3.1. Effect of B4C Addition into (HfMoTaTi)C
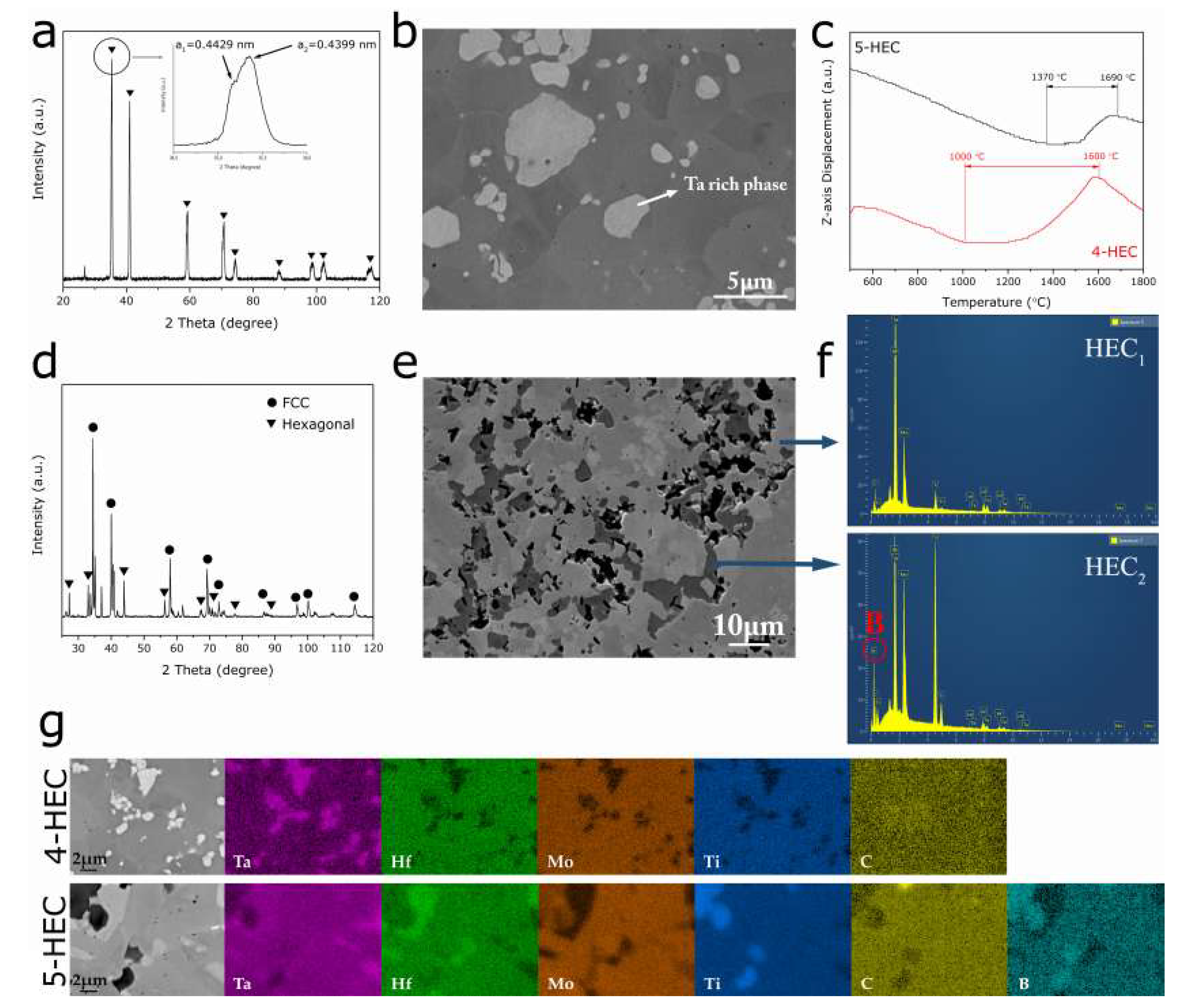
The microstructure of the quaternary HEC composite (4-HEC) sintered from HfC, Mo
2C, TaC, and TiC shows two distinct phases, as shown in
Figure 1. The bright phase with a size of 1–5 µm is dispersed uniformly in the dark matrix phase in
Figure 1b. Based on the energy-dispersive X-ray spectroscopy (EDS) mapping analysis in
Figure 1g, the bright phase is rich in Ta whilst the dark phase contains all constitutional elements Hf, Mo, Ta, Ti and C. Because of the sensitivity of backscattered electron detector (BED) to the atomic number, phases with different densities appear with different contrast in the BED microstructure. The constituent carbides show a vast difference in the densities, from 14.62 g/cm
3 for TaC, 12.2 g/cm
3 for HfC, 9.18 g/cm
3 for Mo
2C to 4.93 g/cm
3 for TiC, therefore the consistent contrast of the dark region in the
Figure 1b implies that it represents the quaternary ceramic phase containing all constitutional elements Hf, Mo, Ta, Ti, and C. The average atomic ratio of each phase obtained by performing EDS point analysis on several point locations are listed in
Table 1. As EDS lacks the accuracy of quantitative analysis of light elements [
16], the atomic ratio of the four metals is normalized to Ta. The results show that the bright phase contains Ta and C as the dominating elements and a minor amount of Mo and trace amount of Hf and Ti, while the dark phase contains all four metal elements with a relatively lower amount of Ta. According to the X-ray diffraction (XRD) data in
Figure 1a, the PCP 4-HEC composite consists of two face-centered cubic (FCC) crystal structures with similar lattice parameters (a
1 = 0.4429 nm, a
2 = 0.4399 nm), as marked in the inset in
Figure 1a. The BED microstructure, EDS analysis and XRD data,
Figure 1 and
Table 1, suggest the formation of high-entropy FCC solid solution containing all constitutional elements. In the previous work on high-entropy ceramic B
4(HfMo
2TaTi)C [
14], it has been reported that TaC has the lowest metal vacancy formation energy among the precursor carbides, thus it acts as the solvent FCC lattice during the formation of the high-entropy phase [
7], i.e., constituent atoms except Ta diffuse into the vacancies in the TaC lattice to form the multicomponent solid solution. Therefore, the Ta-rich phase and the high-entropy phase can be regarded as intermediates in the phase transformation from constitutional carbides to the hexagonal high-entropy ceramic phase. The multicomponent interdiffusion induces TaC lattice distortion, which in this case results in the reduction in the initial lattice parameter of TaC, 0.4460 nm (ICDD reference pattern of TaC: No. 03-065-0282). Based on the quantitative results shown in
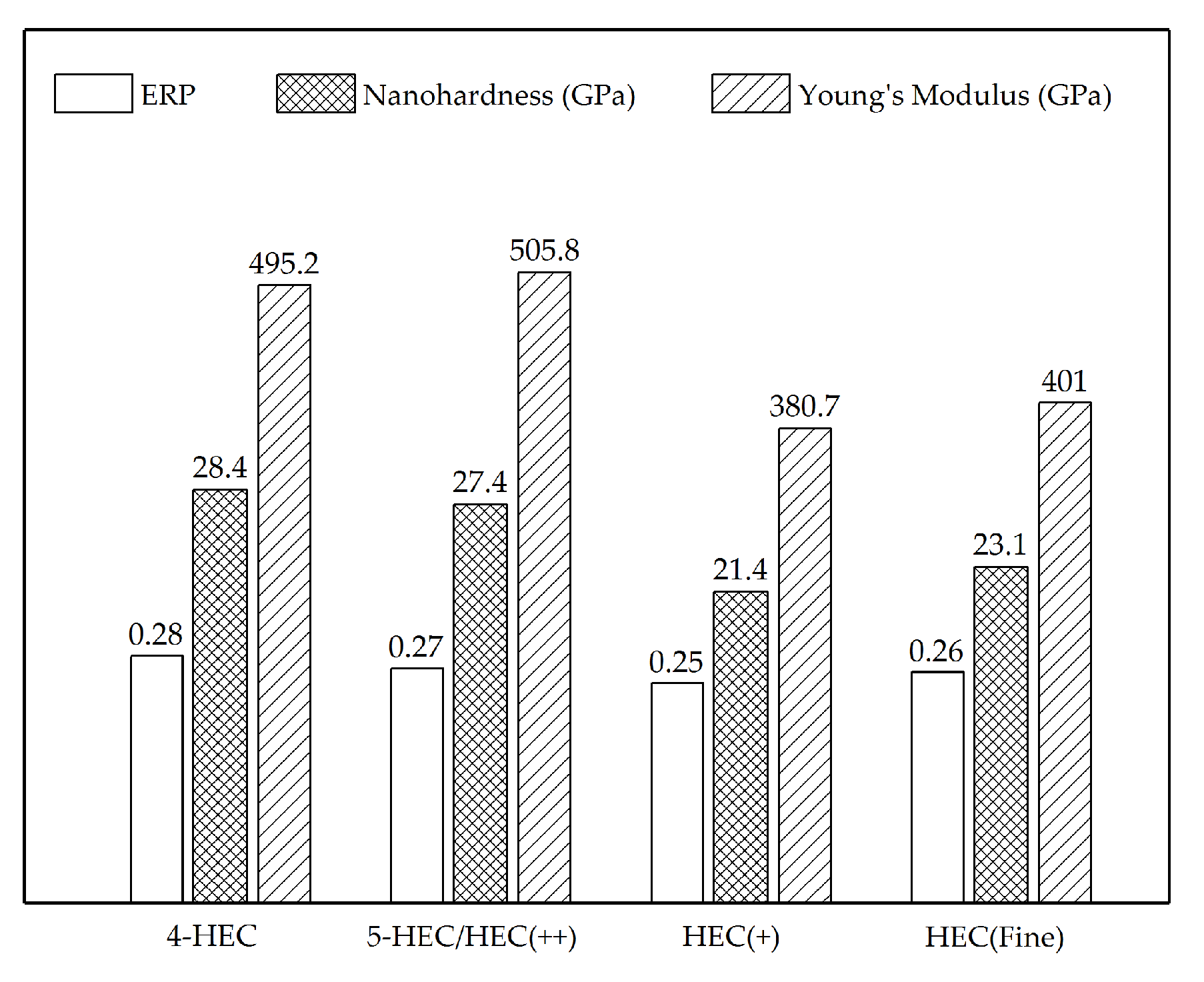
Table 1, the high-entropy phase with higher content of Hf, Mo, and Ti metal atoms experience intense atomic diffusion compared to the Ta-rich phase. Hence, in the quaternary high-entropy phase, the atomic position exchange between Ta and other metal atoms with similar or smaller atomic radii results in a smaller lattice parameter (0.4399 nm) than the TaC-rich phase (0.4429 nm). Furthermore, the high-entropy phase shows a hardness of 28.4 GPa, which is 23.5% higher than that of the Ta-rich phase (23 GPa) as shown in
Figure 2, due to the lattice distortion induced strain strengthening effect. This result is in line with the previous reports on high-entropy materials [
6,
7].
The curve of
Z-axis displacement as a function of temperature was recorded during the PCP. As shown in
Figure 1c, the decline of
Z-axis refers to the thermal expansion of the material, while the up-climbing region corresponds to the shrinkage of the bulk volume. The reduction of the volume typically refers to the occurrence of sintering phenomenon where the powder material becomes compacted and forms a densified solid mass. Since the sintering temperature of powder material is normally 2/3–3/4 of the melting point [
17], the theoretical sintering temperature for current quaternary refractory carbide mixture should be above 1800 °C.
Figure 1c shows that the shrinkage of the four-component carbide system 4-HEC takes place from 1000 °C to 1600 °C, while the same phenomenon for the 5-HEC composite was postponed to a higher temperature range 1370 °C–1690 °C. This indicates that the addition of B
4C to the carbide system hinders the solid-state atomic diffusion required for sintering in the multicomponent carbides, leading to a delay of the formation of the high-entropy ceramic phase. A detailed investigation on the sintering behavior of ceramic precursors to form high-entropy ceramic composites will be reported later, elsewhere.
The addition of B
4C to the precursor carbides resulted in the formation of multiple phases during PCP. According to the backscattered electron microstructure in
Figure 1e, the PCP 5-HEC composite exhibits three distinct phases. Similar to the 4-HEC, the brightest phase in 5-HEC is rich in Ta, which is coordinated with the fact that Ta has the highest atomic number among the constituent elements, therefore, the Ta-rich phase appears as the brightest phase in the BED microstructure. Two high-entropy solid solutions with different elemental compositions were formed in the five-component system, 5-HEC. The atomic ratio of metal atoms Hf, Mo, Ta and Ti in the gray (HEC
1) and dark phase (HEC
2) in
Figure 1e are shown in
Table 1, with HEC
2 showing higher content of the solute metal atoms (Hf, Mo, and Ti) in the structure. The higher content of Ti in HEC
2 agrees more with the darker contrast of HEC
2 than HEC
1 in the microstructure, as Ti has the smallest atomic number among the constitutional metal elements. According to the bond dislocation enthalpy (BDE) of transition metal carbides at 298 K [
18], Ti-C has the lowest BDE of 423 ± 30 KJ/mol among the solute carbides, while Mo-C
2 and Hf-C have a BDE of 500 and 540 ± 25 KJ/mol, respectively, and the covalent atomic radii vary as Ti < Mo < Hf. These factors might contribute to preferable diffusion of Ti over Mo and Hf during the formation of the multicomponent solid solution
, resulting in the metal content ratio in HEC
2 phase as Ti > Mo > Hf in
Table 1. A pronounced boron diffraction peak in the EDS pattern was revealed at the HEC
2 phase (
Figure 1f), suggesting that HEC
2 experienced a more intensive diffusion of B atoms than HEC
1. Additionally, the diffractions peaks of B
4C were not detected in the XRD diffractogram of the 5-HEC composite in
Figure 1d, suggesting the participation of B
4C in the formation of high-entropy solid solutions. However, the diffusion priority of Ti, Mo and Hf was not observed in the high-entropy phase in the 4-HEC composite and HEC
1 phase in 5-HEC, suggesting that the incorporation of B
4C to the transition metal carbides might have promoted the diffusion process of the metal atoms towards a more energetically favorable state.
The XRD data in
Figure 1d shows that the 5-HEC composite contains both FCC and hexagonal structured phases. Similar with the 4-HEC, the FCC pattern is generated from diffraction of two FCC crystal structures with similar lattice parameters, including the presence of an FCC Ta-rich phase. Based on the aforementioned discussions, the HEC
1 phase that contains a lower content of the solute atoms should have a closely-matched crystal structure and lattice constant with the solvent TaC lattice due to the reduced atomic position change in the host lattice corresponding to an FCC structure, while the HEC
2 phase corresponds to the hexagonal structure. The HEC
1 phase shows a lattice parameter of 0.4499 nm, which is slightly greater than that of TaC (0.4460 nm). Assuming that the HEC
1 is formed from only transition metal carbides, the lattice parameter should decline as observed in the FCC high-entropy solid solutions in 4-HEC composite (
Figure 1a). It is known that the metal-boron bond length is longer than metal-carbon bond length, for example, Ta-C = 2.22 Å [
19] and Ta-B = 2.41 Å [
20], the expansion of the lattice can be contributed by the addition of B atoms in the formation of an HEC
2 solid solution. The formation of high-entropy solid solutions HEC
1 and HEC
2 that contain all constitutional elements confirms the possibility of processing high-entropy ceramics from a precursor system containing more than one nonmetal atoms and with different crystal structures. The formation of hexagonal structure is likely to be attributed to more B atoms diffusing in the FCC lattice, which induces more severe lattice distortion and consequently leads to the crystal structure change from FCC to a hexagonal structure. In the 5-HEC composite, the FCC structured HEC
1 solid solution shows a nanohardness of 27.4 GPa and Young’s modulus of 505.8 GPa, which is close to the FCC solid solution phase in 4-HEC (28.4 GPa and 495.2 GPa for nanohardness and Young’s modulus, respectively).
3.2. Effect of Different Starting Particle Sizes
It is well known that the particle size of the precursors is an essential parameter influencing the atomic diffusion and phase evolution during solid-state sintering in PCP [
15]. Fine particles promote the solid-state atomic diffusion by reducing the diffusion distance and promote the kinetics of phase transformation [
21,
22]. To investigate the effect of particle size on the formation of high-entropy ceramics, the same carbide systems with different particle sizes are sintered in PCP (as listed in
Table 2). 5-HEC that utilized relative larger particle sizes of solute metal carbides (HfC, Mo
2C, and TiC) is discussed in the previous section and is denoted as HEC(++) in the following discussion. HEC(fine) and HEC(+) contains precursor carbides with the finest particle sizes and a larger particle size of the solvent carbide (TaC), respectively.
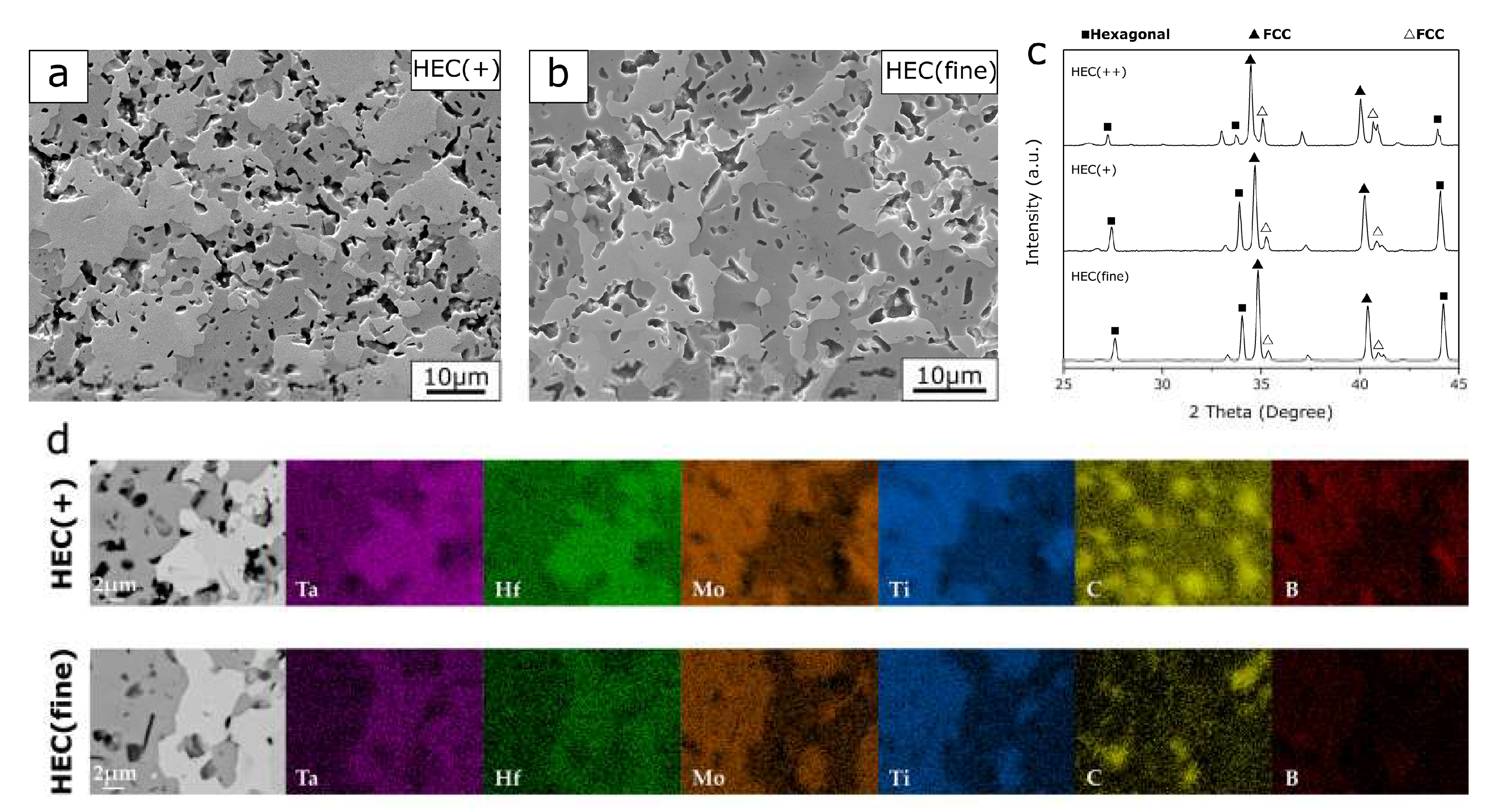
The microstructure of the PCP HEC(+) and HEC(fine) show the presence of two phases in
Figure 3. According to the compositional mapping analysis in
Figure 3d, all four metal elements are distributed in bright and dark phases. The spot-shaped mapping for C is attributed to porosity in the samples, which possibly caused the diamond polishing agents being introduced during the sample preparation procedure. For both PCP HEC(+) and HEC(fine) sample, the bright phase is rich in Ta and Hf while the dark phase contains a greater amount of Mo and Ti. The quantitative analysis results of the selected areas in
Table 3 show that these two phases are high-entropy solid solutions with different elemental compositions. The average atomic ratios are normalized to Ta. The bright phase is rich in Ta, while the dark phase has a higher content of other metals (Ti > Mo > Hf ≈ Ta). Based on the discussion about BED microstructure of different transition metal carbides, the dark region with a higher solute metal content refers to more equilibrium composition and a higher extent of phase transformation towards the high-entropy solid solution.
The XRD patterns of HEC(+) and HEC(fine) show high similarity (
Figure 3c). Similar to the diffractogram of HEC(++), the PCP HEC(+) and HEC(fine) composite reveal diffraction patterns from two FCC and one hexagonal structure. Since the elemental mapping from each region with a constant contrast shows a homogenous distribution of constituent elements, a possible reason why the third phase is not distinguishable in the microstructure is that the two FCC phases have indistinguishable contrast in BED images, which suggests that these phases have a similar atomic composition. As the bright phase with less content of foreign atoms (except Ta), it refers to the component with less lattice distortion, therefore it is extrapolated to retain a FCC crystal structure, whilst the dark phase exhibits a hexagonal structure.
For the solid-state phase transformations, the starting particle size has been reported to have a strong influence on the reaction kinetics by tuning the contact area between the solid particles [
23,
24,
25]. Therefore, it was expected that PCP HEC(fine) with finest starting particle size should have a more promoted phase transformation than HEC(+) and HEC(++) which were fabricated with the same sintering route and sintering conditions. Comparing HEC(fine) with HEC(+), the results show high similarity in the microstructures and phase composition (
Figure 3). Both composites consist of a FCC and a hexagonal crystal structure, with high-entropy solid solutions. With HfC having the lowest formation enthalpy (−1.826 eV) [
14] and highest BDE (9.18 g/cm
3) among the precursors [
18], the promotion of the solid-state diffusion can be observed by the increased Hf content in the FCC phase in HEC(fine) than HEC(+) (Hf/Ta = 1 and 0.4, respectively) according to the EDS quantitative results in
Table 3. On the other hand, HEC(++) has a complicated phase composition compared to HEC(fine). The Ta-rich phase, representing the least diffusion, was observed in HEC(++) (
Figure 1e). A lower fraction of the hexagonal structured high-entropy solid solution in HEC(++) composite can be concluded from the microstructure and lower intensity of X-ray diffraction peaks (for example at 2
θ = 44.2°) in
Figure 3c. Therefore, the degree of diffusion is assumed to be in the order of HEC(++) < HEC(+) ≈ HEC(fine). The results suggest that the particle size of solvent carbide TaC is not as essential as the solute carbides in terms of tailoring the phase composition in the multicomponent carbide system. Due to the porosity and small grain size, the nanoindentation testing of HEC(+) and HEC(fine) composites were obtained from 30 indentations, therefore the data represents the overall mechanical properties of the bulk materials instead of each individual high-entropy phase. Although the PCP HEC(fine) and HEC(+) show the same phase composition and similar microstructure, the HEC(fine) composite sintered from precursors with finest grain size shows improved nanoindentation hardness of 23.1 GPa compared to the PCP HEC(+) composite (21.4 GPa). The experimental hardness value is close to the theoretical hardness calculated from the rule of mixture (23.2 GPa). The enhancement of the hardness caused by utilizing small starting particle size during sintering has been reported before [
26]. Moreover, Young’s modulus of HEC(fine) improves from 380.7 GPa for HEC(+) to 401 GPa, suggesting stronger atomic bonding in the HEC(fine) composite.