Identification of Functional Bioprocess Model for Recombinant E. Coli Cultivation Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Strains
2.2. Medium and Culture Conditions
3. Basis of Biomass and Product Model Fitting
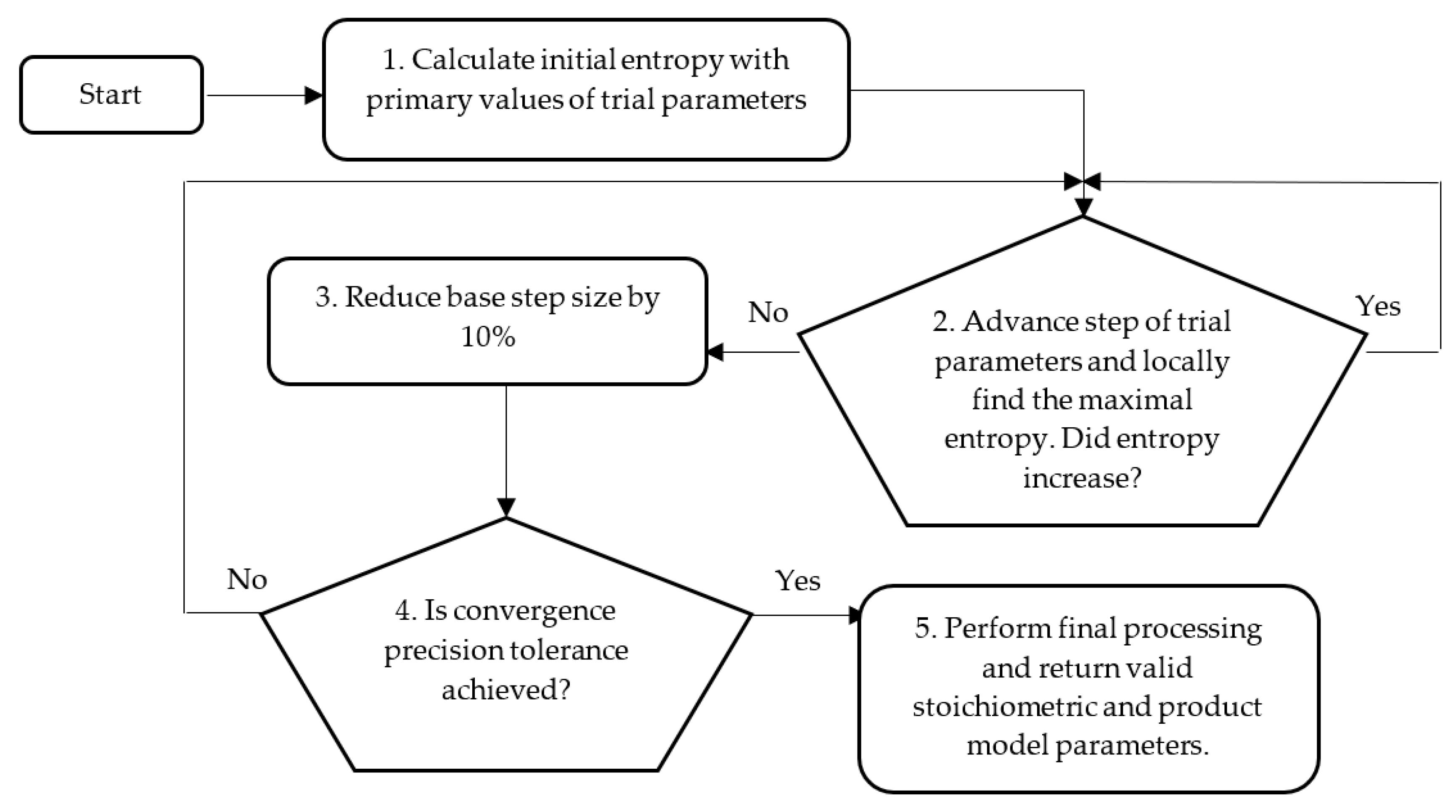
4. System Identification and Parameter Estimation
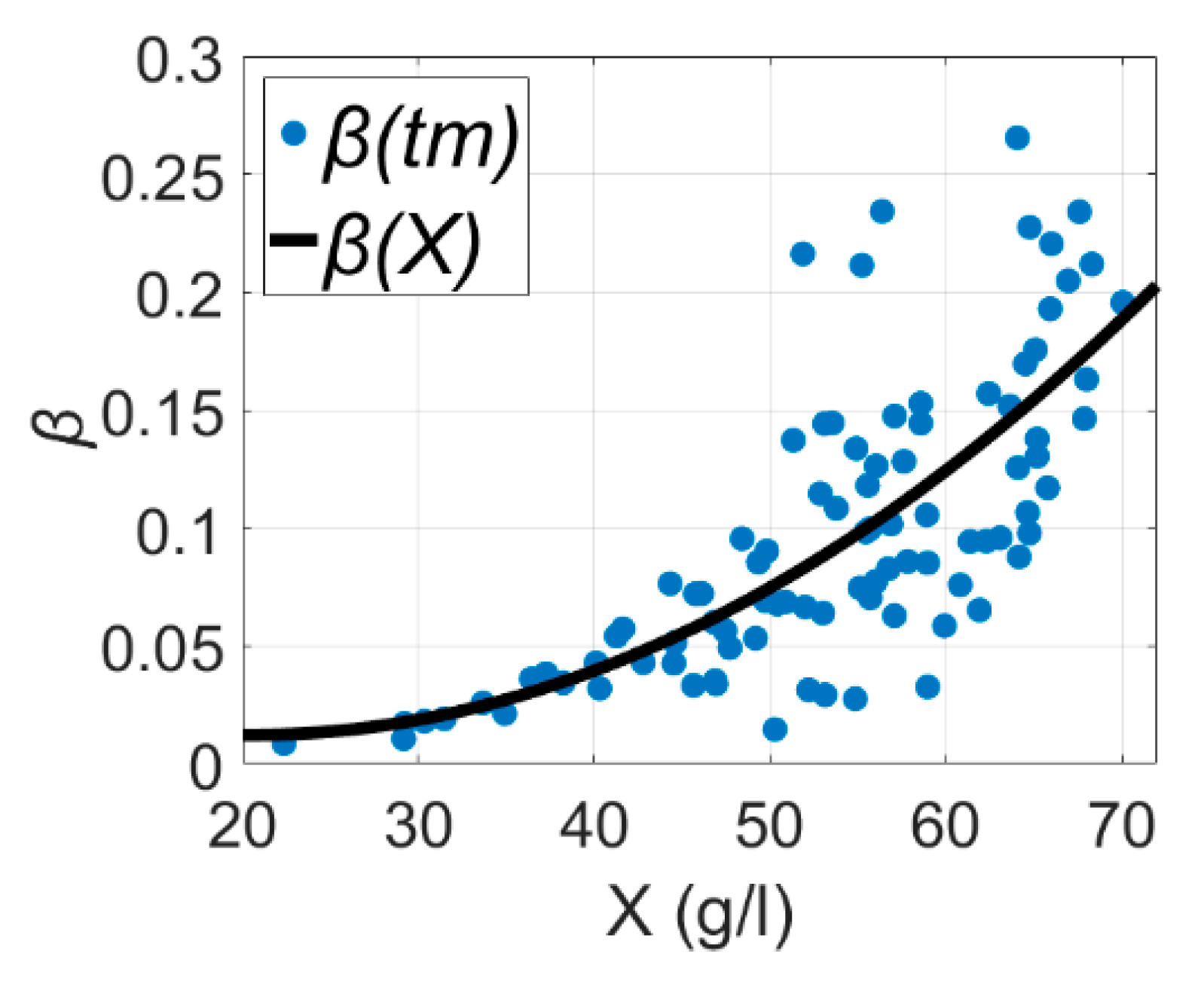
4.1. Stoichiometric Parameter Estimation
4.2. Procedure for Offline Analysis of Stoichiometry Parameters
4.3. Model of Product Model Fitting
4.4. Identification of E. Coli Parameters by Convex Optimization
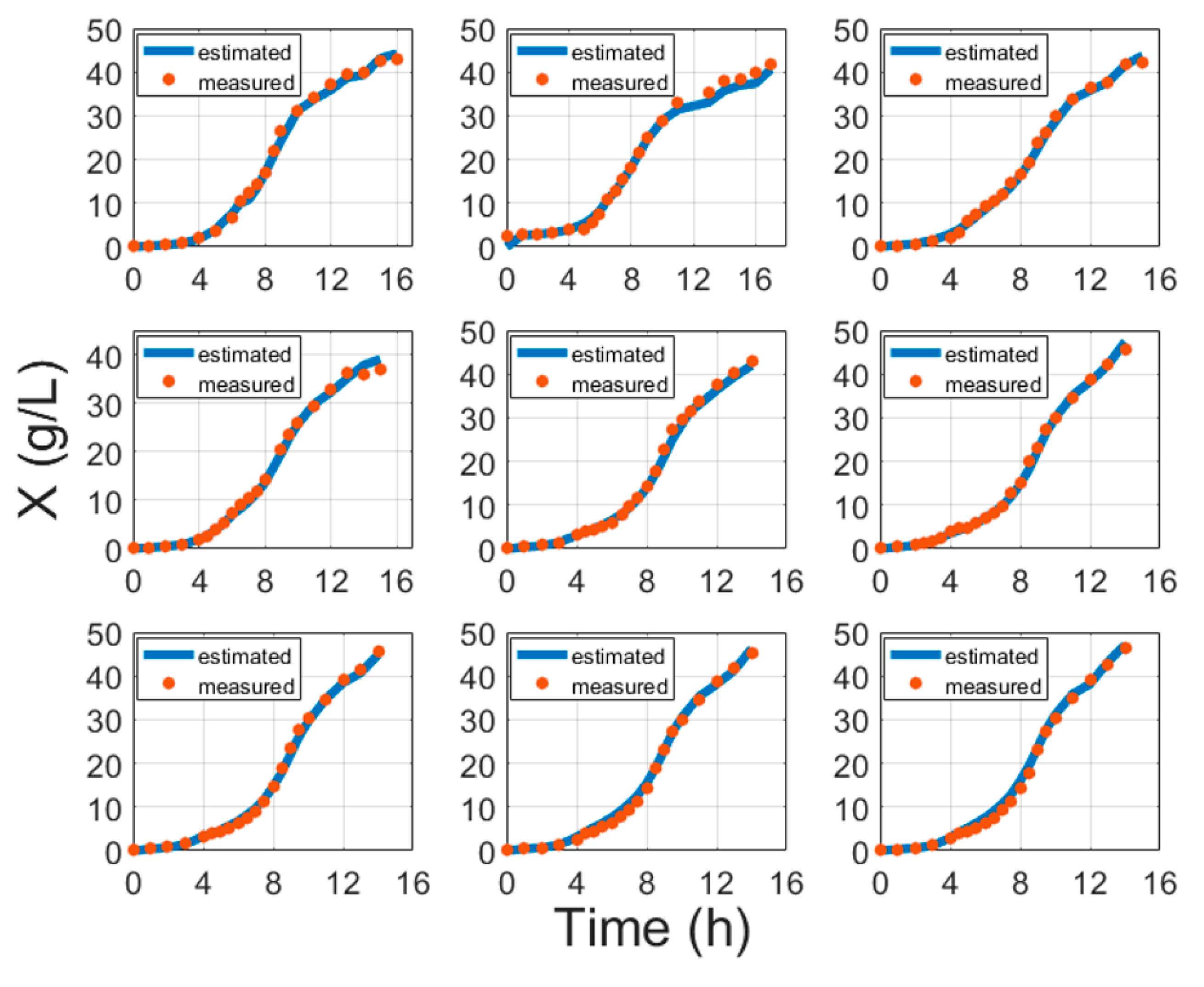
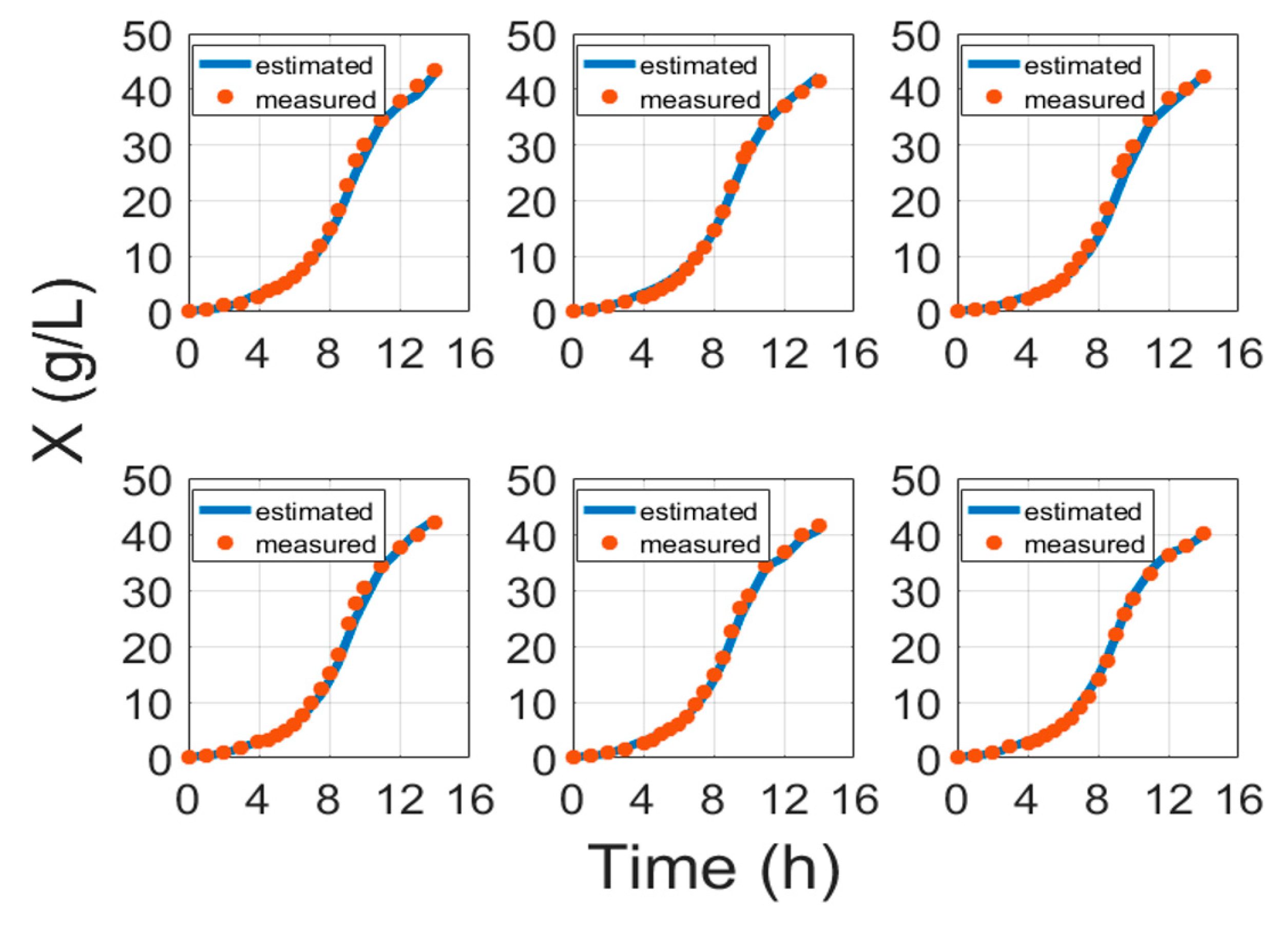
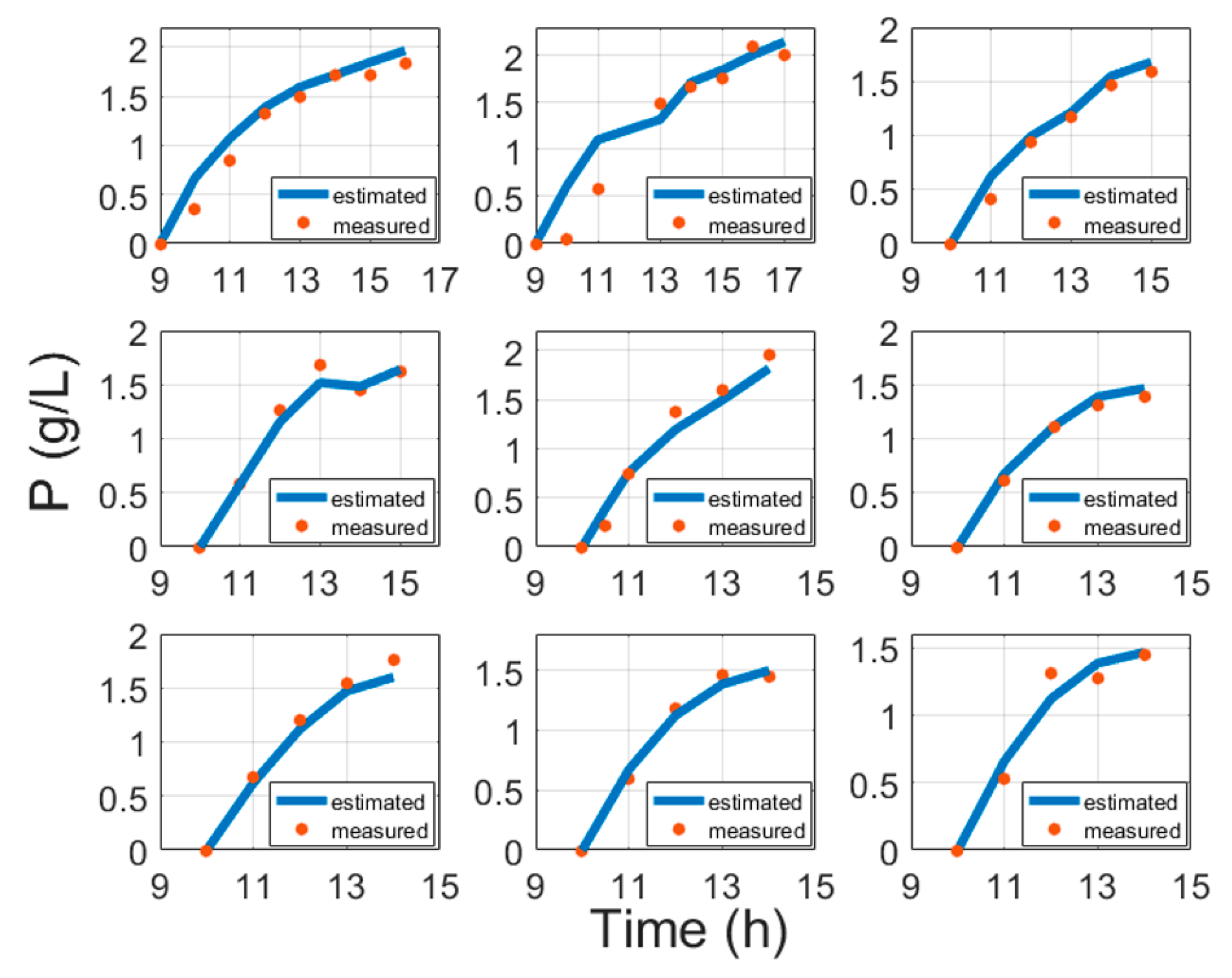
5. Experimental Validation
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goodwin, G.C. Predicting the performance of soft sensors as a route to low cost automation. Annu. Rev. Control 2000, 24, 55–66. [Google Scholar] [CrossRef]
- Mansano, R.; Godoy, E.; Porto, A. The Benefits of Soft Sensor and Multi-Rate Control for the Implementation of Wireless Networked Control Systems. Sensors 2014, 14, 24441–24461. [Google Scholar] [CrossRef]
- Urniezius, R.; Survyla, A.; Paulauskas, D.; Bumelis, V.A.; Galvanauskas, V. Generic estimator of biomass concentration for Escherichia coli and Saccharomyces cerevisiae fed-batch cultures based on cumulative oxygen consumption rate. Microb. Cell Fact. 2019, 18, 190. [Google Scholar] [CrossRef]
- Garcia-Ochoa, F.; Gomez, E.; Santos, V.E.; Merchuk, J.C. Oxygen uptake rate in microbial processes: An overview. Biochem. Eng. J. 2010, 49, 289–307. [Google Scholar] [CrossRef]
- Luedeking, R.; Piret, E.L. A kinetic study of the lactic acid fermentation. Batch process at controlled pH. Biotechnol. Bioeng. 1959, 1, 393–412. [Google Scholar] [CrossRef]
- Gnoth, S.; Simutis, R.; Lübbert, A. Selective expression of the soluble product fraction in Escherichia coli cultures employed in recombinant protein production processes. Appl. Microbiol. Biotechnol. 2010, 87, 2047–2058. [Google Scholar] [CrossRef] [PubMed]
- Jenzsch, M.; Simutis, R.; Luebbert, A. Generic model control of the specific growth rate in recombinant Escherichia coli cultivations. J. Biotechnol. 2006, 122, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Schaepe, S.; Kuprijanov, A.; Simutis, R.; Lübbert, A. Avoiding overfeeding in high cell density fed-batch cultures of E. coli during the production of heterologous proteins. J. Biotechnol. 2014, 192, 146–153. [Google Scholar] [CrossRef]
- Kuprijanov, A.; Gnoth, S.; Simutis, R.; Lübbert, A. Advanced control of dissolved oxygen concentration in fed batch cultures during recombinant protein production. Appl. Microbiol. Biotechnol. 2009, 82, 221–229. [Google Scholar] [CrossRef]
- Gnoth, S.; Kuprijanov, A.; Simutis, R.; Lübbert, A. Simple adaptive pH control in bioreactors using gain-scheduling methods. Appl. Microbiol. Biotechnol. 2010, 85, 955–964. [Google Scholar] [CrossRef]
- Linko, P.; Zhu, Y. Neural network programming in bioprocess variable estimation and state prediction. J. Biotechnol. 1991, 21, 253–269. [Google Scholar] [CrossRef]
- Simutis, R.; Lübbert, A. Bioreactor control improves bioprocess performance. Biotechnol. J. 2015, 10, 1115–1130. [Google Scholar] [CrossRef] [PubMed]
- Schaepe, S.; Kuprijanov, A.; Sieblist, C.; Jenzsch, M.; Simutis, R.; Lübbert, A. Current Advances in Tools Improving Bioreactor Performance. Curr. Biotechnol. 2013, 3, 133–144. [Google Scholar] [CrossRef]
- Lübbert, A.; Bay Jørgensen, S. Bioreactor performance: A more scientific approach for practice. J. Biotechnol. 2001, 85, 187–212. [Google Scholar] [CrossRef]
- Simutis, R.; Galvanauskas, V.; Levisauskas, D.; Repsyte, J.; Vaitkus, V. Comparative Study of Intelligent Soft-Sensors for Bioprocess State Estimation. JOLST 2013, 163–167. [Google Scholar] [CrossRef]
- Wechselberger, P.; Sagmeister, P.; Herwig, C. Real-time estimation of biomass and specific growth rate in physiologically variable recombinant fed-batch processes. Bioprocess Biosyst. Eng. 2013, 36, 1205–1218. [Google Scholar] [CrossRef] [PubMed]
- Gnoth, S.; Jenzsch, M.; Simutis, R.; Lübbert, A. Process Analytical Technology (PAT): Batch-to-batch reproducibility of fermentation processes by robust process operational design and control. J. Biotechnol. 2007, 132, 180–186. [Google Scholar] [CrossRef]
- Caramihai, M.; Severi, I. Bioprocess Modeling and Control. In Biomass Now—Sustainable Growth and Use; Matovic, M.D., Ed.; InTech: Vienna, Austria, 2013; ISBN 978-953-51-1105-4. [Google Scholar]
- Çalik, P.; Yilgör, P.; Demir, A.S. Influence of controlled-pH and uncontrolled-pH operations on recombinant benzaldehyde lyase production by Escherichia coli. Enzyme Microb. Technol. 2006, 38, 617–627. [Google Scholar] [CrossRef]
- Kocabaş, P.; Çalık, P.; Özdamar, T.H. Fermentation characteristics of l-tryptophan production by thermoacidophilic Bacillus acidocaldarius in a defined medium. Enzyme Microb. Technol. 2006, 39, 1077–1088. [Google Scholar] [CrossRef]
- Sivashanmugam, A.; Murray, V.; Cui, C.; Zhang, Y.; Wang, J.; Li, Q. Practical protocols for production of very high yields of recombinant proteins using Escherichia coli. Protein Sci. 2009, 18, 936–948. [Google Scholar] [CrossRef]
- Zymnis, A.; Boyd, S.; Gorinevsky, D. Mixed linear system estimation and identification. Signal Process. 2010, 90, 966–971. [Google Scholar] [CrossRef]
- Babaeipour, V.; Shojaosadati, S.A.; Maghsoudi, N. Maximizing Production of Human Interferon-γ in HCDC of Recombinant E. coli. Iran. J. Pharm Res. 2013, 12, 563–572. [Google Scholar] [PubMed]
- Jenzsch, M.; Gnoth, S.; Kleinschmidt, M.; Simutis, R.; Lübbert, A. Improving the batch-to-batch reproducibility of microbial cultures during recombinant protein production by regulation of the total carbon dioxide production. J. Biotechnol. 2007, 128, 858–867. [Google Scholar] [CrossRef] [PubMed]
- Jenzsch, M.; Simutis, R.; Eisbrenner, G.; Stückrath, I.; Lübbert, A. Estimation of biomass concentrations in fermentation processes for recombinant protein production. Bioprocess Biosyst. Eng. 2006, 29, 19–27. [Google Scholar] [CrossRef]
- Petkov, S.B.; Davis, R.A. On-line biomass estimation using a modified oxygen utilization rate. Bioprocess Eng. 1996, 15, 43–45. [Google Scholar] [CrossRef]
- Brand, L. TotalBoox. TBX Advanced Calculus; Dover Publications: Mineola, NY, USA, 2013; ISBN 978-0-486-15799-3. [Google Scholar]
- Swokowski, E.W. Calculus with Analytic Geometry, 2nd ed.; Prindle, Weber & Schmidt: Boston, MA, USA, 1979; ISBN 978-0-87150-268-1. [Google Scholar]
- Shiloach, J.; Fass, R. Growing E. coli to high cell density—A historical perspective on method development. Biotechnol. Adv. 2005, 23, 345–357. [Google Scholar] [CrossRef]
- Bohlin, T. Practical Grey-Box Process Identification: Theory and Applications; Advances in Industrial Control; Springer: London, UK, 2006; ISBN 978-1-84628-402-1. [Google Scholar]
- Urniezius, R.; Galvanauskas, V.; Survyla, A.; Simutis, R.; Levisauskas, D. From Physics to Bioengineering: Microbial Cultivation Process Design and Feeding Rate Control Based on Relative Entropy Using Nuisance Time. Entropy 2018, 20, 779. [Google Scholar] [CrossRef]
- Giffin, A.; Urniezius, R. The Kalman Filter Revisited Using Maximum Relative Entropy. Entropy 2014, 16, 1047–1069. [Google Scholar] [CrossRef]
- Levisauskas, D.; Galvanauskas, V.; Henrich, S.; Wilhelm, K.; Volk, N.; Lübbert, A. Model-based optimization of viral capsid protein production in fed-batch culture of recombinant Escherichia coli. Bioprocess Biosyst. Eng. 2003, 25, 255–262. [Google Scholar] [CrossRef]
- Galvanauskas, V.; Volk, N.; Simutis, R.; Lübbert, A. Design of recombinant protein production processes. Chem. Eng. Commun. 2004, 191, 732–748. [Google Scholar] [CrossRef]
- Miao, F.; Kompala, D.S. Overexpression of cloned genes using recombinant Escherichia coli regulated by a T7 promoter: I. Batch cultures and kinetic modeling. Biotechnol. Bioeng. 1992, 40, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Schuler, M.M.; Marison, I.W. Real-time monitoring and control of microbial bioprocesses with focus on the specific growth rate: Current state and perspectives. Appl. Microbiol. Biotechnol. 2012, 94, 1469–1482. [Google Scholar] [CrossRef] [PubMed]
- Urniezius, R. Convex programming for semi-globally optimal resource allocation. In AIP Conference Proceedings; AIP Publishing: Beirut, Lebanon, 2016; p. 040002. [Google Scholar]
- Giffin, A.; Urniezius, R. Simultaneous State and Parameter Estimation Using Maximum Relative Entropy with Nonhomogenous Differential Equation Constraints. Entropy 2014, 16, 4974–4991. [Google Scholar] [CrossRef]
- Willmott, C.; Matsuura, K. Advantages of the mean absolute error (MAE) over the root mean square error (RMSE) in assessing average model performance. Clim. Res. 2005, 30, 79–82. [Google Scholar] [CrossRef]
- de Myttenaere, A.; Golden, B.; Le Grand, B.; Rossi, F. Mean Absolute Percentage Error for regression models. Neurocomputing 2016, 192, 38–48. [Google Scholar] [CrossRef]
- Gnoth, S.; Jenzsch, M.; Simutis, R.; Lübbert, A. Control of cultivation processes for recombinant protein production: A review. Bioprocess Biosyst. Eng. 2008, 31, 21–39. [Google Scholar] [CrossRef]
- Galvanauskas, V.; Simutis, R.; Lübbert, A. Hybrid process models for process optimisation, monitoring and control. Bioprocess Biosyst. Eng. 2004, 26, 393–400. [Google Scholar] [CrossRef]
- Gnoth, S.; Jenzsch, M.; Simutis, R.; Lübbert, A. Product formation kinetics in genetically modified E. coli bacteria: Inclusion body formation. Bioprocess Biosyst. Eng. 2008, 31, 41–46. [Google Scholar] [CrossRef]

| Model | α | MAE | MAPE | ||||
|---|---|---|---|---|---|---|---|
| Equation (3) | 0.996 | 0.07 | 0.00084 | 0 | — | 1.422 | 8.85% |
| Equation (12) | 0.997 | 0 | 0 | 0 | 2.705 | 0.68 | 6.92% |
| E. Coli BL21 (DE3) pET28a |
|---|
| Dry Biomass Concentration (Dry Cell Weight, DCW) | Product | |||||
|---|---|---|---|---|---|---|
| No. | MAE (g/L) | MAPE (%) | RMSE (g) | MAE (g/L) | MAPE (%) | RMSE (g) |
| 1 | 0.728 | 6.802 | 5.212 | 0.139 | 5.378 | 0.571 |
| 2 | 0.762 | 4.997 | 6.621 | 0.231 | 6.095 | 0.647 |
| 3 | 0.860 | 11.022 | 6.172 | 0.473 | 52.526 | 2.91 |
| 4 | 0.388 | 4.458 | 3.085 | 0.184 | 13.265 | 1.248 |
| 5 | 0.798 | 8.02 | 6.107 | 0.527 | 82.075 | 3.258 |
| 6 | 0.512 | 8.82 | 3.703 | 0.113 | 6.7898 | 0.608 |
| 7 | 0.595 | 4.787 | 4.605 | 0.127 | 6.957 | 0.84 |
| 8 | 0.311 | 4.433 | 2.191 | 0.629 | 35.36 | 3.757 |
| 9 | 0.576 | 6.046 | 4.266 | 0.178 | 11.250 | 1.471 |
| 10 | 0.873 | 9.017 | 6.166 | 0.634 | 33.844 | 4.147 |
| 11 | 0.582 | 5.248 | 4.468 | 0.1407 | 8.286 | 0.872 |
| 12 | 0.61 | 5.884 | 5.264 | 0.31 | 19.407 | 1.946 |
| 13 | 0.7642 | 5.477 | 4.962 | 0.318 | 39.614 | 1.834 |
| 14 | 0.404 | 3.862 | 3.563 | 0.056 | 7.001 | 0.594 |
| 15 | 0.531 | 5.724 | 3.726 | 0.137 | 9.681 | 0.914 |
| 16 | 0.628 | 7.532 | 4.503 | 0.066 | 4.504 | 0.401 |
| 17 | 0.86 | 7.057 | 6.685 | 0.16 | 17.13 | 1.042 |
| 18 | 1.262 | 11.767 | 9.218 | 0.134 | 10.328 | 1.026 |
| 19 | 0.862 | 10.582 | 5.933 | 0.111 | 8.15 | 0.738 |
| Dry Biomass Concentration (DCW) | Product | |||||
|---|---|---|---|---|---|---|
| No. | MAE (g/L) | MAPE (%) | RMSE (g) | MAE (g/L) | MAPE (%) | RMSE (g) |
| 1 | 0.769 | 8.594 | 5.279 | 0.128 | 11.947 | 0.7222 |
| 2 | 0.481 | 7.39 | 2.916 | 0.0813 | 6.565 | 0.491 |
| 3 | 0.843 | 8.107 | 6.354 | 0.0563 | 7.86 | 0.397 |
| 4 | 0.727 | 5.25 | 5.975 | 0.05 | 4.996 | 0.323 |
| 5 | 0.596 | 7.199 | 4.17 | 0.134 | 8.715 | 0.821 |
| 6 | 0.402 | 6.033 | 2.768 | 0.149 | 9.26 | 1.185 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urniezius, R.; Survyla, A. Identification of Functional Bioprocess Model for Recombinant E. Coli Cultivation Process. Entropy 2019, 21, 1221. https://doi.org/10.3390/e21121221
Urniezius R, Survyla A. Identification of Functional Bioprocess Model for Recombinant E. Coli Cultivation Process. Entropy. 2019; 21(12):1221. https://doi.org/10.3390/e21121221
Chicago/Turabian StyleUrniezius, Renaldas, and Arnas Survyla. 2019. "Identification of Functional Bioprocess Model for Recombinant E. Coli Cultivation Process" Entropy 21, no. 12: 1221. https://doi.org/10.3390/e21121221
APA StyleUrniezius, R., & Survyla, A. (2019). Identification of Functional Bioprocess Model for Recombinant E. Coli Cultivation Process. Entropy, 21(12), 1221. https://doi.org/10.3390/e21121221





