Entropy Analysis of Temperature Swing Adsorption for CO2 Capture Using the Computational Fluid Dynamics (CFD) Method
Abstract
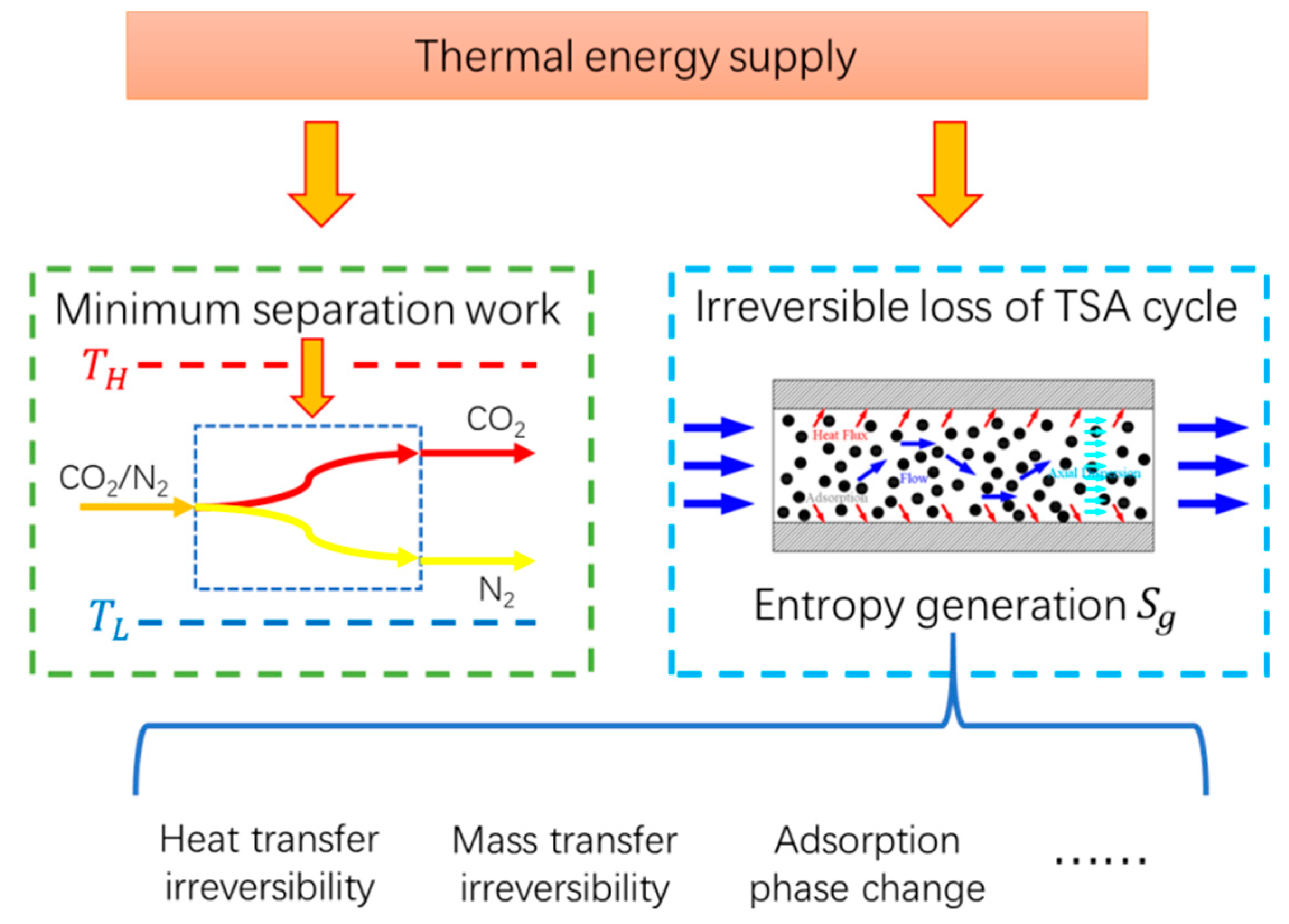
1. Introduction
2. Research Methodology
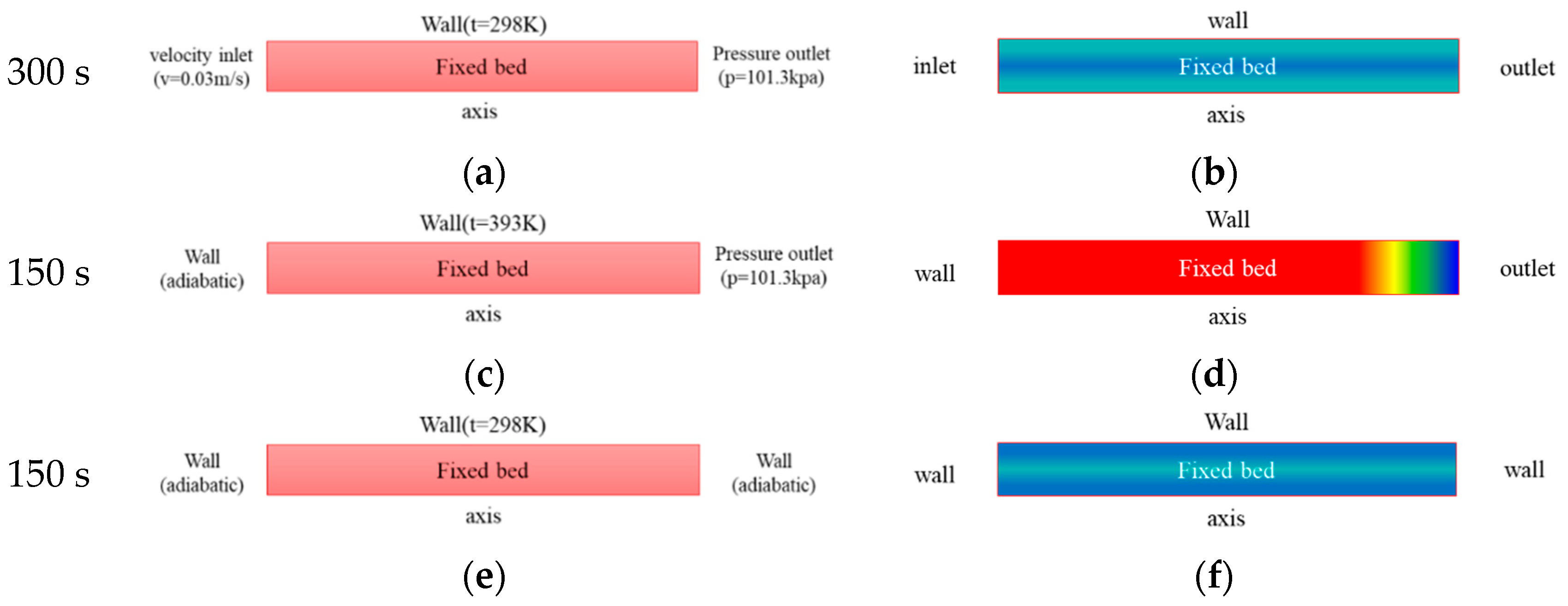
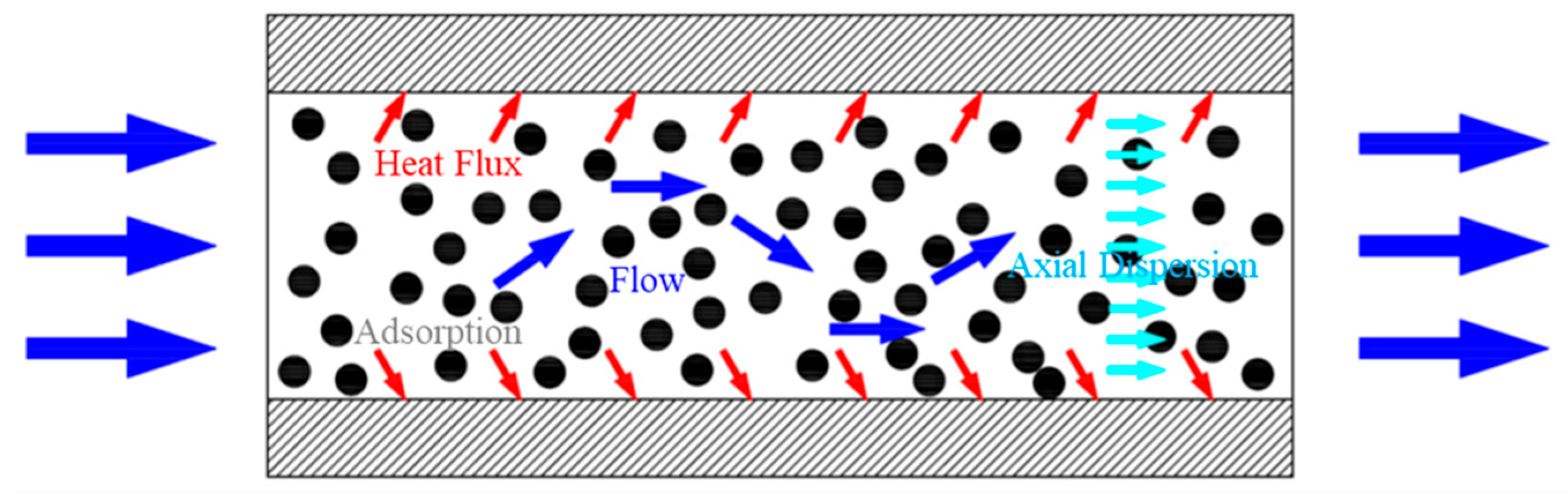
2.1. Physical Model
- (1)
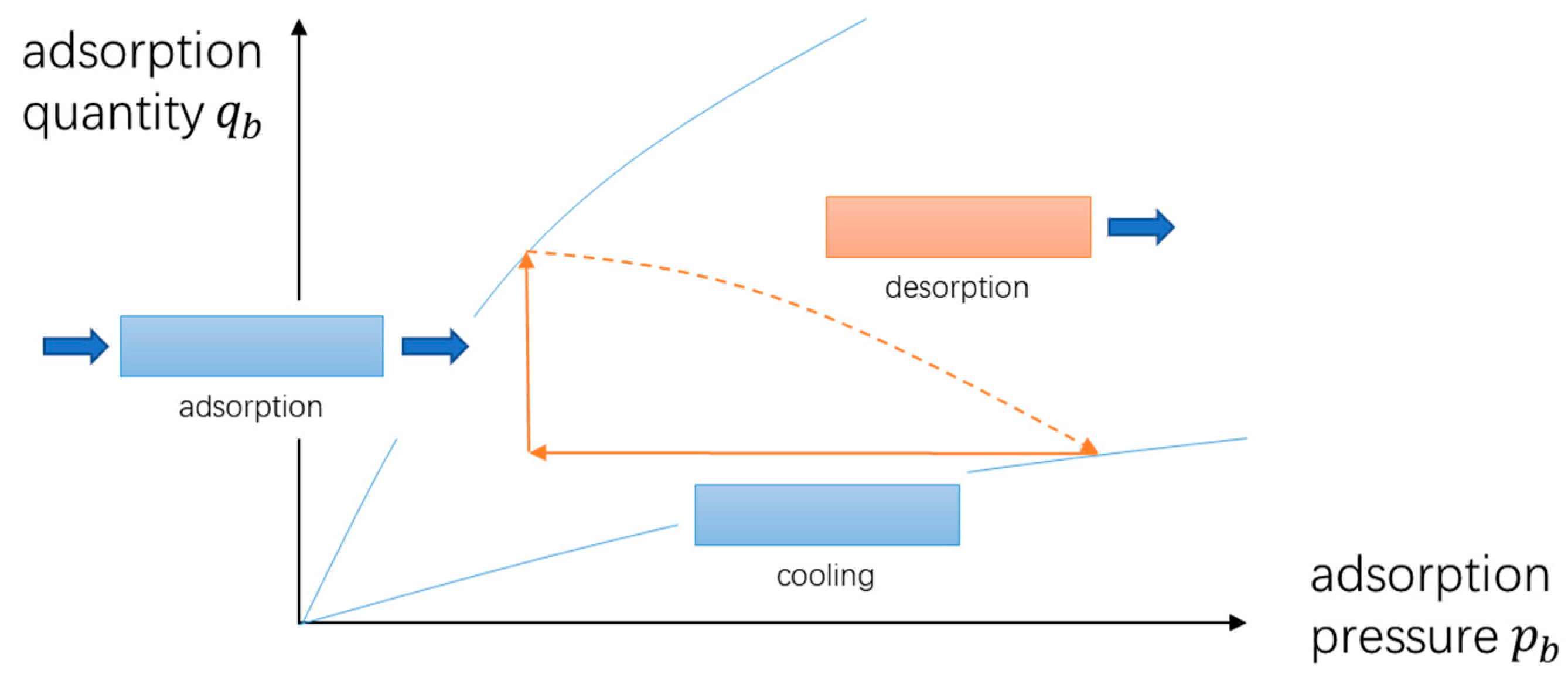
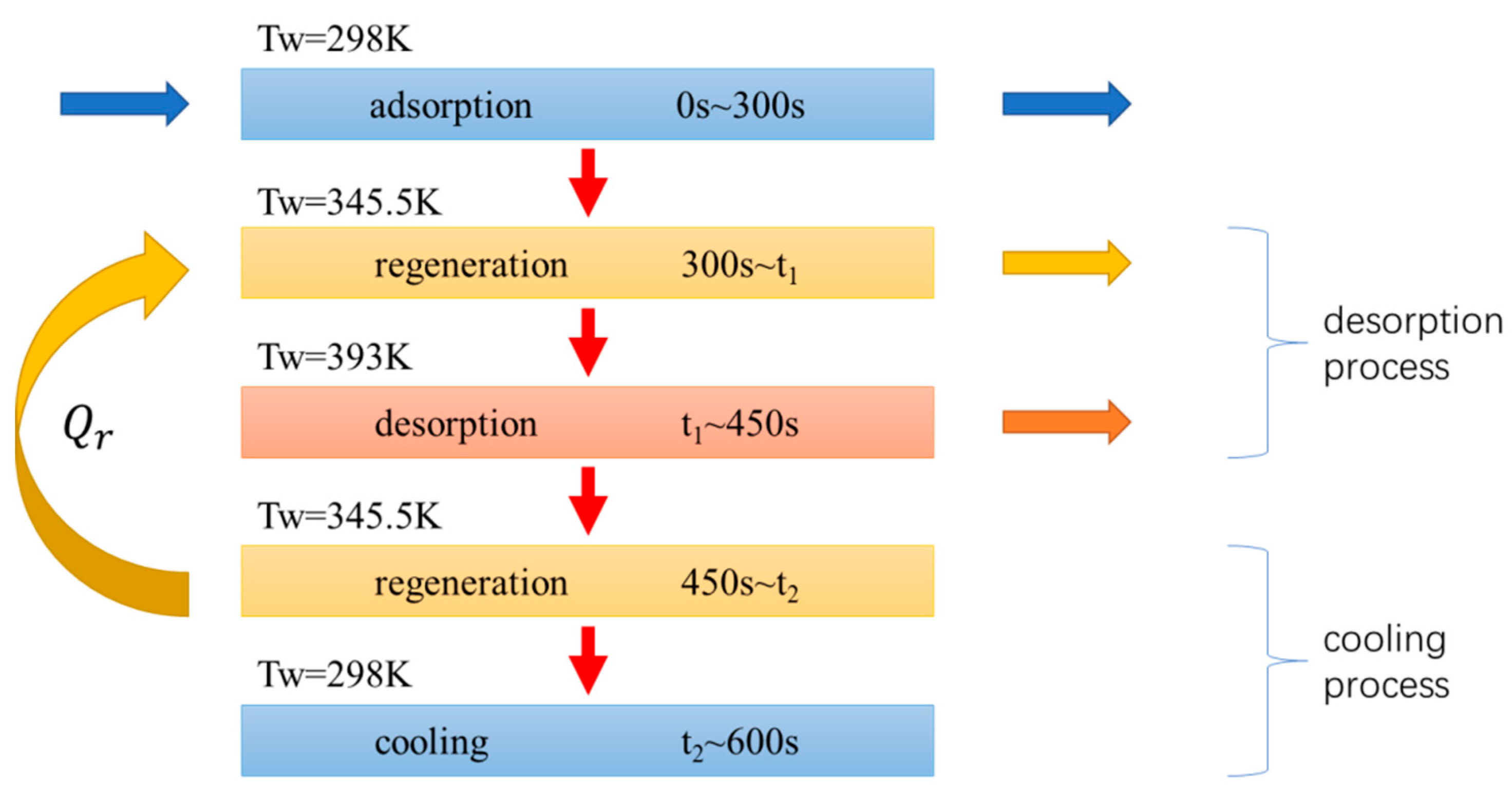
- Adsorption process: the mixture of CO2 and N2 continuously enters the adsorption bed from the inlet. CO2 gas is adsorbed in the adsorption bed through the selective adsorbent material, and N2 flows out of the adsorption bed from the outlet.
- (2)
- Desorption process: the adsorption bed is heated, and the adsorbed gas is desorbed and flows out of the adsorption bed.
- (3)
- Cooling process: the adsorption bed is cooled, during which the adsorption bed outlet and inlet are closed.
- (1)
- The porous media filling layer is regarded as continuous medium.
- (2)
- The mixture of CO2 and N2 is assumed to be an ideal gas
- (3)
- The flow process is considered as laminar flow
- (4)
- The physical properties of the adsorbent material are assumed to be constant
- (5)
- The mass transfer phenomenon in the adsorption process was described by the LDF model
- (6)
- Coupling effect in heat transfer and diffusion process is not considered
- (7)
- It is assumed that the heat capacity of the metal outer wall of the adsorption bed is very small and negligible, and only the internal process of the adsorption bed is simulated
- (8)
- Assume that the velocity of the external heat transfer fluid is very fast and the heat capacity is very large, and the temperature of the external heat transfer fluid is approximately constant. Therefore, the interior wall temperature of adsorption bed is also assumed to be constant during the process of adsorption, desorption and cooling.
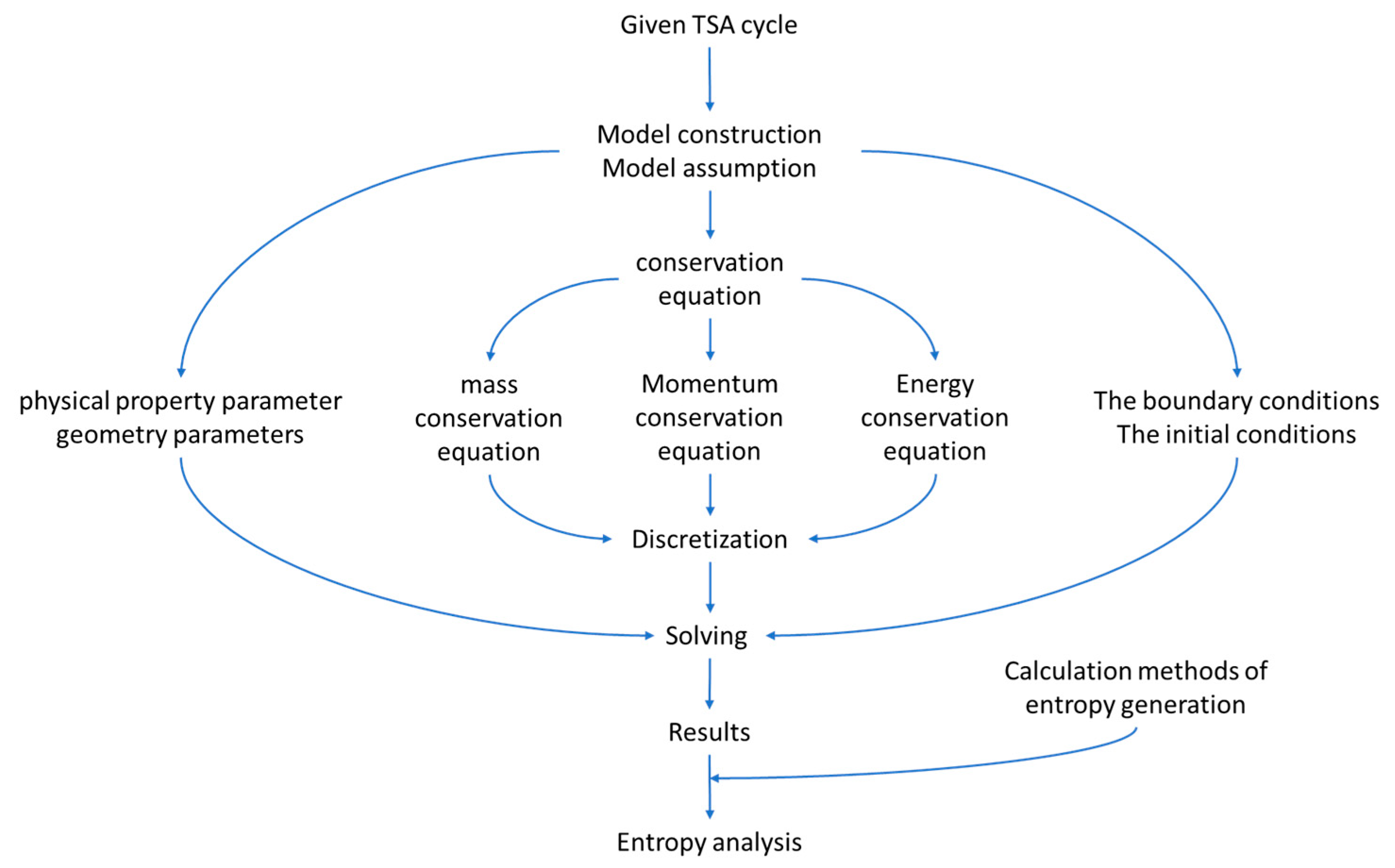
2.2. Mesh and Conservation Equation
2.3. Computing Method of the Entropy Generation and Energy Consumption
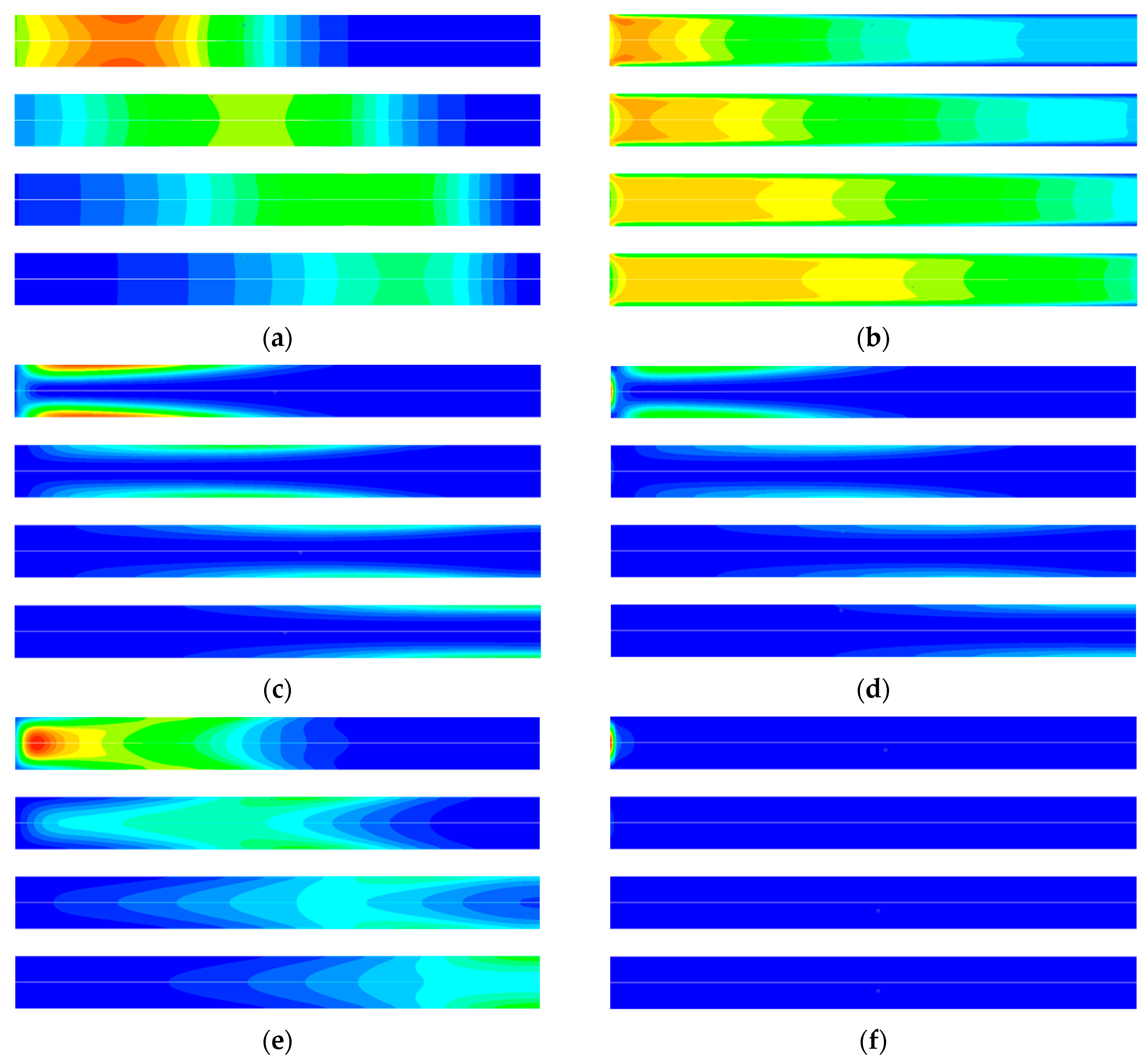
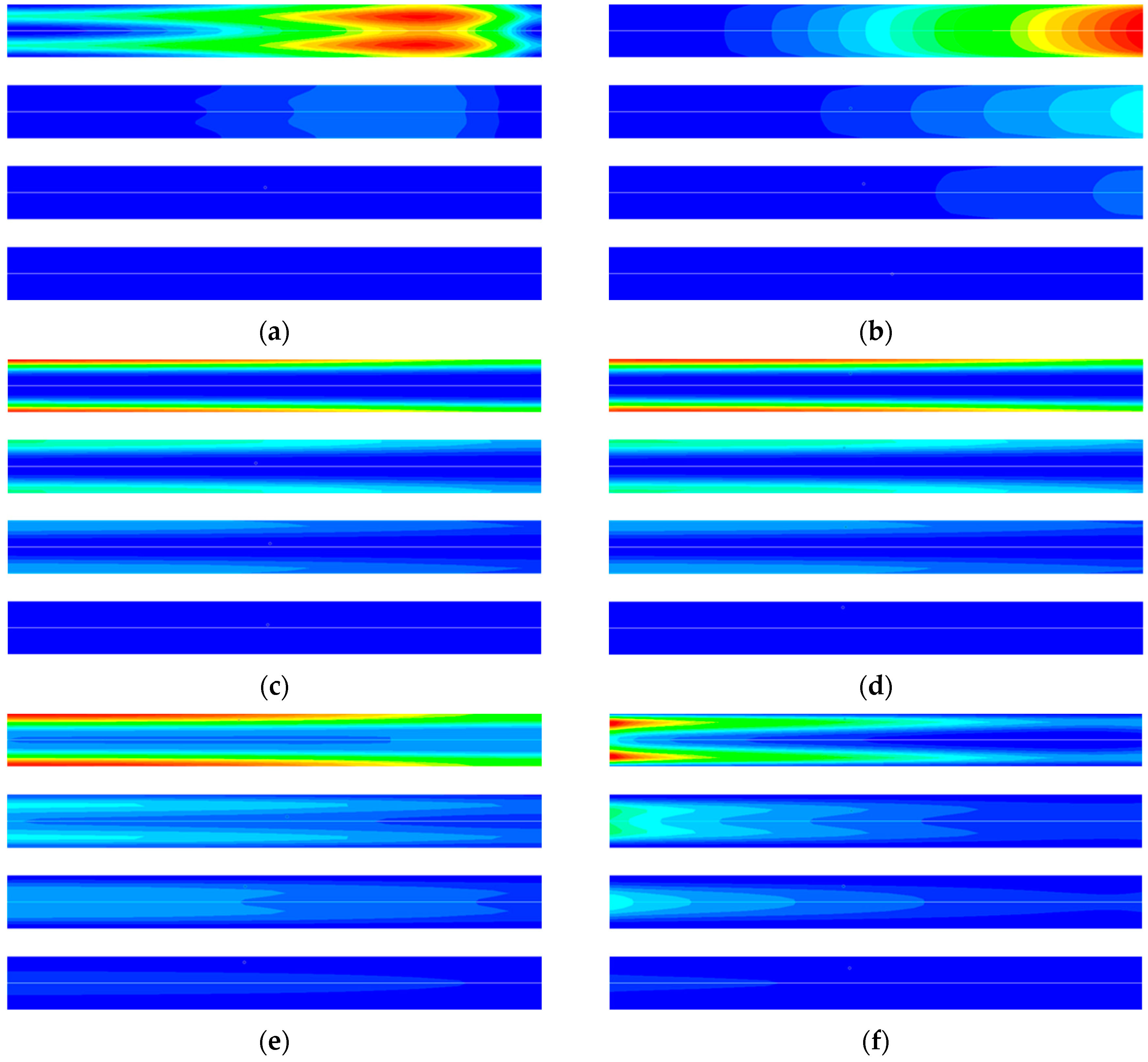
3. Result and Discussion
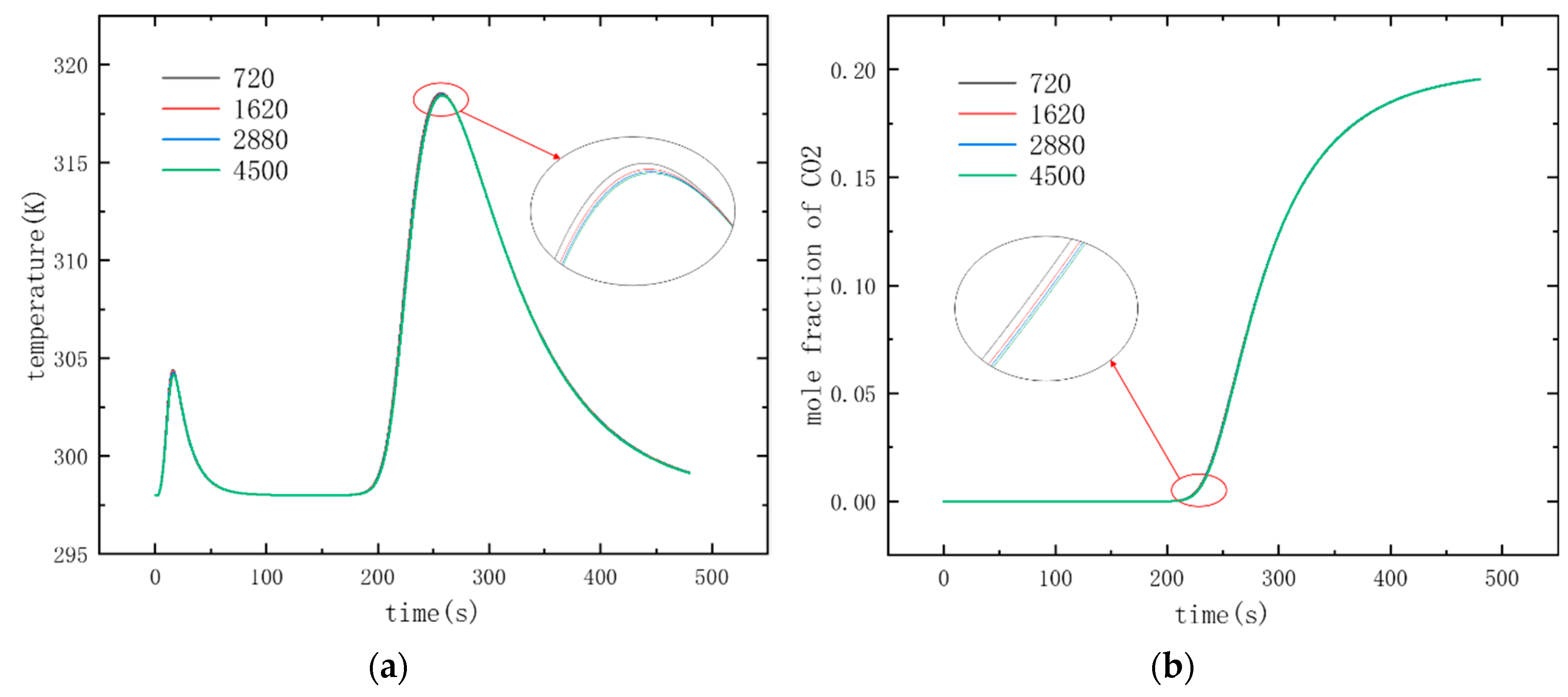
3.1. Mesh Independence
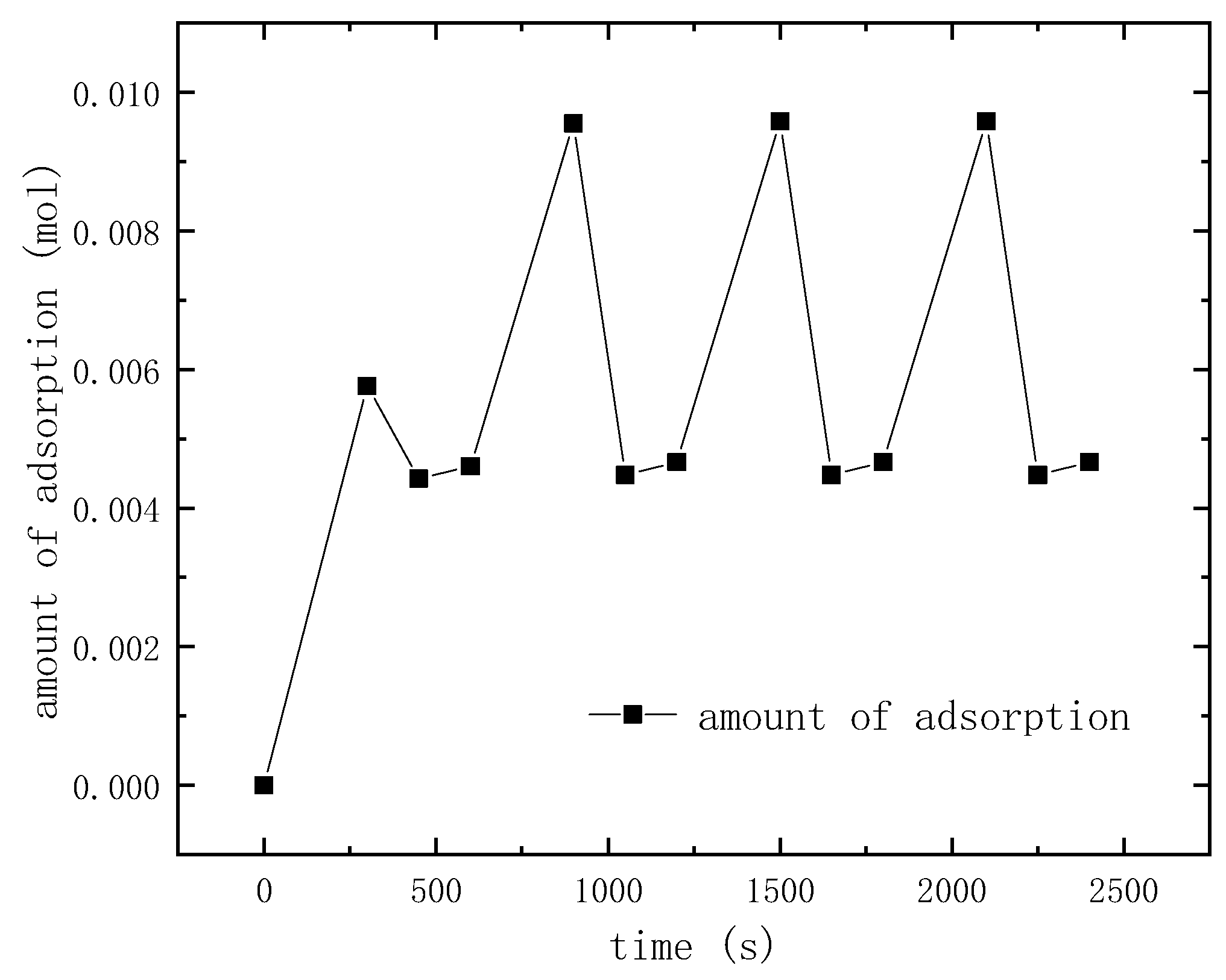
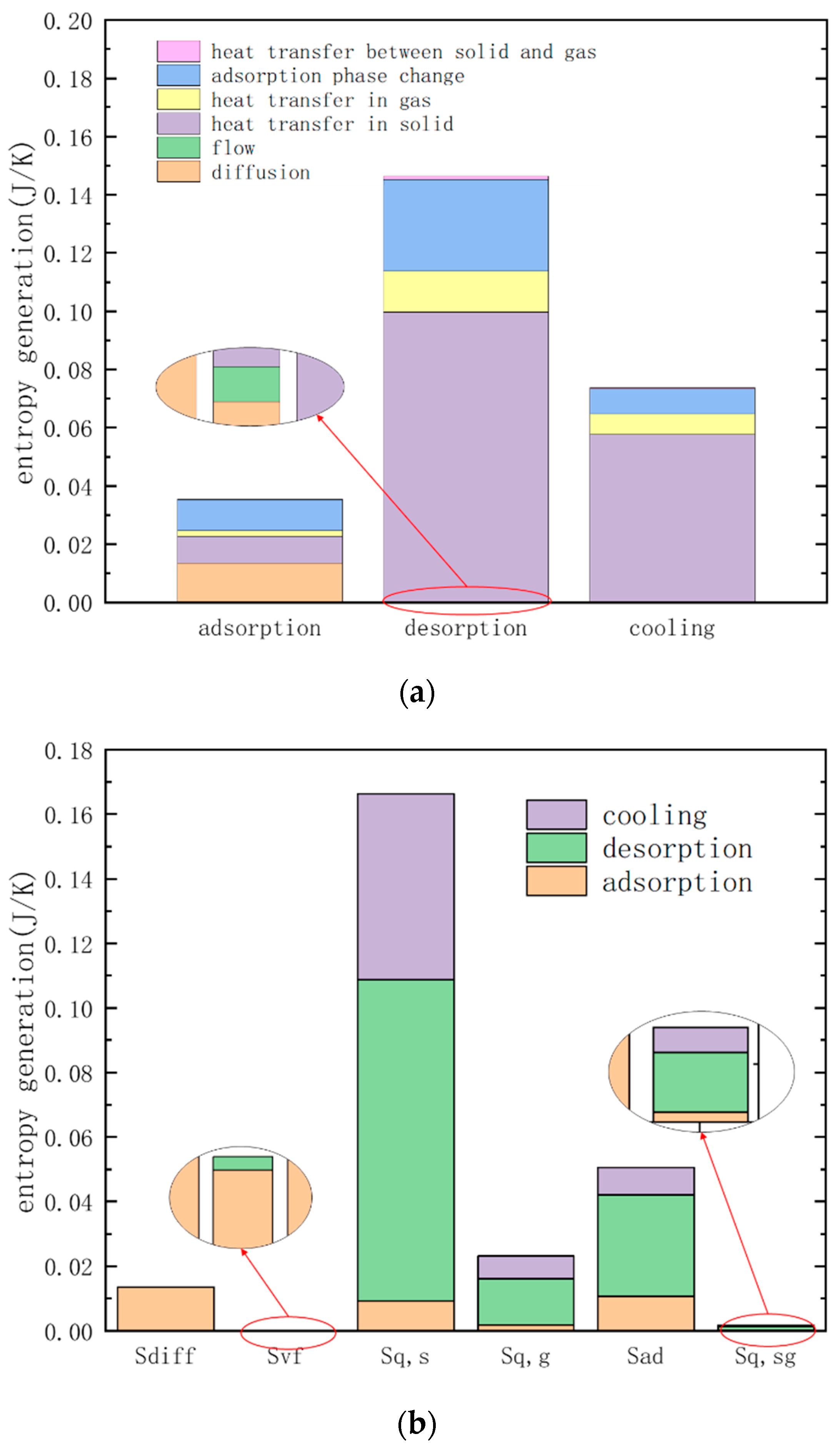
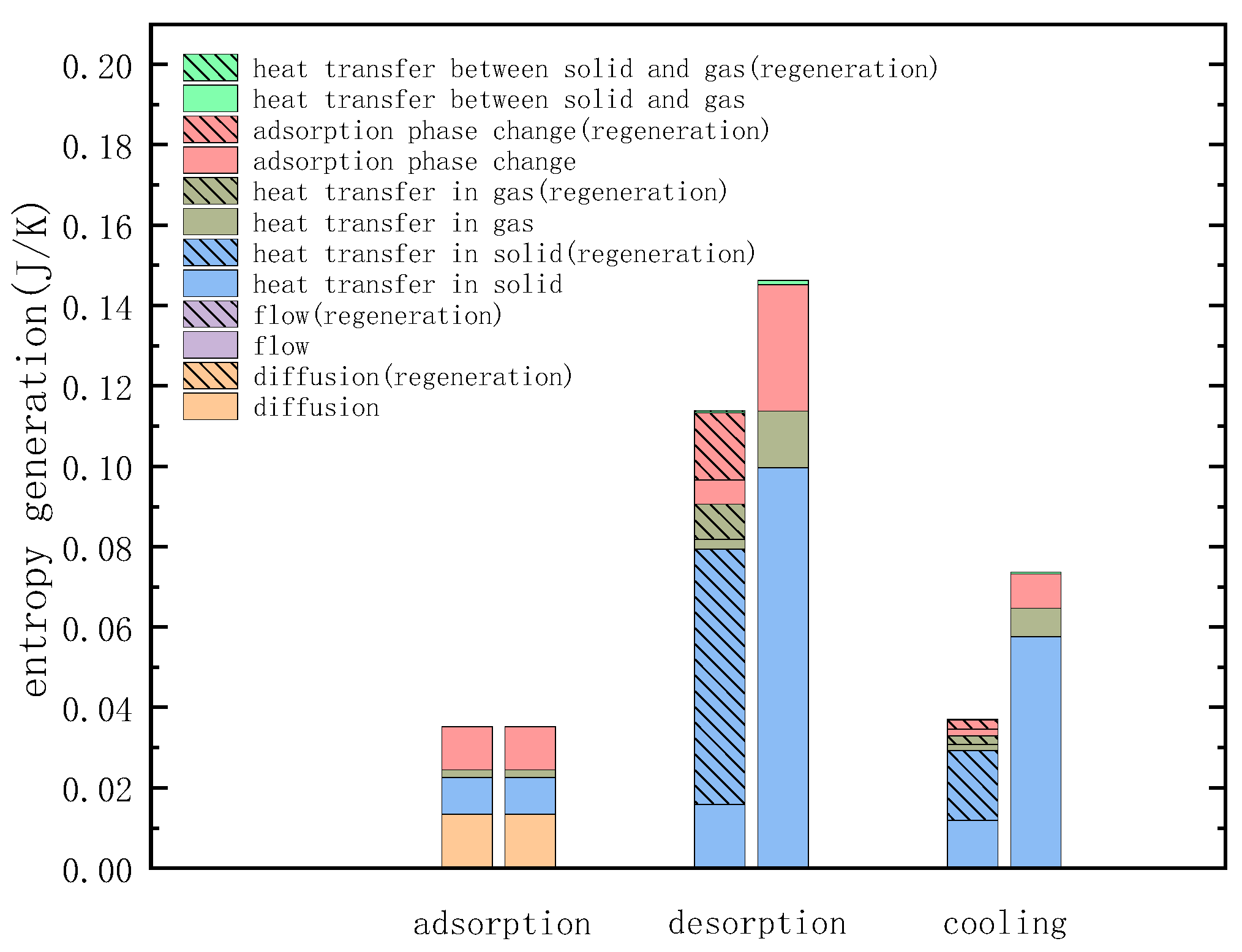
3.2. Entropy Analysis of TSA
3.3. Parameter Analysis of Heating Process
3.3.1. Thermal Conductivity of the Adsorbent
3.3.2. Adsorption Time Constant
3.3.3. Specific Heat Capacity of the Adsorbent
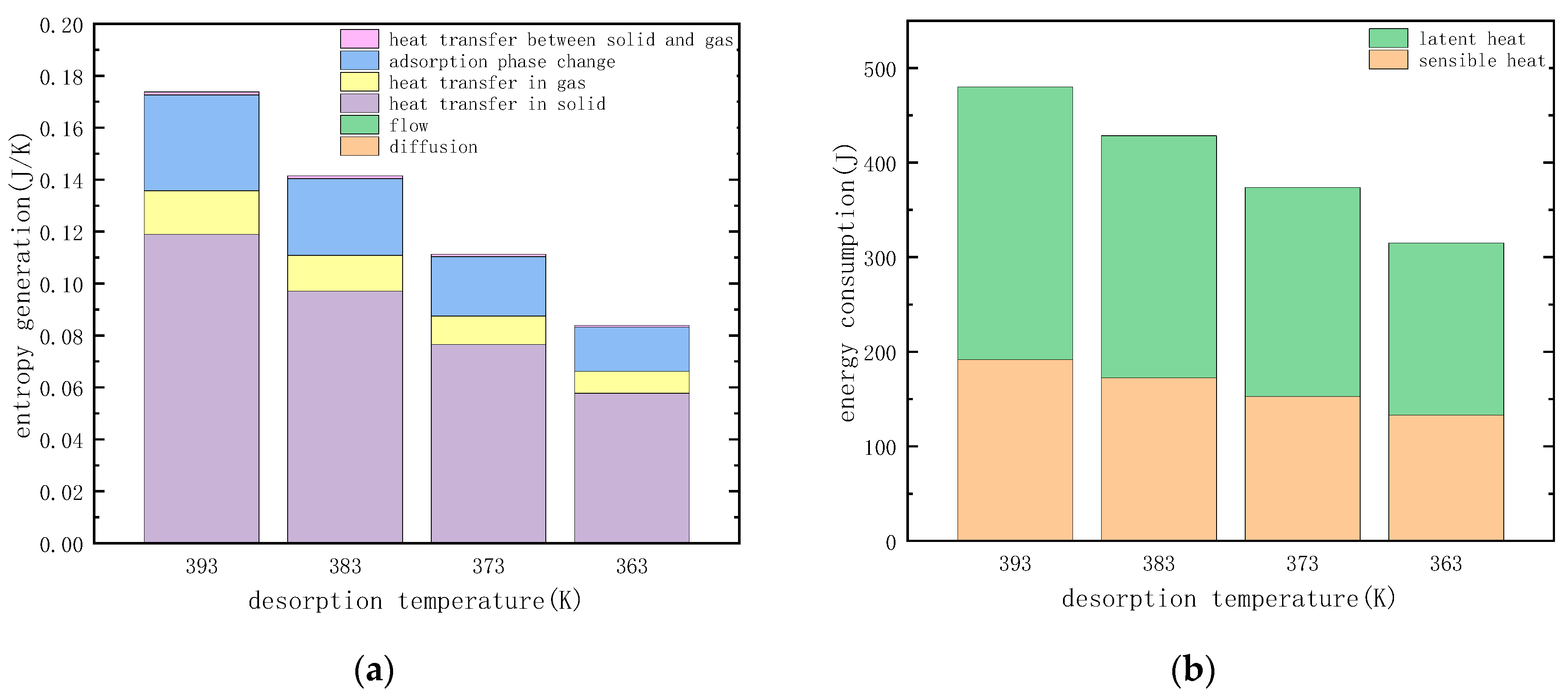
3.3.4. Temperature of Desorption
3.4. TSA Cycle with Heat Regeneration
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| Symbol | |
| C2 | inertial resistance factor, m−1 |
| Cp,s | adsorbent specific heat capacity, J kg−1 k−1 |
| dp | particle diameter, m |
| D | diameter, m |
| Di,m | effective diffusion coefficient for component i, m2 s−1 |
| Di,j | diffusion coefficient of component i in component j, m2 s−1 |
| E | total energy of gas, J m−3 |
| Et1~t2 | energy consumption between t1 and t2 |
| h | enthalpy |
| hf | heat transfer coefficient between gas phase and solid, W m−2 K−1 |
| ∆H | the heat of adsorption, J mol−1 |
| J | diffusion flux, kg m−2 s−1 |
| keq | isotherm adsorption constant, Pa−1 |
| k0 | adsorption constant at infinite dilution, Pa−1 |
| kL | LDF adsorption time constant, s−1 |
| k | thermal conductivity of gas |
| ks | adsorbent thermal conductivity, W m−1 K−1 |
| L | length, m |
| M | molecular weight, mol kg−1 |
| m | mass of captured, kg |
| n | heterogeneity parameter |
| p | Pressure, Pa |
| q | adsorption uptake, mol kg−1 |
| q* | equilibrium adsorption uptake, mol kg−1 |
| qm | maximum adsorption uptake, mol kg−1 |
| R | universal gas constant, J mol−1 K−1 |
| Sg | entropy generation, J K−1 |
| Smomentum | momentum source term |
| T | temperature, K |
| t | time, s |
| x | mole fraction |
| y | mass fraction |
| Greek letters | |
| α | permeability m2 |
| ε | porosity |
| ρ | density, kg m−3 |
| σ | entropy generation rate, W m3 K−1 |
| μ | gas dynamic viscosity, Pa s |
| μa | chemical potential of adsorbed phase, J kg−1 |
| μg | chemical potential of gas, J kg−1 |
| δ | bed wall thickness, m |
| τ | stress tensor |
| χ | chemical potential of gas in diffusion process, J kg−1 |
| Subscripts | |
| a | adsorbed phase |
| ad | adsorption |
| b | bed |
| c | critical |
| d | diffusion |
| g | gas |
| i | components |
| in | inlet |
| p | particle |
| q | heat transfer |
Appendix A
References
- Special Report on Global Warming of 1.5 °C (Report). Incheon, South Korea; Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 7 October 2018.
- Baena-Moreno, F.M.; Rodríguez-Galán, M.; Vega, F.; Alonso-Fariñas, B.; Vilches Arenas, L.F.; Navarrete, B. Carbon capture and utilization technologies: A literature review and recent advances. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 41, 1403–1433. [Google Scholar] [CrossRef]
- Cuéllar-Franca, R.M.; Azapagic, A. Carbon capture, storage and utilisation technologies: A critical analysis and comparison of their life cycle environmental impacts. J. CO2 Util. 2015, 9, 82–102. [Google Scholar] [CrossRef]
- Ben-Mansour, R.; Habib, M.A.; Bamidele, O.E.; Basha, M.; Qasem, N.A.A.; Peedikakkal, A.; Laoui, T.; Ali, M. Carbon capture by physical adsorption: Materials, experimental investigations and numerical modeling and simulations—A review. Appl. Energy 2016, 161, 225–255. [Google Scholar] [CrossRef]
- Al-Mamoori, A.; Krishnamurthy, A.; Rownaghi, A.A.; Rezaei, F. Carbon Capture and Utilization Update. Energy Technol. 2017, 5, 834–849. [Google Scholar] [CrossRef]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef]
- Zhao, R.; Liu, L.; Zhao, L.; Deng, S.; Li, S.; Zhang, Y.; Li, H. Techno-economic analysis of carbon capture from a coal-fired power plant integrating solar-assisted pressure-temperature swing adsorption (PTSA). J. Clean. Prod. 2019, 214, 440–451. [Google Scholar] [CrossRef]
- Zhao, R.; Zhao, L.; Wang, S.; Deng, S.; Li, H.; Yu, Z. Solar-assisted pressure-temperature swing adsorption for CO2 capture: Effect of adsorbent materials. Sol. Energy Mater. Sol. Cells 2018, 185, 494–504. [Google Scholar] [CrossRef]
- Wang, F.; Deng, S.; Zhao, J.; Zhao, J.; Yang, G.; Yan, J. Integrating geothermal into coal-fired power plant with carbon capture: A comparative study with solar energy. Energy Convers. Manag. 2017, 148, 569–582. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, S.; Zhao, R.; He, J.; Zhao, L. Energy-saving pathway exploration of CCS integrated with solar energy: A review of innovative concepts. Renew. Sustain. Energy Rev. 2017, 77, 652–669. [Google Scholar] [CrossRef]
- Ben-Mansour, R.; Qasem, N.A. An efficient temperature swing adsorption (TSA) process for separating CO2 from CO2/N2 mixture using Mg-MOF-74. Energy Convers. Manag. 2018, 156, 10–24. [Google Scholar] [CrossRef]
- Qasem, N.A.; Ben-Mansour, R. Energy and productivity efficient vacuum pressure swing adsorption process to separate CO2 from CO2/N2 mixture using Mg-MOF-74: A CFD simulation. Appl. Energy 2018, 209, 190–202. [Google Scholar] [CrossRef]
- Shen, C.; Liu, Z.; Li, P.; Yu, J. Two-Stage VPSA Process for CO2 Capture from Flue Gas Using Activated Carbon Beads. Ind. Eng. Chem. Res. 2012, 51, 5011–5021. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, L.; Kong, X.; Li, P.; Yu, J.; Rodrigues, A.E. Onsite CO2 Capture from Flue Gas by an Adsorption Process in a Coal-Fired Power Plant. Ind. Eng. Chem. Res. 2012, 51, 7355–7363. [Google Scholar] [CrossRef]
- Zhao, R.; Deng, S.; Liu, Y.; Zhao, Q.; He, J.; Zhao, L. Carbon pump: Fundamental theory and applications. Energy 2017, 119, 1131–1143. [Google Scholar] [CrossRef]
- Zhao, R.; Deng, S.; Zhao, L.; Li, S.; Zhang, Y.; Liu, B. Performance analysis of temperature swing adsorption for CO2 capture using thermodynamic properties of adsorbed phase. Appl. Ther. Eng. 2017, 123, 205–215. [Google Scholar] [CrossRef]
- Zhao, R.; Zhao, L.; Deng, S.; Song, C.; He, J.; Shao, Y.; Li, S. A comparative study on CO2 capture performance of vacuum-pressure swing adsorption and pressure-temperature swing adsorption based on carbon pump cycle. Energy 2017, 137, 495–509. [Google Scholar] [CrossRef]
- Zhao, R.; Deng, S.; Zhao, L.; Zhao, Y.; Li, S.; Zhang, Y.; Yu, Z. Experimental study and energy-efficiency evaluation of a 4-step pressure-vacuum swing adsorption (PVSA) for CO2 capture. Energy Convers. Manag. 2017, 151, 179–189. [Google Scholar] [CrossRef]
- Zhao, R.; Liu, L.; Zhao, L.; Deng, S.; Li, H. Thermodynamic analysis on carbon dioxide capture by Electric Swing Adsorption (ESA) technology. J. CO2 Util. 2018, 26, 388–396. [Google Scholar] [CrossRef]
- Meunier, F.; Poyelle, F.; LeVan, M.D. Second-law analysis of absorptive refrigeration cycles: The role of thermal coupling entropy production. Appl. Ther. Eng. 1997, 17, 43–55. [Google Scholar] [CrossRef]
- Myat, A.; Thu, K.; Choon, N.K. The experimental investigation on the performance of a low temperature waste heat-driven multi-bed desiccant dehumidifier (MBDD) and minimization of entropy generation. Appl. Ther. Eng. 2012, 39, 70–77. [Google Scholar] [CrossRef]
- Guoxiang, L.; Liping, P.; Meng, L.; Dongsheng, Y.; Qingni, Y.; Rong, A. Multi objective Optimal Method for Carbon Dioxide Removal Assembly in Manned Spacecraft. J. Aerosp. Eng. 2016, 29, 04016052. [Google Scholar] [CrossRef]
- Chua, H.T.; Ng, K.C.; Malek, A.; Kashiwagi, T.; Akisawa, A.; Saha, B.B. Entropy generation analysis of two-bed, silica gel-water, non-regenerative adsorption chillers. J. Phys. D Appl. Phys. 1999, 31, 1471. [Google Scholar] [CrossRef]
- Chua, H.T.; Ng, K.C.; Malek, A.; Oo, N.M. General thermodynamic framework for understanding temperature-entropy diagram of batchwise operating thermodynamic cooling cycles. J. Appl. Phys. 2001, 89, 5151–5158. [Google Scholar] [CrossRef]
- Myat, A.; Thu, K.; Ng, K.C.; Kim, Y.D. An entropy generation and genetic algorithm optimization of two-bed adsorption cooling cycle. Proc. Inst. Mech. Eng. Part E J. Process Mech. Eng. 2012, 226, 142–156. [Google Scholar] [CrossRef]
- Thu, K.; Kim, Y.D.; Myat, A.; Chun, W.G.; Ng, K.C. Entropy generation analysis of an adsorption cooling cycle. Int. J. Heat Mass Trans. 2013, 60, 143–150. [Google Scholar] [CrossRef]
- Li, A.; Ismail, A.B.; Thu, K.; Ng, K.C.; Loh, W.S. Performance evaluation of a zeolite-water adsorption chiller with entropy analysis of thermodynamic insight. Appl. Energy 2014, 130, 702–711. [Google Scholar] [CrossRef]
- Meunier, F. Second law analysis of a solid adsorption heat pump operating on reversible cascade cycles: Application to the Zeolite-water pair. J. Heat Recov. Syst. 1985, 5, 133–141. [Google Scholar] [CrossRef]
- Meunier, F.; Kaushik, S.C.; Neveu, P.; Poyelle, F. A comparative thermodynamic study of sorption systems: Second law analysis. Int. J. Refrig. 1996, 19, 414–421. [Google Scholar] [CrossRef]
- Okunev, B.N.; Safonov, M.S. A detailed analysis of entropy production and improvement of the thermodynamic cycle of an adsorption refrigerating plant. J. Eng. Phys. Thermophys. 2006, 79, 781–787. [Google Scholar] [CrossRef]
- Sharafianardakani, A.; Bahrami, M. A quasi steady state model for adsorption cooling systems: Automotive applications. In Proceedings of the ASME 2012 6th International Conference on Energy Sustainability collocated with the ASME 2012 10th International Conference on Fuel Cell Science, Engineering and Technology, San Diego, CA, USA, 23–26 July 2012; American Society of Mechanical Engineers: New York, NY, USA, 2012. [Google Scholar]
- Qasem, N.A.; Ben-Mansour, R. Adsorption breakthrough and cycling stability of carbon dioxide separation from CO2/N2/H2O mixture under ambient conditions using 13X and Mg-MOF-74. Appl. Energy 2018, 230, 1093–1107. [Google Scholar] [CrossRef]
- Zhen, L. Adsorption Process for CO2 Capture from Flue Gas of Coal-Fire Power Plant; East China University of Science and Technology: Shanghai, China, 2012. (In Chinese) [Google Scholar]
- Dantas, T.L.; Luna, F.M.T.; Silva, I.J., Jr.; de Azevedo, D.C.; Grande, C.A.; Rodrigues, A.E.; Moreira, R.F. Carbon dioxide-nitrogen separation through adsorption on activated carbon in a fixed bed. Chem. Eng. J. 2011, 169, 11–19. [Google Scholar] [CrossRef]
- De Groot, S.R. Non-Equilibrium Thermodynamics; North-Holland Pub. Co: Amsterdam, The Netherlands, 1962; pp. 70–71. [Google Scholar]
- Chu, S.-X.; Liu, L.H. Analysis of Entropy Generation During Methane-air Diffusion Combustion Processes. Proc. Chin. Soc. Electr. Eng. 2008, 28, 34–40. (In Chinese) [Google Scholar]
- van der Ham, L.V.; Gross, J.; Verkooijen, A.; Kjelstrup, S. Efficient Conversion of Thermal Energy into Hydrogen: Comparing Two Methods to Reduce Exergy Losses in a Sulfuric Acid Decomposition Reactor. Ind. Eng. Chem. Res. 2009, 48, 8500–8507. [Google Scholar] [CrossRef]
- Shoude, L. Study on the Appilcation of Irreversible Thermodynamics in the Problems of Porous Media Seepage; Zhejiang University: Zhejiang, China, 2003. [Google Scholar]
- Chua, H.T.; Ng, K.C.; Chakraborty, A.; Oo, N.M. Thermodynamic Property Fields of an Adsorbate-Adsorbent System. Langmuir 2003, 19, 2254–2259. [Google Scholar] [CrossRef]
- Qasem, N.A.; El-Shaarawi, M.A. Thermal analysis and modeling study of an activated carbon solar adsorption icemaker: Dhahran case study. Energy Convers. Manag. 2015, 100, 310–323. [Google Scholar] [CrossRef]
- Qasem, N.A.; El-Shaarawi, M.A. Improving ice productivity and performance for an activated carbon/methanol solar adsorption ice-maker. Sol. Energy 2013, 98, 523–542. [Google Scholar] [CrossRef]



| Parameters | Value | Unit |
|---|---|---|
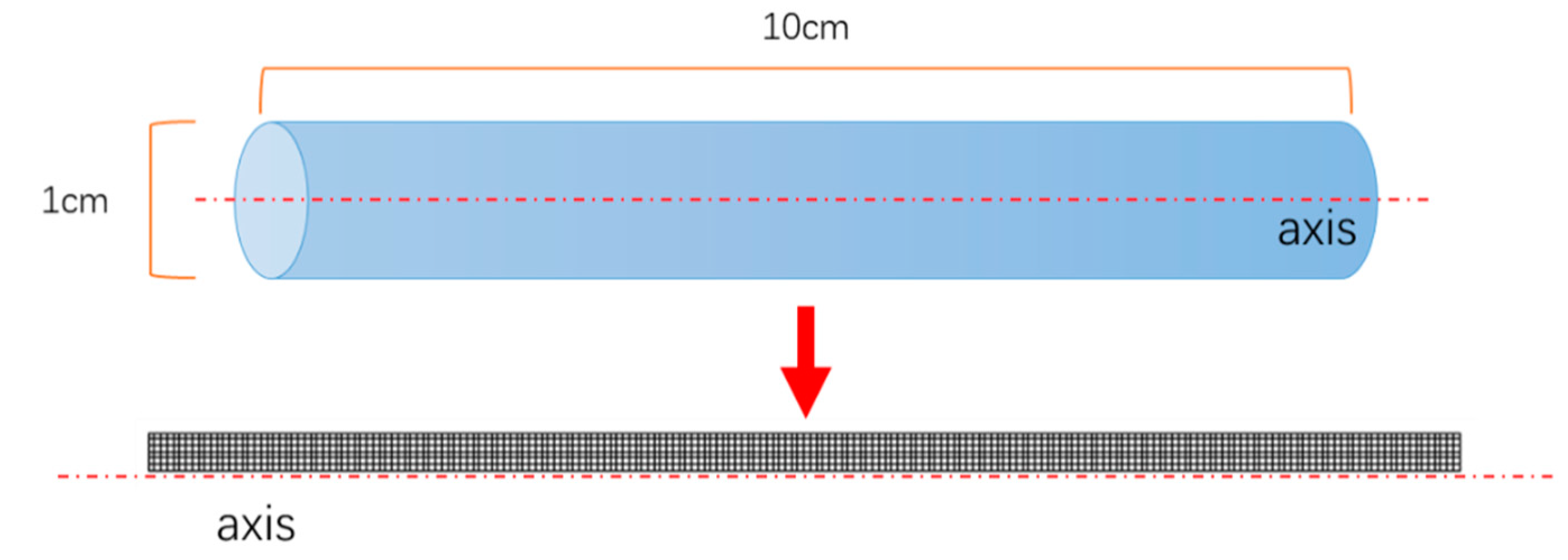
| Length of adsorption bed, L | 10 | cm |
| Inner diameter of adsorption bed, D | 1 | cm |
| Porosity, ε | 0.7417 | |
| Adsorbent specific heat capacity, Cp,s | 911 | J/kg K |
| Absorbent thermal conductivity, ks | 900 | W/m K |
| Inlet velocity, vin | 0.03 | m/s |
| Inlet mole fraction, xin | 0.2 | |
| Adsorption temperature, Tads | 298 | K |
| Desorption temperature, Tdes | 393 | K |
| Operation pressure, pb | 101,300 | Pa |
| qm (mol kg−1) | K0 (Pa−1) | n | ΔH (kJ mol−1) | KL (s−1) | |
|---|---|---|---|---|---|
| CO2 | 11.4048 | 3.089 × 10−11 | 0.4217 | −42,000 | 0.1182 |
| N2 | 6.7072 | 9.36 × 10−10 | 1 | −18,000 | 0.3043 |
| Different Factors of Entropy Generation | Cause of Entropy Generation |
|---|---|
| Entropy generation of heat transfer | 1. Entropy generation of heat transfer in solids 2. Entropy generation of heat transfer in gas 3. Entropy generation of heat transfer between gas and solid |
| Entropy generation of diffusion | 1. Entropy generation of diffusion between different components of the mixture |
| Entropy generation of flow friction | 1. Entropy generation of flow friction dissipation between the wall and the mixture 2. Entropy generation of flow friction dissipation between adsorbent particles and mixture |
| Entropy generation of phase change | 1. Entropy generation of adsorption phase change between adsorbent and mixture |
| Cycle | Productivity of CO2 (kg) | Specific Energy Consumption (GJ t−1) | Specific Entropy Generation (J kg−1) | Purity of Product Gas |
|---|---|---|---|---|
| Cycle with regeneration | 0.000202 | 1.672 | 924 | 0.842 |
| Cycle without regeneration | 0.000223 | 1.906 | 1147 | 0.844 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Z.; Deng, S.; Li, S.; Lian, Y.; Zhao, L.; Yuan, X. Entropy Analysis of Temperature Swing Adsorption for CO2 Capture Using the Computational Fluid Dynamics (CFD) Method. Entropy 2019, 21, 285. https://doi.org/10.3390/e21030285
Guo Z, Deng S, Li S, Lian Y, Zhao L, Yuan X. Entropy Analysis of Temperature Swing Adsorption for CO2 Capture Using the Computational Fluid Dynamics (CFD) Method. Entropy. 2019; 21(3):285. https://doi.org/10.3390/e21030285
Chicago/Turabian StyleGuo, Zhihao, Shuai Deng, Shuangjun Li, Yahui Lian, Li Zhao, and Xiangzhou Yuan. 2019. "Entropy Analysis of Temperature Swing Adsorption for CO2 Capture Using the Computational Fluid Dynamics (CFD) Method" Entropy 21, no. 3: 285. https://doi.org/10.3390/e21030285
APA StyleGuo, Z., Deng, S., Li, S., Lian, Y., Zhao, L., & Yuan, X. (2019). Entropy Analysis of Temperature Swing Adsorption for CO2 Capture Using the Computational Fluid Dynamics (CFD) Method. Entropy, 21(3), 285. https://doi.org/10.3390/e21030285





