Limits and Optimization of Power Input or Output of Actual Thermal Cycles
Abstract
:1. Introduction
2. Thermodynamic Analysis
- (i)
- Power cycles.
- (ii)
- Heat pump and refrigeration cycles.

- Assumptions are listed for the cycles.
- For irreversible Rankine, heat pump and refrigeration cycles.
- All processes are irreversible.
- Systems follow a continuous pattern.
- Heat exchangers’ dimensions are limited and convection coefficients are constant.
- All processes are irreversible.
- Specific heats are constant and average specific heats are used.
- Piston friction is neglected.
- Powering fluid is air, which is the ideal gas.
- Polytrophic coefficients are constant and an average polytrophic coefficient is identified for each cycle.
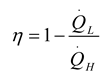

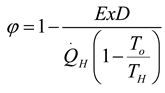
2.1. Power Generation Cycles
2.1.1. Thermodynamic Analysis of Irreversible Rankine Cycle

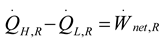
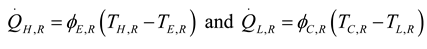




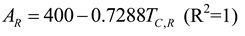
2.1.2. Thermodynamic Analysis of Irreversible Spark Injection (SI) and Compression Injection (CI) Cycles
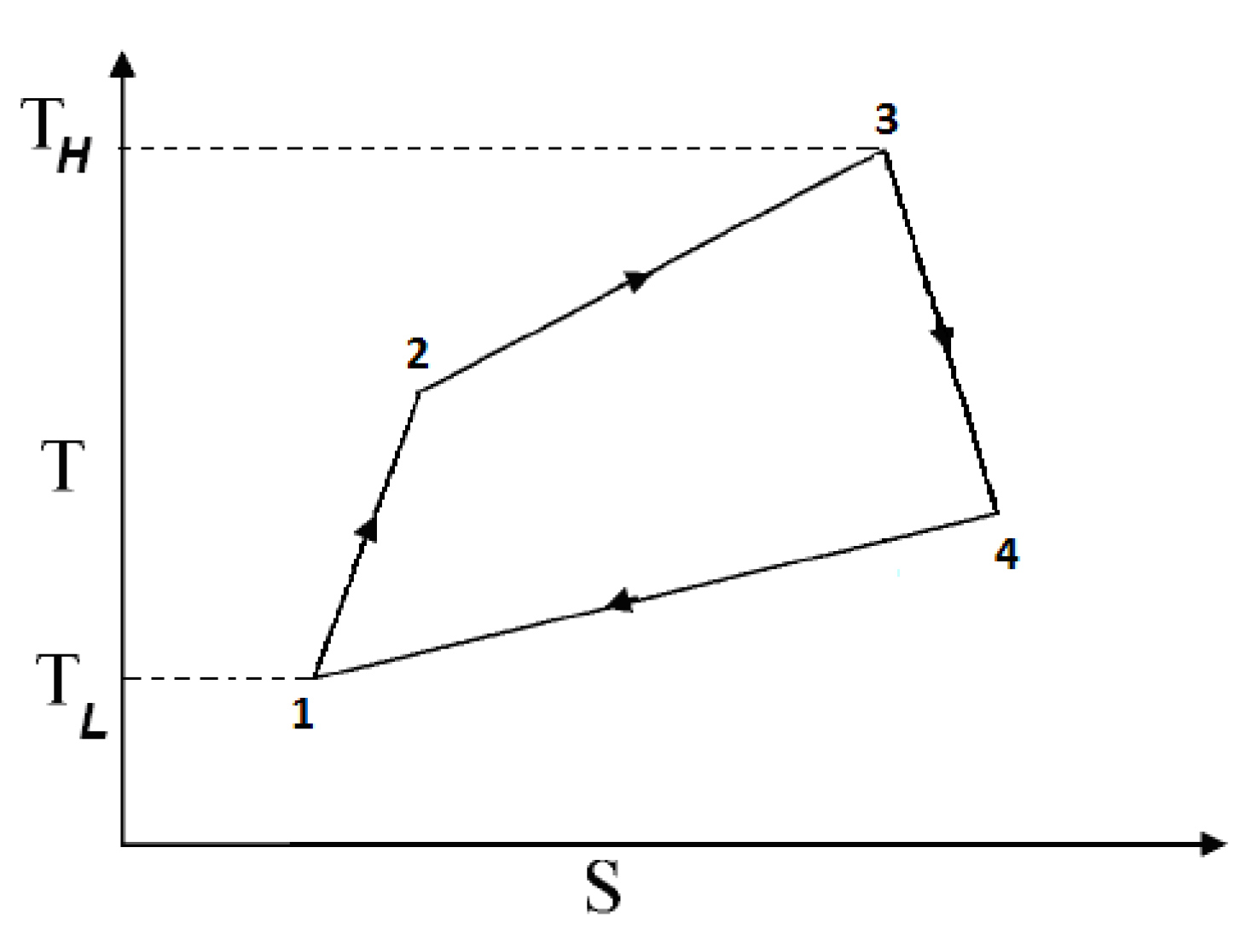
2.1.2.1. Irreversible SI Engine
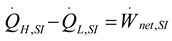
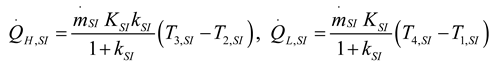
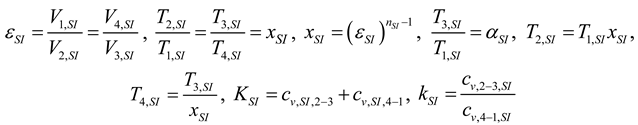

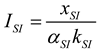
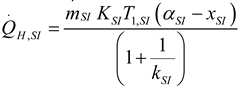
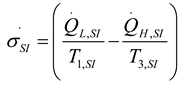
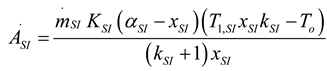
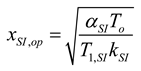
2.1.2.2. Irreversible CI Engine
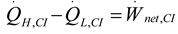

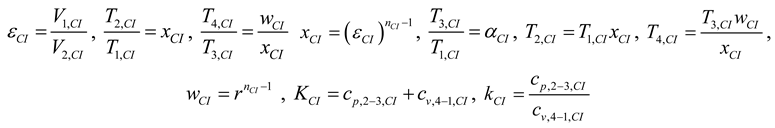
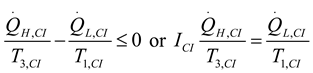

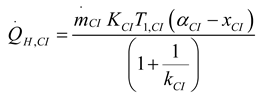
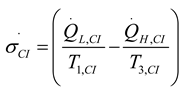


2.1.3. Thermodynamic Analysis of Irreversible Brayton Cycles

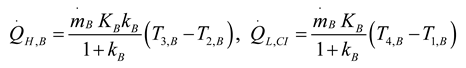
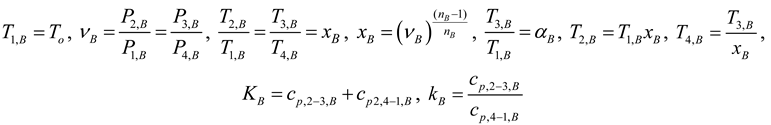


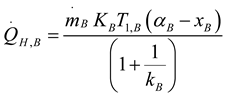
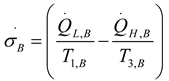


2.1.4. Thermodynamic Analysis of Irreversible Stirling and Ericsson Cycles

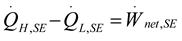
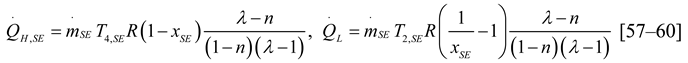
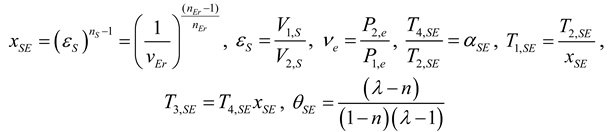
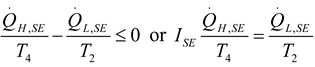
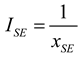

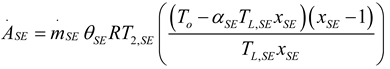
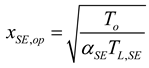
2.2. Heat Pump and Refrigeration Cycles

2.2.1. Thermodynamic Analysis of General Irreversible Heat Pump and Irreversible Refrigerator Systems
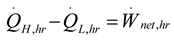
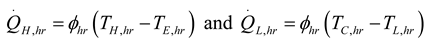
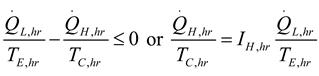
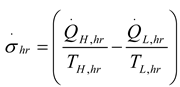
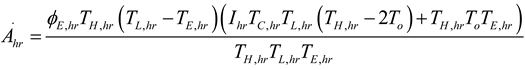

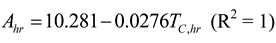
3. Results and Numerical Examples
3.1. Irreversible Rankine Cycle
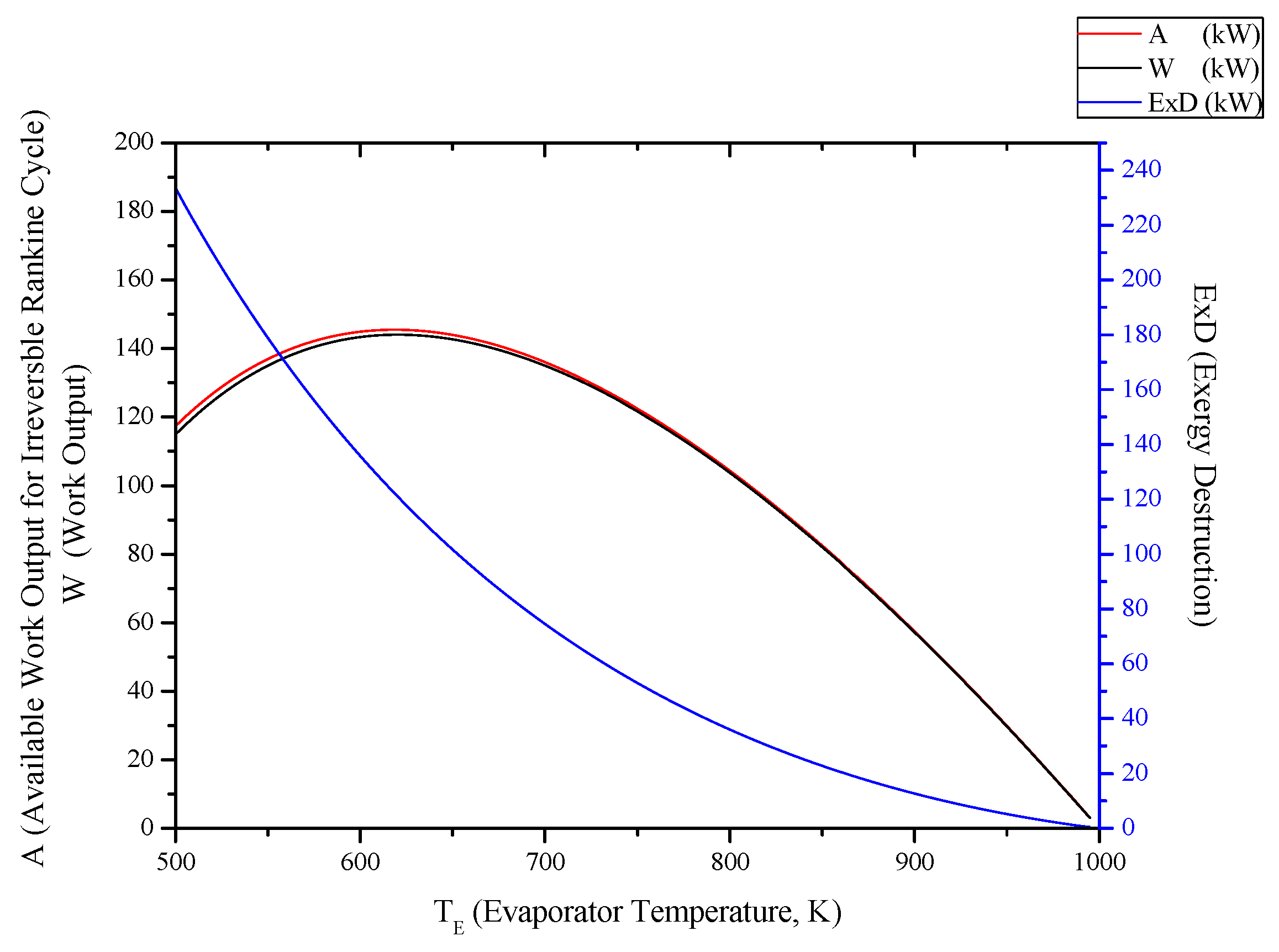

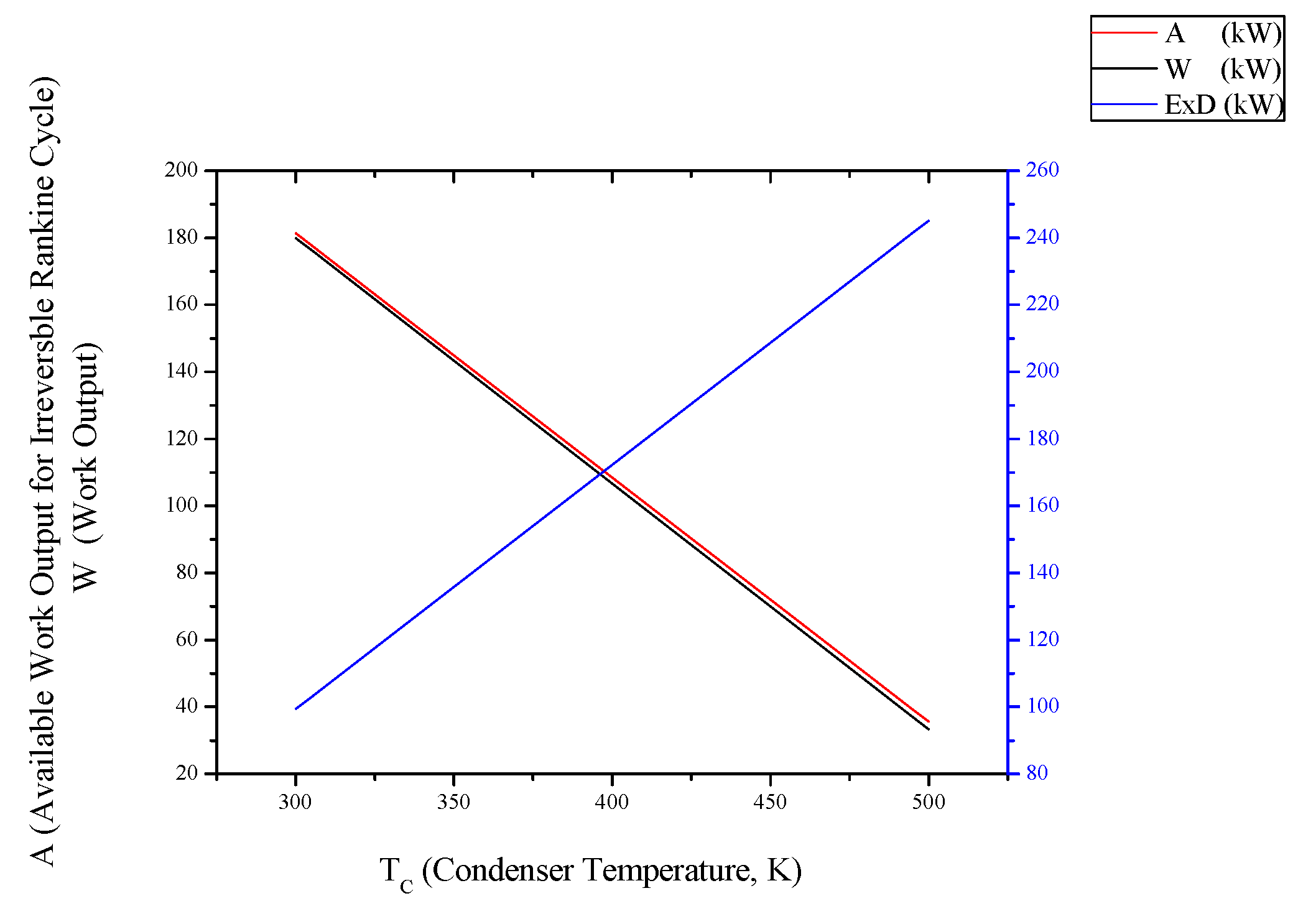

3.2. Irreversible SI Engine
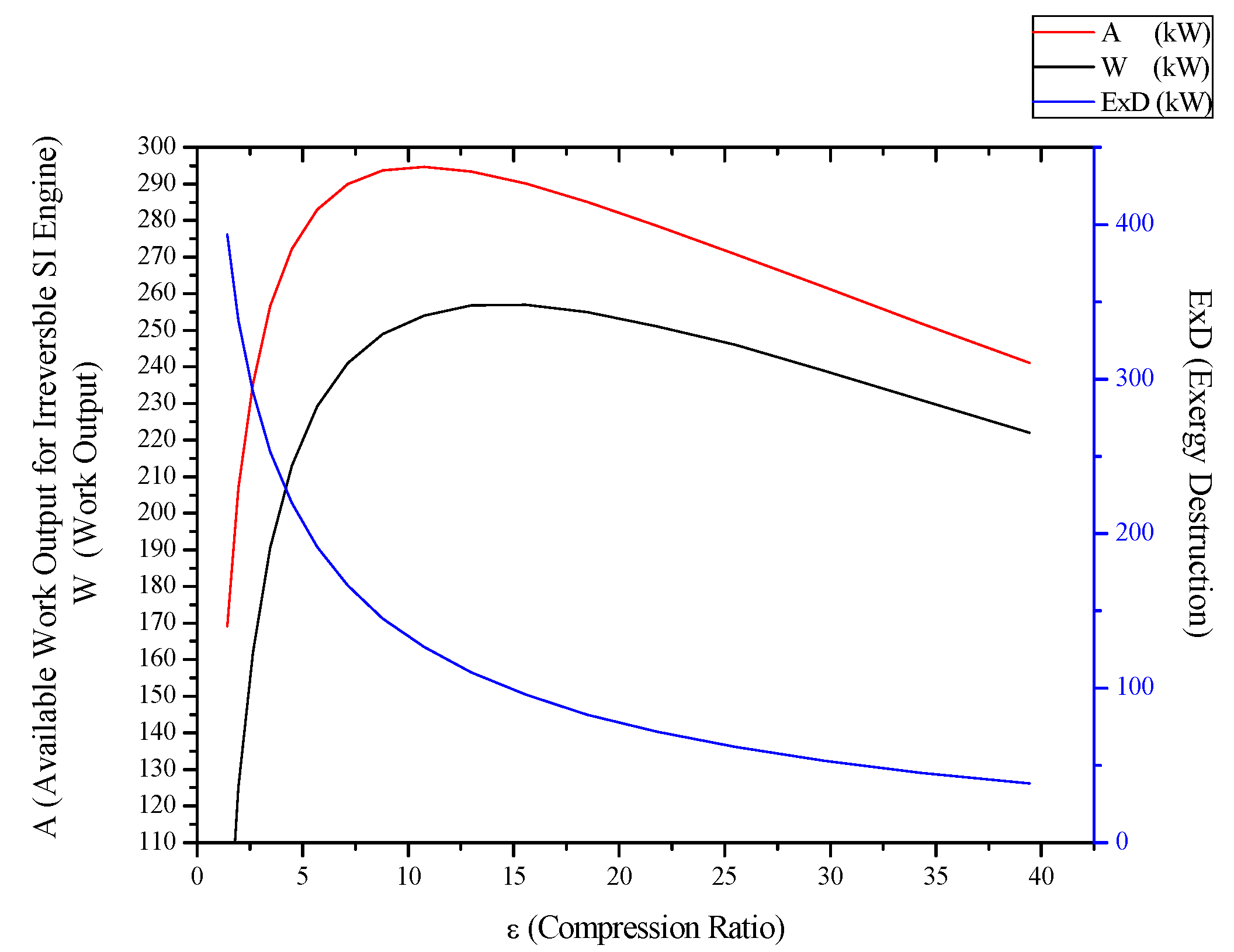
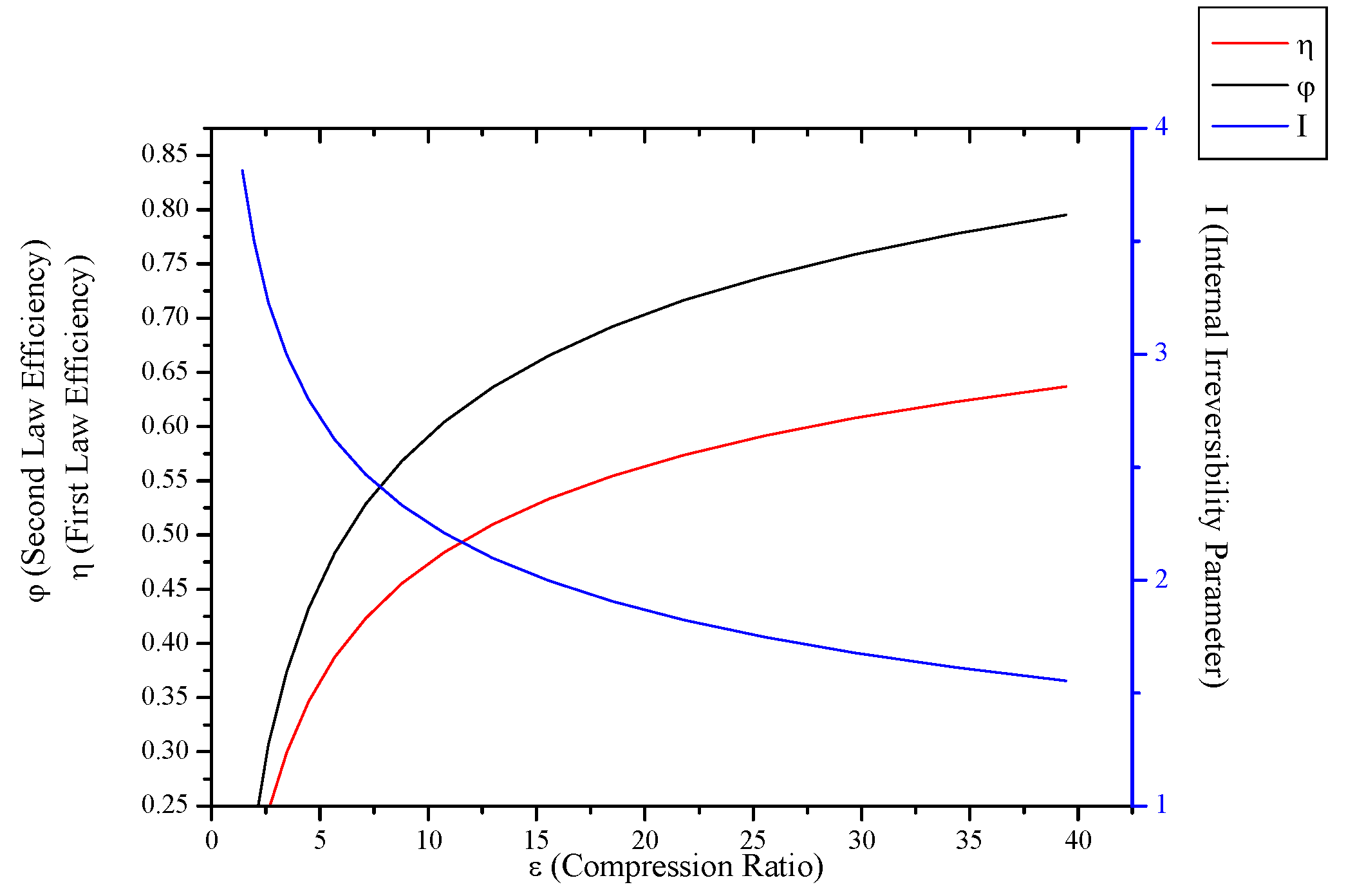
3.3. Irreversible CI Engines

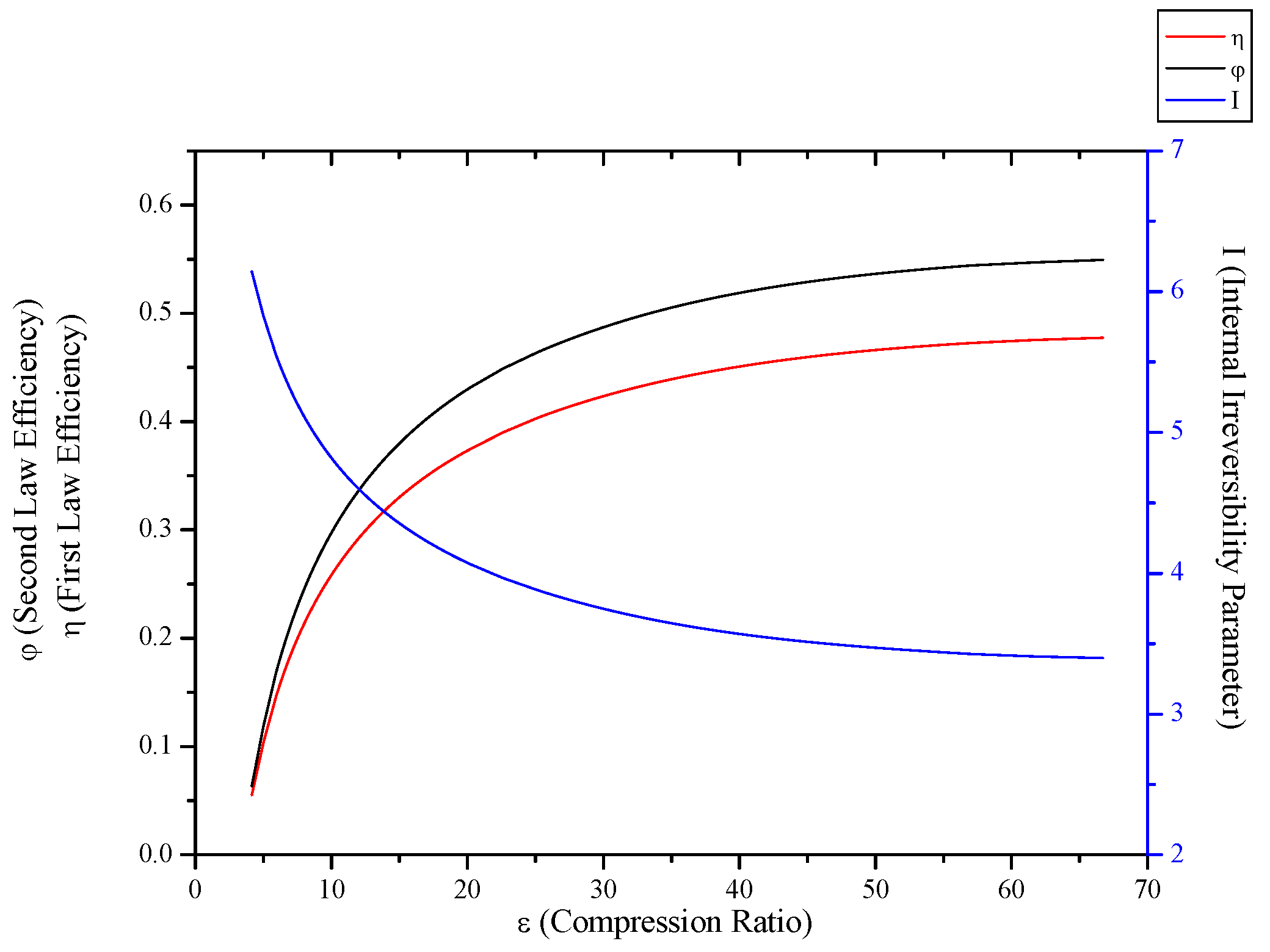
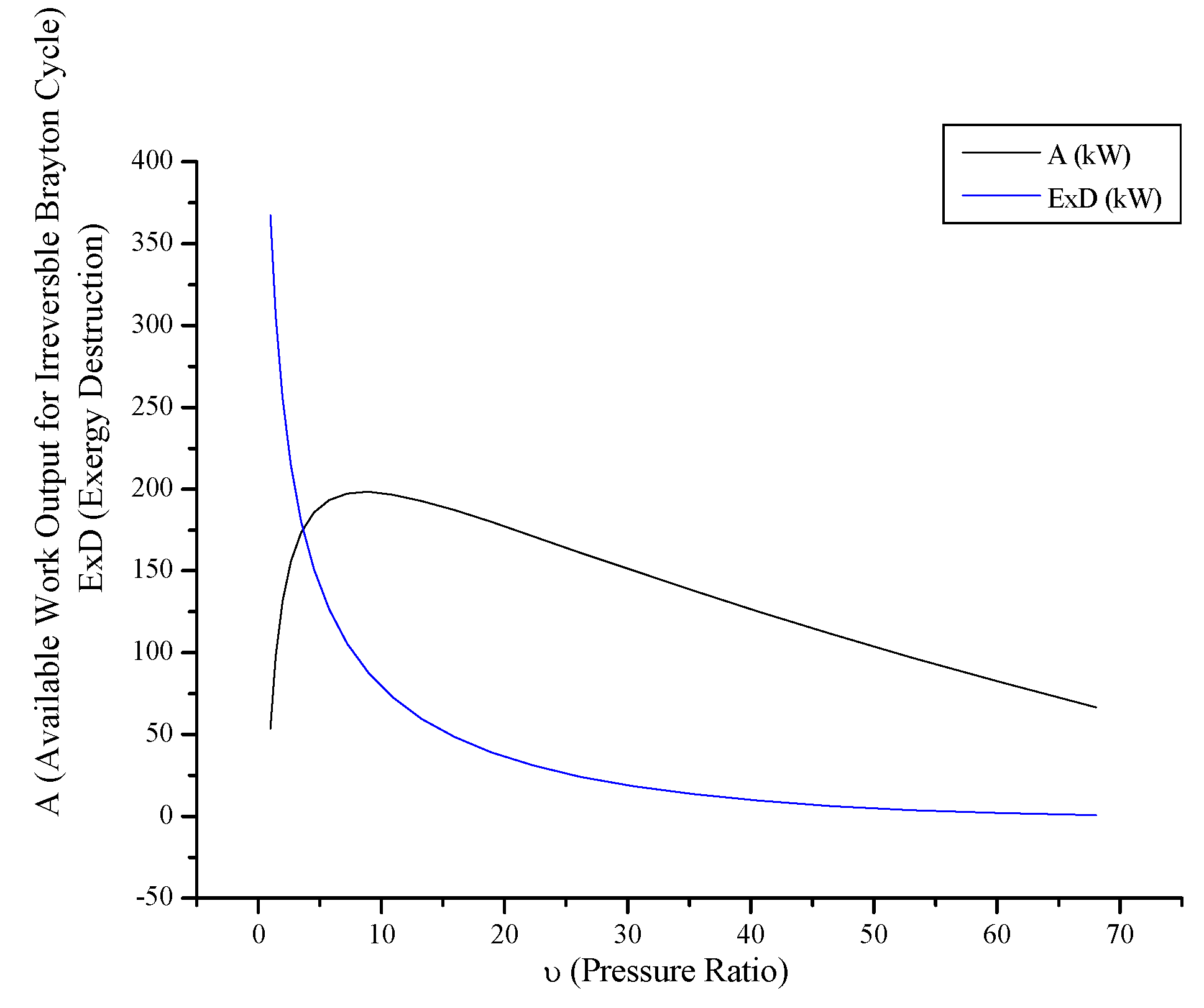
3.4. Irreversible Brayton Cycles

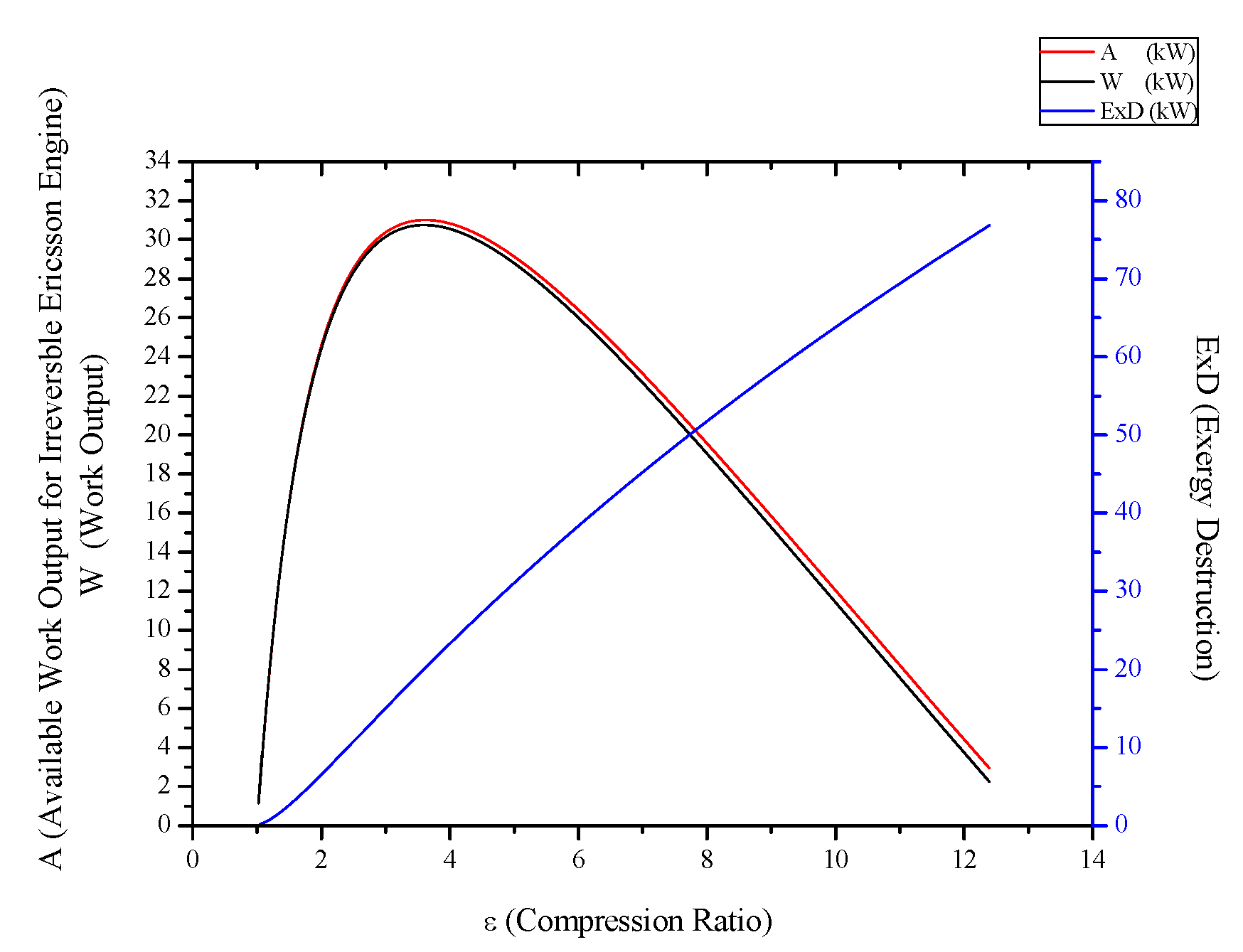
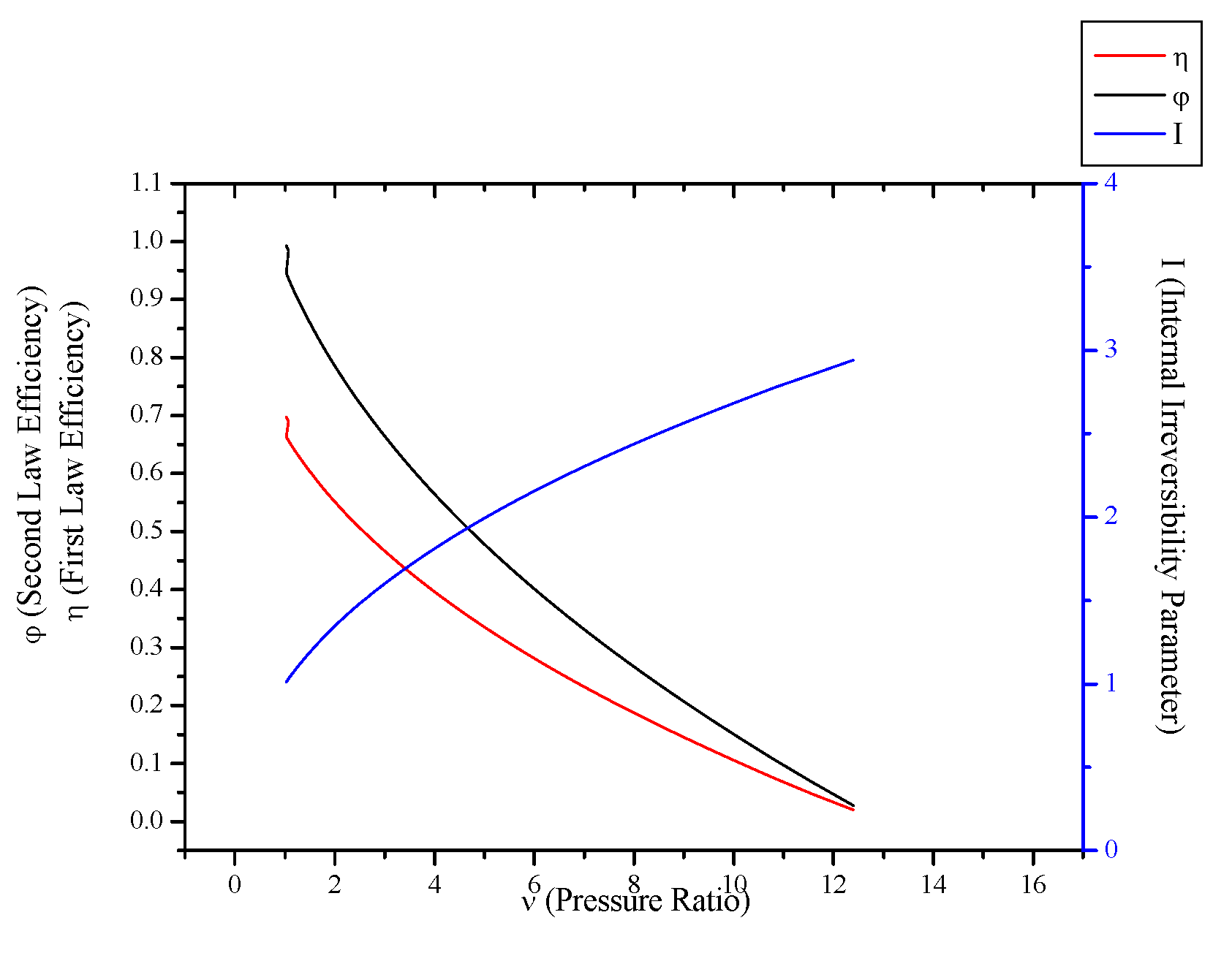
3.5. Irreversible Stirling and Ericsson Engines


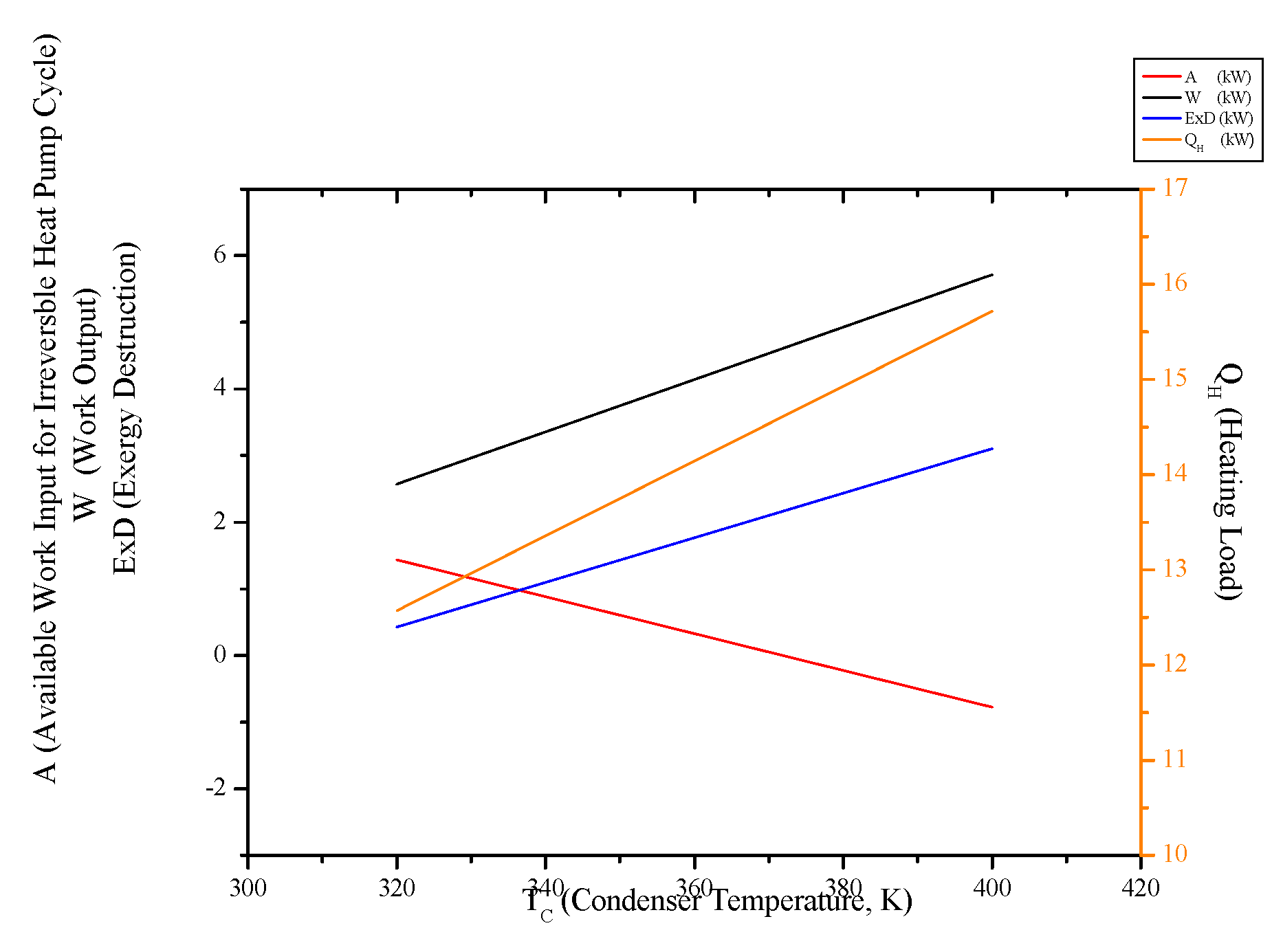
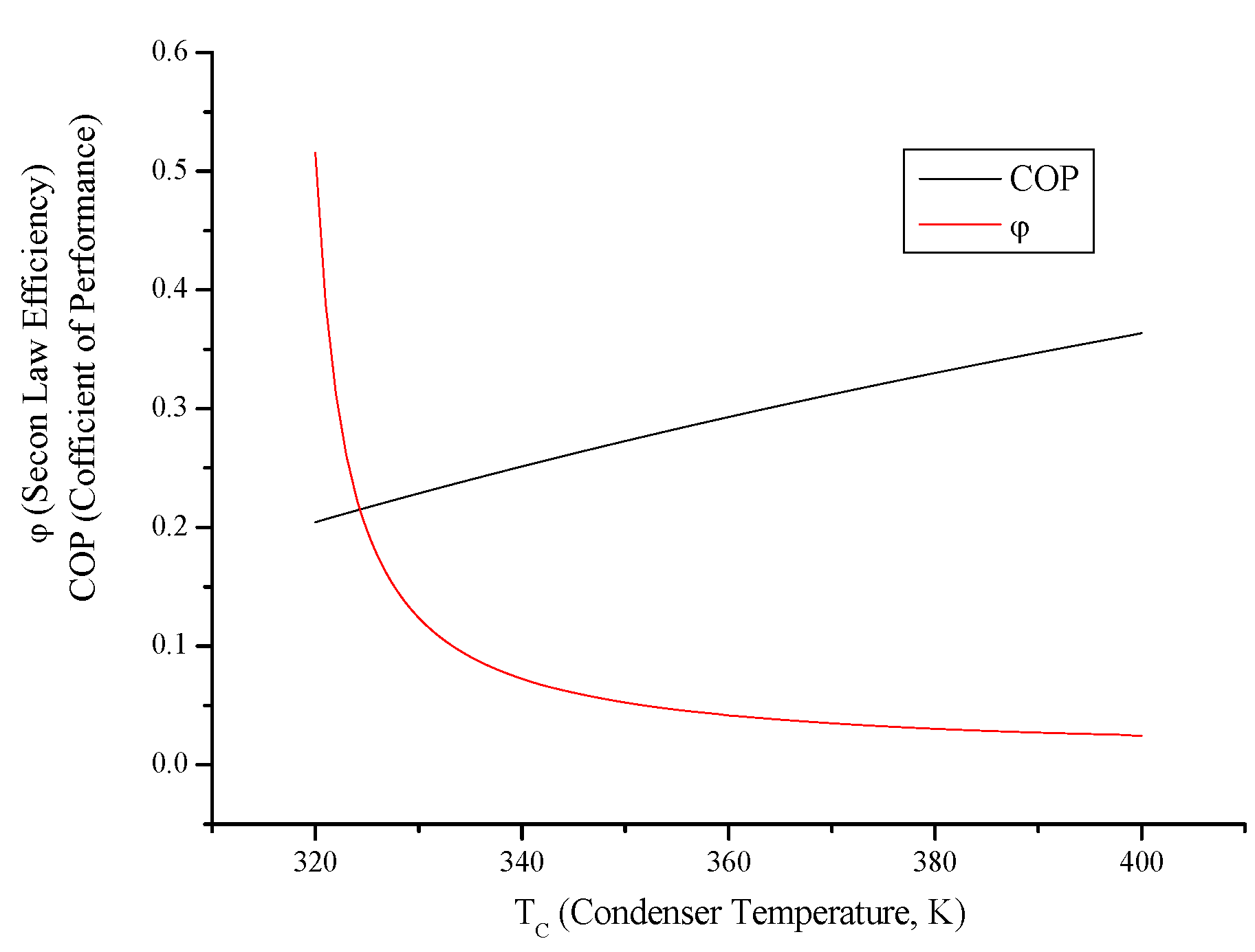
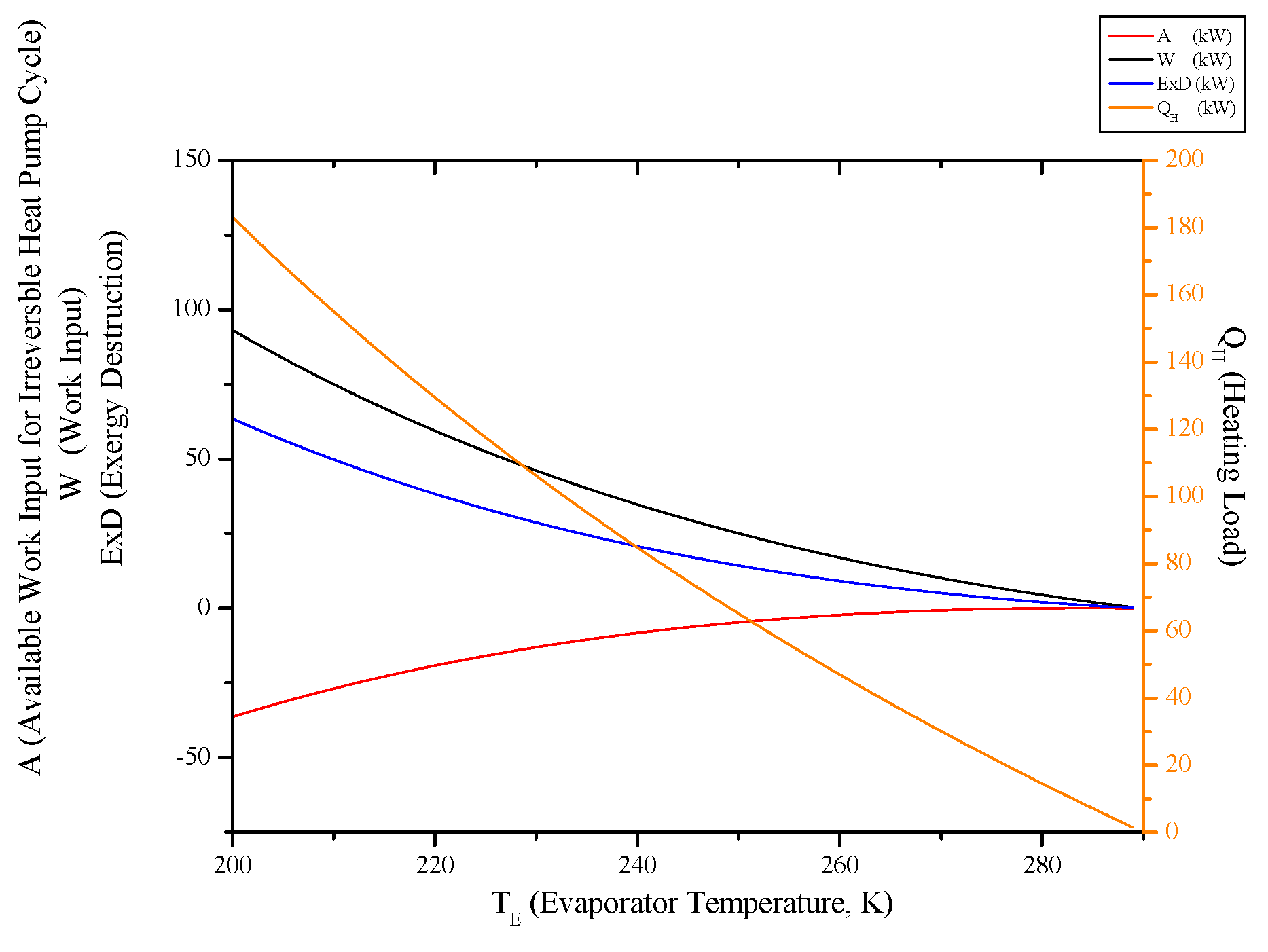
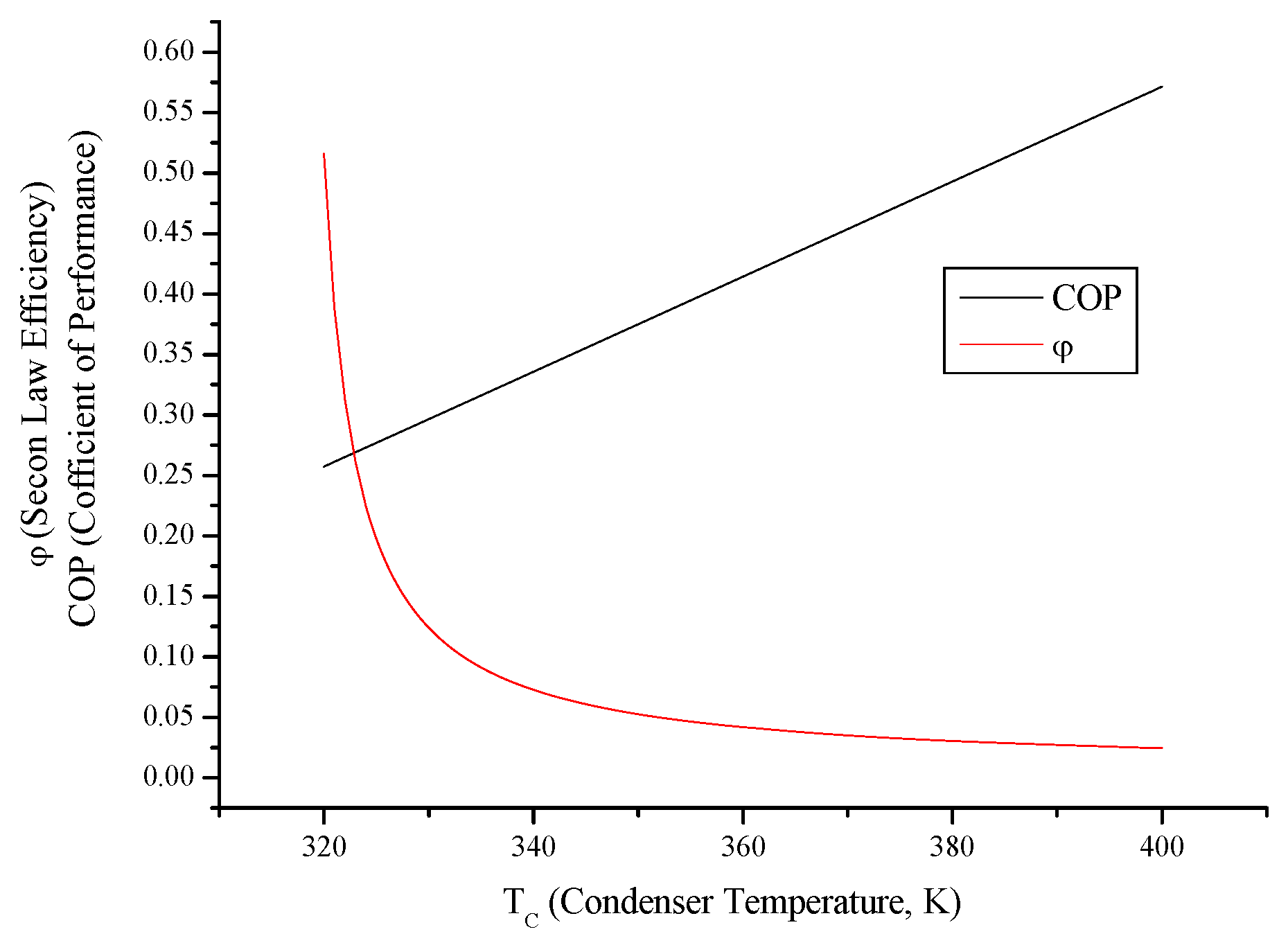
3.6. Irreversible Heat Pump and Refrigeration Cycles


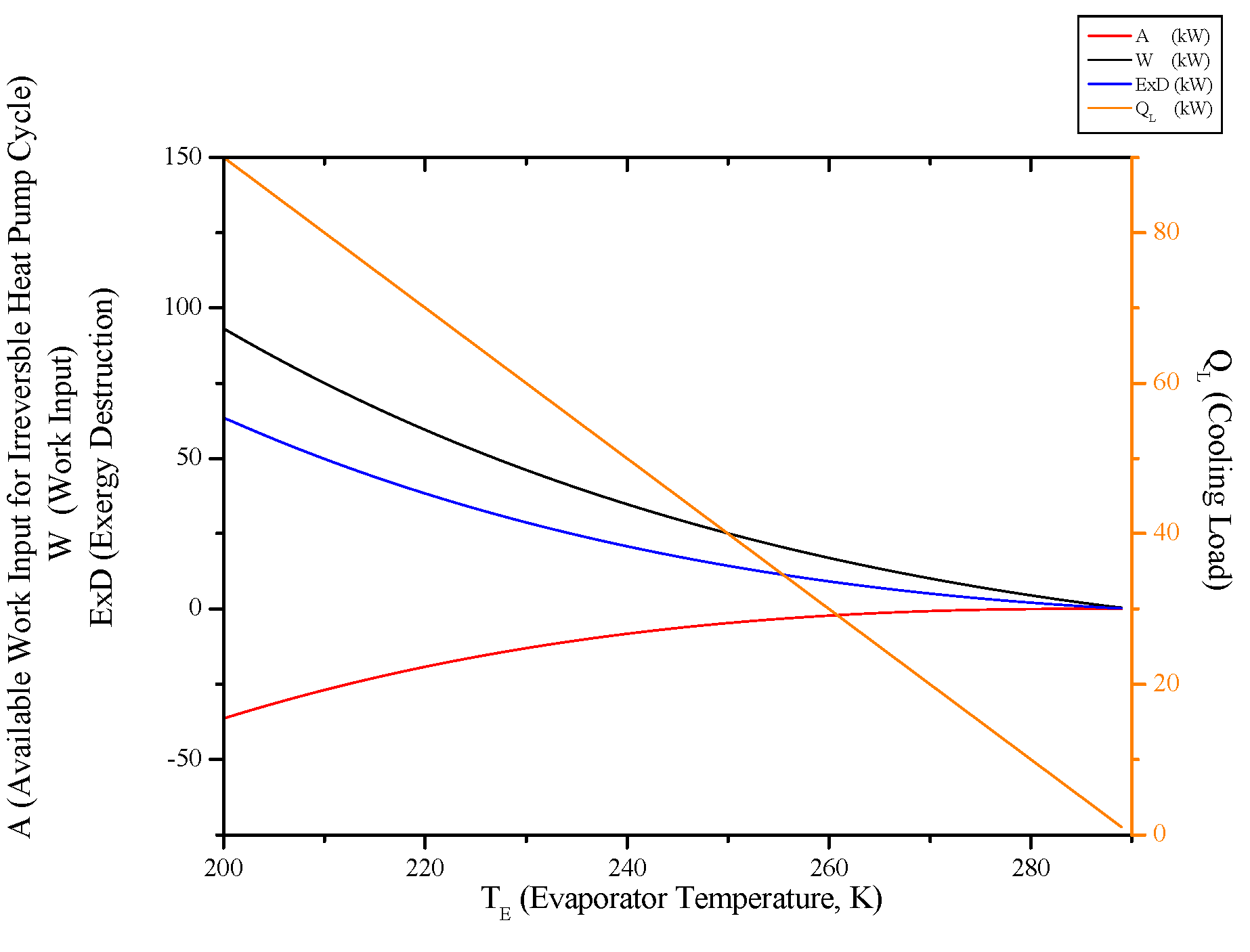
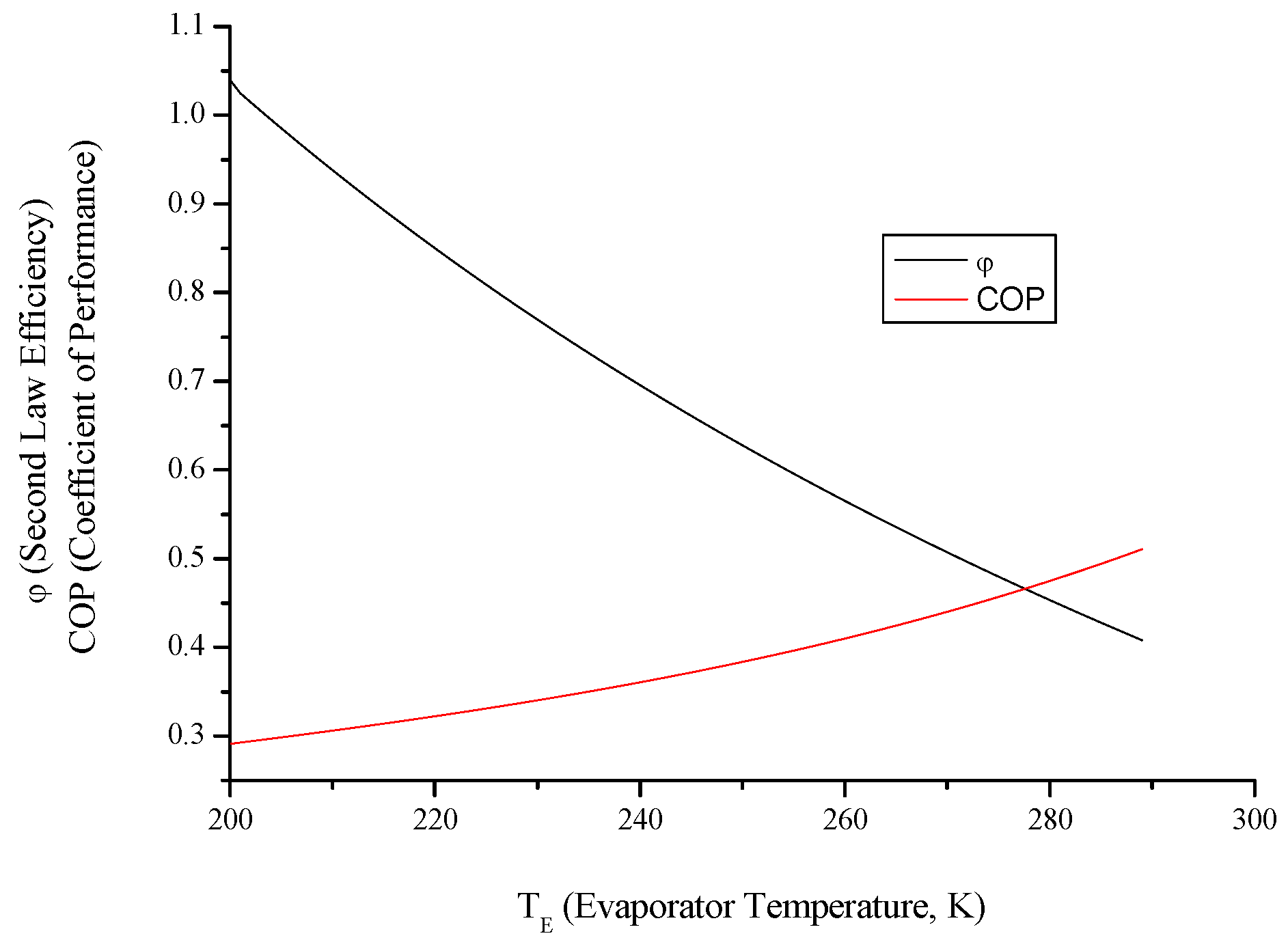
4. Conclusions
- For Rankine, Stirling and Ericsson cycles, which are external combustion engines, available work output and power output has the very close values. On the contrary, difference of these values is much higher at SI, CI and Brayton cycles.
- While available work output is maximum, power output is maximum too for the all power generation cycles.
- When optimization parameters are increased, first and second law efficiencies decrease and exergy destruction internal irreversibility parameter increase for Rankine, Stirling and Ericsson cycles.
- When optimization parameters are increased, first and second law efficiencies increase and exergy destruction internal irreversibility parameter decrease for SI, CI and Brayton cycles.
- It can be seen that TC parameter should be minimum for Rankine cycle to improve its performance.
- Both cycles’ work input, COP and exergy destruction increase with condenser temperature while available work output and second law efficiency decrease. In addition to those, heating load increase wit condenser temperature for the heat pump and cooling load decreases for the refrigerator.
- Evaluating evaporator temperature for both cycle, cooling load for the refrigeration cycle heating load for the heat pump cycle, exergy destructions and second law efficiencies diminish with evaporator temperature, while available work outputs and COP rise up. This point of view, it can be said that evaporator temperature should be at low temperature. available power input.
Conflict of Interest
Nomenclature
available work for the actual cycles (kW) | |
specific heat (kJ/kgK) | |
coefficient of performance | |
exergy destruction (kW) | |
internal irreversibility parameter | |
ratio of specific heats | |
sum of specific heats (kJ/kgK) | |
| m | mass flow (kg/s) |
polytrophic coefficient | |
cut-off ratio | |
ideal gas constant (kJ/kgK) | |
heat (kW) | |
temperature(K) | |
power (kW) | |
dimensionless compression ratio or dimensionless pressure ratio parameter | |
| Subscripts | |
Brayton | |
condenser | |
compression injection engine | |
critical point | |
Ericsson engine | |
Stirling | |
heat pump | |
high | |
heat pump or refrigeration | |
low | |
refrigerator | |
Rankine | |
Stirling | |
Stirling or Ericsson engine | |
spark injection engine | |
optimum point | |
| Greek letters | |
ratio of the highest and the lowest temperature of the cycle | |
compression ratio | |
heat conductance (kW/K) | |
isentropic coefficient | |
efficiency | |
pressure ratio | |
a coefficient for Ericsson and Stirling engines | |
| σ | entropy generation (kW/K) |
References
- Chen, L.; Wu, C.; Sun, F. Finite time thermodynamic optimization or entropy generation minimization of energy systems. J. Non-Equilib. Thermodyn. 1999, 24, 327–359. [Google Scholar] [CrossRef]
- Wu, C. Recent Advances in Finite Time Thermodynamics; Wu, C., Chen, L., Chen, J., Eds.; Nova Science Publishers: New York, NY, USA, 1999. [Google Scholar]
- Chen, L.; Sun, F. (Eds.) Advances in Finite Time Thermodynamics: Analysis and Optimization; Nova Science Publishers: New York, NY, USA, 2004.
- Andresen, B.; Rubin, M.H.; Berry, R.S. Availability for finite-time processes. General theory and a model. J. Chem. Phys. 1983, 87, 2704–2713. [Google Scholar] [CrossRef]
- Mironova, V.A.; Tsirlin, A.M.; Kazakov, V.A.; Berry, R.S. Finite–time thermodynamics: Exergy and optimization of time-constrained processes. J. Appl. Phys. 1994, 76, 629–636. [Google Scholar] [CrossRef]
- Sieniutycz, S.; Spakovsky, M.R.V. Finite time generalization of thermal exergy. Energy Convers. Manag. 1997, 39, 1432–1447. [Google Scholar] [CrossRef]
- Sieniutycz, S. Generalized Carnot problem of maximum power in finite time via Hamilton-Jacobi-Bellman theory. Energy Convers. Manag. 1998, 39, 1735–1743. [Google Scholar] [CrossRef]
- Sieniutycz, S. Carnot problem of maximum power from a finite resource interacting with environment in a finite time. Physica A 1999, 264, 234–263. [Google Scholar] [CrossRef]
- Sieniutycz, S. Hamilton-Jacobi-Bellman theory of dissipative thermal availability. Phys. Rev. E 1997, 56, 5051–5064. [Google Scholar] [CrossRef]
- Xia, S.; Chen, L.; Sun, F. Finite-time exergy with a finite heat reservoir and generalized radiative heat transfer law. Revista Mexicana De Fisica 2010, 56, 287–296. [Google Scholar]
- Song, H.; Chen, L.; Sun, F. Optimal expansion of heated powering fluid for maximum power output with generalized radiative heat transfer law. J. Appl. Phys. 2007, 102, 094901. [Google Scholar] [CrossRef]
- Denton, J.C. Thermal cycles in classical thermodynamics and nonequilibrium thermodynamics in contrast with finite time thermodynamics. Energy Convers. Manag. 2002, 43, 1583–1617. [Google Scholar] [CrossRef]
- Li, J.; Chen, L.; Sun, F. Optimal configuration of a class of endoreversible heat-engines for maximum power-output with linear phenomenological heat-transfer law. Appl. Energy 2007, 84, 944–957. [Google Scholar] [CrossRef]
- Li, J.; Chen, L.; Sun, F. Optimum work in real systems with a class of finite thermal capacity reservoirs. Math. Comput. Model. 2009, 49, 542–547. [Google Scholar] [CrossRef]
- Li, J.; Chen, L.; Sun, F. Maximum work output of multistage continuous Carnot heat engine system with finite reservoirs of thermal capacity and radiation between heat source and working fluid. Therm. Sci. 2010, 14, 1–9. [Google Scholar] [CrossRef]
- Li, J.; Chen, L.; Sun, F. Extremal work of an endoreversible system with two finite thermal capacity reservoirs. J. Energy Inst. 2009, 82, 53–56. [Google Scholar] [CrossRef]
- Xia, S.; Chen, L.; Sun, F. Optimal paths for minimizing entransy dissipation during heat transfer processes with generalized radiative heat transfer law. Appl. Math. Model. 2010, 34, 2242–2255. [Google Scholar] [CrossRef]
- Chen, L.; Song, H.; Sun, F. Endoreversible radiative heat engines for maximum efficiency. Appl. Math. Model. 2010, 34, 1710–1720. [Google Scholar] [CrossRef]
- Wu, F.; Chen, L.; Sun, F. Exergetic efficiency optimization for an irreversible quantum Brayton refrigerator with spin systems. Appl. Math. Model. 2010, 34, 617–625. [Google Scholar] [CrossRef]
- Xia, D.; Chen, L.; Sun, F. Optimal performance of an endoreversible three-mass- reservoir chemical pump with diffusive mass transfer law. Appl. Math. Model. 2010, 34, 140–145. [Google Scholar] [CrossRef]
- Xia, S.; Chen, L.; Sun, F. Effects of mass transfer laws on finite-time exergy. J. Energy Inst. 2010, 83, 210–216. [Google Scholar] [CrossRef]
- Xia, S.; Chen, L.; Sun, F. Power-optimization of non-ideal energy converters under generalized convective heat transfer law via Hamilton-Jacobi-Bellman theory. Energy 2011, 36, 633–646. [Google Scholar] [CrossRef]
- Xia, S.; Chen, L.; Sun, F. Finite-time exergy with a generalized heat transfer law. J. Energy Inst. 2012, 85, 70–77. [Google Scholar] [CrossRef]
- Xia, S.; Chen, L.; Sun, F. Endoreversible modeling and optimization of a multistage heat engine system with a generalized heat transfer law via Hamilton-Jacobi-Bellman equations and dynamic programming. Acta Phys. Pol. A 2011, 119, 747–760. [Google Scholar]
- Xia, S.; Chen, L.; Sun, F. Hamilton-Jacobi-Bellman equations and dynamic programming for power-optimization of radiative law multistage heat engine system. Int. J. Energy Environ. 2012, 3, 359–382. [Google Scholar]
- Chen, L.; Li, J.; Sun, F. Optimal temperatures and maximum power output of a complex system with linear phenomenological heat transfer law. Therm. Sci. 2009, 13, 33–40. [Google Scholar] [CrossRef]
- Baierlein, R. Thermal Physics; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Wu, F.; Chen, L.; Sun, F. Ecological optimization performance of an irreversible quantum SI engine powering with an ideal Fermi gas. Open Syst. Inf. Dyn. 2006, 13, 55–66. [Google Scholar] [CrossRef]
- Sogut, S.S.; Ust, Y.; Sahin, B. The effects of intercooling and regeneration on thermo–ecological performance analysis of an irreversible–closed Brayton heat engine with variable–temperature thermal reservoirs. J. Phys. D: Appl. Phys. 2006, 39, 4713–4721. [Google Scholar] [CrossRef]
- Wu, C.; Kiang, R.L. Work and power optimization of a finite-time Brayton cycle. Int. J. Ambient Energy 1990, 11, 129–136. [Google Scholar] [CrossRef]
- Wu, C.; Kiang, R.L. Power performance of a nonisentropic Brayton cycle. ASME J. Eng. Gas Turbines Power 1991, 113, 501–504. [Google Scholar] [CrossRef]
- Sahin, B.; Kodal, A.; Yavuz, H. Efficiency of a Joule-Brayton engine at maximum power density. J. Phys. D: Appl. Phys. 1995, 28, 1309–1313. [Google Scholar] [CrossRef]
- Sahin, B.; Kodal, A.; Yilmaz, T.; Yavuz, H. Maximum power density analysis of an irreversible Joule–Brayton engine. J. Phys. D: Appl. Phys. 1996, 29, 1162–1167. [Google Scholar] [CrossRef]
- Sahin, B.; Kodal, A.; Kaya, S.S. A comparative performance analysis of irreversible regenerative reheating Joule-Brayton engines under maximum power density and maximum power conditions. J. Phys. D: Appl. Phys. 1998, 31, 2125–2131. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Chen, C.K. Power optimization of an irreversible Brayton heat engine. Energy Sources 1997, 1, 461–474. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Chen, C.K. Power optimization of an endoreversible regenerative Brayton cycle. Energy 1996, 2, 241–247. [Google Scholar] [CrossRef]
- Chen, L.; Sun, F.; Wu, C.; Kiang, R.L. Theoretical analysis of the performance of a regenerative closed Brayton cycle with internal irreversibilities. Energy Convers. Manag. 1997, 3, 871–877. [Google Scholar] [CrossRef]
- Roco, J.M.M.; Velasco, S.; Medina, A.; Hernandez, A.C. Optimum performance of a regenerative Brayton thermal cycle. J. Appl. Phys. 1997, 8, 2735–2741. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, J.L.; Sun, F.; Wu, C. Power density analysis and optimization of a regenerated closed variable-temperature heat reservoir Brayton cycle. J. Phys. D: Appl. Phys. 2001, 3, 1727–1739. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, J.L.; Sun, F.; Wu, C. Performance comparison of an endoreversible closed variable temperature heat reservoir Brayton cycle under maximum power density and maximum power conditions. Energy Convers. Manag. 2002, 4, 33–43. [Google Scholar] [CrossRef]
- Ust, Y.; Sahin, B.; Kodal, A.; Akcay, I.H. Ecological coefficient of performance analysis and optimization of an irreversible regenerative-Brayton heat engine. Appl. Energy 2006, 83, 558–572. [Google Scholar] [CrossRef]
- Chen, L.; Sun, F.; Wu, C. Performance analysis of an irreversible Brayton heat engine. J. Energy Inst. 1997, 70, 2–8. [Google Scholar]
- Chen, L.; Ni, N.; Cheng, G.; Sun, F.; Wu, C. Performance analysis for a real closed regenerated Brayton cycle via methods of finite time thermodynamics. Int. J. Ambient Energy 1999, 20, 95–104. [Google Scholar] [CrossRef]
- Chen, L.; Ni, N.; Wu, C.; Sun, F. Performance analysis of a closed regenerated Brayton heat pump with internal irreversibilities. Int. J. Energy Res. 1999, 23, 1039–1050. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, J.; Sun, F.; Wu, C. Performance comparison of an irreversible closed Brayton cycle under maximum power density and maximum power conditions. Exergy 2002, 2, 345–351. [Google Scholar] [CrossRef]
- Chen, L.; Wang, W.; Sun, F.; Wu, C. Power and efficiency analysis of an endoreversible closed intercooled regenerated Brayton cycle. Exergy 2004, 1, 475–494. [Google Scholar] [CrossRef]
- Wang, W.; Chen, L.; Sun, F.; Wu, C. Heat transfer effect on the performance of an endoreversible closed intercooled regenerated Brayton cycle. Int. J. Ambient Energy 2004, 25, 199–211. [Google Scholar] [CrossRef]
- Chen, L.; Wang, J.; Sun, F.; Wu, C. Power density optimization of an endoreversible closed variable-temperature heat reservoir intercooled regenerated Brayton cycle. Int. J. Ambient Energy 2006, 27, 99–112. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, L.; Sun, F.; Wu, C. Second-law analysis and optimization for combined Brayton and inverse Brayton cycles. Int. J. Ambient Energy 2007, 28, 15–26. [Google Scholar] [CrossRef]
- Zhang, Wi.; Chen, L.; Sun, F. Power and efficiency optimization for combined Brayton and inverse Brayton cycles. Appl. Therm. Eng. 2009, 29, 2885–2894. [Google Scholar] [CrossRef]
- Chen, L.; Wang, J.; Sun, F.; Wu, C. Power density optimization of an irreversible variable-temperature heat reservoir closed intercooled regenerated Brayton cycle. Int. J. Ambient Energy 2009, 30, 9–26. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, L.; Sun, F.; Wu, C. Second law analysis and parametric study for combined Brayton and two parallel inverse Brayton cycles. Int. J. Ambient Energy 2009, 30, 179–192. [Google Scholar] [CrossRef]
- Chen, L.; Yang, B.; Sun, F.; Wu, C. Exergetic performance optimisation of an endoreversible intercooled regenerated Brayton cogeneration plant. Part 1: Thermodynamic model and parametric analysis. Inter. J. Ambient Energy 2011, 32, 116–123. [Google Scholar] [CrossRef]
- Yang, B.; Chen, L.; Sun, F.; Wu, C. Exergetic performance optimisation of an endoreversible intercooled regenerated Brayton cogeneration plant. Part 2: Exergy output rate and exergy efficiency optimis ation. Inter. J. Ambient Energy 2012, 33, 98–104. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Z.; Sun, F. Thermodynamic modelling for open combined regenerative Brayton and inverse Brayton cycles with regeneration before the inverse cycle. Entropy 2012, 14, 58–73. [Google Scholar] [CrossRef]
- Tyagi, S.K.; Kaushik, S.C.; Salhotra, R. Ecological optimization and performance study of irreversible Stirling and Ericsson heat engines. J. Phys. D: Appl. Phys. 2002, 35, 2668–2675. [Google Scholar] [CrossRef]
- Blank, D.A.; Wu, C. Power limit of an endoreversible Ericsson cycle with regeneration. Energy Convers. Mang. 1996, 37, 59–66. [Google Scholar] [CrossRef]
- Chen, J.; Yan, Z.; Chen, L.; Anderson, B. Efficiency bound of a solar-driven Stirling heat engine system. Int. J. Energy Res. 1998, 22, 805–812. [Google Scholar] [CrossRef]
- Blank, D.A.; Wu, C. Finite time power limit for solar-radiant Ericsson engines for space applications. Appl. Therm. Eng. 1996, 18, 1347–1357. [Google Scholar] [CrossRef]
- Erbay, L.B.; Yavuz, H. The maximum cooling density of a realistic Stirling refrigerator. J. Phys. D: Appl. Phys. 1998, 31, 291–293. [Google Scholar] [CrossRef]
- Erbay, L.B.; Yavuz, H. Analysis of Stirling heat engine at maximum power conditions. Energy 1997, 22, 645–650. [Google Scholar] [CrossRef]
- Erbay, L.B.; Yavuz, H. Analysis of an irreversible Ericsson engine with a realistic regenerator. Appl. Energy 1999, 62, 155–167. [Google Scholar] [CrossRef]
- Erbay, L.B.; Yavuz, H. Optimization of the irreversible Stirling heat engine. Int. J. Energy Res. 1999, 23, 863–873. [Google Scholar] [CrossRef]
- Ust, Y.; Sahin, B.; Sogut, O.S. Performance analysis and optimization of an irreversible dual-cycle based on an ecological coefficient of performance criterion. Appl. Energy 2005, 82, 23–39. [Google Scholar] [CrossRef]
- Ge, Y.; Chen, L.; Sun, F. Optimal paths of piston motion of irreversible diesel cycle for minimum entropy generation. Therm. Sci. 2011, 15, 975–993. [Google Scholar] [CrossRef]
- Wu, F.; Yang, Z.; Chen, L.; Liu, X.; Wu, S. Power output and efficiency of a reversible quantum Otto cycle. Therm. Sci. 2010, 14, 879–886. [Google Scholar] [CrossRef]
- Ge, Y.; Chen, L.; Sun, F. Finite time thermodynamic modeling and analysis for an irreversible Atkinson cycle. Therm. Sci. 2010, 14, 887–896. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, J. Optimum performance analysis of an irreversible Diesel heat engine affected by variable heat capacities of powering fluid. Energy Convers. Manag. 2007, 48, 2595–2603. [Google Scholar] [CrossRef]
- Zhao, Y.; Lin, B.; Zhang, Y.; Chen, J. Performance analysis and parametric optimum design of an irreversible Diesel heat engine. Energy Convers. Manag. 2006, 47, 3383–3392. [Google Scholar] [CrossRef]
- Chen, L.; Wu, C.; Sun, F.; Cao, S. Heat transfer effects on the net power output and efficiency characteristics for an air standard Otto cycle. Energy Convers. Manag. 1998, 39, 643–648. [Google Scholar] [CrossRef]
- Chen, L.; Ge, Y.; Sun, F.; Wu, C. Effects of heat transfer, friction and variable specific heats of powering fluid on performance of an irreversible dual cycle. Energy Convers. Manag. 2006, 47, 3224–3234. [Google Scholar] [CrossRef]
- Sanchez Salas, N.; Velasco, S.; Hernandez, A.C. Unified working regime of irreversible Carnot-like heat engines with nonlinear heat transfer laws. Energy Convers. Manag. 2002, 43, 2341–2348. [Google Scholar] [CrossRef]
- Hou, S.S. Comparison of performances of air standard Atkinson and Otto cycles with heat transfer considerations. Energy Convers. Manag. 2007, 48, 1683–1690. [Google Scholar] [CrossRef]
- Hou, S.S. Heat transfer effects on the performance of an air standard Dual cycle. Energy Convers. Manag. 2004, 45, 3003–3015. [Google Scholar] [CrossRef]
- Bhattacharyya, S. Optimizing an irreversible Diesel cycle-fine tuning of compression ratio and cut-off ratio. Energy Convers. Manag. 2000, 41, 847–854. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, T.; Sun, F.; Wu, C. The power and efficiency characteristics for an irreversible Otto cycle. Int. J. Ambient Energy 2003, 24, 195–200. [Google Scholar] [CrossRef]
- Ge, Y.; Chen, L.; Sun, F.; Wu, C. Thermodynamic simulation of performance of an Otto cycle with heat transfer and variable specific heats for the powering fluid. Int. J. Therm. Sci. 2005, 44, 506–511. [Google Scholar] [CrossRef]
- Ge, Y.; Chen, L.; Sun, F.; Wu, C. The effects of variable specific-heats of the powering fluid on the performance of an irreversible Otto cycle. Int. J. Exergy 2005, 2, 274–283. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, J. An irreversible heat-engine model including three typical thermodynamic cycles and the optimal performance analysis. Int. J. Therm. Sci. 2007, 46, 605–613. [Google Scholar] [CrossRef]
- Parlak, A. Comparative performance analysis of irreversible dual and Diesel cycles under maximum power conditions. Energy Convers. Manag. 2007, 46, 351–359. [Google Scholar] [CrossRef]
- Al-Sarkhi, A.; Jaber, J.O.; Abu-Qudais, M.; Probert, S.D. Effects of friction and temperature-dependent specific-heat of the powering fluid on the performance of a Diesel-engine. Appl. Energy 2006, 83, 153–165. [Google Scholar] [CrossRef]
- Klein, S.A. An explanation for observed compression ratios in internal combustion engines. J. Eng. Gas Turbines Power 1991, 113, 511–513. [Google Scholar] [CrossRef]
- Orlov, V.N.; Berry, R.S. Power and efficiency limits for internal combustion engines via methods of finite-time thermodynamics. J. Appl. Phys. 1993, 74, 4317–4322. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, Y.; He, J. Optimization criteria for the important parameters of an irreversible Otto heat-engine. Appl. Energy 2006, 83, 228–238. [Google Scholar] [CrossRef]
- Angulo-Brown, F.; Rocha-Martinez, J.A.; Navarrete-Gonzalez, T.D. A non-endoreversible Otto cycle model: Improving power output and efficiency. J. Phys. D: Appl. Phys. 1996, 29, 80–83. [Google Scholar] [CrossRef]
- Curto-Risso, P.L.; Medina, A.; Hernández, A.C. Theoretical and simulated models for an irreversible Otto cycle. J. Appl. Phys. 2008, 104, 094911. [Google Scholar] [CrossRef]
- Curto-Risso, P.L.; Medina, A.; Hernández, A.C. Optimizing the operation of a spark ignition engine: Simulation and theoretical tools. J. Appl. Phys. 2009, 105, 094904. [Google Scholar] [CrossRef]
- Kolchin, A.; Demidov, V. Design of Automotive Engines; MIR Publishers: Moscow, Russia, 1984. [Google Scholar]
- Ust, Y.; Sahin, B. Performance optimization of irreversible refrigerators based on a new thermo-ecological criterion. Int. J. Refrig. 2007, 30, 527–534. [Google Scholar] [CrossRef]
- Sun, F.; Chen, W.; Chen, L.; Wu, C. Optimal performance of an endoreversible Carnot heat pump. Energy Convers. Manag. 1997, 38, 1439–1443. [Google Scholar] [CrossRef]
- Wu, C.; Chen, L.; Sun, F. Effects of heat transfer law on finite-time Exergoeconomic performance of Carnot heat pump. Energy Convers. Manag. 1998, 39, 579–588. [Google Scholar] [CrossRef]
- Bhardwaj, P.K.; Kaushik, S.C.; Jain, S. Finite time optimization of an endoreversible and irreversible vapour absorption refrigeration system. Energy Convers. Manag. 2003, 44, 1131–1144. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, X.; Sun, F.; Wu, C. Ecological optimization for generalized irreversible Carnot refrigerators. J. Phys. D: Appl. Phys. 2005, 38, 113–118. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, L.; Sun, F.; Wu, C. Exergy based ecological optimization for a generalized irreversible Carnot refrigerator. J. Energy Inst. 2006, 79, 42–46. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, X.; Sun, F.; Wu, C. Ecological optimization of a generalized irreversible Carnot refrigerator for a generalized heat transfer law. Int. J. Ambient Energy 2007, 28, 213–219. [Google Scholar] [CrossRef]
- Li, J.; Chen, L.; Sun, F.; Wu, C. Ecological performance of an endoreversible Carnot refrigerator with complex heat transfer law. Int. J. Ambient Energy 2011, 32, 31–36. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Sun, F. Ecological optimization of a generalized irreversible Carnot refrigerator in case of Q ∝ (ΔTn)m. Int. J. Sustain. Energy 2012, 31, 59–72. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, L.; Sun, F.; Wu, C. Effect of heat transfer law on the ecological optimization of a generalized irreversible Carnot heat pump. Int. J. Exergy 2005, 2, 423–436. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, L.; Sun, F.; Wu, C. The ecological optimization of a generalized irreversible Carnot heat pump for a generalized heat transfer law. J. Energy Inst. 2005, 78, 5–10. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Sun, F.; Wu, C. Effect of a complex generalized heat transfer law on ecological performance of an endoreversible Carnot heat pump. Int. J. Ambient Energy 2009, 30, 102–108. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Sun, F. Optimal ecological performance of a generalized irreversible Carnot heat pump with a generalized heat transfer law. Termotehnica 2009, 13, 61–68. [Google Scholar]
- Ust, Y.; Akkaya, A.V.; Safa, A. Analysis of a vapour compression refrigeration system via exergetic performance coefficient criterion. J. Energy Inst. 2011, 84, 66–72. [Google Scholar] [CrossRef]
- Chen, L.; Tu, Y.; Sun, F. Exergetic efficiency optimization for real regenerated air refrigerators. Appl. Therm. Eng. 2011, 31, 3161–3167. [Google Scholar] [CrossRef]
- Khaliq, A. Finite-time heat-transfer analysis and generalized power-optimization of an endoreversible Rankine heat-engine. Appl. Energy 2004, 79, 27–40. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Açıkkalp, E.; Yamık, H. Limits and Optimization of Power Input or Output of Actual Thermal Cycles. Entropy 2013, 15, 3219-3248. https://doi.org/10.3390/e15083309
Açıkkalp E, Yamık H. Limits and Optimization of Power Input or Output of Actual Thermal Cycles. Entropy. 2013; 15(8):3219-3248. https://doi.org/10.3390/e15083309
Chicago/Turabian StyleAçıkkalp, Emin, and Hasan Yamık. 2013. "Limits and Optimization of Power Input or Output of Actual Thermal Cycles" Entropy 15, no. 8: 3219-3248. https://doi.org/10.3390/e15083309
APA StyleAçıkkalp, E., & Yamık, H. (2013). Limits and Optimization of Power Input or Output of Actual Thermal Cycles. Entropy, 15(8), 3219-3248. https://doi.org/10.3390/e15083309



