Effect of Mn Addition on the Microstructures and Mechanical Properties of CoCrFeNiPd High Entropy Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Material Characterization
3. Results
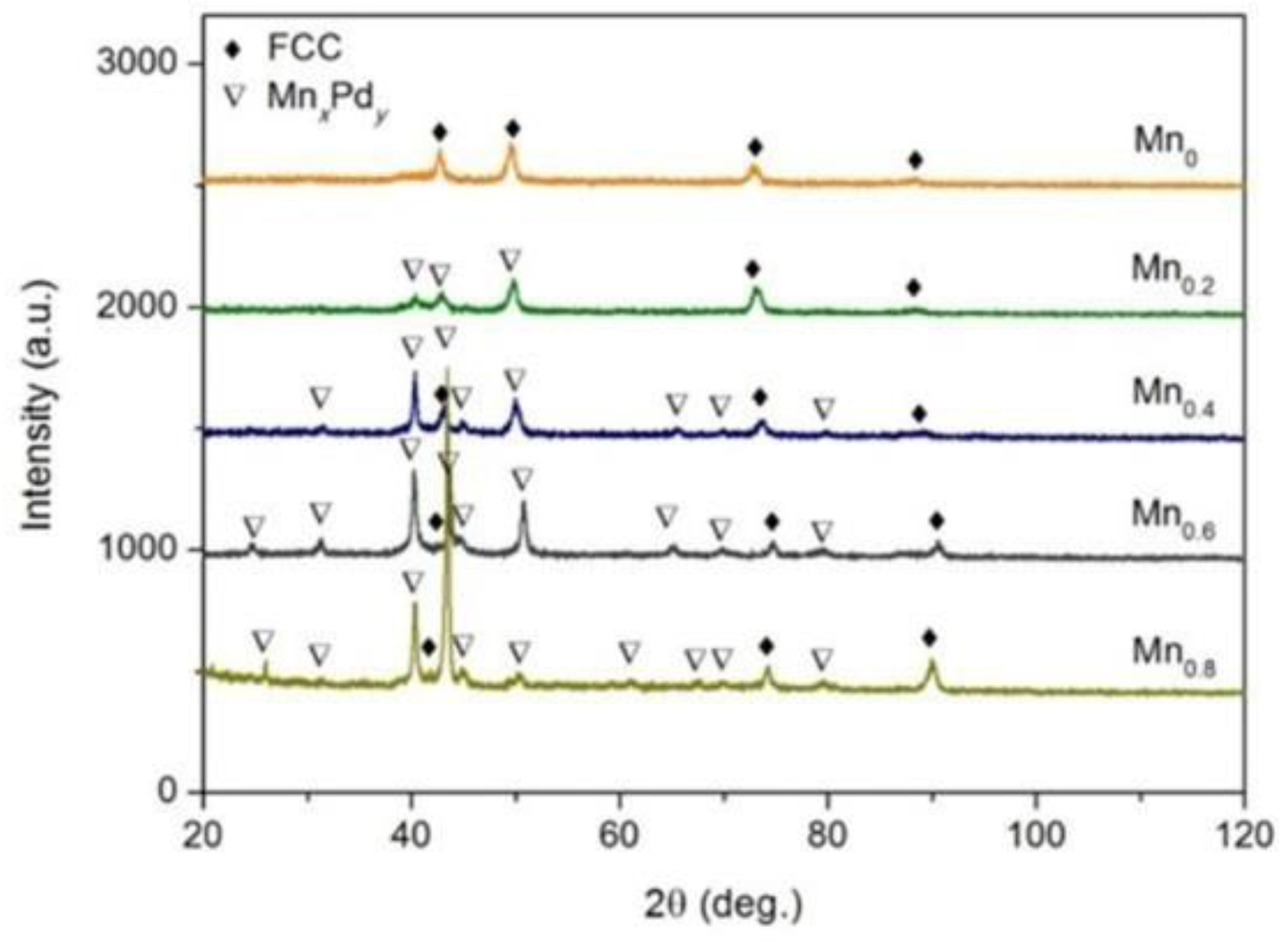
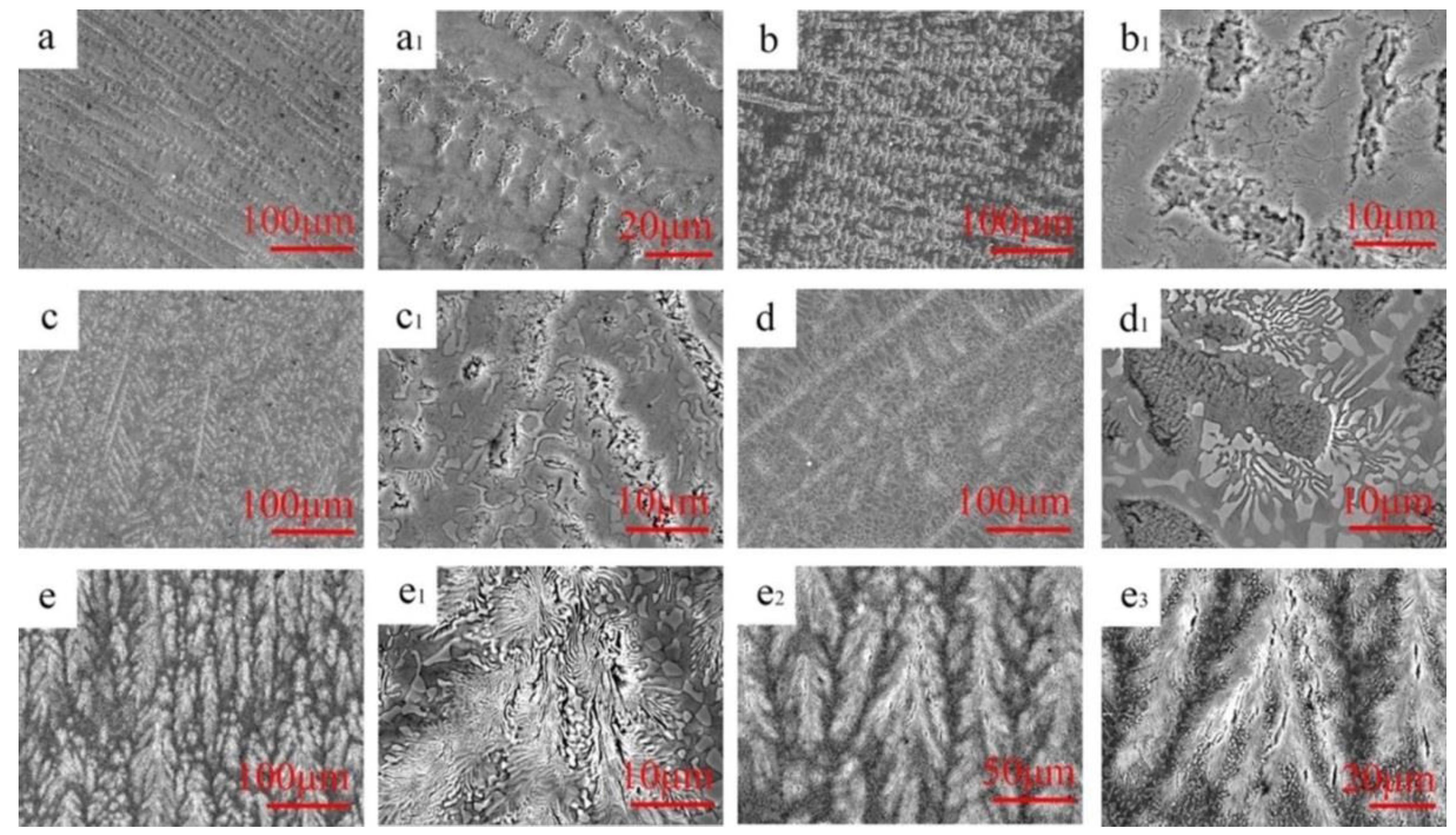
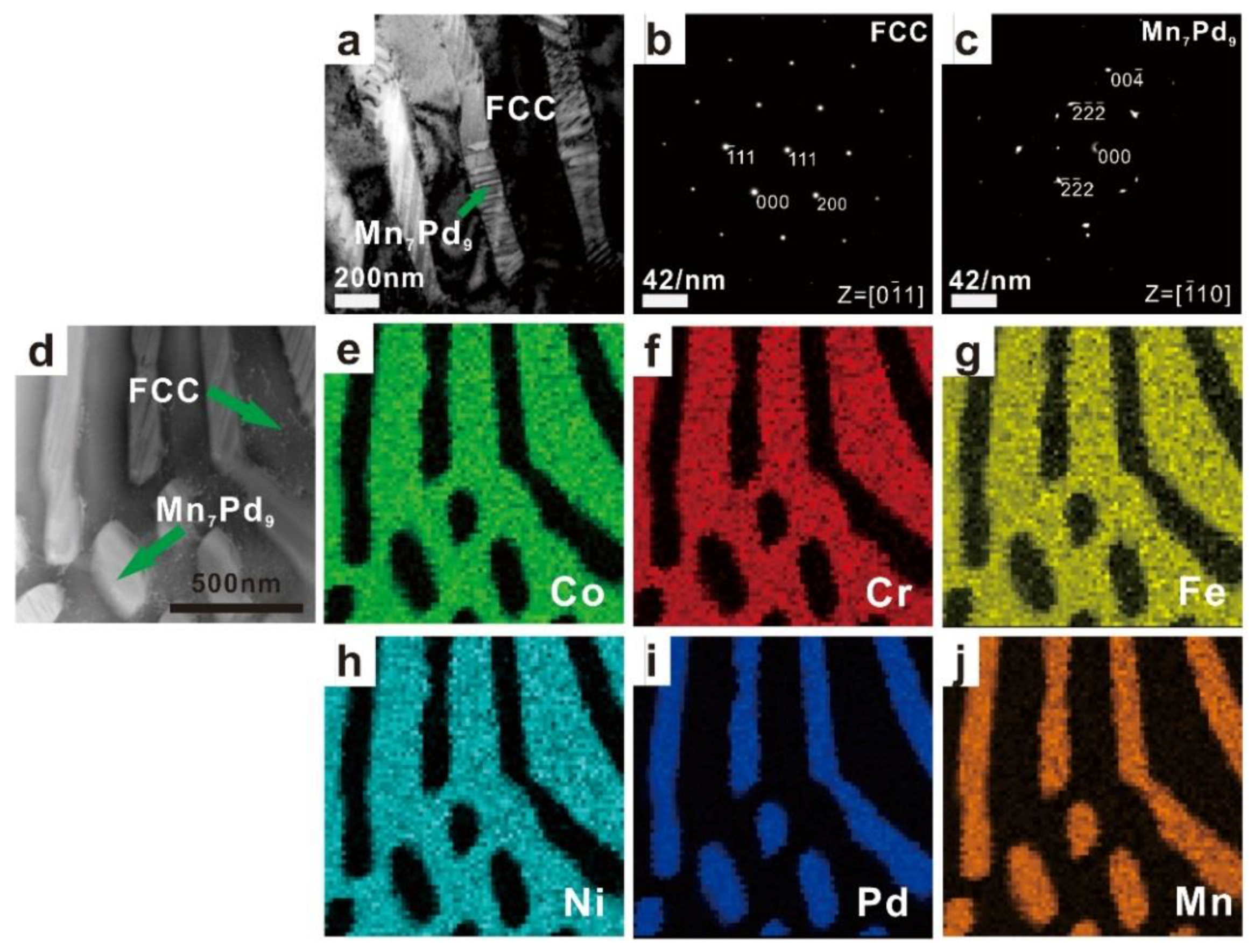
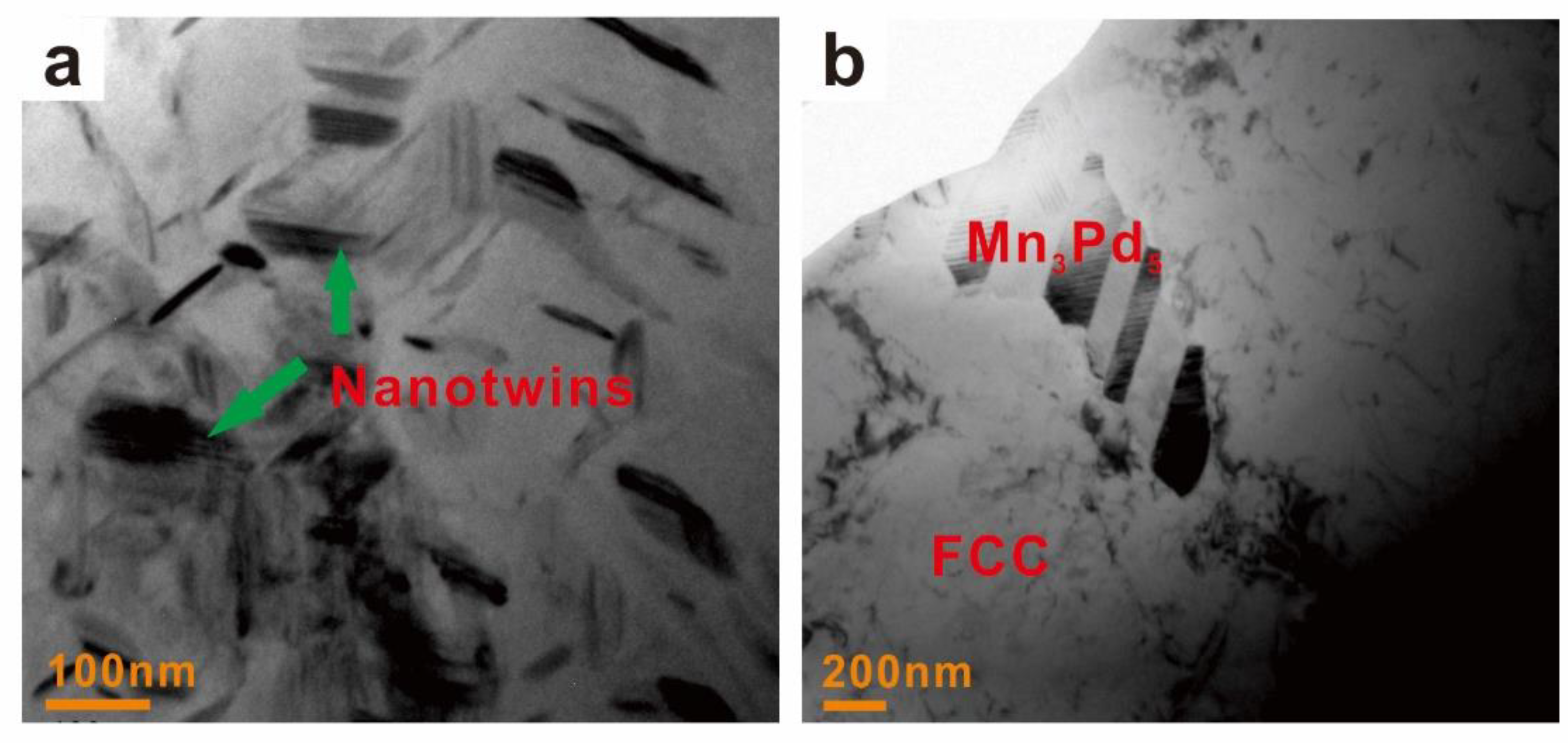
3.1. Crystal Structures and Microstructures
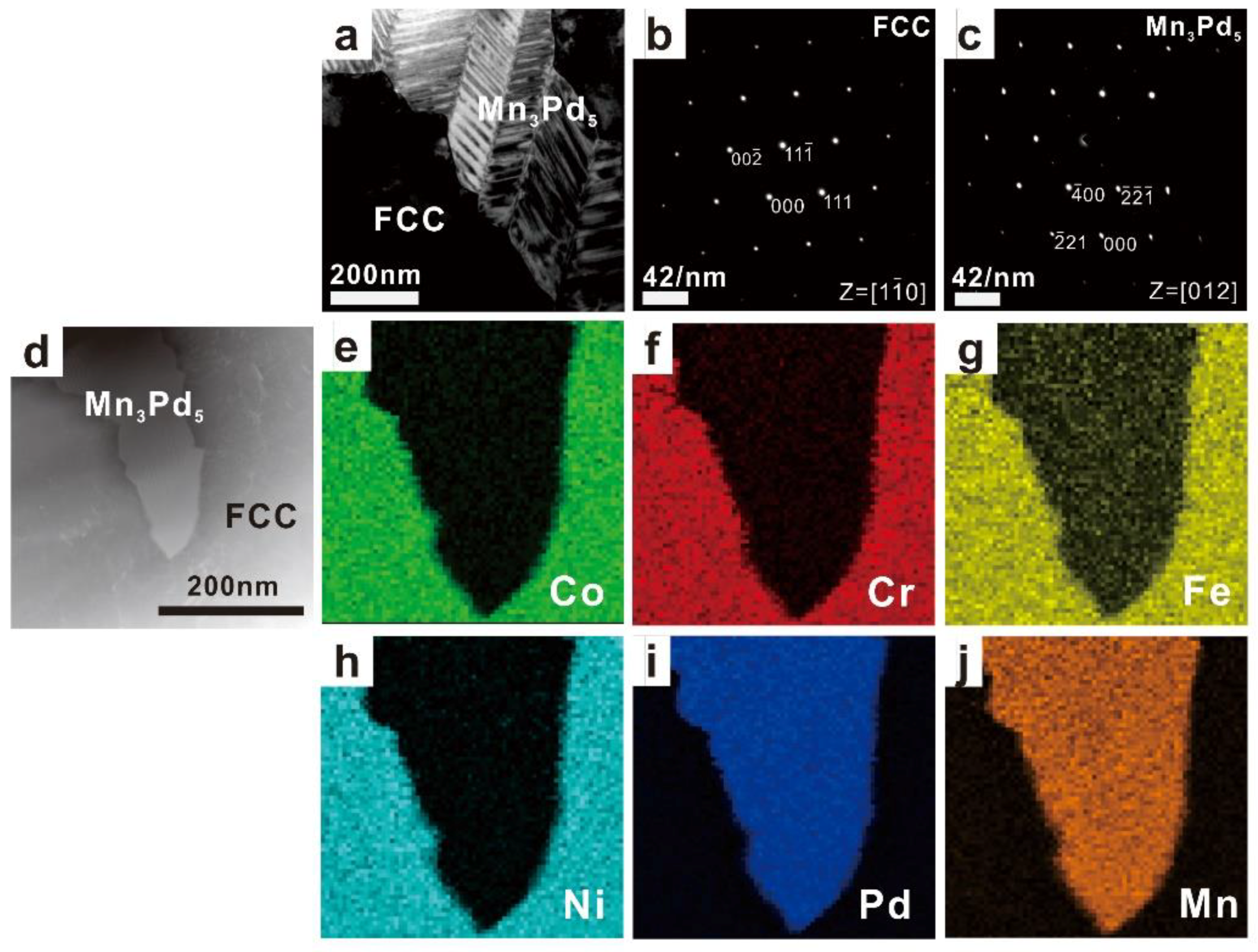
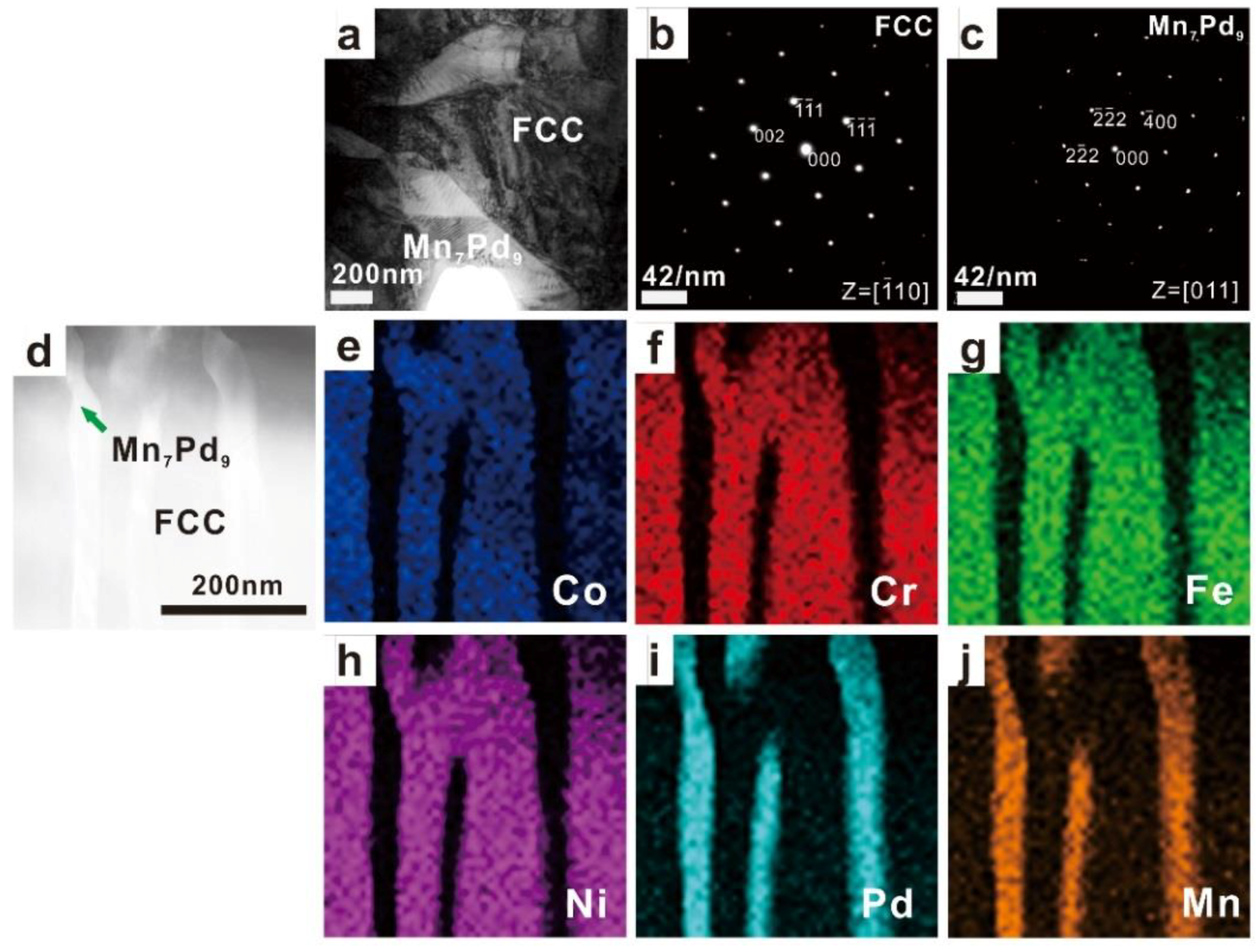
3.2. Phase Identification
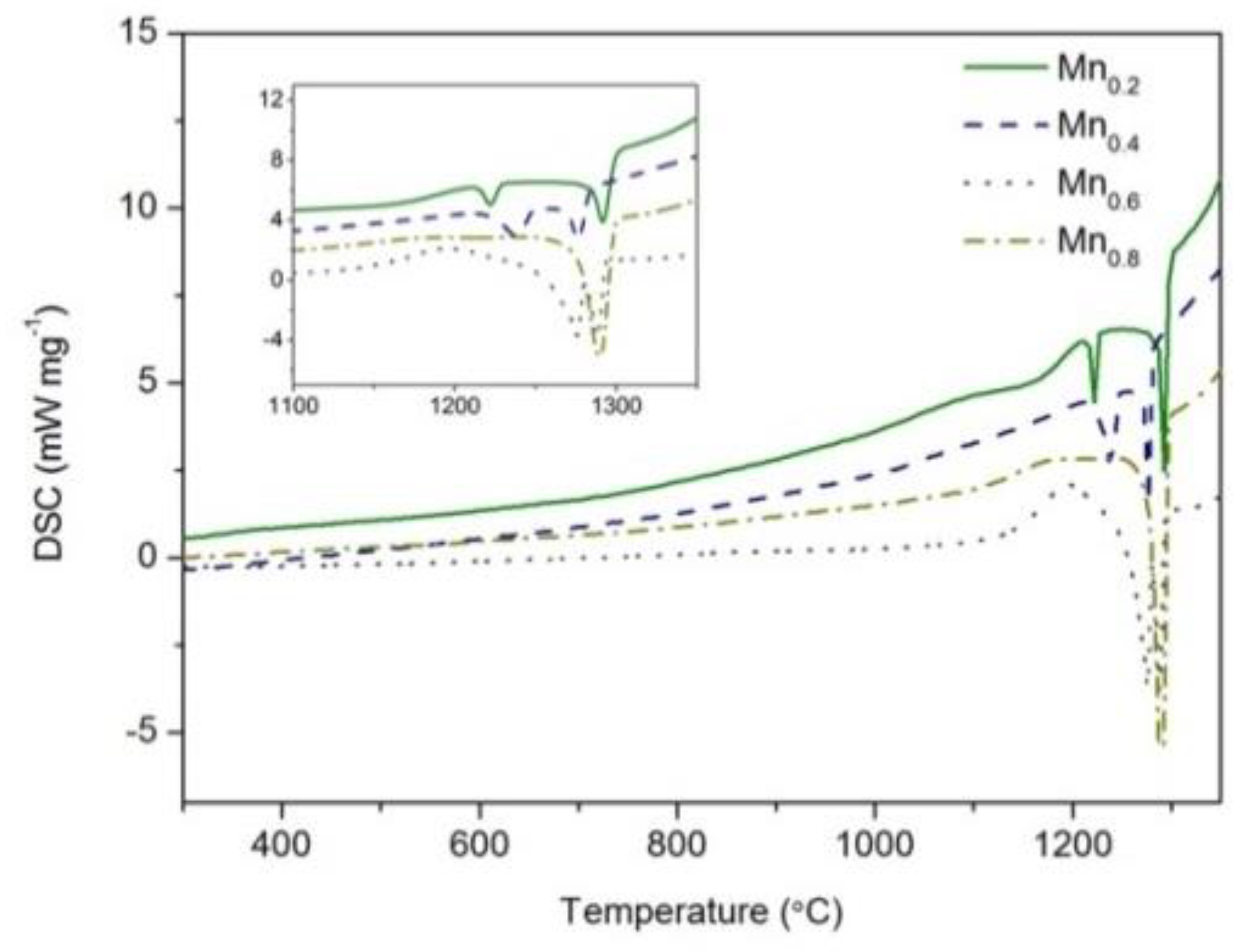
3.3. Solidification Path
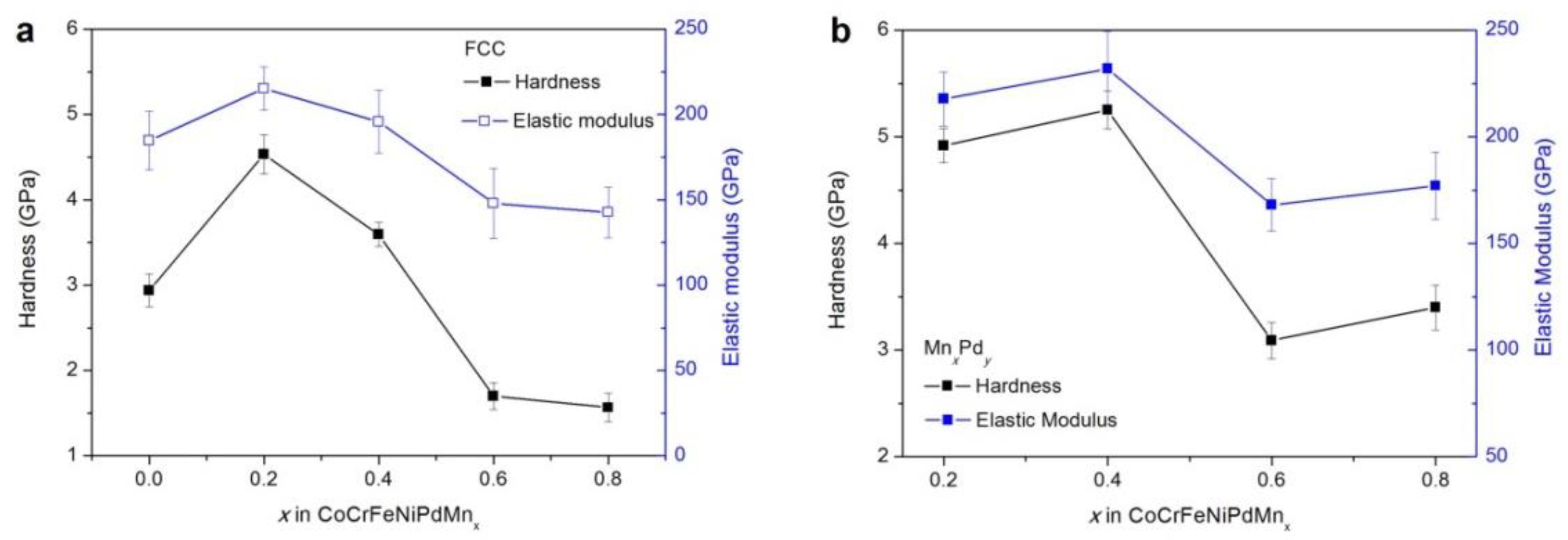
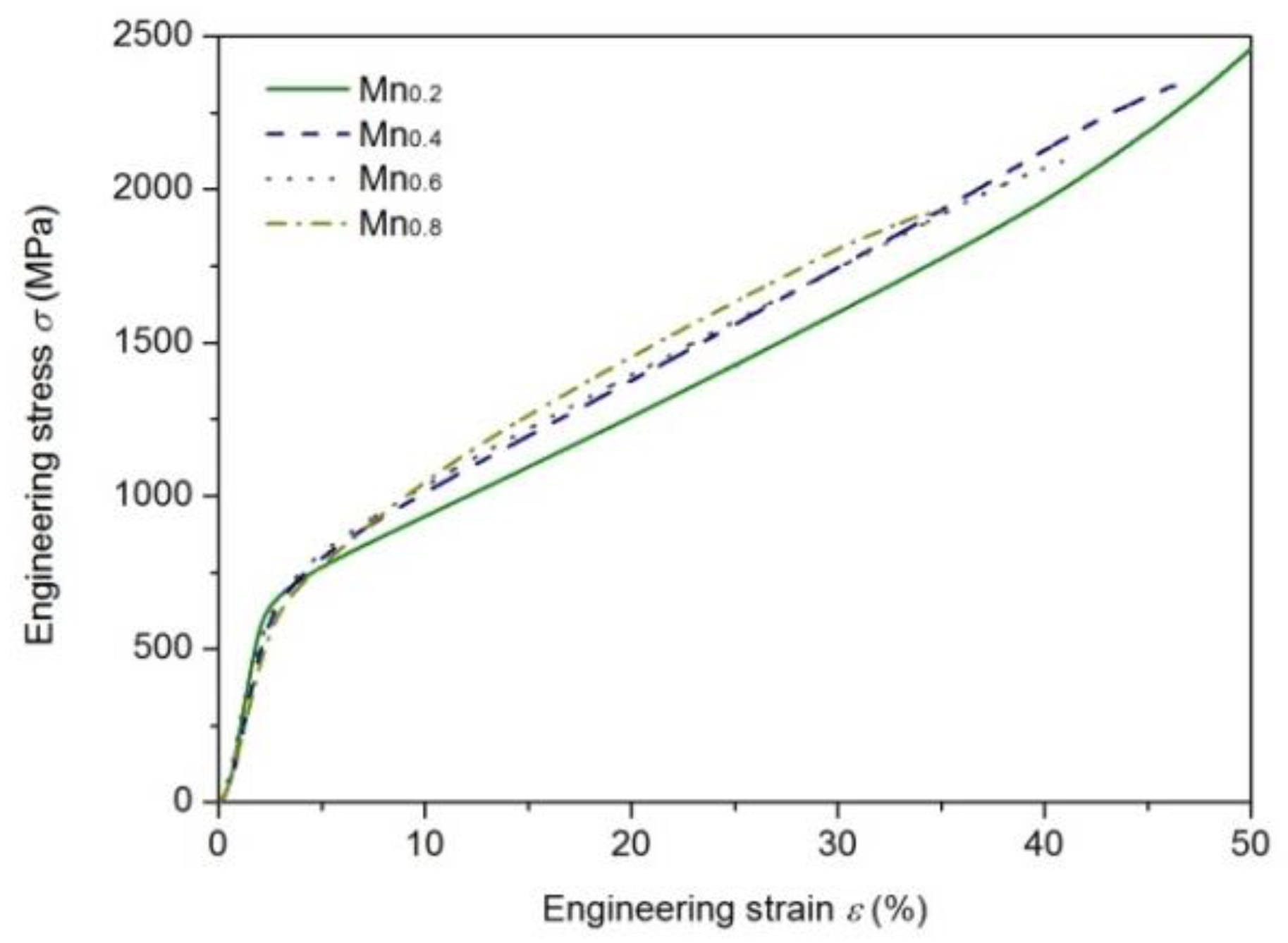
3.4. Mechanical Properties
4. Discussion
4.1. Effect of Mn Addition on Microstructures
4.2. Effect of Mn Addition on Mechanical Properties
4.3. Designing Rules for EHEAs
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructures high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Tsai, M.H.; Yeh, J.W. High-entropy alloys: A critical review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Tsai, M.H. Three Strategies for the Design of Advanced High-Entropy Alloys. Entropy 2016, 18, 252. [Google Scholar] [CrossRef]
- Yeh, J.W. Physical metallurgy of high-entropy alloys. JOM 2015, 67, S499–S503. [Google Scholar] [CrossRef]
- Pickering, E.J.; Jones, N.G. High-entropy alloys: A critical assessment of their founding principles and future prospects. Int. Mater. Rev. 2016, 61, 183–202. [Google Scholar] [CrossRef]
- Ye, Y.F.; Wang, Q.; Lu, J.; Liu, C.T.; Yang, Y. High entropy alloy: Challenges and prospects. Mater. Today 2016, 19, 349–362. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Gorsse, S.; Miracle, D.B.; Senkov, O.N. Mapping the world of complex concentrated alloys. Acta Mater. 2017, 135, 177–187. [Google Scholar] [CrossRef]
- Gludovatz, B.; Hohenwarter, A.; Catoor, D.; Chang, E.H.; George, E.P.; Rirtchie, R.O. A fracture-resistant high-entropy alloy for cryogenic applications. Science 2014, 345, 1153. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.M.; Pradeep, K.G.; Deng, Y.; Raabe, D.; Tasan, C.C. Metastable high-entropy dual-phase alloys overcome the strength–ductility trade-off. Nature 2016, 534, 227–230. [Google Scholar] [CrossRef]
- Lei, Z.F.; Liu, X.J.; Wu, Y.; Wang, H.; Jiang, S.; Wang, S.; Hui, X.; Wu, Y.; Gault, B.; Kontis, P. Enhanced strength and ductility in a high-entropy alloy via ordered oxygen complexes. Nature 2018, 563, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Zhao, Y.L.; Tong, Y.; Jiao, Z.B.; Wei, J.; Cai, J.X.; Han, X.D.; Chen, D.; Hu, A.; Kai, J.J. Multicomponent intermetallic nanoparticles and superb mechanical behaviors of complex alloys. Science 2018, 362, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Senkov, O.N.; Wilks, G.B.; Scott, J.M.; Miracle, D.B. Mechanical properties of Nb25Mo25Ta25W25 and V20Nb20Mo20Ta20W20 refractory high entropy alloys. Intermetallics 2011, 19, 698–706. [Google Scholar] [CrossRef]
- Chen, S.Y.; Yang, X.; Dahmen, K.A.; Liaw, P.K.; Zhang, Y. Microstructures and Crackling Noise of AlxNbTiMoV High Entropy Alloys. Entropy 2014, 16, 870–884. [Google Scholar] [CrossRef]
- Yao, H.W.; Qiao, J.W.; Gao, M.C.; Hawk, J.A.; Ma, S.G.; Zhou, H.F. MoNbTaV Medium-Entropy Alloy. Entropy 2016, 18, 189. [Google Scholar] [CrossRef]
- Ye, Y.X.; Liu, C.Z.; Wang, H.; Nieh, T.G. Friction and wear behavior of a single-phase equiatomic TiZrHfNb high-entropy alloy studied using a nanoscratch technique. Acta Mater. 2018, 147, 78–89. [Google Scholar] [CrossRef]
- Tang, Z.; Yuan, T.; Tsai, C.W.; Yeh, J.W.; Lundin, C.D.; Liaw, P.K. Fatigue behavior of a wrought Al0.5CoCrCuFeNi two-phase high-entropy alloy. Acta Mater. 2015, 99, 247–258. [Google Scholar] [CrossRef]
- Zou, Y.; Ma, H.; Spolenak, R. Ultrastrong ductile and stable high-entropy alloys at small scales. Nat. Commun. 2015, 6, 7748. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.P.; Dong, Y.; Guo, S.; Jiang, L.; Kang, H.J.; Wang, T.M.; Wen, B.; Wang, Z.J.; Jie, J.C.; Cao, Z.Q.; et al. A promising new class of high-temperature alloys: Eutectic high entropy alloys. Sci. Rep. 2014, 4, 6200. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; He, F.; Qiao, Z.H.; Wang, Z.J.; Liu, W.M.; Yang, J. Effects of temperature and microstructure on the triblogical properties of CoCrFeNiNbx eutectic high entropy alloys. J. Alloys Compd. 2019, 775, 1376–1385. [Google Scholar] [CrossRef]
- Li, D.Y.; Li, C.X.; Feng, T.; Zhang, Y.D.; Sha, G.; Lewandowski, J.J.; Liaw, P.K.; Zhang, Y. High-entropy AlCoCrFeNi alloy fibers with high tensile strength and ductility at ambient and cryogenic temperatures. Acta Mater. 2017, 123, 285–294. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Cheng, Y.Q.; Liaw, P.K. High-entropy alloys with high saturation magnetization, electrical resistivity, and malleability. Sci. Rep. 2013, 3, 1455. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wu, Y.; Tong, X.; Zhang, H.; Wang, H.; Liu, X.J.; Ma, L.; Suo, H.L.; Lu, Z.P. Rare-earth high-entropy alloys with giant magnetocaloric effect. Acta. Mater. 2017, 125, 481–489. [Google Scholar] [CrossRef]
- Zhou, Q.; Du, Y.; Han, W.C.; Ren, Y.; Zhai, H.M.; Wang, H.F. Identifying the origin of strain rate sensitivity in a high entropy bulk metallic glass. Scr. Mater. 2019, 164, 121–125. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L.; Liaw, P.K. Solid-solution phase-formation rules for multi-component alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y. Prediction of high entropy stabilized solid solution in multi-component alloys. Mater. Chem. Phys. 2012, 132, 233–238. [Google Scholar] [CrossRef]
- Otto, F.; Yang, Y.; Bei, H.; George, E.P. Relative effects of enthalpy and entropy on the phase stability of equiatomic high entropy alloys. Acta Mater. 2013, 61, 2628–2638. [Google Scholar] [CrossRef]
- Tsai, M.H.; Li, J.H.; Fan, A.C.; Tsai, P.H. Incorrect predictions of simple solid solution high entropy alloys: Cause and possible solution. Scr. Mater. 2017, 127, 6–9. [Google Scholar] [CrossRef]
- King, D.J.M.; Middle burgh, S.C.; Mcgregor, A.G.; Cortie, M.B. Predicting the formation and stability of single phase high entropy alloys. Acta. Mater. 2016, 104, 172–179. [Google Scholar] [CrossRef]
- Sohn, S.; Liu, Y.H.; Liu, J.B.; Gong, P.; Prades-Rodel, S.; Blatter, A.; Scanley, B.E.; Broadbridge, C.C.; Schroers, J. Noble metal high entropy alloys. Scr. Mater. 2017, 126, 29–32. [Google Scholar] [CrossRef]
- Stepanov, N.D.; Shaysultanov, D.G.; Salishchev, G.A.; Tikhonovsky, M.A.; Oleynik, E.E.; Tortika, A.S.; Senkov, O.N. Effect of V content on microstructure and mechanical properties of the CoCrFeMnNiVx high entropy alloys. J. Alloys Compd. 2015, 628, 170–185. [Google Scholar] [CrossRef]
- Xu, X.D.; Liu, P.; Guo, S.; Hirata, A.; Fujita, T.; Nieh, T.G. Nanoscale phase separation in a fcc-based CoCrCuFeNiAl high entropy alloy. Acta. Mater. 2015, 84, 145–152. [Google Scholar] [CrossRef]
- Lu, Y.P.; Gao, X.Z.; Jiang, L.; Chen, Z.M.; Wang, T.; Jie, J.; Kang, H.; Zhang, Y.; Guo, S.; Ruan, H. Directly cast bulk eutectic and near-eutectic high entropy alloys with balanced strength and ductility in a wide temperature range. Acta Mater. 2017, 124, 143–150. [Google Scholar] [CrossRef]
- Guo, N.N.; Wang, L.; Luo, L.S.; Li, X.Z.; Su, Y.Q.; Guo, J.J.; Fu, H.Z. Microstructure and mechanical properties of refractory MoNbHfZrTi high-entropy alloy. Mater. Des. 2015, 81, 87–94. [Google Scholar] [CrossRef]
- Chen, R.; Qin, G.; Zheng, H.T.; Wang, L.; Su, Y.Q.; Chiu, Y.L.; Ding, H.S.; Guo, J.J.; Fu, H.Z. Composition design of high entropy alloy using the valence electron concentration to balance strength and ductility. Acta Mater. 2018, 144, 129–137. [Google Scholar] [CrossRef]
- Lu, Y.P.; Gao, X.X.; Dong, Y.; Wang, T.M.; Chen, H.; Maob, H.; Zhao, Y.; Jiang, H.; Cao, Z.; Li, T. Preparing bulk ultrafine-microstructure high entropy alloys via direct solidification. Nanoscale 2017, 10, 1039. [Google Scholar] [CrossRef]
- Ai, C.; He, F.; Guo, M.; Zhou, J.; Wang, Z.J.; Yuan, Z.W.; Guo, Y.J.; Liu, Y.L.; Liu, L. Alloy design, mechanical and macromechanical properties of CoCrFeNiTax eutectic high entropy alloys. J. Alloys Compd. 2018, 735, 2653–2662. [Google Scholar] [CrossRef]
- Wani, I.S.; Bhattacharjee, T.; Sheikh, S.; Lu, Y.P.; Chatterjee, S.; Bhattacharjee, P.P.; Guo, S.; Tsuji, N. Ultrafine-grained AlCoCrFeNi eutectic high entropy alloy. Mater. Res. Lett. 2016, 4, 174–179. [Google Scholar] [CrossRef]
- Bhattacharjee, T.; Wani, I.S.; Sheikh, S.; Clark, I.T.; Okawa, T.; Guo, S.; Bhattacharjee, P.P.; Tsuji, N. Simultaneous strength-ductility enhancement of a nano-lamellar AlCoCrFeNi2.1 eutectic high entropy alloy by cryo-rolling and annealing. Sci. Rep. 2018, 8, 3276. [Google Scholar] [CrossRef]
- He, F.; Wang, Z.J.; Cheng, P.; Wang, Q.; Li, J.J.; Dang, Y.Y.; Wang, J.C.; Liu, C.T. Designing eutectic high entropy alloys of CoCrFeNiNbx. J. Alloys Compd. 2016, 656, 284–289. [Google Scholar] [CrossRef]
- He, F.; Wang, Z.J.; Shang, X.L.; Leng, C.; Li, J.J.; Wang, J.C. Stability of lamellar structures in CoCrFeNiNbx eutectic high entropy alloys at elevated temperatures. Mater. Des. 2016, 104, 259–264. [Google Scholar] [CrossRef]
- Huo, W.Y.; Zhou, H.; Fang, F.; Xie, Z.H.; Jiang, J.Q. Microstructure and mechanical properties of CoCrFeNiZrx eutectic high entropy alloys. Mater. Des. 2017, 134, 226–233. [Google Scholar] [CrossRef]
- Lucas, M.S.; Mauger, L.; Muoz, J.A.; Xiao, Y.M.; Sheets, A.O.; Semiatin, S.L.; Horwath, J.; Turgut, Z. Magnetic and vibrational properties of high-entropy alloys. J. Appl. Phys. 2011, 109, 07E307. [Google Scholar] [CrossRef]
- Karadeniz, E.P.; Lang, P.; Moszner, F.; Pogatscher, S.; Ruban, A.V.; Uggowitzer, P.J.; Kozeschnik, E. Thermodynamics of Pd-Mn phases and extension to the Fe-Mn-Pd system. Calphad 2015, 51, 314–333. [Google Scholar] [CrossRef]
- Tan, Y.M.; Li, J.S.; Wang, J.; Kou, H.C. Seaweed eutectic-dendritic solidification pattern in a CoCrFeNiMnPd eutectic high-entropy alloy. Intermetallics 2017, 85, 74–79. [Google Scholar] [CrossRef]
- Tan, Y.M.; Li, J.S.; Wang, J.; Kolbe, M.; Kou, H.C. Microstructure characterization of CoCrFeNiMnPdx eutectic high entropy alloys. J. Alloys Compd. 2018, 731, 600–611. [Google Scholar] [CrossRef]
- Wang, J.; Guo, T.; Li, J.S.; Jia, W.J.; Kou, H.C. Microstructure and mechanical properties of non-equilibrium solidified CoCrFeNi high entropy alloy. Mater. Chem. Phys. 2018, 200, 192–196. [Google Scholar] [CrossRef]
- Ben-Jacob, E.; Garik, P. The formation of patterns in non-equilibrium growth. Nature 1990, 343, 523–530. [Google Scholar] [CrossRef]
- Friedli, J.; Fife, J.L.; Di Napoli, P.; Rappaz, M. Dendritic Growth Morphologies in Al-Zn Alloys—Part I: X-ray Tomographic Microscopy. Metall. Mater. Trans. A 2013, 44, 5522–5531. [Google Scholar] [CrossRef]
- Dantzig, J.A.; Di Napoli, P.; Friedli, J.; Rappaz, M. Dendritic Growth Morphologies in Al-Zn Alloys—Part II: Phase-Field Computations. Metall. Mater. Trans. A 2013, 44, 5532–5543. [Google Scholar] [CrossRef]
- Castle, E.G.; Mullis, A.M.; Cochrane, R.F. Evidence for an extensive, undercooling-mediated transition in growth orientation, and novel dendritic seaweed microstructures in Cu–8.9 wt.% Ni. Acta Mater. 2014, 66, 378–387. [Google Scholar] [CrossRef]
- Castle, E.G.; Mullis, A.M.; Cochrane, R.F. Mechanism selection for spontaneous grain refinement in undercooled metallic melts. Acta Mater. 2014, 77, 76–84. [Google Scholar] [CrossRef]
- Liu, L.; Li, J.F.; Zhou, Y.H. Solidification interface morphology pattern in the undercooled Co-24.0 at.% Sn eutectic melt. Acta Mater. 2011, 59, 5558–5567. [Google Scholar] [CrossRef]
- Yuan, F.P.; Wu, X.L. Size effects of primary/secondary twins on the atomistic deformation mechanisms in hierarchically nanotwinned metals. J. Appl. Phys. 2013, 113, 203516. [Google Scholar] [CrossRef]
- Lu, Y.P.; Jiang, H.; Guo, S.; Wang, T.M.; Cao, Z.Q.; Li, T.J. A new strategy to design eutectic high entropy alloys using mixing enthalpy. Intermetallics 2017, 91, 124–128. [Google Scholar] [CrossRef]
- Jiang, H.; Han, K.M.; Gao, X.X.; Lu, Y.P.; Cao, Z.Q.; Gao, M.C.; Hawk, J.A.; Li, T.J. A new strategy to design eutectic high-entropy alloys using simple mixture method. Mater. Des. 2018, 142, 101–105. [Google Scholar] [CrossRef]
- Jin, X.; Zhou, Y.; Zhang, L.; Du, X.Y.; Li, B.S. A new pseudo binary strategy to design eutectic high entropy alloys using mixing enthalpy and valence electron concentration. Mater. Des. 2018, 143, 49–55. [Google Scholar] [CrossRef]

| HEA | Co | Cr | Fe | Ni | Pd | Mn | FCC Phase |
|---|---|---|---|---|---|---|---|
| Mn0.2 | 22.32 ± 0.91 | 21.23 ± 0.48 | 19.30 ± 0.41 | 19.79 ± 0.28 | 13.49 ± 0.66 | 2.09 ± 0.36 | CoCrFeNiPd-rich |
| Mn0.4 | 22.61 ± 0.53 | 21.92 ± 0.39 | 20.41 ± 0.90 | 20.02 ± 0.60 | 11.75 ± 0.67 | 3.56 ± 0.25 | CoCrFeNiPd-rich |
| Mn0.6 | 22.29 ± 0.51 | 22.12 ± 0.76 | 21.32 ± 0.36 | 21.22 ± 0.97 | 8.09 ± 0.89 | 4.22 ± 0.45 | CoCrFeNi-rich |
| Mn0.8 | 20.83 ± 0.36 | 21.44 ± 0.45 | 20.84 ± 0.53 | 24.46 ± 0.59 | 6.67 ± 0.70 | 5.12 ± 0.30 | CoCrFeNi-rich |
| HEA | Co | Cr | Fe | Ni | Pd | Mn | MnxPdy |
|---|---|---|---|---|---|---|---|
| Mn0.2 | 3.87 ± 0.85 | 7.48 ± 0.51 | 8.55 ± 0.17 | 5.49 ± 0.41 | 47.32 ± 0.65 | 27.29 ± 0.65 | Mn3Pd5 |
| Mn0.4 | 4.49 ± 0.83 | 7.32 ± 0.21 | 8.71 ± 0.21 | 5.53 ± 0.31 | 46.01 ± 0.76 | 29.44 ± 0.57 | Mn3Pd5 |
| Mn0.6 | 1.60 ± 0.09 | 4.78 ± 0.36 | 4.27 ± 0.45 | 3.39 ± 0.34 | 43.66 ± 0.93 | 42.31 ± 0.17 | Mn7Pd9 |
| Mn0.8 | 2.56 ± 0.38 | 6.28 ± 0.11 | 4.69 ± 0.79 | 3.81 ± 0.44 | 40.35 ± 0.67 | 41.71 ± 0.23 | Mn7Pd9 |
| EHEA (MnxPdy) | Spacing of Primary Twins λ1 (nm) | Spacing of Secondary Twins λ2 (nm) |
|---|---|---|
| Mn0.2 (Mn3Pd5) | 242.10 ± 26.63 | 10.02 ± 1.10 |
| Mn0.4 (Mn3Pd5) | 180.33 ± 19.84 | 9.99 ± 1.22 |
| Mn0.6 (Mn7Pd9) | 14.96 ± 16.46 | 2.22 ± 0.24 |
| Mn0.8 (Mn7Pd9) | 15.02 ± 1.65 | 1.46 ± 0.16 |
| Co | Cr | Ni | Mn | Pd | Al | Nb | Ta | Zr | Hf | |
|---|---|---|---|---|---|---|---|---|---|---|
| Fe | −1 | −1 | −2 | 0 | −4 | −11 | −16 | −15 | −25 | −21 |
| Co | −4 | 0 | −5 | −1 | −19 | −25 | −24 | −41 | −35 | |
| Cr | −7 | 2 | −15 | −10 | −9 | −7 | −12 | −9 | ||
| Ni | −8 | 0 | −22 | −30 | −29 | −49 | −42 | |||
| Mn | −23 | −19 | −4 | −4 | −15 | −12 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Y.; Li, J.; Wang, J.; Kou, H. Effect of Mn Addition on the Microstructures and Mechanical Properties of CoCrFeNiPd High Entropy Alloy. Entropy 2019, 21, 288. https://doi.org/10.3390/e21030288
Tan Y, Li J, Wang J, Kou H. Effect of Mn Addition on the Microstructures and Mechanical Properties of CoCrFeNiPd High Entropy Alloy. Entropy. 2019; 21(3):288. https://doi.org/10.3390/e21030288
Chicago/Turabian StyleTan, Yiming, Jinshan Li, Jun Wang, and Hongchao Kou. 2019. "Effect of Mn Addition on the Microstructures and Mechanical Properties of CoCrFeNiPd High Entropy Alloy" Entropy 21, no. 3: 288. https://doi.org/10.3390/e21030288
APA StyleTan, Y., Li, J., Wang, J., & Kou, H. (2019). Effect of Mn Addition on the Microstructures and Mechanical Properties of CoCrFeNiPd High Entropy Alloy. Entropy, 21(3), 288. https://doi.org/10.3390/e21030288







