Entropy Generation during Turbulent Flow of Zirconia-water and Other Nanofluids in a Square Cross Section Tube with a Constant Heat Flux
Abstract
:1. Introduction
2. Methodology
2.1. Thermophysical Properties of Nanofluid
2.2. Governing Equations
2.3. Description of the Problem
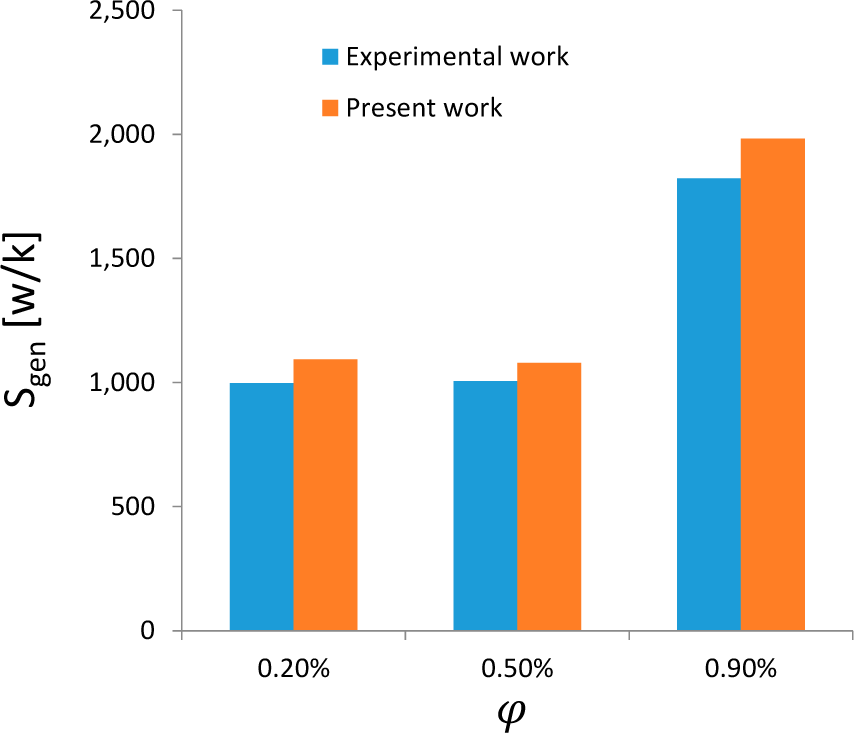
2.4. Validation
3. Results and Discussion
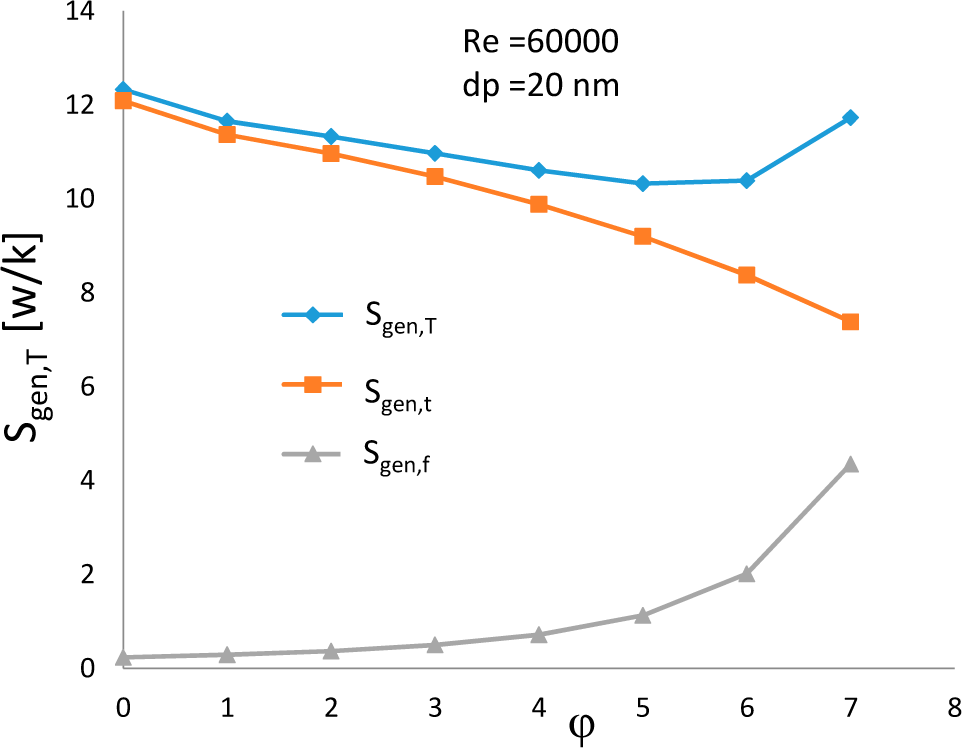
3.1. Effects of Nanoparticle Concentration
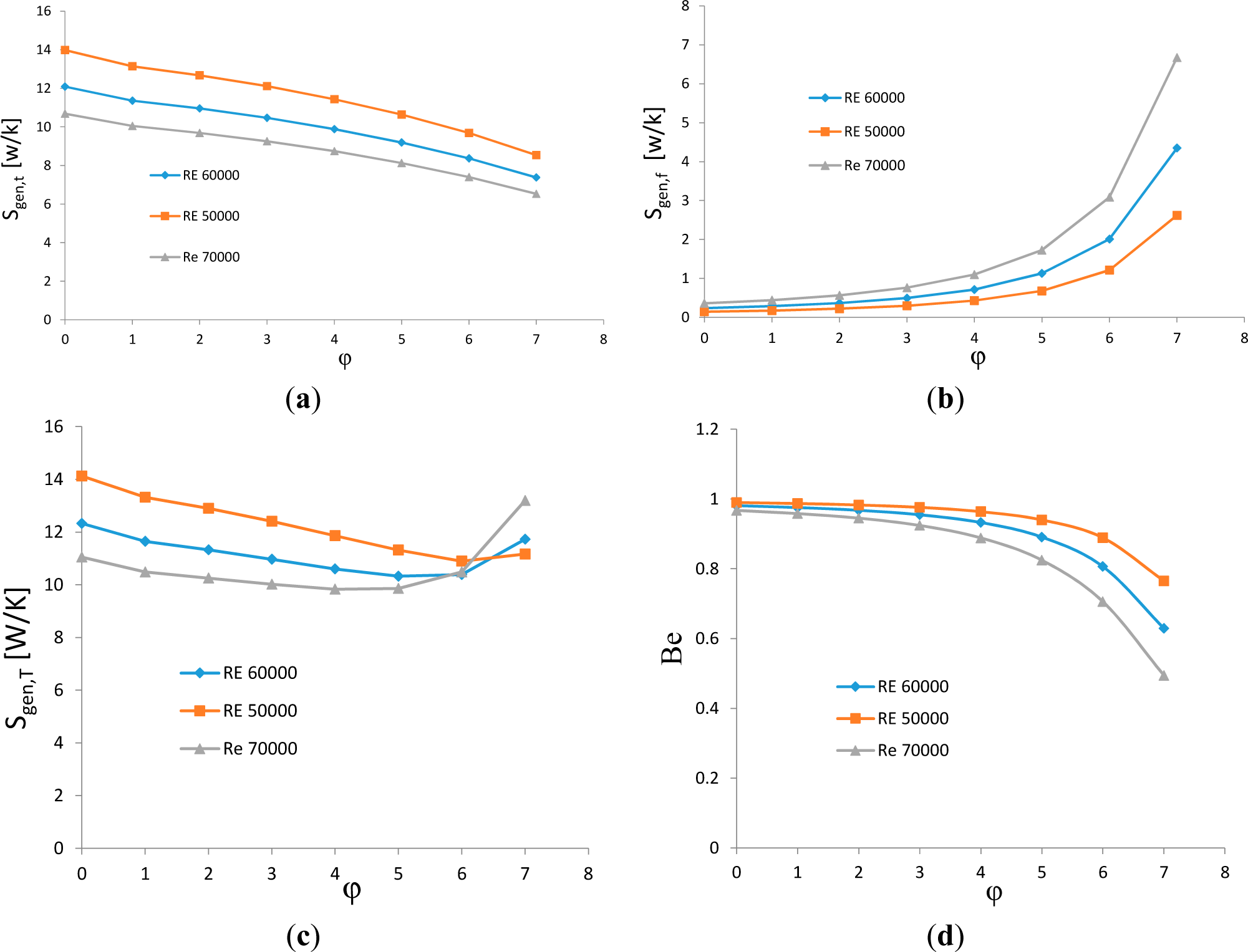
3.2. Effects of Reynolds Number
3.3. Effect of Nanoparticle Diameter
3.4. Effect of Different Nanoparticles
4. Conclusions
- At a constant Reynolds number and for a given nanoparticle size, the thermal entropy generation decreases with the increase of concentration, while the frictional entropy generation has an opposite trend. The total entropy generation decreases to a minimum at 6% solid volume fraction and then increases with further increase of volume concentration.
- With an increase in Reynolds number, the total and the thermal entropy generation decrease, while the frictional entropy generation increases. The optimal volume concentration for minimum entropy generation decreases with the increase in Reynolds number.
- As nanoparticle size increases, the thermal and the total entropy generation increase, but the frictional entropy production decreases. The optimal concentration for minimum total entropy generation becomes higher for larger nanoparticle diameters.
- Al2O3-water nanofluid has the lowest thermal entropy generation, and the ZrO2-water nanofluid has the highest. In terms of total entropy generation, the SiO2-water nanofluid generated the highest entropy generation among the tested nanofluids.
Acknowledgments
Nomenclature
| A | Area (m2) |
| Be | Bejan number |
| Cp | Specific heat (kJ kg−1·K−1) |
| D | Tube diameter (m) |
| dp | Particle diameter (nm) |
| f | Friction factor |
| k | Thermal conductivity (Wm−1 K−1) |
| L | Tube length (m) |
| Nu | Nusselt number |
| m | Mass flow rate (kg s−1) |
| p | Pressure (Pa) |
| P | Perimeter (m) |
| Pr | Prandtl number, (μ Cp/k) |
| q | Heat flux (Wm−2) |
| q | Heat transfer per unit of length (Wm−1) |
| Re | Reynolds number |
| Sgen | Entropy generation (WK−1) |
| T | Temperature (K) |
| x | Axial coordinate (m) |
| V | Velocity (ms−1) |
| Greek Symbols | |
|---|---|
| φ | Particles concentration |
| μ | Dynamic viscosity (Nsm−2) |
| ρ | Density (kgm−3) |
| Subscripts | |
|---|---|
| Ave | Average |
| bf | Base fluid |
| d | Nanoparticle diameter |
| eff | Effective |
| f | Frictional |
| g | Generation |
| h | Hydraulic |
| in | Inlet |
| nf | Nanofluids |
| out | Outlet |
| p | Particles |
| t | Thermal |
| T | Total |
Author Contributions
Conflicts of Interest
References
- Maxwell, J.C. A Treatise on Electricity and Magnetism; Clarendon press: Oxford, UK, 1881; Volume 1. [Google Scholar]
- Choi, S. Enhancing thermal conductivity of fluids with nanoparticles. In Developments and Applications of Nonnewtonian Flows; American Society of Mechanical Engineers; New York, NY, USA, 1995; pp. 99–106. [Google Scholar]
- Mahian, O.; Kianifar, A.; Kleinstreuer, C.; Al-Nimr, M.A.; Pop, I.; Sahin, A.Z.; Wongwises, S. A review of entropy generation in nanofluid flow. Int. J. Heat Mass Transf 2013, 65, 514–532. [Google Scholar]
- Duangthongsuk, W.; Wongwises, S. Heat transfer enhancement and pressure drop characteristics of TiO2—Water nanofluid in a double-tube counter flow heat exchanger. Int. J. Heat Mass Transf 2009, 52, 2059–2067. [Google Scholar]
- Huminic, G.; Huminic, A. Heat transfer characteristics in double tube helical heat exchangers using nanofluids. Int. J. Heat Mass Transf 2011, 54, 4280–4287. [Google Scholar]
- Duangthongsuk, W.; Selim Dalkilic, A.; Wongwises, S. Convective heat transfer of Al2O3-water nanofluids in a microchannel heat sink. Curr. Nanosci 2012, 8, 317–322. [Google Scholar]
- Li, J.; Kleinstreuer, C. Thermal performance of nanofluid flow in microchannels. Int. J. Heat Fluid Flow 2008, 29, 1221–1232. [Google Scholar]
- Li, J.; Kleinstreuer, C. Microfluidics analysis of nanoparticle mixing in a microchannel system. Microfluid. Nanofluidics 2009, 6, 661–668. [Google Scholar]
- Liu, Z.-H.; Li, Y.-Y. A new frontier of nanofluid research—application of nanofluids in heat pipes. Int. J. Heat Mass Transf 2012, 55, 6786–6797. [Google Scholar]
- Mohammadi, M.; Mohammadi, M.; Shafii, M. Experimental investigation of a pulsating heat pipe using ferrofluid (magnetic nanofluid). J. Heat Transf. 2012, 134, 014504. [Google Scholar]
- Alizad, K.; Vafai, K.; Shafahi, M. Thermal performance and operational attributes of the startup characteristics of flat-shaped heat pipes using nanofluids. Int. J. Heat Mass Transf 2012, 55, 140–155. [Google Scholar]
- Shafahi, M.; Bianco, V.; Vafai, K.; Manca, O. An investigation of the thermal performance of cylindrical heat pipes using nanofluids. Int. J. Heat Mass Transf 2010, 53, 376–383. [Google Scholar]
- Shafahi, M.; Bianco, V.; Vafai, K.; Manca, O. Thermal performance of flat-shaped heat pipes using nanofluids. Int. J. Heat Mass Transf 2010, 53, 1438–1445. [Google Scholar]
- Yousefi, T.; Shojaeizadeh, E.; Veysi, F.; Zinadini, S. An experimental investigation on the effect of pH variation of MWCNT-H2O nanofluid on the efficiency of a flat-plate solar collector. Sol. Energy 2012, 86, 771–779. [Google Scholar]
- Gharehkhani, S.; Nouri-Borujerdi, A.; Kazi, S.N.; Yarmand, H. Extension of weighted sum of gray gas data to mathematical simulation of radiative heat transfer in a boiler with gas-soot media. Sci. World J 2014, 2014, 504601. [Google Scholar]
- Kulkarni, D.P.; Das, D.K.; Chukwu, G.A. Temperature dependent rheological property of copper oxide nanoparticles suspension (nanofluid). J. Nanosci. Nanotechnol 2006, 6, 1150–1154. [Google Scholar]
- Liu, M.-S.; Lin, M.C.-C.; Tsai, C.; Wang, C.-C. Enhancement of thermal conductivity with Cu for nanofluids using chemical reduction method. Int. J. Heat Mass Transf 2006, 49, 3028–3033. [Google Scholar]
- Haghshenas Fard, M.; Esfahany, M.N.; Talaie, M. Numerical study of convective heat transfer of nanofluids in a circular tube two-phase model versus single-phase model. Int. Commun. Heat Mass Transf 2010, 37, 91–97. [Google Scholar]
- Mahmoudi, A.H.; Shahi, M.; Raouf, A.H.; Ghasemian, A. Numerical study of natural convection cooling of horizontal heat source mounted in a square cavity filled with nanofluid. Int. Commun. Heat Mass Transf 2010, 37, 1135–1141. [Google Scholar]
- Yoo, D.-H.; Hong, K.; Yang, H.-S. Study of thermal conductivity of nanofluids for the application of heat transfer fluids. Thermochim. Acta 2007, 455, 66–69. [Google Scholar]
- Xie, H.; Wang, J.; Xi, T.; Liu, Y.; Ai, F.; Wu, Q. Thermal conductivity enhancement of suspensions containing nanosized alumina particles. J. Appl. Phys 2002, 91, 4568–4572. [Google Scholar]
- Maïga, S.E.B.; Nguyen, C.T.; Galanis, N.; Roy, G.; Maré, T.; Coqueux, M. Heat transfer enhancement in turbulent tube flow using Al2O3 nanoparticle suspension. Int. J. Numer. Methods Heat Fluid Flow 2006, 16, 275–292. [Google Scholar]
- Vajjha, R.S.; Das, D.K.; Kulkarni, D.P. Development of new correlations for convective heat transfer and friction factor in turbulent regime for nanofluids. Int. J. Heat Mass Transf 2010, 53, 4607–4618. [Google Scholar]
- Yarmand, H.; Gharehkhani, S.; Kazi, S.N.; Sadeghinezhad, E.; Safaei, M.R. Numerical investigation of heat transfer enhancement in a rectangular heated pipe for turbulent nanofluid. Sci. World J 2014, 2014. [Google Scholar] [CrossRef]
- Mahian, O.; Mahmud, S.; Pop, I. Analysis of first and second laws of thermodynamics between two isothermal cylinders with relative rotation in the presence of MHD flow. Int. J. Heat Mass Transf 2012, 55, 4808–4816. [Google Scholar]
- Bejan, A. Entropy Generation Minimization: The Method of Thermodynamic Optimization of Finite-size Systems and Finite-time Processes; CRC Press: New York, NY, USA, 1995. [Google Scholar]
- Oztop, H.F.; Al-Salem, K. A review on entropy generation in natural and mixed convection heat transfer for energy systems. Renew. Sustain. Energy Rev 2012, 16, 911–920. [Google Scholar]
- Şahin, A.Z. Entropy generation in turbulent liquid flow through a smooth duct subjected to constant wall temperature. Int. J. Heat Mass Transf 2000, 43, 1469–1478. [Google Scholar]
- Mahian, O.; Mahmud, S.; Heris, S.Z. Analysis of entropy generation between co-rotating cylinders using nanofluids. Energy 2012, 44, 438–446. [Google Scholar]
- Mahian, O.; Mahmud, S.; Heris, S.Z. Effect of uncertainties in physical properties on entropy generation between two rotating cylinders with nanofluids. J. Heat Transf. 2012, 134, 101704. [Google Scholar]
- Ko, T.; Ting, K. Entropy generation and optimal analysis for laminar forced convection in curved rectangular ducts: A numerical study. Int. J. Therm. Sci 2006, 45, 138–150. [Google Scholar]
- Leong, K.; Saidur, R.; Mahlia, T.; Yau, Y. Entropy generation analysis of nanofluid flow in a circular tube subjected to constant wall temperature. Int. Commun. Heat Mass Transf 2012, 39, 1169–1175. [Google Scholar]
- Bianco, V.; Manca, O.; Nardini, S. Second law analysis of-water nanofluid turbulent forced convection in a circular cross section tube with constant wall temperature. Adv. Mech. Eng 2013, 2013. [Google Scholar] [CrossRef]
- Moghaddami, M.; Mohammadzade, A.; Esfehani, S.A.V. Second law analysis of nanofluid flow. Energy Convers. Manag 2011, 52, 1397–1405. [Google Scholar]
- Moghaddami, M.; Shahidi, S.; Siavashi, M. Entropy generation analysis of nanofluid flow in turbulent and laminar regimes. J. Comput. Theor. Nanosci 2012, 9, 1586–1595. [Google Scholar]
- Bianco, V.; Nardini, S.; Manca, O. Enhancement of heat transfer and entropy generation analysis of nanofluids turbulent convection flow in square section tubes. Nanoscale Res. Lett 2011, 6, 1–12. [Google Scholar]
- Bianco, V.; Manca, O.; Nardini, S. Entropy generation analysis of turbulent convection flow of Al2O3–water nanofluid in a circular tube subjected to constant wall heat flux. Energy Convers. Manag 2014, 77, 306–314. [Google Scholar]
- Das, S.K.; Putra, N.; Thiesen, P.; Roetzel, W. Temperature dependence of thermal conductivity enhancement for nanofluids. J. Heat Transf 2003, 125, 567–574. [Google Scholar]
- Hoffmann, K.A.; Chiang, S.T. Computational Fluid Dynamics; Volume 1, Wichita, K.S., Ed.; Engineering Education System: Washington, DC, USA, 2000. [Google Scholar]
- Corcione, M. Heat transfer features of buoyancy-driven nanofluids inside rectangular enclosures differentially heated at the sidewalls. Int. J. Therm. Sci 2010, 49, 1536–1546. [Google Scholar]
- Ratts, E.B.; Raut, A.G. Entropy generation minimization of fully developed internal flow with constant heat flux. J. Heat Transf 2004, 126, 656–659. [Google Scholar]
- Pak, B.C.; Cho, Y.I. Hydrodynamic and heat transfer study of dispersed fluids with submicron metallic oxide particles. Exp. Heat Transf. Int. J 1998, 11, 151–170. [Google Scholar]
- Williams, W.; Hu, L.-W.; Buongiorno, J. Experimental investigation of turbulent convective heat transfer and pressure loss of alumina/water and zirconia/water nanoparticle colloids (nanofluids) in horizontal tubes. J. Heat Transf 2008, 130, 1–7. [Google Scholar]
- Teng, T.-P.; Hung, Y.-H.; Teng, T.-C.; Mo, H.-E.; Hsu, H.-G. The effect of alumina/water nanofluid particle size on thermal conductivity. Appl. Therm. Eng 2010, 30, 2213–2218. [Google Scholar]
- Kleinstreuer, C.; Feng, Y. Experimental and theoretical studies of nanofluid thermal conductivity enhancement: A review. Nanoscale Res. Lett 2011, 6, 1–13. [Google Scholar]



© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yarmand, H.; Ahmadi, G.; Gharehkhani, S.; Kazi, S.N.; Safaei, M.R.; Alehashem, M.S.; Mahat, A.B. Entropy Generation during Turbulent Flow of Zirconia-water and Other Nanofluids in a Square Cross Section Tube with a Constant Heat Flux. Entropy 2014, 16, 6116-6132. https://doi.org/10.3390/e16116116
Yarmand H, Ahmadi G, Gharehkhani S, Kazi SN, Safaei MR, Alehashem MS, Mahat AB. Entropy Generation during Turbulent Flow of Zirconia-water and Other Nanofluids in a Square Cross Section Tube with a Constant Heat Flux. Entropy. 2014; 16(11):6116-6132. https://doi.org/10.3390/e16116116
Chicago/Turabian StyleYarmand, Hooman, Goodarz Ahmadi, Samira Gharehkhani, Salim Newaz Kazi, Mohammad Reza Safaei, Maryam Sadat Alehashem, and Abu Bakar Mahat. 2014. "Entropy Generation during Turbulent Flow of Zirconia-water and Other Nanofluids in a Square Cross Section Tube with a Constant Heat Flux" Entropy 16, no. 11: 6116-6132. https://doi.org/10.3390/e16116116
APA StyleYarmand, H., Ahmadi, G., Gharehkhani, S., Kazi, S. N., Safaei, M. R., Alehashem, M. S., & Mahat, A. B. (2014). Entropy Generation during Turbulent Flow of Zirconia-water and Other Nanofluids in a Square Cross Section Tube with a Constant Heat Flux. Entropy, 16(11), 6116-6132. https://doi.org/10.3390/e16116116





