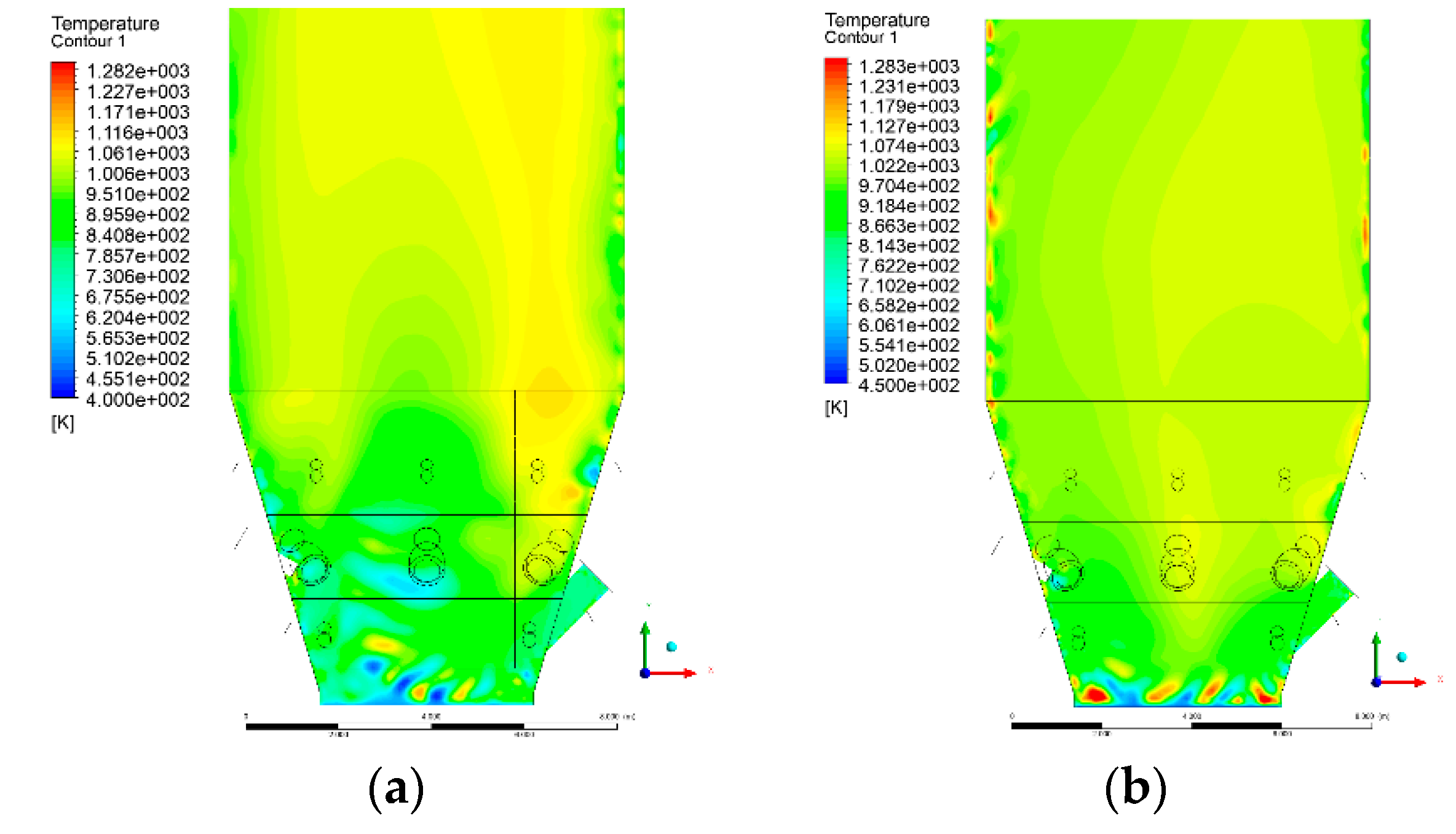
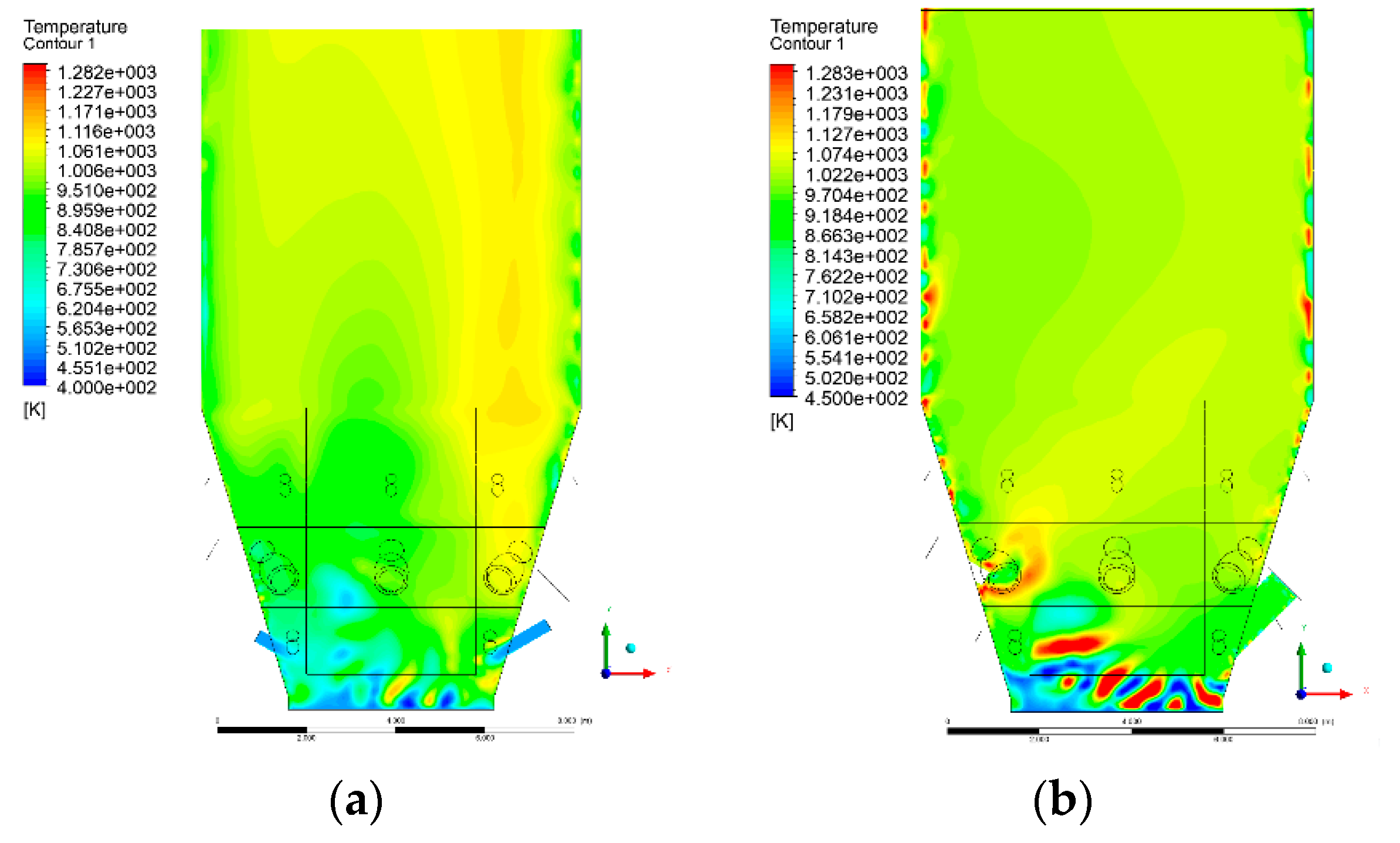
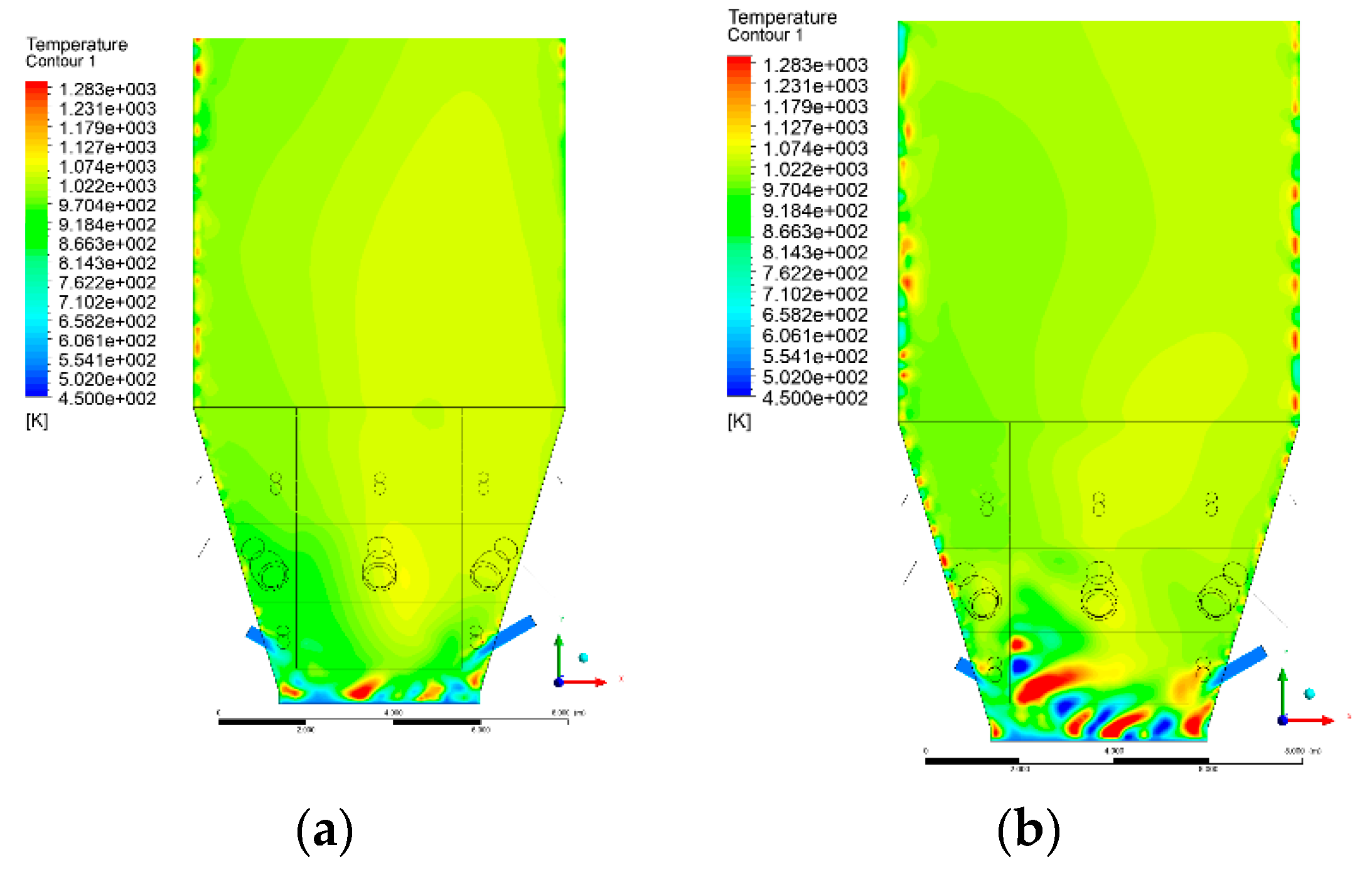
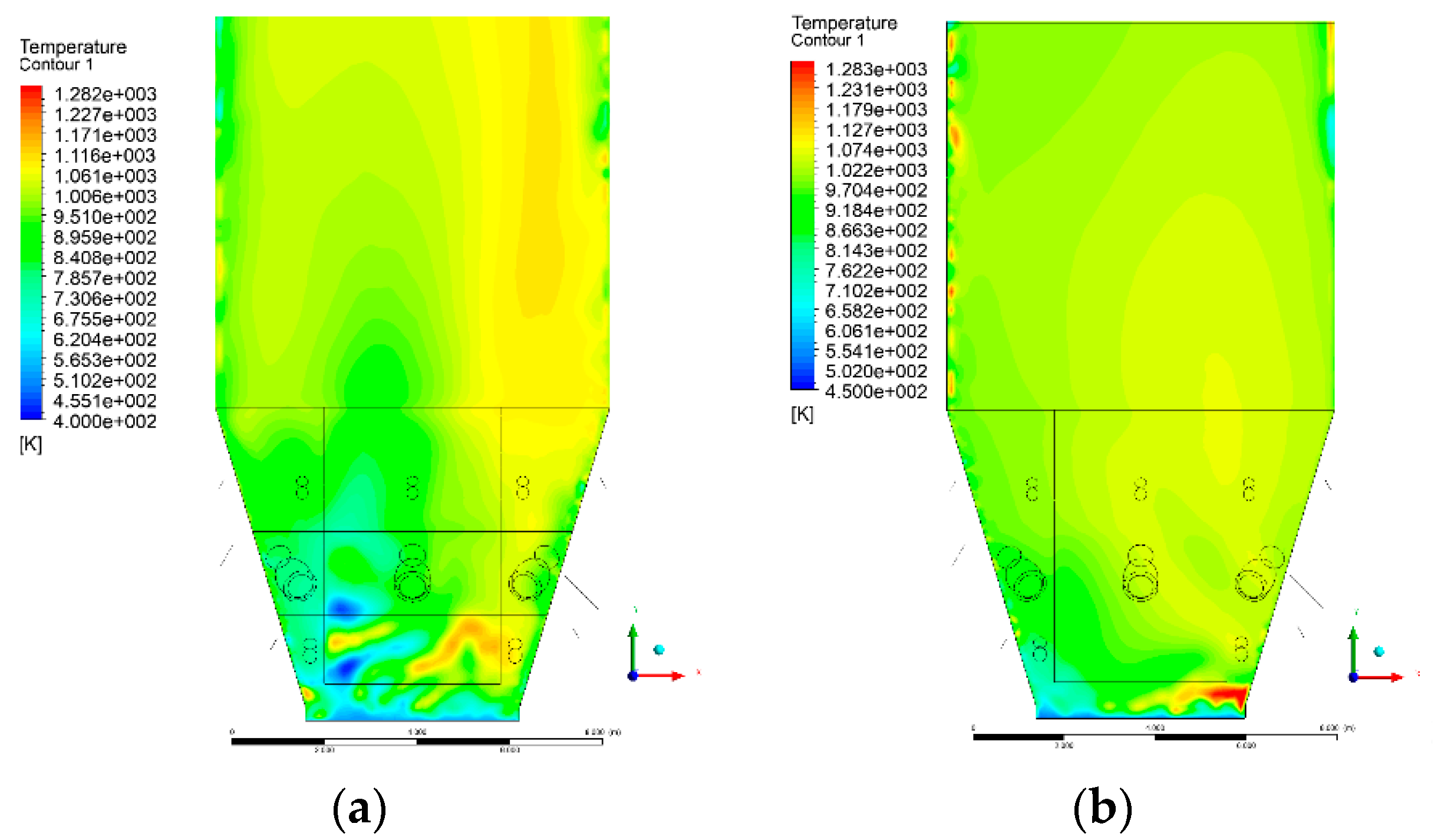
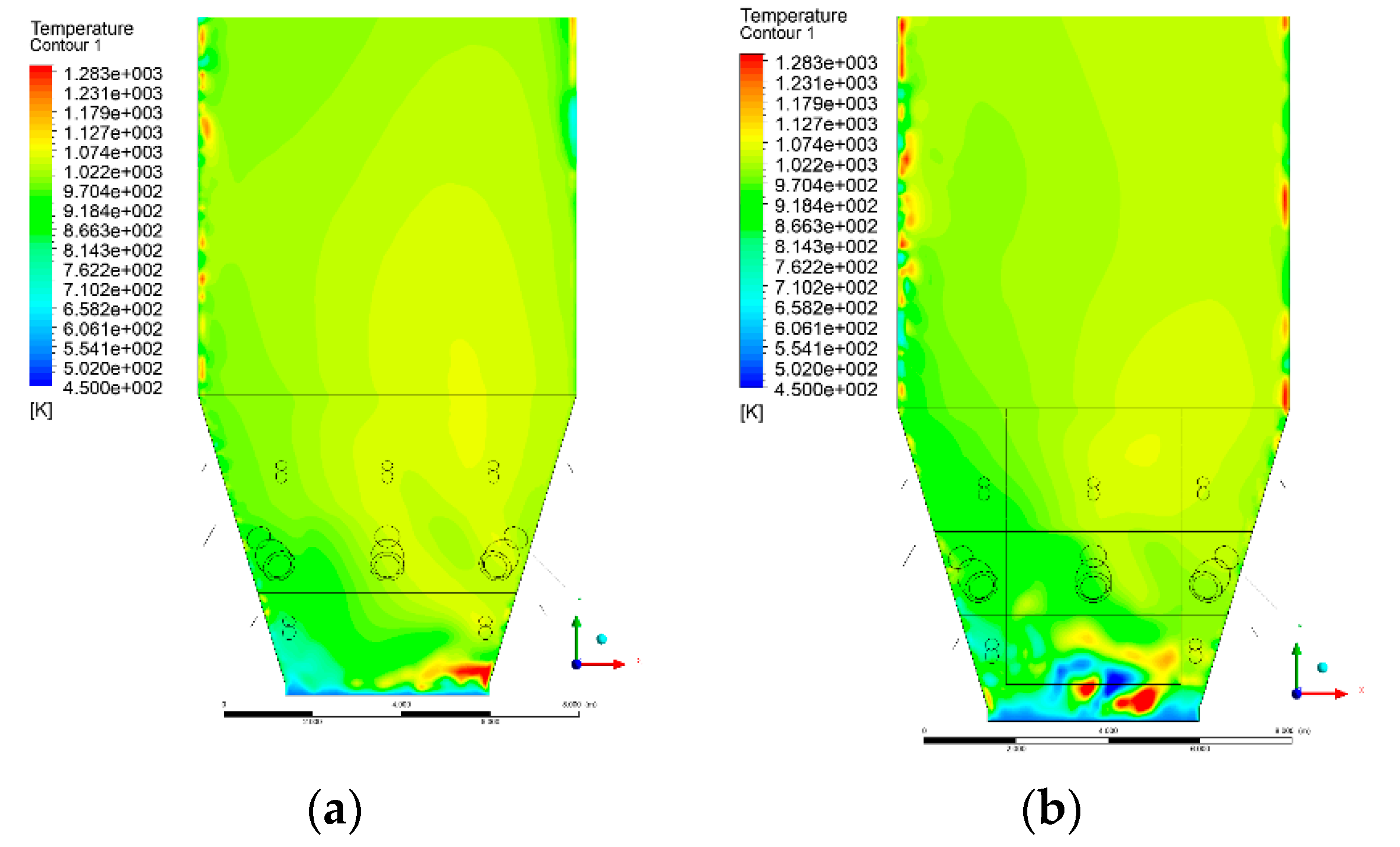
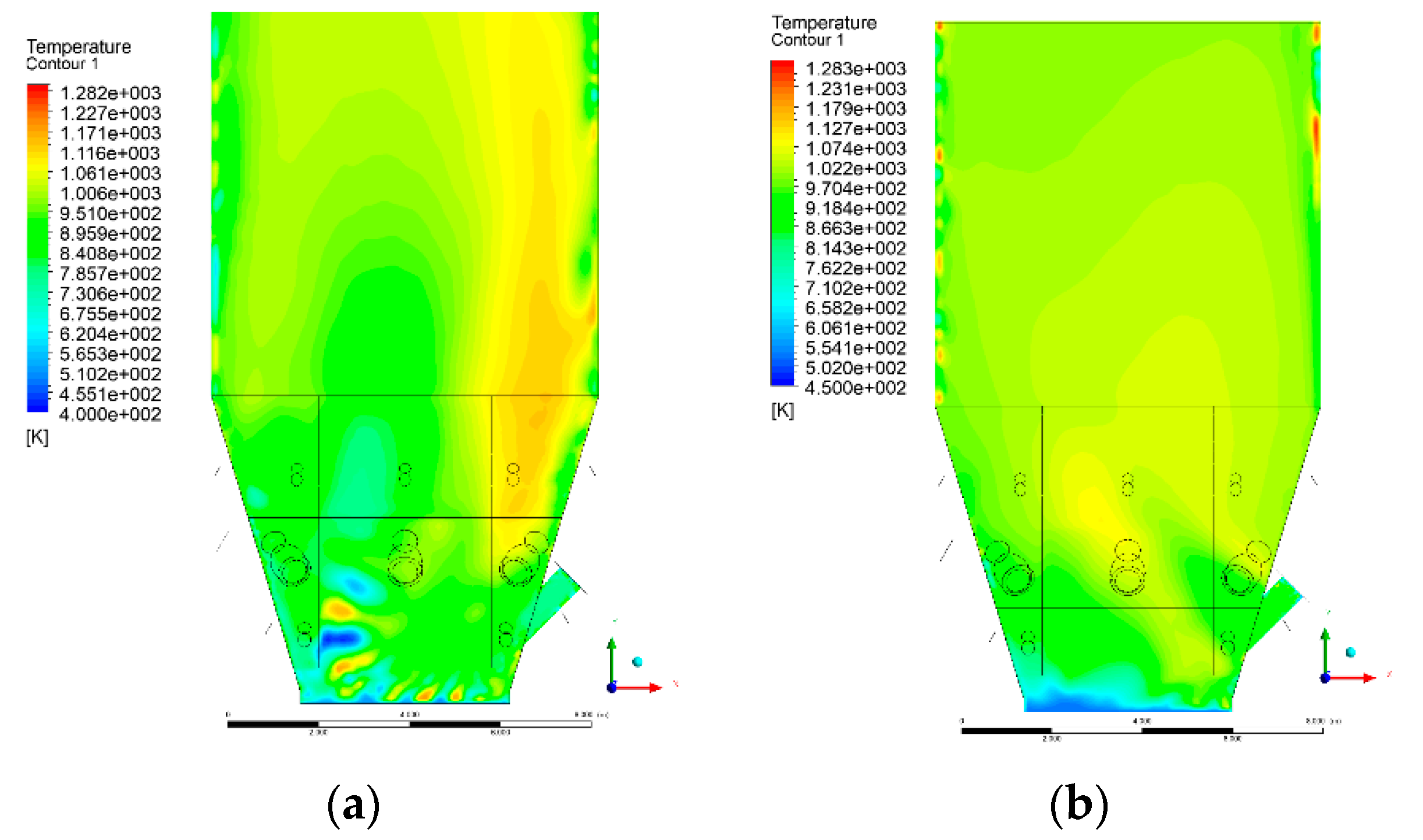
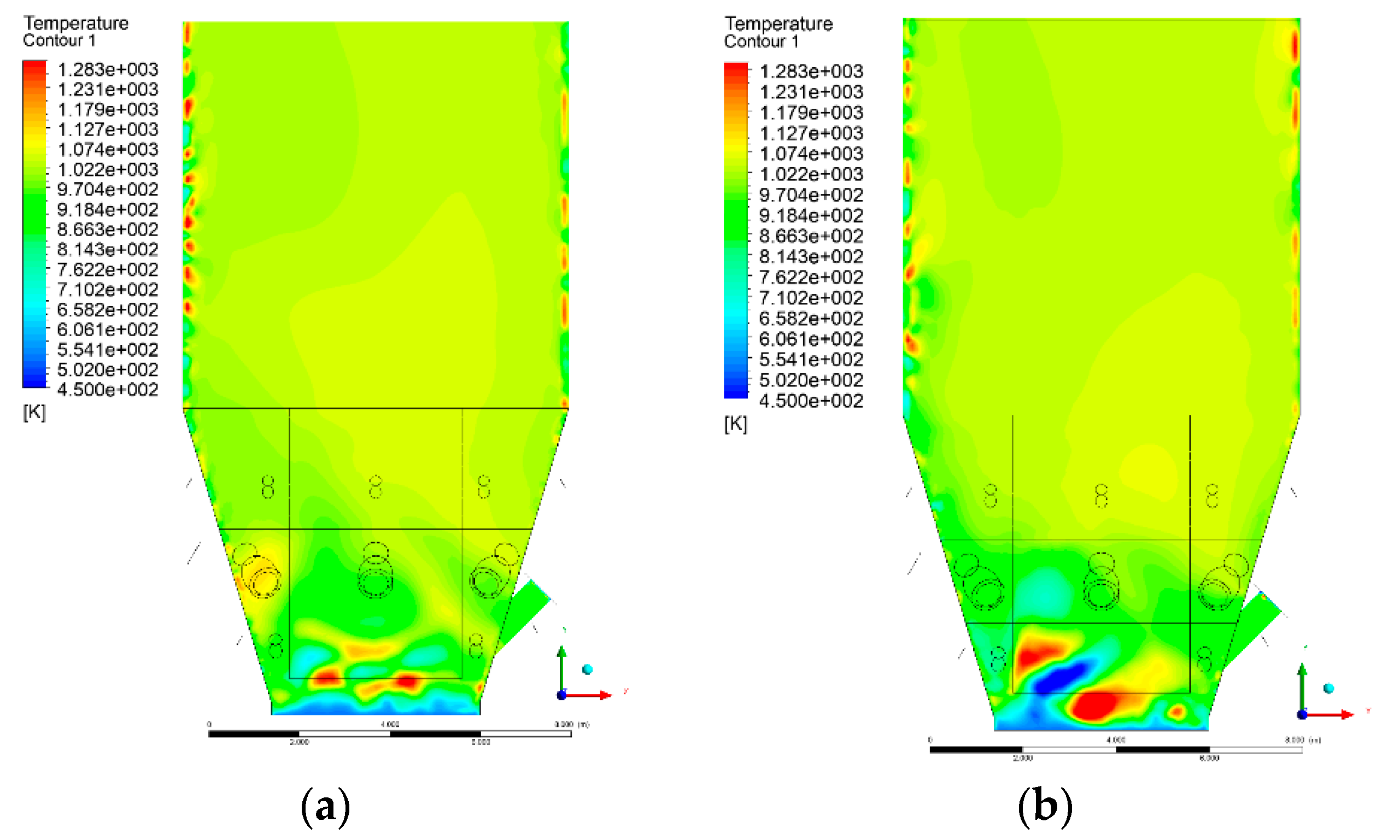
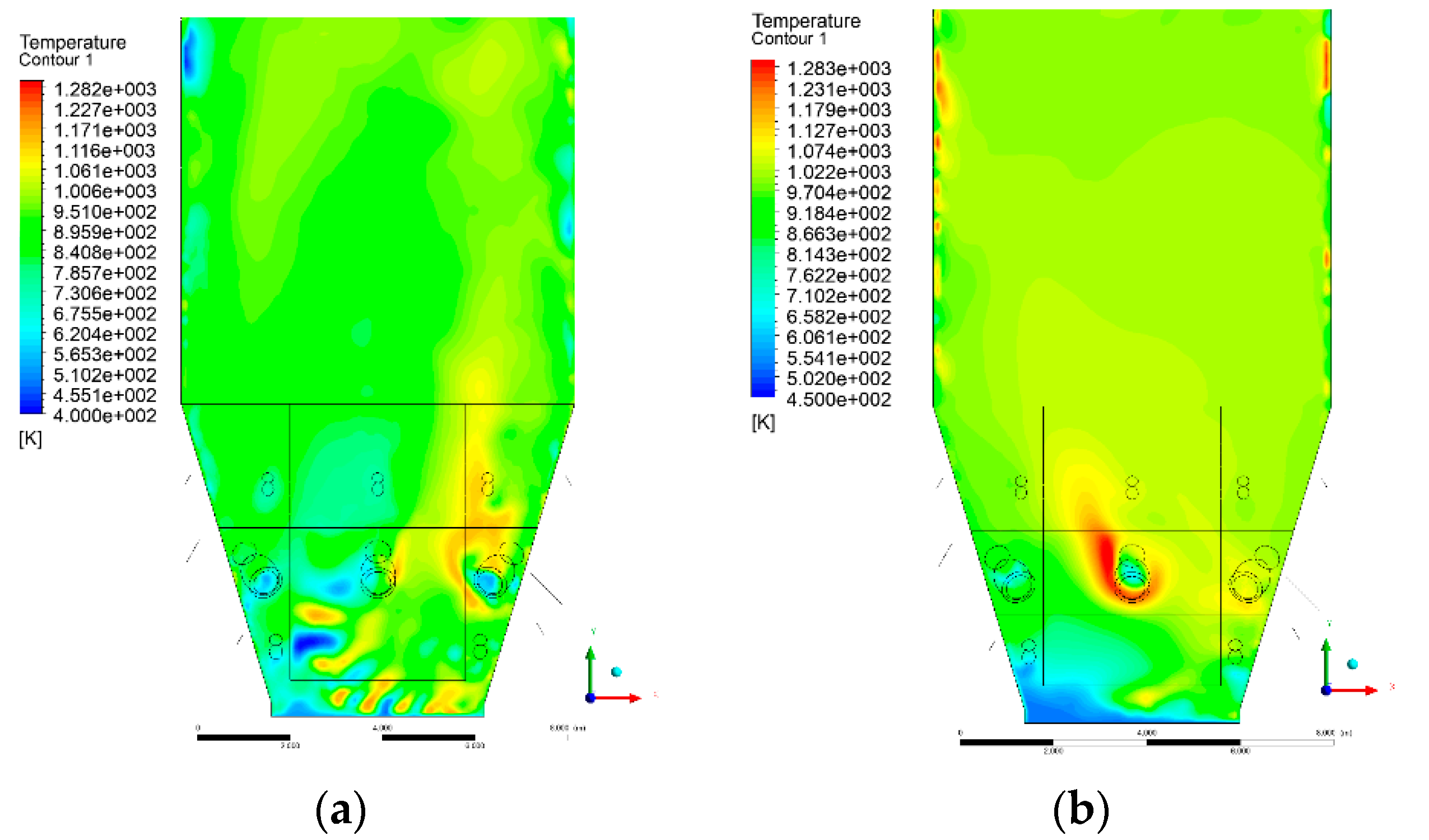
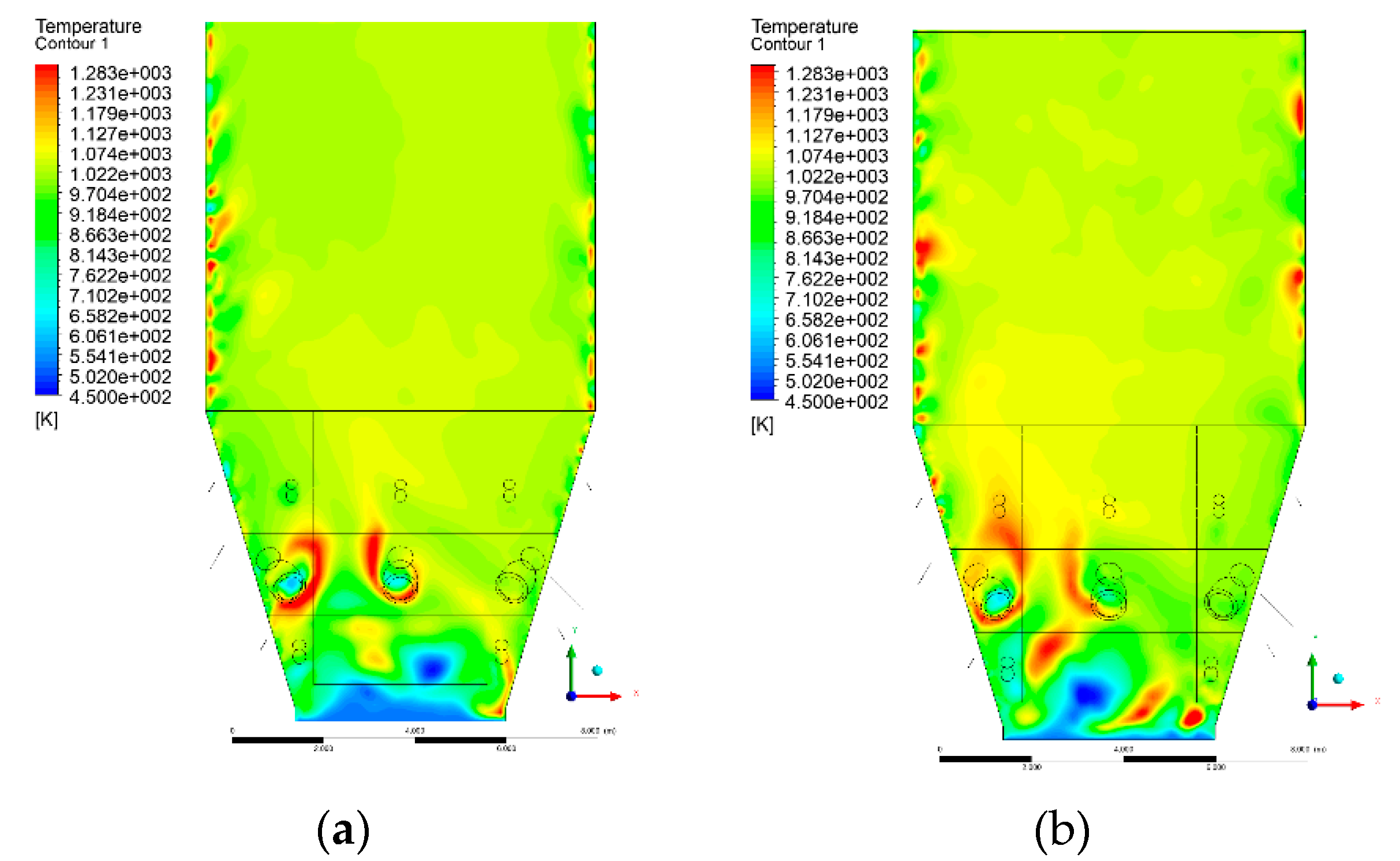
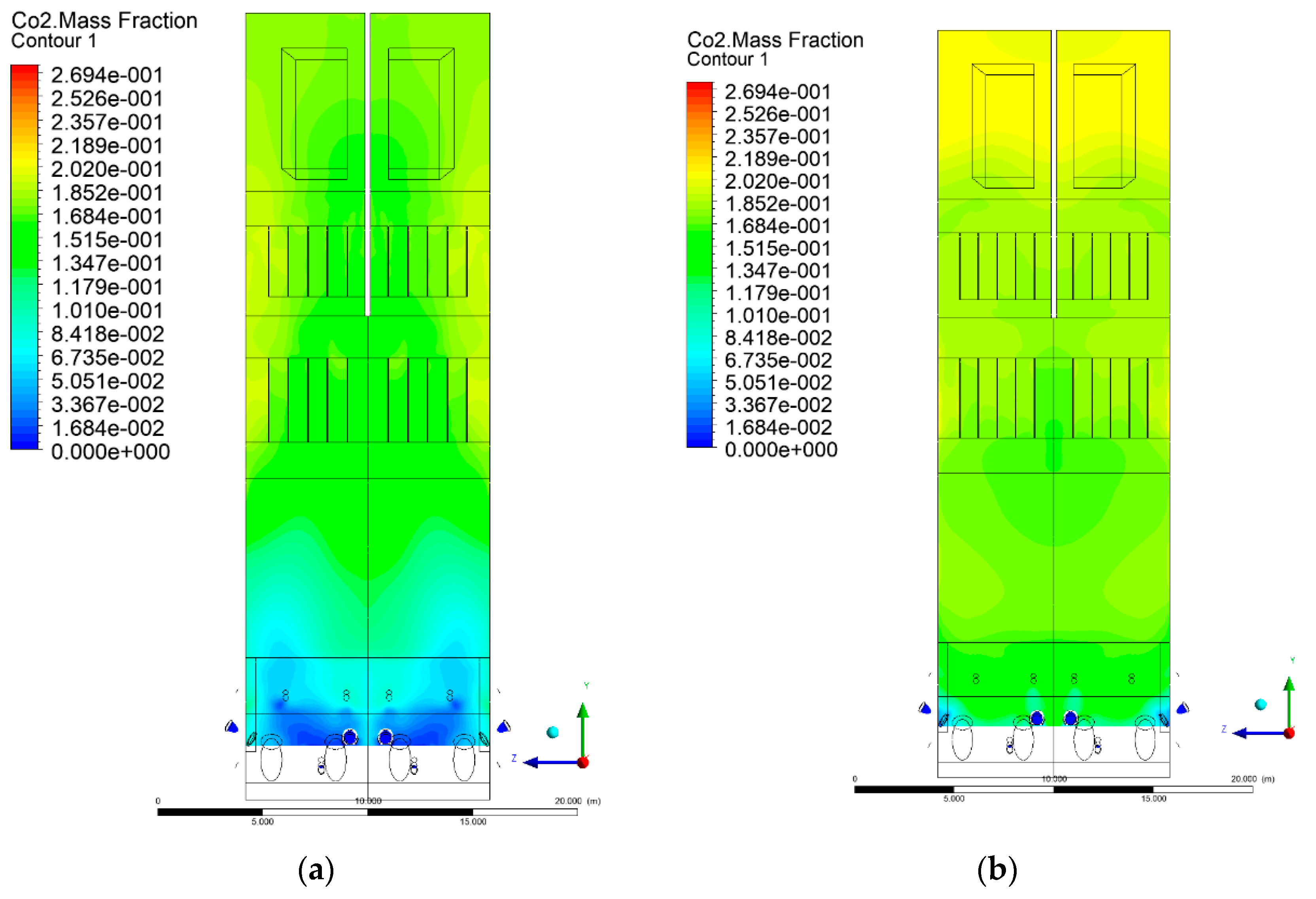
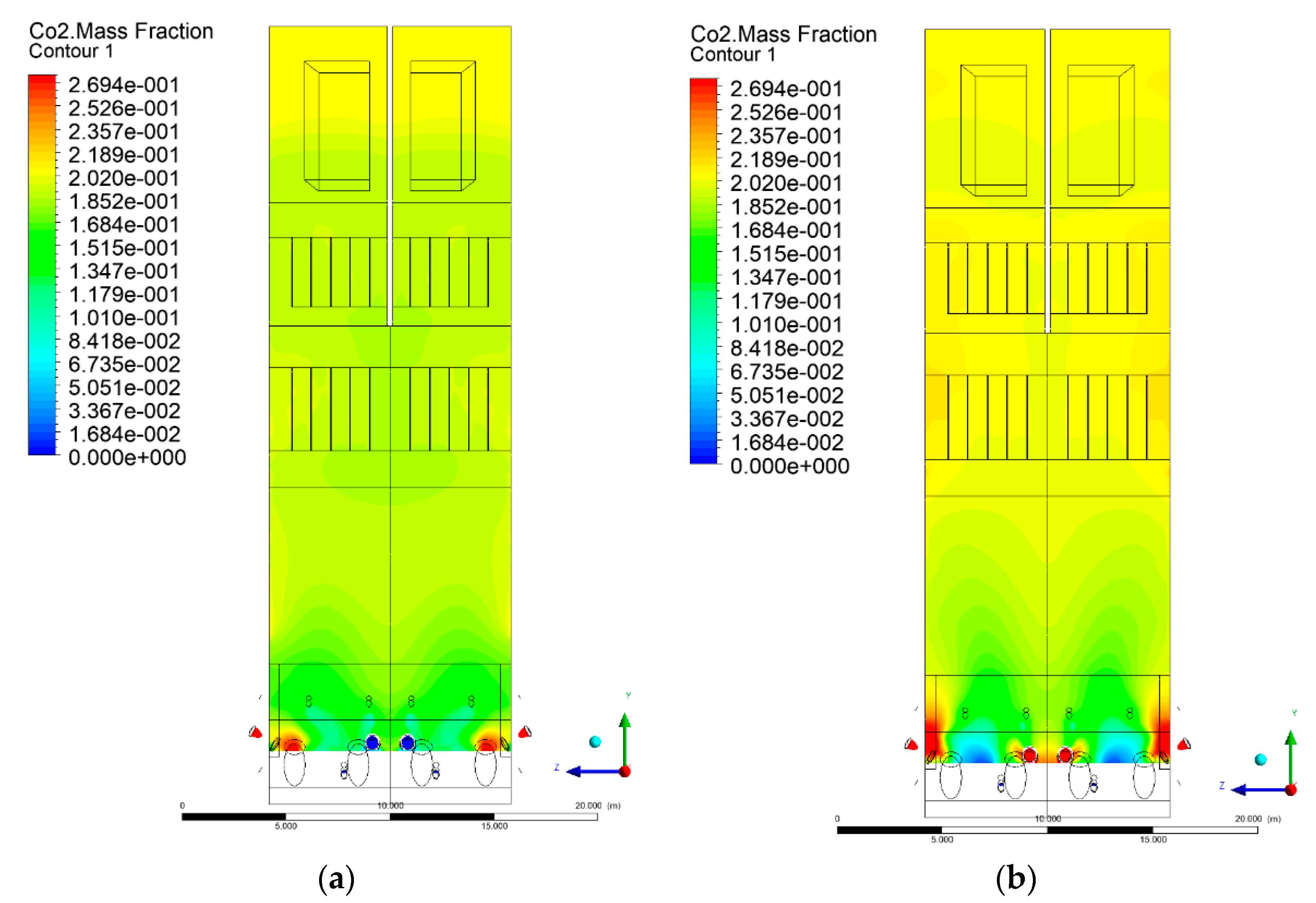
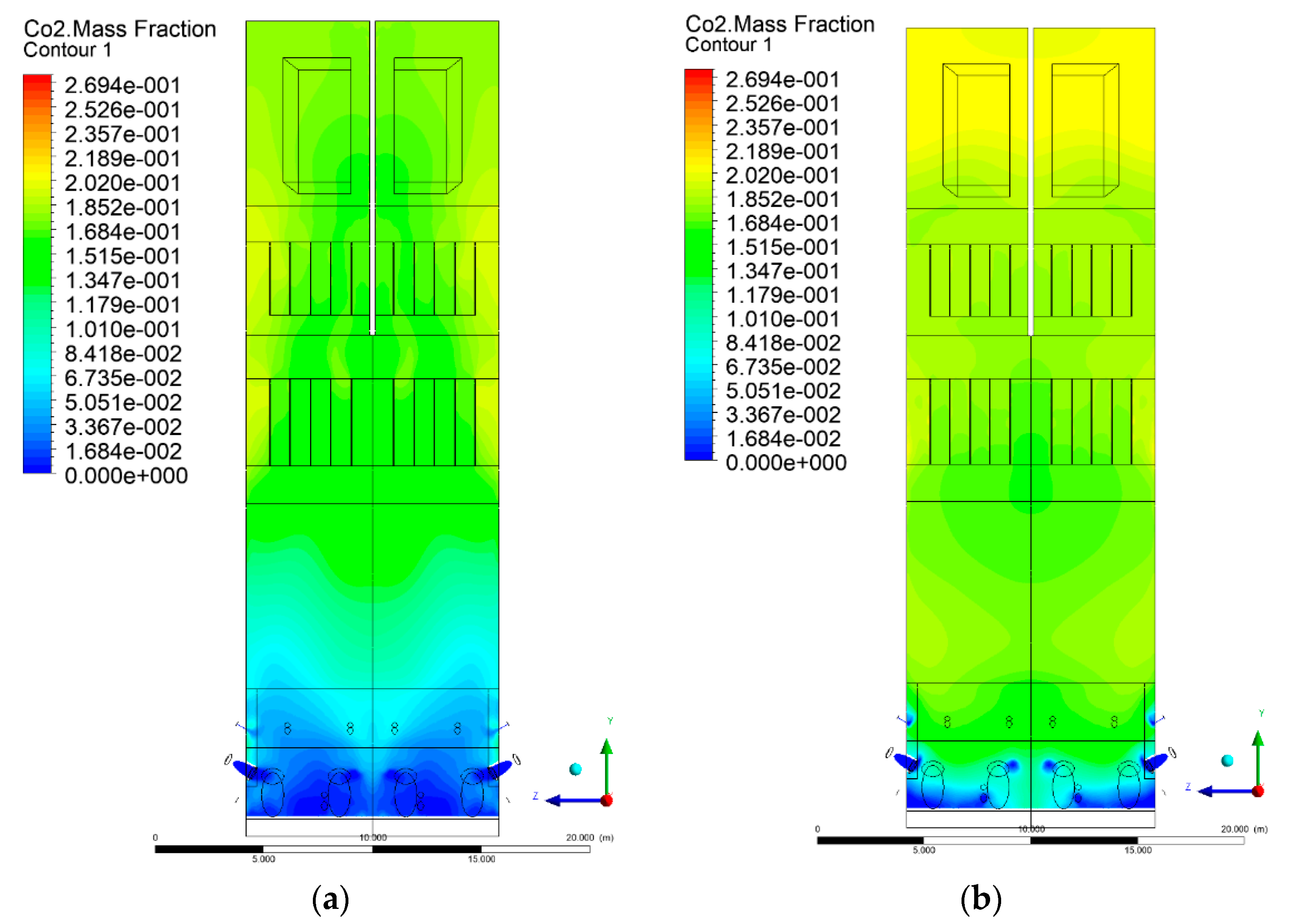
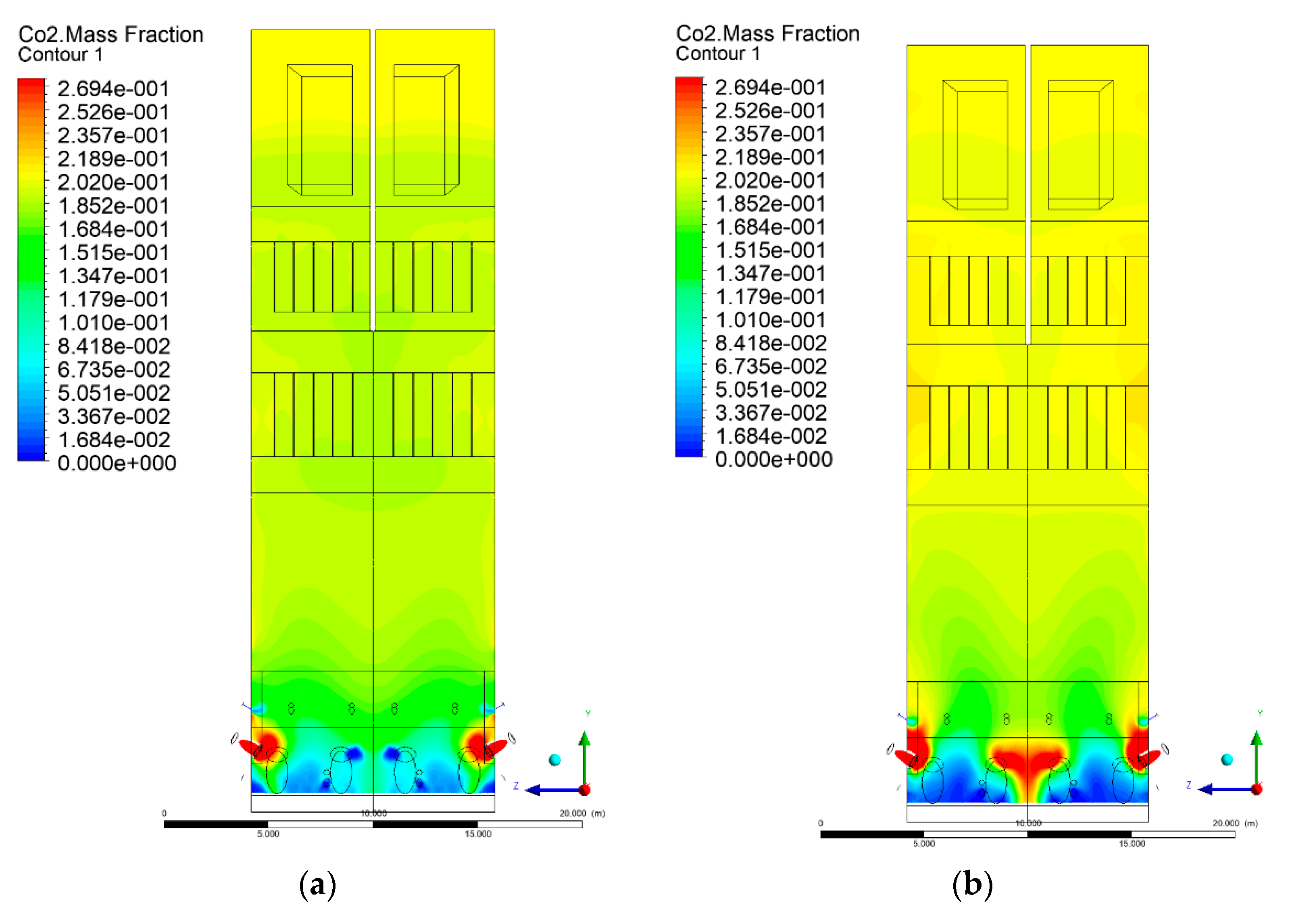
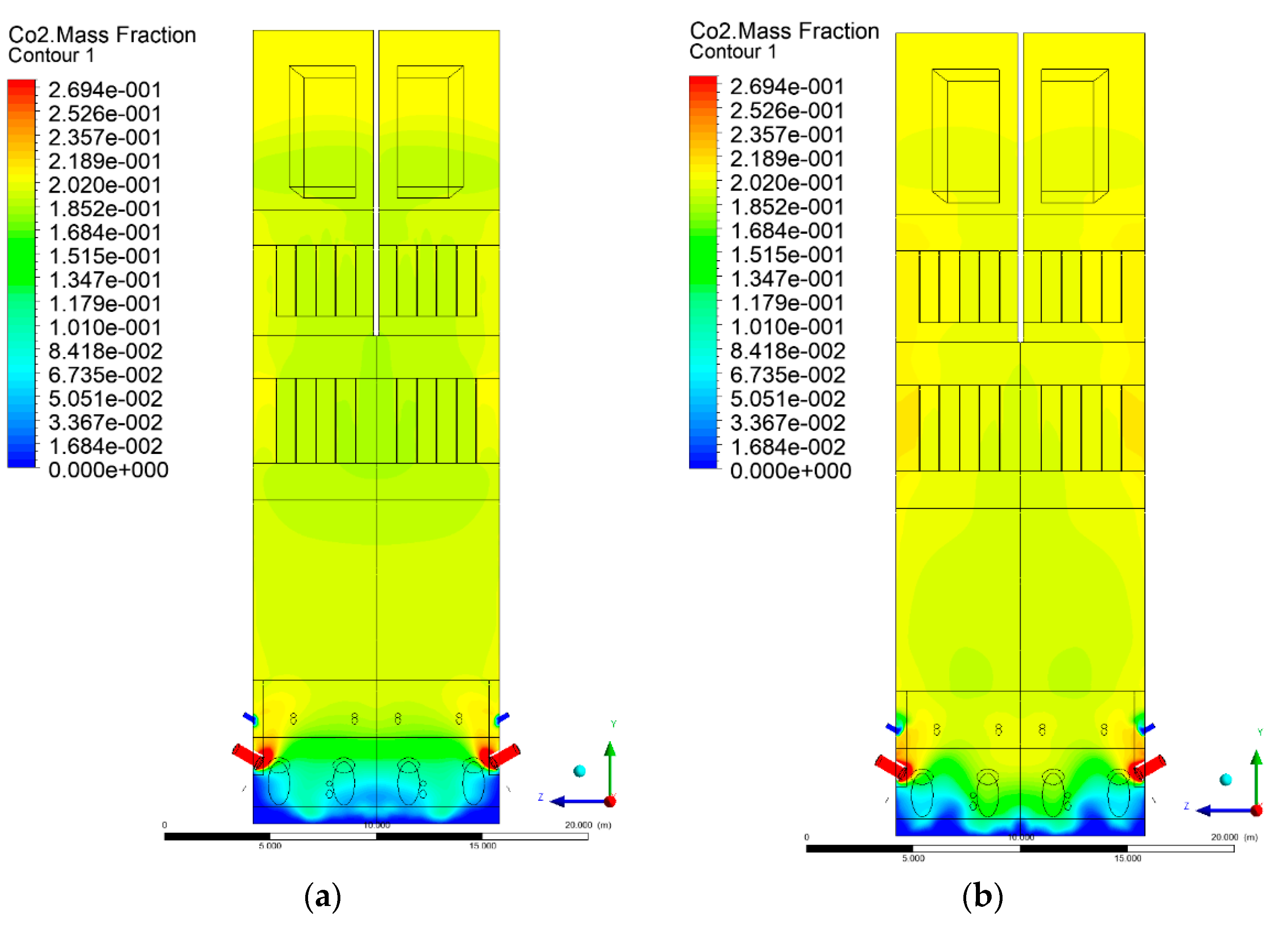
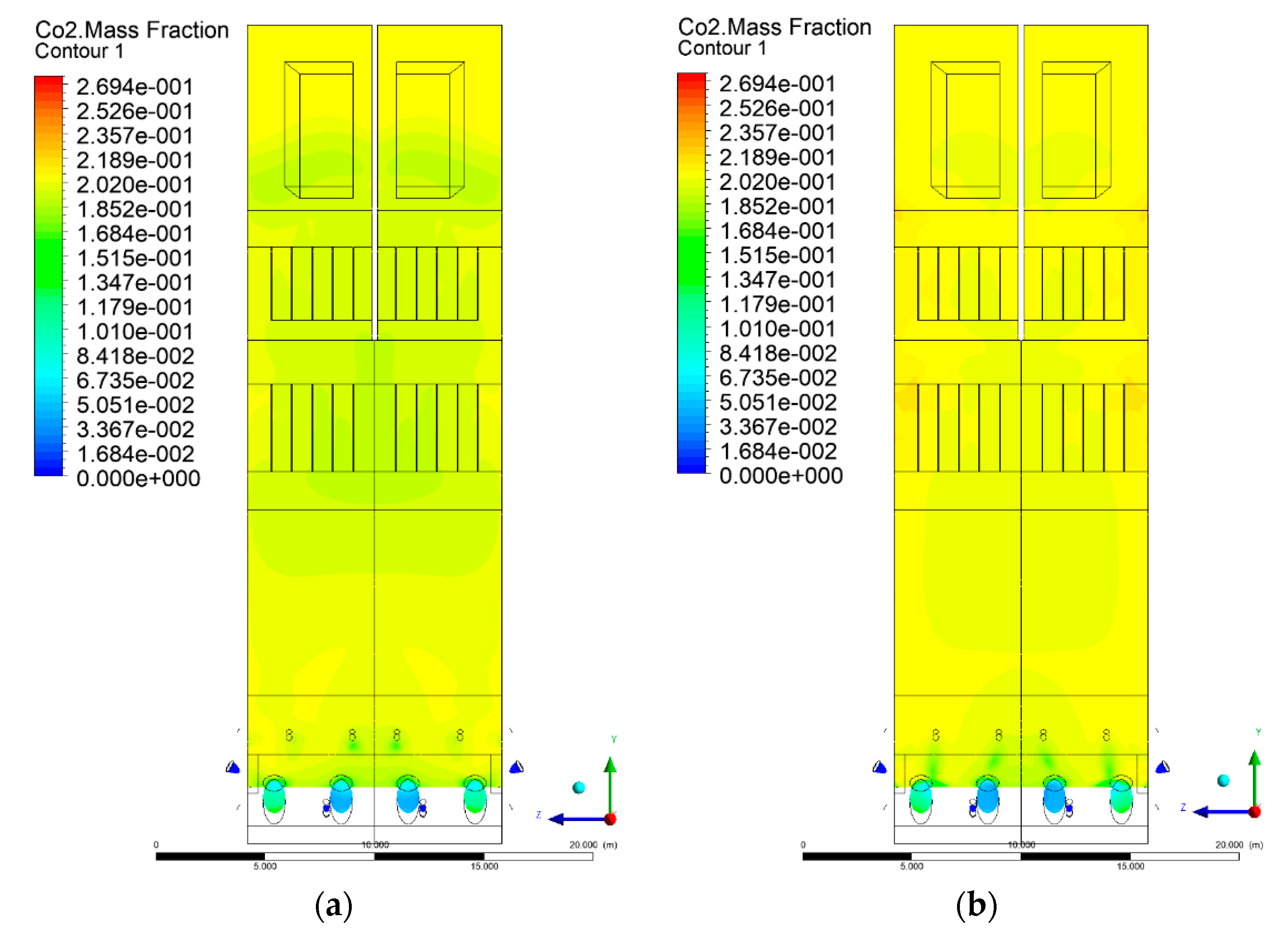
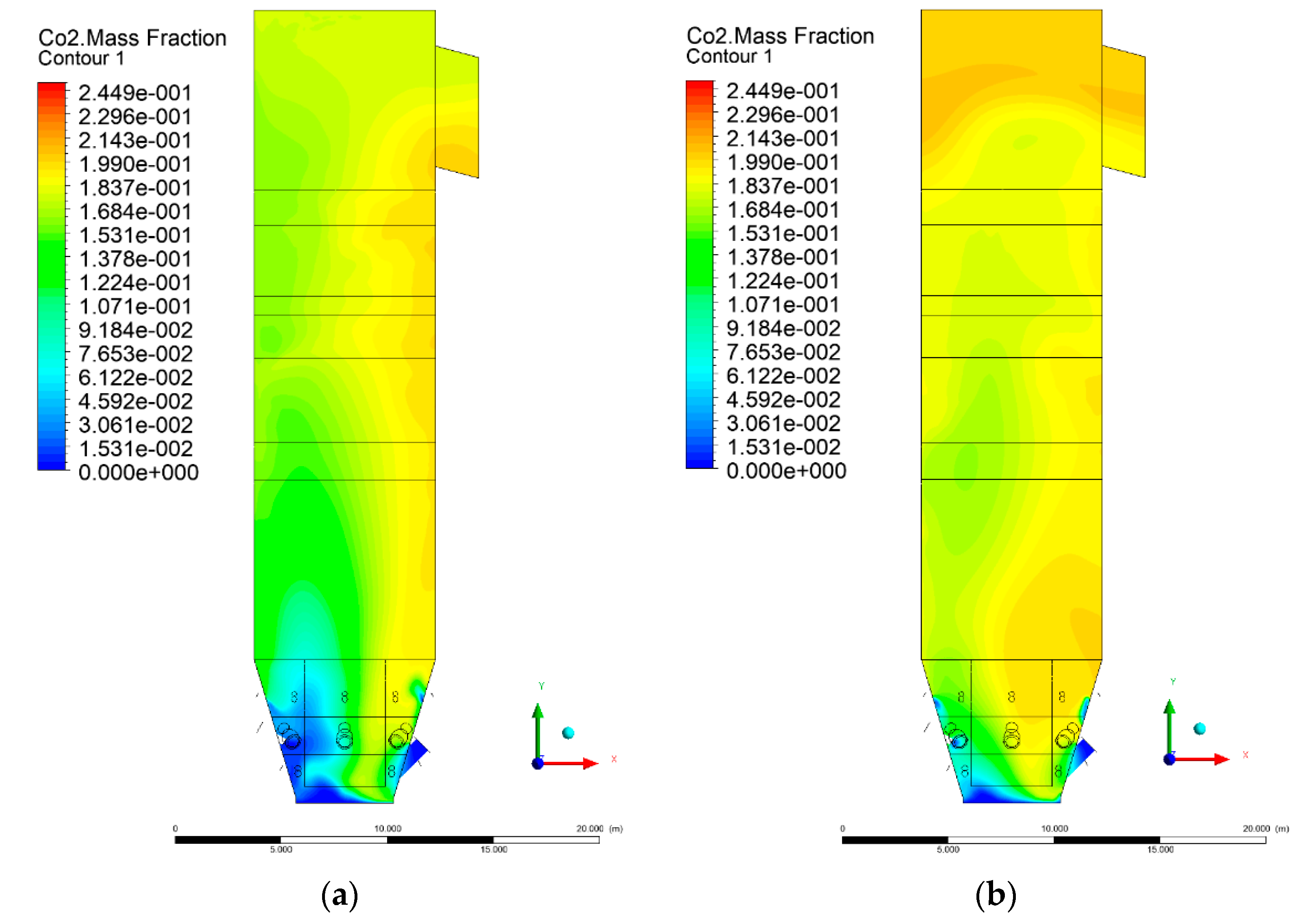
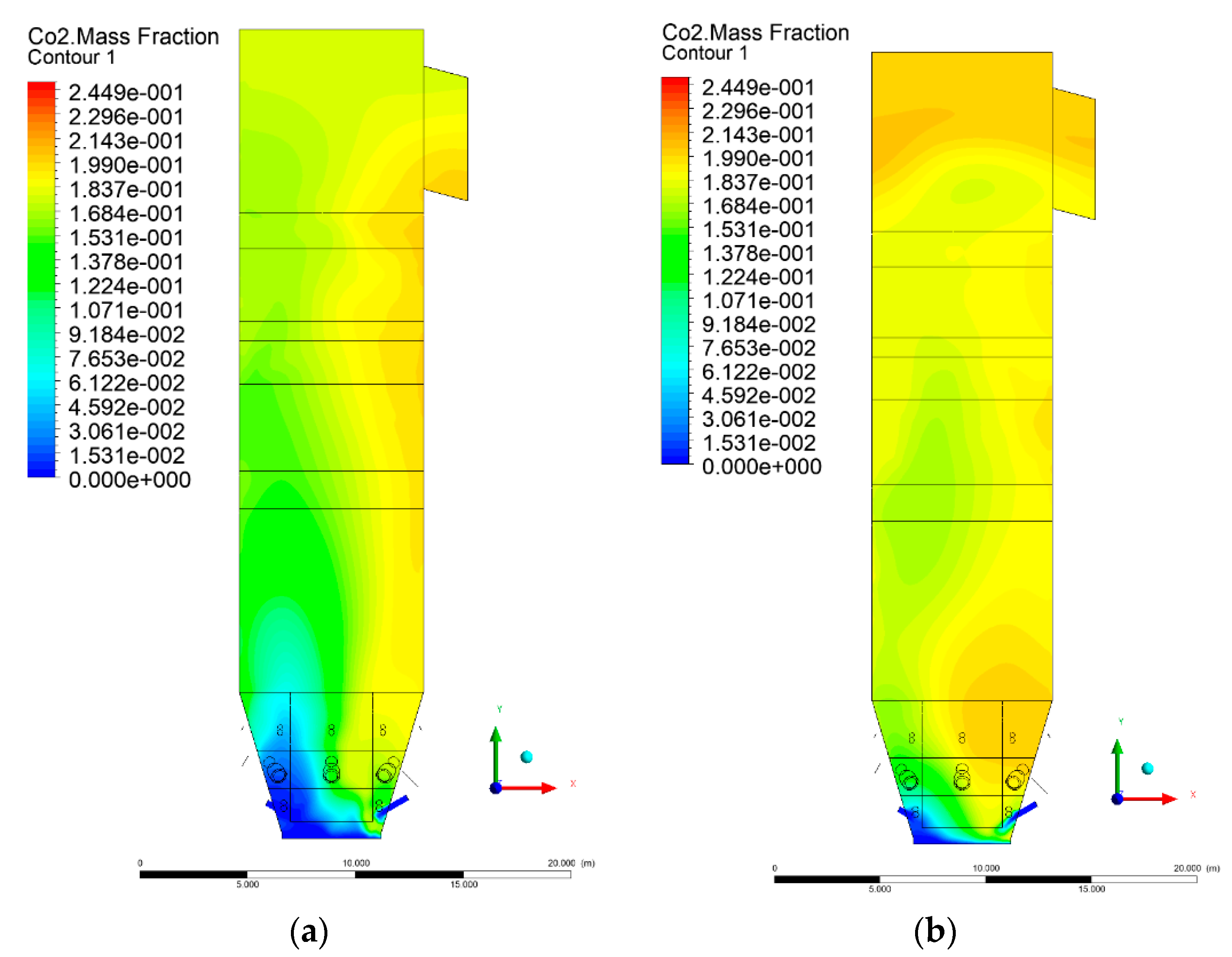
- Sectional view X-Y parallel to the model’s plane of symmetry, cut X-Y-1, 1.2 m away from the plane of symmetry, then subsequent cuts every 1 m,
- Sectional view Y-Z parallel to the front/rear wall of the boiler, cut Y-Z-1 1.26 m away from the front wall, then cuts arranged accordingly: 1.76 m; 4.26 m (at equal distance from the front and rear wall), 6.76 m and 7.26 m from the front wall.
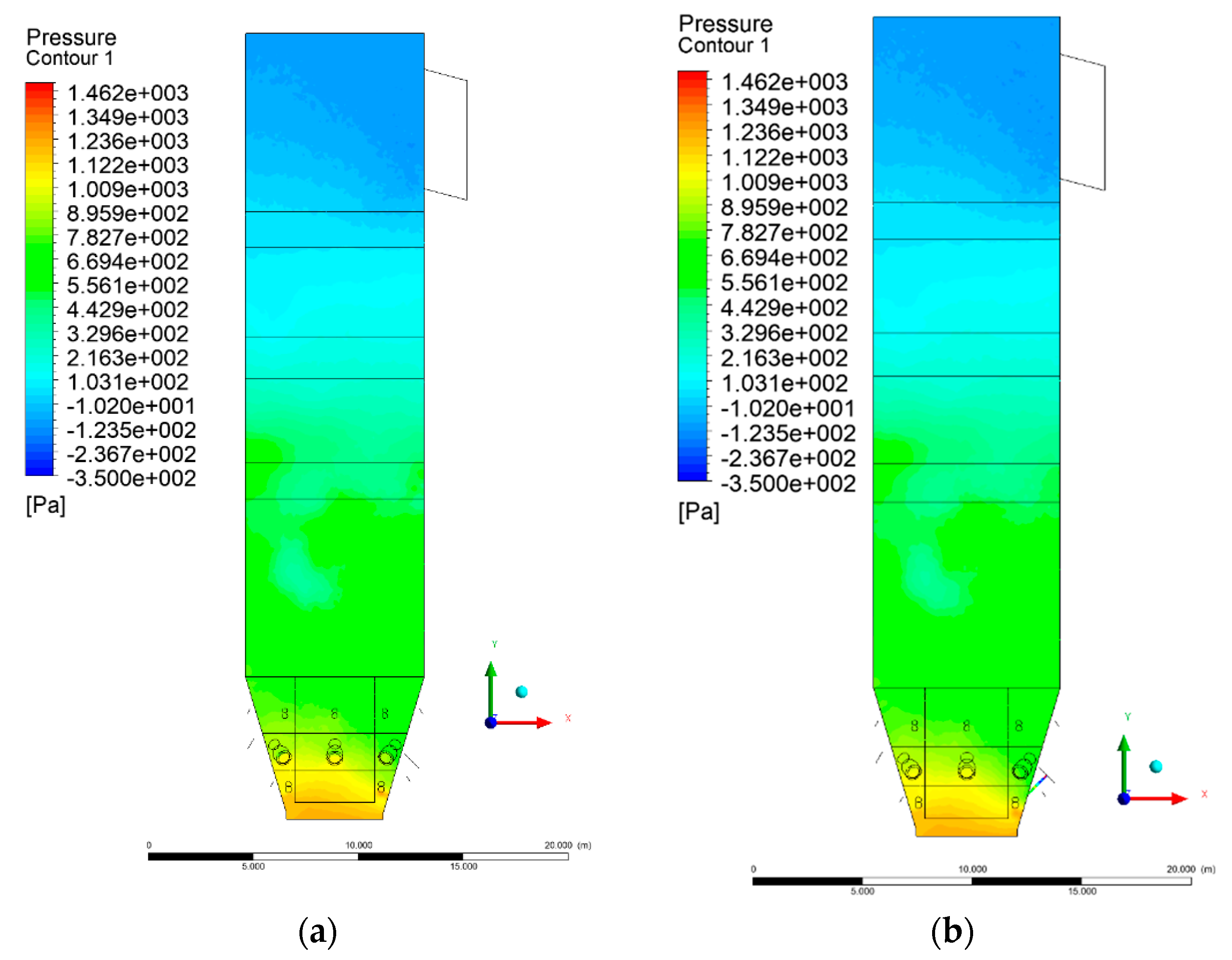
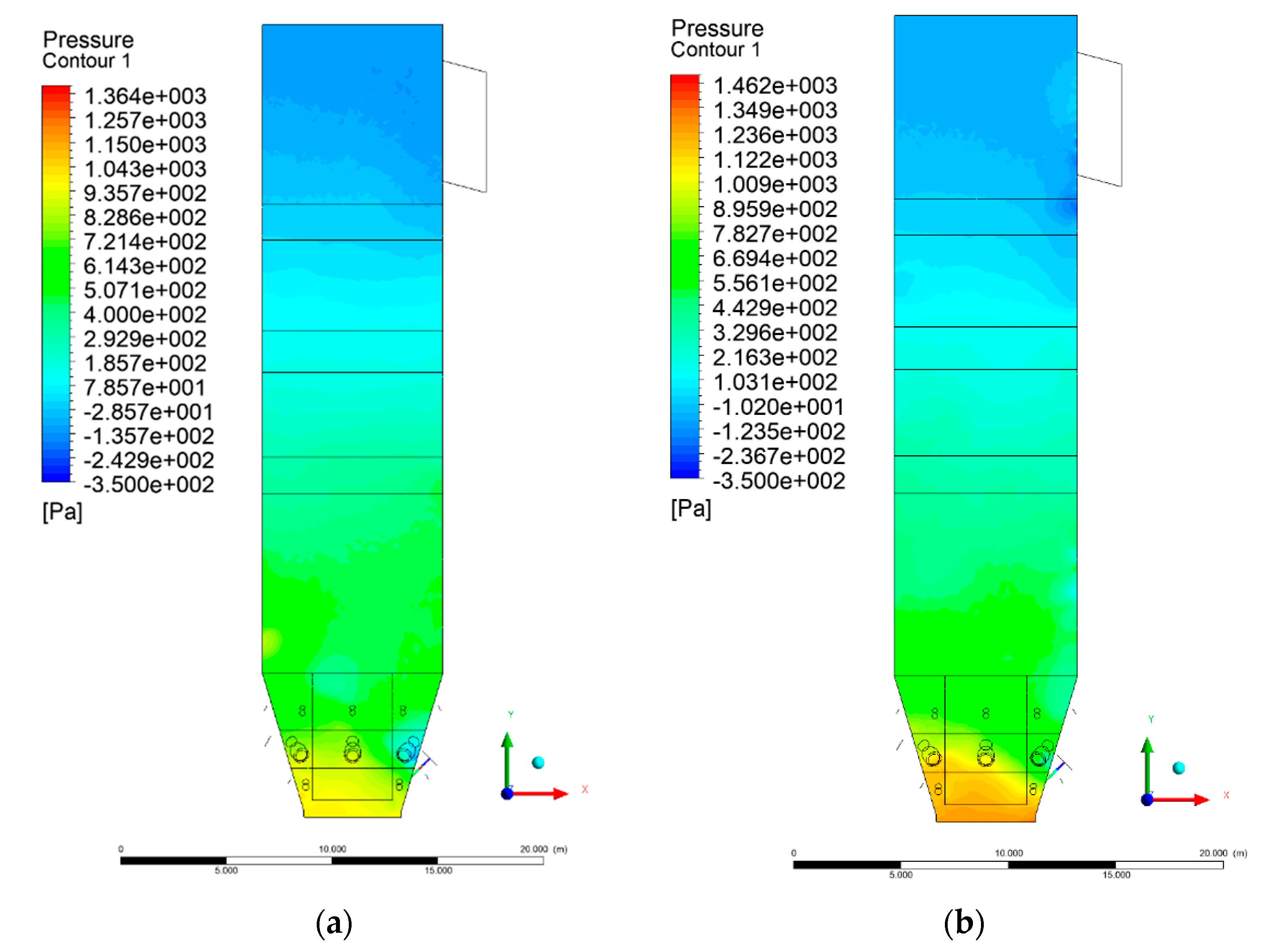
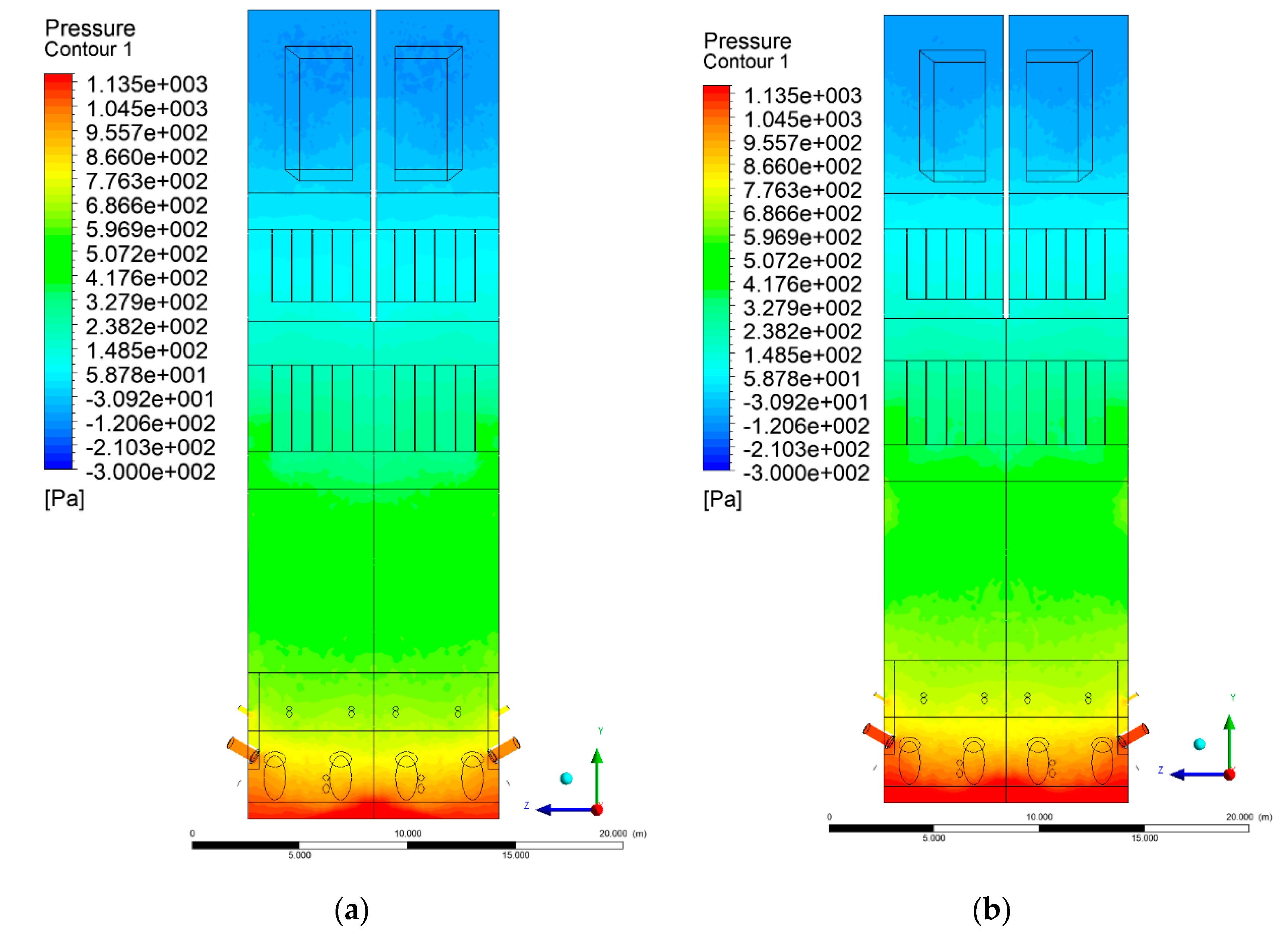
3.2. Pressure Distribution in the Combustion Chamber
3.3. Velocity Distribution in the Combustion Chamber
3.4. Combustion Chamber Temperature Distribution
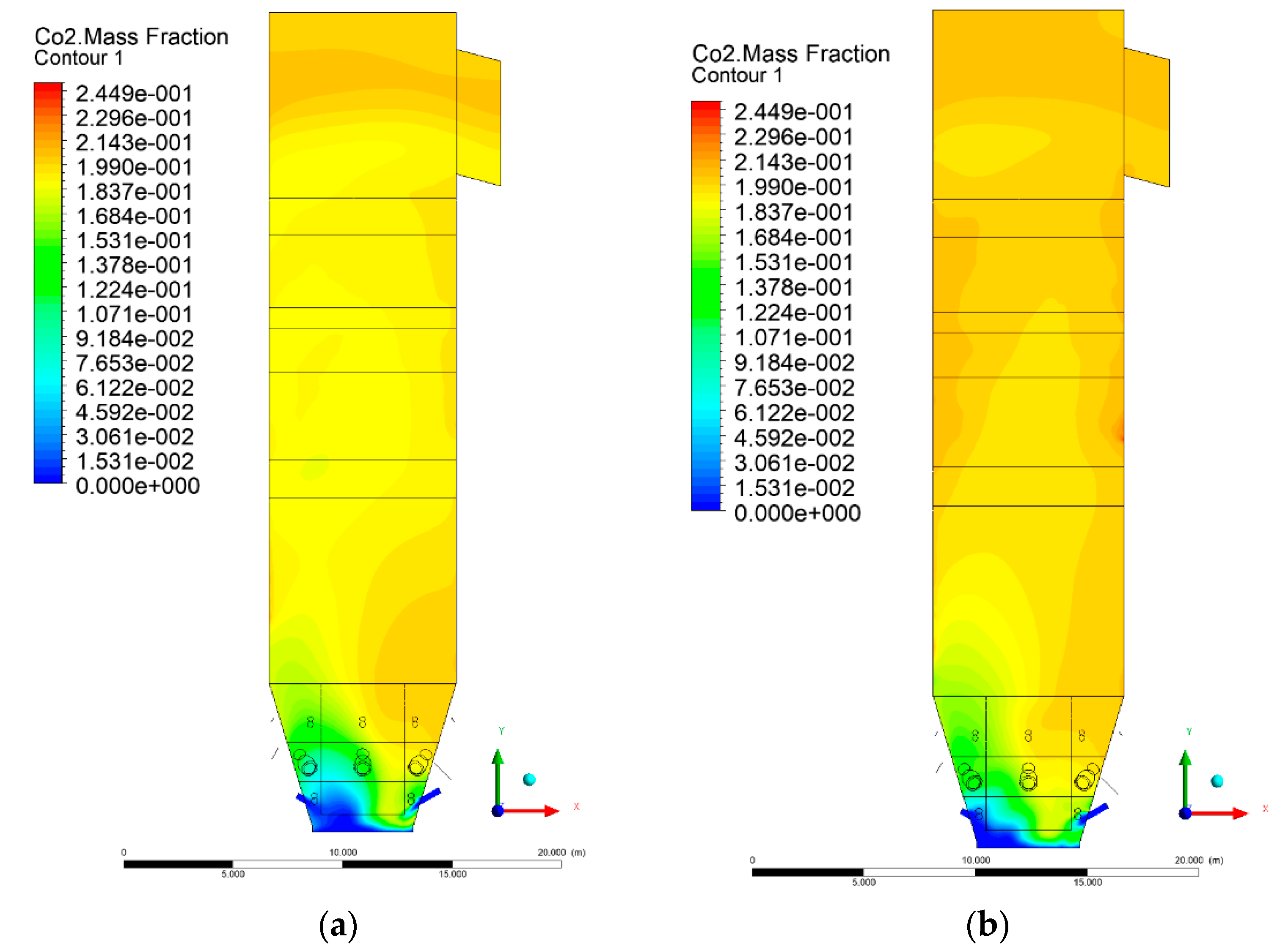
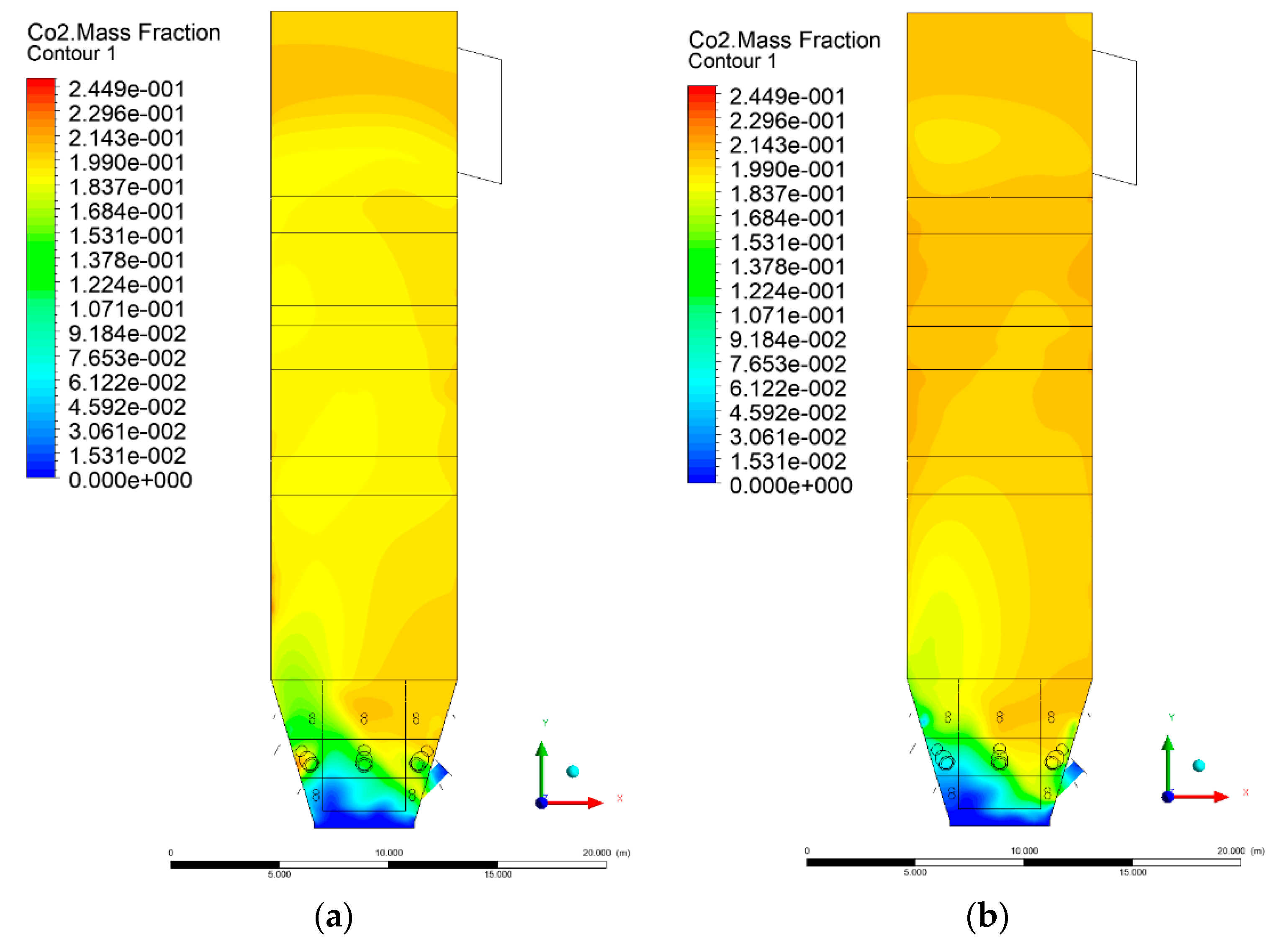
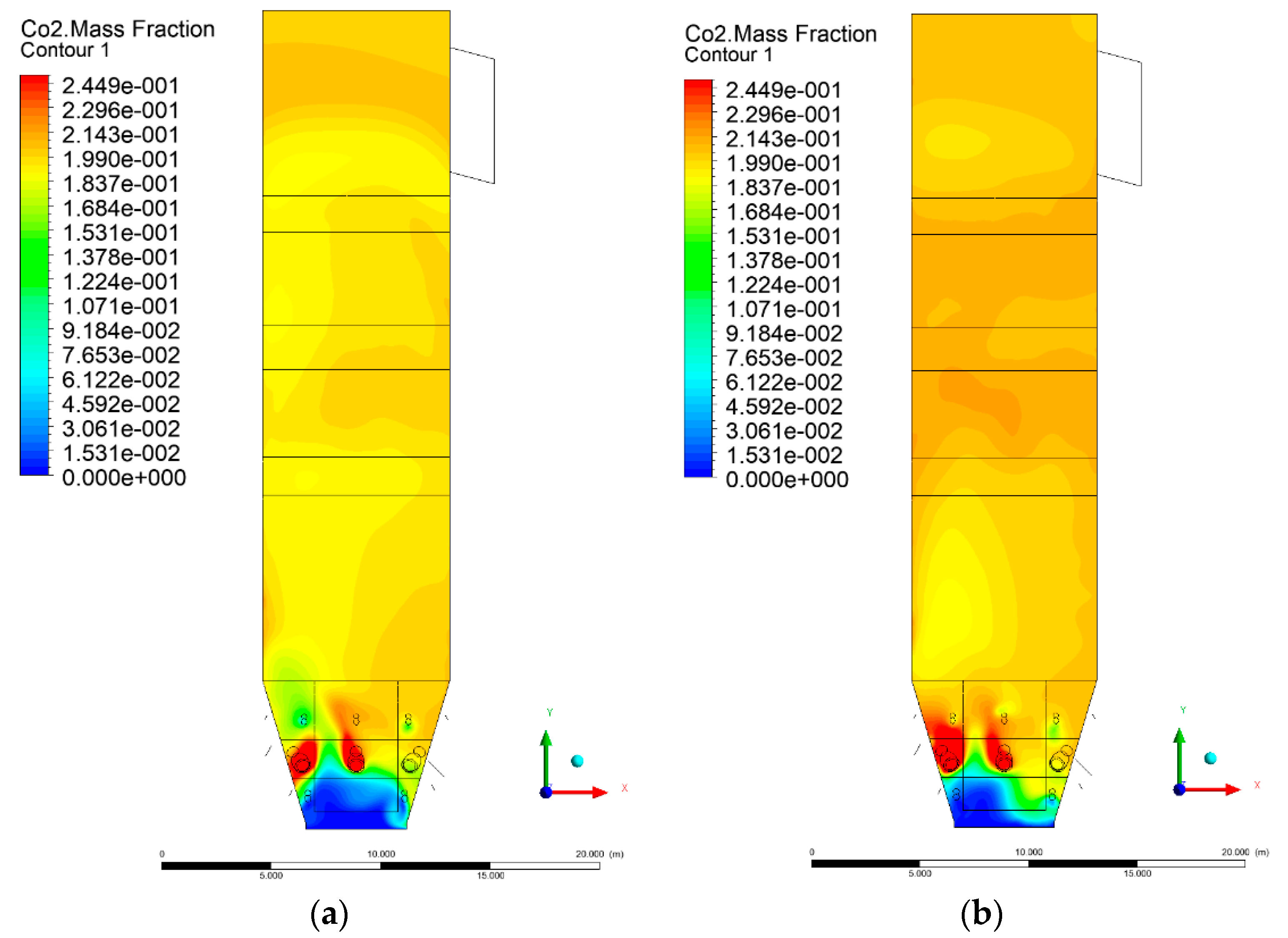
3.5. Combustion Chamber Carbon Dioxide Concentrations
4. Discussion and Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Cengel, Y.A.; Boles Michael, A. THERMODYNAMICS: An Engineering Approach, 8th ed; McGraw-Hill Education, 2 Penn Plaza: New York, NY, USA, 2015; p. 10121. [Google Scholar]
- Krzywanski, J. Heat Transfer Performance in a Superheater of an Industrial CFBC Using Fuzzy Logic-Based Methods. Entropy 2019, 21, 919. [Google Scholar] [CrossRef]
- Kotowicz, J.; Brzęczek, M. Comprehensive multivariable analysis of the possibility of an increase in the electrical efficiency of a modern combined cycle power plant with and without a CO2 capture and compression installations study. Energy 2019, 175, 1100–1120. [Google Scholar] [CrossRef]
- Kotowicz, J.; Brzęczek, M. Analysis of increasing efficiency of modern combined cycle power plant: A case study. Energy 2018, 153, 90–99. [Google Scholar] [CrossRef]
- Gungor, A.; Eskin, N. Two-dimensional coal combustion modeling of CFB. Int. J. Therm. Sci. 2008, 47, 157–174. [Google Scholar] [CrossRef]
- Hupa, M. Interaction of fuels in co-firing in FBC. Fuel 2005, 84, 1312–1319. [Google Scholar] [CrossRef]
- Yue, G.; Cai, R.; Lu, J.; Zhang, H. From a CFB reactor to a CFB boiler—The review of R&D progress of CFB coal combustion technology in China. Powder Technol. 2017, 316, 18–28. [Google Scholar] [CrossRef]
- Leckner, B. Co-combustion: A summary of technology. Therm. Sci. 2007, 11, 5–40. [Google Scholar] [CrossRef]
- Werther, J. Potentials of Biomass Co-Combustion in Coal-Fired Boilers. In Proceedings of the 20th International Conference on Fluidized Bed Combustion; Yue, G., Zhang, H., Zhao, C., Luo, Z., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 27–42. [Google Scholar]
- Wischnewski, R.; Ratschow, L.; Hartge, E.-U.; Werther, J. Reactive gas–solids flows in large volumes—3D modeling of industrial circulating fluidized bed combustors. Particuology 2010, 8, 67–77. [Google Scholar] [CrossRef]
- Knoebig, T.; Luecke, K.; Werther, J. Mixing and reaction in the circulating fluidized bed—A three-dimensional combustor model. Chem. Eng. Sci. 1999, 54, 2151–2160. [Google Scholar] [CrossRef]
- Xu, L.; Cheng, L.; Ji, J.; Wang, Q.; Fang, M. A comprehensive CFD combustion model for supercritical CFB boilers. Particuology 2019, 43, 29–37. [Google Scholar] [CrossRef]
- Wan, Z.; Yang, S.; Wei, Y.; Hu, J.; Wang, H. CFD modeling of the flow dynamics and gasification in the combustor and gasifier of a dual fluidized bed pilot plant. Energy 2020, 198, 117366. [Google Scholar] [CrossRef]
- Liu, Q.; Zhong, W.; Gu, J.; Yu, A. Three-dimensional simulation of the co-firing of coal and biomass in an oxy-fuel fluidized bed. Powder Technol. 2020, 373, 522–534. [Google Scholar] [CrossRef]
- Yan, J.; Lu, X.; Xue, R.; Lu, J.; Zheng, Y.; Zhang, Y.; Liu, Z. Validation and application of CPFD model in simulating gas-solid flow and combustion of a supercritical CFB boiler with improved inlet boundary conditions. Fuel Process. Technol. 2020, 208, 106512. [Google Scholar] [CrossRef]
- Arjunwadkar, A.; Basu, P.; Acharya, B. A review of some operation and maintenance issues of CFBC boilers. Appl. Therm. Eng. 2016, 102, 672–694. [Google Scholar] [CrossRef]
- Taler, J. Thermal and flow processes in large steam boilers. In Modeling and Monitoring; PWN: Warsaw, Poland, 2011. [Google Scholar]
- Bis, Z. Fluidized Bed Boilers. Theory and Practice. Czestochowa; University of Technology Press: Czestochowa, Poland, 2010; pp. 215–227. [Google Scholar]
- Kruczek, S. Boilers. In Constructions and Calculations; Wroclaw University of Technology: Wroclaw, Poland, 2001. [Google Scholar]
- Krzywanski, J.; Żyłka, A.; Czakiert, T.; Kulicki, K.; Jankowska, S.; Nowak, W. A 1.5D model of a complex geometry laboratory scale fuidized bed clc equipment. Powder Technol. 2017, 316, 592–598. [Google Scholar] [CrossRef]
- Muskała, W.; Krzywański, J.; Rajczyk, R.; Cecerko, M.; Kierzkowski, B.; Nowak, W.; Gajewski, W. Investigation of erosion in CFB boilers. Rynek Energii 2010, 87, 97–102. [Google Scholar]
- Krzywanski, J.; Wesolowska, M.; Blaszczuk, A.; Majchrzak, A.; Komorowski, M.; Nowak, W. Fuzzy logic and bed-to-wall heat transfer in a large-scale CFBC. Int. J. Numer. Methods Heat Fluid Flow 2018, 28, 254–266. [Google Scholar] [CrossRef]
- Sosnowski, M.; Krzywanski, J.; Scurek, R. A Fuzzy Logic Approach for the Reduction of MeshInduced Error in CFD Analysis: A Case Study of an Impinging Jet. Entropy 2019, 21, 1047. [Google Scholar] [CrossRef]
- Grabowska, K.; Sosnowski, M.; Krzywanski, J.; Sztekler, K.; Kalawa, W.; Zylka, A.; Nowak, W. The Numerical Comparison of Heat Transfer in a Coated and Fixed Bed of an Adsorption Chiller. J. Therm. Sci. 2018, 27, 421–426. [Google Scholar] [CrossRef]
- Versteeg, H.K.; Malalasekera, W. An Introduction to Computational Fluid Dynamics: The Finite Volume Method; Pearson Education: London, UK, 2007; ISBN 978-0-13-127498-3. [Google Scholar]
- ANSYS, Inc. Ansys Fluent Theory Guide; ANSYS Inc.: Canonsburg, PA, USA, 2013. [Google Scholar]
- Zhou, W.; Zhao, C.; Duan, L.; Chen, X.; Liu, D. A simulation study of coal combustion under O2/CO2 and O2/RFG atmospheres in circulating fluidized bed. Chem. Eng. J. 2013, 223, 816–823. [Google Scholar] [CrossRef]
- Muskała, W.; Krzywański, J.; Czakiert, T.; Nowak, W. The research of CFB boiler operation for oxygen-enhanced dried lignite combustion. Rynek Energii 2011, 1, 172–176. [Google Scholar]
- Muskała, W.; Krzywański, J.; Sekret, R.; Nowak, W. Model research of coal combustion in circulating fluidized bed boilers. Chem. Process Eng. 2008, 29, 473–492. [Google Scholar]
- Basu, P. Circulating Fluidized Bed Boilers: Design, Operation and Maintenance; Springer: Berlin/Heidelberger, Germany, 2015; ISBN 978-3-319-06172-6. [Google Scholar]
- Basu, P. Combustion and Gasification in Fluidized Beds; CRC Press: New York, NY, USA, 2006; ISBN 978-1-4200-0515-8. [Google Scholar]
- Chen, Z.; Lin, M.; Ignowski, J.; Kelly, B.; Linjewile, T.M.; Agarwal, P.K. Mathematical modeling of fluidized bed combustion. 4: N2O and NOX emissions from the combustion of char. Fuel 2001, 80, 1259–1272. [Google Scholar] [CrossRef]
- Krzywanski, J.; Czakiert, T.; Shimizu, T.; Majchrzak-Kuceba, I.; Shimazaki, Y.; Zylka, A.; Grabowska, K.; Sosnowski, M. NOx Emissions from Regenerator of Calcium Looping Process. Energy Fuels 2018, 32, 6355–6362. [Google Scholar] [CrossRef]
- Miller, J.A.; Bowman, C.T. Mechanism and modeling of nitrogen chemistry in combustion. Prog. Energy Combust. Sci. 1989, 15, 287–338. [Google Scholar] [CrossRef]
- Hayhurst, A.N.; Lawrence, A.D. The amounts of NOx and N2O formed in a fluidized bed combustor during the burning of coal volatiles and also of char. Combust. Flame 1996, 105, 341–357. [Google Scholar] [CrossRef]
- Klajny, T.; Krzywanski, J.; Nowak, W. Mechanism and kinetics of coal combustion in oxygen enhanced conditions. In Proceedings of the 6th Internationa Symposium on Coal Combustion. Huazhong University of Science and Technology, Wuhan, China, 1–4 December 2007; pp. 148–153. [Google Scholar]
- Lehtonen, P.; Stroemdahl, J. CFB. Multi-fuel design features and operating experience ETDEWEB. VGB PowerTech 2012, 92, 40. [Google Scholar]
- Yang, W.-C. Handbook of Fluidization and Fluid-Particle Systems; CRC Press: Basel, Switzerland, 2003; ISBN 978-0-8247-4836-4. [Google Scholar]
- Breitholtz, C.; Leckner, B.; Baskakov, A.P. Wall average heat transfer in CFB boilers. Powder Technol. 2001, 120, 41–48. [Google Scholar] [CrossRef]
- Krzywanski, J. A General Approach in Optimization of Heat Exchangers by Bio-Inspired Artificial Intelligence Methods. Energies 2019, 12, 4441. [Google Scholar] [CrossRef]
- Mehmet, K.; Cengel, Y.A.; Cimbala, J.M. Fundamentals and Applications of Renewable Energy; Mac Graw Hill: New York, USA, 2019. [Google Scholar]
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction. Practical Design and Theory; II; Elsevier: San Diego, CA, USA, 2013. [Google Scholar]
- Grabowska, K.; Krzywanski, J.; Nowak, W.; Wesolowska, M. Construction of an innovative adsorbent bed configuration in the adsorption chiller—Selection criteria for effective sorbent-glue pair. Energy 2018, 151, 317–323. [Google Scholar] [CrossRef]















| Height above the Grid m | Measured Temperature °C | Calculated Temperature °C | Relative Error % |
|---|---|---|---|
| 0.2 | 834.1 | 859.1 | −3.0 |
| 1.2 | 817.3 | 830.2 | −1.6 |
| 6.0 | 779.2 | 795.9 | −2.1 |
| Height above the Grid m | Measured Pressure kPa | Calculated Pressure kPa | Relative Error % |
|---|---|---|---|
| 0.2 | 7.03 | 7.38 | −4.99 |
| 1.2 | 2.57 | 2.70 | −5.06 |
| 6.0 | 0.32 | 0.34 | −6.25 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzywanski, J.; Sztekler, K.; Szubel, M.; Siwek, T.; Nowak, W.; Mika, Ł. A Comprehensive, Three-Dimensional Analysis of a Large-Scale, Multi-Fuel, CFB Boiler Burning Coal and Syngas. Part 2. Numerical Simulations of Coal and Syngas Co-Combustion. Entropy 2020, 22, 856. https://doi.org/10.3390/e22080856
Krzywanski J, Sztekler K, Szubel M, Siwek T, Nowak W, Mika Ł. A Comprehensive, Three-Dimensional Analysis of a Large-Scale, Multi-Fuel, CFB Boiler Burning Coal and Syngas. Part 2. Numerical Simulations of Coal and Syngas Co-Combustion. Entropy. 2020; 22(8):856. https://doi.org/10.3390/e22080856
Chicago/Turabian StyleKrzywanski, Jaroslaw, Karol Sztekler, Mateusz Szubel, Tomasz Siwek, Wojciech Nowak, and Łukasz Mika. 2020. "A Comprehensive, Three-Dimensional Analysis of a Large-Scale, Multi-Fuel, CFB Boiler Burning Coal and Syngas. Part 2. Numerical Simulations of Coal and Syngas Co-Combustion" Entropy 22, no. 8: 856. https://doi.org/10.3390/e22080856
APA StyleKrzywanski, J., Sztekler, K., Szubel, M., Siwek, T., Nowak, W., & Mika, Ł. (2020). A Comprehensive, Three-Dimensional Analysis of a Large-Scale, Multi-Fuel, CFB Boiler Burning Coal and Syngas. Part 2. Numerical Simulations of Coal and Syngas Co-Combustion. Entropy, 22(8), 856. https://doi.org/10.3390/e22080856







