Self-oscillating Water Chemiluminescence Modes and Reactive Oxygen Species Generation Induced by Laser Irradiation; Effect of the Exclusion Zone Created by Nafion
Abstract
:1. Introduction
2. Results and Discussion
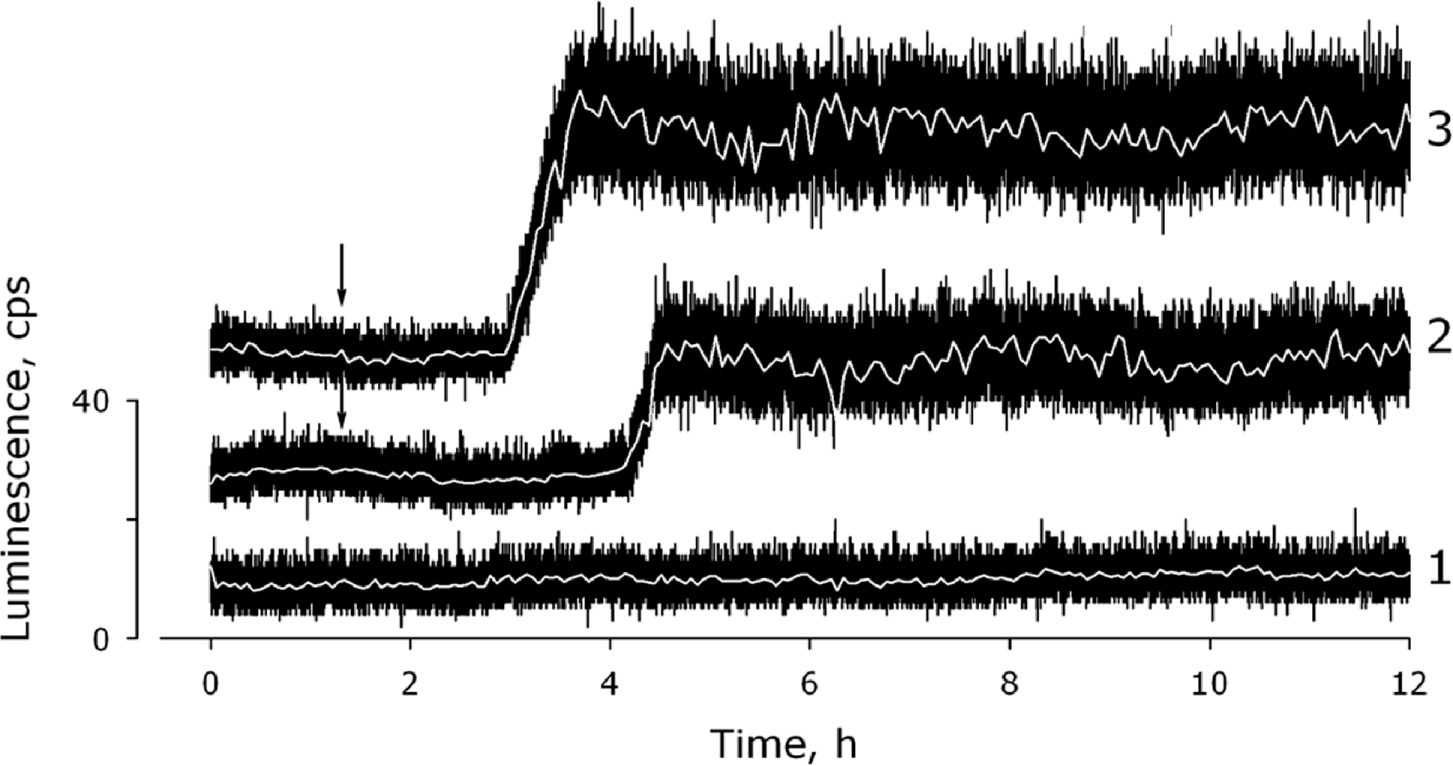
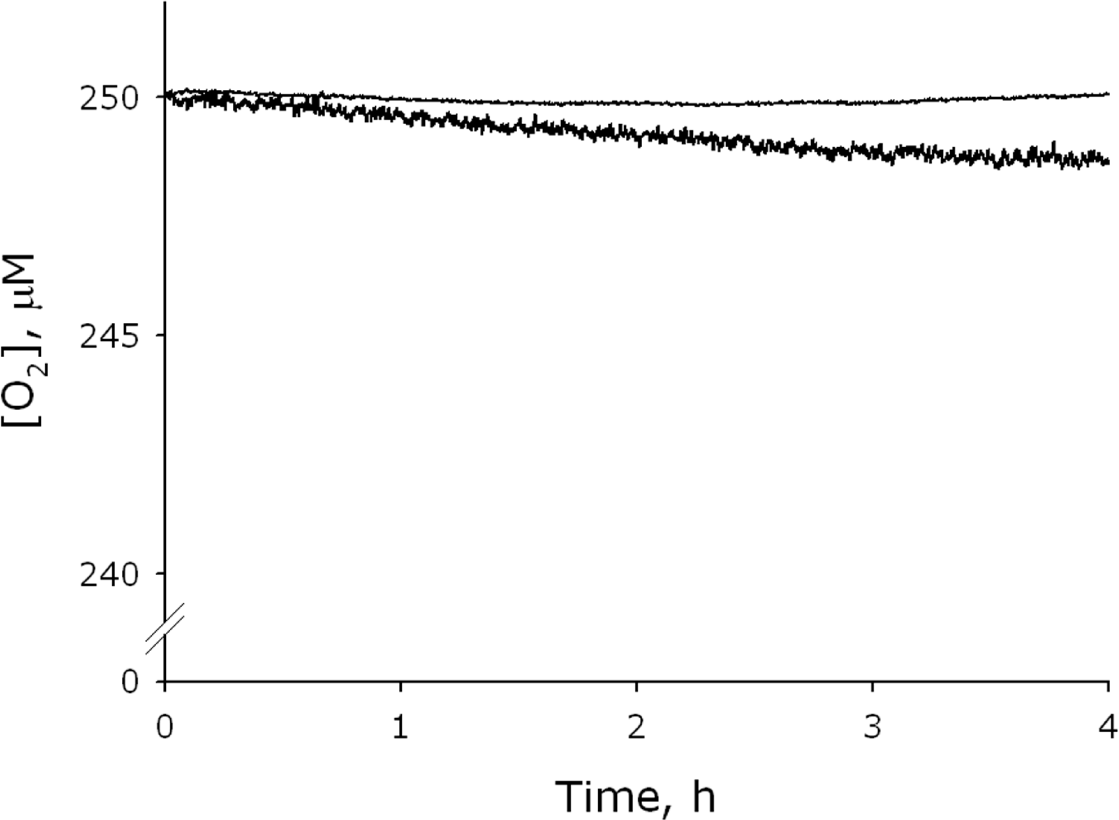
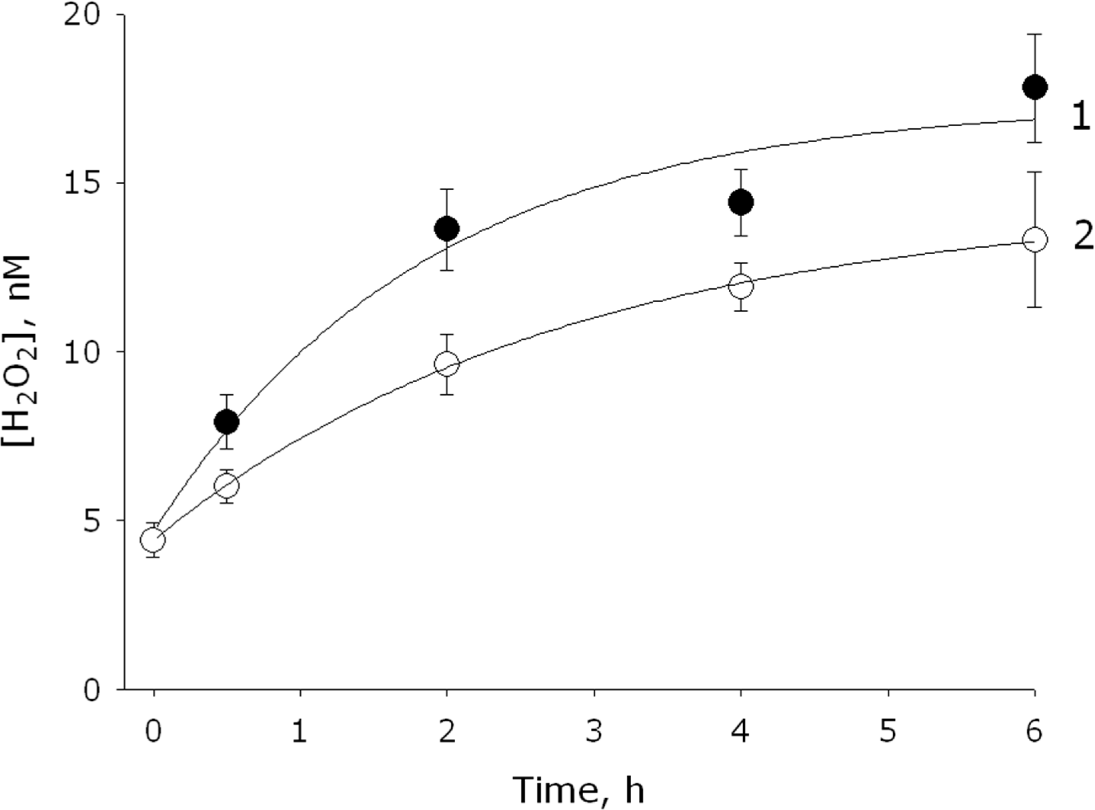
2.1. Measurement of the Chemiluminescence Intensity and the Hydrogen Peroxide Concentration
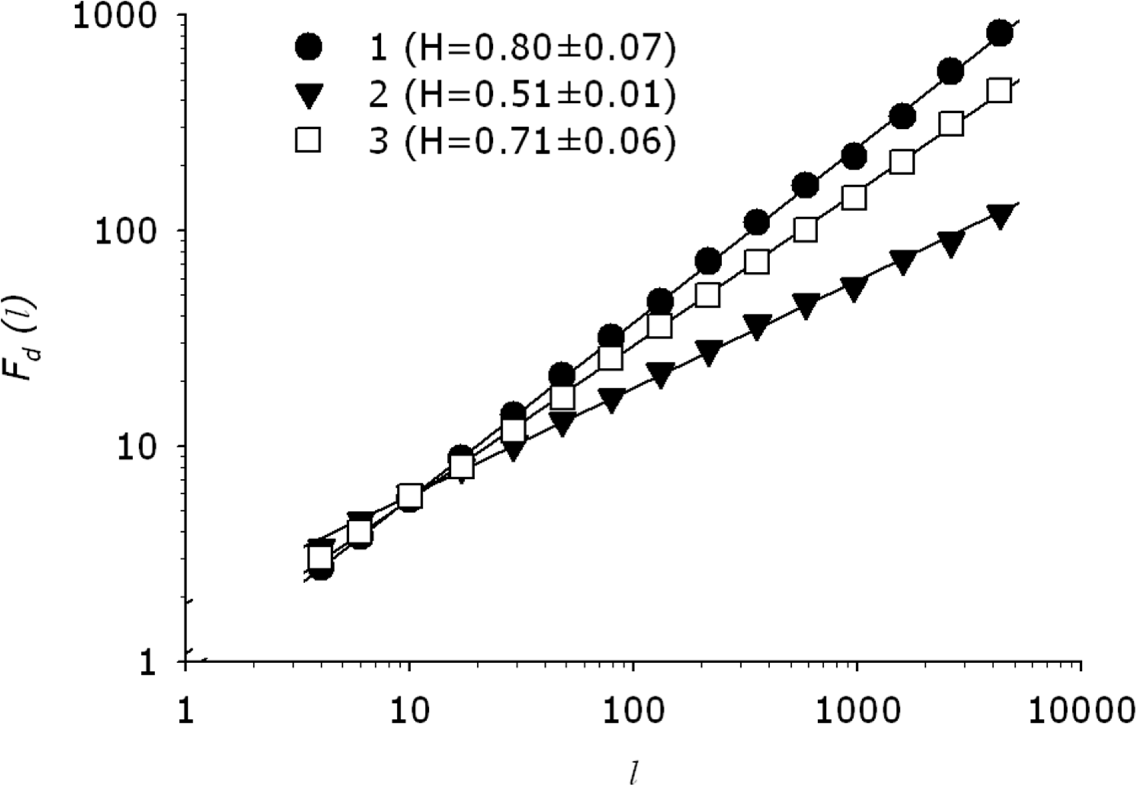
2.2. Time Series Analysis Using Detrended Fluctuation Analysis (DFA)
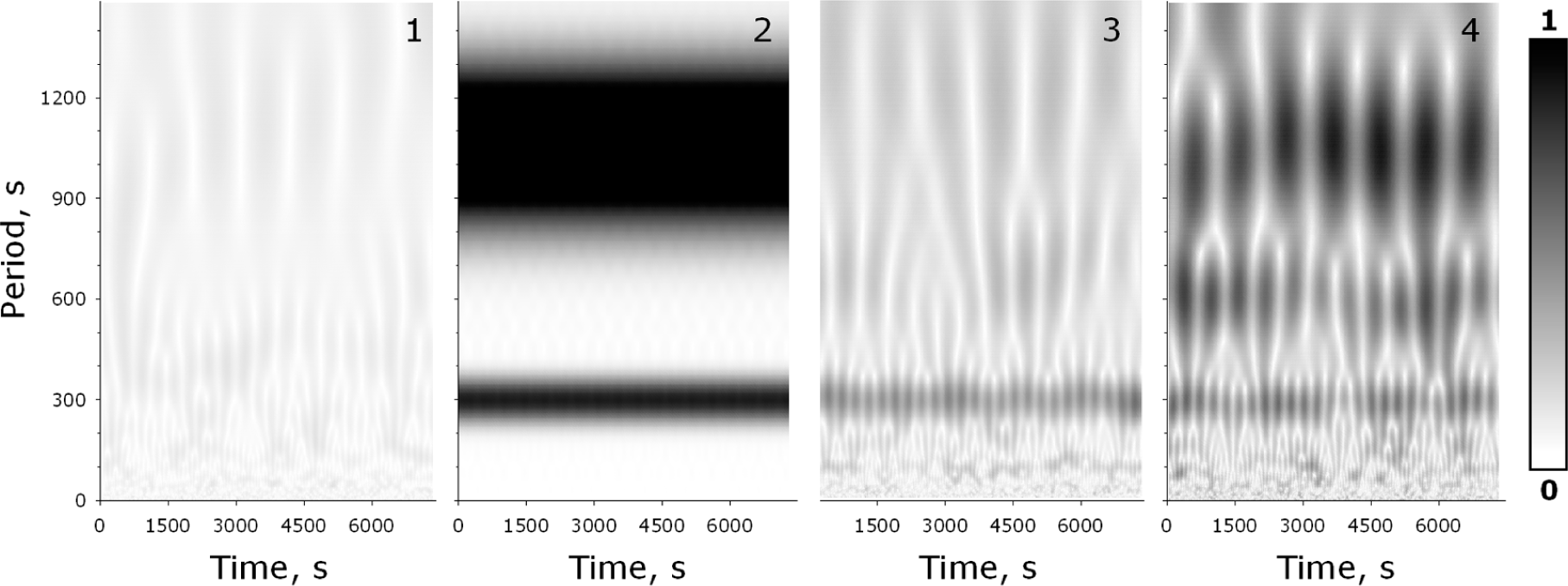
2.3. Time Series Analysis by Using the Wavelet Transform
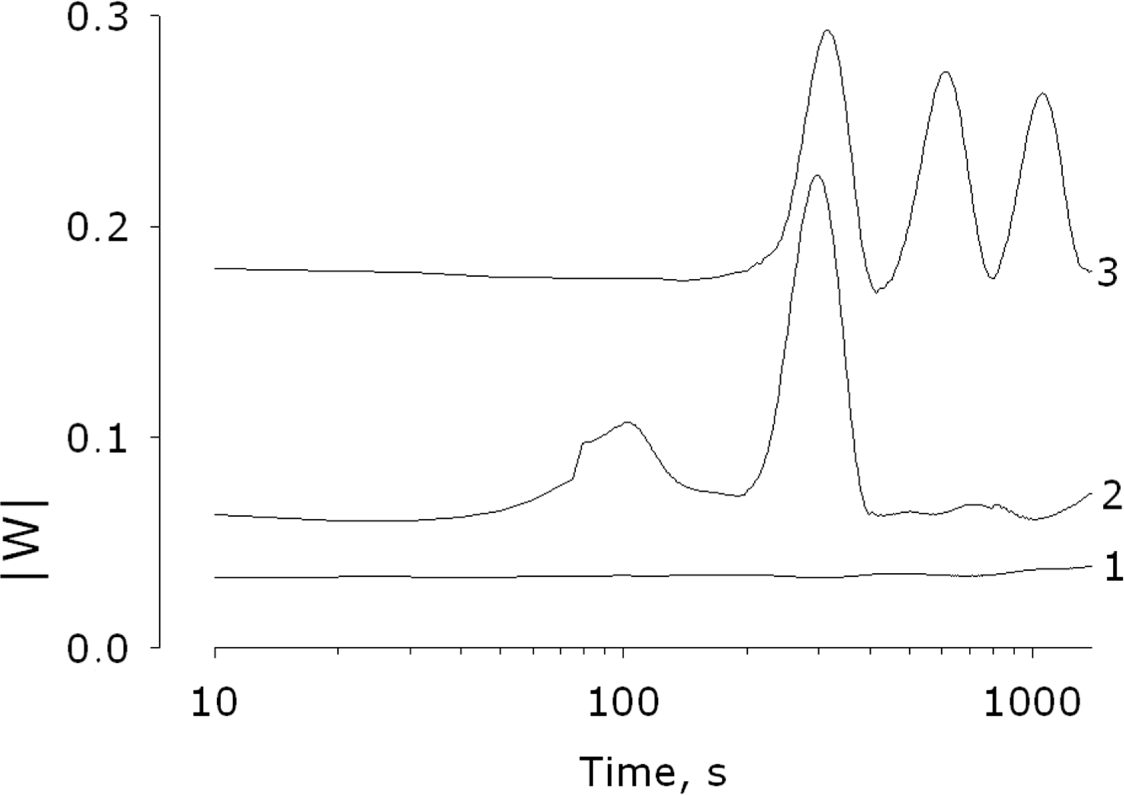
2.4. Analysis of the Coherence of the Self-Oscillating Modes
3. Experimental Section
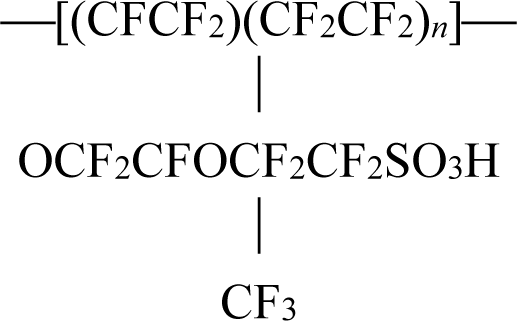
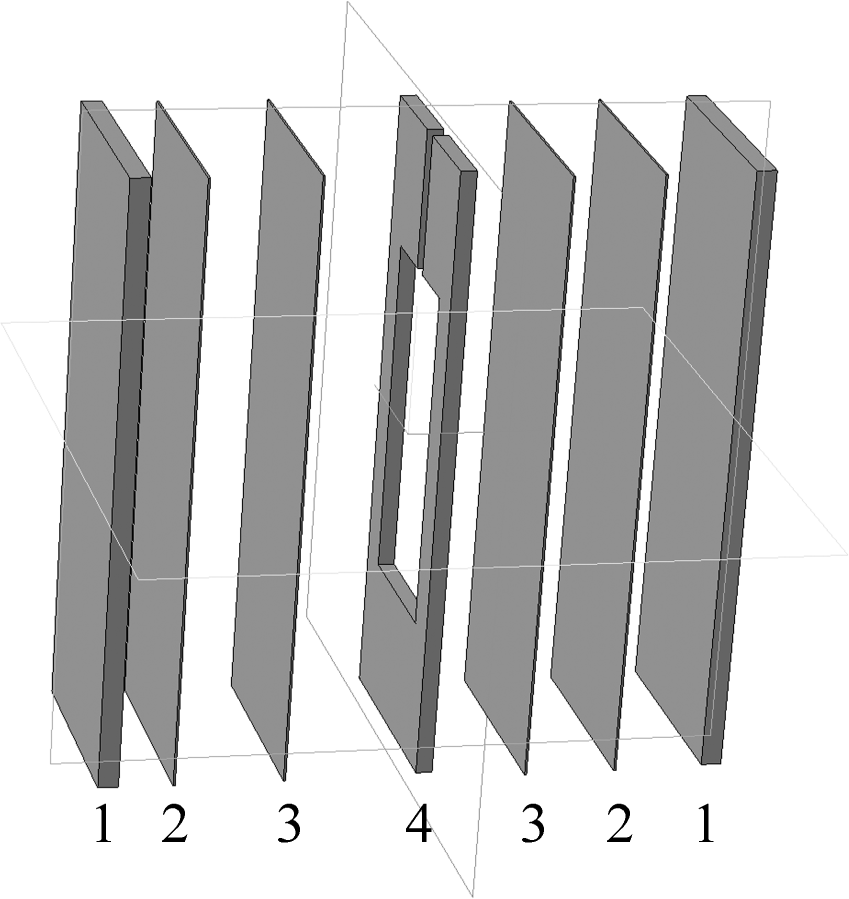
3.1. Sample Preparation
3.2. Irradiation
3.3. Measurement of Luminescence
3.4. Measurement of Hydrogen Peroxide
3.5. Method of Detrended Fluctuation Analysis
- Two integer random numbers (a and b) in the range 1/l were generated.
- The values of the series Xa and Xb were interchanged.
- Steps 2 and 3 were repeated 1000 × l times, which provided a complete mixing of the initial series.
3.6. Wavelet Transform
3.7. The Method of Cross-Correlation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Heitner-Wirguin, C. Recent advances in perfluorinated ionomer membranes: Structure, properties and applications. J. Membr. Sci. 1996, 120, 1–33. [Google Scholar]
- Mauritz, K.A.; Moore, R.B. State of understanding of Nafion. Chem. Rev. 2004, 104, 4535–4586. [Google Scholar]
- Ivanov, V.G.; Berestovskii, T.N. Lipidnyi Bisloi biologicheskih Membrane; Nauka: Moscow, USSR, 1982; In Russian. [Google Scholar]
- Ninham, B.W.; Lo Nostro, P. Intermolecular Forces and Self Assembly in Colloid, Nano Sciences and Biology; Cambridge University Press: New York, NY, USA, 2010. [Google Scholar]
- Chai, B.; Yoo, H.; Pollack, G.H. Effect of radiant energy on near-surface water. J. Phys. Chem. B. 2009, 113, 13953–13958. [Google Scholar]
- Chai, B.; Pollack, G.H. Solute-free interfacial zones in polar liquids. J. Phys. Chem. B. 2010, 114, 5371–5375. [Google Scholar]
- Bhalerao, A.S.; Pollack, G.H. Light-induced effects on Brownian displacements. J. Biophotonics. 2011, 4, 172–177. [Google Scholar]
- Yoo, H.; Paranji, R.; Pollack, G.H. Impact of hydrophilic surfaces on interfacial water dynamics probed with NMR spectroscopy. J. Phys. Chem. Lett. 2011, 2, 532–536. [Google Scholar]
- Yoo, H.; Baker, D.R.; Pirie, C.M.; Hovakeemian, B.; Pollack, G.H. Water: The Forgotten Biological Molecule; Pan Stanford Publishing: Denver, CO, USA, 2011. [Google Scholar]
- Zheng, J.; Pollack, G.H. Long-range forces extending from polymer-gel surfaces. Phys. Rev. E. 2003, 68, 031408. [Google Scholar]
- Chai, B.; Zheng, J.; Zhao, Q.; Pollack, G.H. Spectroscopic studies of solutes in aqueous solution. J. Phys. Chem. A. 2008, 112, 2242–2247. [Google Scholar]
- Zheng, J.; Chin, W.C.; Khijniak, E.; Khijniak, E., Jr.; Pollack, G.H. Surfaces and interfacial water: Evidence that hydrophilic surfaces have long-range impact. Adv. Colloid Interface 2006, 127, 19–27. [Google Scholar]
- Pollack, G.H. The Fourth Phase of Water: Beyond Solid, Liquid, and Vapor; Ebner and Sons Publishers: Seattle, WA, USA, 2013. [Google Scholar]
- Bunkin, N.F.; Kozlov, V.A.; Ignat’ev, P.S.; Suiazov, N.V.; Shkirin, A.V.; Atakhodzhaev, I.A. Refraction Coefficient of Water and Aqueous Solutions in the Optical Frequency Range in the Vicinity of Nafion. Biophysics 2012, 57, 733–749. [Google Scholar]
- Bunkin, N.F.; Ignatiev, P.S.; Kozlov, V.A.; Shkirin, A.V.; Zakharov, S.D.; Zinchenko, A.A. Study of the phase states of water close to Nafion interface. Water 2013, 4, 129–154. [Google Scholar]
- Bunkin, N.F.; Gorelik, V.S.; Kozlov, V.A.; Shkirin, A.V.; Suyazov, N.V. Colloidal crystal formation at the “Nafion-Water” interface. J. Phys. Chem. B. 2014, 118, 3372–3377. [Google Scholar]
- Gudkov, S.V.; Bruskov, V.I.; Astashev, M.E.; Chernikov, A.V.; Yaguzhinsky, L.S.; Zakharov, S.D. Oxygen-dependent auto-oscillations of water luminescence triggered by the 1264 nm radiation. J. Phys. Chem. B. 2011, 115, 7693–7698. [Google Scholar]
- Atkinson, R.; Baulch, D.L.; Cox, R.A.; Hampson, R.F., Jr.; Kerr, J.A.; Rossi, M.J.; Troe, J. Evaluated kinetic and photochemical data for atmospheric chemistry: Supplement VI. IUPAC subcommittee on gas kinetic data evaluation for atmospheric chemistry. J. Phys. Chem. Ref. Data. 1997, 26, 1329–1499. [Google Scholar]
- Schweitzer, C.; Schmidt, R. Physical mechanisms of generation and deactivation of singlet oxygen. Chem. Rev. 2003, 103, 1685–1758. [Google Scholar]
- Zakharov, S.D.; Ivanov, A.V. Light-oxygen effect in cells and its potential applications in tumour therapy (review). Quantum Electron 1999, 29, 1031–1053. [Google Scholar]
- Kuznetsova, L.A.; Kuz’menko, N.E.; Kozakov, W.E.; Plastinin, Y.A. The Probability of Optical Transitions of Diatomic Molecules; Nauka: Moscow, USSR, 1980; In Russian. [Google Scholar]
- Margulis, M.A. Sonochemistry and Cavitation; Gordon and Breach Science Publishers: Langhorne, PA, USA, 1995. [Google Scholar]
- Saksena, T.K.; Nyborg, W.L. Sonoluminescence from Stable Cavitation. J. Chem. Phys. 1970, 53, 1722–1734. [Google Scholar]
- Bruskov, V.I.; Gudkov, S.V.; Chalkin, S.F.; Smirnova, E.G.; Yaguzhinskii, L.S. Self-oscillating water luminescence induced by laser irradiation. Dokl. Biochem. Biophys. 2009, 425, 114–116. [Google Scholar]
- Morre, D.J.; Orczyk, J.; Hignite, H.; Kim, C. Regular oscillatory behavior of aqueous solutions of Cull salts related to edects on equilibrium dynamics of ortho/para hydrogen spin isomers of water. J. Inorg. Biochem. 2008, 102, 260–267. [Google Scholar]
- Bunkin, N.F.; Ninham, B.W.; Babenko, V.A.; Suyazov, N.V.; Sychev, A.A. Role of dissolved gas in optical breakdown of water: Differences between effects due to helium and other gases. J. Phys. Chem. B. 2010, 114, 7743–7752. [Google Scholar]
- Belosludtsev, K.N.; Belosludtseva, N.V.; Agafonov, A.V.; Astashev, M.E.; Kazakov, A.S.; Saris, N.E.; Mironova, G.D. Ca(2+)-dependent permeabilization of mitochondria and liposomes by palmitic and oleic acids: A comparative study. Biochim. Biophys. Acta. 2600–2606.
- Belosludtsev, K.N.; Belosludtseva, N.V.; Kondratyev, M.S.; Agafonov, A.V.; Purtov, Y.A. Interaction of phospholipase A of the E. coli outer membrane with the inhibitors of eucaryotic phospholipases A2 and their effect on the Ca2+-induced permeabilization of the bacterial membrane. J. Membr. Biol. 2014, 247, 281–288. [Google Scholar]
- Feder, J. Fractals; Plenum Press: New York, NY, USA, 1988. [Google Scholar]
- Peng, C.-K.; Buldyrev, S.V.; Havlin, S.; Simons, M.; Stanley, H.E.; Golberger, A.L. On the mosaic organization of DNA sequences. Phys. Rev. E. 1994. [Google Scholar]
- Rock, P.A. Chemical Thermodynamics; MacMillan: New York, NY, USA, 1969. [Google Scholar]
- Mössbauer, R.L. Kernresonanzfluoreszenz von Gammastrahlung in Ir191. Zeitschrift für Physik 1958, 151, 124–143, In German. [Google Scholar]
- Gudkov, S.V.; Shtarkman, I.N.; Chernikov, A.V.; Usacheva, A.M.; Bruskov, V.I. Guanosine and inosine (riboxin) eliminate the long-lived protein radicals induced X-ray radiation. Dokl Biochem. Biophys. 2007, 413, 50–53. [Google Scholar]
- Bruskov, V.I.; Popova, N.R.; Ivanov, V.E.; Karp, O.E.; Chernikov, A.V.; Gudkov, S.V. Formation of long-lived reactive species of blood serum proteins by the action of heat. Biochem. Biophys. Res. Commun. 2014, 443, 957–961. [Google Scholar]
- Garmash, S.A.; Smirnova, V.S.; Karp, O.E.; Usacheva, A.M.; Berezhnov, A.V.; Ivanov, V.E.; Chernikov, A.V.; Bruskov, V.I.; Gudkov, S.V. Pro-oxidative, genotoxic and cytotoxic properties of uranyl ions. J. Environ. Radioact. 2014, 127, 163–170. [Google Scholar]
- Shtarkman, I.N.; Gudkov, S.V.; Chernikov, A.V.; Bruskov, V.I. Effect of amino acids on X-ray-induced hydrogen peroxide and hydroxyl radical formation in water and 8-oxoguanine in DNA. Biochemistry-Moscow 2008, 73, 470–478. [Google Scholar]
- Chernikov, A.V.; Gudkov, S.V.; Shtarkman, I.N.; Bruskov, V.I. Oxygen effect in heat-mediated damage to DNA. Biofizika 2007, 52, 244–251. [Google Scholar]
- Gudkov, S.V.; Garmash, S.A.; Shtarkman, I.N.; Chernikov, A.V.; Karp, O.E.; Bruskov, V.I. Long-lived protein radicals induced by X-ray irradiation are the source of reactive oxygen species in aqueous medium. Dokl Biochem. Biophys. 2010, 430, 1–4. [Google Scholar]
- Kantelhardt, J.W.; Zschiegner, S.A.; Koscielny-Brude, E.; Bande, A.; Halvin, S.; Stanley, E. Multifractal detrended fluctuation analysis of nonstationary time series. Physica A 2002, 316, 87–114. [Google Scholar]
- Moskalev, A.; Shaposhnikov, M.; Snezhkina, A.; Kogan, V.; Plyusnina, E.; Peregudova, D.; Melnikova, N.; Uroshlev, L.; Mylnikov, S.; Dmitriev, A.; et al. Mining gene expression data for pollutants (dioxin, toluene, formaldehyde) and low dose of gamma-irradiation. PLoS One 2014, 9, e86051. [Google Scholar]
- Astaf’eva, N.M. Wavelet analysis: Basic theory and some applications. Phys. Usp. 1996, 39, 1085–1096. [Google Scholar]
- Bandrivskyy, A.; Bernjak, A.; Mcclintock, P.; Stefanovska, A. Wavelet phase coherence analysis: Application to skin temperature and blood flow. Cardiovasc. Eng. 2004, 4, 89–93. [Google Scholar]
- Haar, A. Zur Theorie der orthogonalen Funktionensysteme. Mathematische Annalen 1910, 69, 331–371. [Google Scholar]

© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gudkov, S.V.; Astashev, M.E.; Bruskov, V.I.; Kozlov, V.А.; Zakharov, S.D.; Bunkin, N.F. Self-oscillating Water Chemiluminescence Modes and Reactive Oxygen Species Generation Induced by Laser Irradiation; Effect of the Exclusion Zone Created by Nafion. Entropy 2014, 16, 6166-6185. https://doi.org/10.3390/e16116166
Gudkov SV, Astashev ME, Bruskov VI, Kozlov VА, Zakharov SD, Bunkin NF. Self-oscillating Water Chemiluminescence Modes and Reactive Oxygen Species Generation Induced by Laser Irradiation; Effect of the Exclusion Zone Created by Nafion. Entropy. 2014; 16(11):6166-6185. https://doi.org/10.3390/e16116166
Chicago/Turabian StyleGudkov, Sergey V., Maxim E. Astashev, Vadim I. Bruskov, Valeriy А. Kozlov, Stanislav D. Zakharov, and Nikolai F. Bunkin. 2014. "Self-oscillating Water Chemiluminescence Modes and Reactive Oxygen Species Generation Induced by Laser Irradiation; Effect of the Exclusion Zone Created by Nafion" Entropy 16, no. 11: 6166-6185. https://doi.org/10.3390/e16116166
APA StyleGudkov, S. V., Astashev, M. E., Bruskov, V. I., Kozlov, V. А., Zakharov, S. D., & Bunkin, N. F. (2014). Self-oscillating Water Chemiluminescence Modes and Reactive Oxygen Species Generation Induced by Laser Irradiation; Effect of the Exclusion Zone Created by Nafion. Entropy, 16(11), 6166-6185. https://doi.org/10.3390/e16116166






