Thermodynamic Analysis about Nucleation and Growth of Cubic Boron Nitride Crystals in the hBN-Li3N System under High Pressure and High Temperature †
Abstract
:1. Introduction
2. Experiments and Calculation
2.1. Experiments Section
2.2. Calculations
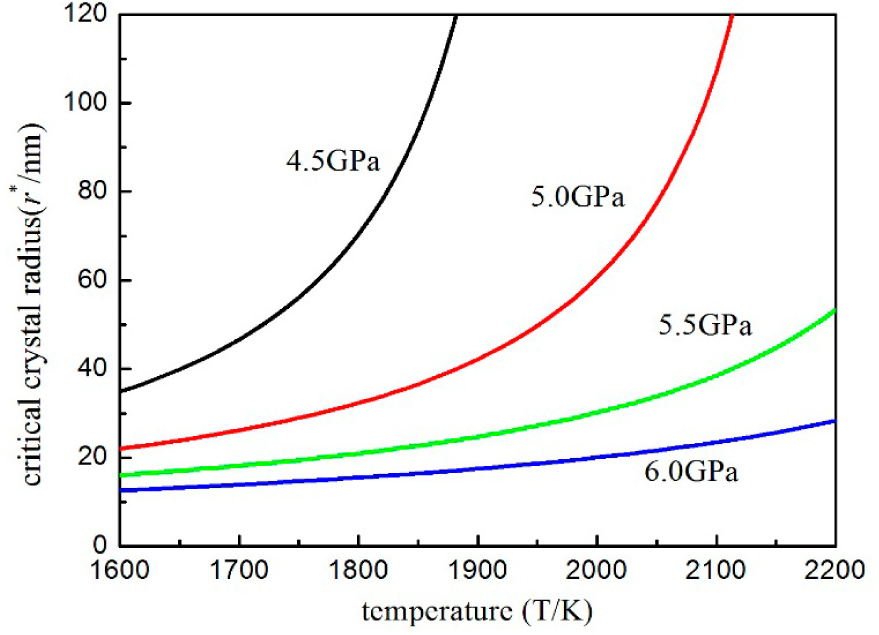
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Devries, R.C.; Fleischer, J.F. The system Li3BN2 at high pressures and temperatures. Mater. Res. Bull. 1969, 4, 433–441. [Google Scholar]
- Solozhenko, V.L.; Lazarenko, A.G.; Petitet, J.P.; Kanaev, A.V. Bandgap energy of graphite-like hexagonal boron nitride. J. Phys. Chem. Solids. 2001, 62, 1331–1334. [Google Scholar]
- Solozhenko, V.L.; Gregoryanz, E. Synthesis of superhard materials. Mater. Today. 2005, 8, 44–51. [Google Scholar]
- Wang, X.C.; Jia, X.P.; Zhang, T.C. cBN synthesis in the system of hBN-Mg and bonded water. Diam. Relat. Mater. 2003, 12, 57–60. [Google Scholar]
- Taniguchi, T.; Yamaoka, S. Spontaneous nucleation of cubic boron nitride single crystal by temperature gradient method under high pressure. J. Cryst. Growth. 2001, 222, 549–557. [Google Scholar]
- Bocquillon, G.; Loriers-Susse, C.; Loriers, J. Synthesis of cubic boron nitride using Mg and pure or M’-doped Li3N, Ca3N2 and Mg3N2 with M’ = Al, B, Si, Ti. J. Mater. Sci. 1993, 28, 3547–3556. [Google Scholar]
- Lorenz, H.; Orgzall, I.; Hinze, E. Rapid formation of cubic boron nitride in the system Mg3N2-BN. Diam. Relat. Mater. 1995, 4, 1050–1055. [Google Scholar]
- Taniguchi, T.; Watanabe, K. Synthesis of high-purity boron nitride single crystals under high pressure by using Ba-BN solvent. J. Cryst. Growth. 2007, 303, 525–529. [Google Scholar]
- Bundy, F.P.; Wentorf, R.H. Direct transformation of hexagonal boron nitride to denser forms. J. Chem. Phys. 1963, 38, 1144–1149. [Google Scholar]
- Fukunaga, O. The equilibrium phase boundary between hexagonal and cubic boron nitride. Diam. Relat. Mater. 2000, 9, 7–12. [Google Scholar]
- Mullin, J.W. Crystallization, 3rd ed; World Publishing Corporation: Beijing, China, 2000; pp. 182–190. [Google Scholar]
- Solozhenko, V.L.; Turkevich, V.Z.; Holzapfel, W.B. Refined phase diagram of boron nitride. J. Phys. Chem. B 1999, 103, 2903–2905. [Google Scholar]
- Lorenz, H.; Lorzen, B.; Kuhune, U. The kinetics of cubic boron nitride formation in the system BN-Mg3N2. J. Mater. Sci. 1988, 23, 3254–3257. [Google Scholar]
- Xu, B.; Li, M.S.; Cui, J.J.; Gong, J.H.; Wang, S.H. An investigation of a thin metal film covering on HPHT as-grown diamond from Fe–Ni–C system. Mater. Sci. Eng. A 2005, 396, 352–359. [Google Scholar]
- Fukunaga, O.; Nakano, S.; Taniguchi, T. Nucleation and growth of cubic boron nitride using a Ca-B-N solvent. Diam. Relat. Mater. 2004, 13, 1709–1713. [Google Scholar]
- Li, S.; Guo, X.F.; Xu, B.; Wang, H. Fracture morphology and XRD layered characterization of cBN cake. J. Synth. Cryst. 2012, 41, 15–19, In Chinese. [Google Scholar]
- Xu, B.; Lv, M.Z.; Yang, H.M.; Wen, Z.X. Thermodynamic Analysis of the V-Shaped Area of High Pressure and High Temperature in Cubic Boron Nitride Synthesis with Li3N as a Catalyst. Entropy 2014, 16, 912–920. [Google Scholar]
- Yamane, H.; Kikkawa, S.; Koizumi, M. High-and low-temperature phases of lithium boron nitride, Li3BN2: Preparation, phase relation, crystal structure, and ionic conductivity. J. Solid State Chem 1987, 71, 1–11. [Google Scholar]
- Guo, W. Effects of Additive on the Synthesis of High Quality cBN Single Crystal in the Li3N-hBN System. Ph.D. Thesis, Jilin University, Changchun, China, 2011. [Google Scholar]



© 2015 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.-F.; Xu, B.; Zhang, W.; Lv, M.-Z.; Yang, H.-M.; Fan, X.-H. Thermodynamic Analysis about Nucleation and Growth of Cubic Boron Nitride Crystals in the hBN-Li3N System under High Pressure and High Temperature. Entropy 2015, 17, 755-762. https://doi.org/10.3390/e17020755
Guo X-F, Xu B, Zhang W, Lv M-Z, Yang H-M, Fan X-H. Thermodynamic Analysis about Nucleation and Growth of Cubic Boron Nitride Crystals in the hBN-Li3N System under High Pressure and High Temperature. Entropy. 2015; 17(2):755-762. https://doi.org/10.3390/e17020755
Chicago/Turabian StyleGuo, Xiao-Fei, Bin Xu, Wen Zhang, Mei-Zhe Lv, Hong-Mei Yang, and Xiao-Hong Fan. 2015. "Thermodynamic Analysis about Nucleation and Growth of Cubic Boron Nitride Crystals in the hBN-Li3N System under High Pressure and High Temperature" Entropy 17, no. 2: 755-762. https://doi.org/10.3390/e17020755
APA StyleGuo, X.-F., Xu, B., Zhang, W., Lv, M.-Z., Yang, H.-M., & Fan, X.-H. (2015). Thermodynamic Analysis about Nucleation and Growth of Cubic Boron Nitride Crystals in the hBN-Li3N System under High Pressure and High Temperature. Entropy, 17(2), 755-762. https://doi.org/10.3390/e17020755



