1. Introduction
Optimal control of physical and chemical systems, and of the processes taking place in such systems, has been a major goal since the beginning of scientific investigations [
1,
2]. Essentially any application of scientific insights to practical problems has constituted such an effort in optimal control of some kind—even if not formulated as a mathematical control problem—, with the objective ranging from minimizing the difference of the values of characteristic parameters and quantities between the ideal theory and the real experiment [
3,
4], to maximizing the amount or quality of the desired output for given material and technical constraints. One can distinguish between those controls that are based on practical limitations due to the availability of tools and materials or lack thereof, and those based on the laws of physics.
Perhaps the classical examples of optimal control based on laws of physics have been the analyses of thermodynamic cycles [
5,
6,
7], where the famous formulas for maximal efficiencies of hypothetical engines are sometimes given a status nearly equal to the fundamental laws of thermodynamics [
6]. Such formulas have typically been derived under the assumption of infinite time available for each step of the cycles, allowing us to move from equilibrium state to equilibrium state. About fifty years ago, engineers and scientists began to question this assumption and re-formulated the optimal control problem by demanding that the cycle should be performed in a given finite amount of time, leading to the development of the field of finite-time thermodynamics [
8,
9,
10,
11].
A second aspect of optimal control based on the laws of physics is to reduce the engine under consideration to the most elementary physical systems that are stripped of all weaknesses and complications which are associated with the macroscopic aspects of the experimental apparatus employed in their realization, resulting in the creation and investigation of molecular machines [
12]. Typically, this involves reducing the size of the system in the sense that we are dealing with a macroscopic system as a (non-interacting) ensemble of elementary but microscopic systems. Of course, in practice, isolating the individual elementary system often requires a very large experimental apparatus, but for the purpose of the analysis of the physics and the optimal control of the system, this counts as “environment” and “control tools”, whose optimality in themselves are usually irrelevant to the issue of optimally controlling the (ensemble of) elementary system(s) as such. Many experiments have been performed where single atoms or ions in vacuum [
13,
14,
15] or single defects in solids [
16,
17] have been studied and controlled in some fashion.
A third feature of achieving optimal control of systems on the level of physical laws is dealing with the quantum nature of these elementary systems, which is forced upon us when reducing the elementary systems to atomic dimensions. While the quantum aspects are unavoidable, one can nevertheless often separate them from the issue of optimal control one investigates, especially if the time resolution of the control is so large that many quantum aspects can be captured by, e.g., effective decay rates.
From the point of view of a theorist, such a reduction to elementary physical systems is often a desirable feature because it allows us to focus on the elementary system itself—which can, in many cases, be analyzed analytically to a certain point—while assigning all other aspects to a generic environment. Examples are the optimal control of harmonic oscillator systems, which have been studied both on the classical and on the quantum mechanical level [
18,
19,
20,
21,
22,
23,
24,
25,
26], spin systems [
26,
27,
28], particles in a box [
26], and generic two-level systems [
29].
In this study, we are going to analyze the basic concepts of optimal control at the example of a hydrogen atom-like system, which we employ as an engine, or more specifically, the working fluid of such an engine, to perform a thermodynamic cycle within finite time. On the technical mathematical level, the electronic Hamiltonian possesses a very high degree of symmetry allowing at least some analytical treatment. On the level of possible applications, there exist systems equivalent to hydrogen-like atoms, e.g., excitons inside a solid [
30], that can be controlled by modifying the environment while the hydrogen atom-like system remains in its state. Controlling such a system might allow us to construct nano-size engines inside solids. Alternatively, we can consider isolated hydrogen atoms inside a cavity [
31], where also the conditions can be controlled such that the hydrogen atom becomes a modified hydrogen atom.
We will analyze the stages of the thermodynamic control cycle on the general level of probability distributions, both for the ideal (infinite-time) cycle and the cycle at finite time, derive an optimality criterion for minimal waste of work/minimal excess production of heat (dissipation)/minimal production of entropy during finite-time processes for general statistical mechanical systems, and apply these formulas to the example of the hydrogen atom-like system. Note that we are dealing with an “electronic” states-based engine, not with a standard thermal atom movement-based engine as studied, e.g., in [
13].
2. Background
2.1. Aspects of the Hydrogen Atom-Like System
Due to the high symmetry of the Coulomb potential, the electronic Schrödinger equation for the hydrogen atom can be solved analytically, and we get for the energy eigenvalues
where
. The degeneracy of the
’th energy level is
. In principle, we need to add a factor 2 for the spin degeneracy of the electron, but this factor is not relevant for this study and will be dropped.
If we consider hydrogen atom-like systems, the formula for the energy is modified by, e.g., changing the mass of the electron or quasi-particle
, i.e.,
, by changing the effective charge that generates the Coulomb field
and/or the effective charge of the quasi-particle
, i.e.,
or by introducing a shielding dielectric constant
, such that
, or combinations thereof. Quite generally, we note that these modifications can be incorporated in a straightforward fashion by introducing a modification factor
such that
If we can apply external forces or fields to the system such that
(i.e.,
,
and/or
) can be smoothly varied, then we can modify the energy levels in a controlled fashion. In that case, the hydrogen atom-like system is used as the working fluid of an engine, where the changing of the energy levels leads to a change in energy that may be extracted as work. A change of the statistical occupation of the various energy levels at given temperature
is associated with the entropy content and the heat exchanges of the system, and we can define work cycles in, e.g.,
-space. Examples for individual legs (or branches) of such a cycle would be adiabatic or isothermal changes in
from some initial value
to a final value
, and iso-
changes in temperature where we keep
constant (see
Figure 1,
Figure 2 and
Figure 3 below).
In this context, one should note that, e.g., the dielectric constant
depends on both temperature and pressure, complicating the issue considerably; for small ranges of temperature and pressure,
commonly changes with temperature and pressure in an approximately linear fashion in a solid [
32,
33], i.e.,
. Depending on the type of system, we may have
and
, respectively [
32], or the opposite [
33], or other combinations. Thus, e.g., we find that
increases with pressure and decreases with temperature for CdF
2 [
32], and we could translate our analysis from using
to
as control variables, in principle.
Of course, all energy levels of the hydrogen atom-like system will exhibit nonzero occupancy in thermodynamic equilibrium in general—in principle, even including the continuous distribution of energy levels associated with modified plane–wave states beyond the ionization energy (although these will most likely be irrelevant due to the assumed localization or confinement of the electron). However, for practical calculations, one can often simplify the system by considering, e.g., only the two lowest energy levels
and
. Frequently, such simplified models can yield some quantitative results and help to better understand the qualitative behavior of the full system [
34]. Finally, we note that there are other ways to modify the electronic states of the hydrogen atom-like system, e.g., by breaking the spherical symmetry of the Hamiltonian, such that Equations (1)–(3) no longer apply. Since the corresponding changes in the eigenvalue spectrum of such a modified Hamiltonian are very specific to the way we break the symmetry and need to be treated on a case-by-case basis, we are not going to discuss such systems and only allow modifications of the hydrogen atom-like system that preserve the spherical symmetry and the
dependence of the Coulomb potential.
2.2. Thermodynamic Cycles for the Hydrogen Atom-Like System
In general, we can distinguish two ways to change the occupation of the energy levels: directly via radiation with suitable frequencies corresponding to energy differences between specific energy levels, and stochastically by contact to heat baths at various temperatures. However, in the case of a radiation driven a-thermal cycle, we are not dealing with a system close to thermodynamic equilibrium, and the classical thermodynamic optimal control might not be suitable. Thus, in this study, we focus on the more familiar cycles involving interactions with heat reservoirs.
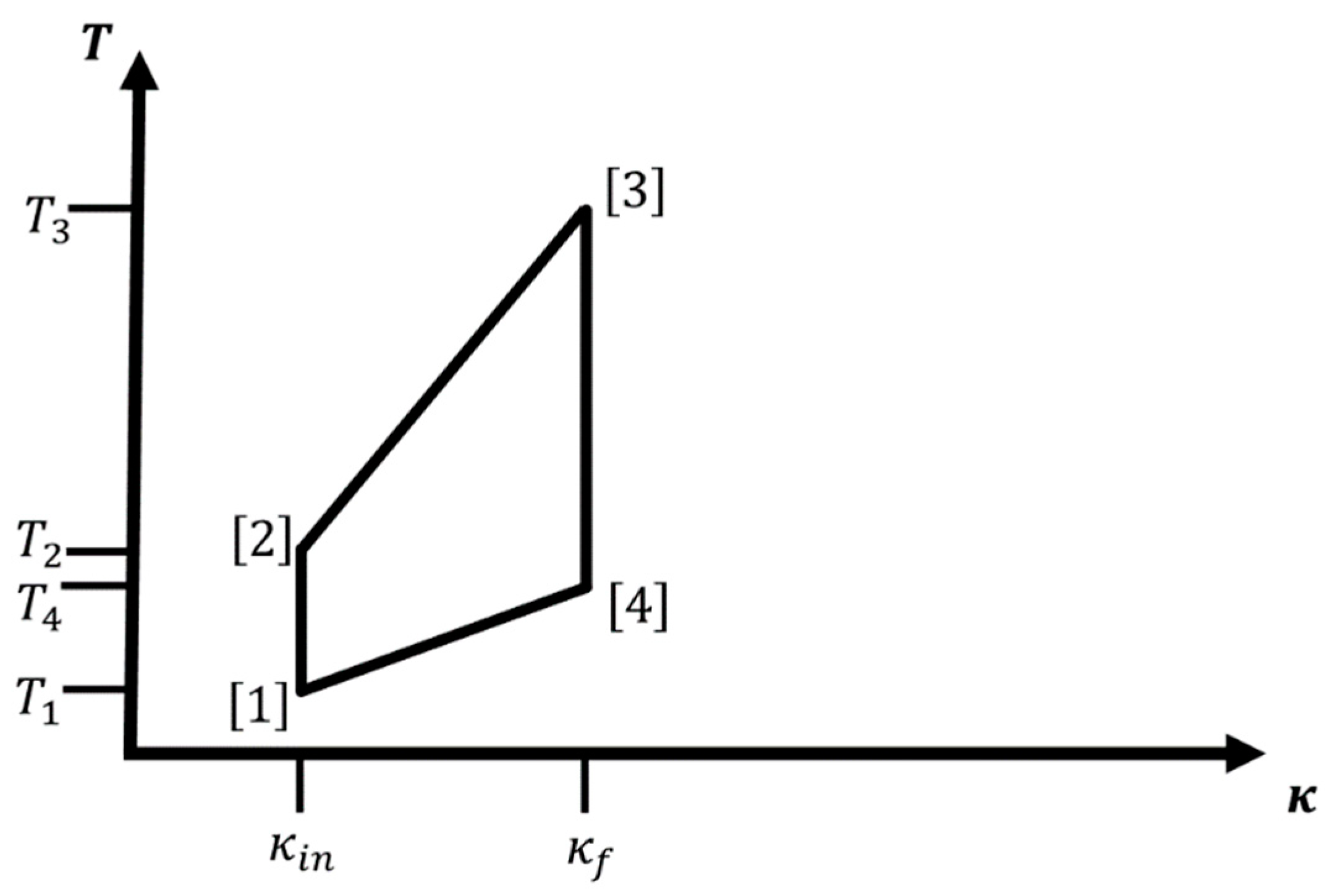
Regarding the notation, we write or to indicate that the variable, parameter, or quantity belongs to leg which runs from corner to corner of the four-leg cycle (corner = corner , of course). Furthermore, quantities associated with corner are referred to as . This notation is introduced to keep the labeling of legs and corners distinct from the one for quantities such as the probability distribution at step along each branch when analyzing the effect of finite-time processes. Finally, the subscripts , , and indicate that the quantity is associated with an iso--isothermal, iso--adiabatic, or adiabatic-isothermal cycle, respectively.
If we allow interaction with a heat bath, we need to consider an ensemble of identical isolated localized hydrogen atom-like systems such that we can apply statistical mechanics to evaluate the (equilibrium) probabilities of occupation of the individual energy levels. From a statistical point of view, the microstates correspond to the eigenstates of the hydrogen-like atom. For concreteness, we always define (without loss of generality) and to denote the smallest and largest value of on the cycle, and as the lowest temperature in the cycle. Starting point of the cycle is always . Together with the assignment of path types to the legs (adiabatic, isothermal, iso-), there is only one additional temperature, which we can choose, e.g., or ; all other temperatures and values of the cycle are then fixed. We always assume smooth changes in both and along the legs of the cycle, i.e., for example, the system is exposed to an infinite number of heat baths with intermediary temperatures when changing .
Note that we use the terms adiabatic and isentropic essentially interchangeably for thermodynamic equilibrium paths, since we assume that the system is in thermodynamic equilibrium both at the starting and at the end point of a leg. In the general case of such an adiabatic path, only the entropy remains constant along the path. However, for some physical systems, such as the hydrogen atom-like ones we are going to discuss, not only the entropy but also the equilibrium Boltzmann distribution remains unchanged along an adiabatic path, and we call such paths “special adiabatic”.
For such a macroscopic system,
Figure 1 and
Figure 2 show cycles combining two iso-
legs with two isothermal or adiabatic legs, respectively. We excite the ensemble of atoms via change in temperature
, while we keep
at the value
:
. Next, we perform/extract work while we increase
from
to
. This can be done adiabatically, thus involving a concomitant change in temperature during the process (
), or isothermally keeping the temperature constant at
. In leg 3, we de-excite the atom ensemble via change in temperature
, while we keep
at the value
, and finally, we decrease
from
back to
; again, this can be done either adiabatically (
) or isothermally at
. Note that for cycles containing two iso-
branches, the only condition on
is
. In the literature, one sometimes calls the iso-
-adiabatic cycle an Otto cycle, in analogy to the isochore-adiabatic cycle, which is the underlying cycle of an Otto engine [
7], and similarly, we could call the iso-
-isothermal cycle a Stirling cycle in analogy to the isochore-isothermal cycle belonging to the Stirling engine [
7].
An alternative type of cycle shown in
Figure 3 would include no legs with constant
; instead, we combine two adiabatic and two isothermal legs to the analogue of a Carnot cycle [
7]. We adiabatically increase
from
to
,
, appropriately changing the temperature during the process (
), followed by an isothermal leg where we increase
from
to
while keeping the temperature constant at
. Next, we adiabatically decrease
from
to
,
, while appropriately changing the temperature (
), and finally, we decrease
from
back to
while keeping the temperature constant
.
Note that a feasible isothermal-adiabatic cycle usually will have an additional condition on
. For the special adiabatic legs of a hydrogen atom-like system, feasibility requires that the temperature at the second corner fulfills the condition
where
otherwise, leg 3 would have a larger slope than leg 1, violating the condition that
.
When analyzing the work performed and the heat exchanged along the legs of the cycles, we do not include the work done on the environment of the hydrogen atom-like system (e.g., the solid or the cavity), which is needed to vary . Of course, we can take that into account, e.g., via replacing by the pressure as control variable if depends on pressure in a monotonic fashion, and then compute the work needed to establish such a pressure inside the solid. However, the focus is on the hydrogen-atom like system, and not on the whole solid or cavity where the system might be realized in the experiment. Thus, we will stay with using as the control variable, and only consider the change in the hydrogen atom-like system itself.
There are several general aspects that need to be considered regarding the equilibrium distributions, relaxation, and entropy/excess heat production along the cycles. How close are the instantaneous probability distributions over the states of the system to the (equilibrium) Boltzmann distributions at the various temperatures along its iso- legs? Similarly, for branches where is changed, how does the finite time available to vary affect our ability to keep the probability distribution close to the appropriate Boltzmann distribution for isothermal processes, and how close can the adiabatic path in -space which is realized in experiment, be to the ideal adiabatic path? All these questions directly lead to the issue of relaxation to the appropriate Boltzmann distributions in finite time, for which we can hope to derive general formulas as long as we can assume that the processes are slow enough for the system to always remain close to thermodynamic equilibrium throughout the cycle.
2.3. Statistical Mechanics and Thermodynamics of Cycles
Regarding the statistical mechanics and thermodynamics of cycles, the starting point of our analysis will be the first law of thermodynamics,
along a piece of path in thermodynamic space, which is equivalent to
. Here,
means that the internal energy of the system is increased along the leg, and
means that heat is added to the atom from the reservoir(s) the atom is in contact with along the path, thus increasing the system’s internal energy.
means that the system does work on the apparatus, radiation field or the environment in general, along the leg, and reduces the system’s internal energy in the process. To avoid confusion, we note that in the literature
is frequently defined in an alternative fashion to refer to the amount of work done by the apparatus on the system increasing its internal energy; as a consequence, one then would write the first law as
and
.
The connection to statistical mechanics appears via the information theoretic definition of the entropy
where
is the probability for the microstate
to be occupied, and the sum is over all
microstates [
5]. In equilibrium at given temperature
, these probabilities
correspond to the equilibrium Boltzmann probability distribution,
for the case of the canonical ensemble, where the sum over states
serves as the normalization of the probability distribution, and
is the energy of microstate
. Keep in mind that the equilibrium distribution maximizes the entropy, for a given set of boundary conditions that are, e.g., defining the ensemble.
If we consider the energy levels
of the states instead of the microstates themselves in the formula of the entropy, then we need to include the degeneracies
of the energies
. As far as the equilibrium probabilities are concerned, we can assume that the probabilities of occupying two states
and
with the same energy
are equal; for convenience, we define
, and thus
is the equilibrium probability to find the system with energy
. In general, we can write the equilibrium entropy as
where now the summation goes over the
energy levels and
are the equilibrium probabilities to find the system in a state with energy
. Note that for non-degenerate states, i.e.,
, this expression reverts to the original microstate-based formula, and for the special case
for
while
for
, we obtain the formula for the entropy in the microcanonical ensemble,
.
Finally, for those probability distributions where we deviate from the equilibrium distribution but nevertheless can assume that the probabilities of being in a state with energy are the same, we can define , and thus . Then, we get for the entropy the expression and the normalization . Note that this is a substantial restriction in the set of allowed probability distributions; thus, unless this assumption is valid, we need to employ the microstate formulation of the problem.
As a shorthand notation, we can represent the probability distributions by vectors
, and expressions like
can be written as
where
. Similarly,
, and we can relate the equilibrium probabilities of the Boltzmann distribution to the energies via
where
is a constant that can be related to an overall shift of the energy scale, and
[
35]. The expectation value of the energy is then given by
or, for the case of the expectation value in equilibrium,
. Note that some of these energies will appear many times in this vector if the energy level is degenerate. Another example is the expectation value of the square of the energy,
, where
. If appropriate, we can replace the sums over the
microstates by sums over the
energies, i.e.,
, where
and
, or for the entropy
where
. In the case of the single atom-like system, the microstates correspond to the eigenfunctions of the Hamiltonian of an electron (or a quasi-particle) in a (shielded) Coulomb potential. Note that we are not considering the degeneracy due to the multiple copies of the atom-like system in the ensemble—the ensemble is only introduced to allow us to visualize the occupation probabilities of the single atom-like system with which we are dealing.
Since the energy is a state function, the change of its equilibrium expectation value
along a leg
of the path does not depend on the choice of path, i.e., the total change in energy can be computed directly from taking the difference between the two states,
For the heat delivered/absorbed by the atom along a leg
(parametrized by
), we can use the formula
if we stay at the equilibrium distribution along the path, and an analogous expression in
if we deviate from the equilibrium distribution because of, e.g., finite-time effects:
Finally, the work performed by the atom can be computed via the first law by taking the difference between
and
along the path. Along the legs where
is constant, both
and
will vary as they are both functions of the probability distribution, which in turn evolves as the temperature changes. To compute
is no problem, but the expression for
is nontrivial. We can switch coordinates to follow the change in temperature instead of the change in entropy,
However, we note that this way of writing
does not necessarily make the integral easier to perform in general either, even though
, and thus all the energy levels
, are constant along these legs of the cycle. However, since the work
would usually be associated with changes in
, we expect that
along thermodynamic equilibrium paths with fixed
, and therefore,
. Using Equations (16) and (12), we find:
since
along a thermodynamic equilibrium path because of probability conservation. Note that we have not exploited the fact that we are dealing with a hydrogen atom-like system, and thus this result is general for any statistical mechanical system along legs with
= constant, even for the most general case of many control parameters
.
If we consider the complete cycle, the changes in energy will sum to zero, and thus
over the whole cycle, i.e., the net heat added to the atom over the cycle must be converted into net work done by the atom on the apparatus. The discussion in this subsection is standard procedure from equilibrium thermodynamics [
5,
6], but has been included to establish the concepts and notation needed for analyzing the thermodynamics of the cycles of statistical mechanical systems in finite time in the next sections.
4. Application to the Hydrogen Atom-Like System
Regarding the hydrogen atom-like system, we first note that
and
enter the formulas for the equilibrium probability distribution everywhere in the combination
such that
In particular, this means that the ratio
remains constant along the adiabatic legs of the cycle, ensuring that
does not change along this branch. Thus, the adiabatic branches for cycles involving the hydrogen atom-like system belong into the special category where not only the entropy but the whole equilibrium probability distribution is constant along the path. Note that in the cycle diagram (
Figure 2 and
Figure 3), an adiabatic branch
for the hydrogen atom-like system is always a straight line (that can be continued through the origin) with slope
. Of course, the actual value of the ratio
will be different for each adiabatic leg. For an adiabatic leg
, it follows that
. For the combination of iso-
and adiabatic legs, we have
and
; therefore, we get
Analogously, for the combination of adiabatic and isothermal legs, we have the two adiabatic branches (legs 1 and 3) with
, for which
and
, and the isothermal legs yield the conditions
and
. Thus, we have the relations
Regarding the expressions for the thermodynamic lengths of the various legs in this section, we remark that the main effect of dealing with the hydrogen atom-like system is the special adiabaticity of the isentropic paths, such that the contribution of the adiabatic paths to the excess heat or entropy production can be set equal to zero unless we include the “off-target” contributions discussed in
Section 3.4.2. In addition, we can exploit Equation (3) to rewrite the specific heat as
where both the variance
and the specific heat
are functions of
. Furthermore, we can rewrite Equation (35) as
where
is a function of
, and thus Equation (36) becomes
Note that the variance of in Equation (36) is now transformed into a variance of the energy of the unmodified hydrogen atom.
For each cycle discussed below, we first write down a formula for the total work done by the atom and the heat absorbed by the atom in the quasi-static approximation, where everywhere along the cycle, and subsequently, we provide formulas for the efficiency, and the optimality criterion for the minimal excess heat production.
4.1. Iso--Adiabatic Cycle
In the case of the iso-
-adiabatic cycle where the legs 2 and 4 are adiabatic and do not contribute to the heat production, we can focus on legs 1 and 3 when computing the heat exchange with the environment. Using Equations (18) and (19), we obtain therefore for the total heat exchange over the full cycle:
Since , , and furthermore, the equilibrium entropy at higher temperatures is always larger than the one at lower temperatures, the term belonging to leg 1 transfers heat into the system, while along leg 3, the system gives off heat.
Since the legs 2 and 4 represent adiabatic processes, we have, furthermore,
and
, and also
and
. Using Equation (18) and shifting variables back and forth from
to
and
, this implies that
since
. This shows that the hydrogen atom-like system gives off heat to the environment, when the cycle is run as described.
Of course, this equals the total work around the cycle, which needs to balance the total heat by energy conservation. If we want to compute the expressions for the work along each of the four legs—e.g., in order to talk about the efficiency of the hydrogen atom-like system as working fluid in an engine, we need to add the contribution for the energy change. We then find:
,
, and
. This shows that we reduce the internal energy of the hydrogen atom-like system by a certain amount along leg 2 and raise it along leg 4 but by a much larger amount. Taking the sum, we obtain
since
and
because
. Thus, during this cycle, the apparatus performs net work on the system over the legs 2 and 4.
For the efficiency, a possible definition is to take the ratio between the net work along the legs where
is varied, and total heat added to/extracted from the system. With this definition, we find
Of course, one can define many other efficiencies, depending on the quantities and processes of interest.
Regarding the excess heat, we note that due to Equation (52), the adiabatic legs are special. We do not include the effect of being “off-target” during the adiabatic legs, and thus we only consider the two terms from the iso-
legs 1 and 3:
Using Equations (42) and (56), we get for the whole cycle
Switching variables to
for the integration, keeping in mind that
is a function of
, and using
and
since legs (4) and (2) are adiabatic, we find
since
.
In the case of the adiabatic legs considered here, we do have the problem that, to first order, the thermodynamic length vanishes as long as we are perfectly on target with the thermodynamic controls. Thus, the formula for yields zero time for the adiabatic legs of the path, which essentially means we could jump directly from the end of the first leg (= beginning of the second leg) to the beginning of the third leg (= end of the second leg), and analogously for the fourth branch. In practice, this does not make too much sense, and we would want to employ the off-target formula derived above, even though this requires additional information from experiment, or make assumptions about the location of the virtual intermediary points discussed above. Alternatively, we can make heuristic assumptions about the (hopefully short, i.e., ) minimal time needed to move along the adiabatic leg without noticeable deviations from the ideal adiabatic curve in space and subtract these times from the total available time , , before assigning and according the principle of constant thermodynamic speed.
Since
, the optimal time allocation for the two legs must be
and thus we obtain
and
. We remark that when we minimize the entropy production and thus use the metric
, we find
and, after subtracting
from
, we obtain
.
Due to the sum over microstates for the heat capacity with nontrivial energy eigenvalues under the square root and the nontrivial temperature dependence of , no further analytical simplifications appear to be possible. Thus, the allocation along the individual legs is an open question, but should depend on the variance as function of .
4.2. Iso--Isothermal Cycle
For the iso-
-isothermal cycle (
and
), we have a non-vanishing heat term along all legs, where the integrals over temperature along the isothermal branches vanish, of course,
Using the fact that along the iso-
branches
, we get for the total heat produced on the cycle,
For the work along the legs, we find
,
and
. The possible net extracted work from the isothermal legs 2 and 4 is now:
Now, and similarly , and thus the probability distribution is more concentrated at low energies than , leading to the conclusion that , and analogously . Similarly, and . Since , the energy contribution to the work along these legs should be negative, resulting in an overall negative work, i.e., the environment performs net work on the hydrogen atom-like system.
Again, we compute the efficiency as
Regarding the excess heat production, all four legs contribute in this case. Thus, we have
Note that
NT and
NK of the individual legs will be determined from the ratios
, where we again will assume that the relaxation times are constant. Employing Equations (37), (42) and (56)–(58), the total thermodynamic length of the cycle is then
Keeping in mind that
depends only on
, we switch variables in all integrals from
and
to
, to obtain
Note that since all legs contribute already to first order to the total thermodynamic length, the times spent in each leg in the optimal case are proportional to the thermodynamic lengths of each leg,
. Since the values of
at the corners are all different, no simple general estimate of assigning times to the various legs appears possible. However, for the special case of (approximately) constant variance
, we find
,
,
and
. From this follows
and thus the optimal times for the four legs we obtain
,
,
and
. Analogous results are obtained using the metric
for the case of minimum entropy production. In particular, if we again assume an approximately constant variance
, we obtain
,
,
and
. From this follows for the total thermodynamic length of the path
, yielding the optimal time assignments to the legs as
,
,
and
.
4.3. Isothermal-Adiabatic Cycle
In the case of the isothermal-adiabatic cycle where the legs 1 and 3 are adiabatic and do not contribute to the heat exchange, we have from legs 2 and 4:
Here, we have used the fact that the temperature does not change along legs 2 and 4, and , and that the entropy does not change along the adiabatic legs, i.e., and .
Since , and furthermore, for equal temperatures, the equilibrium entropy decreases with , i.e., , we have a rejection of heat over the cycle, i.e., the hydrogen atom-like system converts work performed on it into heat transferred to the environment. Furthermore, the term belonging to leg 2 absorbs heat in the system, while along leg 3, we are removing heat from the system.
For the work performed by the system along the four legs, we find
,
,
, and
. Taking the sum along the two isothermal legs, we obtain
where we have used
,
,
, and
. We note that
and thus
as long as
; note that this is exactly the feasibility condition for the special adiabatic-isothermal cycle, Equation (5). Furthermore,
and thus
, suggesting that the net work for these two legs is likely to be negative, i.e., the apparatus does work on the atom and increases its internal energy in the process.
For the “efficiency”, we again compute the ratio between the net work along the isothermal legs and the total heat exchanged
Alternatively, we could use the two adiabatic legs to extract/perform work, i.e.,
in that case, we get for the efficiency
Clearly, , since .
Regarding minimizing the excess heat, we do not include the effect of being “off-target” during the adiabatic legs, and thus we only have the two terms from the isothermal legs 2 and 4:
We can use Equations (37) and (58) to write an expression for the thermodynamic length of the cycle:
We note that for the hydrogen atom-like system, the integrand in the thermodynamic length integrals of the isothermal legs is the square root of the heat capacity, just as in the case of the iso-
legs. We again switch to integration over
, and find for the thermodynamic lengths
since
,
, and
along the special adiabatic legs, and
depends only on
.
As far as distributing the available time over the four legs, we again have to face the problem of the adiabatic legs being of zero thermodynamic length as long as we can keep the controls on target along these legs. We assume a short (i.e.,
) time
needed to move along the adiabatic leg without noticeable deviations from the ideal adiabatic curve in
space and subtract these times from the total available time
,
, before assigning
and
according the principle of constant thermodynamic speed. Assuming constant relaxation times, we determine the optimal times using the ratio of the thermodynamic lengths,
to be
and
. Analogously, when minimizing the entropy production instead of the excess heat, we obtain
, and thus with
, the optimal time assignment to the two legs is
.
Again, due to the sum over microstates with nontrivial energy eigenvalues under the square root, further analytical calculations require additional simplifications; in particular, the optimal placement of the steps along each of the isothermal paths remains open but is expected to vary according to the specific heat.
6. Summary and Discussion
In the previous sections, we have presented three thermodynamic cycles for a hydrogen atom-like system in space, where allows us to control the electronic energy levels of the system: iso--isothermal, iso--adiabatic, and adiabatic-isothermal. We have written down expressions for heat and work along the legs of these cycles and derived conditions that yield optimal ways to run through the cycles in finite time such that the entropy production or the excess heat production is minimal. In particular, we found that the optimal allocation of time—in units of relaxation-to-equilibrium times—including the allocation of discrete steps along the cycle, should take place in such a way that a) the path in space, for given corners and prescribed types of branches, should be chosen such that the total thermodynamic length of the cycle is a minimum, b) the time allocated to each leg of the cycle should be proportional to the thermodynamic length of each leg separately, and c) the discrete steps along each leg should be spaced in such a way that the thermodynamic lengths between all pairs of consecutive points along the branch are identical. We showed that the thermodynamic length could be evaluated using an appropriate metric, or , in probability distribution space, which was obtained as part of the optimal control analysis.
We note that condition a) is trivial for the cycles chosen in the present study, since each leg is completely determined by the assignment of its end points and the type of path, i.e., whether it is iso-, isothermal, or adiabatic. If there were several control parameters that influence the change in the energy levels of the system, then step a) would be a major part of the optimal control problem of minimizing entropy or excess heat production, of course.
Furthermore, we discussed the minimization of excess heat along special adiabatic branches, which by construction equals zero due to the condition that the equilibrium probability distribution is constant along such a special adiabatic path, and thus never an imbalance between actual and equilibrium distribution can build up; for example, by construction, all two-state statistical mechanical systems constitute examples where adiabatic paths are special. Thus, only our inability in practice to keep the control parameters on-target while moving along a special adiabatic path generates deviations from the equilibrium occupation of the microstates of the system, which are the main source of excess heat or entropy production. We derived approximate expressions for the thermodynamic length of generic off-target paths that still remain close to the ideal adiabatic path.
We note that the issue of on-target path control arises for every leg of any cycle, but that one usually ignores these contributions to the entropy or excess heat production. The reason for discounting them is twofold; for one, they tend to be overwhelmed by the effects of having only finite time available to run through the legs of the cycle. Perhaps more important is the fact that an analysis would require information about the apparatus employed to move the system in the space, which is specific to each experiment, and thus usually not within the purview of the theoretical study.
Related finite-time analyses have been performed for thermodynamic engines in the past by assuming, e.g., a generic heat leakage or (inefficient) heat conduction during the processes of the cycle, which are described by phenomenological laws [
43,
44,
45]. However, this leakage was not connected to the issue of being “on-target” vs. “off-target”; instead, the thermodynamic controls were assumed to be perfect, and the inefficiencies associated with, e.g., friction or heat conduction were considered part of the working of the engine.
These optimality criteria and the associated thermodynamic metrics are very general and apply to essentially all statistical mechanical systems, as long as the energy levels can be controlled by a generic parameter
or set of parameters
. Thus, there are connections to other general results [
35,
46,
47,
48,
49,
50]. For example, the excess heat we consider corresponds to the excess work investigated by Sivak and Crooks [
47], and thus Equations (28) and (29) for the thermodynamic lengths
correspond to the generalized thermodynamic length they define via the time-integrated force covariance matrix [
47]. Furthermore, the relationship
for statistical mechanical systems observed in this study corresponds to the conformal equivalence of the energy and entropy metric demonstrated by Salamon and co-workers for thermodynamic equilibrium systems [
50]. This also shows that the excess heat metric
measures the dissipation or loss of availability when proceeding along the path in finite time, as had been shown earlier in the context of optimally measuring free energy differences in statistical mechanical systems [
35] and computing thermodynamic lengths within computer simulations [
46].
Specific to the hydrogen atom-like system is the observation that
for all energy levels
. From this follow certain simplifications, such as the fact that
and
appear only in the combination
in the equilibrium probability distribution
, and therefore all adiabatic paths in
space are special and lie on straight lines that contain the origin
. In particular, we observed that for the isothermal-adiabatic cycle the time allocation to the two isothermal branches should be proportional to the square roots of the temperatures associated with these branches when minimizing the excess heat production. Similarly, for the iso-
-adiabatic cycle, the time allocation for the two iso-
branches should be proportional to the square roots of the
values associated with these branches. In contrast, when minimizing the entropy production of the cycles that contain two adiabatic branches, the optimal times assigned to the two isothermal or iso-
-legs should be equal. Here, we note that the results obtained in
Section 4 would be applicable to any system whose energy spectrum scales with the control parameter
according to Equation (3), such as, e.g., a quantum harmonic oscillator if one modifies only the basic frequency,
, or a spin system with
energy levels in a magnetic field as long as we only change the strength of the applied magnetic field,
. For an analysis of the influence of the eigenvalue spectrum of a system on the finite-time performance, we refer to [
51].
For the case of the hydrogen atom-like system, we have also considered a two-state approximation, which allows us to perform further analytical evaluations of the optimal control conditions. When minimizing excess heat, we can analytically solve the isothermal-adiabatic cycle, while for minimal entropy production, all three cycles can now be solved analytically. These results are quite general and hold for any two-state system, as can be seen from the agreement with results obtained from, e.g., a spin-1/2 system [
28]. Furthermore, the result for the Carnot cycle is reminiscent to the outcome of some finite time optimal control calculations for heat engines, working between two reservoirs [
10], and agrees with corresponding results for the spin 1/2 system [
28].
However, thermal interactions with the environment affect all energy levels, making the two-state approximation of the hydrogen atom somewhat artificial. On the other hand, enforcing transitions via narrow band radiation allows us to focus on single pairs of energy levels, thus providing a more realistic example of a two-state system at the price of dealing with an a-thermal cycle.
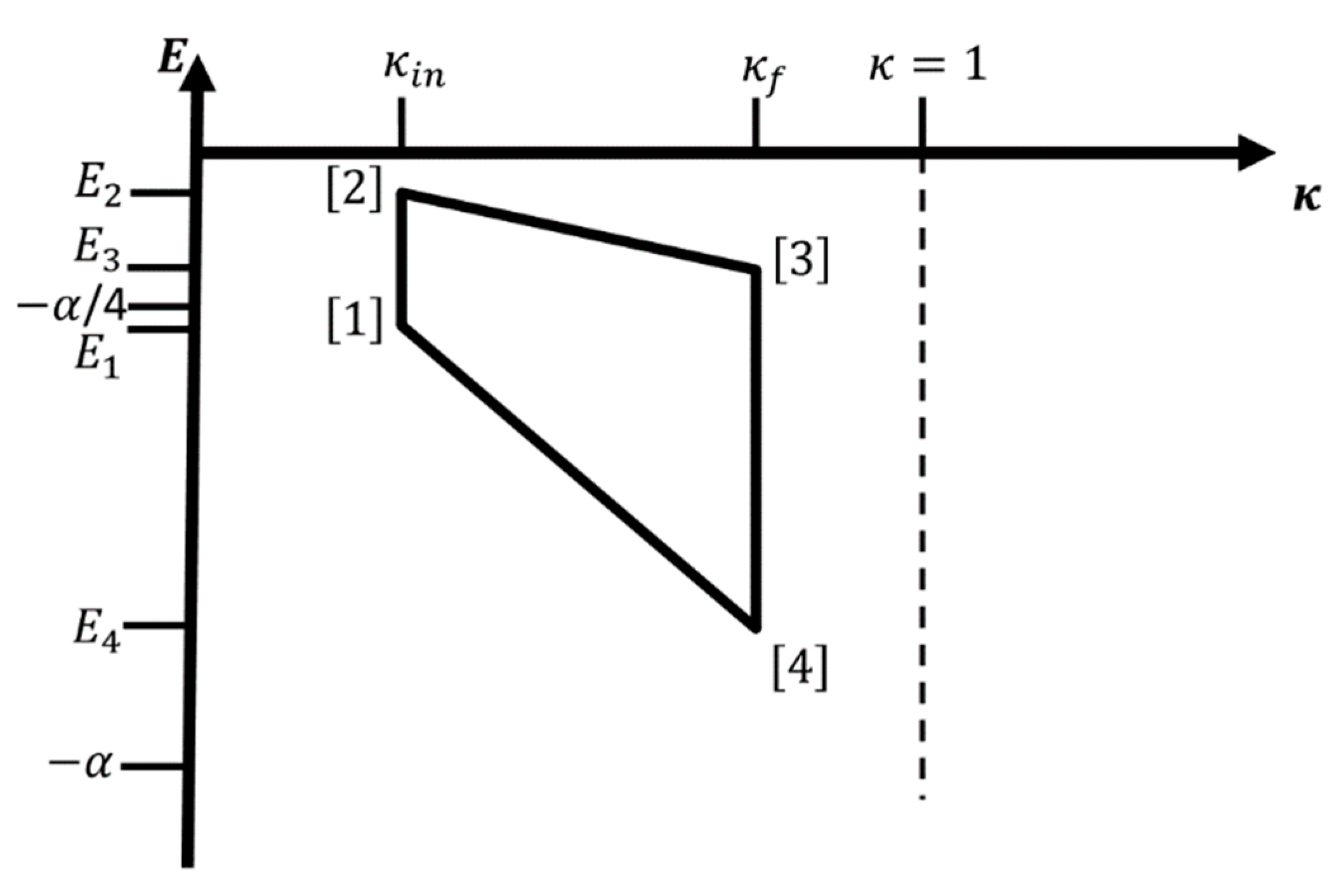
A possible four-leg cycle for the two-state version of a single hydrogen atom-like system (i.e., only two of the electronic energy levels
and
participate in the process) without contact to a heat bath is shown in
Figure 5 for the case
and
. No temperature is involved, and the cycle runs as follows, where we take as starting point the atom in the state
with energy
for
. In leg 1, we excite the atom from
to
via irradiation at frequency
(
), while we keep
at the value
,
. Next, we increase
to
, while keeping the atom in the excited state
, i.e.,
. This is followed by the reverse operations, i.e., we de-excite the atom back to
via irradiation at frequency
(
), while keeping
at the value
,
, followed by the decrease of
to
, while keeping the atom in the state
, thus closing the cycle.
Note that legs 2 and 4 would be the analogues to adiabatic branches in the thermal cycle. There, we perform or extract work on the system by changing the energy content of the hydrogen atom-like system from
to
and from
to
, respectively. The total amount of work (done by the atom) associated with these two legs would be
since
and
. Thus, our external apparatus, which changes
, performs a net amount of work on the atom along these two legs.
Concerning legs 1 and 3, which would be the analogues to the heating and cooling branches of a typical thermodynamic cycle, it is not obvious how to account for the equivalent of heat transferred from and to the heat reservoir at different temperatures, and thus make a connection to thermodynamic cycles. If the radiation fields had a black-body frequency distribution, the formulas of radiation thermodynamics would apply [
52,
53,
54], but this would weaken the desired approximation of the system through only two energy levels. What we can consider is a “reservoir” of photons with frequencies
and
, which the hydrogen atom-like system is in contact with during the excitation and de-excitation processes in legs 1 and 3, respectively. However, such a radiation field that consists of narrow frequency bands does not act as a standard thermal heat source as far as the hydrogen atom-like system is concerned. Instead, we treat the radiation field as part of the external apparatus for the purpose of this discussion, analogous to the pressure we might apply to the material to change the properties of the excitons representing the hydrogen atom-like system. As a consequence, the net work done by the radiation field on the atom equals
i.e., the energy of the radiation field shows a net increase along legs 1 and 3 by the same amount as the atom gained in energy when we applied work along legs 2 and 4.
We can thus visualize the two-state approximation of our hydrogen atom-like system as an “engine” that acts as a “frequency conversion pump” while keeping the number of photons conserved. A possible co-efficient of performance would be
i.e., the a-thermal cycle of the single controllable hydrogen atom-like system represents a perfect energy conversion apparatus from photons with one frequency to photons with another frequency. We note that this perfect restriction of the hydrogen atom-like system to a two-state system would also work if the modification of the Hamiltonian breaks the symmetry of the Coulomb field, as might be the case by exposing the hydrogen atom to an anisotropic external electric field inside a cavity.
Of course, in practice, this a-thermal cycle will encounter finite-time losses, too. The sources of possible finite-time losses have to be identified in an analysis of the quantum processes involved when changing the Hamiltonian and exciting/de-exciting the electrons between the two levels, including the degree of coherence maintained in the system along each leg. An analysis of these issues would go beyond the purview of this study, however. Possible approaches on how to analyze these issues can be found in some of the cited references dealing explicitly with the quantum aspects of heat engines [
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
48,
49,
51].
A final question is whether there are experimental systems where the thermodynamic or a-thermal cycles discussed in this study might be realized. The most straightforward suggestion would be to take an individual hydrogen atom inside a cavity whose shape can be changed, such that the energy levels can be modified in some way [
31]. This system should allow an a-thermal cycle, but the momentum transfer during absorption would start injecting kinetic energy, which would re-appear as a source of heat to the system. In the case of a thermodynamic cycle, where we were to expose the atom to a heat reservoir over a sufficiently large temperature range for exciting the hydrogen atom(s) to a noticeable degree, the system’s translational degrees of freedom would quickly establish thermal movement with all that implies, possibly overwhelming the thermodynamic quantities of interest in the electronic two-state system. However, since the experimental control of the hydrogen atom as a thermal engine based on the translational degrees of freedom has been quite successful already [
13], a quasi-a-thermal or a thermodynamic engine based on the occupation of the electronic states and on the modification of their energy levels should be feasible.
An alternative would be an excitonic defect in a solid, since this is usually more localized (but still mobile, in principle) and thus probably less susceptible to the kinetic thermal contributions. Furthermore, the reference value of , , should be rather small, and thus small changes in temperature would already lead to noticeable changes in the occupation probabilities of the excitonic states. On the other hand, as mentioned earlier, varying in a solid can be nontrivial, since the quantities entering will depend on both pressure—which would be a suitable control variable—and temperature. As a consequence, in a space representation, the iso- branches would involve closely coordinated changes both in pressure and temperature. Additionally, an a-thermal cycle might be difficult to achieve, due to the strong thermal coupling of the exciton to the rest of the solid. Nevertheless, it appears that a variety of systems exist which might serve as substrates for the realization of an electronic states-based engine with a single hydrogen atom-like system as the working fluid.