On the Rarefied Gas Experiments
Abstract
1. Introduction
2. Experiments
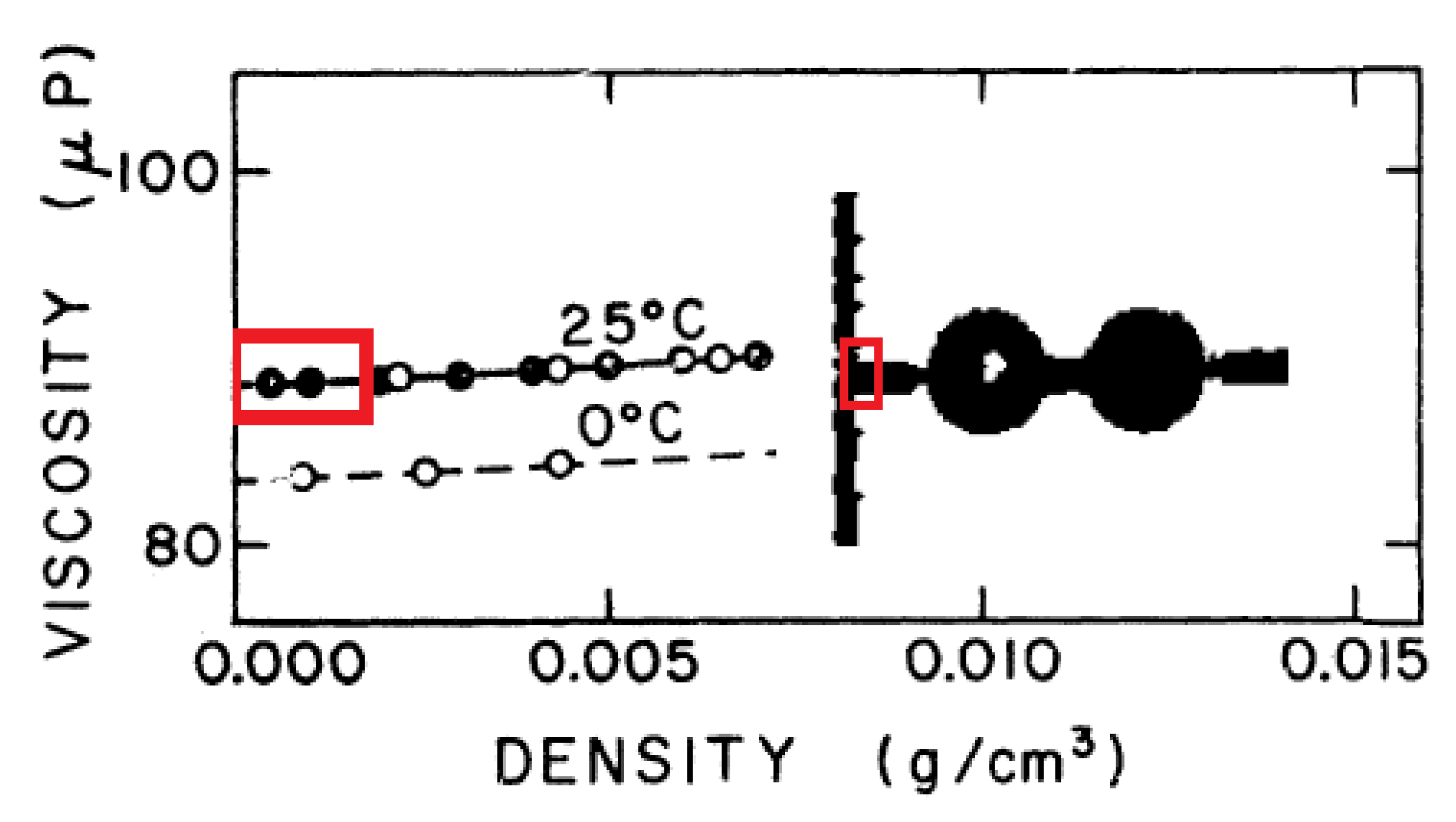
2.1. Density Dependence of Material Parameters
- the viscosities are constant: for shear viscosity and for the bulk part,
- the thermal conductivity is also constant: (in which c being the specific heat),
- as a consequence of the above, the relaxation times are inversely proportional with mass density: .
- both viscosities and the thermal conductivity are constant:Pas, Pas and W/(mK), respectively.
- all relaxation times and the coupling coefficients have dependence.
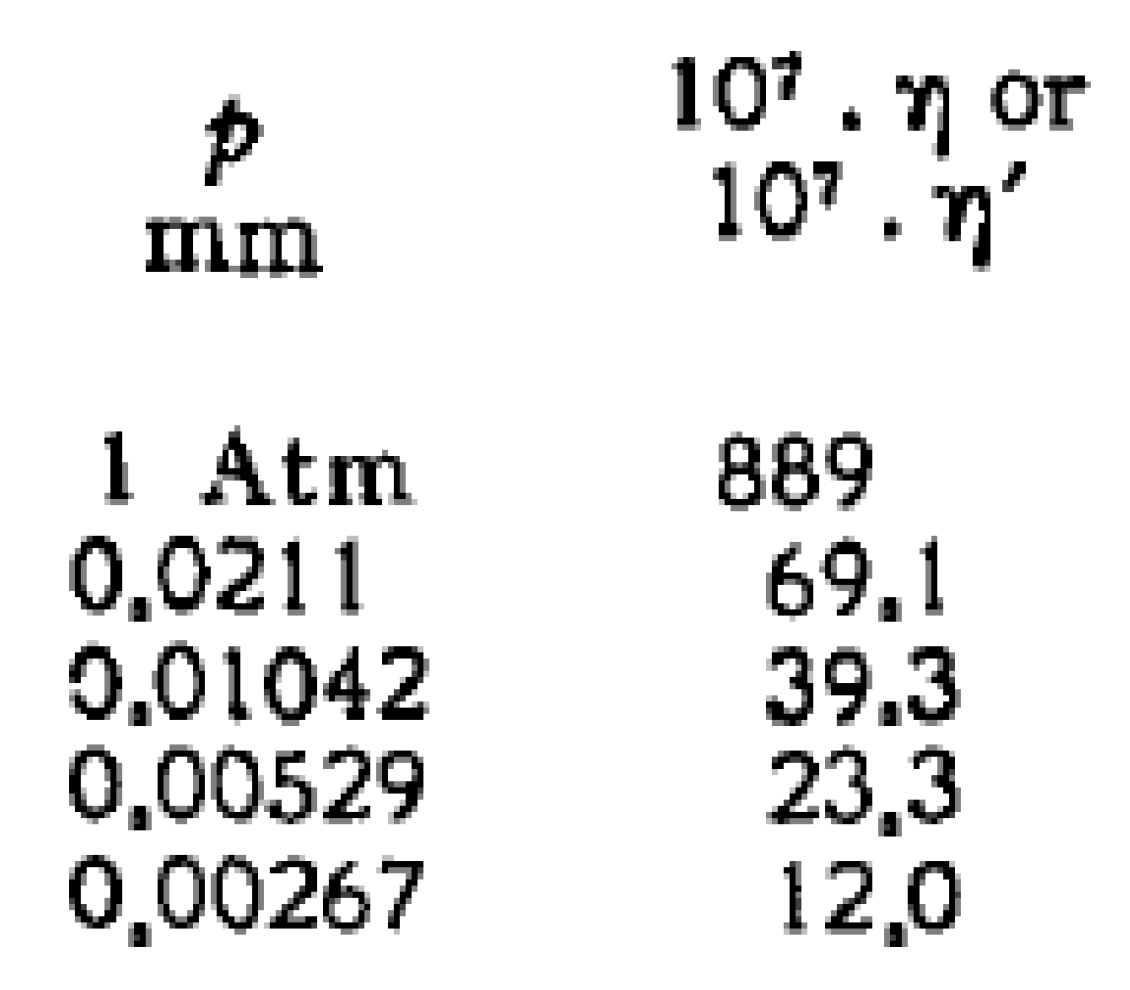
2.2. Frequency-Pressure Dependence
2.3. Evaluations, Comparisons and Conclusions
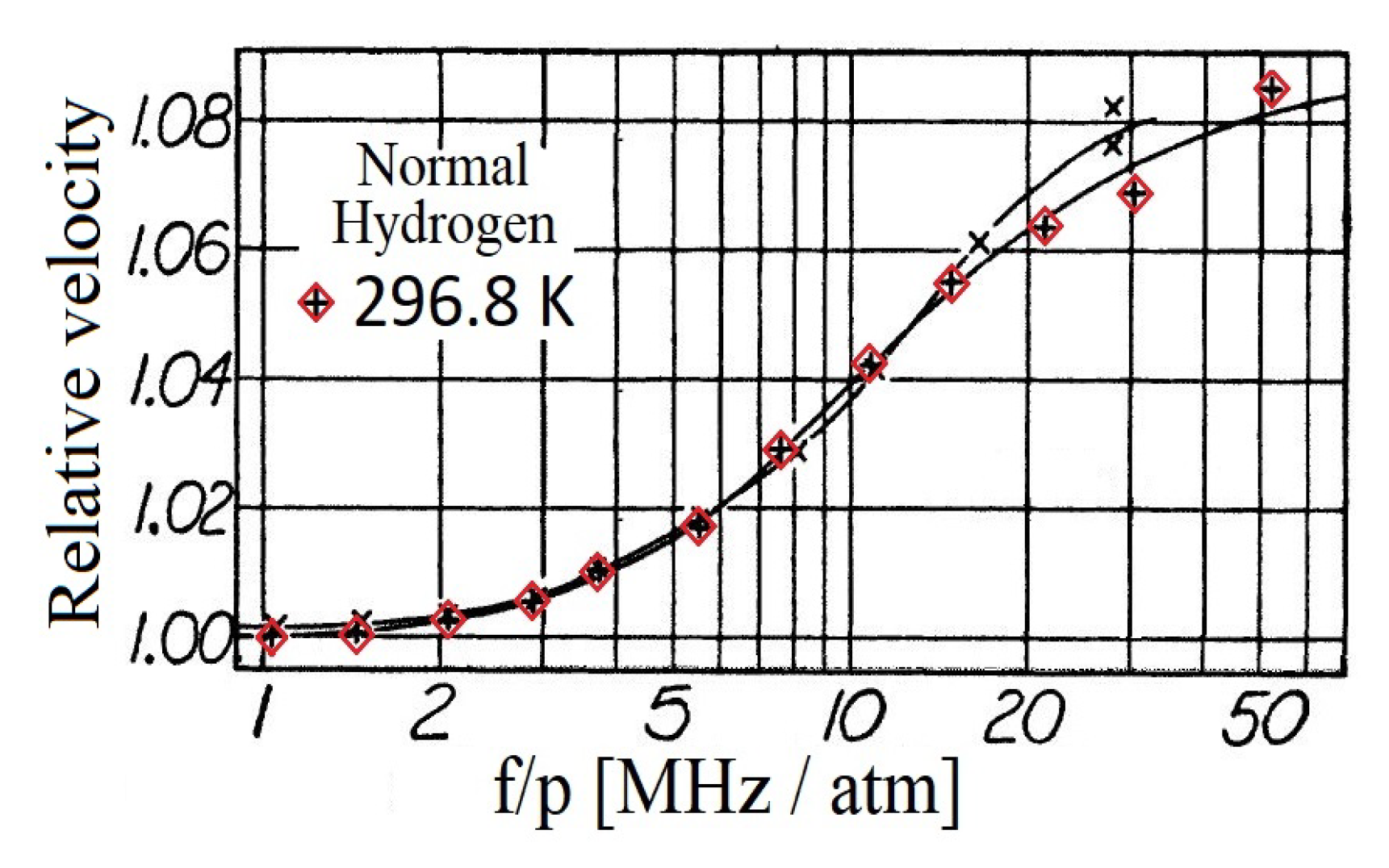
- Dimensionless frequency is used instead of , moreover, hence the shear viscosity is used to rescale the experimental data. Moreover, the papers [24,43] do not reflect the fact that the frequency was constant. It could be confusing hence it hides that the mass density is the real independent variable in the equations in which all new coefficients (relaxation times and coupling parameters) depend on .
- Regarding the temperature, K is used instead of K. It is seemingly a small difference; however, when one attempts to calculate the mass density from the data , it leads to a different value, i.e., slightly inaccurate the data in [24]. As a consequence, even the speed of sound is slightly inaccurate that used for vertical scale.
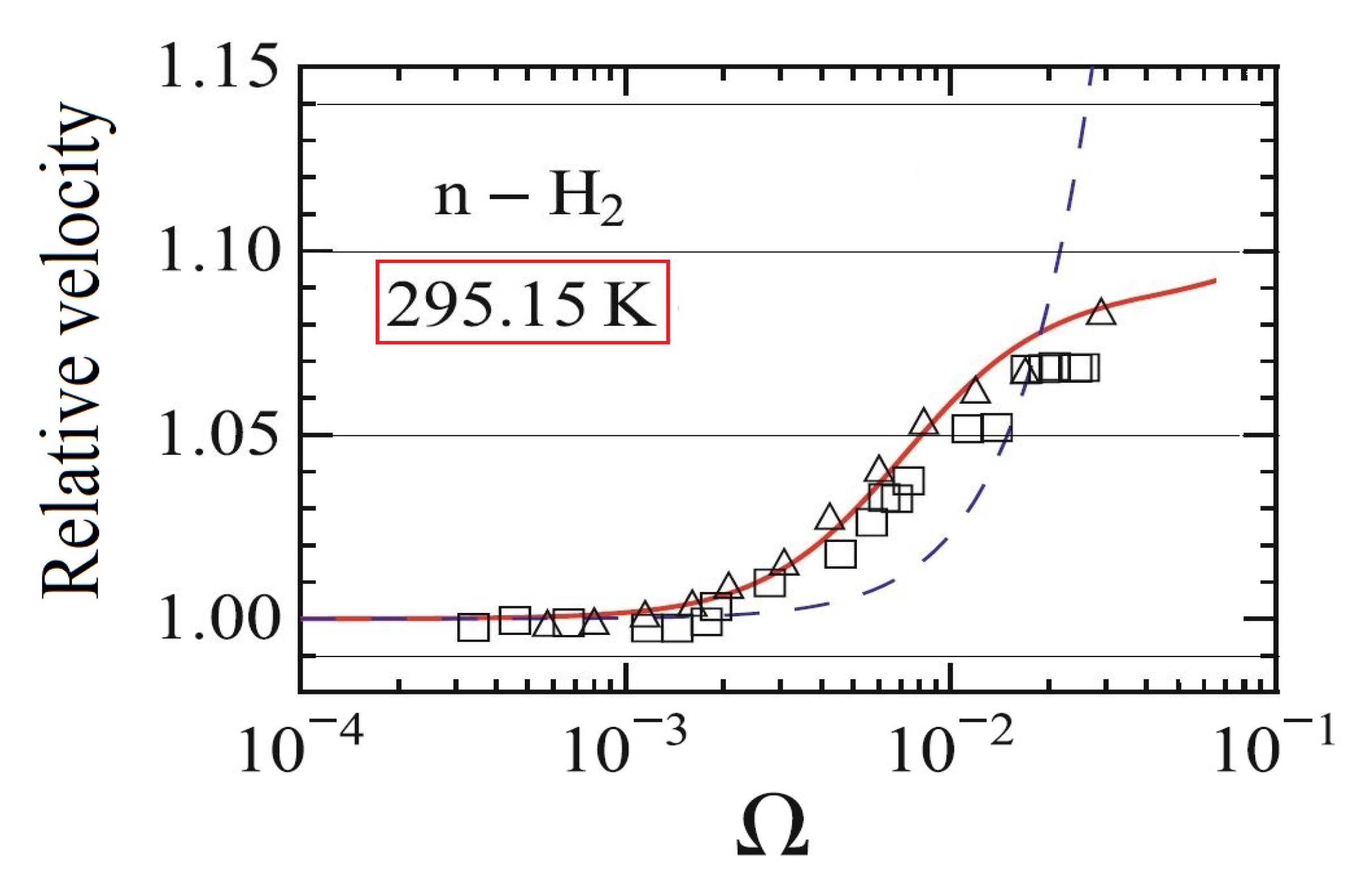
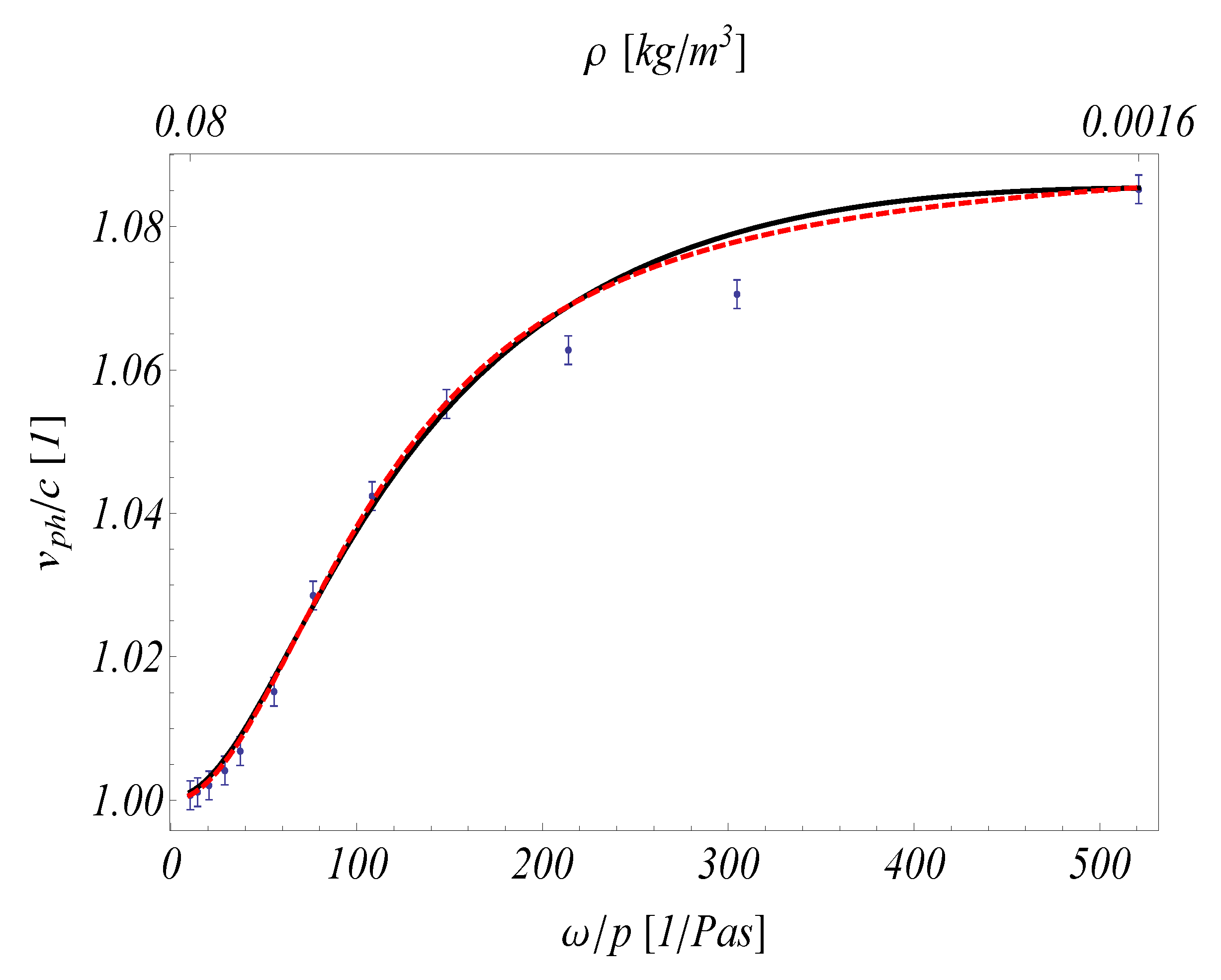
- One single parameter set is used to fit the data from papers [50,53,80] (Figure 4). The relevant data is the following: , , since the coupling coefficients are also proportional with the relaxation times. It is apparent from Figure 4 that for other measurements, even on the same material, these ratios may be different.
3. Discussion
Funding
Acknowledgments
Conflicts of Interest
References
- Chester, M. Second sound in solids. Phys. Rev. 1963, 131, 2013–2015. [Google Scholar] [CrossRef]
- Guyer, R.A.; Krumhansl, J.A. Thermal Conductivity, Second Sound, and Phonon Hydrodynamic Phenomena in Nonmetallic Crystals. Phys. Rev. 1966, 148, 778–788. [Google Scholar] [CrossRef]
- Jackson, H.E.; Walker, C.T.; McNelly, T.F. Second sound in NaF. Phys. Rev. Lett. 1970, 25, 26–28. [Google Scholar] [CrossRef]
- Rogers, S.J. Transport of heat and approach to second sound in some isotropically pure alkali-halide crystals. Phys. Rev. B 1971, 3, 1440. [Google Scholar] [CrossRef]
- Narayanamurti, V.; Dynes, R.C. Observation of second sound in bismuth. Phys. Rev. Lett. 1972, 28, 1461–1465. [Google Scholar] [CrossRef]
- Gyarmati, I. On the wave approach of thermodynamics and some problems of non-linear theories. J. Non-Equilib. Thermodyn. 1977, 2, 233–260. [Google Scholar] [CrossRef]
- Joseph, D.D.; Preziosi, L. Heat waves. Rev. Mod. Phys. 1989, 61, 41. [Google Scholar] [CrossRef]
- Joseph, D.D.; Preziosi, L. Addendum to the paper on heat waves. Rev. Mod. Phys. 1990, 62, 375–391. [Google Scholar] [CrossRef]
- Dreyer, W.; Struchtrup, H. Heat pulse experiments revisited. Contin. Mech. Thermodyn. 1993, 5, 3–50. [Google Scholar] [CrossRef]
- McNelly, T.F.; Rogers, S.J.; Channin, D.J.; Rollefson, R.J.; Goubau, W.M.; Schmidt, G.E.; Krumhansl, J.A.; Pohl, R.O. Heat pulses in NaF: Onset of second sound. Phys. Rev. Lett. 1970, 24, 100–102. [Google Scholar] [CrossRef]
- McNelly, T.F. Second Sound and Anharmonic Processes in Isotopically Pure Alkali-Halides. Ph.D. Thesis, Cornell University, Ithaca, NY, USA, 1974. [Google Scholar]
- Kovács, R.; Ván, P. Generalized heat conduction in heat pulse experiments. Int. J. Heat Mass Transf. 2015, 83, 613–620. [Google Scholar] [CrossRef]
- Ván, P. Weakly nonlocal irreversible thermodynamics—The Guyer-Krumhansl and the Cahn-Hilliard equations. Phys. Lett. A 2001, 290, 88–92. [Google Scholar] [CrossRef]
- Ván, P.; Fülöp, T. Universality in heat conduction theory—Weakly nonlocal thermodynamics. Annalen der Physik (Berlin) 2012, 524, 470–478. [Google Scholar] [CrossRef]
- Chen, G. Ballistic-diffusive heat-conduction equations. Phys. Rev. Lett. 2001, 86, 2297–2300. [Google Scholar] [CrossRef]
- Cimmelli, V.A. Different thermodynamic theories and different heat conduction laws. J. Non-Equilib. Thermodyn. 2009, 34, 299–333. [Google Scholar] [CrossRef]
- Ván, P. Theories and heat pulse experiments of non-Fourier heat conduction. Commun. Appl. Ind. Math. 2016, 7, 150–166. [Google Scholar] [CrossRef]
- Jou, D.; Casas-Vázquez, J.; Lebon, G. Extended Irreversible Thermodynamics, 4th ed.; Springer: Berlin, Germany, 2010. [Google Scholar]
- Sellitto, A.; Cimmelli, V.A.; Jou, D. Mesoscopic Theories of Heat Transport in Nanosystems; Springer: Berlin, Germany, 2016. [Google Scholar]
- Szücs, M.; Fülöp, T. Kluitenberg-Verhás rheology of solids in the GENERIC framework. arXiv 2019, arXiv:1812.07052. [Google Scholar] [CrossRef]
- Müller, I.; Ruggeri, T. Rational Extended Thermodynamics; Springer: Berlin, Germany, 1998. [Google Scholar]
- Kovács, R.; Ván, P. Models of Ballistic Propagation of Heat at Low Temperatures. Int. J. Thermophys. 2016, 37, 95. [Google Scholar] [CrossRef]
- Kovács, R.; Ván, P. Second sound and ballistic heat conduction: NaF experiments revisited. Int. J. Heat Mass Transf. 2018, 117, 682–690. [Google Scholar] [CrossRef]
- Ruggeri, T.; Sugiyama, M. Rational Extended Thermodynamics Beyond the Monatomic Gas; Springer: Berlin, Germany, 2015. [Google Scholar]
- Kovács, R. Heat Conduction beyond Fourier’s Law: Theoretical Predictions and Experimental Validation. Ph.D. Thesis, Budapest University of Technology and Economics (BME), Budapest, Hungary, 2017. [Google Scholar]
- Kovács, R.; Madjarević, D.; Simić, S.; Ván, P. Theories of rarefied gases. arXiv 2018, arXiv:1812.10355. [Google Scholar]
- Both, S.; Czél, B.; Fülöp, T.; Gróf, G.; Gyenis, Á.; Kovács, R.; Ván, P.; Verhás, J. Deviation from the Fourier law in room-temperature heat pulse experiments. J. Non-Equilib. Thermodyn. 2016, 41, 41–48. [Google Scholar] [CrossRef]
- Ván, P.; Berezovski, A.; Fülöp, T.; Gróf, G.; Kovács, R.; Lovas, Á.; Verhás, J. Guyer-Krumhansl-type heat conduction at room temperature. EPL 2017, 118, 50005. [Google Scholar] [CrossRef]
- Fülöp, T.; Kovács, R.; Lovas, Á.; Rieth, Á.; Fodor, T.; Szücs, M.; Ván, P.; Gróf, G. Emergence of non-Fourier hierarchies. Entropy 2018, 20, 832. [Google Scholar] [CrossRef]
- Struchtrup, H. Macroscopic Transport Equations for Rarefied Gas Flows; Springer: Berlin, Germany, 2005. [Google Scholar]
- Nyíri, B. On the entropy current. J. Non-Equilib. Thermodyn. 1991, 16, 179–186. [Google Scholar] [CrossRef]
- Jou, D.; Casas-Vázquez, J.; Lebon, G. Extended Irreversible Thermodynamics. Rep. Prog. Phys. 1988, 51, 1105. [Google Scholar] [CrossRef]
- Alvarez, F.X.; Jou, D.; Sellitto, A. Phonon hydrodynamics and phonon- boundary scattering in nanosystems. J. Appl. Phys. 2009, 105, 014317. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, M. Phonon hydrodynamics and its applications in nanoscale heat transport. Phys. Rep. 2015, 595, 1–44. [Google Scholar] [CrossRef]
- Struchtrup, H. Resonance in rarefied gases. Contin. Mech. Thermodyn. 2012, 24, 361–376. [Google Scholar] [CrossRef]
- Berezovski, A.; Ván, P. Microinertia and internal variables. arXiv 2015, arXiv:1504.03485. [Google Scholar]
- Berezovski, A.; Ván, P. Internal Variables in Thermoelasticity; Springer: Basel, Switzerland, 2017. [Google Scholar]
- Verhás, J. Thermodynamics and Rheology; Springer: Dordrecht, The Netherlands, 1997. [Google Scholar]
- Meixner, J. Absorption und Dispersion des Schalles in Gasen mit Chemisch Reagierenden und Anregbaren Komponenten. I. Teil. Annalen der Physik 1943, 435, 470–487. (In German) [Google Scholar] [CrossRef]
- Sellitto, A.; Cimmelli, V.A.; Jou, D. Entropy flux and anomalous axial heat transport at the nanoscale. Phys. Rev. B 2013, 87, 054302. [Google Scholar] [CrossRef]
- Arima, T.; Taniguchi, S.; Ruggeri, T.; Sugiyama, M. Extended thermodynamics of dense gases. Contin. Mech. Thermodyn. 2012, 24, 271–292. [Google Scholar] [CrossRef]
- Arima, T.; Taniguchi, S.; Ruggeri, T.; Sugiyama, M. Extended thermodynamics of real gases with dynamic pressure: An extension of Meixner’s theory. Phys. Lett. A 2012, 376, 2799–2803. [Google Scholar] [CrossRef]
- Arima, T.; Taniguchi, S.; Ruggeri, T.; Sugiyama, M. Dispersion relation for sound in rarefied polyatomic gases based on extended thermodynamics. Contin. Mech. Thermodyn. 2013, 25, 727–737. [Google Scholar] [CrossRef]
- Pavić, M.; Ruggeri, T.; Simić, S. Maximum entropy principle for rarefied polyatomic gases. Phys. A Stat. Mech. Appl. 2013, 392, 1302–1317. [Google Scholar] [CrossRef]
- Lebon, G.; Cloot, A. Propagation of ultrasonic sound waves in dissipative dilute gases and extended irreversible thermodynamics. Wave Motion 1989, 11, 23–32. [Google Scholar] [CrossRef]
- Carrasi, M.; Morro, A. A modified Navier-Stokes equation, and its consequences on sound dispersion. Il Nuovo Cimento B 1972, 9, 321–343. [Google Scholar] [CrossRef]
- Carrasi, M.; Morro, A. Some remarks about dispersion and absorption of sound in monatomic rarefied gases. Il Nuovo Cimento B 1973, 13, 281–289. [Google Scholar] [CrossRef]
- Meyer, E.; Sessler, G. Schallausbreitung in gasen bei hohen frequenzen und sehr niedrigen drucken. Zeitschrift für Physik 1957, 149, 15–39. (In German) [Google Scholar] [CrossRef]
- Klimontovich, Y.L. Statistical Theory of Open Systems: A Unified Approach to Kinetic Description of Processes in Active Systems; Springer: Dordrecht, The Netherlands, 1995. [Google Scholar]
- Rhodes, J.E., Jr. The velocity of sound in hydrogen when rotational degrees of freedom fail to be excited. Phys. Rev. 1946, 70, 932. [Google Scholar] [CrossRef]
- Sette, D.; Busala, A.; Hubbard, J.C. Energy transfer by collisions in vapors of chlorinated methanes. J. Chem. Phys. 1955, 23, 787–793. [Google Scholar] [CrossRef]
- Greenspan, M. Propagation of sound in five monatomic gases. J. Acoust. Soc. Am. 1956, 28, 644–648. [Google Scholar] [CrossRef]
- Sluijter, C.G.; Knaap, H.F.P.; Beenakker, J.J.M. Determination of rotational relaxation times of hydrogen isotopes by sound absorption measurements at low temperatures I. Physica 1964, 30, 745–762. [Google Scholar] [CrossRef]
- Sluijter, C.G.; Knaap, H.F.P.; Beenakker, J.J.M. Determination of rotational relaxation times of hydrogen isotopes by sound absorption measurements at low temperatures II. Physica 1965, 31, 915–940. [Google Scholar] [CrossRef]
- Stewart, J.L. A variable path ultrasonic interferometer for the four megacycle region with some measurements on air, co2, and h2. Rev. Sci. Instrum. 1946, 17, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Truesdell, C.; Muncaster, R.G. Fundamentals of Maxwel’s Kinetic Theory of a Simple Monatomic Gas: Treated as a Branch of Rational Mechanics; Academic Press: Cambridge, MA, USA, 1980; Volume 83. [Google Scholar]
- Pollard, W.G.; Present, R.D. On gaseous self-diffusion in long capillary tubes. Phys. Rev. 1948, 73, 762. [Google Scholar] [CrossRef]
- Chapman, S.; Cowling, T. The Mathematical Theory of Non-uniform Gases, 3rd ed.; Cambridge University Press: New York, NY, USA, 1970. [Google Scholar]
- Michalis, V.K.; Kalarakis, A.N.; Skouras, E.D.; Burganos, V.N. Rarefaction effects on gas viscosity in the Knudsen transition regime. Microfluid. Nanofluidics 2010, 9, 847–853. [Google Scholar] [CrossRef]
- Cohen, Y.; Sandler, S.I. The viscosity and thermal conductivity of simple dense gases. Ind. Eng. Chem. Fundam. 1980, 19, 186–188. [Google Scholar] [CrossRef]
- van der Gulik, P.S.; Trappeniers, N.J. The viscosity of argon at high densities. Phys. A Stat. Mech. Appl. 1986, 135, 1–20. [Google Scholar] [CrossRef]
- van der Gulik, P.S.; Trappeniers, N.J. Application of Enskog theory on the viscosity of argon. Physica B + C 1986, 139, 137–139. [Google Scholar] [CrossRef]
- van der Gulik, P.S.; ten Seldam, C.A. Density dependence of the viscosity of some noble gases. Int. J. Thermophys. 2002, 23, 15–26. [Google Scholar] [CrossRef]
- Dymond, J.H. Corrections to the Enskog theory for viscosity and thermal conductivity. Physica B 1987, 144, 267–276. [Google Scholar] [CrossRef]
- Umla, R.; Vesovic, V. Viscosity of liquids—Enskog-2σ model. Fluid Phase Equilib. 2014, 372, 34–42. [Google Scholar] [CrossRef]
- Gracki, J.A.; Flynn, G.P.; Ross, J. Viscosity of Nitrogen, Helium, Hydrogen, and Argon from −100 to 25 c up to 150–250 Atmospheres; Project SQUID Technical Report; Purdue University: Lafayette, IN, USA, 1969; 33p. [Google Scholar]
- Gracki, J.A.; Flynn, G.P.; Ross, J. Viscosity of Nitrogen, Helium, Hydrogen, and Argon from −100 to 25 c up to 150–250 atm. J. Chem. Phys. 1969, 9, 3856–3863. [Google Scholar] [CrossRef]
- Haynes, W.M. Viscosity of gaseous and liquid argon. Physica 1973, 67, 440–470. [Google Scholar] [CrossRef]
- Liboff, R.L. Kinetic Theory: Classical, Quantum, and Relativistic Descriptions; Springer Science & Business Media: Basel, Switzerland, 2003. [Google Scholar]
- van Itterbeek, A.; Keesom, W.H. Measurements on the viscosity of helium gas between 293 and 1.6 k. Physica 1938, 5, 257–269. [Google Scholar] [CrossRef]
- van Itterbeek, A.; Claes, A. Measurements on the viscosity of hydrogen-and deuterium gas between 293 K and 14 K. Physica 1938, 5, 938–944. [Google Scholar] [CrossRef]
- van Itterbeek, A.; van Paemel, O. Measurements on the viscosity of gases for low pressures at room temperature and at low temperatures. Physica 1940, 7, 273–283. [Google Scholar] [CrossRef]
- Beskok, A.; Karniadakis, G.E. Report: A model for flows in channels, pipes, and ducts at micro and nano scales. Microscale Thermophys. Eng. 1999, 3, 43–77. [Google Scholar]
- Roohi, E.; Darbandi, M. Extending the Navier–Stokes solutions to transition regime in two-dimensional micro-and nanochannel flows using information preservation scheme. Phys. Fluids 2009, 21, 082001. [Google Scholar] [CrossRef]
- Galindo-Rosales, F.J.; Rubio-Hernández, F.J.; Sevilla, A. An apparent viscosity function for shear thickening fluids. J. Non-Newton. Fluid Mech. 2011, 166, 321–325. [Google Scholar] [CrossRef]
- Møller, P.C.F.; Fall, A.; Bonn, D. Origin of apparent viscosity in yield stress fluids below yielding. Europhys. Lett. 2009, 87, 38004. [Google Scholar] [CrossRef]
- Falls, A.H.; Musters, J.J.; Ratulowski, J. The apparent viscosity of foams in homogeneous bead packs. SPE Reserv. Eng. 1989, 4, 155–164. [Google Scholar] [CrossRef]
- Lipowsky, H.H.; Usami, S.; Chien, S. In vivo measurements of “apparent viscosity” and microvessel hematocrit in the mesentery of the cat. Microvasc. Res. 1980, 19, 297–319. [Google Scholar] [CrossRef]
- Metzner, A.B.; Otto, R.E. Agitation of non-Newtonian fluids. AIChE J. 1957, 3, 3–10. [Google Scholar] [CrossRef]
- Winter, T.G.; Hill, G.L. High-temperature ultrasonic measurements of rotational relaxation in hydrogen, deuterium, nitrogen, and oxygen. J. Acoust. Soc. Am. 1967, 42, 848–858. [Google Scholar] [CrossRef]
| , | , | , | ||
|---|---|---|---|---|
| 144 |
| , | , | , | , |
|---|---|---|---|
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovács, R. On the Rarefied Gas Experiments. Entropy 2019, 21, 718. https://doi.org/10.3390/e21070718
Kovács R. On the Rarefied Gas Experiments. Entropy. 2019; 21(7):718. https://doi.org/10.3390/e21070718
Chicago/Turabian StyleKovács, Róbert. 2019. "On the Rarefied Gas Experiments" Entropy 21, no. 7: 718. https://doi.org/10.3390/e21070718
APA StyleKovács, R. (2019). On the Rarefied Gas Experiments. Entropy, 21(7), 718. https://doi.org/10.3390/e21070718





