Association between Multiscale Entropy Characteristics of Heart Rate Variability and Ischemic Stroke Risk in Patients with Permanent Atrial Fibrillation
Abstract
:1. Introduction
2. Data and Methods
2.1. Patients and HRV Time Series
2.2. Multiscale Entropy (MSE) Analysis
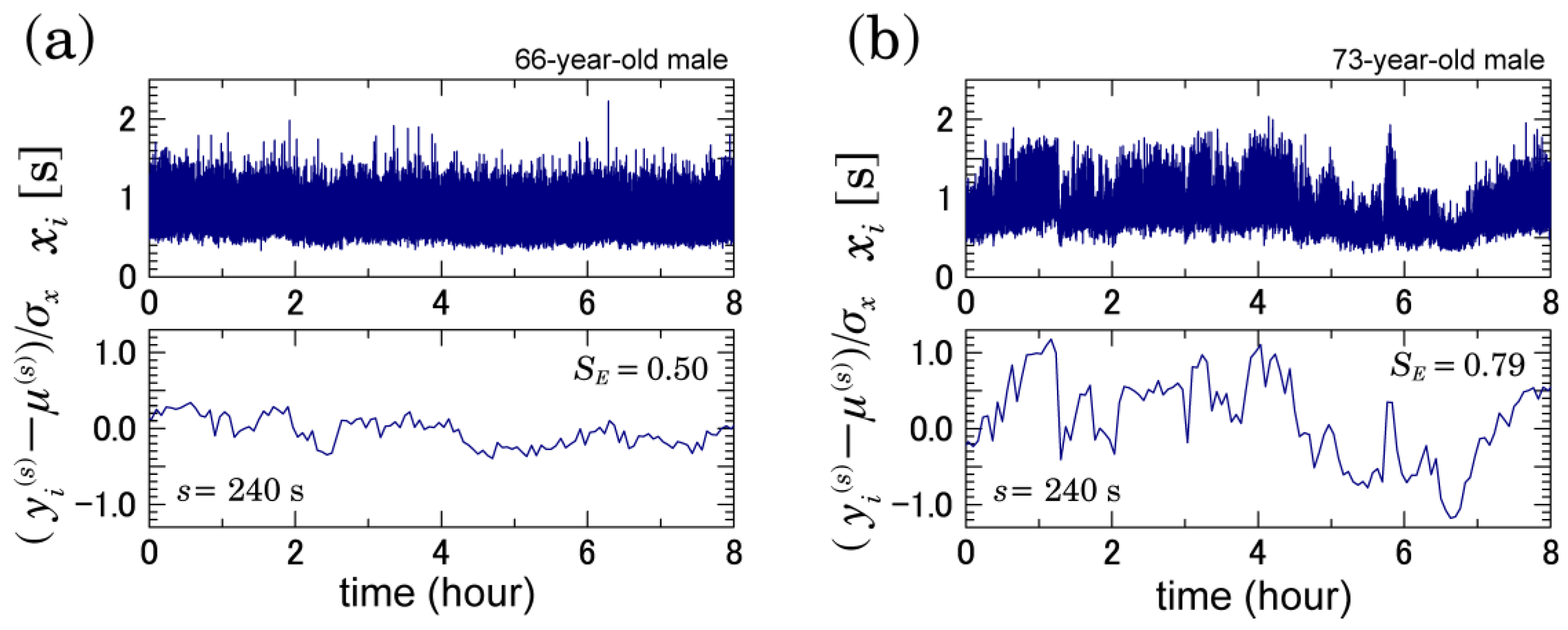
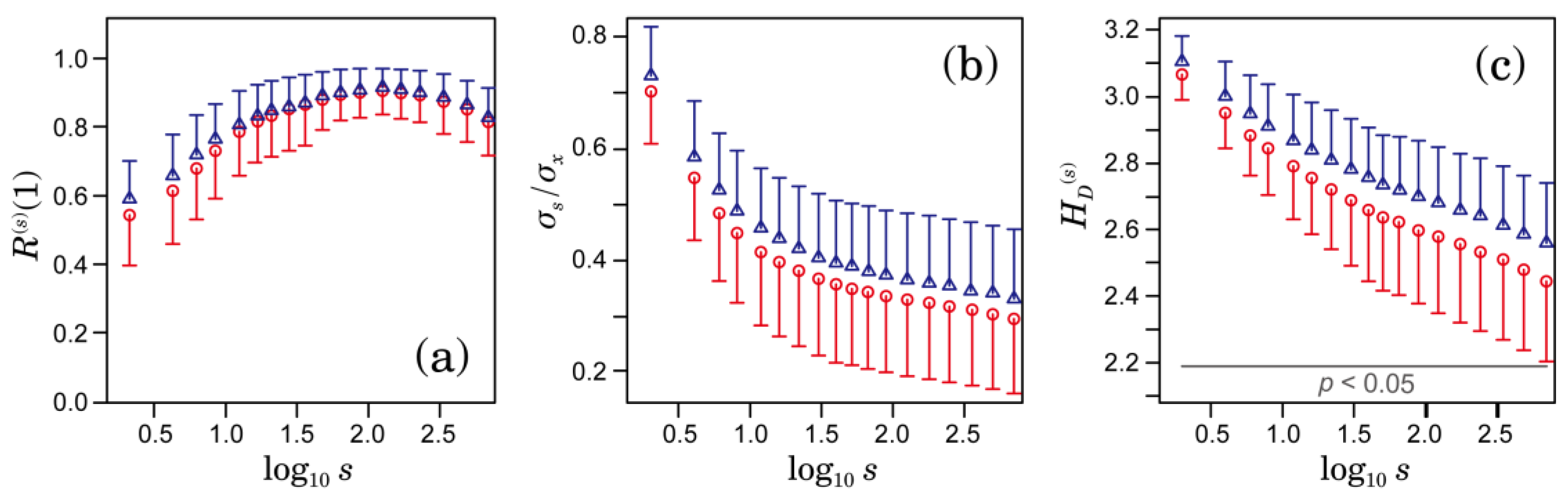
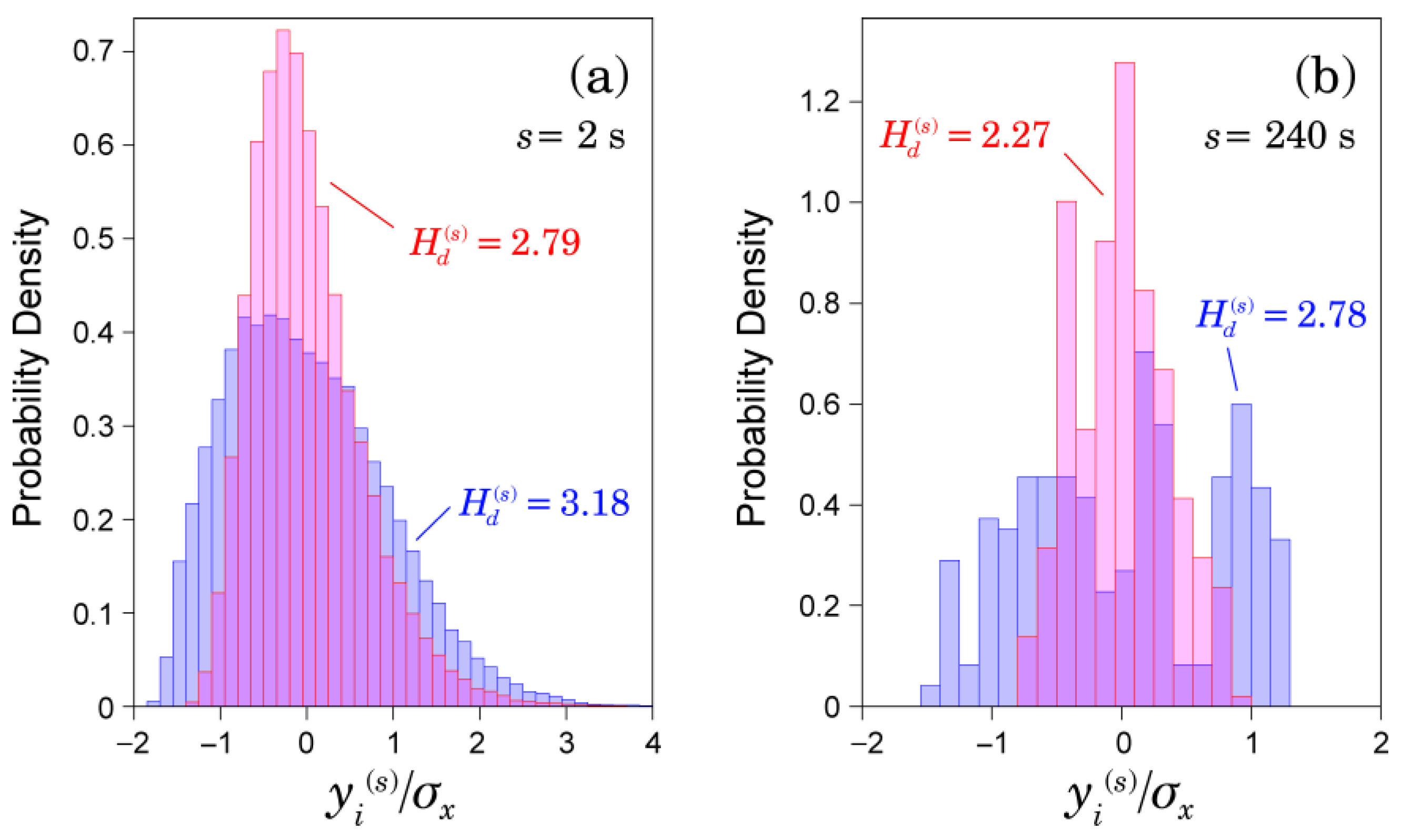
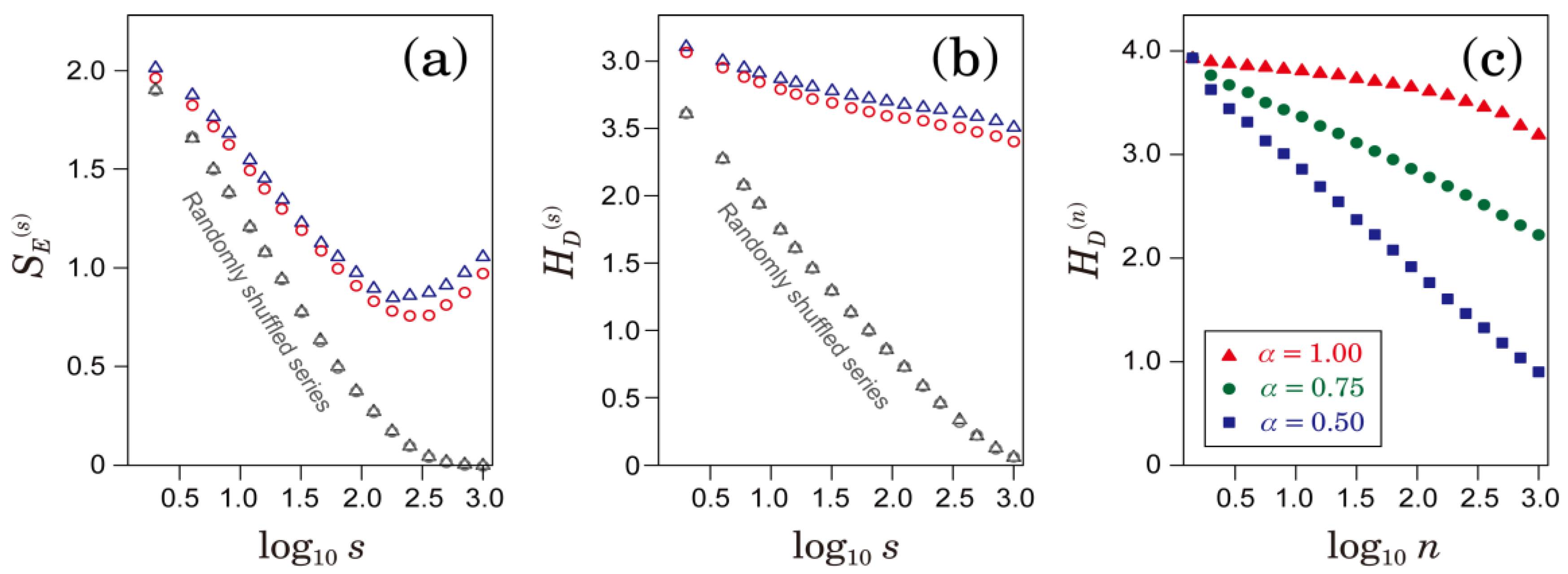
2.3. Multiscale Characterizations of Time Series
2.4. Statistical Analysis
3. Results
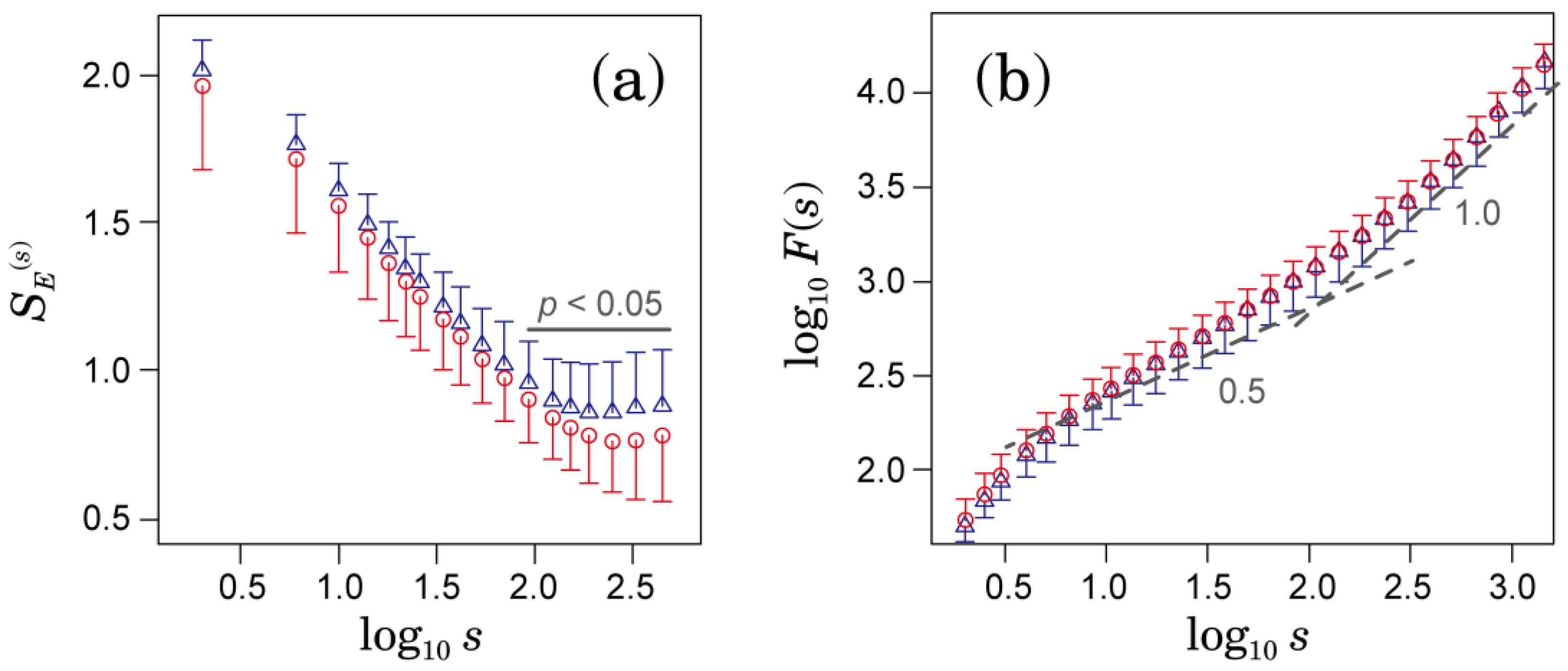
3.1. Multiscale Characteristics of HRV in Patients with AFib
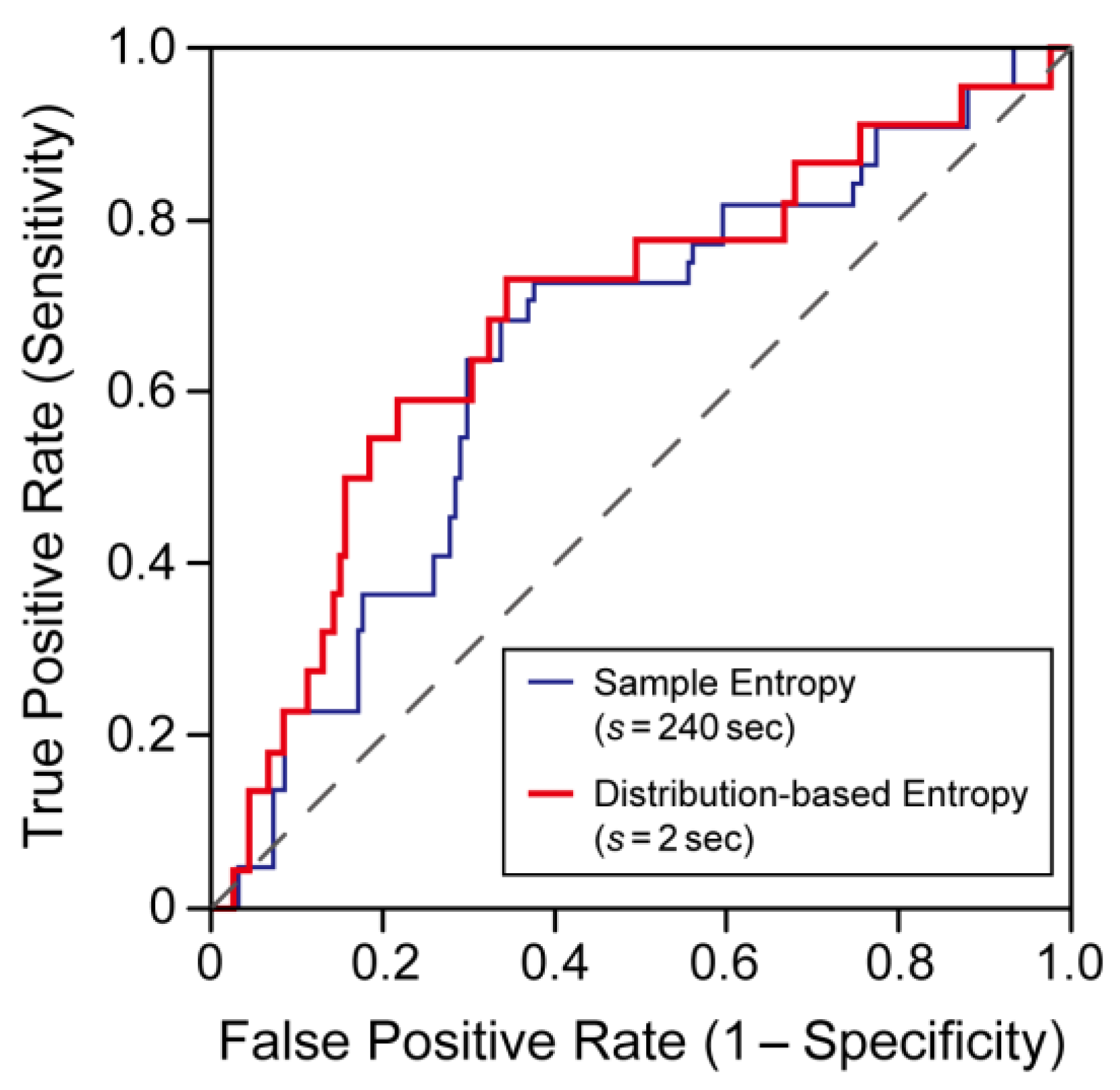
3.2. Comparison of Predictive Performance for Ischemic Stroke
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kobayashi, M.; Musha, T. 1/f fluctuation of heartbeat period. IEEE Trans. Biomed. Eng. 1982, 6, 456–457. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Goldberger, A.L.; Peng, C.K. Multiscale entropy analysis of complex physiologic time series. Phys. Rev. Lett. 2002, 89, 068102. [Google Scholar] [CrossRef] [PubMed]
- Pincus, S.M. Assessing serial irregularity and its implications for health. Ann. N. Y. Acad. Sci. 2001, 954, 245–267. [Google Scholar] [CrossRef] [PubMed]
- Lipsitz, L.A.; Goldberger, A.L. Loss of “complexity” and aging: Potential applications of fractals and chaos theory to senescence. JAMA 1992, 267, 1806–1809. [Google Scholar] [CrossRef] [PubMed]
- Richman, J.S.; Moorman, J.R. Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H2039–H2049. [Google Scholar] [PubMed]
- Costa, M.; Goldberger, A.L.; Peng, C.K. Multiscale entropy analysis of biological signals. Phys. Rev. E 2005, 71, 021906. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, E.; Kiyono, K.; Hayano, J.; Yamamoto, Y.; Inamasu, J.; Yamamoto, M.; Ichikawa, T.; Sobue, Y.; Harada, M.; Ozaki, Y. Multiscale entropy of the heart rate variability for the prediction of an ischemic stroke in patients with permanent atrial fibrillation. PLoS ONE 2015, 10, e0137144. [Google Scholar] [CrossRef] [PubMed]
- Marini, C.; De Santis, F.; Sacco, S.; Russo, T.; Olivieri, L.; Totaro, R.; Carolei, A. Contribution of atrial fibrillation to incidence and outcome of ischemic stroke. Stroke 2005, 36, 1115–1119. [Google Scholar] [CrossRef] [PubMed]
- Hayano, J.; Sakata, S.; Okada, A.; Mukai, S.; Fujinami, T. Circadian rhythms of atrioventricular conduction properties in chronic atrial fibrillation with and without heart failure. J. Am. Coll. Cardiol. 1998, 31, 158–166. [Google Scholar] [CrossRef]
- Hayano, J.; Ishihara, S.; Fukuta, H.; Sakata, S.; Mukai, S.; Ohte, N.; Kimura, G. Circadian rhythm of atrioventricular conduction predicts long-term survival in patients with chronic atrial fibrillation. Chronobiol. Int. 2002, 19, 633–648. [Google Scholar] [CrossRef] [PubMed]
- Hayano, J.; Yamasaki, S.; Sakata, S.; Okada, A.; Mukai, S.; Fujinami, T. Spectral characteristics of ventricular response to atrial fibrillation. Am. J. Physiol. Heart Circ. Physiol. 1997, 273, H2811–H2816. [Google Scholar]
- Ducharme, S.W. Quantifying Gait Adapatablity: Fractality, Complexity, and Stability during Asymmetric Walking. Ph.D. Thesis, University of Massachusetts Amherst, Amherst, MA, USA, 2017. [Google Scholar]
- Goldberger, A.L.; Amaral, L.A.; Hausdorff, J.M.; Ivanov, P.C.; Peng, C.K.; Stanley, H.E. Fractal dynamics in physiology: Alterations with disease and aging. Proc. Natl. Acad. Sci. USA 2002, 99, 2466–2472. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, C.; Udhayakumar, R.K.; Li, P.; Venkatesh, S.; Palaniswami, M. Stability, consistency and performance of distribution entropy in analyzing short length heart rate variability (HRV) signal. Front. Physiol. 2017, 8, 720. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.K.; Havlin, S.; Stanley, H.E.; Goldberger, A.L. Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series. Chaos 1995, 5, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Kiyono, K. Establishing a direct connection between detrended fluctuation analysis and Fourier analysis. Phys. Rev. E 2015, 92, 042925. [Google Scholar] [CrossRef] [PubMed]
- Sassi, R.; Cerutti, S.; Lombardi, F.; Malik, M.; Huikuri, H.V.; Peng, C.K.; Schmidt, G.; Yamamoto, Y. Advances in heart rate variability signal analysis: joint position statement by the e-Cardiology ESC Working Group and the European Heart Rhythm Association co-endorsed by the Asia Pacific Heart Rhythm Society. Europace 2015, 17, 1341–1353. [Google Scholar] [CrossRef] [PubMed]
- Kiyono, K. Log-amplitude statistics of intermittent and non-Gaussian time series. Phys. Rev. E 2009, 79, 031129. [Google Scholar] [CrossRef] [PubMed]
- Kiyono, K.; Tsujimoto, Y. Time and frequency domain characteristics of detrending-operation-based scaling analysis: Exact DFA and DMA frequency responses. Phys. Rev. E 2016, 94, 012111. [Google Scholar] [CrossRef] [PubMed]
- Leonarduzzi, R.; Abry, P.; Wendt, H.; Kiyono, K.; Yamamoto, Y.; Watanabe, E.; Hayano, J. Scattering Transform of Heart Rate Variability for the Prediction of Ischemic Stroke in Patients with Atrial Fibrillation. Methods Inf. Med. 2016, in press. [Google Scholar]
- Huang, N.; Shen, Z.; Long, S.R.; Wu, M.L.C.; Shih, H.H.; Zheng, Q.; Yen, N.C.; Tung, C.-C.; Liu, H.H. The empirical mode decomposition and the Hilbert spectrum for nonlinear and non-stationary time series analysis. Proc. R. Soc. Lond. A 1998, 454, 903. [Google Scholar] [CrossRef]
- Goldstein, L.B.; Bushnell, C.D.; Adams, R.J.; Appel, L.J.; Braun, L.T.; Chaturvedi, S.; Creager, M.A.; Culebras, A.; Eckel, R.H.; Hart, R.G.; et al. Guidelines for the primary prevention of stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011, 42, 517–584. [Google Scholar] [CrossRef] [PubMed]
| Clinical Characteristics | Ischemic Stroke (n = 22) | Non-Ischemic Stroke (n = 151) | p-Value |
|---|---|---|---|
| Age | 71 ± 8 | 69 ± 11 | 0.35 |
| Female, n (%) | 7 (32) | 43 (28) | 0.26 |
| Underlying disease, n (%) | - | - | - |
| Congestive heart failure | 9 (41) | 56 (37) | 0.72 |
| Hypertension | 15 (68) | 88 (58) | 0.38 |
| Diabetes | 1 (5) | 14 (9) | 0.46 |
| Stroke or TIA | 9 (41) | 40 (26) | 0.16 |
| Vascular disease | 2 (9) | 15 (10) | 0.90 |
| Medications, n (%) | - | - | - |
| Beta-blocker | 4 (18) | 24 (16) | 0.79 |
| Digitalis | 8 (36) | 65 (43) | 0.55 |
| Ca-channnel blocker | 7 (32) | 35 (23) | 0.38 |
| ACE inhibitor | 9 (41) | 37 (25) | 0.47 |
| Diuretics | 5 (23) | 56 (37) | 0.75 |
| Warfarin | 10 (45) | 83 (55) | 0.40 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuoka, R.; Yoshino, K.; Watanabe, E.; Kiyono, K. Association between Multiscale Entropy Characteristics of Heart Rate Variability and Ischemic Stroke Risk in Patients with Permanent Atrial Fibrillation. Entropy 2017, 19, 672. https://doi.org/10.3390/e19120672
Matsuoka R, Yoshino K, Watanabe E, Kiyono K. Association between Multiscale Entropy Characteristics of Heart Rate Variability and Ischemic Stroke Risk in Patients with Permanent Atrial Fibrillation. Entropy. 2017; 19(12):672. https://doi.org/10.3390/e19120672
Chicago/Turabian StyleMatsuoka, Ryo, Kohzoh Yoshino, Eiichi Watanabe, and Ken Kiyono. 2017. "Association between Multiscale Entropy Characteristics of Heart Rate Variability and Ischemic Stroke Risk in Patients with Permanent Atrial Fibrillation" Entropy 19, no. 12: 672. https://doi.org/10.3390/e19120672
APA StyleMatsuoka, R., Yoshino, K., Watanabe, E., & Kiyono, K. (2017). Association between Multiscale Entropy Characteristics of Heart Rate Variability and Ischemic Stroke Risk in Patients with Permanent Atrial Fibrillation. Entropy, 19(12), 672. https://doi.org/10.3390/e19120672





