Entropy Generation and Heat Transfer in Drilling Nanoliquids with Clay Nanoparticles
Abstract
1. Introduction
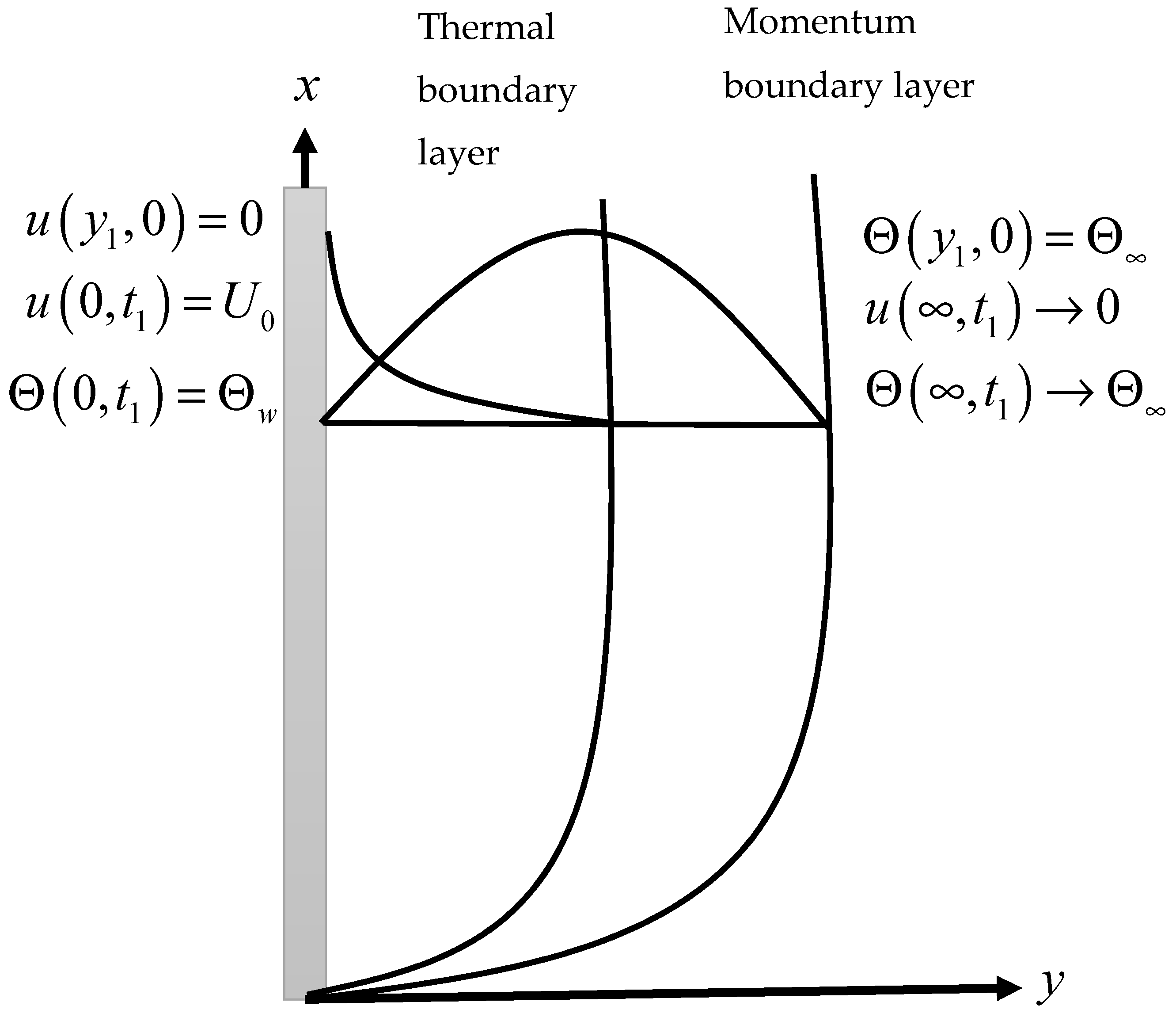
2. Model Formulation
3. Entropy Generation (Irreversibility Analysis)
4. Solution of the Model
4.1. Solutions for Conventional Base Fluids (Water, Engine Oil and Kerosene Oil)
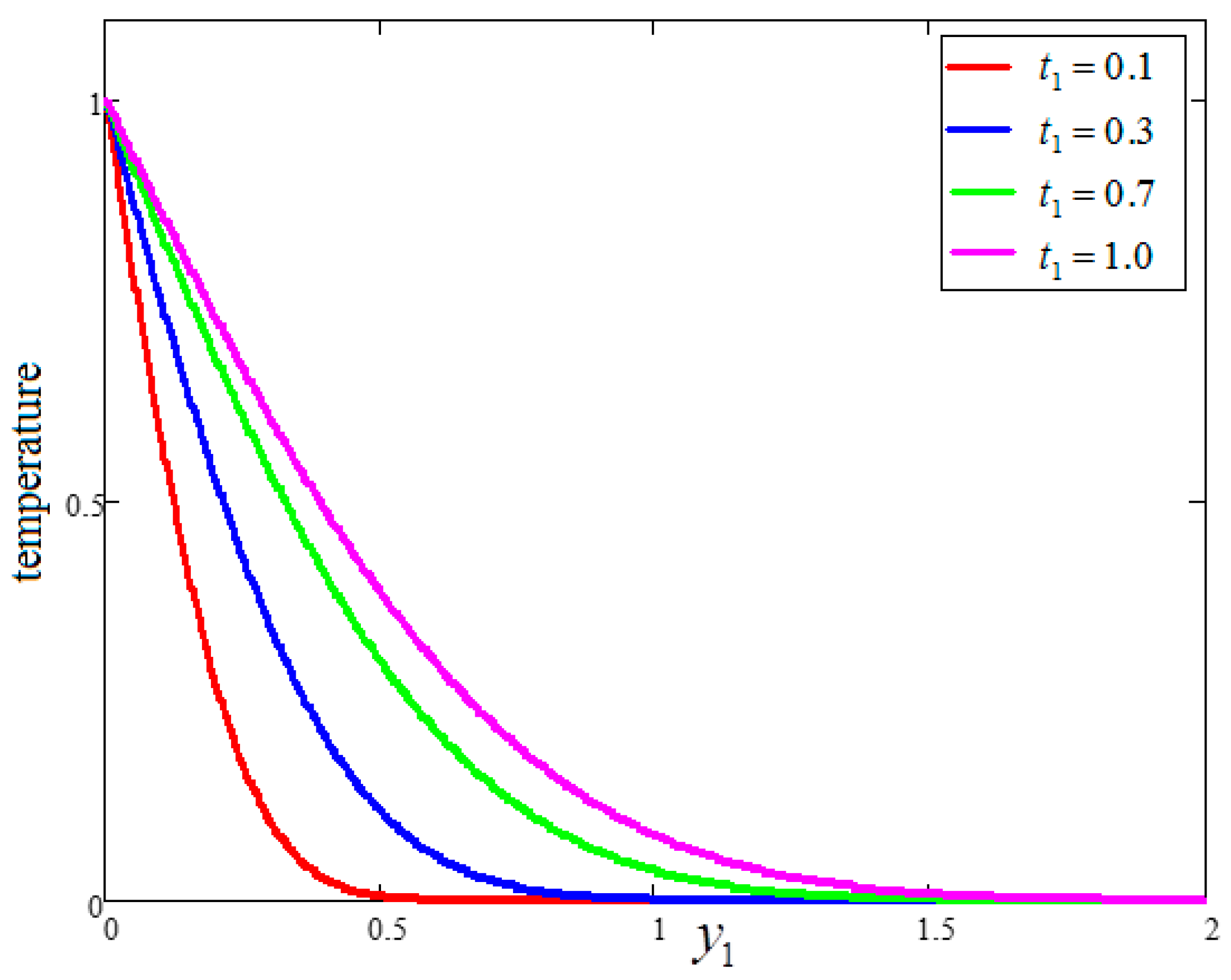
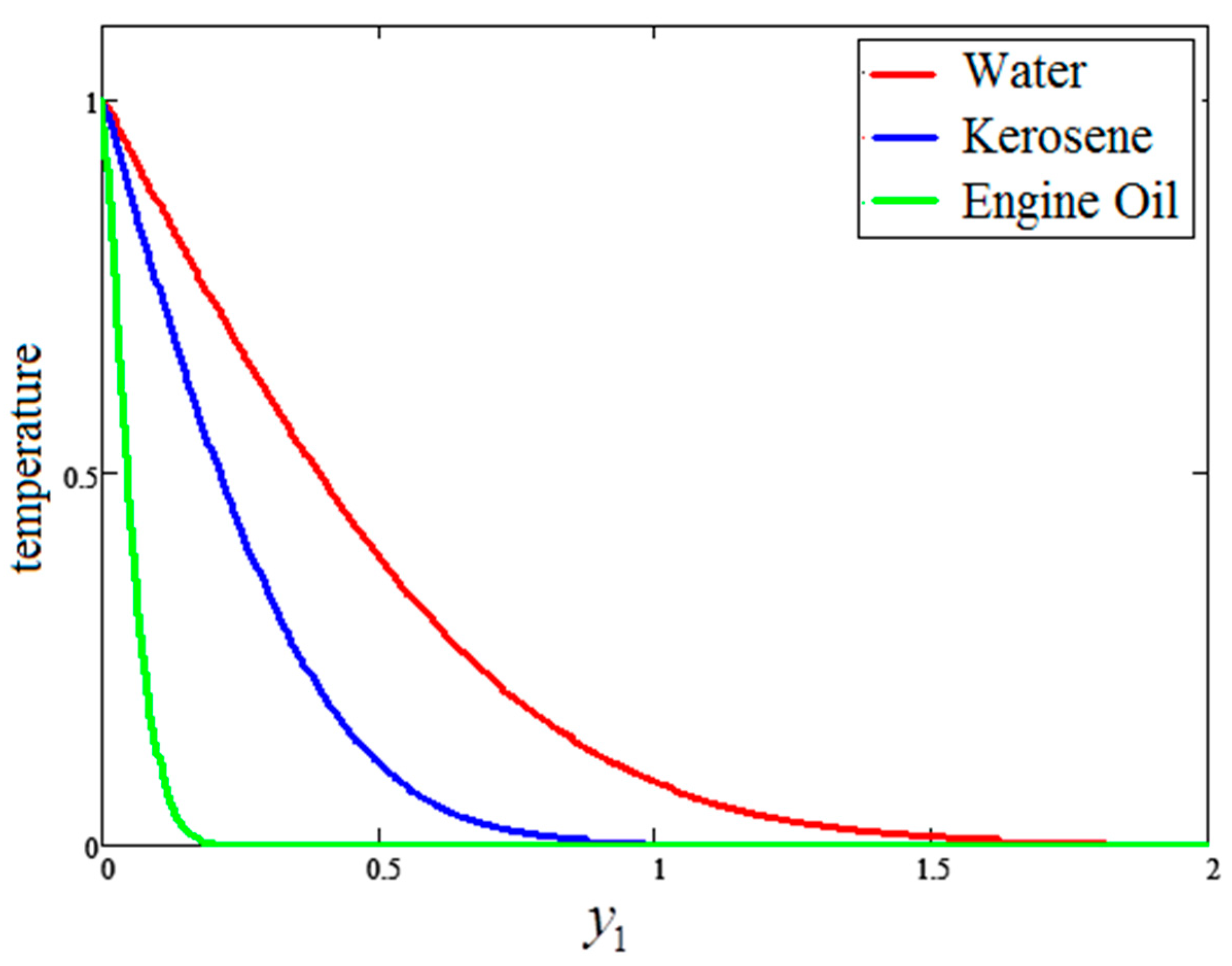
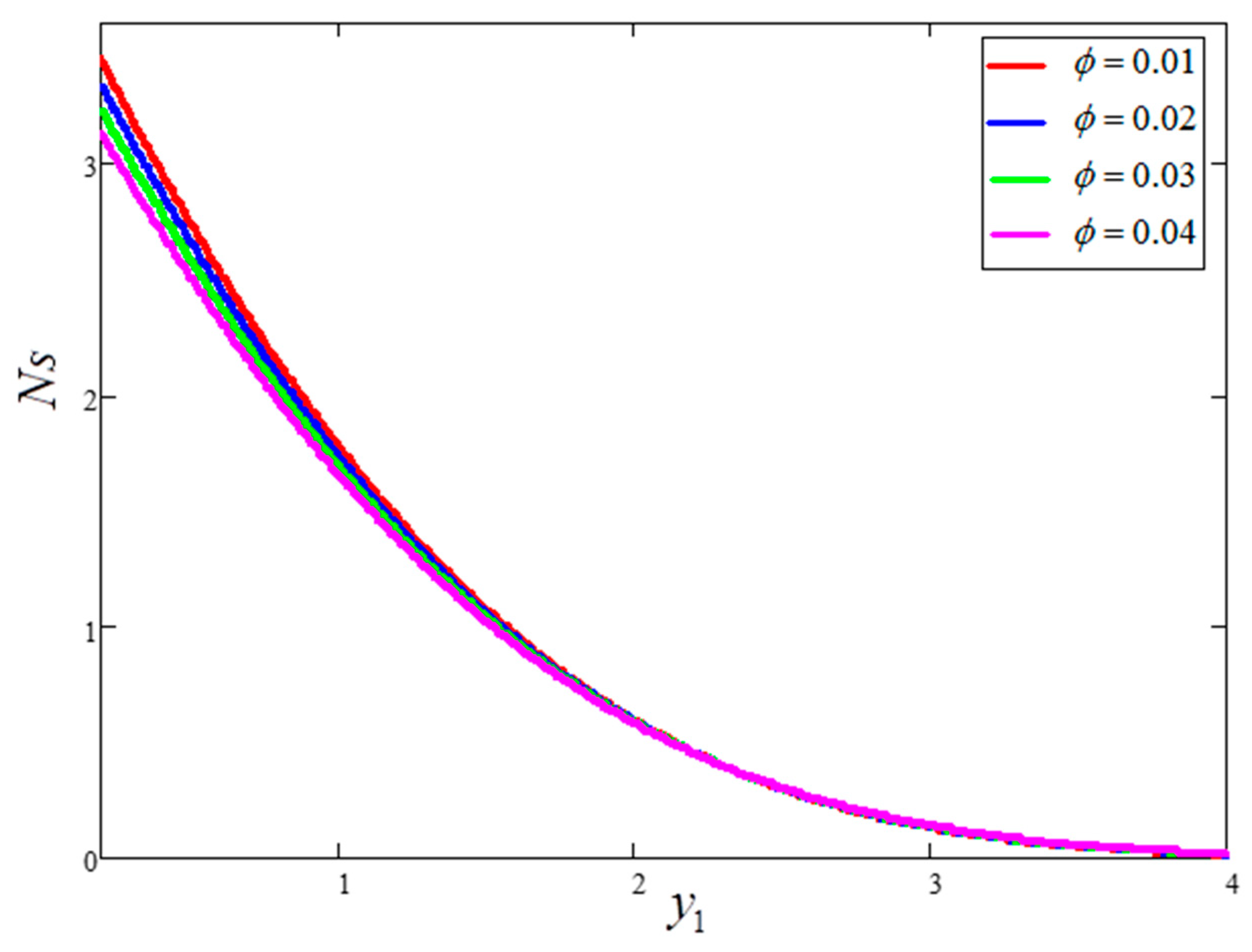
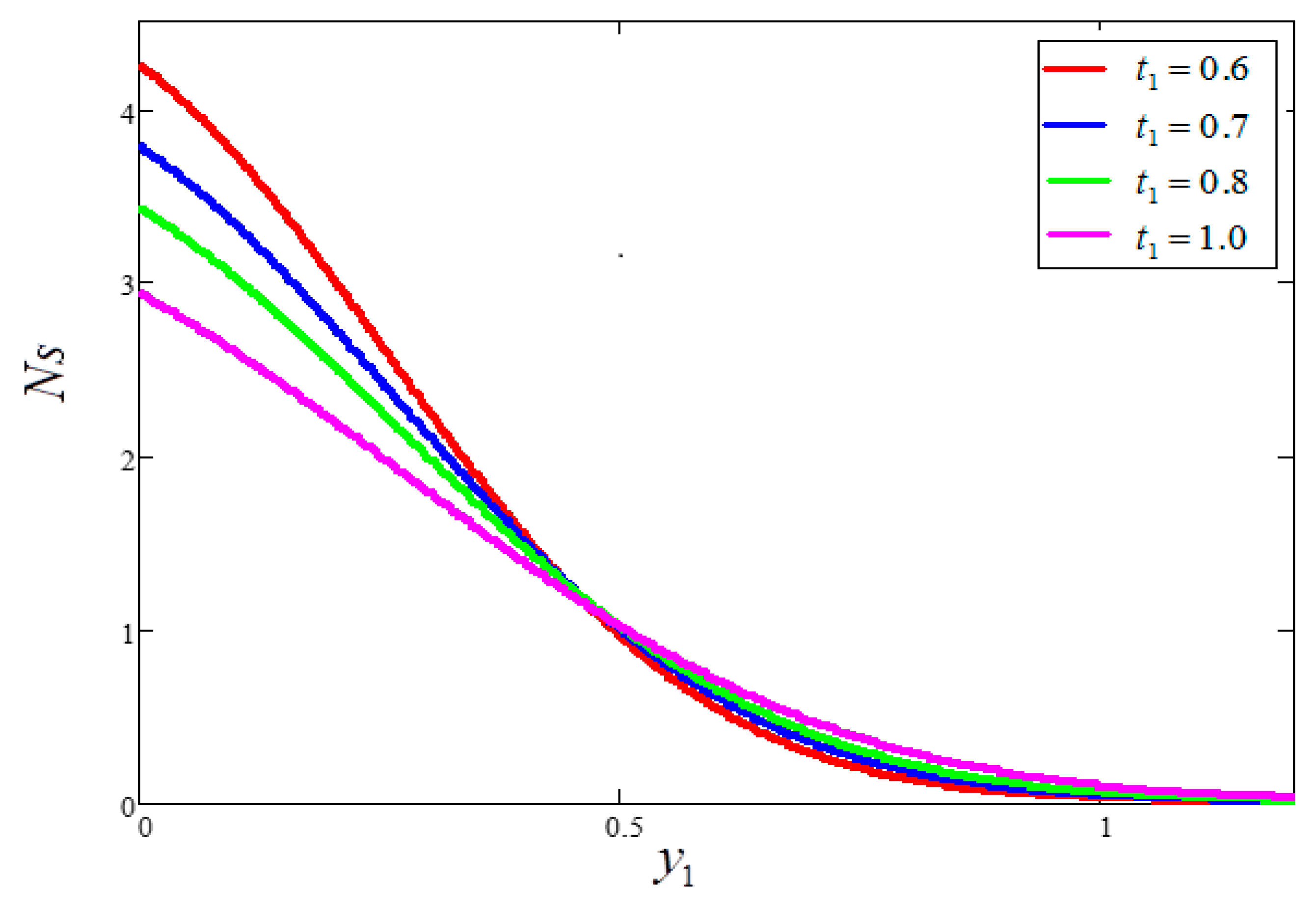
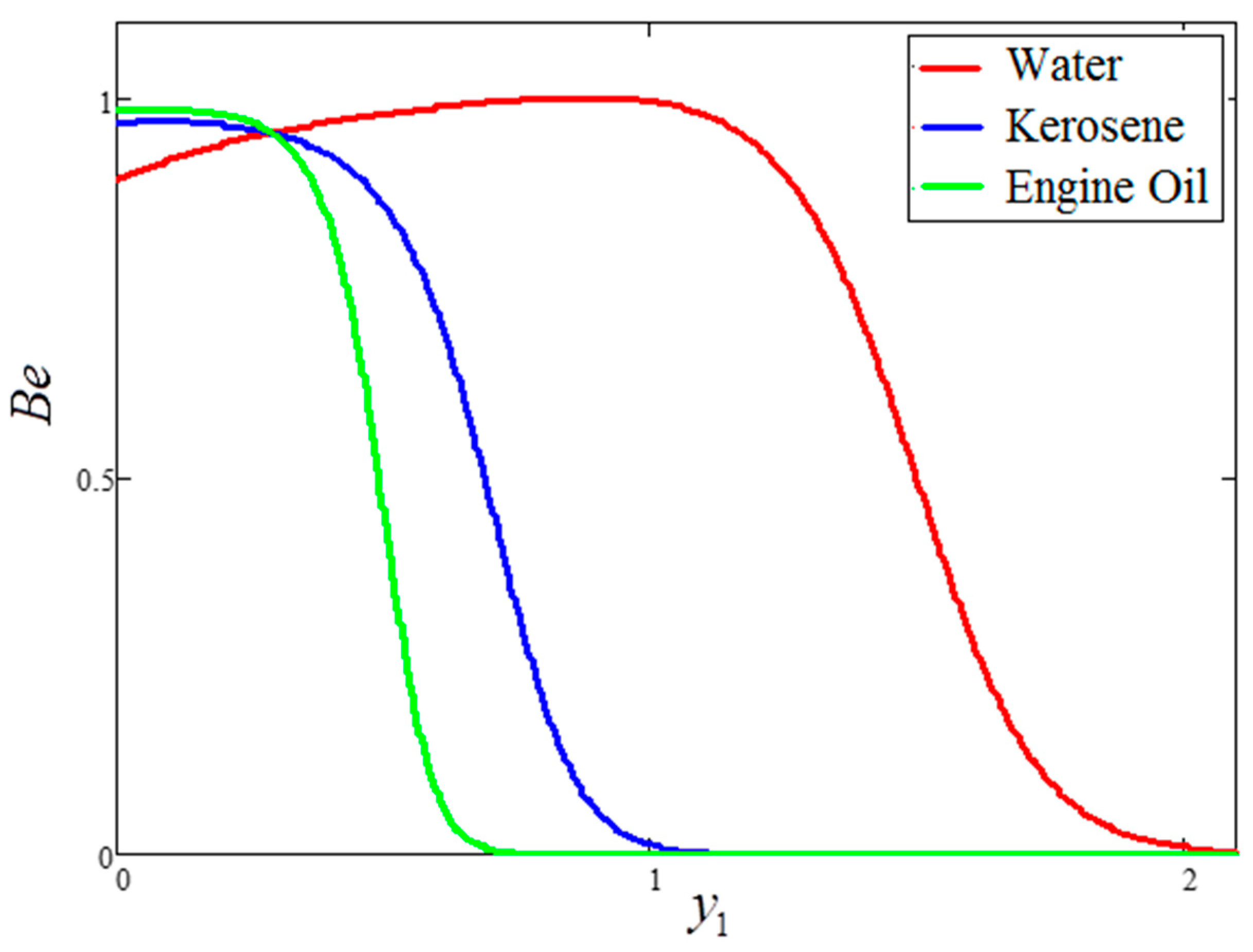
5. Graphical Results and Physical Interpretations
6. Conclusions
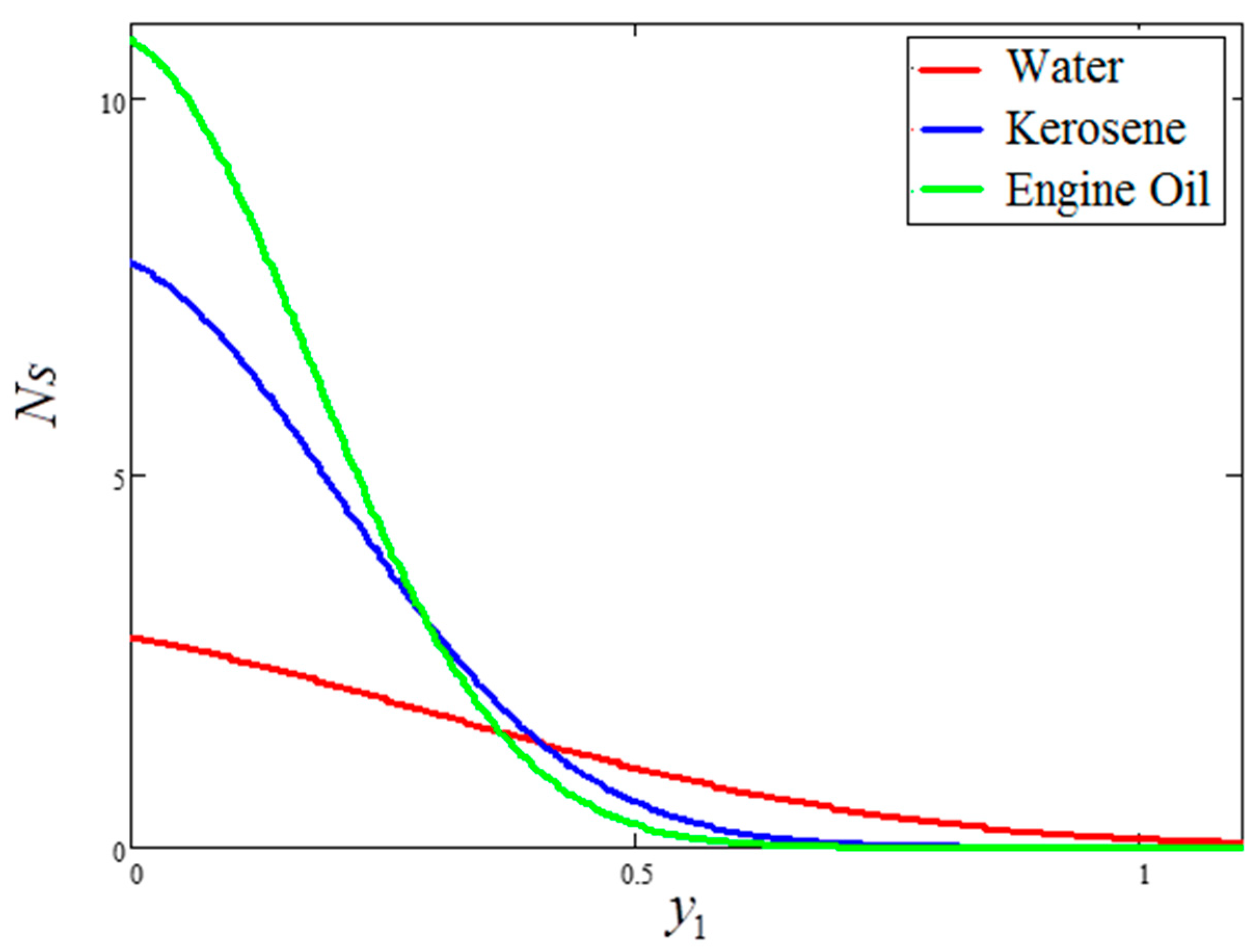
- For the water-based clay nanofluid, the velocity, temperature and Bejan number were higher compared to those obtained for the kerosene oil and engine oil-based nanofluids, but lower in the case of entropy generation.
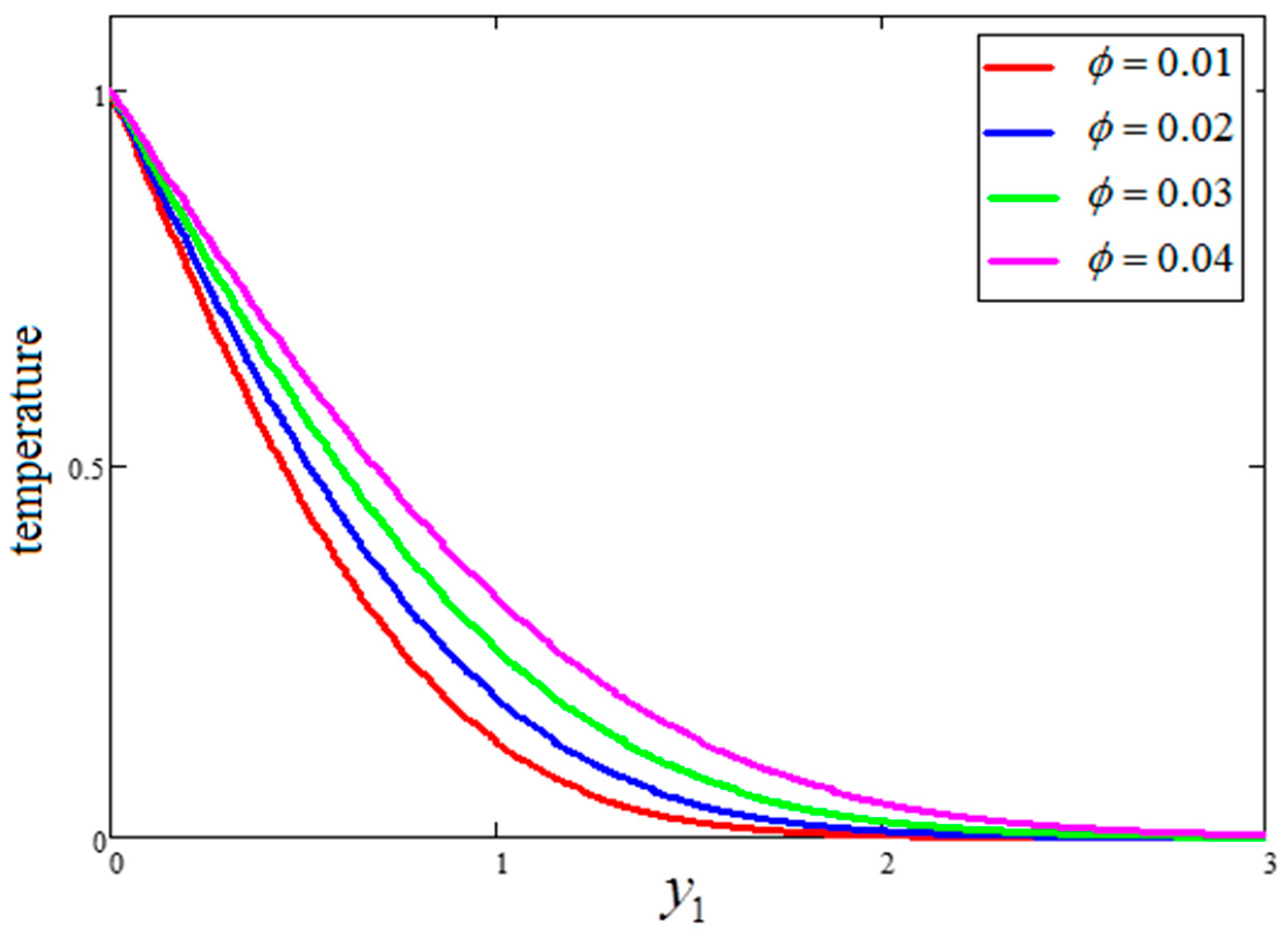
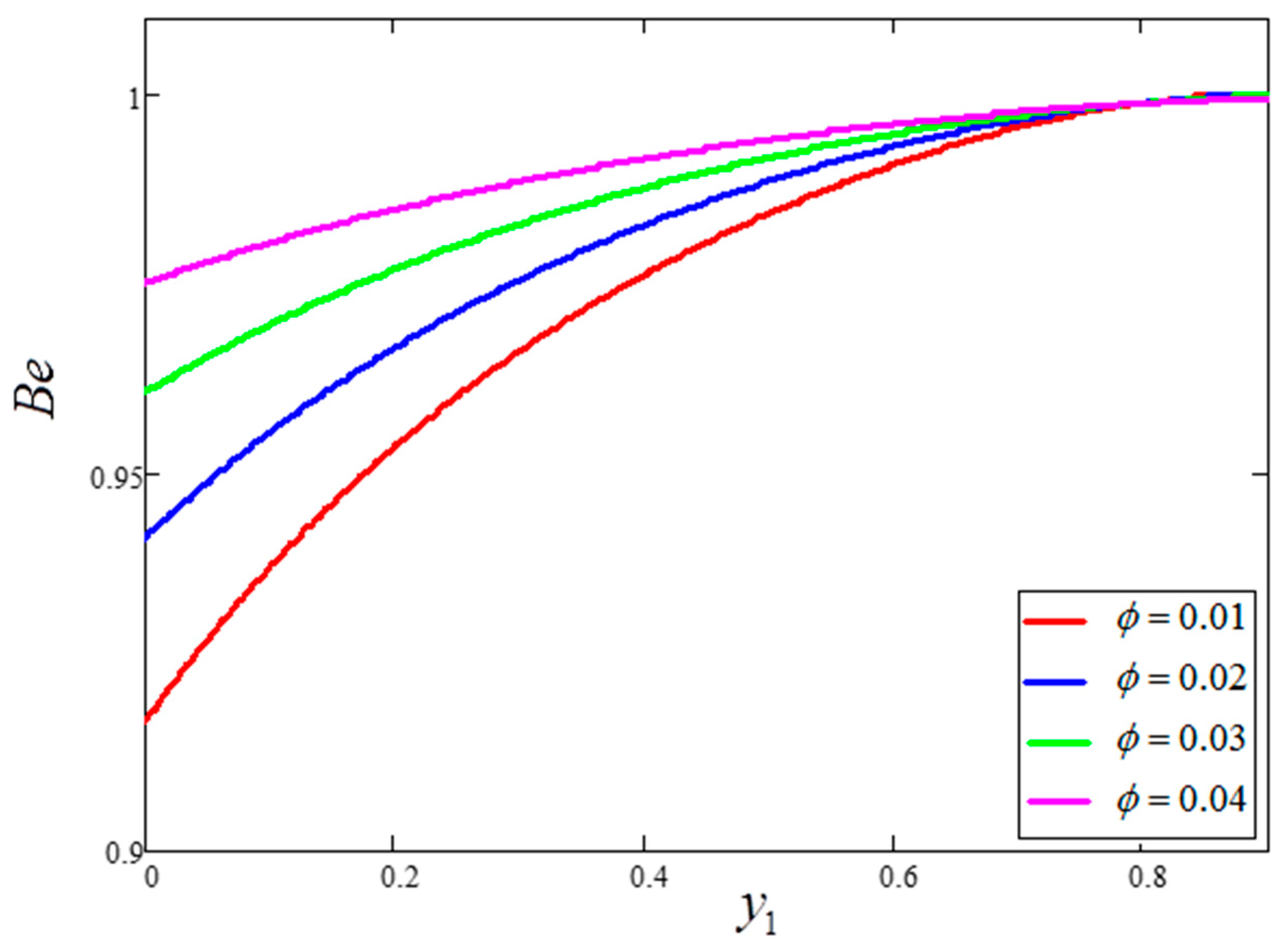
- The behavior of temperature and Bejan number decreased for greater clay nanoparticle volume fractions “”. However, velocity and entropy generation showed the opposite behavior.
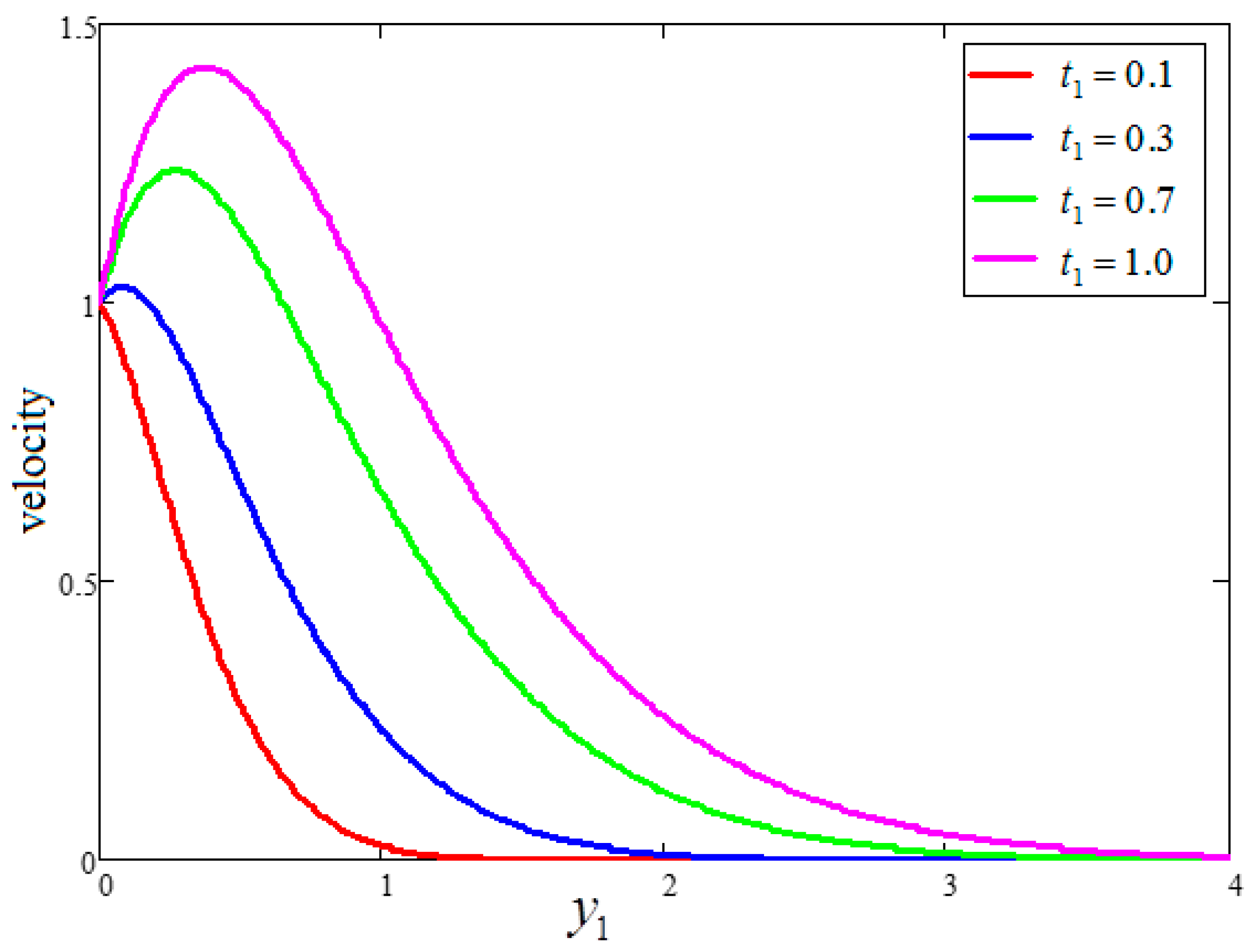
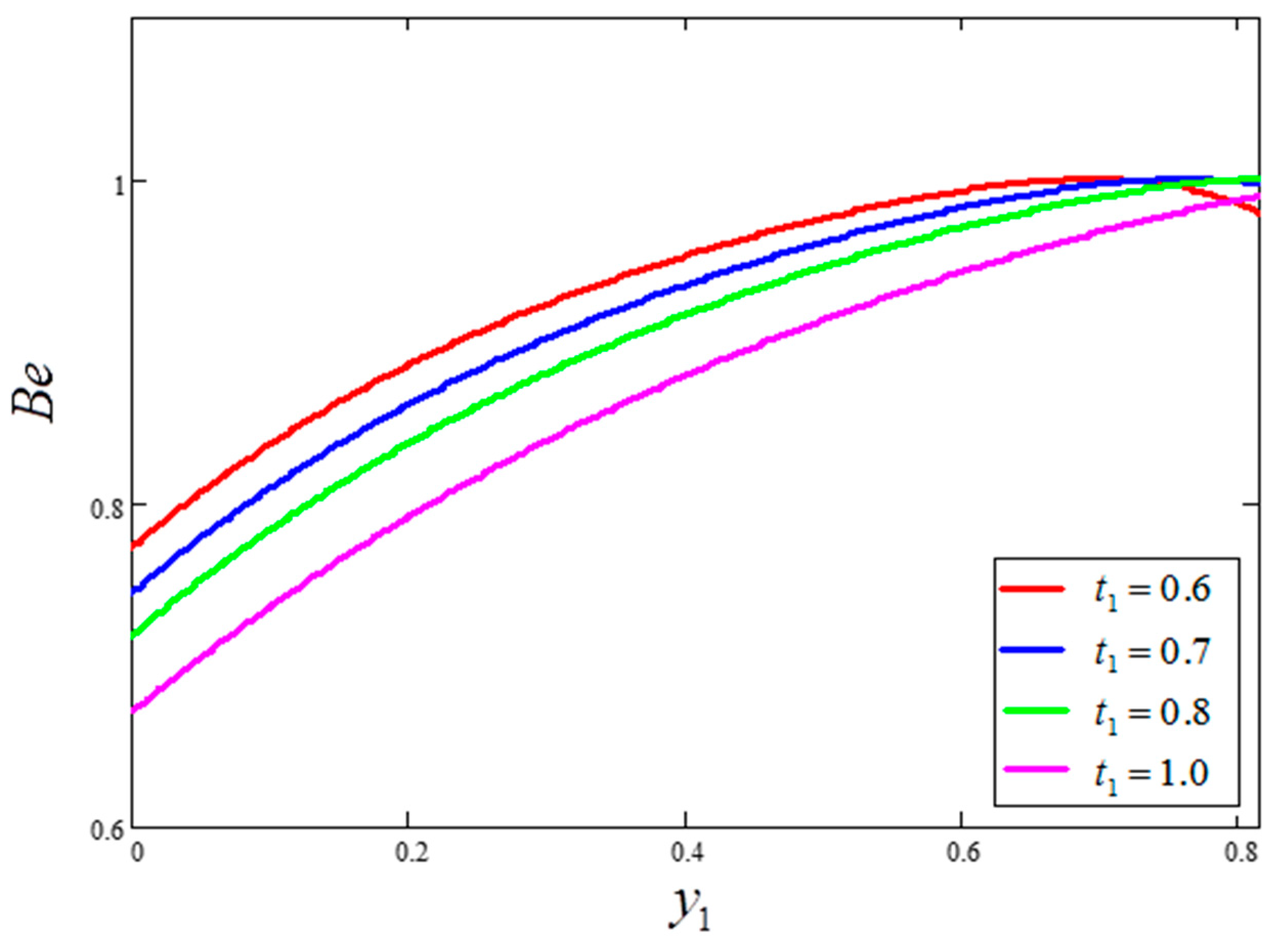
- An increase in time “” increased the velocity and temperature value, as well as reduced the Bejan number and entropy generation value.
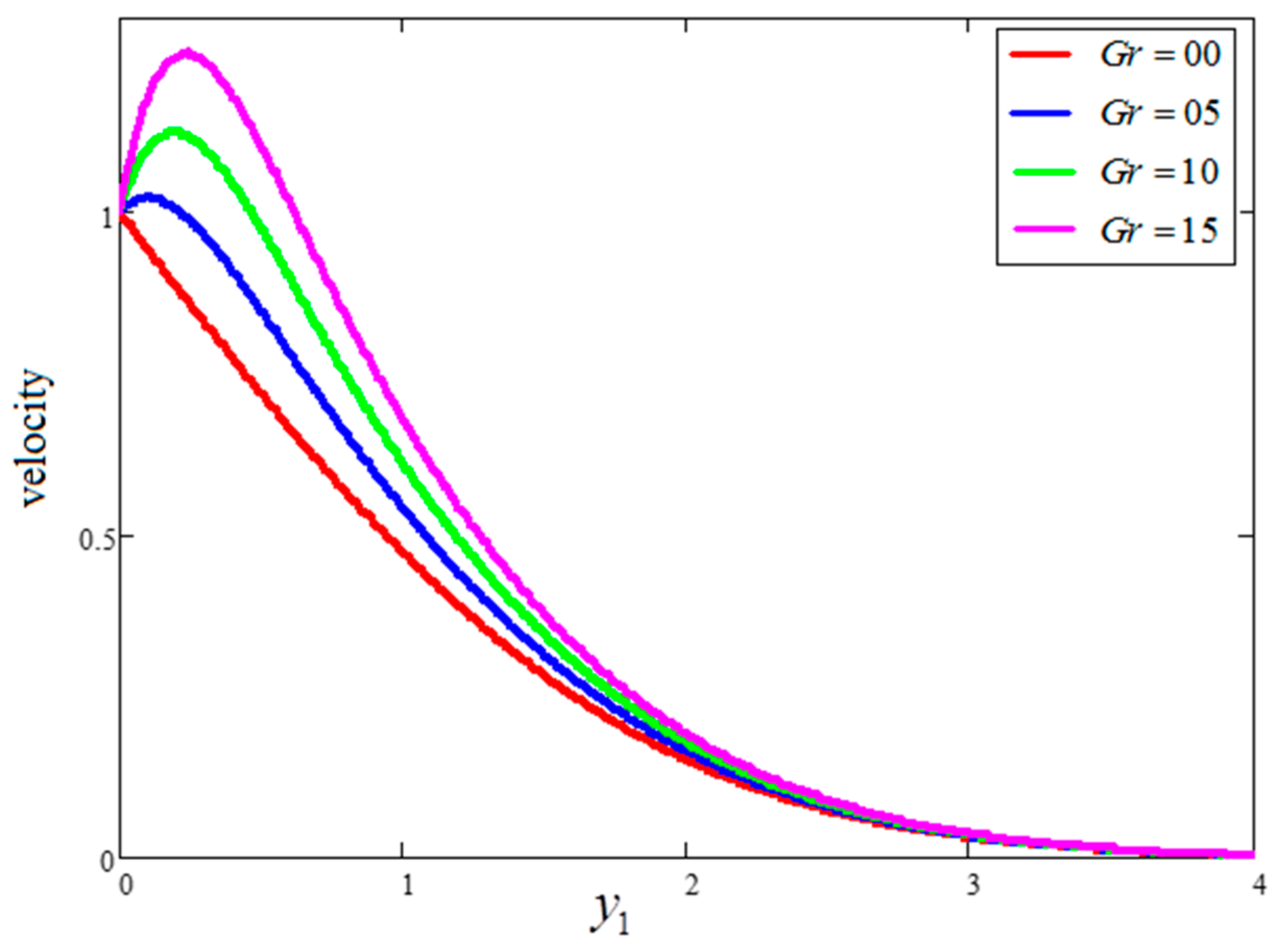
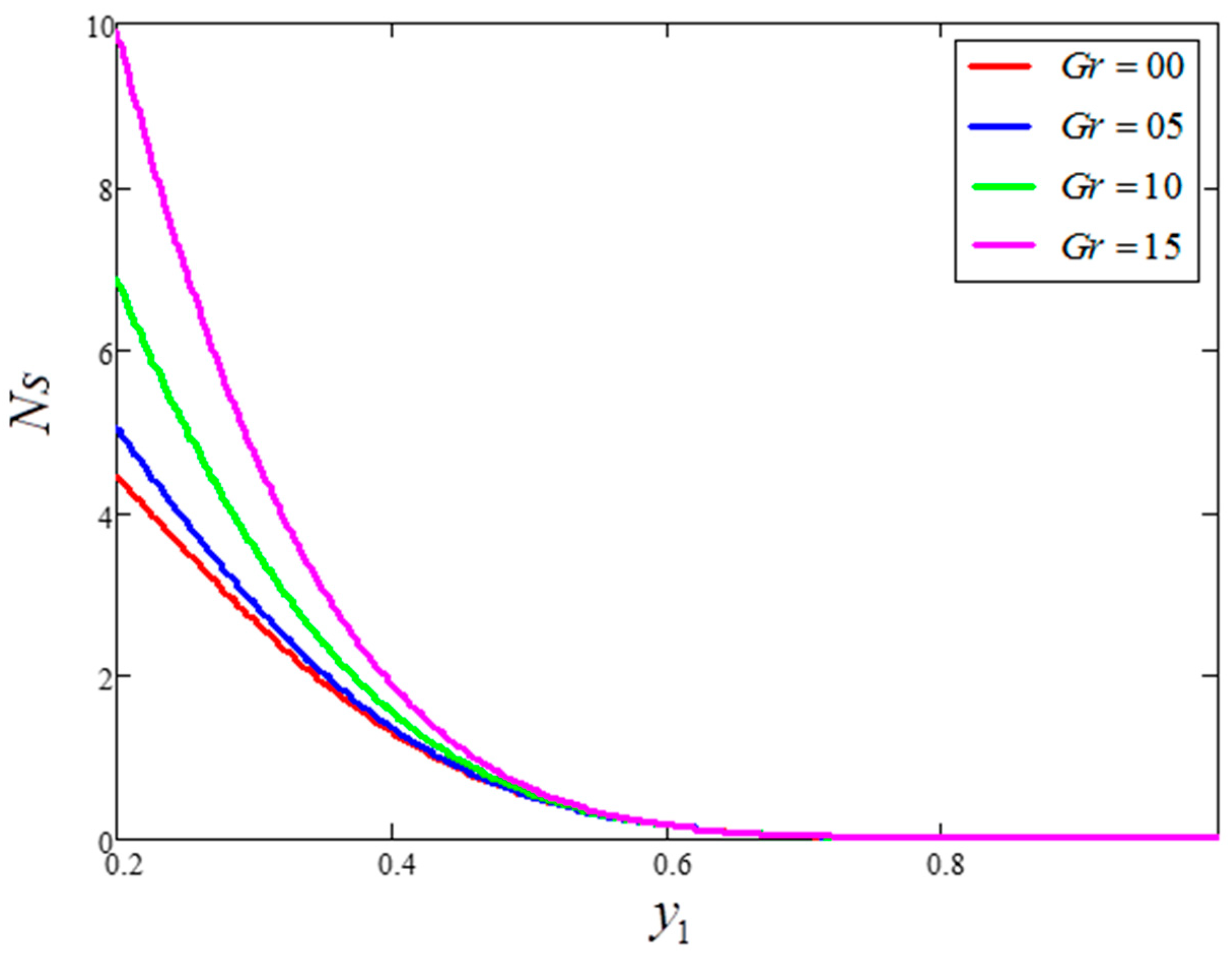
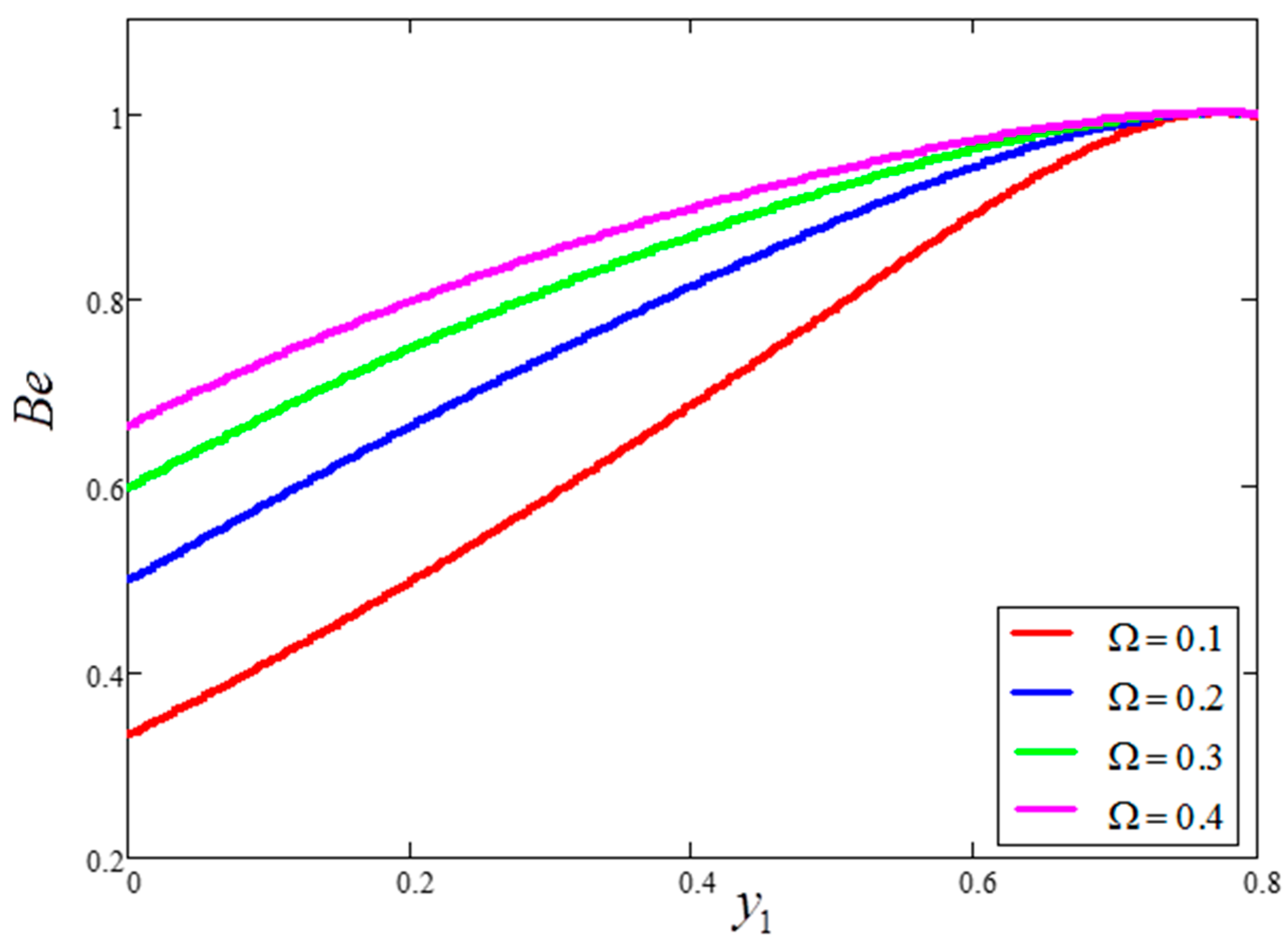
- is a major source of enhancement to the velocity and entropy generation value, although it decreases the Bejan number.
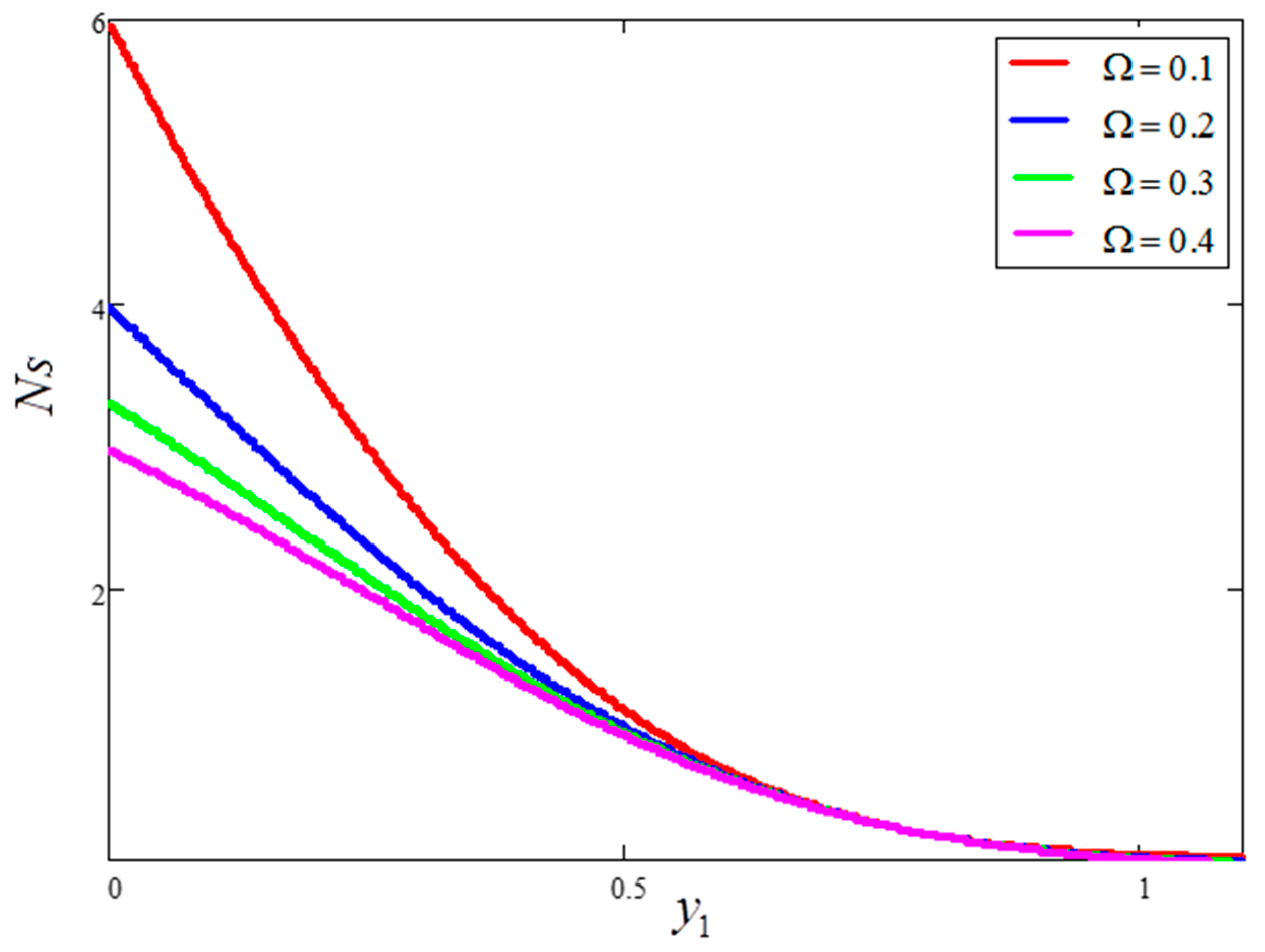
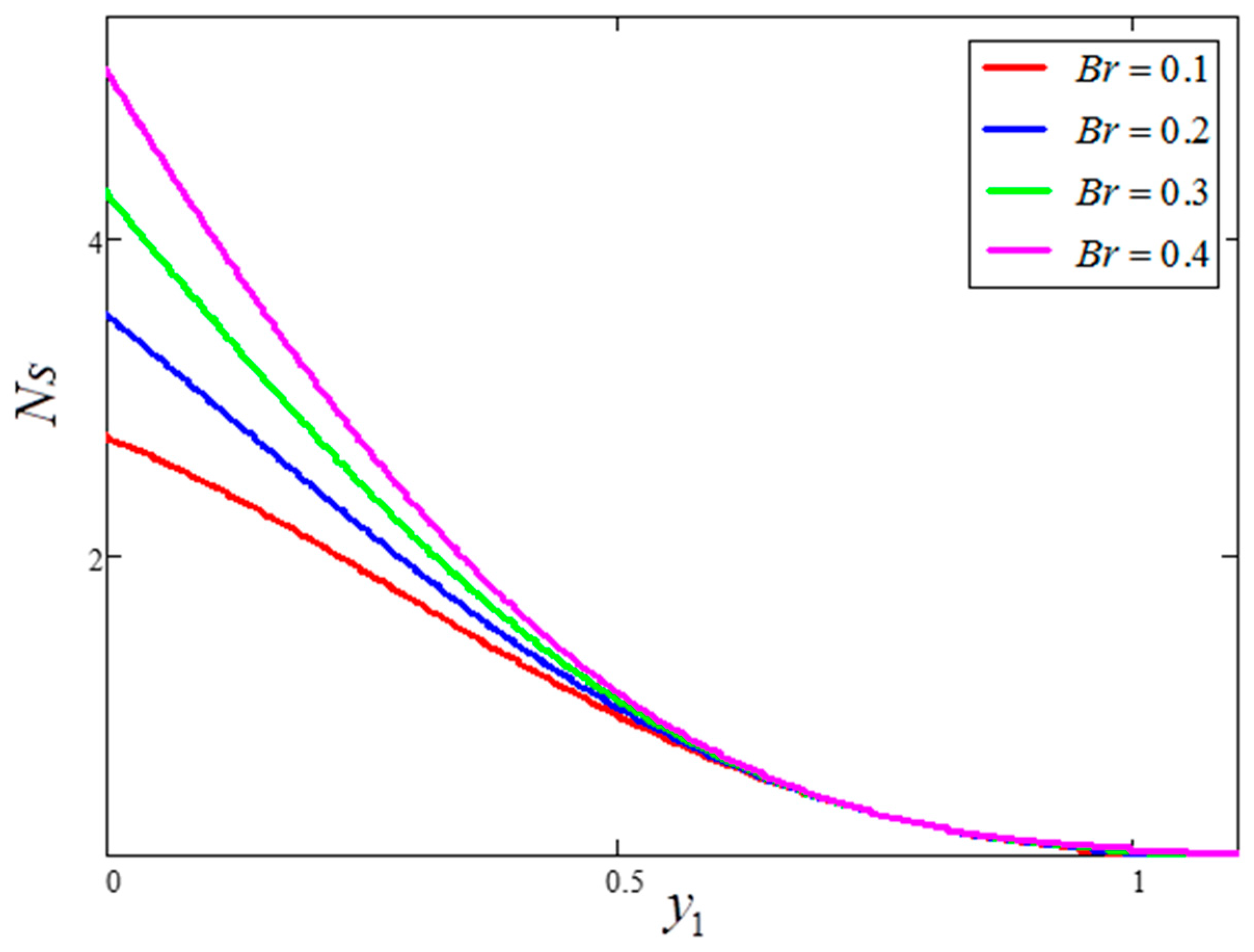
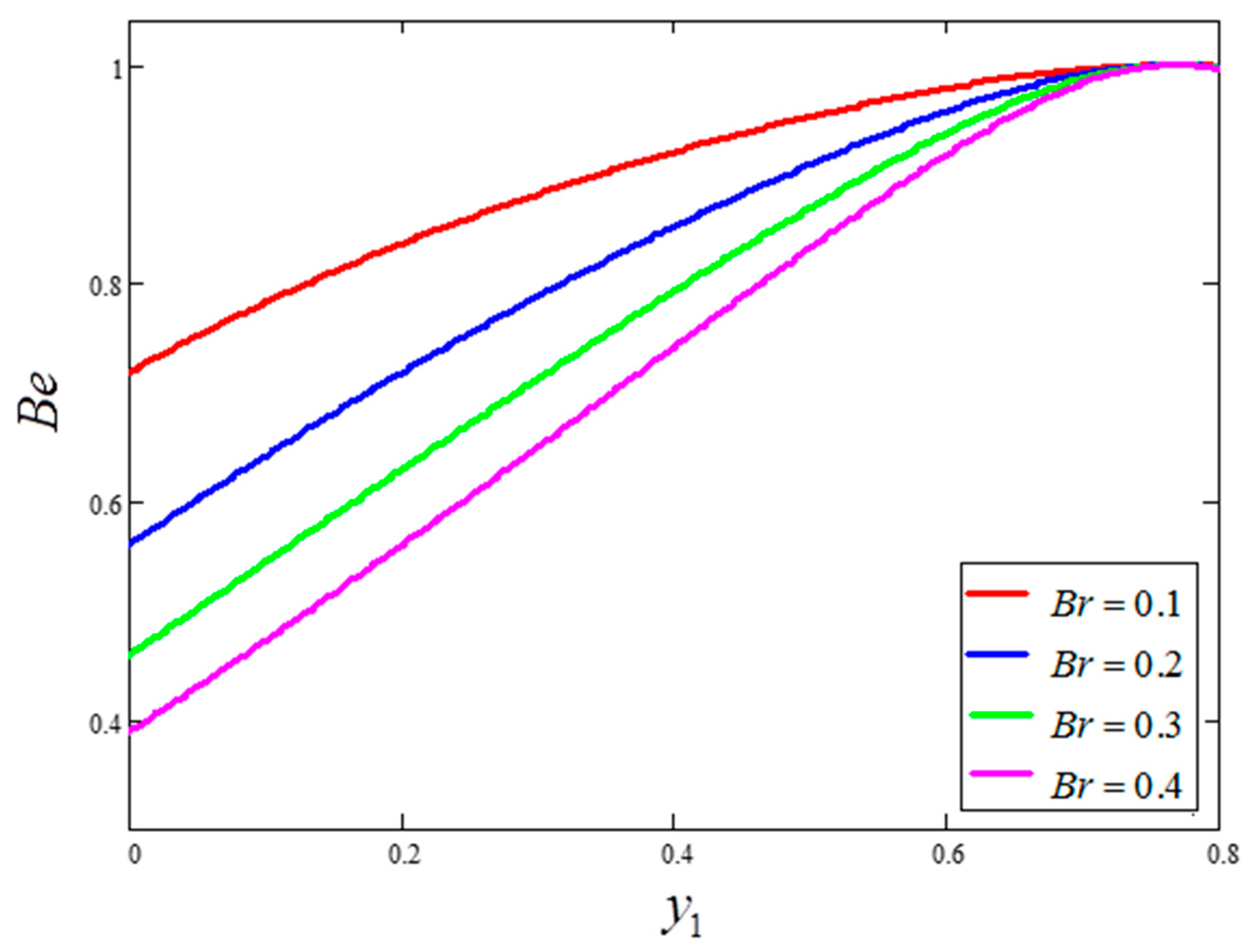
- Entropy generation is smaller for greater values of and larger for greater values of .
- Bejan number is smaller for greater values of and larger for greater values of .
Author Contributions
Funding
Conflicts of Interest
| Nomenclature | Greek symbols | ||||
| Nanofluid heat capacity at a constant pressure | Volumetric coefficient of thermal expansion | ||||
| Complementary error function. | Dimensionless temperature | ||||
| Thermal Grashof number | Temperature of the fluid | K | |||
| Acceleration due to gravity | Ambient temperature | K | |||
| Base fluid thermal conductivity | Wall temperature | K | |||
| Nanofluid thermal conductivity | Base fluid density | ||||
| Solid particle thermal conductivity | Nanofluid density | ||||
| Prandtl number | Solid particle density | ||||
| Time | Nanoparticle volume fraction | ||||
| Velocity of the fluid | Base fluid dynamic viscosity | ||||
| Nanofluid dynamic viscosity | |||||
References
- Bejan, A. Second-law analysis in heat transfer and thermal design. Adv. Heat Transf. 1982, 15, 1–58. [Google Scholar]
- Bejan, A. Entropy Generation Minimization; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Bejan, A. A study of entropy generation in fundamental convective heat transfer. J. Heat Transf. 1979, 101, 718–725. [Google Scholar] [CrossRef]
- Bejan, A. The thermodynamic design of heat and mass transfer processes and devices. Int J. Heat Fluid Flow. 1987, 8, 259–276. [Google Scholar] [CrossRef]
- Khan, A.; Khan, I.; Alkanhal, A.T.; Ali, F.; Khan, D.; Nisar, S.K. Entropy Generation in MHD Conjugate Flow with Wall Shear Stress over an Infinite Plate: Exact Analysis. Entropy 2019, 21, 359. [Google Scholar] [CrossRef]
- Awad, M.M. A new definition of Bejan number. Therm. Sci. 2012, 16, 1251–1253. [Google Scholar] [CrossRef]
- Awad, M.M.; Lage, J.L. Extending the Bejan number to a general form. Therm. Sci. 2013, 17, 631–633. [Google Scholar] [CrossRef]
- Saouli, S.; Aïboud-Saouli, S. Second law analysis of laminar falling liquid film along an inclined heated plate. Int. Comm. Heat Mass Transf. 2004, 3, 879–886. [Google Scholar] [CrossRef]
- Mahmud, S.; Tasnim, S.H.; Mamun, H.A.A. Thermodynamic analysis of mixed convection in a channel with transverse hydromagnetic effect. Int. J. Therm. Sci. 2003, 42, 731–740. [Google Scholar] [CrossRef]
- Selimefendigil, F.; Öztop, H.; Abu-Hamdeh, N. Natural convection and entropy generation in nanofluid filled entrapped trapezoidal cavities under the influence of magnetic field. Entropy 2016, 18, 43. [Google Scholar] [CrossRef]
- Sheremet, M.; Oztop, H.; Pop, I.; Abu-Hamdeh, N. Analysis of entropy generation in natural convection of nanofluid inside a square cavity having hot solid block: Tiwari and Das’ model. Entropy 2015, 18, 9. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, C.H.; Yang, X.; Shi, L. Entropy generation analysis and performance evaluation of turbulent forced convective heat transfer to nanofluids. Entropy 2017, 19, 108. [Google Scholar] [CrossRef]
- Qing, J.; Bhatti, M.; Abbas, M.; Rashidi, M.; Ali, M. Entropy generation on MHD Casson nanofluid flow over a porous stretching/shrinking surface. Entropy 2016, 18, 123. [Google Scholar] [CrossRef]
- Hayat, T.; Khan, I.M.; Qayyum, S.; Alsaedi, A. Entropy generation in flow with silver and copper nanoparticles. Colloid Surf. A Physicochem. Eng. Asp. 2018, 539, 335–346. [Google Scholar] [CrossRef]
- Farshad, A.S.; Sheikholeslami, M. Turbulent nanofluid flow through a solar collector influenced by multi-channel twisted tape considering entropy generation. Eur. Phys. J 2019, 134, 149. [Google Scholar] [CrossRef]
- Saqib, M.; Ali, F.; Khan, I.; Sheikh, A.N.; Khan, A. Entropy generation in different types of fractionalized nanofluids. Arab. J. Sci. Eng. 2019, 44, 531–540. [Google Scholar] [CrossRef]
- Sheikholeslami, M.; Jafaryar, M.; Shafee, A.; Li, Z.; Haq, U.R. Heat transfer of nanoparticles employing innovative turbulator considering entropy generation. Int. J. Heat Mass Transf. 2019, 136, 1233–1240. [Google Scholar] [CrossRef]
- Aziz, A. Entropy generation in pressure gradient assisted Couette flow with different thermal boundary conditions. Entropy 2006, 8, 50–62. [Google Scholar] [CrossRef]
- Aïboud-Saouli, S.; Saouli, S.; Settou, N.; Meza, N. Thermodynamic analysis of gravity-driven liquid film along an inclined heated plate with hydromagnetic and viscous dissipation effects. Entropy 2006, 8, 188–199. [Google Scholar] [CrossRef]
- Aiboud-Saouli, S.; Settou, N.; Saouli, S.; Meza, N. Second-law analysis of laminar fluid flow in a heated channel with hydro-magnetic and viscous dissipation effects. Appl. Energy 2007, 84, 279–289. [Google Scholar] [CrossRef]
- Aïboud-Saouli, S.; Saouli, S. Entropy analysis for viscoelastic magnetohydrodynamic flow over a stretching surface. Int. J. Non-Linear Mech. 2010, 45, 482–489. [Google Scholar] [CrossRef]
- Ahmed, N.T.; Khan, I. Mixed convection flow of sodium alginate (SA-NaAlg) based molybdenum disulphide (MoS2) nanofluids: Maxwell Garnetts and Brinkman models. Results Phys. 2018, 8, 752–757. [Google Scholar] [CrossRef]
- Ali, F.; Khan, I.; Sheikh, A.N.; Gohar, M.; Tlili, I. Effects of Different Shaped Nanoparticles on the Performance of Engine-Oil and Kerosene-Oil: A generalized Brinkman-Type Fluid model with Non-Singular Kernel. Sci. Rep. 2018, 8, 15285. [Google Scholar] [CrossRef]
- Hussanan, A.; Salleh, Z.M.; Khan, I.; Shafie, S. Convection heat transfer in micropolar nanofluids with oxide nanoparticles in water, kerosene and engine oil. J. Mol. Liq. 2017, 229, 482–488. [Google Scholar] [CrossRef]
- Rashidi, S.; Mahian, O.; Languri, M.E. Applications of nanofluids in condensing and evaporating systems. J. Therm. Anal. Calorim. 2018, 131, 2027–2039. [Google Scholar] [CrossRef]
- Elsheikh, H.A.; Sharshir, W.S.; Mostafa, E.M.; Essa, A.F.; Ali, A.M.K. Applications of nanofluids in solar energy: a review of recent advances. Renew. Sustain. Energy Rev. 2018, 82, 3483–3502. [Google Scholar] [CrossRef]
- Khan, I.; Hussanan, A.; Saqib, M.; Shafie, S. Convective Heat Transfer in Drilling Nanofluid with Clay Nanoparticles: Applications in Water Cleaning Process. BioNanoScience 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Brinkman, H.C. The viscosity of concentrated suspensions and solution. J. Chem. Phys. 1952, 20, 571–581. [Google Scholar] [CrossRef]
- Khan, A.; Khan, D.; Khan, I.; Ali, F.; Karim, U.F.; Imran, M. MHD flow of Sodium Alginate-based Casson type nanofluid passing through a porous medium with Newtonian heating. Sci. Rep. 2018, 8, 8645. [Google Scholar] [CrossRef]
- Matin, H.M.; Pop, I. Forced convection heat and mass transfer flow of a nanofluid through a porous channel with a first order chemical reaction on the wall. Int. Commun. Heat Mass Transf. 2013, 46, 134–141. [Google Scholar] [CrossRef]
- Qasim, M.; Khan, H.Z.; Khan, I.; Al-Mdallal, Q. Analysis of entropy generation in flow of methanol-based nanofluid in a sinusoidal wavy channel. Entropy 2017, 19, 490. [Google Scholar] [CrossRef]
- Khan, A.; Karim, U.F.; Khan, I.; Ali, F.; Khan, D. Irreversibility analysis in unsteady flow over a vertical plate with arbitrary wall shear stress and ramped wall temperature. Results Phys. 2018, 8, 1283–1290. [Google Scholar] [CrossRef]
- Khan, A.; Khan, I.; Ali, F.; Shafie, S. A note on entropy generation in MHD flow over a vertical plate embedded in a porous medium with arbitrary shear stress and ramped temperature. J. Porous Media 2016, 19, 175–187. [Google Scholar] [CrossRef]



| Material | Base Fluids | Nanoparticles | ||
|---|---|---|---|---|
| Engine Oil | Kerosene Oil | Water | Clay | |
| 884 | 783 | 997 | 6320 | |
| 1910 | 2090 | 4179 | 531.8 | |
| 0.114 | 0.145 | 0.613 | 76.5 | |
| 70 | 99 | 21 | 1.80 | |
| 500 | 21 | 6.2 | - | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sooppy Nisar, K.; Khan, D.; Khan, A.; Khan, W.A.; Khan, I.; Aldawsari, A.M. Entropy Generation and Heat Transfer in Drilling Nanoliquids with Clay Nanoparticles. Entropy 2019, 21, 1226. https://doi.org/10.3390/e21121226
Sooppy Nisar K, Khan D, Khan A, Khan WA, Khan I, Aldawsari AM. Entropy Generation and Heat Transfer in Drilling Nanoliquids with Clay Nanoparticles. Entropy. 2019; 21(12):1226. https://doi.org/10.3390/e21121226
Chicago/Turabian StyleSooppy Nisar, Kottakkaran, Dolat Khan, Arshad Khan, Waqar A Khan, Ilyas Khan, and Abdullah Mohammed Aldawsari. 2019. "Entropy Generation and Heat Transfer in Drilling Nanoliquids with Clay Nanoparticles" Entropy 21, no. 12: 1226. https://doi.org/10.3390/e21121226
APA StyleSooppy Nisar, K., Khan, D., Khan, A., Khan, W. A., Khan, I., & Aldawsari, A. M. (2019). Entropy Generation and Heat Transfer in Drilling Nanoliquids with Clay Nanoparticles. Entropy, 21(12), 1226. https://doi.org/10.3390/e21121226






