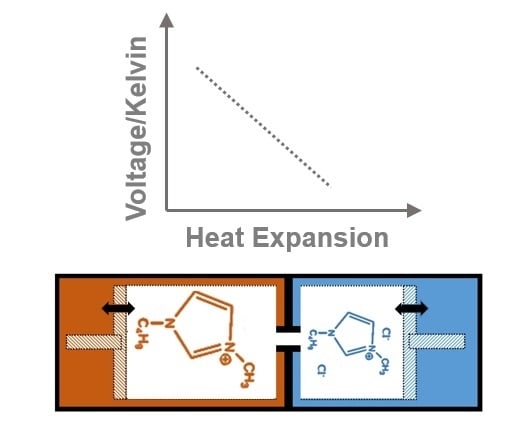
Role of Heat Expansion with a Series of Ionic Liquids: The Case for Isochoric Thermoelectric Generators and Minimal Steric Repulsion
Abstract
1. Introduction
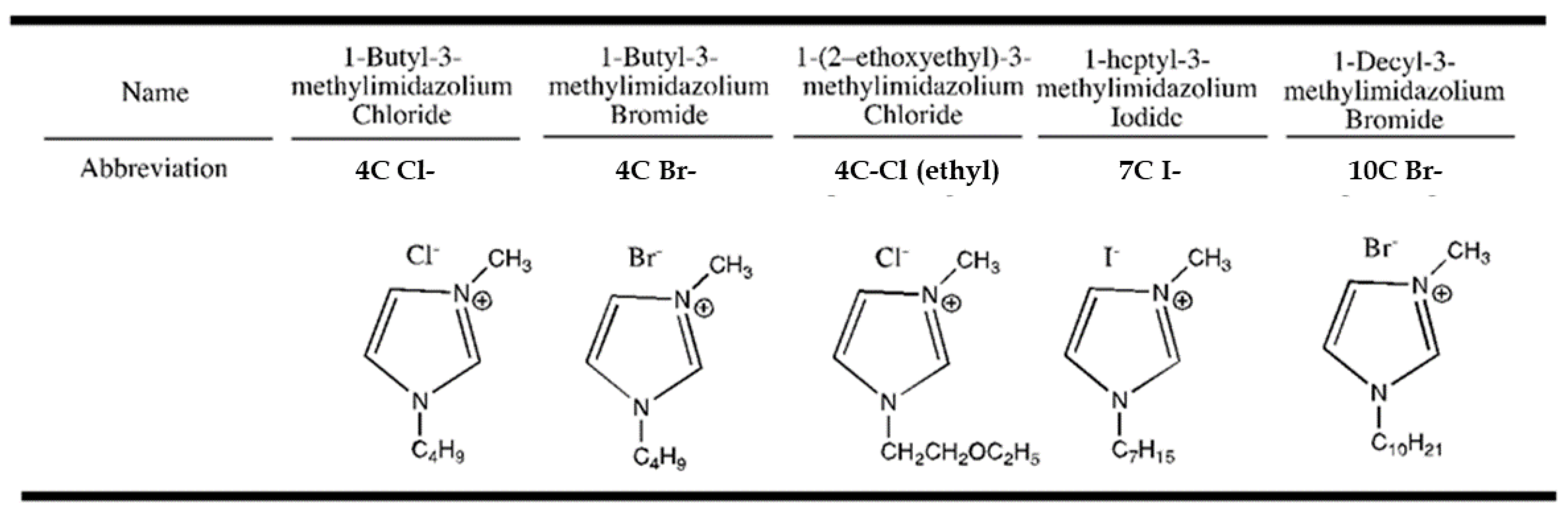
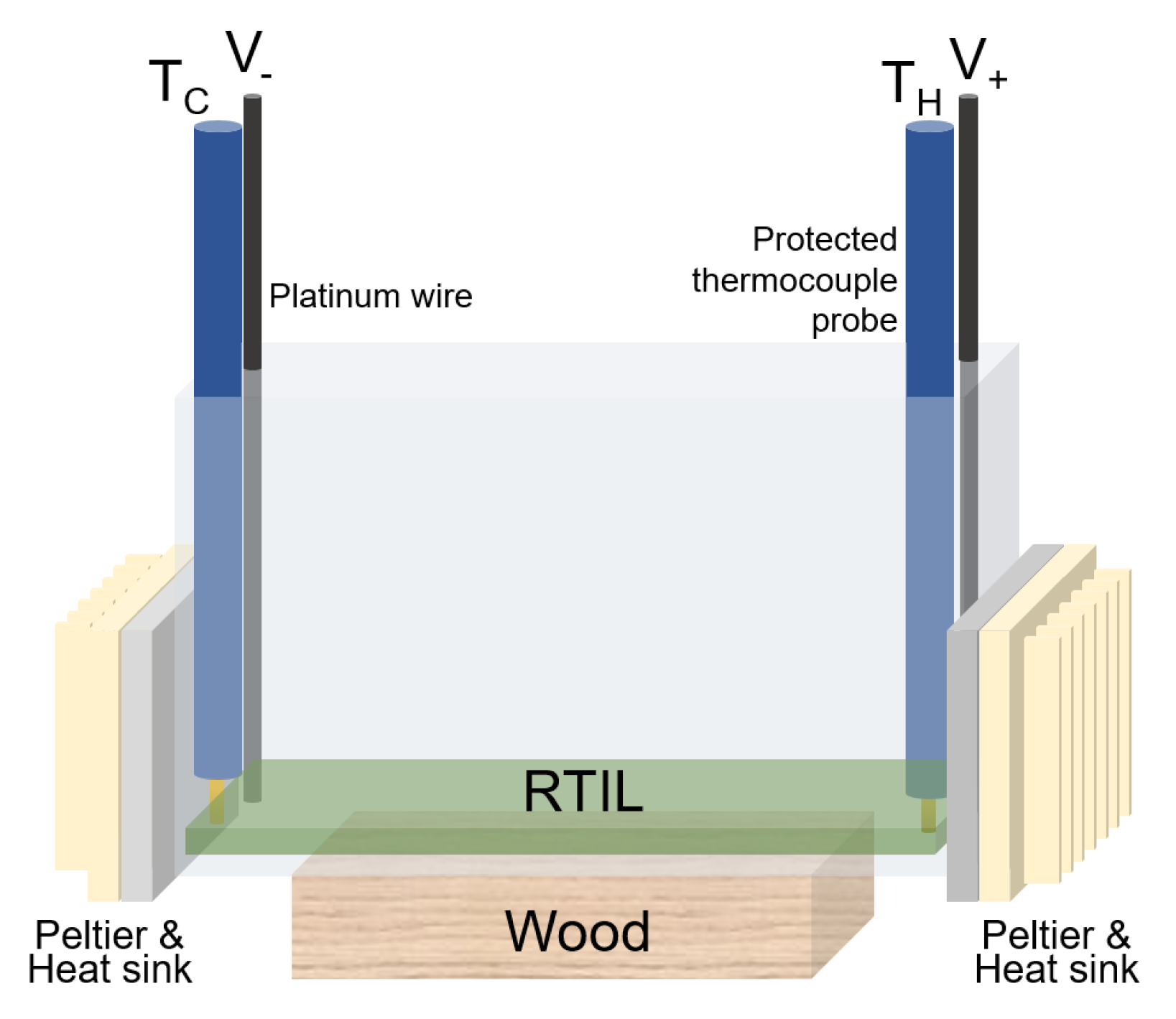
2. Materials and Methods
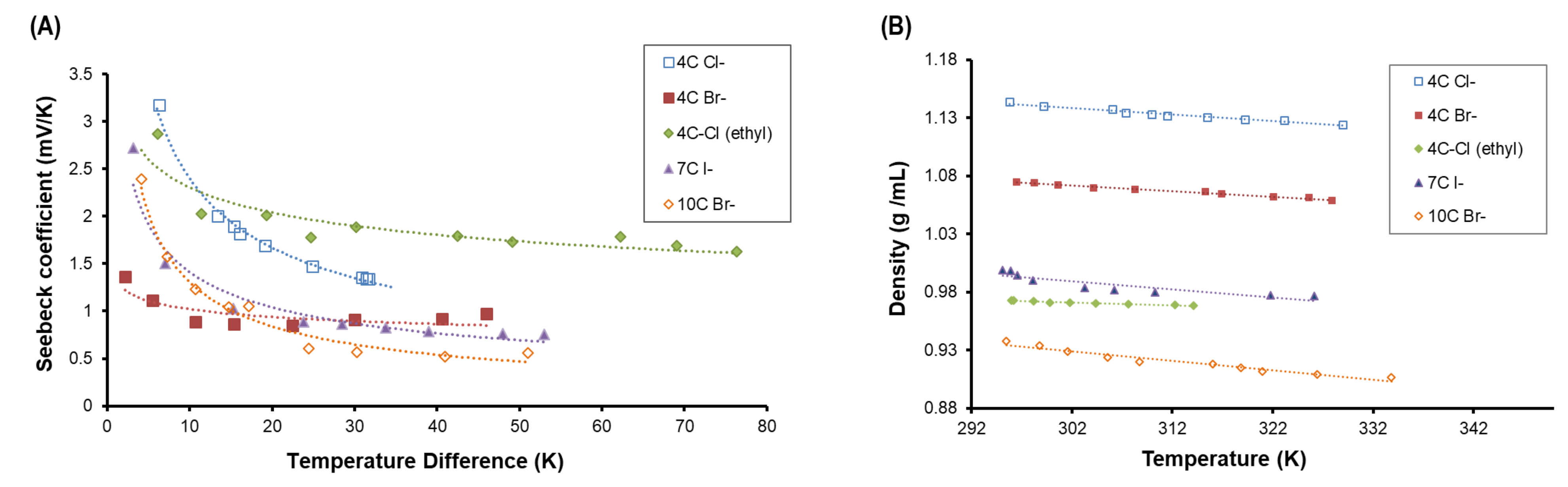
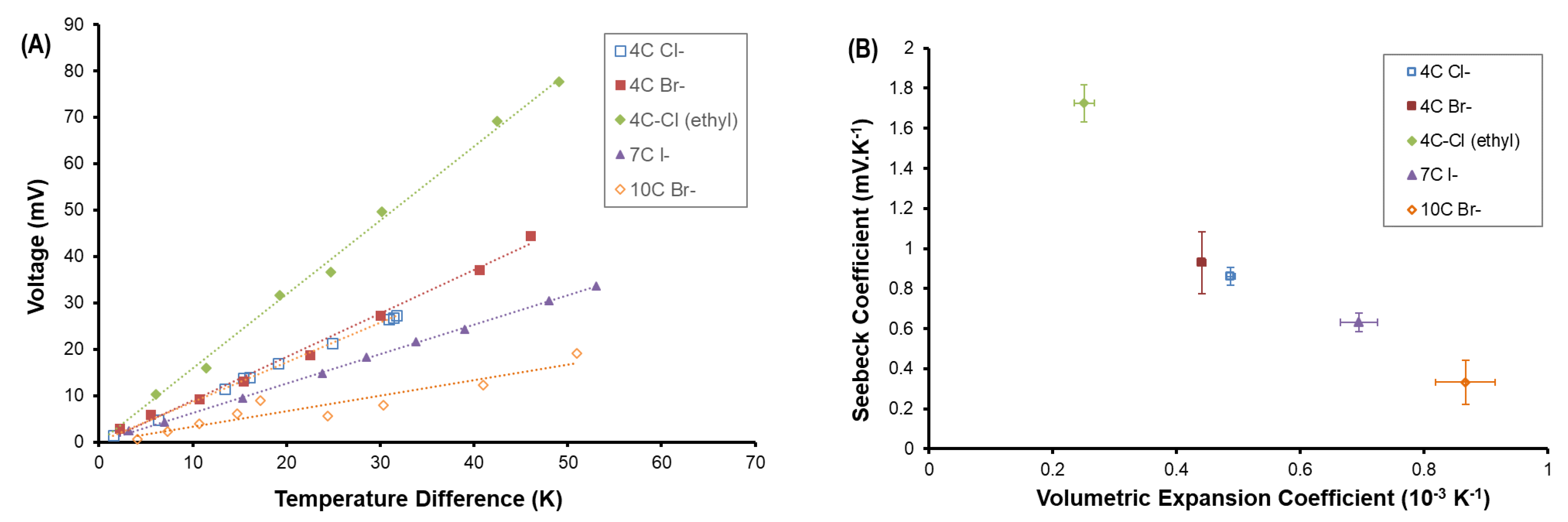
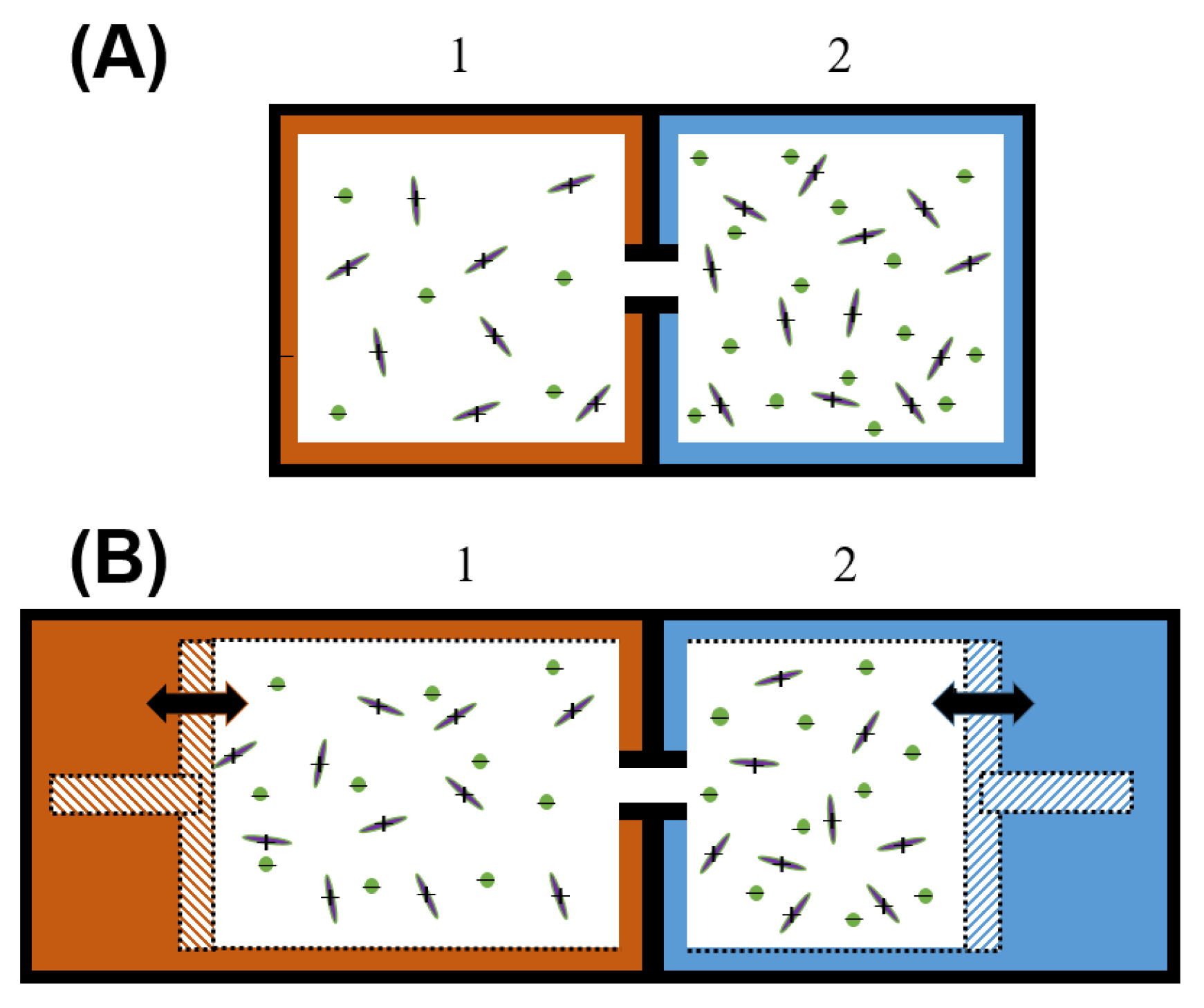
3. Results and Analysis
4. Discussion and Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bonetti, M.; Nakamae, S.; Huang, B.T.; Salez, T.J.; Wiertel-Gasquet, C.; Roger, M. Thermoelectric energy recovery at ionic-liquid/electrode interface. J. Chem. Phys. 2015, 142. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, S. Thermal diffusion in liquid mixtures and polymer solutions. J. Phys. Condens. Matter 2004, 16, R357–R379. [Google Scholar] [CrossRef]
- Samsonidze, G.; Kozinsky, B. Accelerated Screening of Thermoelectric Materials by First-Principles Computations of Electron-Phonon Scattering. Adv. Energy Mater. 2018, 8, 1800246. [Google Scholar] [CrossRef]
- Laux, E.; Uhl, S.; Jeandupeux, L.; López, P.P.; Sanglard, P.; Vanoli, E.; Marti, R.; Keppner, H. Thermoelectric Generators Based on Ionic Liquids. J. Electron. Mater. 2018, 47, 3193–3197. [Google Scholar] [CrossRef]
- Duan, J.; Feng, G.; Yu, B.; Li, J.; Chen, M.; Yang, P.; Feng, J.; Liu, K.; Zhou, J. Aqueous thermogalvanic cells with a high Seebeck coefficient for low-grade heat harvest. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Salez, T.J.; Huang, B.T.; Rietjens, M.; Bonetti, M.; Wiertel-Gasquet, C.; Roger, M.; Filomeno, C.L.; Dubois, E.; Perzynski, R.; Nakamae, S. Can charged colloidal particles increase the thermoelectric energy conversion efficiency? Phys. Chem. Chem. Phys. 2017, 19, 9409–9416. [Google Scholar] [CrossRef] [PubMed]
- Apertet, Y.; Ouerdane, H.; Goupil, C.; Lecæur, P. Internal convection in thermoelectric generator models. J. Phys. Conf. Ser. 2012, 395, 012103. [Google Scholar] [CrossRef]
- Gunawan, A.; Li, H.; Lin, C.H.; Buttry, D.A.; Mujica, V.; Taylor, R.A.; Prasher, R.S.; Phelan, P.E. The amplifying effect of natural convection on power generation of thermogalvanic cells. Int. J. Heat Mass Transf. 2014, 78, 423–434. [Google Scholar] [CrossRef]
- Novev, J.K.; Compton, R.G. Natural convection effects in electrochemical systems. Curr. Opin. Electrochem. 2018, 7, 118–129. [Google Scholar] [CrossRef]
- Salez, T.; Nakamae, S.; Perzynski, R.; Mériguet, G.; Cebers, A.; Roger, M. Thermoelectricity and Thermodiffusion in Magnetic Nanofluids: Entropic Analysis. Entropy 2018, 20, 405. [Google Scholar] [CrossRef]
- Li, X.; Batchelor-McAuley, C.; Novev, J.K.; Compton, R.G. A thermostated cell for electrochemistry: minimising natural convection and investigating the role of evaporation and radiation. Phys. Chem. Chem. Phys. 2018, 20, 11794–11804. [Google Scholar] [CrossRef] [PubMed]
- Laux, E.; Uhl, S.; Gauthier, N.; Jeandupeux, L.; Keppner, H.; López, P.P.; Pauline, S.; Vanoli, E.; Marti, R. Development of Thermoelectric generator based on Ionic Liquids for high temperature applications. Mater. Today Proc. 2018, 5, 10195–10202. [Google Scholar] [CrossRef]
- Vigolo, D.; Buzzaccaro, S.; Piazza, R. Thermophoresis and Thermoelectricity in Surfactant Solutions. Langmuir 2010, 26, 7792–7801. [Google Scholar] [CrossRef] [PubMed]
- Shukla, M.; Srivastava, N.; Saha, S. Interactions and Transitions in Imidazolium Cation Based Ionic Liquids. In Ionic Liquids; Handy, S.T., Ed.; IntechOpen: Rijeka, Croatia, 2011; Chapter 7. [Google Scholar] [CrossRef]
- Petra, C.; Attila, G.; Katalin, B.B.; Laszlo, G. Dielectric Properties of Ionic Liquids Proposed to Be Used in Batteries. In Ionic Liquids - Classes and Properties; IntechOpen: Rijeka, Croatia, 2011. [Google Scholar] [CrossRef][Green Version]
- Keppner, H.; Uhl, S.; Laux, E.; Jeandupeux, L.; Tschanz, J.; Journot, T. Ionic Liquid-based Thermoelectric Generator: Links between Liquid Data and Generator Characteristics. Mater. Today Proc. 2015, 2, 680–689. [Google Scholar] [CrossRef]
- Alcalde, R.; García, G.; Atilhan, M.; Aparicio, S. Systematic Study on the Viscosity of Ionic Liquids: Measurement and Prediction. Ind. Eng. Chem. Res. 2015, 54, 10918–10924. [Google Scholar] [CrossRef]
- Endres, F. Physical chemistry of ionic liquids. Phys. Chem. Chem. Phys. 2010, 12, 1648. [Google Scholar] [CrossRef]
- Abraham, T.J.; MacFarlane, D.R.; Pringle, J.M. Seebeck coefficients in ionic liquids—prospects for thermo-electrochemical cells. Chem. Commun. 2011, 47, 6260. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Tachikawa, N.; Forsyth, M.; Pringle, J.M.; Howlett, P.C.; Elliott, G.D.; Davis, J.H.; Watanabe, M.; Simon, P.; Angell, C.A. Energy applications of ionic liquids. Energy Environ. Sci. 2014, 7, 232–250. [Google Scholar] [CrossRef]
- Lall, S.I.; Mancheno, D.; Castro, S.; Behaj, V.; Cohen, J.I.; Engel, R. Polycations. Part X. LIPs, a new category of room temperature ionic liquid based on polyammonium salts. Chem. Commun. 2000, 2413–2414. [Google Scholar] [CrossRef]
- Patra, R.N.; Gardas, R.L. Effect of Nitro Groups on Desulfurization Efficiency of Benzyl-Substituted Imidiazolium-Based Ionic Liquids: Experimental and Computational Approach. Energy Fuels 2019, 33, 7659–7666. [Google Scholar] [CrossRef]
- Chandran, D.; Khalid, M.; Walvekar, R.; Mubarak, N.M.; Dharaskar, S.; Wong, W.Y.; Gupta, T.C.S.M. Deep eutectic solvents for extraction-desulphurization: A review. J. Mol. Liq. 2019, 275, 312–322. [Google Scholar] [CrossRef]
- Zhang, Z.; Kang, N.; Wang, J.; Sui, H.; He, L.; Li, X. Synthesis and application of amino acid ionic liquid-based deep eutectic solvents for oil-carbonate mineral separation. Chem. Eng. Sci. 2018, 181, 264–271. [Google Scholar] [CrossRef]
- Pérez, P.; Uhl, S.; Laux, E.; Sanglard, P.; Marti, R.; Keppner, H.; Vanoli, E. Synthesis and Structure modification of Ionic Liquids to optimize their Thermoelectric Properties. Mater. Today Proc. 2018, 5, 10298–10305. [Google Scholar] [CrossRef]
- Bonetti, M.; Nakamae, S.; Roger, M.; Guenoun, P. Huge Seebeck coefficients in nonaqueous electrolytes. J. Chem. Phys. 2011, 134, 114513. [Google Scholar] [CrossRef] [PubMed]
- Giglio, M.; Vendramini, A. Thermal-Diffusion Measurements near a Consolute Critical Point. Phys. Rev. Lett. 1975, 34, 561–564. [Google Scholar] [CrossRef]
- Gunawan, A.; Lin, C.H.; Buttry, D.A.; Mujica, V.; Taylor, R.A.; Prasher, R.S.; Phelan, P.E. Liquid Thermoelectrics: Review of Recent And Limited New Data of Thermogalvanic Cell Experiments. Nanosc. Microsc. Therm. Eng. 2013, 17, 304–323. [Google Scholar] [CrossRef]
- Uhl, S.; Laux, E.; Journot, T.; Jeandupeux, L.; Charmet, J.; Keppner, H. Development of Flexible Micro-Thermo-electrochemical Generators Based on Ionic Liquids. J. Electron. Mater. 2014, 43, 3758–3764. [Google Scholar] [CrossRef]
- Perkin, S.; Crowhurst, L.; Niedermeyer, H.; Welton, T.; Smith, A.M.; Gosvami, N.N. Self-assembly in the electrical double layer of ionic liquids. Chem. Commun. 2011, 47, 6572. [Google Scholar] [CrossRef]
- Fedorov, M.; Georgi, N.; Kornyshev, A. Double layer in ionic liquids: The nature of the camel shape of capacitance. Electrochem. Commun. 2010, 12, 296–299. [Google Scholar] [CrossRef]
- Abdel-Aal, S.K.; Kocher-Oberlehner, G.; Ionov, A.; Mozhchil, R.N. Effect of organic chain length on structure, electronic composition, lattice potential energy, and optical properties of 2D hybrid perovskites [(NH3)(CH2) n (NH3)]CuCl4, n = 2–9. Appl. Phys. A 2017, 123. [Google Scholar] [CrossRef]
- Ferreira, A.R.; Freire, M.G.; Ribeiro, J.C.; Lopes, F.M.; Crespo, J.G.; Coutinho, J.A.P. An Overview of the Liquid-Liquid Equilibria of (Ionic Liquid Hydrocarbon) Binary Systems and Their Modeling by the Conductor-like Screening Model for Real Solvents. Ind. Eng. Chem. Res. 2011, 50, 5279–5294. [Google Scholar] [CrossRef]
- Krossing, I.; Slattery, J.M.; Daguenet, C.; Dyson, P.J.; Oleinikova, A.; Weingärtner, H. Why Are Ionic Liquids Liquid? A Simple Explanation Based on Lattice and Solvation Energies. J. Am. Chem. Soc. 2006, 128, 13427–13434. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, Y.; Ning, H.; Lei, J.; Hu, G. Synthesis, structure and properties of imidazolium-based energetic ionic liquids. RSC Adv. 2017, 7, 33231–33240. [Google Scholar] [CrossRef]
- Chiou, J.Y.Z.; Chen, J.N.; Lei, J.S.; Lin, I.J.B. Ionic liquid crystals of imidazolium salts with a pendant hydroxyl group. J. Mater. Chem. 2006, 16, 2972. [Google Scholar] [CrossRef]
- Getsis, A.; Mudring, A.V. Imidazolium based ionic liquid crystals: structure, photophysical and thermal behaviour of [Cnmim]BrxH2O (n = 12, 14; x = 0, 1). Cryst. Res. Technol. 2008, 43, 1187–1196. [Google Scholar] [CrossRef]
- Wang, Y.; Voth, G.A. Unique Spatial Heterogeneity in Ionic Liquids. J. Am. Chem. Soc. 2005, 127, 12192–12193. [Google Scholar] [CrossRef]
- Luettmer-Strathmann, J. Two-chamber lattice model for thermodiffusion in polymer solutions. J. Chem. Phys. 2003, 119, 2892–2902. [Google Scholar] [CrossRef]
- Apertet, Y.; Ouerdane, H.; Goupil, C.; Lecoeur, P. A note on the electrochemical nature of the thermoelectric power. Eur. Phys. J. Plus 2016, 131. [Google Scholar] [CrossRef]
- Borukhov, I.; Andelman, D.; Orland, H. Steric Effects in Electrolytes: A Modified Poisson-Boltzmann Equation. Phys. Rev. Lett. 1997, 79, 435–438. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jackson, M.; Engel, R.R.; Vuong, L.T. Role of Heat Expansion with a Series of Ionic Liquids: The Case for Isochoric Thermoelectric Generators and Minimal Steric Repulsion. Entropy 2019, 21, 1086. https://doi.org/10.3390/e21111086
Jackson M, Engel RR, Vuong LT. Role of Heat Expansion with a Series of Ionic Liquids: The Case for Isochoric Thermoelectric Generators and Minimal Steric Repulsion. Entropy. 2019; 21(11):1086. https://doi.org/10.3390/e21111086
Chicago/Turabian StyleJackson, Marcus, Robert R. Engel, and Luat T. Vuong. 2019. "Role of Heat Expansion with a Series of Ionic Liquids: The Case for Isochoric Thermoelectric Generators and Minimal Steric Repulsion" Entropy 21, no. 11: 1086. https://doi.org/10.3390/e21111086
APA StyleJackson, M., Engel, R. R., & Vuong, L. T. (2019). Role of Heat Expansion with a Series of Ionic Liquids: The Case for Isochoric Thermoelectric Generators and Minimal Steric Repulsion. Entropy, 21(11), 1086. https://doi.org/10.3390/e21111086