1. Introduction
High-Entropy Alloys (HEAs) are defined according to the atomic percentage of their principal elements, between 5 and 35 at %, or according to their configurational entropy at random state, ΔS > 1.5 R (R = 8.314 J·K
−1·mol
−1). Their high compositional complexity draws a hyper-dimensional space whose limits have yet to be explored, for its investigation has mostly focused on equiatomic or near-equiatomic compositions [
1,
2]. In fact, the study of HEAs is driven by the search of new single-phase systems, whose formation is opposed by the diverse mixing enthalpies, atomic size, and valence electron concentration of the constituent elements. Consequently, single-phase HEAs are rare, whereas systems consisting of multiple solid solutions and ordered intermetallic phases are more common [
3].
Following the pioneering publications in 2004 by Yeh [
4] and Cantor [
5], HEAs have attracted growing research interest due to their outstanding mechanical and thermal properties; including high compression yield, fracture strength, ductility and toughness, as well as extreme corrosion, wear and fatigue resistance. HEAs have thus been proposed as candidates for applications in which high temperature stability is pivotal; e.g., as replacements for conventional binders after liquid-phase or spark-plasma sintering, for liquefied gas storage and for high-temperature thermoelectrics [
1,
6]. Thermoelectric properties can be tuned with respect to valence electron concentration (VEC) and a dimensionless thermoelectric figure of merit (
ZT) of 0.012 at 505 °C was reached for Al
2CoCrFeNi. The phase evolution of HEAs has been extensively studied both ex situ and in situ [
7,
8,
9,
10,
11].
Following a traditional trend in alloy development, HEAs have seen the addition of selected secondary phases to further tune their mechanical properties. This approach has led to the development of a new class of metal-matrix composites, containing oxides [
12], silicon carbide [
13] or nano-diamonds [
14]. Alloying with elements in low concentrations, on the other hand, has resulted in the precipitation of intermetallic compounds in the matrix phase. However, while intermetallics deeply affect yield strength, hardness, tensile properties, and matrix stabilization—due to the competition between mixing enthalpy of atom pairs and mixing entropy—their proper distribution, size, shape, and volume fraction represent a cause for concern [
1]. The synthesis of precipitation-hardened HEAs following the introduction of intermetallics has proven largely unsuccessful when binary compounds are concerned, and no studies have been performed on the formation of stable ternary inclusions as pinning centers in complex multi-principal component alloys [
15,
16,
17]. This is mostly due to the difficulties in choosing appropriate alloying elements. Their selection should be guided by the following considerations: miscibility for most or all HEA constitutive elements in liquid state, low formation enthalpy for ternary compounds and crystallization in common structure types (e.g., σ-phase, Laves phase).
We recently highlighted the compound-forming ability of scandium and its outstanding effect on the mechanical properties of multicomponent alloys [
18]. Scandium forms over three hundred binary and ternary phases with most elements of the periodic table, many of which crystallize in highly symmetrical structures (e.g., space groups
Pmm,
Fmm,
P/mmc). Scandium-based intermetallics (i.e., Al
3Sc,
V- and
W-phases) are responsible for the enhanced properties of several commercial aluminum alloys and newly developed multicomponent systems [
19]. These features make scandium a perfect candidate to achieve precipitation-hardened HEAs. Moreover, its low density makes it ideal in combination with HEA based on 3
d-elements, which make up to 85% of the known systems [
1]. The Al
xCoCrCu
yFeNi HEA can be considered a model alloy. The nature of the solid solution can be tuned by changing the aluminum and copper content, since Al and Cu act as
bcc- and
fcc-stabilizers respectively [
20]. Thus, Al
2CoCrFeNi and AlCoCrCu
0.5FeNi have been widely reported as pure
bcc phases, while Al
0.5CoCrCuFeNi as purely
fcc-structured [
21,
22,
23].
We herein report on the effects of 0.3–5 wt.% scandium addition to the microstructure, mechanical and transport properties, thermal stability, and phase evolution upon temperature of the model Al2CoCrFeNi, AlCoCrCu0.5FeNi, and Al0.5CoCrCuFeNi HEAs. We show that scandium forms the same stable ternary intermetallic in all three alloys, but that the compound interacts differently with bcc- and fcc-structured alloys.
2. Materials and Methods
The target alloys were prepared using induction melting from pure metallic powders, in a BN crucible in an Ar-filled Customised DAB01 glove-box (Saffron Scientific Equipment Limited, Knaresborough, UK). Complete melting of the samples was achieved above 1300 °C. After 5 min at the melting temperature, the sample was cooled down naturally to room temperature. The samples were re-melted three times to assure homogeneity. A brief rationale of the synthesized specimens and their performed analysis is reported in
Supplementary Materials Table S1.
Differential scanning calorimetry (DSC) measurements were performed on small pieces of sintered samples (50 mg) placed in an Al2O3 crucible and heated in a Netzsch STA 449 F1 Jupiter. Heating (Selb, Germany) and cooling were performed in flowing Ar gas with a temperature ramp of 10 K·min−1 from 35 to 1300 °C.
The transition temperatures highlighted by DSC were used to decide annealing conditions. Samples were heat-treated above their first reversible or irreversible transition temperature with the following specifics: Al2CoCrFeNi, Al0.5CoCrCuFeNi and AlCoCrCu0.5FeNi at 850 °C, 12 h; Al2CoCrFeNi + 3 wt.% Sc at 900 °C, 12 h; Al0.5CoCrCuFeNi + 3 wt.% Sc and AlCoCrCu0.5FeNi + 3 wt.% Sc at 930 °C for 6 h. During annealing, pellets of each sample were sealed in a silica tube under vacuum (<10−7 Pa) and heated in a furnace. After annealing, the tubes were quenched in ice-cold water.
For microstructure and elemental analysis, all samples were mounted in carbonized resin, ground and polished using MetaDiTM Supreme Polycrystalline Diamond Suspension (1 μm) (Coventry, UK).
The morphology and elemental compositions were analyzed using a Hitachi S-4800 Field Emission scanning-electron microscope (SEM) equipped with energy dispersive X-ray (EDX) analyzer (Tokio, Japan). The average elemental composition was obtained from 2.5 mm maps (
Table S2).
The Vickers hardness was measured on a WilsonR VH3100 Automatic Knoop/Vickers Hardness tester (Buehler, Lake Bluff, IL, USA); 25 individual points under a 9.81 N (1 kg) testing load were measured to get statistically significant results.
The density was measured according to Archimedes’ principle in water, in the ATTENSION equipment (Biolin Scientific, Stockholm, Sweden). Six measurements were taken for each sample to obtain statistically relevant results
The small punch tests were performed on discs of diameter 12.5 mm and thickness circa 0.8 mm. Measurements were performed with a properly modified Tinius Olsen H25KS Benchtop Tester (Salfords, UK). The setup included a lower die (diameter of 8 mm) and a punch (4 mm diameter). Each experiment was reproduced twice for statistical significance; however, since each specimen had a slightly different thickness, the final results were normalized for the standard 0.5 mm thickness as described in [
24].
For powder X-ray diffraction (PXRD), samples were powdered using a Fritsch mini-mill Pulverisette 23 (Idar-Oberschtein, Germany) (steel vial and ball, 10 min at 50 rpm). PXRD data for the annealed powdered samples were collected at ID06B-LVP beam-line at the European Synchrotron Research Facility, ESRF (room temperature, λ = 0.22542 Å) using position sensitive detector. LaB6 (NIST SRM 660c) was used as external standard for calibration.
In situ high-temperature PXRD patterns were collected at the I-11 beam-line at the DIAMOND light source (λ = 0.494984 Å). LaB
6 (NIST SRM 660c) was used as external standard for wavelength and sample to detector distance calibration. A wide-angle Mythen-2 Si position sensitive detector. The detector was moved at constant angular speed with 10 s scan time at each temperature and 60 s waiting time to let the temperature stabilize. The powdered alloys were sealed in a 0.5 mm silica glass capillary in vacuum and heated in the capillary furnace from 25 to 1200 °C with axial rotation [
25]. In all samples, oxidation was detected above 1000 °C, which can be due to the reaction of metallic alloy with silica at high temperature, resulting in capillary destruction. In situ low-temperature PXRD profiles were collected at the P02.1 beam-line at the PETRA III synchrotron (λ = 0.207150 Å). LaB
6 (NIST SRM 660c) was used as external standard for calibration. A wide-angle position sensitive detector based on Mythen-2 Si strip modules was used. The detector was moved at constant angular speed with 10 s scan time at each temperature and 60 s waiting time to let the temperature stabilize. The powdered alloys were sealed in 0.5 mm silica glass capillaries in vacuum and cooled in nitrogen flow from 300 to 100 K. Temperature was directly measured with a thermocouple during the experiment; error arising from the distance between sensor and capillary was estimated to be below 5%.
Temperature dependent PXRD patterns were analyzed using Powder3D software [
26]. Phase composition has been verified using the Powder Diffraction File database [
27]. Parametric sequential refinements were performed using the TOPAS 5.0 software [
28]. Profile parameters for the Lorentzian function, cell parameters, and phase fractions were refined simultaneously for all phases.
The Seebeck coefficient S (µV·K
−1) and electrical conductivity σ (kS·cm
−1) were measured simultaneously under He atmosphere with a Linseis – Seebeck and Electric Resistivity Unit (LSR-3 1100, Linseis, Selb, Germany) four-point setup with PtRh/Pt and Pt contacts and a continuous reverse of the polarity of the thermocouples (bipolar setup, measurement current: 100 mA) using cuboid samples (ca. 8 × 2 × 3 mm). Three heating cycles up to 875 °C (10 K·min
−1, 3 data points per temperature) were performed. Thermal diffusivity was measured up to 875 °C (heating/cooling rate 10 K·min
−1) under He atmosphere with a Linseis LFA1000 (Selb, Germany) apparatus. Simultaneous heat loss and finite pulse corrections were applied using Dusza’s model [
29]. Values were averaged from five measurement points at each temperature. For calculation of thermal conductivity κ, they were multiplied with the Dulong−Petit heat capacity C
p and the density as derived by the weight and the volume determined by Archimedes’ principle. The single values of each sample are given in
Table S3. According to experimental C
p values of materials with similar compositions (e.g., 0.60 J·g
−1·K
−1 for AlCoCrFeNi at 25 °C) [
30], the room temperature heat capacity of these materials is about 20% higher than the Dulong–Petit value of 0.49 J·g
−1·K
−1; this probably adds this uncertainty to the values of κ and thus
ZT.
3. Results and Discussions
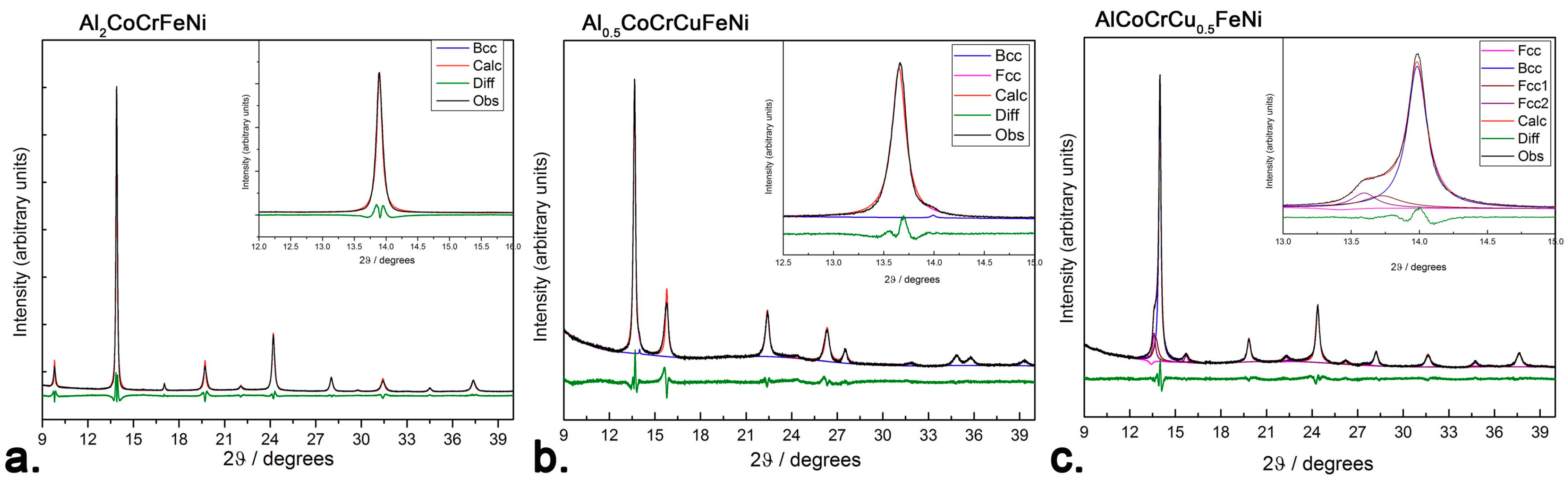
Al
2CoCrFeNi, Al
0.5CoCrCuFeNi and AlCoCrCu
0.5FeNi were synthesized via induction melting in the atomic compositions reported in the
Table S1. While all three systems are widely reported as single-phase [
1], refinements of PXRD data obtained with synchrotron radiation highlight the presence of a secondary
fcc phase in the
bcc-structured AlCoCrCu
0.5FeNi alloy (
Figure 1c) and of a very small secondary
bcc phase in the mainly
fcc-structured Al
0.5CoCrCuFeNi alloy (
Figure 1b). Unlike Al
0.5CoCrCuFeNi and AlCoCrCu
0.5FeNi, Al
2CoCrFeNi appears to be a solid solution forming a disordered CsCl structure-type. (
Figure 1a). This is consistent with a previously reported investigation on CoCrCuFeNi-based systems—which displayed a phase separation due to the positive mixing enthalpy between copper and other elements [
31]—and with the Hume–Rotary classification maps in ref. [
32].
The presence of the (100) diffraction line in the
bcc-structured PXRD pattern indicates an ordered superstructure, generally attributed to Al and Ni ordering [
22,
33,
34]. Lattice parameters of the as-cast alloys are the following:
aB2 = 2.877(2) Å for Al
2CoCrFeNi;
afcc = 3.601(1) Å for Al
0.5CoCrCuFeNi and
abcc = 2.891(3) Å for AlCoCrCu
0.5FeNi. All values are consistent with the literature within the experimental error [
20,
22,
35,
36,
37]. Small differences between the reported results arise from the high sensitivity of the HEA to synthetic pathway and to minor compositional variations. Therefore, nominally equivalent starting materials can in turn display profoundly different crystal structures, microstructures, and phase transitions.
The microstructures and elemental distributions of as-cast Al
2CoCrFeNi, Al
0.5CoCrCuFeNi and AlCoCrCu
0.5FeNi are reported by means of EDX element mapping in
Figures S1–S3, respectively. Two phases are present in the as-cast AlCoCrCu
0.5FeNi alloy, whose microstructure is characterized by a matrix and a circular secondary phase of darker color. Elemental distributions appear completely homogeneous (
Figure S3). Annealing results in the growth of a darker secondary phase and the slight segregation of Al from the rest of the elements. The as-cast Al
0.5CoCrCuFeNi HEA consists of dendritic-like and interdendritic-like regions, the first being richer in Co, Cr and Fe; and the latter Cu-rich (
Figure S2). Both the dendrite-like and interdendrite-like matrix have been previously linked to a simple
fcc phase, and copper segregation has been explained through its high mixing enthalpy with cobalt, chromium, iron, and nickel [
20]. The microstructure changes drastically after annealing, as the two phases cannot be easily differentiated. Nevertheless, element segregation persists, with Cu and Al separating from Co, Cr, Fe, and Ni. The as cast Al
2CoCrFeNi microstructure is dominated by large (∼100 μm) unstructured grains which, unlike previous studies, show no trace of non-equiaxed dendrites [
21,
37]. With respect to other elements, chromium segregation is clearly visible, though it can be reduced by annealing (see
Figure S1). On the other hand, annealing causes the formation of a homogeneously dispersed secondary phase, which appears as black dots.
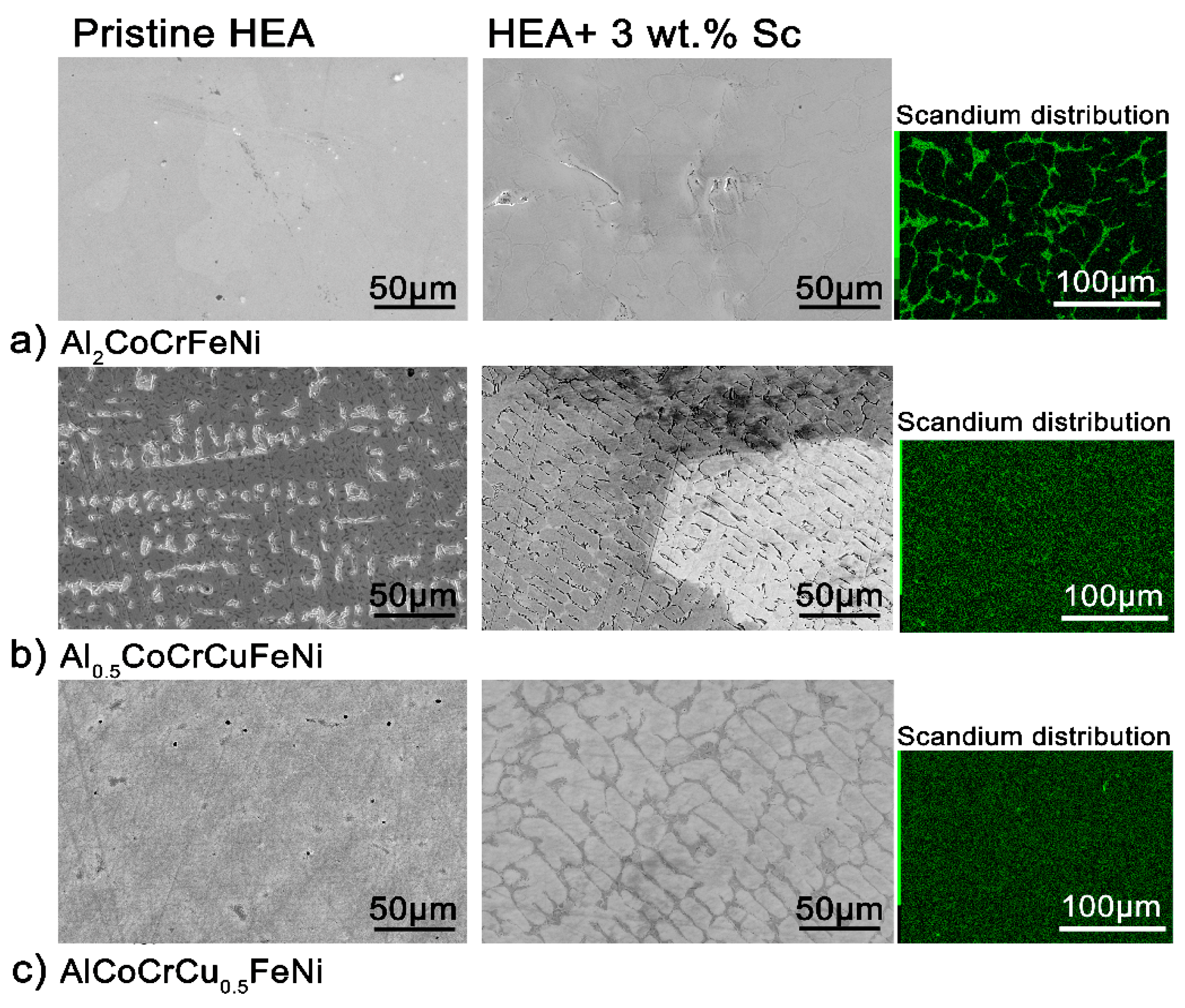
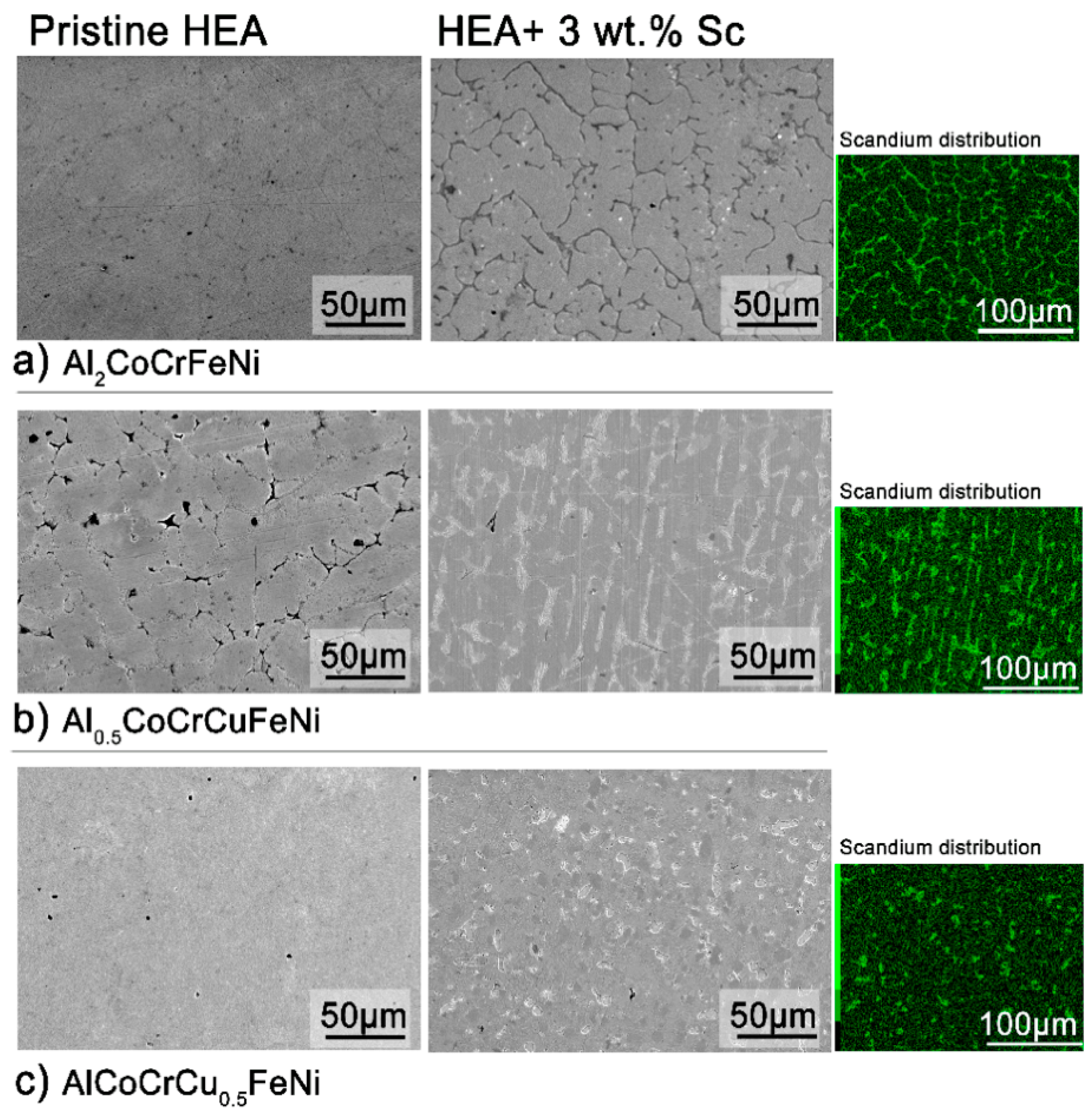
The microstructure of all alloys is strongly affected by even a 3 wt.% addition of scandium. In the case of Al
2CoCrFeNi (
Figure 2a,
Figure S4), the large grains of homogeneous compositions are refined and scandium segregates in the inter-grain volume. The scandium-based intermetallic forming in the inter-granular region appears to contain all elements in the same relative fractions as the main phase—with the notable exception of chromium (
Table S1). In Al
0.5CoCrCuFeN (
Figure 2b), scandium is dispersed more homogeneously and aids the formation of a globular microstructure. Nevertheless, most of it segregates in the inter-granular region, with copper- and nickel-rich areas (
Figure S5). Lastly, following scandium addition the original columnar cellular microstructure of the AlCoCrCu
0.5FeNi HEA turns into equiaxed non-dendritic-like grains (
Figure 2c). The chemical inhomogeneity due to scandium segregation appears surprising, considering the strongly negative mixing enthalpy of the metal with iron, nickel, cobalt and aluminum [
38,
39,
40]. The driving force of the segregation is thus the formation of the secondary phase.
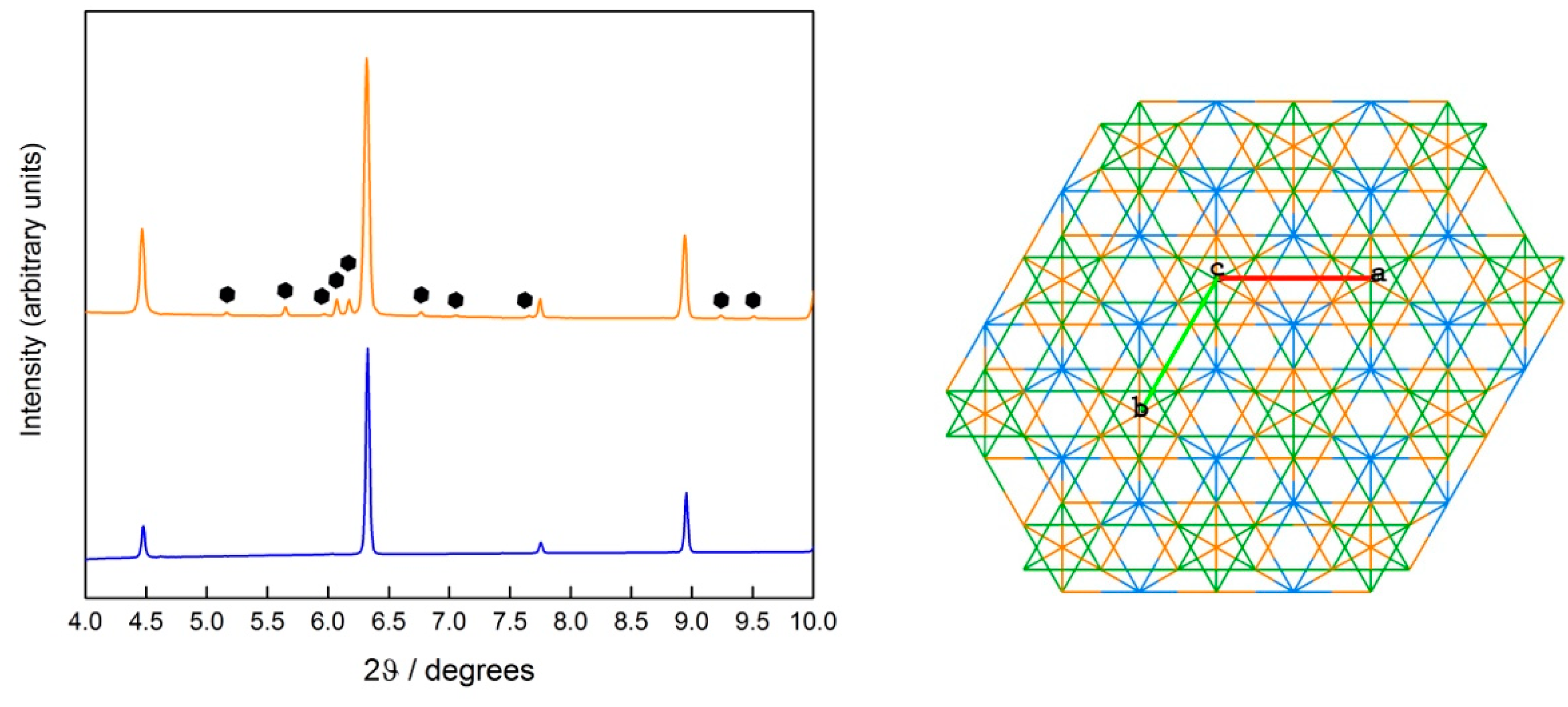
The secondary phase is the same in all systems and can be indexed as a ternary intermetallic analogous of MgZn
2-type (
Figure 3). Compounds of this structure type have been reported for scandium with several metals, in compositions such as AlCuSc, AlCoSc, Al
1.06Cr
0.94Sc, AlFeSc, and AlNiSc [
18]. As confirmed by elemental composition maps (
Figures S4–S6), the ternary phase is a highly disordered structure containing all five elements and scandium.
Knowledge about scandium-containing ternary compounds is still fragmentary. Out of all the cited MgZn
2-type intermetallic phases, only AlCuSc has been thoroughly investigated, due to its effect on the mechanical properties of Al-based alloys, as part of the so-called
W-phase. In particular, it was shown that the microhardness of the
W-phase is much higher than the one of the Al
3Sc phase (5150–5170 MPa against 3900–4300 MPa) [
18].
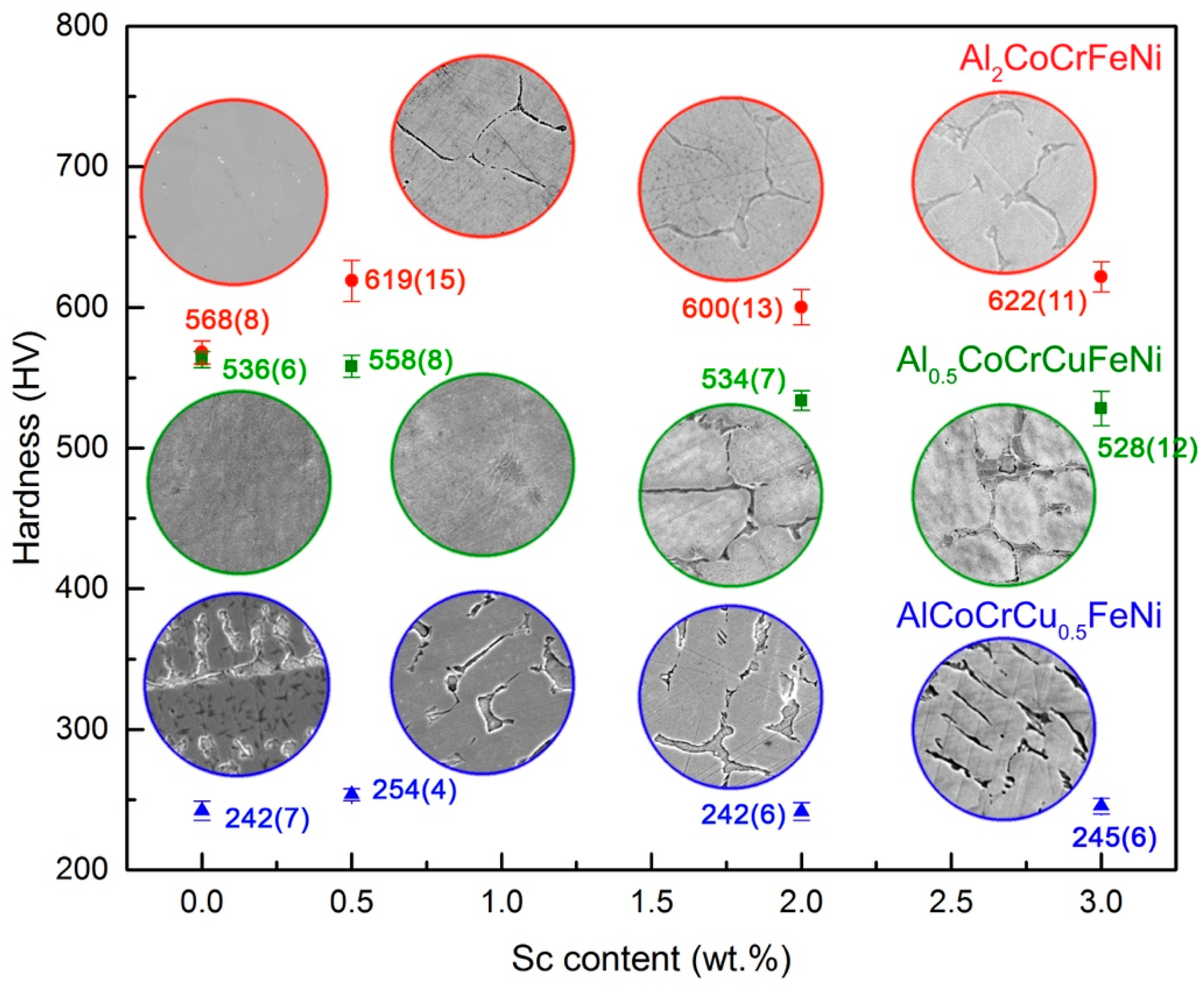
The formation of a very hard phase in the HEA matrix affects its mechanical properties. As shown in
Figure 4, single phase
bcc alloys are harder than duplex-structured and
fcc alloys. This is hardly surprising, considering the stronger interatomic forces involved in the
bcc vs.
fcc packing of alloys. On the other hand, increasing scandium content in Al
0.5CoCrCuFeNi does not affect hardness. Indeed, it is even detrimental to the hardness of the AlCoCrCu
0.5FeNi alloy and is not accompanied by an increase in ductility (as shown by the disk punch tests presented in
Figure S7). Only in the originally hard Al
2CoCrFeNi HEA the formation of the intermetallic results in an increase in hardness. The addition of 0.5 wt.% Sc causes a 20% hardness enhancement, as well as visible grain refinement. Disc punch tests performed on the HEA with 0, 0.5 and 2 wt.% scandium additions show a decisive increment in brittleness, proportional to the concentration of scandium (
Figure S8).
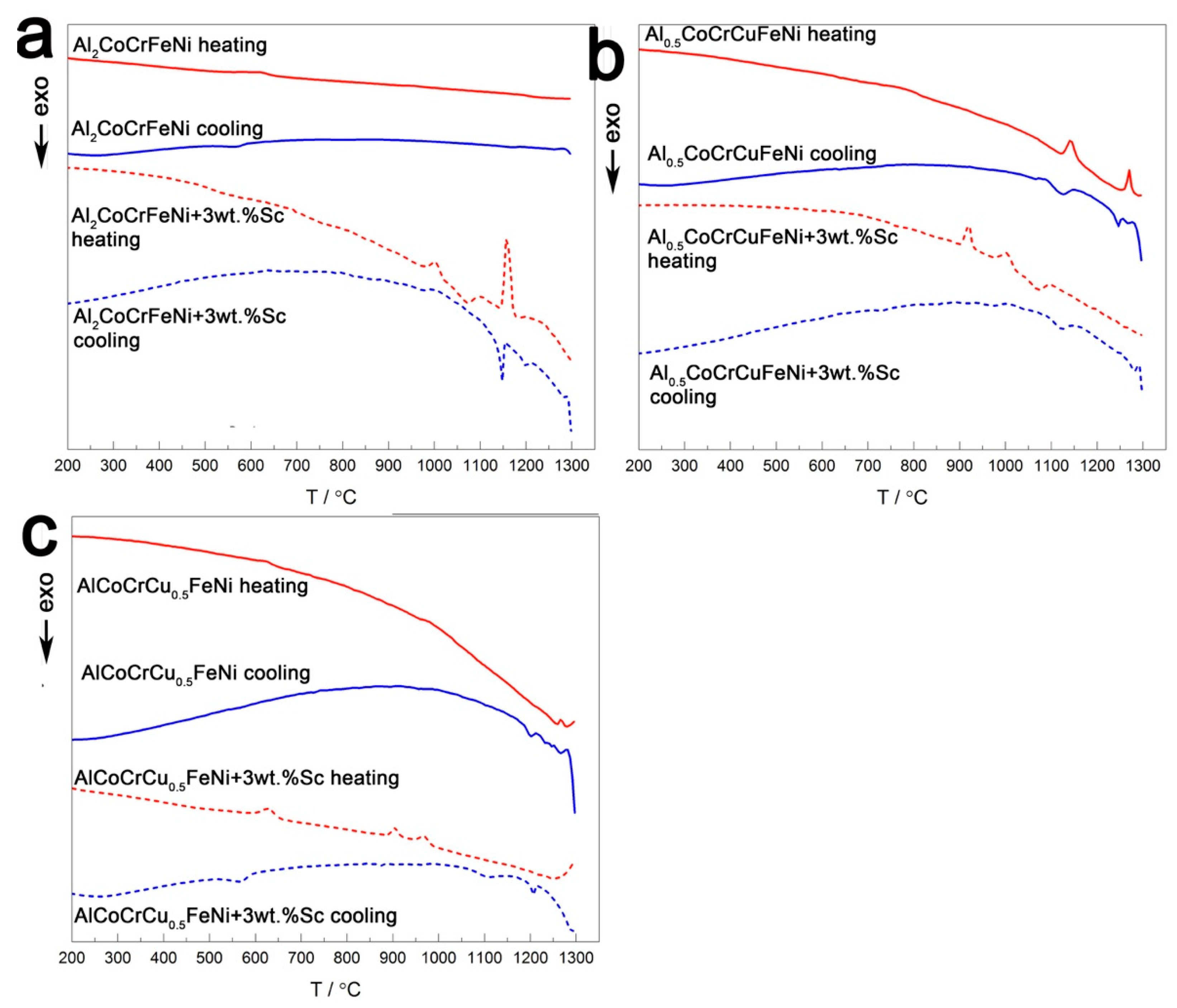
Differential scanning calorimetry (DSC) was performed on alloys from room temperature to 1300 °C with a 10 K·min
−1 heating rate. The second cycle of heating and cooling, which is less influenced by effects of the synthetic route on the specimens—i.e., internal stress-strain, magnetic ordering—is reported in
Figure 5.
The DSC profile of Al
2CoCrFeNi (
Figure 5a) has a sigmoid-like deviation between 600 and 700 °C. In a theoretical work, Gao associates this feature with the transition
bcc1 + bcc2 + CsCl →
bcc+CsCl, but this is incompatible with the crystal structure of the Al
2CoCrFeNi alloy as per
Figure 1a. The divergence between the results reported here and Gao’s interpretation might arise from the profound differences in the crystal structures of the nominally equivalent starting material [
41]. To unequivocally identify the cause of the transition, a more detailed investigation of the effect of temperature on phase stability is needed. The corresponding Sc-containing sample displays a sharp reversible peak at 1150 °C, probably corresponding to the melting and crystallization of the intermetallic phase, preceded by irreversible peaks. These two endothermic peaks, located at 906 °C and 966 °C, might correspond to phase transitions occurring in the scandium-phase. The sigmoid-like deviation clearly visible in the pristine alloy is hardly distinguishable from the background line in the Sc-containing specimen but is located at higher temperature (between 700 and 800 °C).
Al
0.5CoCrCuFeNi shows a slight reversible peak centered at 760 °C, as well as two reversible peaks above 1150 °C (
Figure 5b). In previously reported DSC curves, the two endothermic peaks of Al
0.5CoCrCuFeNi at 1140 and 1270 °C have been linked to the melting of interdendritic-like and dendritic-like material, respectively. The results of Jones et al. highlight a third reversible peak, appearing at 850 °C and related to the dissolution and recrystallization of the L1
2 phase, which might form during prolonged heat treatment below 850 °C and is dependent from the sample cooling rate [
42,
43]. Its absence is indicative of the purity of the as-cast
fcc sample, which is confirmed by high-resolution PXRD data (
Figure 1b). The scandium containing specimen displays three irreversible signals upon heating (the first two, endothermic, at 921 and 1000 °C; the latter, exothermic, at 1073 °C). Upon cooling, the Sc-containing specimen behaves very similarly to its corresponding pristine alloy. The irreversible transitions occurring in the sample might thus relate solely to the intermetallic, and have little impact on the matrix.
Finally, AlCoCrCu
0.5FeNi displays a reversible transition at circa 624 °C, which is maintained in the Sc-containing sample (
Figure 5c). Irreversible phenomena occur in the pristine alloy above 1150 °C, as in the previous sample. The scandium-containing alloy presents two irreversible endothermic peaks (at 906 and 966 °C) upon heating and two exothermic peaks upon cooling (at 1102 and 1206 °C), which are too far from the heating ones to be considered part of the same phenomenon.
Reversible phenomena thus occur in all systems above 600 °C; whereas irreversible peaks appear in the scandium-containing alloys at ca. 900 °C. To investigate the nature of these transitions, the pristine alloys were annealed above their average first reversible transition temperature, and the scandium-containing specimens at the temperatures of their first irreversible transition. Annealing time was shortened for the samples at the highest temperature (930 °C) in order not to lose aluminum. Therefore, Al
2CoCrFeNi, Al
0.5CoCrCuFeNi, and AlCoCrCu
0.5FeNi were annealed at 850 °C for 12 h. Al
2CoCrFeNi + 3 wt.% Sc was treated at 900 °C for 12 h, while Al
0.5CoCrCuFeNi + 3 wt.% Sc and AlCoCrCu
0.5FeNi + 3 wt.% Sc at 930 °C for 6 h. The corresponding element distribution and microstructures are presented in the following pages.
Figure 6 reports the microstructure and element distribution of the annealed Al
2CoCrFeNi + 3 wt.% Sc alloy. With respect to
Figure S1b (the annealed pristine alloy), microstructure is refined and the intermetallic scandium phase grows in a dendritic-like structure. The secondary phase which that have appeared in the pristine alloy (in the form of black dots) is not displayed by the matrix. A comparison with the scandium-containing alloy prior to annealing (
Figure S4) shows a more homogeneous distribution of chromium, even though the metal still visibly segregates along the grain boundaries.
The heat treatment of the pristine Al
0.5CoCrCuFeNi (
Figure 6b) causes the disruption of the original dendritic- and interdentritic-like microstructure. However, the microstructure of the scandium-containing alloy after annealing is quite similar to the as-cast pristine alloy: a columnar cellular structure displaying strong element segregation (
Figure S2b). The light phase consists mostly of Cu and Sc, and within it regions rich in Al and Ni. The darker areas are rich in Co, Cr, and Fe. It is of interest to note that this element segregation is different from the one prior annealing (
Figure S5), as Ni is depleted from the matrix.
The annealed AlCoCrCu
0.5FeNi + 3 wt.% Sc has a complex microstructure (
Figure 6c). Acicular particles grow in an unstructured matrix, the bigger of those being easily removed from the specimen during polishing (and leaving behind elongated valleys). Elemental segregation occurs to form several diverse regions: A Co-rich matrix, areas rich in Al and Ni, and Cu-Sc precipitates. Iron clearly segregates from Al and Ni, but does not follow the trend of other elements. Finally, Cr coalesces in circular well-shaped areas. Combining this information, we can note that the brittle phase is, as expected, rich in Cu and Sc. The annealed scandium-containing alloy is very different not only from the pristine alloy, but also from the original as cast specimen. Indeed, the annealed pristine alloy shows no clustering of Cr, Fe, and Cu (
Figure S3); and the scandium-containing as cast alloy clearly highlights the coexistence of Co, Cr, and Fe (
Figure S6).
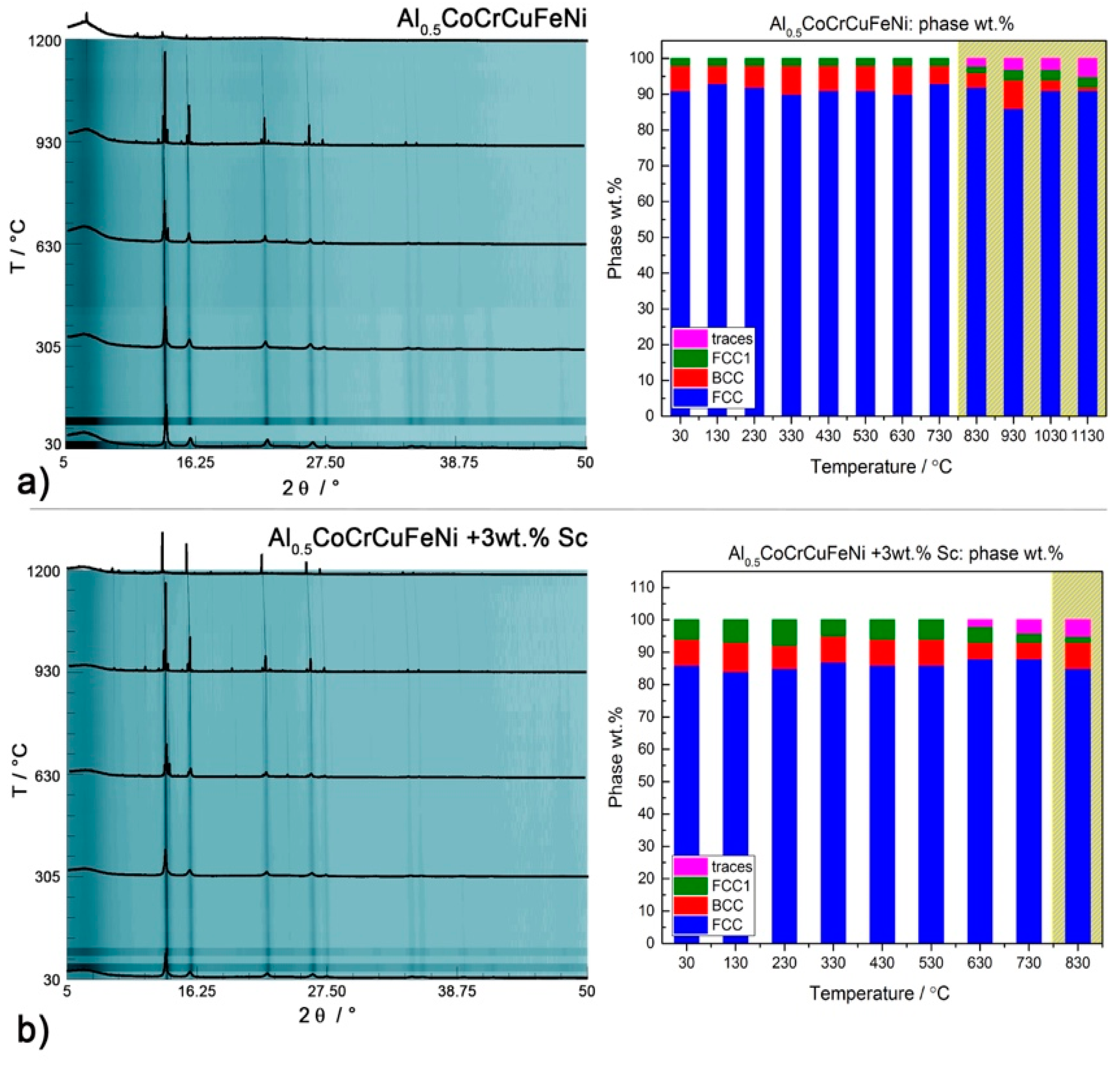
To further investigate the nature of the transitions occurring in our HEA systems and how they are affected by the intermetallic phases, we performed in situ PXRD of disordered CsCl-type Al2CoCrFeNi and the fcc-structured Al0.5CoCrFeNi with and without 3 wt.% scandium. Quantitative data are available only for the main phases, whose reflections had enough intensity to be detected and refined. The development of scandium-rich phases could not be followed directly.
Several works have highlighted that Al
0.5CoCrCuFeNi consists of a mixture of two
fcc phases of similar cell parameters, corresponding to dendritic and interdendritic phase [
44,
45,
46]. Chen reported a single
fcc structure below 500 °C, and a duplex
fcc/bcc structure above 600 °C [
47]. According to Jones, on the other hand, two
fcc phases of similar cell parameters co-exist and are stable up until their melting temperatures (1150 °C for the Cu based solid solution and 1350 °C for the multi-component phase) [
44]. As shown in
Figure 1b, our system consists of an
fcc phase (
afcc = 3.601(1) Å) and a small (<10%) amount of
bcc phase (
abcc = 2.867(4)). The second
fcc2 phase (
= 3.609(2) Å) reported in
Figure 7a can be considered a minor admixture in an exsolution equilibrium with the
bcc phase. Heating does not affect the primary phase: reflections become sharper, but their relative intensity remains similar. The addition of scandium (
Figure 7b) has no impact on the
fcc matrix, but influences the
bcc →
fcc2 equilibrium by shifting it towards the formation of
bcc.
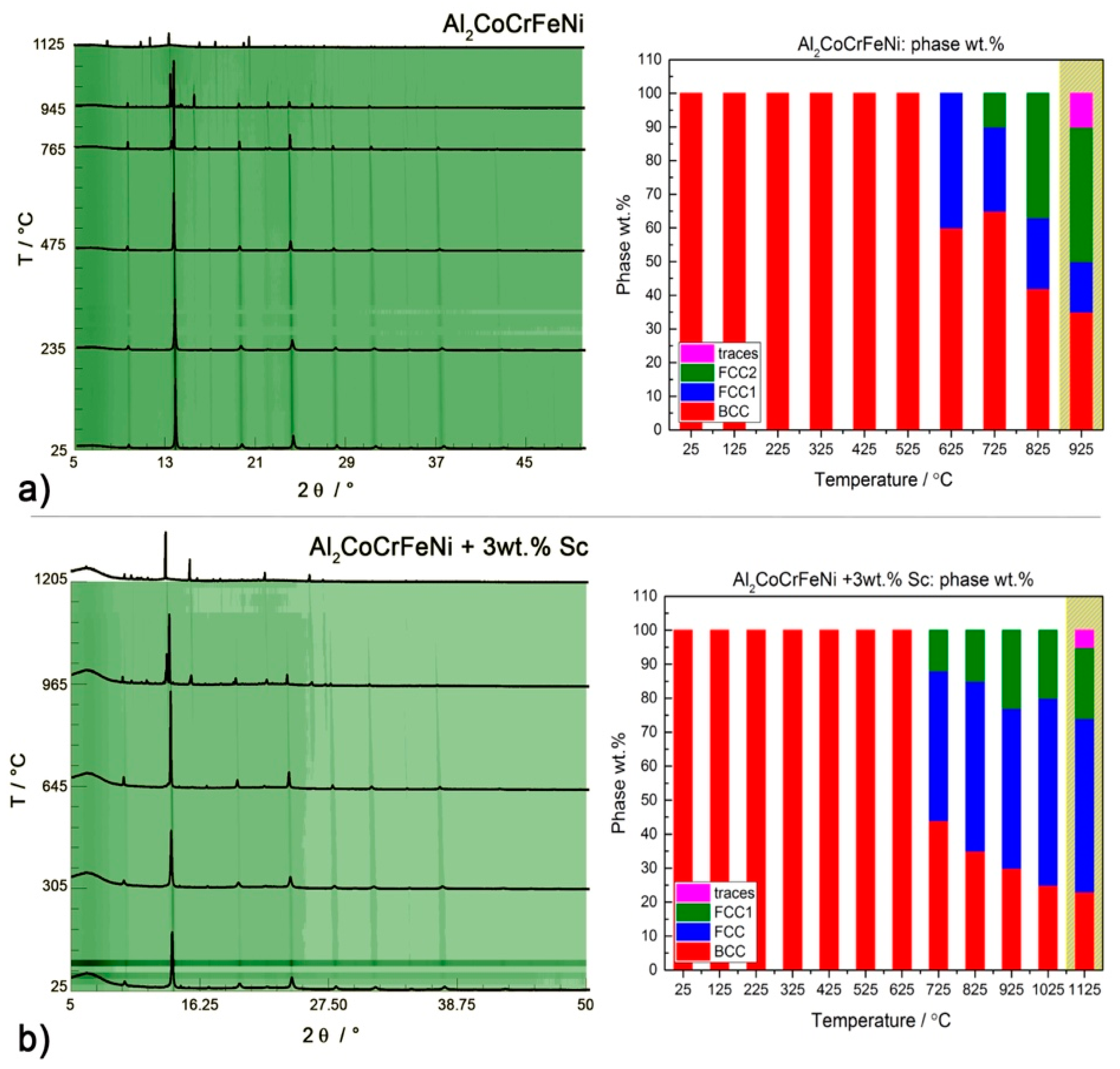
The thermal behavior of the CsCl-type Al
2CoCrFeNi sample is displayed in
Figure 8a. We observe the decomposition of 40 wt.% of the CsCl-type phase above 620 °C and the exsolution of an
fcc phase (
= 3.635(8) Å). At 750 °C a second
fcc2 phase of lattice parameter
= 3.631(9) Å develops from
fcc1 and grows almost linearly with temperature. The continuous presence of the (100) diffraction line in the PXRD pattern of the alloy suggests that the Al-rich sub-structure responsible for the CsCl-type crystal structure might not be involved in the exsolution process. Results for the Sc-containing Al
2CoCrFeNi alloy are presented in
Figure 8b. The exsolution of the
fcc phase from the CsCl-type one follows a different pathway, with
fcc1 and
fcc2 forming at the same time. The transformation occurs at higher temperatures: a 3 wt.% scandium addition is enough to stabilize the main CsCl-type phase for about 150 °C.
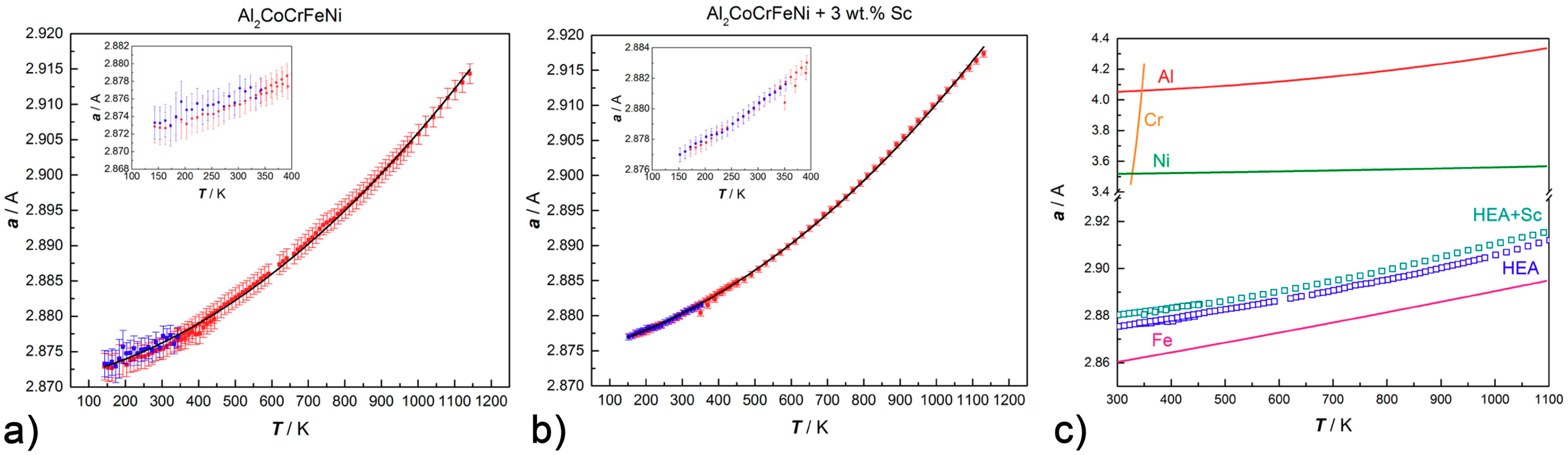
The thermal expansion coefficient of Al
2CoCrFeNi and Al
2CoCrFeNi + 3 wt.% Sc can be evaluated by in situ high-temperature PXRD measurements by fitting the corresponding dataset to:
where
is the cell parameter α at the reference temperature (T
o).
Figure 9 reports the variation of the lattice parameter
upon heating and cooling. A satisfactory fitting can be obtained for Al
2CoCrFeNi for α and β equalling 3.6(2) × 10
−6·K
−1 and 1.69(3) × 10
−8·K
−2, respectively (
Figure 9a); and for Al
2CoCrFeNi + 3 wt.% Sc for α and β equaling 4.2(1) × 10
−6× K
−1 and 1.62(2) × 10
−8 ×K
−2, respectively (
Figure 9b). As highlighted in
Figure 9c, the scandium-containing CsCl-type phase has slightly larger cell parameters than the regular alloy and follows the same trend with temperature. This trend is consistent to the one of pure iron in the investigated temperature range, but differs strongly from the behavior of other cubic metals which constitute the alloy (in particular, from chromium and nickel) [
48,
49,
50,
51].
The addition of scandium to the Al-containing HEAs results in the precipitation of a ternary intermetallic of MgZn2-type along the grain boundaries. Microstructural data as well as high-temperature PXRD suggest an extraordinary stability of Sc-based precipitates, which form in all alloys containing aluminum and first raw transition metals. The matrix coherence following the formation of the secondary phase is maintained and the Vickers hardness increases up to 20% due to precipitation hardening (for 0.5 wt.% Sc addition to Al2CoCrFeNi). The intermetallic phase has high thermal stability and affects the bcc → fcc exsolution equilibrium by stabilizing the body-centered cubic phase with respect to the face-centered cubic one. This effect is likely related to the segregation of part of the fcc-stabilizing elements (i.e., Ni and Co) in the ternary compound. Out of bcc-stabilizers, aluminum segregates in the MgZn2-type phase, but chromium has much lower affinity for the secondary phase and coalesces in the matrix (as highlighted by EDX maps). Lattice thermal expansion data of the CsCl-type alloy with or without scandium show that both systems follow the same trend upon heating. However, the cubic phase in the scandium-containing HEA has slightly larger cell parameters: this is a further indication of the compositional difference between the matrix of the two systems.
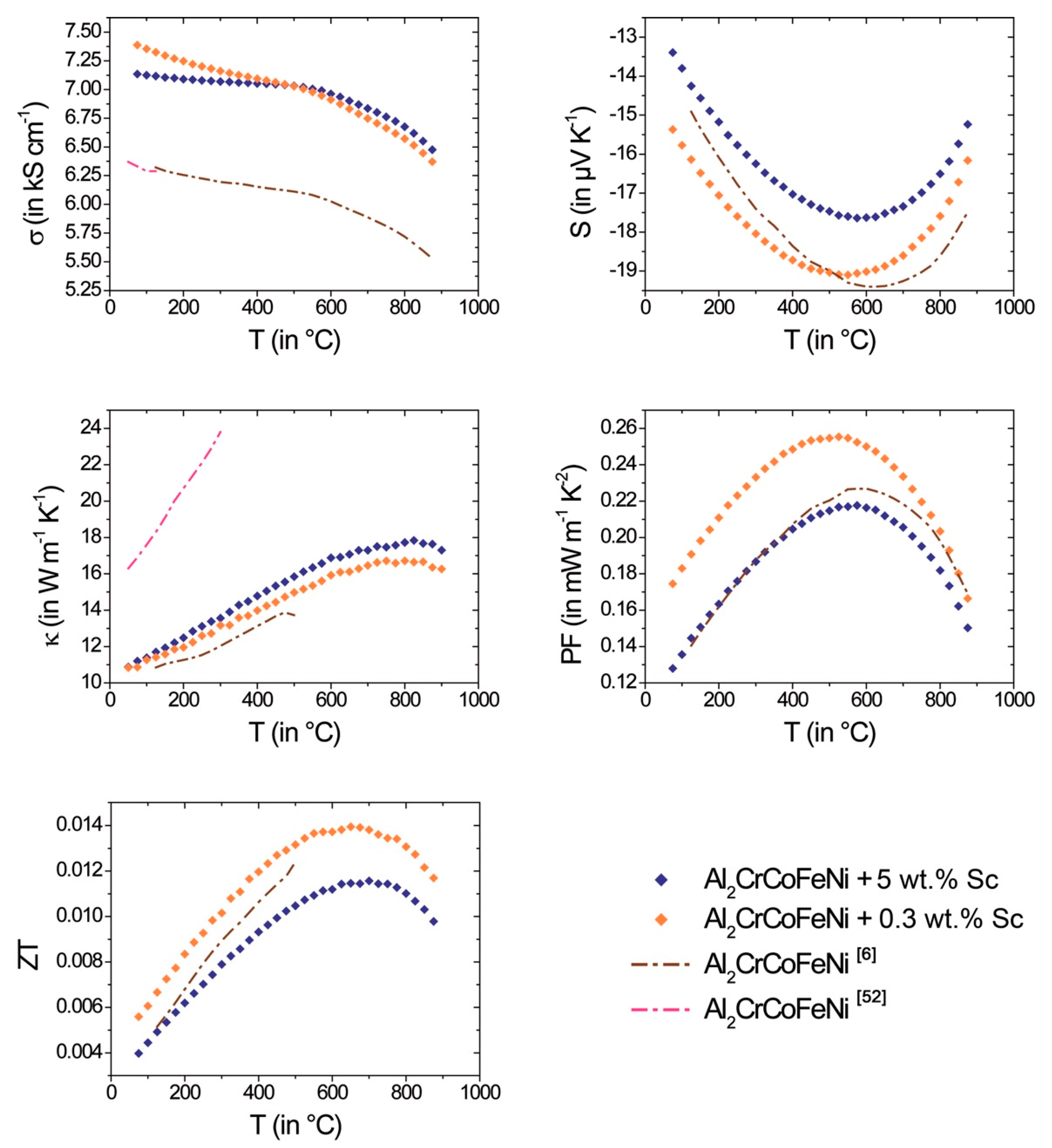
While the thermoelectric properties of Al
xCoCrFeNi (0 ≤
x ≤ 3) [
6,
30,
52] are discussed in detail in literature, the effect of Sc alloying on the transport properties of Al
2CoCrFeNi is herein reported for the first time (
Figure 10). The absolute Seebeck coefficient of
n-type materials decreases slightly with increasing Sc content. The electrical conductivity for both compositions increases by about 1000 S·cm
−1 compared to pure Al
2CoCrFeNi [
6,
52]. In accordance with the higher electrical conductivity, the thermal conductivity shows similar, also slightly increased values compared to ref. [
6]; whereas the thermal conductivity for pure Al
2CrCoFeNi from ref. [
52] is extraordinarily high.
Power factor and
ZT value increase with temperature until a peak
ZT value of 0.012 at 700 °C (for 5 wt.% Sc addition) and 0.014 at 650 °C (for 0.3 wt.% Sc) is reached. The subsequent decrease of
ZT with temperature is mainly attributed to the decrease of the absolute value of
S due to excitation of minority charge carriers. According to PXRD patterns of the samples after thermoelectric measurements, no degradation was observed (
Figure S12). In addition, the samples show a good cyclability within three subsequent heating and cooling cycles (
Figures S13 and S14).