Comparative Analysis of Energy and Exergy Performance of Hydrogen Production Methods
Abstract
1. Introduction
- 1)
- The combustion of hydrogen results in the formation of steam and liquid water. In this respect, the use of hydrogen is practically safe from an environmental standpoint, compared to other combustion processes.
- 2)
- It is nontoxic.
- 3)
- It is easily assimilated into the biosphere; its combustion products are recycled by plants in the form of carbohydrates.
- 4)
- Hydrogen may be produced from the most abundant chemical on earth: water. Hydrogen can be obtained electrolytically, photoelectrochemically, thermochemically, by direct thermal decomposition, or biochemically from water.
2. Materials and Methods
3. Results and Discussion
3.1. Fuel Conversion and Hydrogen (or Syngas) Production
3.2. Energy Analysis
3.3. Exergy Analysis
3.4. Carbon Capture and Sequestration/Storage (CCS)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| Energy efficiency, % | |
| Mass flow, kg s−1 | |
| Lower heating value, MJ kg−1 | |
| Heat of reaction, MJ s−1 | |
| Exergy efficiency, % | |
| Exergy, kJ mol−1 | |
| Pressure, bar | |
| Temperature, K | |
| Kinetic constant, s−1 | |
| Initial molar flow fuel, kmol h−1 |
Abbreviations
| GHG | Greenhouse Gas |
| IPCC | Intergovernmental Panel on Climate Change |
| NGP | Natural Gas Pyrolysis |
| DRM | Dry Reforming of Methane |
| SMR | Steam Reforming of Methane |
| POM | Partial Oxidation of Methane |
| CG | Coal Gasification |
| ATM | Auto-thermal Reforming of Methane |
| WE | Water Electrolysis |
| WGS | Water-Gas Shift |
| PrOx | Preferential Oxidation |
| RWGS | Reverse Water-Gas Shift |
| LHV | Lower Heating Value |
| CCS | Carbon Capture and Sequestration |
References
- Birol, F.; Cozzi, L.; Bromhead, A.; Gould, T.; Baroni, M. World Energy Outlook 2014; International Energy Agency: Paris, France, 2014; p. 726. [Google Scholar]
- International Energy Agency. World Energy Outlook Special Report; International Energy Agency: Paris, France, 2015. [Google Scholar]
- Stocker, T.F.; Qin, D.; Plattner, G.K.; Tignor, M.M.B.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. Climate change 2013 the physical science basis: Working Group I contribution to the fifth assessment report of the intergovernmental panel on climate change. Clim. Chang. 2013 Phys. Sci. Basis Work. Gr. I Contrib. Fifth Assess. Rep. Intergov Panel Clim. Chang. 2013, 9781107057, 1–1535. [Google Scholar]
- Marbán, G.; Valdés-Solís, T. Towards the Hydrogen Economy? Int. J. Hydrogen Energy 2007, 32, 1625–1637. [Google Scholar] [CrossRef]
- Samanta, S.K.; Verma, P. Advanced Hydrogen Production through Methane. Discov. J. 2015, 1, 109–123. [Google Scholar]
- Liu, B.; Liu, S.; Guo, S.; Zhang, S. Economic study of a large-scale renewable hydrogen application utilizing surplus renewable energy and natural gas pipeline transportation in China. Int. J. Hydrogen Energy 2020, 45, 1385–1398. [Google Scholar] [CrossRef]
- Bellocchi, S.; De Falco, M.; Gambini, M.; Manno, M.; Stilo, T.; Vellini, M. Opportunities for power-to-Gas and Power-to-liquid in CO2-reduced energy scenarios: The Italian case. Energy 2019, 175, 847–861. [Google Scholar] [CrossRef]
- Götz, M.; Lefebvre, J.; Mörs, F.; McDaniel Koch, A.; Graf, F.; Bajohr, S.; Reimert, R.; Kolb, T. Renewable Power-to-Gas: A technological and economic review. Renew. Energy 2016, 85, 1371–1390. [Google Scholar] [CrossRef]
- ADEME. Technical Review: The Role of Hydrogen in the Energy Transition; ADEME: Angers, France, 2018; Volume 2018. [Google Scholar]
- Boudries, R.; Dizène, R.; Khellaf, A.; Belhamel, M. Hydrogen as an energy carrier. In Clean Energy: Resources, Production and Developments; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2012; pp. 147–184. ISBN 9781617615092. [Google Scholar]
- Stolten, D.; Emonts, B. Hydrogen Science and Engineering: Materials, Processes, Systems and Technology; Wiley-VCH Verlag: Weinheim, Germany, 2016; Volume 1–2, ISBN 9783527674268. [Google Scholar]
- Nazir, S.M.; Cloete, J.H.; Cloete, S.; Amini, S. Efficient hydrogen production with CO2 capture using gas switching reforming. Energy 2019, 185, 372–385. [Google Scholar] [CrossRef]
- Ogden, J.M. Prospects for building a hydrogen energy infrastructure. Annu. Rev. Energy Environ. 1999, 24, 227–279. [Google Scholar] [CrossRef]
- Kalamaras, C.M.; Efstathiou, A.M. Hydrogen Production Technologies: Current State and Future Developments. Conf. Pap. Energy 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Stadler, P.; Caliandro, P.; Leonardi, C.; Van Herle, J. Cost Evaluation of Large Scale Hydrogen Production for the Aviation Industry; Master Semester Project; École Polytechnique Fédérale de Lausanne: Lausanne, Switzerland, 2014. [Google Scholar]
- Dincer, I.; Acar, C. Review and evaluation of hydrogen production methods for better sustainability. Int. J. Hydrogen Energy 2014, 40, 11094–11111. [Google Scholar] [CrossRef]
- El-Shafie, M.; Kambara, S.; Hayakawa, Y. Hydrogen Production Technologies Overview. J. Power Energy Eng. 2019, 7, 107–154. [Google Scholar] [CrossRef]
- Bhat, S.A.; Sadhukhan, J. Process intensification aspects for steam methane reforming: An overview. AIChE J. 2009, 55, 408–422. [Google Scholar] [CrossRef]
- Maqbool, W.; Park, S.J.; Lee, E.S. Steam methane reforming of natural gas with substantial carbon dioxide contents—Process optimization for gas-to-liquid applications. In Proceedings of the Applied Mechanics and Materials; Trans Tech Publications: Stafa-Zurich, Switzerland, 2014; Volume 548–549, pp. 316–320. [Google Scholar]
- Corbo, P.; Migliardini, F. Hydrogen production by catalytic partial oxidation of methane and propane on Ni and Pt catalysts. Int. J. Hydrogen Energy 2007, 32, 55–66. [Google Scholar] [CrossRef]
- Lin, S.; Harada, M.; Suzuki, Y.; Hatano, H. Hydrogen production from coal by separating carbon dioxide during gasification. Fuel 2002, 81, 2079–2085. [Google Scholar] [CrossRef]
- De Castro, J.; Rivera-Tinoco, R.; Bouallou, C. Hydrogen production from natural gas: Auto-Thermal Reforming and CO2 capture. In Proceedings of the Chemical Engineering Transactions; Italian Association of Chemical Engineering—AIDIC: Milano, Italy, 2010; Volume 21, pp. 163–168. [Google Scholar]
- Shiva Kumar, S.; Himabindu, V. Hydrogen production by PEM water electrolysis—A review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar] [CrossRef]
- Guéret, C.; Daroux, M.; Billaud, F. Methane pyrolysis: Thermodynamics. Chem. Eng. Sci. 1997, 52, 815–827. [Google Scholar] [CrossRef]
- Buelens, L.C.; Galvita, V.V.; Poelman, H.; Detavernier, C.; Marin, G.B. Super-dry reforming of methane intensifies CO2 utilization via le Chatelier’s principle. Science 2016, 354, 449–452. [Google Scholar] [CrossRef]
- Summa, P.; Samojeden, B.; Motak, M. Dry and steam reforming of methane. Comparison and analysis of recently investigated catalytic materials. A short review. Polish J. Chem. Technol. 2019, 21, 31–37. [Google Scholar] [CrossRef]
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An overview of hydrogen production technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.; Shah, S.A.A.; Zameer, H.; Solangi, Y.A.; Walasai, G.D.; Siyal, Z.A. Economic Viability and Environmental Efficiency Analysis of Hydrogen Production Processes for the Decarbonization of Energy Systems. Processes 2019, 7, 494. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C. Energy and exergy analysis of hydrogen production by a proton exchange membrane (PEM) electrolyzer plant. Energy Convers. Manag. 2008, 49, 2748–2756. [Google Scholar] [CrossRef]
- Kurokawa, H.; Shirasaki, Y.; Yasuda, I. Energy-efficient distributed carbon capture in hydrogen production from natural gas. In Proceedings of the Energy Procedia; Elsevier Ltd.: Amsterdam, The Netherlands, 2011; Volume 4, pp. 674–680. [Google Scholar]
- F-Chart-Software EES: Engineering Equation Solver | F-Chart Software: Engineering Software. Available online: http://www.fchart.com/ees/ees-refprop.php (accessed on 3 November 2020).
- Chein, R.Y.; Hsu, W.H. Analysis of syngas production from biogas via the tri-reforming process. Energies 2018, 11, 1075. [Google Scholar] [CrossRef]
- Wang, Z.; Fan, W.; Zhang, G. Comparison of the exergy efficiency of four power generation systems from methane using fuel cells. RSC Adv. 2017, 7, 39391–39402. [Google Scholar] [CrossRef]
- Simpson, A.P.; Lutz, A.E. Exergy analysis of hydrogen production via steam methane reforming. Int. J. Hydrogen Energy 2007, 32, 4811–4820. [Google Scholar] [CrossRef]
- Modeiros, D. DWSIM Open Source Process Simulator. Available online: http://dwsim.inforside.com.br/wiki/index.php?title=DWSIM (accessed on 24 March 2020).
- Benguerba, Y.; Virginie, M.; Dumas, C.; Ernst, B. Methane dry reforming over Ni-Co/Al2O3: Kinetic modelling in a catalytic fixed-bed reactor. Int. J. Chem. React. Eng. 2017, 15, 15. [Google Scholar] [CrossRef]
- Keith, J.M. Hydrogen Education Curriculum Path at Michigan Technological University; MichiganTech: Houghton, MI, USA, 2010. [Google Scholar]
- Hoang, D.L.; Chan, S.H.; Ding, O.L. Kinetic modelling of partial oxidation of methane in an oxygen permeable membrane reactor. Chem. Eng. Res. Des. 2005, 83, 177–186. [Google Scholar] [CrossRef]
- Fernandes, F.A.N.; Sousa, J.F.; Souza, C.P.; Rodrigues, S. Modeling of Partial Oxidation of Methane in a Membrane Reactor. Rev. Eng. Térmica 2018, 5, 40–45. [Google Scholar] [CrossRef]
- Zeng, K.; Zhang, D. Recent progress in alkaline water electrolysis for hydrogen production and applications. Prog. Energy Combust. Sci. 2009, 36, 307–326. [Google Scholar] [CrossRef]
- Solli, K.-A.; Kumar Thapa, R.; Moldestad, B.M.E. Screening of Kinetic Rate Equations for Gasification Simulation Models. In Proceedings of the 9th EUROSIM Congress on Modelling and Simulation, EUROSIM 2016, 57th SIMS Conf. Simul. Model. SIMS 2016, Oulu, Finland, 12–16 September 2016; Volume 142, pp. 105–112. [Google Scholar]
- Umeki, K.; Yamamoto, K.; Namioka, T.; Yoshikawa, K. High temperature steam-only gasification of woody biomass. Appl. Energy 2010, 87, 791–798. [Google Scholar] [CrossRef]
- Zahedi nezhad, M.; Rowshanzamir, S.; Eikani, M.H. Autothermal reforming of methane to synthesis gas: Modeling and simulation. Int. J. Hydrogen Energy 2009, 34, 1292–1300. [Google Scholar] [CrossRef]
- OriginLab—Origin and OriginPro—Data Analysis and Graphing Software. Available online: http://www.originlab.com/ (accessed on 29 April 2020).
- Abánades, A.; Rubbia, C.; Salmieri, D. Thermal cracking of methane into Hydrogen for a CO2-free utilization of natural gas. Int. J. Hydrogen Energy 2013, 38, 8491–8496. [Google Scholar] [CrossRef]
- Thengane, S.K.; Hoadley, A.; Bhattacharya, S.; Mitra, S.; Bandyopadhyay, S. Exergy efficiency improvement in hydrogen production process by recovery of chemical energy versus thermal energy. Clean Technol. Environ. Policy 2016, 18, 1391–1404. [Google Scholar] [CrossRef]
- Wang, Z.; Fan, W.; Zhang, G.; Dong, S. Exergy analysis of methane cracking thermally coupled with chemical looping combustion for hydrogen production. Appl. Energy 2016, 168, 1–12. [Google Scholar] [CrossRef]
- Cormos, C.C. Hydrogen production from fossil fuels with carbon capture and storage based on chemical looping systems. Int. J. Hydrogen Energy 2011, 36, 5960–5971. [Google Scholar] [CrossRef]
- Kocs, E.A. The global carbon nation: Status of CO2 capture, storage and utilization. EPJ Web Conf. 2017, 148, 00002. [Google Scholar] [CrossRef]
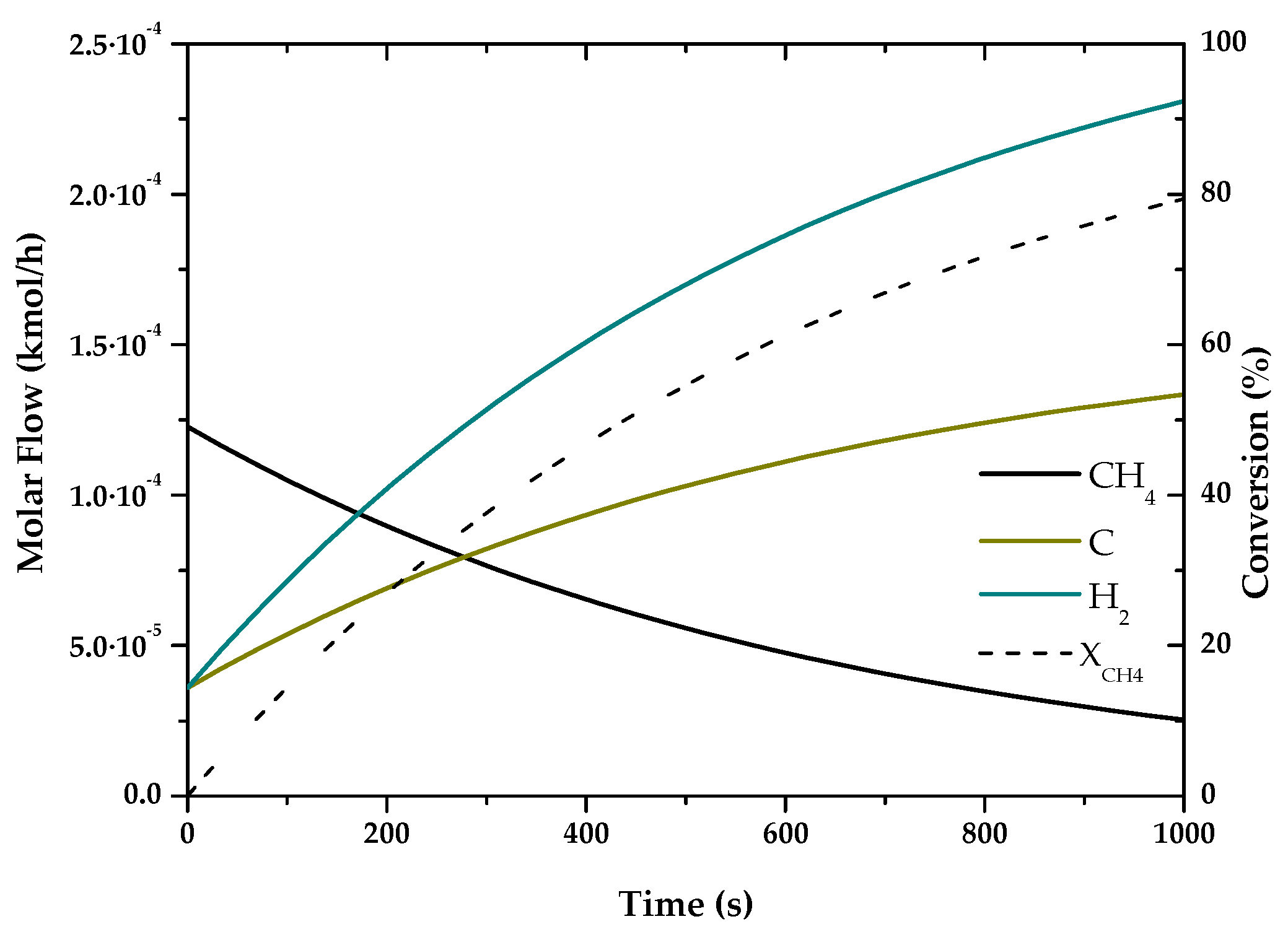

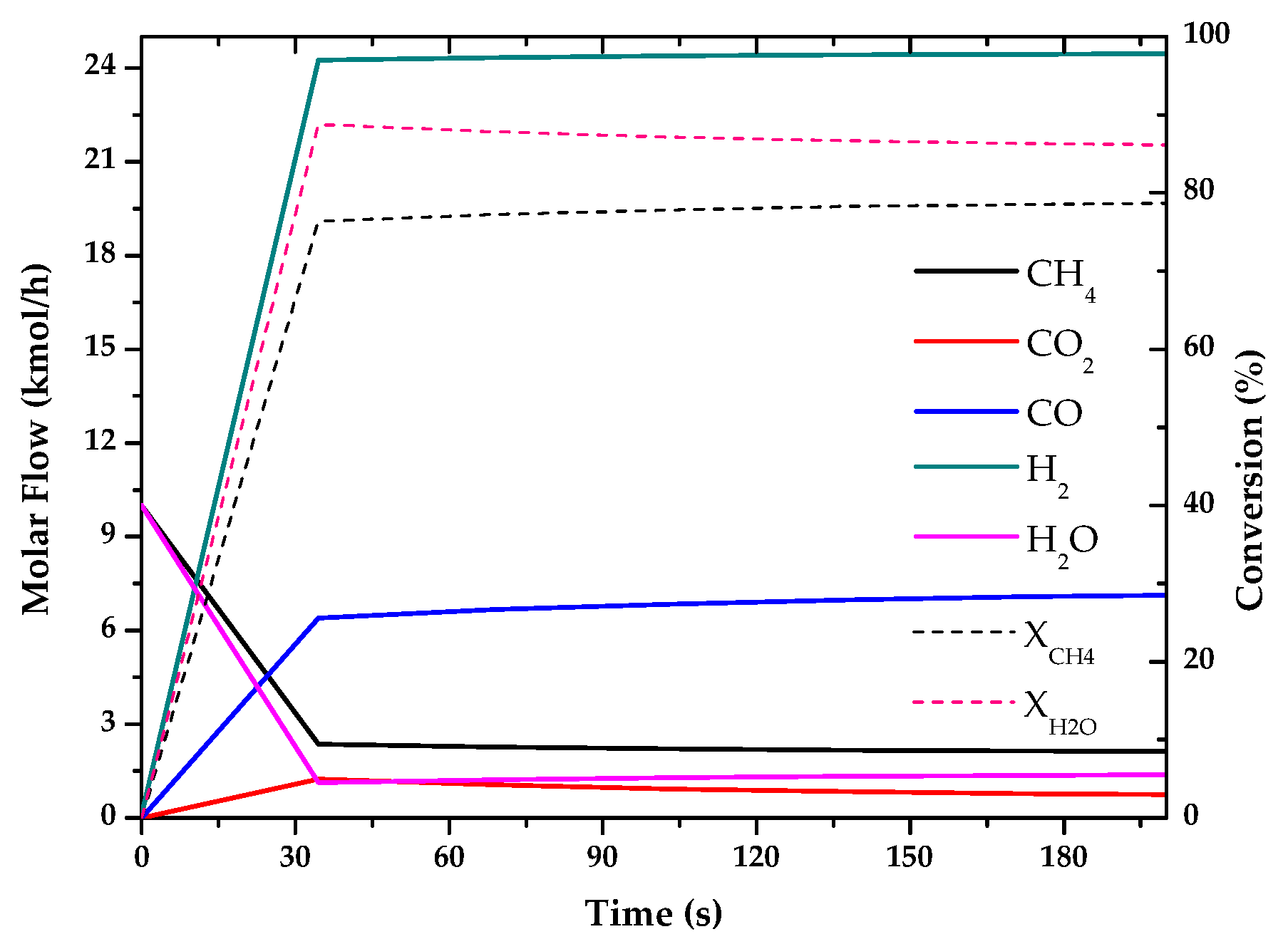
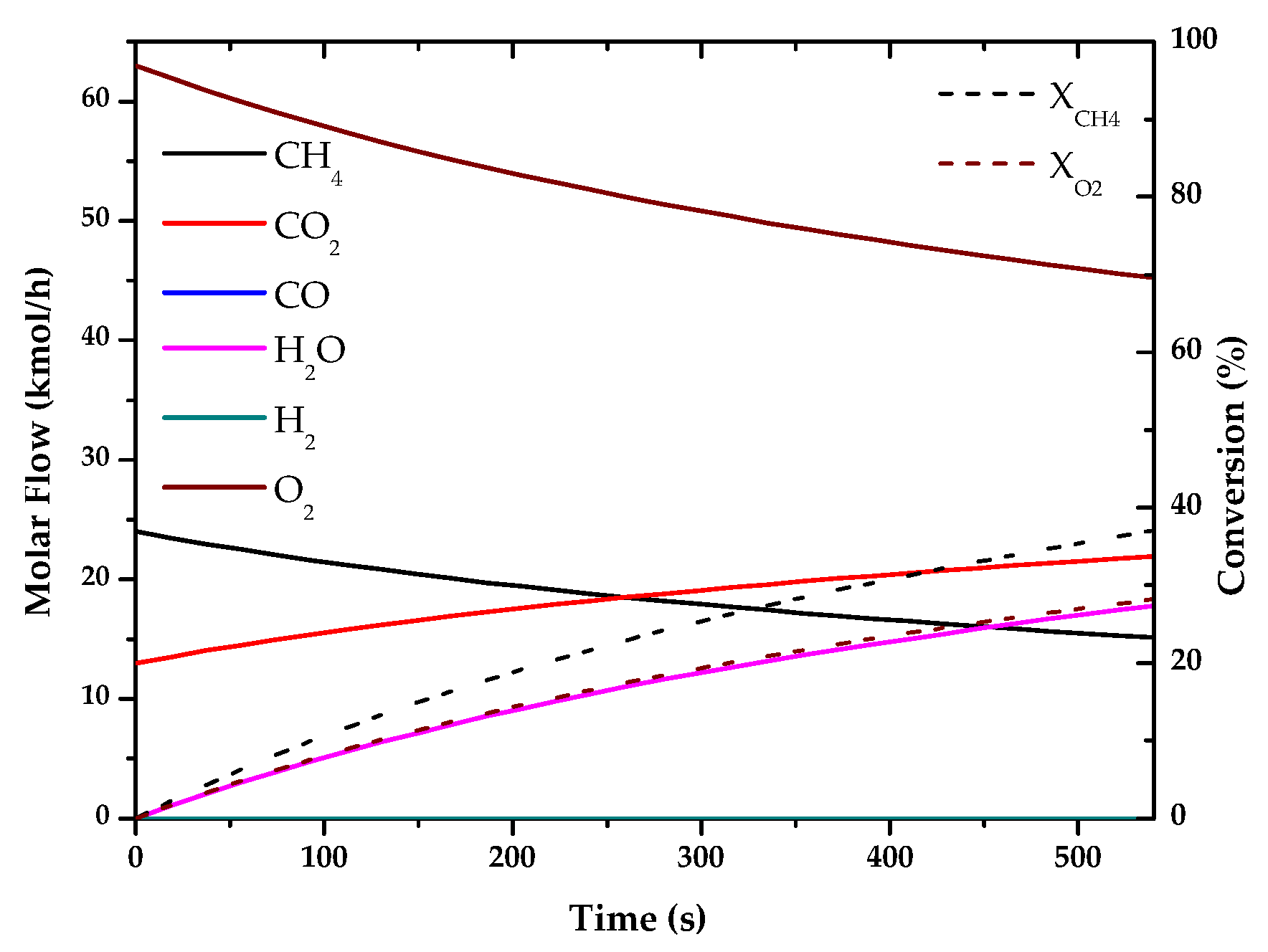
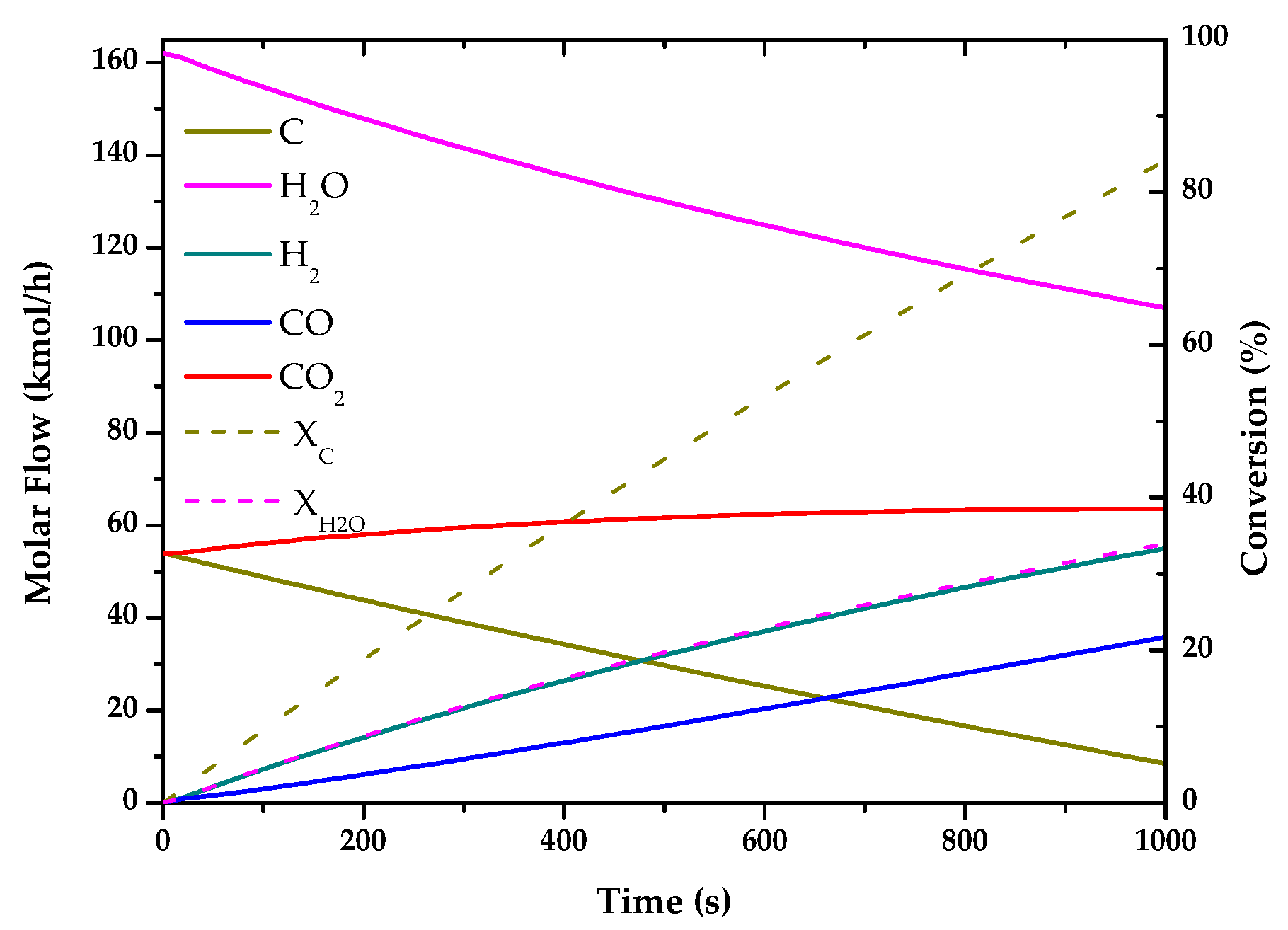
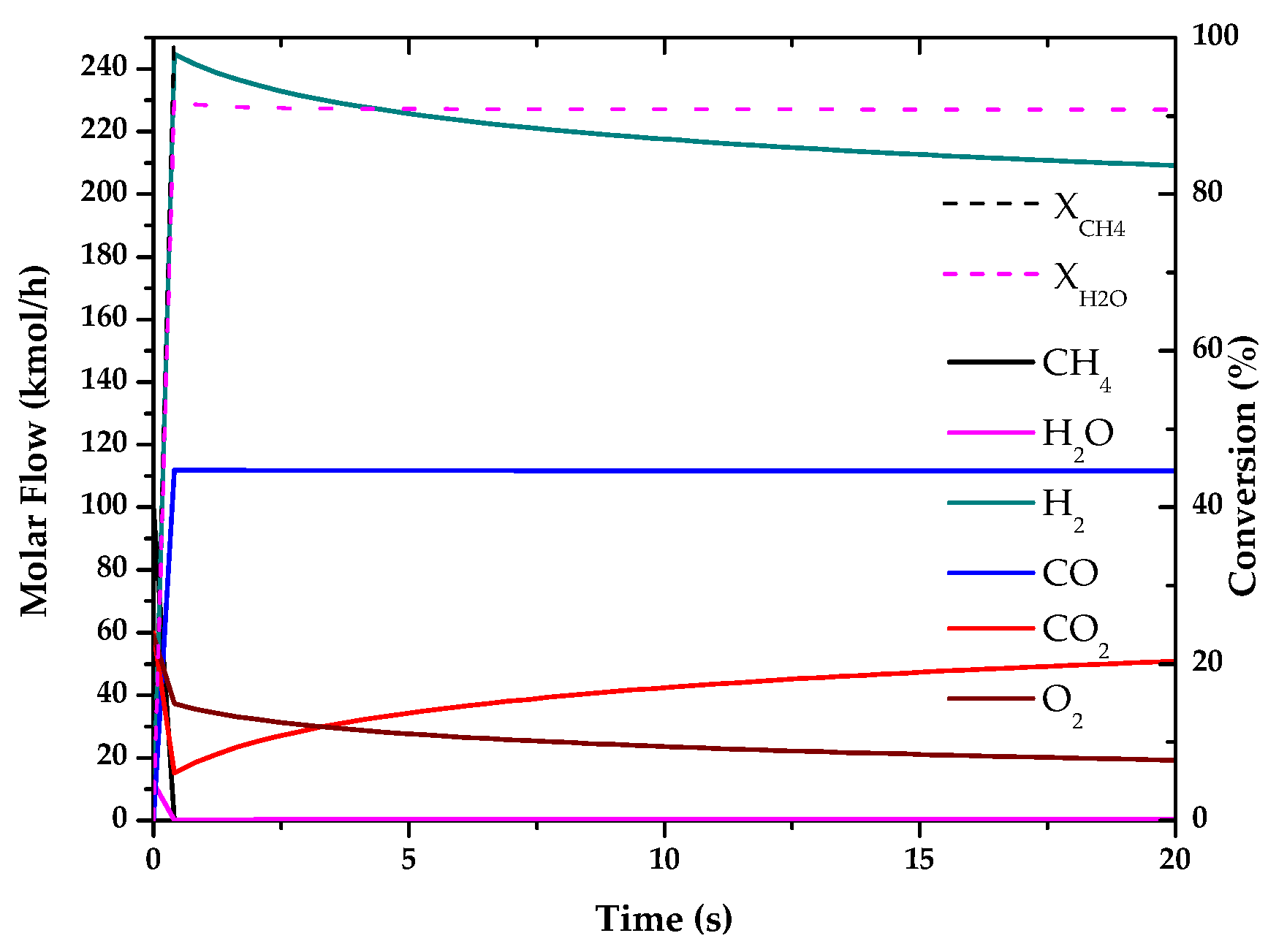
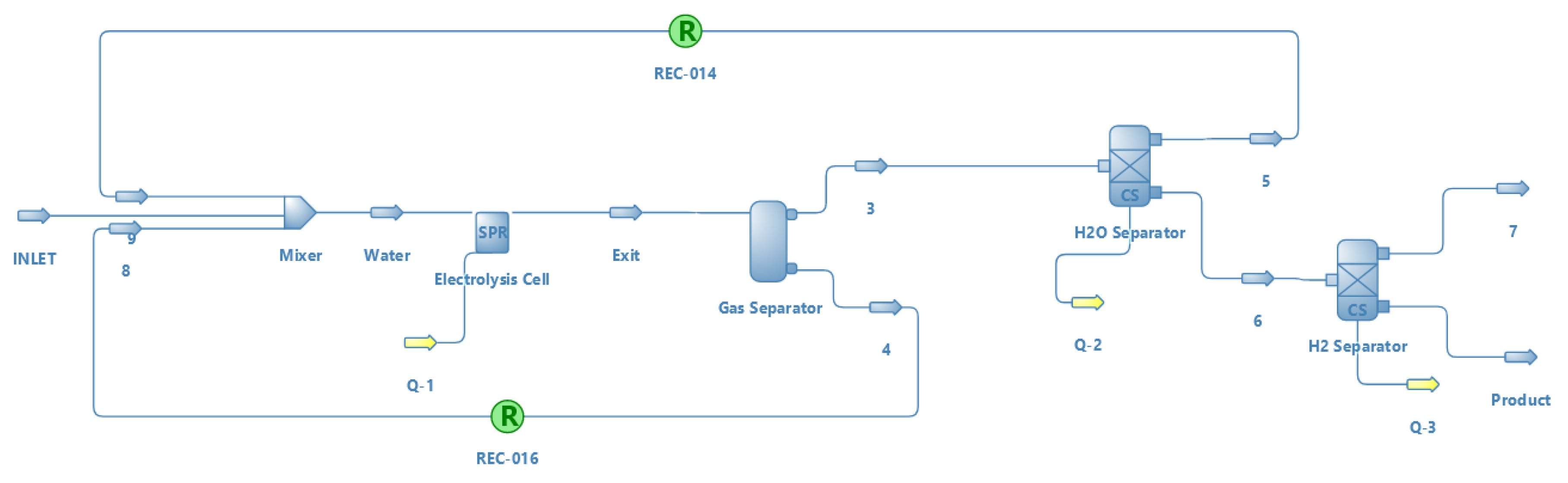
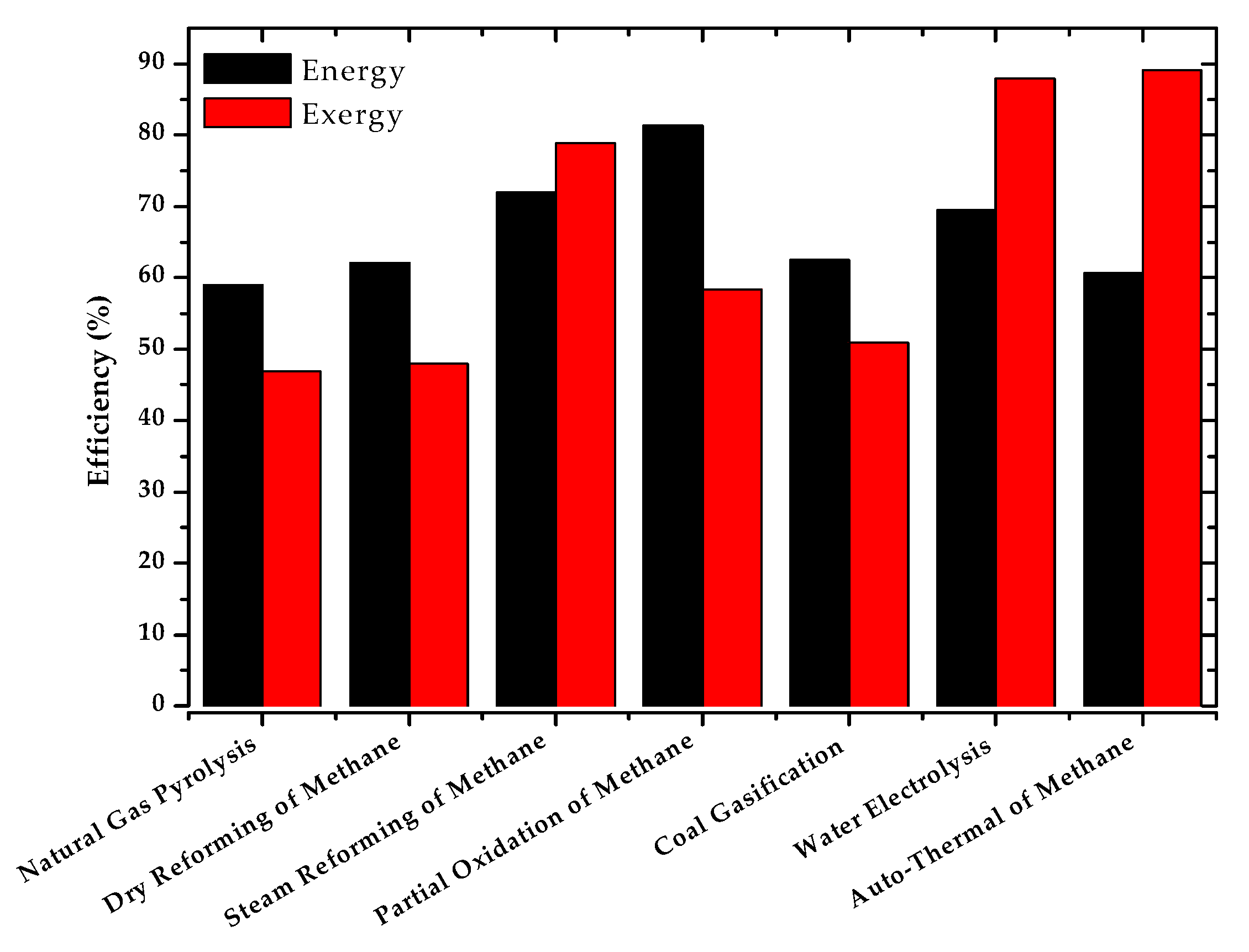
| Method | Source | Brief Description | |
|---|---|---|---|
| Primary Energy | Material | ||
| Electrolysis | Electrical | Water | Direct current is used to split water into O2 and H2 (electrochemical reaction) |
| Plasma arc decomposition | “ | Fossil fuels | Cleaned natural gas is passed through plasma arc to generate H2 and carbon soot |
| Thermolysis | Thermal | Water | Thermal decomposition of water (steam) at temperatures over 2500 K |
| Thermochemical (Water splitting) | “ | Water | Cyclical chemical reactions (net reaction: water splitting into H2) |
| Thermochemical (Biomass conversion) | “ | Biomass | Thermocatalytic conversion |
| Thermochemical (Gasification) | “ | “ | Conversion of biomass into syngas |
| Thermochemical (Reforming) | “ | “ | Conversion of liquid biomass (biofuels) into H2 |
| PV electrolysis | Photonic | Water | PV panels are used to generate electricity |
| Photocatalysis | “ | “ | Water is split into H2 by using the electron-hole pair generated by the photocatalyst |
| Photoelectrochemical method | “ | “ | A hybrid cell simultaneously produces current and voltage upon absorption of light |
| Dark fermentation | Biochemical | Biomass | Biological systems are used to generate H2 in the absence of light |
| High temperature electrolysis | Electrical + Thermal | Water | Electrical and thermal energy are used together to drive water splitting at high temperatures |
| Coal gasification | “ | “ | Conversion of coal into syngas |
| Fossil fuel reforming | “ | “ | Fossil fuels are converted to H2 and CO2 |
| Biophotolysis | Photonic + Biochemical | Biomass + Water | Biological systems (microbes, bacteria, etc.) are used to generate H2 |
| Photofermentation | “ | “ | Fermentation process activated by exposure to light |
| Photoelectrolysis | Electrical + Photonic | Water | Photoelectrodes and external electricity are used to drive water electrolysis |
| Global Reaction | ||||
|---|---|---|---|---|
| Natural Gas Pyrolysis | - | 0.5 | 0 | |
| Dry Reforming of Methane | 0.33 | 0.50 | 0.5 | |
| Steam Reforming of Methane | 0.25 | 0.25 | 1 | |
| Partial Oxidation of Methane | 0.38 | 0.38 | 1 | |
| Coal Gasification | 0.75 | 0.75 | 1 | |
| Water Electrolysis | - | - | - | |
| Autothermal Reforming of Methane | 0.38 | 0.38 | 1 |
| Technology | Advantages | Disadvantages |
|---|---|---|
| Natural Gas Pyrolysis | No emission CO2 and CO Oxygen and water not required Fuel flexibility | Carbon formed High operating temperatures |
| Reforming of Methane | Most extensive industrial experience Oxygen not required Lowest process temperature Best H2/CO ratio for H2 production | Highest air emissions |
| Partial Oxidation of Methane | Reduced desulfurization requirement No catalyst requirement Low methane slip | Low H2/CO ratio High operating temperatures Complex handling process |
| Coal Gasification | Remove impurities before burning the fuel Lower material costs | High coal demands High CO2 production/H2 |
| Electrolysis | No emission CO2 Production of high purity hydrogen | High energy requirement |
| Autothermal Reforming of Methane | Lower process temperature than partial oxidation Low methane slip | Limited commercial experience Air/oxygen requirement |
| Substance | |
|---|---|
| CH4 | 51.12 |
| CO2 | - |
| H2 | 120.00 |
| CO | 10.10 |
| H2O | - |
| C (s) | 32.80 |
| O2 | - |
| Substance | |
|---|---|
| CH4 | 831.65 |
| CO2 | 19.87 |
| H2 | 236.10 |
| CO | 275.10 |
| H2O | 9.50 |
| C (s) | 410.00 |
| O2 | 3.87 |
| Temperature (K) | Pressure (bar) | |||
|---|---|---|---|---|
| Natural Gas Pyrolysis | 1173 | 1 | −1.58·10−3 | 1.23·10−4 |
| Dry Reforming of Methane | 973 | 1 | −1.68·104 | 6.18·10−5 |
| Steam Reforming of Methane | 1000 | 1 | −0.10 | 7.90 |
| Partial Oxidation of Methane | 1223 | 1 | −2.10·10−3 | 12.94 |
| Coal Gasification | 1123 | 1 | −2.77·10−4 | 187.82 |
| Autothermal Reforming of Methane | 1510 | 21 | −53.21 | 100.00 |
| Energy Efficiency (%) | ||||
|---|---|---|---|---|
| in Transformation DWSIM | in Transformation MATLAB | Reference [5,13,14,46] | Process DWSIM | |
| Natural Gas Pyrolysis | 58.99 | 55.19 | ~55–58 | 44.95 |
| Dry Reforming of Methane | 62.13 | 62.32 | ~56–85 | 40.85 |
| Steam Reforming of Methane | 71.98 | 71.66 | ~74 | 64.95 |
| Partial Oxidation of Methane | 81.27 | 81.47 | ~70–80 | 78.39 |
| Coal Gasification | 62.49 | 62.13 | ~60 | 51.75 |
| Electrolysis | 69.46 | - | ~50–70 | 47.64 |
| Autothermal Reforming of Methane | 60.66 | 61.38 | −60–75 | 56.85 |
| Energy Requirement (kWh/kg H2) | ||||
|---|---|---|---|---|
| Natural Gas Pyrolysis | 16.28 | - | 0.5 | - |
| Dry Reforming of Methane | 24.50 | 0.34 | 0.54 | 0.47 |
| Steam Reforming of Methane | 10.84 | 0.32 | 0.32 | 1.00 |
| Partial Oxidation of Methane 2 | - | 4.48 | 4.48 | 1.00 |
| Coal Gasification | 3.76 | 0.77 | 0.77 | 1.00 |
| Electrolysis | 47.99 | - | - | - |
| Autothermal Reforming of Methane | 5.76 | 0.31 | 0.31 | 1.00 |
| Natural Gas Pyrolysis | 46.88 | 53.12 | 40.70 | 93.82 |
| Dry Reforming of Methane | 47.97 | 52.03 | 1.46 | 53.50 |
| Steam Reforming of Methane | 78.87 | 21.13 | 0.26 | 21.39 |
| Partial Oxidation of Methane | 58.35 | 41.65 | 5.51 | 47.16 |
| Coal Gasification | 50.92 | 49.08 | 6.45 | 55.55 |
| Electrolysis | 87.92 | 12.08 | 0.73 | 12.81 |
| Autothermal Reforming of Methane | 89.08 | 10.92 | 0.75 | 11.66 |
| Energy Efficiency (%) | ||
|---|---|---|
| in Transformation | With CCS | |
| Natural Gas Pyrolysis | 58.99 | 58.99 |
| Dry Reforming of Methane | 62.13 | 46.60 |
| Steam Reforming of Methane | 71.98 | 53.99 |
| Partial Oxidation of Methane | 81.27 | 60.95 |
| Coal Gasification | 62.49 | 46.87 |
| Electrolysis | 69.46 | 69.46 |
| Autothermal Reforming of Methane | 60.66 | 45.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Rodríguez, A.; Abánades, A. Comparative Analysis of Energy and Exergy Performance of Hydrogen Production Methods. Entropy 2020, 22, 1286. https://doi.org/10.3390/e22111286
Martínez-Rodríguez A, Abánades A. Comparative Analysis of Energy and Exergy Performance of Hydrogen Production Methods. Entropy. 2020; 22(11):1286. https://doi.org/10.3390/e22111286
Chicago/Turabian StyleMartínez-Rodríguez, Angel, and Alberto Abánades. 2020. "Comparative Analysis of Energy and Exergy Performance of Hydrogen Production Methods" Entropy 22, no. 11: 1286. https://doi.org/10.3390/e22111286
APA StyleMartínez-Rodríguez, A., & Abánades, A. (2020). Comparative Analysis of Energy and Exergy Performance of Hydrogen Production Methods. Entropy, 22(11), 1286. https://doi.org/10.3390/e22111286






