A Parsimonious Granger Causality Formulation for Capturing Arbitrarily Long Multivariate Associations
Abstract
1. Introduction
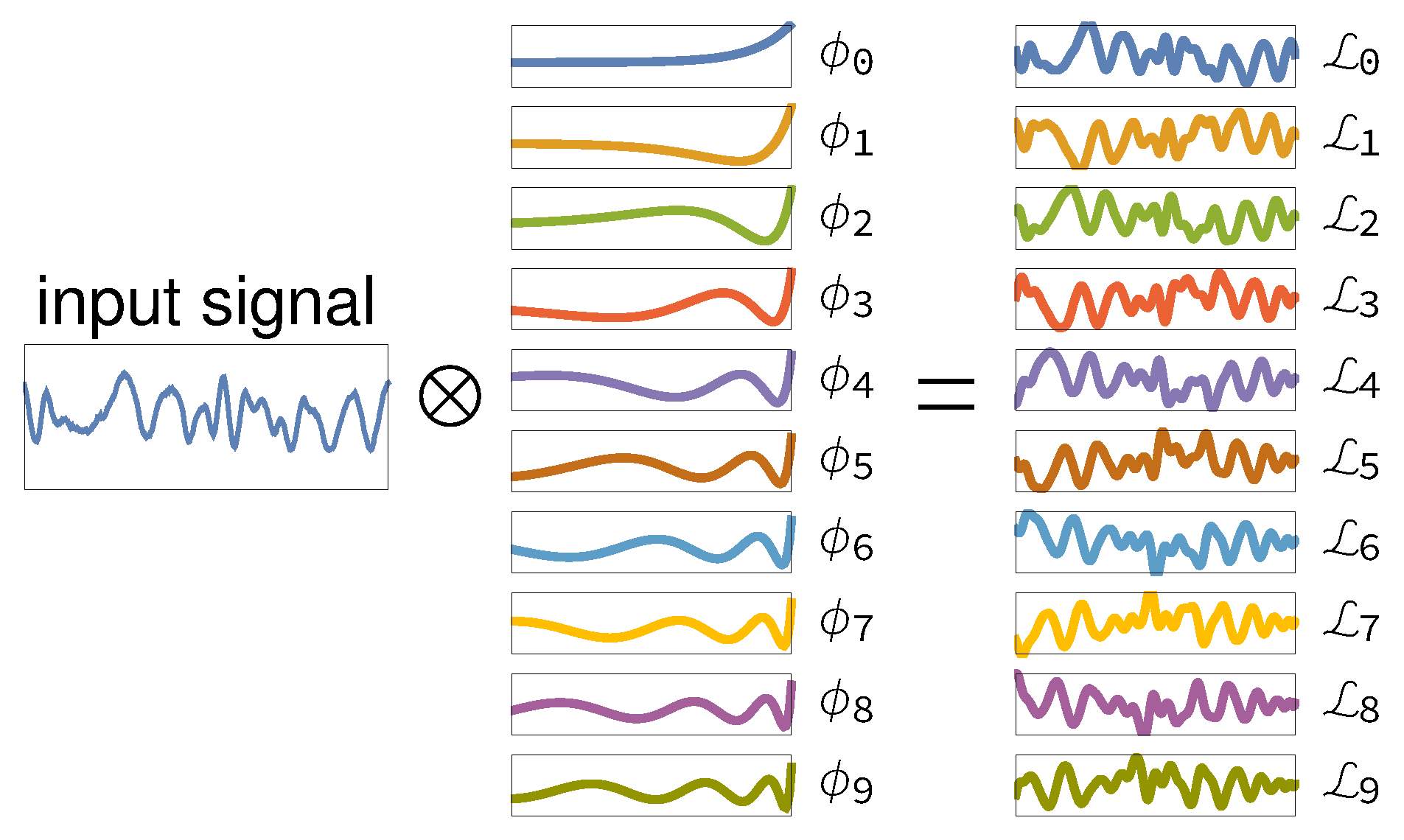
2. Theory
3. Methods
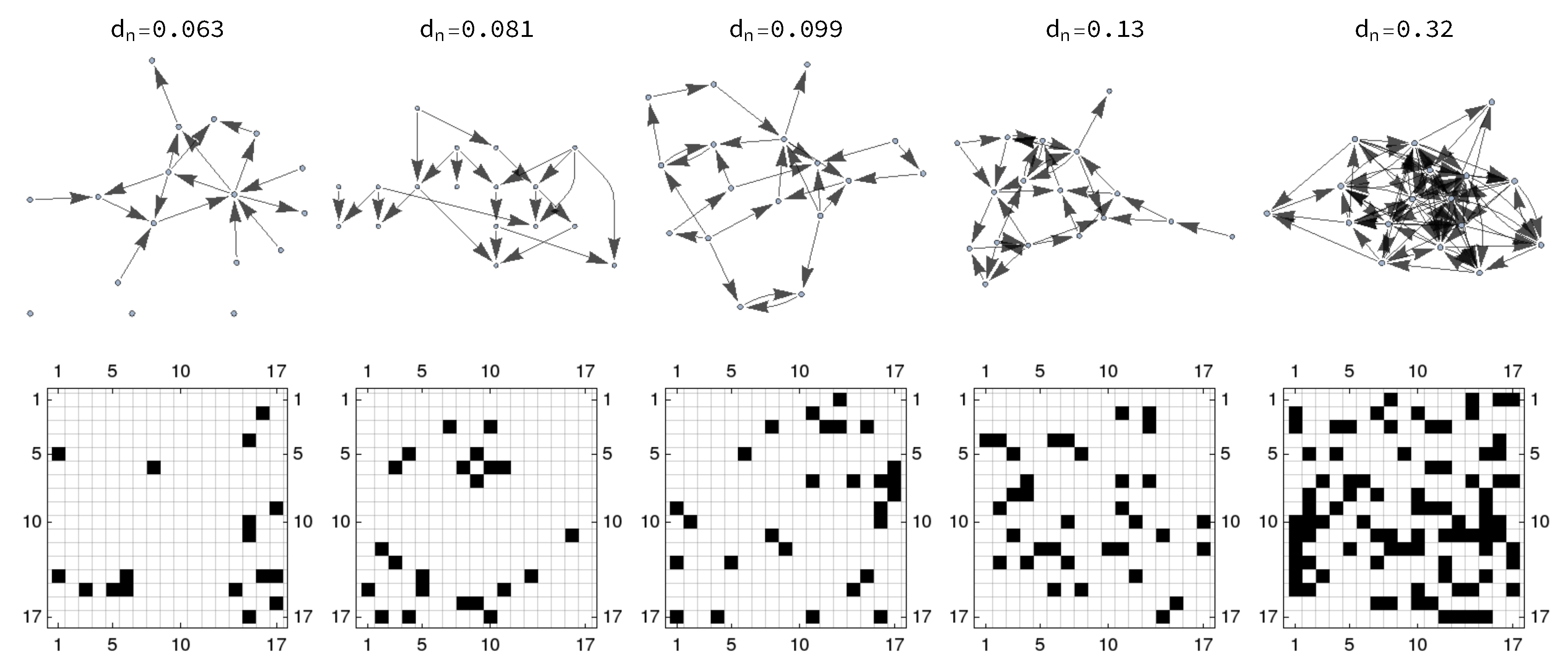
3.1. Network Generation
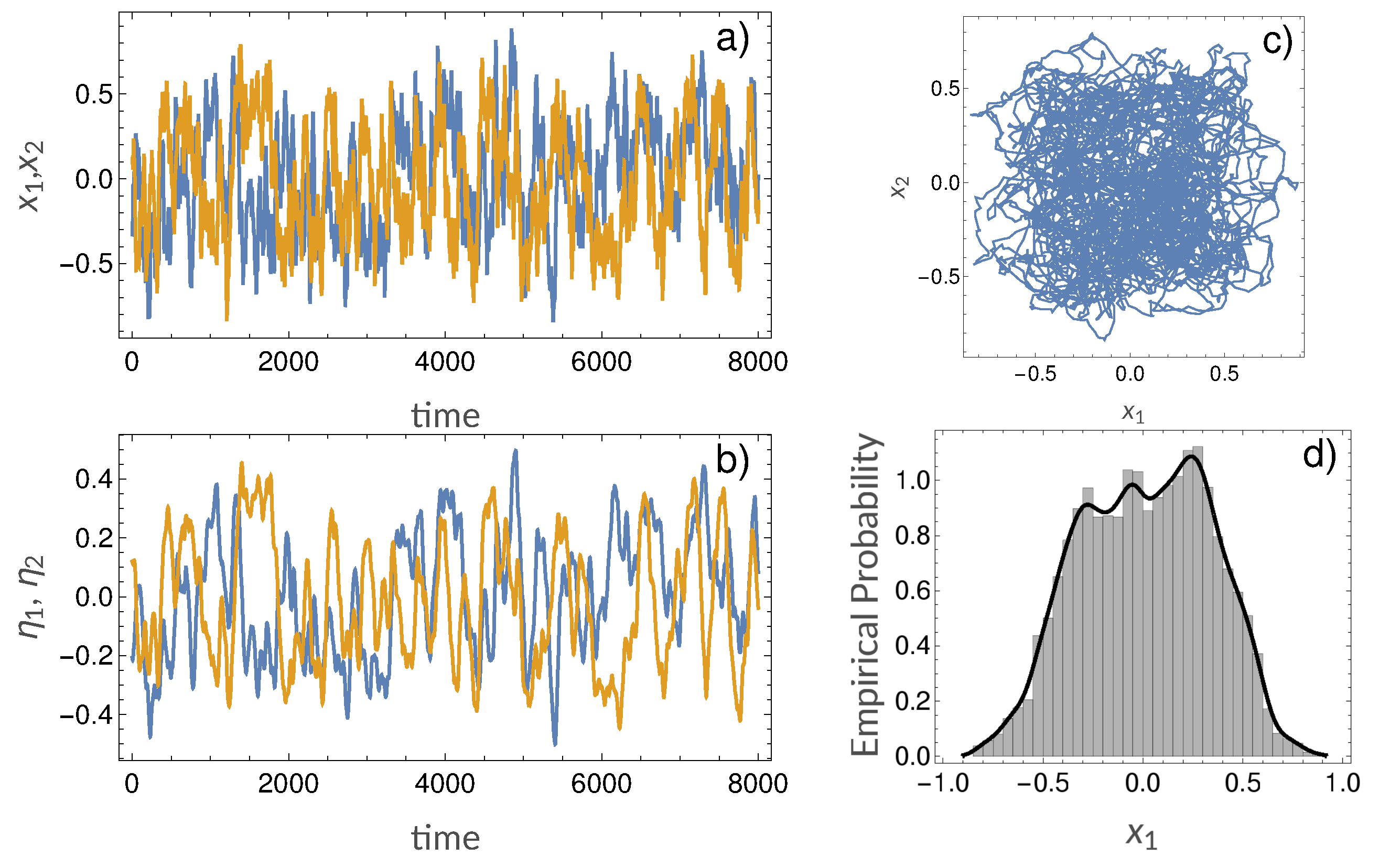
3.2. Synthetic Simulations
3.3. Directed Brain Connectivity Estimation in MEG Data
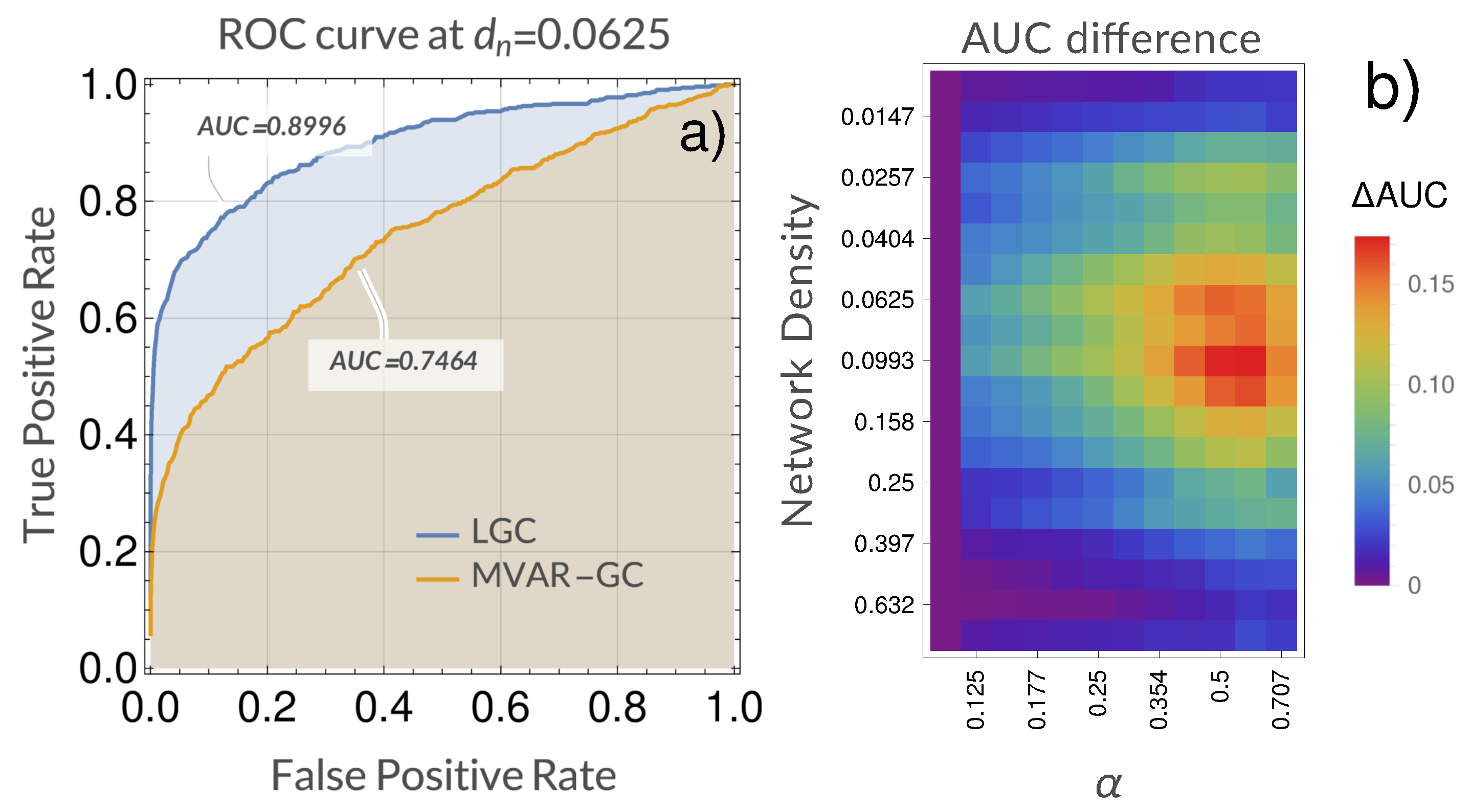
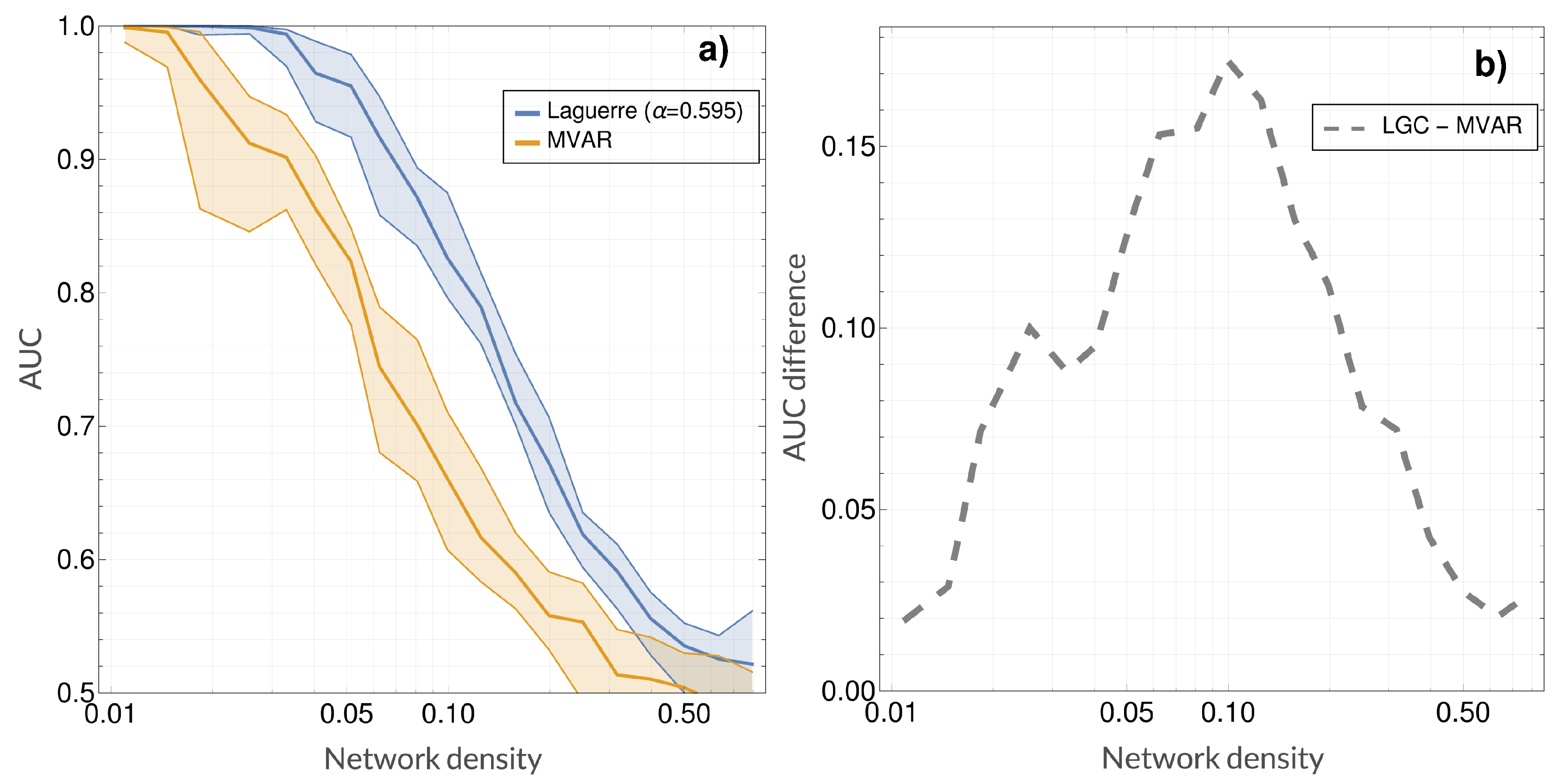
4. Results
5. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AUC | Area under ROC curve |
| ECG | Electrocardiography |
| EEG | Electroencephalography |
| EMG | Electromyography |
| EOG | Electrooculography |
| fMRI | Functional magnetic resonance imaging |
| HCP | Human Connectome Project |
| ICA | Independent component analysis |
| LASSO | Least absolute shrinkage and selection operator |
| LGC | Laguerre Granger causality |
| MEG | Magnetoencephalography |
| MVAR-GC | MultiVARiate Granger causality |
| RM | Restricted model |
| rMEG | Resting-state magnetoencephalography |
| ROC | Receiver operating characteristic |
| RSS | Residual sum of squares |
| UM | Enrestricted model |
References
- Astolfi, L.; Cincotti, F.; Mattia, D.; Babiloni, C.; Carducci, F.; Basilisco, A.; Rossini, P.; Salinari, S.; Ding, L.; Ni, Y.; et al. Assessing cortical functional connectivity by linear inverse estimation and directed transfer function: Simulations and application to real data. Clin. Neurophysiol. 2005, 116, 920–932. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, G.; LaConte, S.; James, G.A.; Peltier, S.; Hu, X. Multivariate Granger causality analysis of fMRI data. Hum. Brain Mapp. 2009, 30, 1361–1373. [Google Scholar] [CrossRef] [PubMed]
- Bressler, S.L.; Seth, A.K. Wiener–Granger causality: A well established methodology. Neuroimage 2011, 58, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Seth, A.K.; Barrett, A.B.; Barnett, L. Granger causality analysis in neuroscience and neuroimaging. J. Neurosci. 2015, 35, 3293–3297. [Google Scholar] [CrossRef] [PubMed]
- Duggento, A.; Passamonti, L.; Valenza, G.; Barbieri, R.; Guerrisi, M.; Toschi, N. Multivariate Granger causality unveils directed parietal to prefrontal cortex connectivity during task-free MRI. Sci. Rep. 2018, 8, 5571. [Google Scholar] [CrossRef]
- Anzolin, A.; Presti, P.; Van De Steen, F.; Astolfi, L.; Haufe, S.; Marinazzo, D. Quantifying the effect of demixing approaches on directed connectivity estimated between reconstructed EEG sources. Brain Topogr. 2019, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Botcharova, M.; Berthouze, L.; Brookes, M.J.; Barnes, G.R.; Farmer, S.F. Resting state MEG oscillations show long-range temporal correlations of phase synchrony that break down during finger movement. Front. Physiol. 2015, 6, 183. [Google Scholar] [CrossRef] [PubMed]
- Palva, J.M.; Zhigalov, A.; Hirvonen, J.; Korhonen, O.; Linkenkaer-Hansen, K.; Palva, S. Neuronal long-range temporal correlations and avalanche dynamics are correlated with behavioral scaling laws. Proc. Natl. Acad. Sci. USA 2013, 110, 3585–3590. [Google Scholar] [CrossRef] [PubMed]
- Bornas, X.; Fiol-Veny, A.; Balle, M.; Morillas-Romero, A.; Tortella-Feliu, M. Long range temporal correlations in EEG oscillations of subclinically depressed individuals: Their association with brooding and suppression. Cognit. Neurodynamics 2015, 9, 53–62. [Google Scholar] [CrossRef]
- Nikulin, V.V.; Brismar, T. Long-range temporal correlations in electroencephalographic oscillations: Relation to topography, frequency band, age and gender. Neuroscience 2005, 130, 549–558. [Google Scholar] [CrossRef]
- Nolte, G.; Aburidi, M.; Engel, A.K. Robust calculation of slopes in detrended fluctuation analysis and its application to envelopes of human alpha rhythms. Sci. Rep. 2019, 9, 6339. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Seth, A.K.; Kendrick, K.M.; Zhou, C.; Feng, J. Partial Granger causality—Eliminating exogenous inputs and latent variables. J. Neurosci. Methods 2008, 172, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Marinazzo, D.; Pellicoro, M.; Stramaglia, S. Causal information approach to partial conditioning in multivariate data sets. Comput. Math. Methods Med. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Stramaglia, S.; Cortes, J.M.; Marinazzo, D. Synergy and redundancy in the Granger causal analysis of dynamical networks. New J. Phys. 2014, 16, 105003. [Google Scholar] [CrossRef]
- Lizier, J.T.; Rubinov, M. Multivariate construction of effective computational networks from observational data. Preprint 2012. Available online: https://www.mis.mpg.de/de/publications/preprints/2012/2012-25.html (accessed on 24 June 2019).
- Faes, L.; Nollo, G.; Stramaglia, S.; Marinazzo, D. Multiscale granger causality. Phys. Rev. E 2017, 96, 042150. [Google Scholar] [CrossRef] [PubMed]
- Faes, L.; Pereira, M.A.; Silva, M.E.; Pernice, R.; Busacca, A.; Javorka, M.; Rocha, A.P. Multiscale information storage of linear long-range correlated stochastic processes. Phys. Rev. E 2019, 99, 032115. [Google Scholar] [CrossRef] [PubMed]
- Stramaglia, S.; Bassez, I.; Faes, L.; Marinazzo, D. Multiscale Granger causality analysis by à trous wavelet transform. In Proceedings of the 7th IEEE International Workshop on Advances in Sensors and Interfaces (IWASI), Vieste, Italy, 15–16 June 2017; pp. 25–28. [Google Scholar]
- Scheines, R.; Spirtes, P.; Glymour, C.; Meek, C.; Richardson, T. The TETRAD project: Constraint based aids to causal model specification. Multivar. Behav. Res. 1998, 33, 65–117. [Google Scholar] [CrossRef]
- Pearl, J. Causality; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Balcombe, K.G. Model selection using information criteria and genetic algorithms. Comput. Econ. 2005, 25, 207–228. [Google Scholar] [CrossRef]
- Yu, S.; Wang, K.; Wei, Y.M. A hybrid self-adaptive Particle Swarm Optimization–Genetic Algorithm–Radial Basis Function model for annual electricity demand prediction. Energy Convers. Manag. 2015, 91, 176–185. [Google Scholar] [CrossRef]
- Huang, C.M.; Huang, C.J.; Wang, M.L. A particle swarm optimization to identifying the ARMAX model for short-term load forecasting. IEEE Trans. Power Syst. 2005, 20, 1126–1133. [Google Scholar] [CrossRef]
- Shojaie, A.; Michailidis, G. Discovering graphical Granger causality using the truncating lasso penalty. Bioinformatics 2010, 26, i517–i523. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Bressler, S.L.; Sylvester, C.M.; Shulman, G.L.; Corbetta, M. Measuring Granger causality between cortical regions from voxelwise fMRI BOLD signals with LASSO. PLoS Comput. Biol. 2012, 8, e1002513. [Google Scholar] [CrossRef] [PubMed]
- Furqan, M.S.; Siyal, M.Y. Random forest Granger causality for detection of effective brain connectivity using high-dimensional data. J. Integr. Neurosci. 2016, 15, 55–66. [Google Scholar] [CrossRef] [PubMed]
- DSouza, A.M.; Abidin, A.Z.; Leistritz, L.; Wismüller, A. Exploring connectivity with large-scale Granger causality on resting-state functional MRI. J. Neurosci. Methods 2017, 287, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Pearl, J. Causality: Models, Reasoning and Inference; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Solomonoff, R.J. A formal theory of inductive inference. Inf. Control 1964, 7, 1–22. [Google Scholar] [CrossRef]
- Zenil, H.; Kiani, N.A.; Zea, A.A.; Tegnér, J. Causal deconvolution by algorithmic generative models. Nat. Mach. Intell. 2019, 1, 58. [Google Scholar] [CrossRef]
- Valenza, G.; Citi, L.; Barbieri, R. Instantaneous nonlinear assessment of complex cardiovascular dynamics by laguerre-volterra point process models. In Proceedings of the 35th Annual International Conference of the Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 6131–6134. [Google Scholar]
- Akay, M. Nonlinear Biomedical Signal Processing Vol. II: Dynamic Analysis and Modeling; Wiley-IEEE Press: Hoboken, NJ, USA, 2000. [Google Scholar]
- Valenza, G.; Citi, L.; Scilingo, E.P.; Barbieri, R. Point-process nonlinear models with laguerre and volterra expansions: Instantaneous assessment of heartbeat dynamics. IEEE Trans. Signal Process. 2013, 61, 2914–2926. [Google Scholar] [CrossRef]
- Marmarelis, V. Identification of nonlinear biological system using Laguerre expansions of kernels. Ann. Biomed. Eng. 1993, 21, 573–589. [Google Scholar] [CrossRef]
- Valenza, G.; Citi, L.; Scilingo, E.P.; Barbieri, R. Using Laguerre expansion within point-process models of heartbeat dynamics: A comparative study. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), San Diego, CA, USA, 28 August–1 September 2012; pp. 29–32. [Google Scholar]
- Duggento, A.; Valenza, G.; Passamonti, L.; Guerrisi, M.; Barbieri, R.; Toschi, N. Reconstructing multivariate causal structure between functional brain networks through a Laguerre-Volterra based Granger causality approach. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 5477–5480. [Google Scholar]
- Wahlberg, B. System identification using Laguerre models. IEEE Trans. Autom. Control 1991, 36, 551–562. [Google Scholar] [CrossRef]
- Watanabe, A.; Stark, L. Kernel method for nonlinear analysis: Identification of a biological control system. Math. Biosci. 1975, 27, 99–108. [Google Scholar] [CrossRef]
- Geweke, J.F. Measures of conditional linear dependence and feedback between time series. J. Am. Stat. Assoc. 1984, 79, 907–915. [Google Scholar] [CrossRef]
- Geweke, J. Measurement of Linear Dependence and Feedback between Multiple Time Series. J. Am. Stat. Assoc. 1982, 77, 304–313. [Google Scholar] [CrossRef]
- Lambert, D.; Hall, W. Asymptotic lognormality of p-values. Ann. Stat. 1982, 10, 44–64. [Google Scholar] [CrossRef]
- Kasdin, N.J. Discrete simulation of colored noise and stochastic processes and 1/f/sup/spl alpha//power law noise generation. Proc. IEEE 1995, 83, 802–827. [Google Scholar] [CrossRef]
- Nakamura, T.; Small, M.; Tanizawa, T. Long-range correlation properties of stationary linear models with mixed periodicities. Phys. Rev. E 2019, 99, 022128. [Google Scholar] [CrossRef] [PubMed]
- Shibata, R. Selection of the order of an autoregressive model by Akaike’s information criterion. Biometrika 1976, 63, 117–126. [Google Scholar] [CrossRef]
- Van Essen, D.C.; Smith, S.M.; Barch, D.M.; Behrens, T.E.; Yacoub, E.; Ugurbil, K.; Consortium, W.M.H. The WU-Minn human connectome project: An overview. Neuroimage 2013, 80, 62–79. [Google Scholar] [CrossRef]
- Winter, W.R.; Nunez, P.L.; Ding, J.; Srinivasan, R. Comparison of the effect of volume conduction on EEG coherence with the effect of field spread on MEG coherence. Stat. Med. 2007, 26, 3946–3957. [Google Scholar] [CrossRef]
- Thomas Yeo, B.; Krienen, F.M.; Sepulcre, J.; Sabuncu, M.R.; Lashkari, D.; Hollinshead, M.; Roffman, J.L.; Smoller, J.W.; Zöllei, L.; Polimeni, J.R.; et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011, 106, 1125–1165. [Google Scholar] [CrossRef]
- Ancona, N.; Marinazzo, D.; Stramaglia, S. Radial basis function approach to nonlinear Granger causality of time series. Phys. Rev. E 2004, 70, 056221. [Google Scholar] [CrossRef] [PubMed]
- Marinazzo, D.; Pellicoro, M.; Stramaglia, S. Kernel method for nonlinear Granger causality. Phys. Rev. Lett. 2008, 100, 144103. [Google Scholar] [CrossRef] [PubMed]
- Faes, L.; Nollo, G.; Porta, A. Information-based detection of nonlinear Granger causality in multivariate processes via a nonuniform embedding technique. Phys. Rev. E 2011, 83, 051112. [Google Scholar] [CrossRef] [PubMed]
- Benhmad, F. Modeling nonlinear Granger causality between the oil price and US dollar: A wavelet based approach. Econ. Modell. 2012, 29, 1505–1514. [Google Scholar] [CrossRef]
- Tank, A.; Covert, I.; Foti, N.; Shojaie, A.; Fox, E. Neural granger causality for nonlinear time series. arXiv 2018, arXiv:1802.05842. [Google Scholar]
- Barnett, N.; Crutchfield, J.P. Computational Mechanics of Input–Output Processes: Structured Transformations and the ϵ-Transducer. J. Stat. Phys. 2015, 161, 404–451. [Google Scholar] [CrossRef]
- James, R.G.; Barnett, N.; Crutchfield, J.P. Information flows? A critique of transfer entropies. Phys. Rev. Lett. 2016, 116, 238701. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.L.; Beer, R.D. Nonnegative decomposition of multivariate information. arXiv 2010, arXiv:1004.2515. [Google Scholar]
- Faes, L.; Marinazzo, D.; Stramaglia, S. Multiscale information decomposition: Exact computation for multivariate Gaussian processes. Entropy 2017, 19, 408. [Google Scholar] [CrossRef]
- Lizier, J.; Bertschinger, N.; Jost, J.; Wibral, M. Information decomposition of target effects from multi-source interactions: Perspectives on previous, current and future work. Entropy 2018, 20, 307. [Google Scholar] [CrossRef]
- Barrett, A.B. Exploration of synergistic and redundant information sharing in static and dynamical Gaussian systems. Phys. Rev. E 2015, 91, 052802. [Google Scholar] [CrossRef] [PubMed]
- Harder, M.; Salge, C.; Polani, D. Bivariate measure of redundant information. Phys. Rev. E 2013, 87, 012130. [Google Scholar] [CrossRef] [PubMed]
- Bertschinger, N.; Rauh, J.; Olbrich, E.; Jost, J. Shared information–New insights and problems in decomposing information in complex systems. In Proceedings of the European Conference on Complex Systems 2012; Gilbert, T., Kirkilionis, M., Nicolis, G., Eds.; Springer: Cham, Switzerland, 2013; pp. 251–269. [Google Scholar]
- Griffith, V.; Koch, C. Quantifying synergistic mutual information. In Guided Self-Organization: Inception; Springer: Berlin/Heidelberg, Germany, 2014; pp. 159–190. [Google Scholar]
- Bertschinger, N.; Rauh, J.; Olbrich, E.; Jost, J.; Ay, N. Quantifying unique information. Entropy 2014, 16, 2161–2183. [Google Scholar] [CrossRef]
- Florin, E.; Baillet, S. The brain’s resting-state activity is shaped by synchronized cross-frequency coupling of neural oscillations. Neuroimage 2015, 111, 26–35. [Google Scholar] [CrossRef] [PubMed]
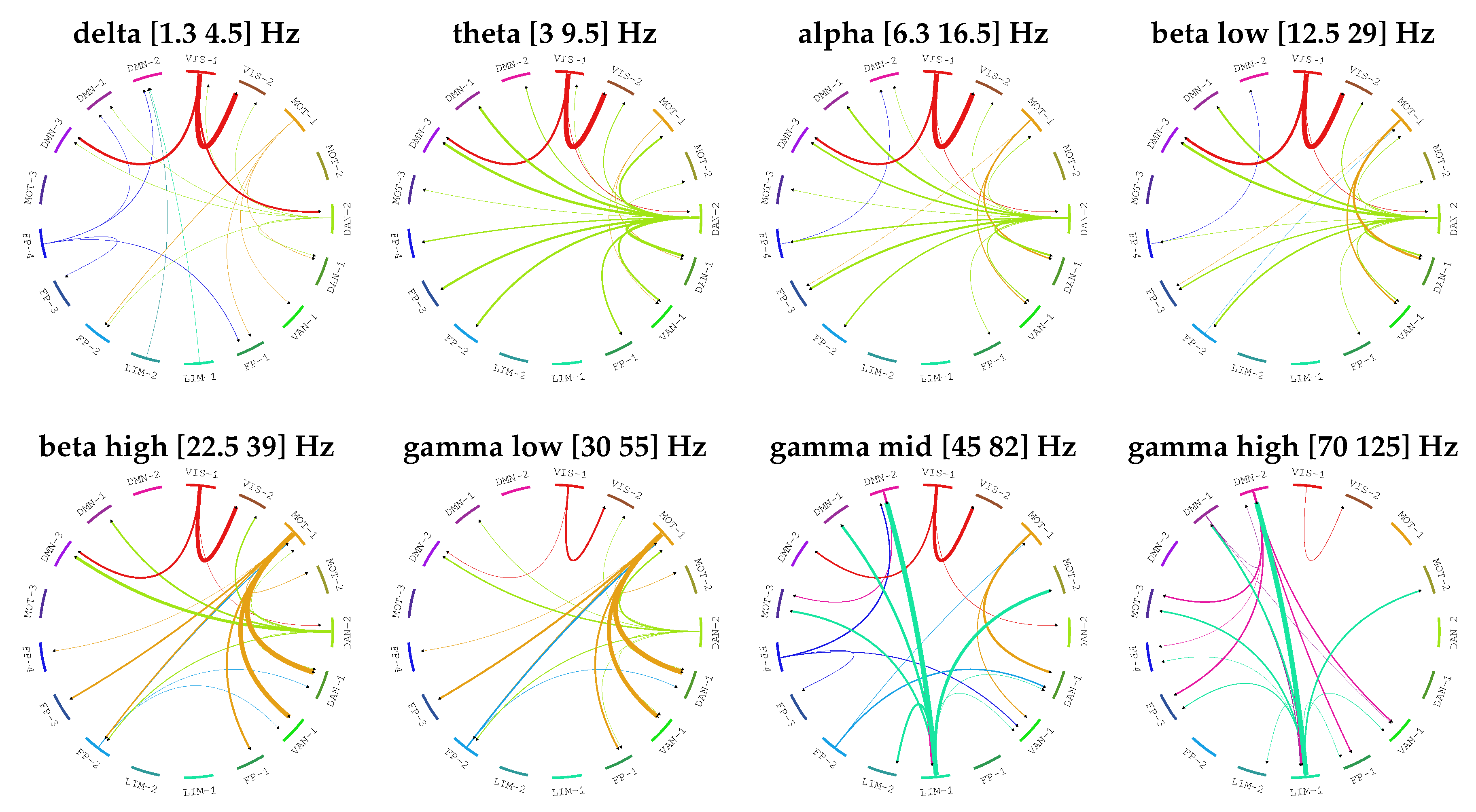
| Frequency Band Name | Frequency Band Ranges |
|---|---|
| delta | [1.3, 4.5] Hz |
| theta | [3, 9.5] Hz |
| alpha | [6.3, 16.5] Hz |
| beta low | [12.5, 29] Hz |
| beta high | [22.5, 39] Hz |
| gamma low | [30, 55] Hz |
| gamma mid | [45, 82] Hz |
| gamma high | [70, 125] Hz |
| Network Name | Physiological Interpretation | |
|---|---|---|
| 1 | VIS-1 | Visual |
| 2 | VIS-2 | |
| 3 | MOT-1 | Motor |
| 4 | MOT-2 | |
| 5 | DAN-2 | Dorsal Attention |
| 6 | DAN-1 | |
| 7 | VAN-1 | Ventral Attention |
| 8 | FP-1 | Frontoparietal |
| 9 | LIM-1 | Limbic |
| 10 | LIM-2 | |
| 11 | FP-2 | Frontoparietal |
| 12 | FP-3 | |
| 13 | FP-4 | |
| 14 | MOT-3 | Motor |
| 15 | DMN-3 | Default Mode Network |
| 16 | DMN-1 | |
| 17 | DMN-2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duggento, A.; Valenza, G.; Passamonti, L.; Nigro, S.; Bianco, M.G.; Guerrisi, M.; Barbieri, R.; Toschi, N. A Parsimonious Granger Causality Formulation for Capturing Arbitrarily Long Multivariate Associations. Entropy 2019, 21, 629. https://doi.org/10.3390/e21070629
Duggento A, Valenza G, Passamonti L, Nigro S, Bianco MG, Guerrisi M, Barbieri R, Toschi N. A Parsimonious Granger Causality Formulation for Capturing Arbitrarily Long Multivariate Associations. Entropy. 2019; 21(7):629. https://doi.org/10.3390/e21070629
Chicago/Turabian StyleDuggento, Andrea, Gaetano Valenza, Luca Passamonti, Salvatore Nigro, Maria Giovanna Bianco, Maria Guerrisi, Riccardo Barbieri, and Nicola Toschi. 2019. "A Parsimonious Granger Causality Formulation for Capturing Arbitrarily Long Multivariate Associations" Entropy 21, no. 7: 629. https://doi.org/10.3390/e21070629
APA StyleDuggento, A., Valenza, G., Passamonti, L., Nigro, S., Bianco, M. G., Guerrisi, M., Barbieri, R., & Toschi, N. (2019). A Parsimonious Granger Causality Formulation for Capturing Arbitrarily Long Multivariate Associations. Entropy, 21(7), 629. https://doi.org/10.3390/e21070629







