The Initial Common Pathway of Inflammation, Disease, and Sudden Death †
Abstract
:1. Introduction
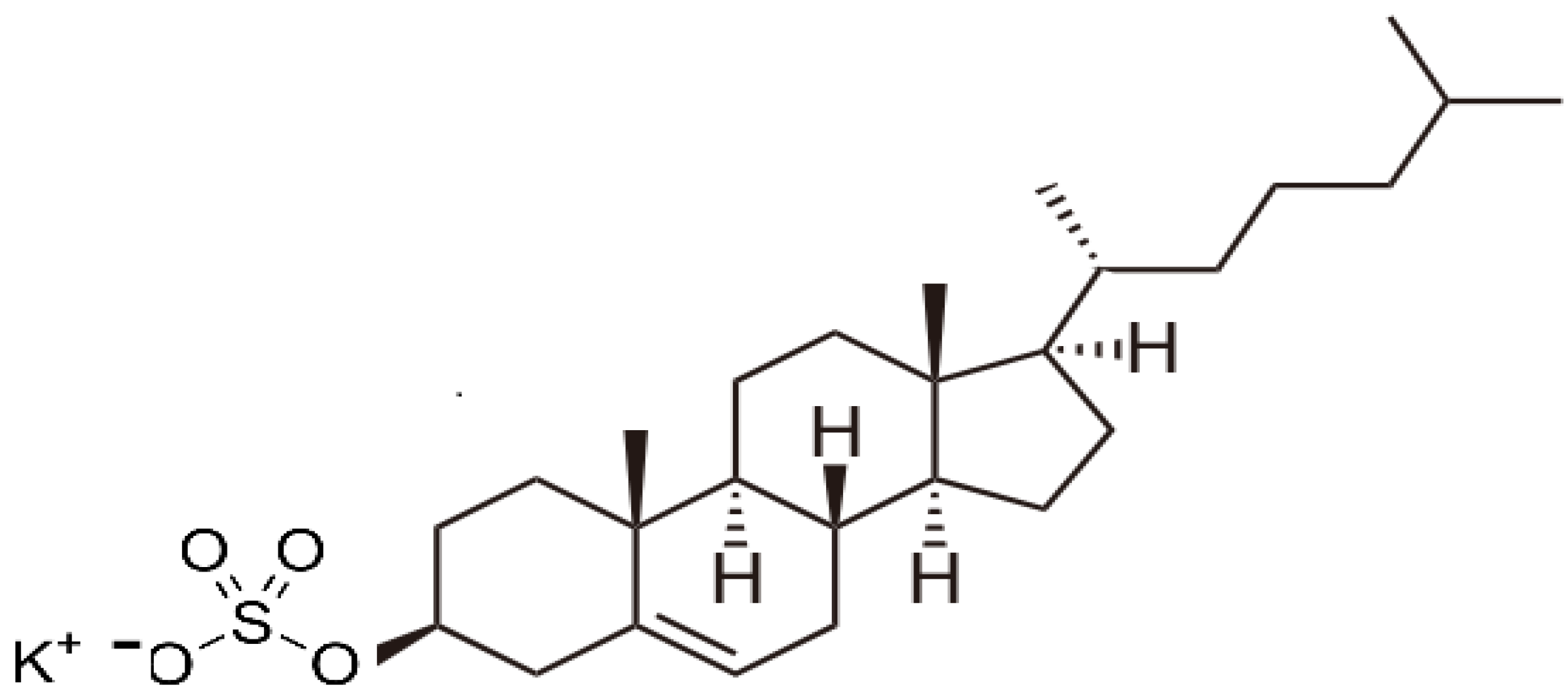
2. Results
2.1. Stress Induced Breathing Patterns Following Vaccination
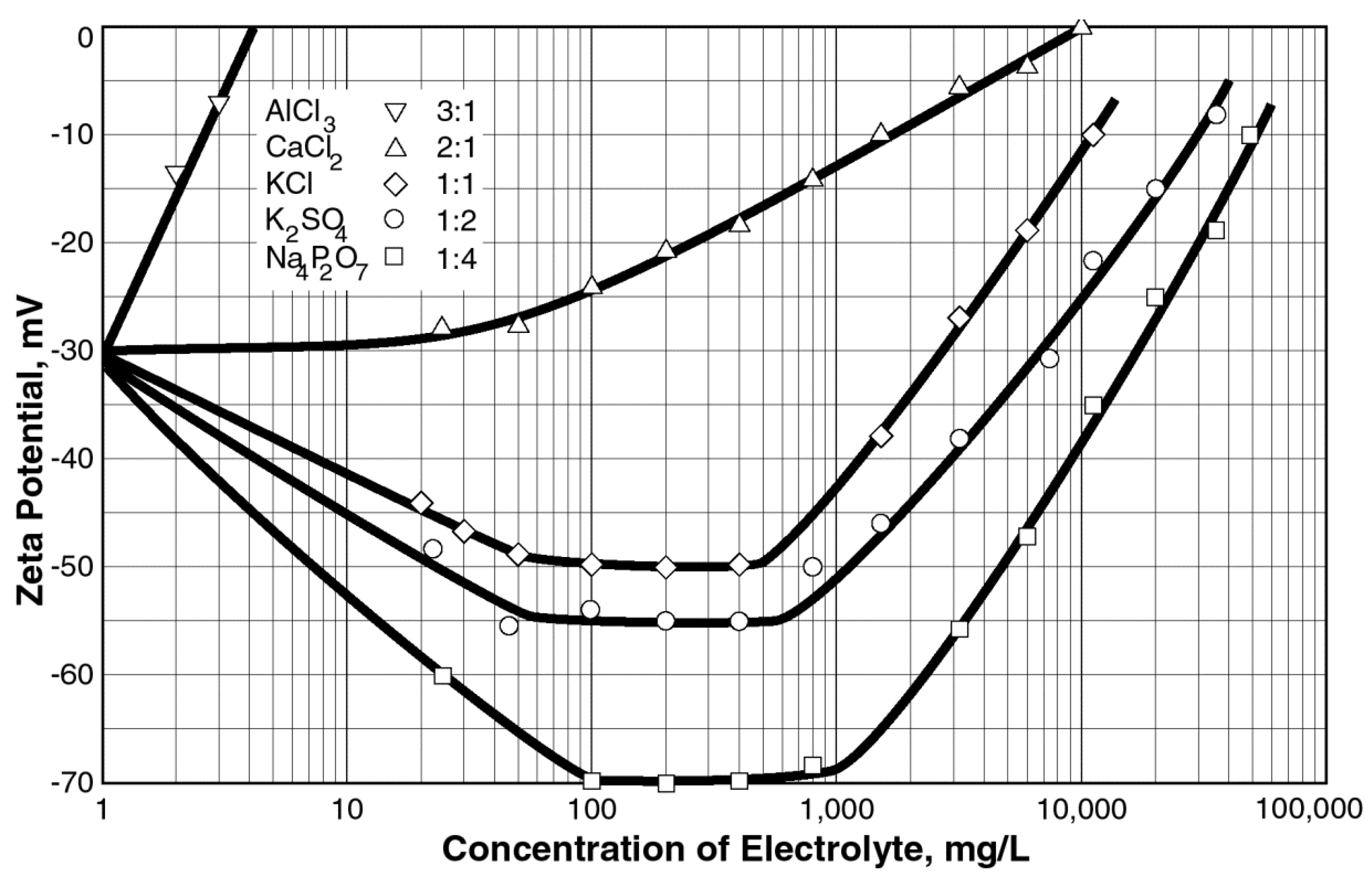
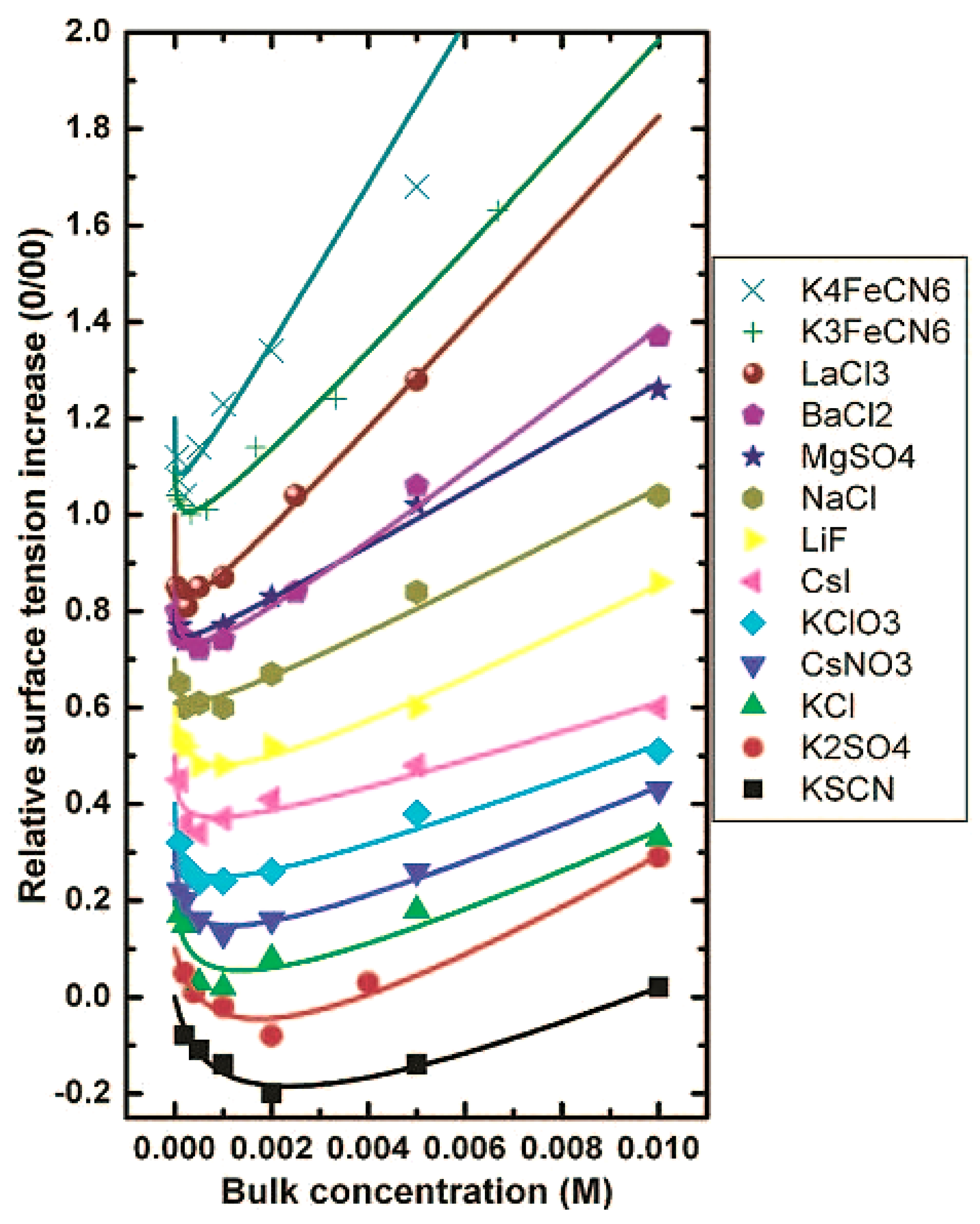
2.2. Hofmeister Effect
2.3. Serum Albumin and Zeta Potential
2.4. Origin of the Surface Charge
2.5. Surfactant-Induced Interfacial Water Stress
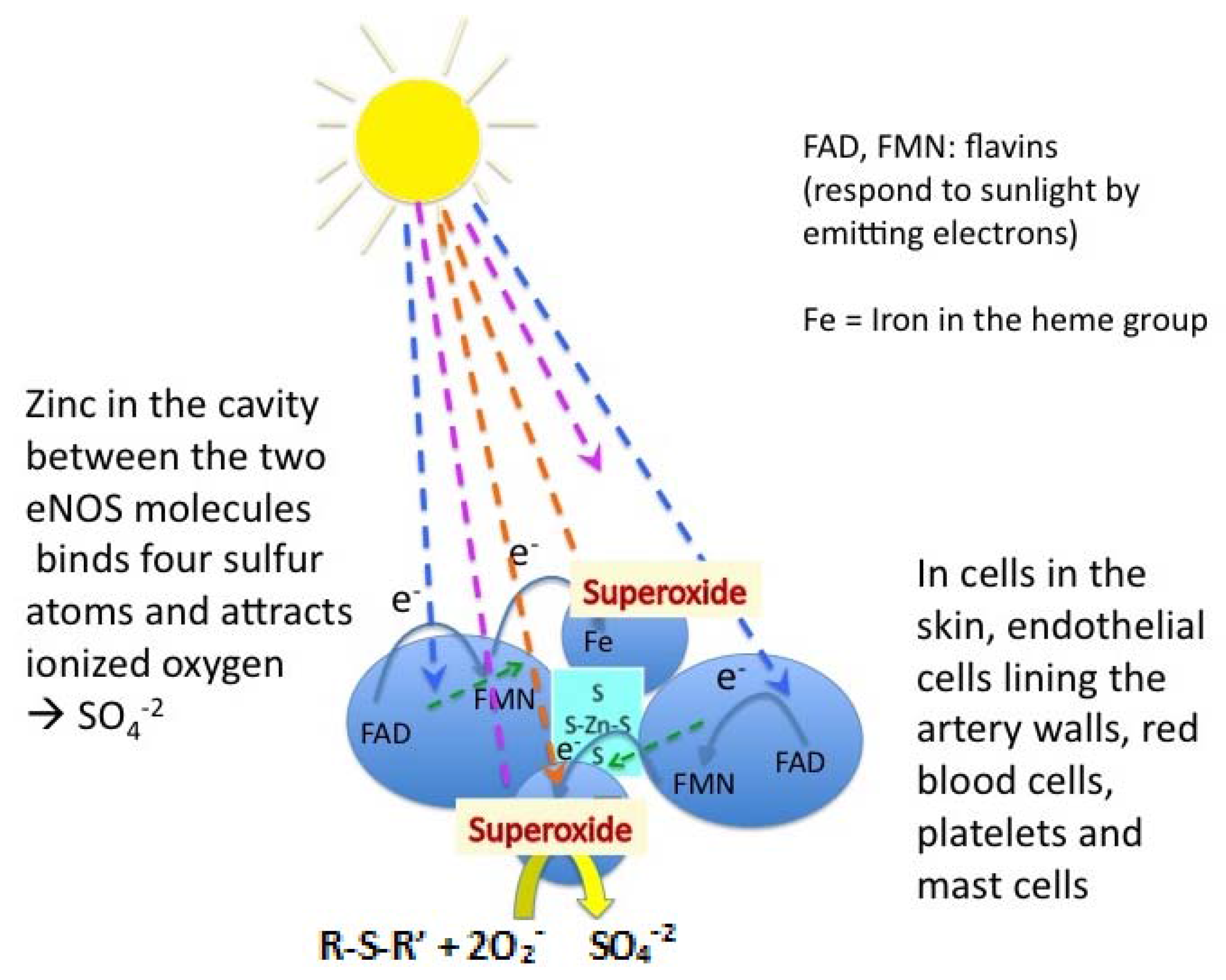
2.6. Zeta Potential and Cardiovascular Disease
2.7. The Role of Bio-sulfates in Maintaining Cell Membrane Function
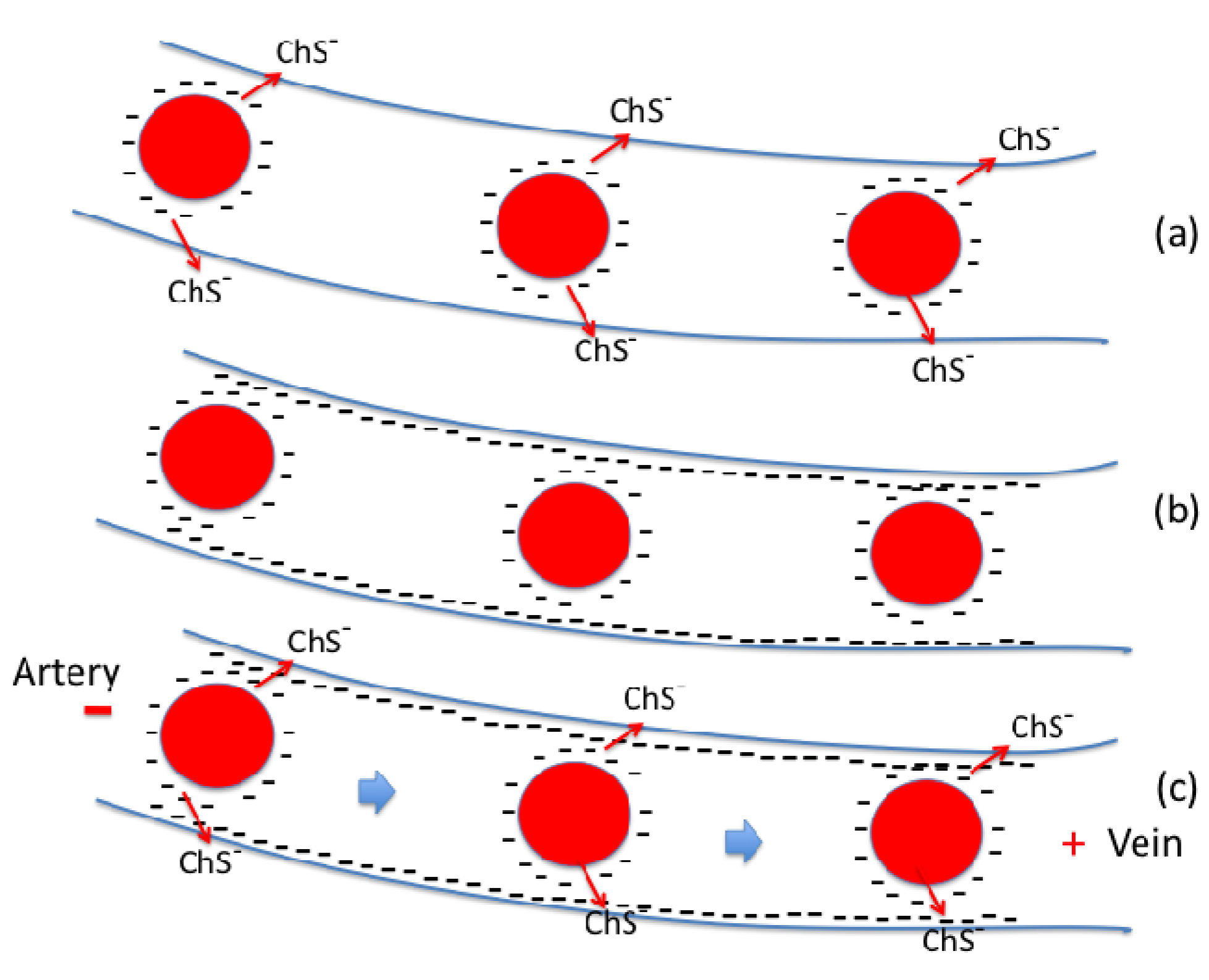
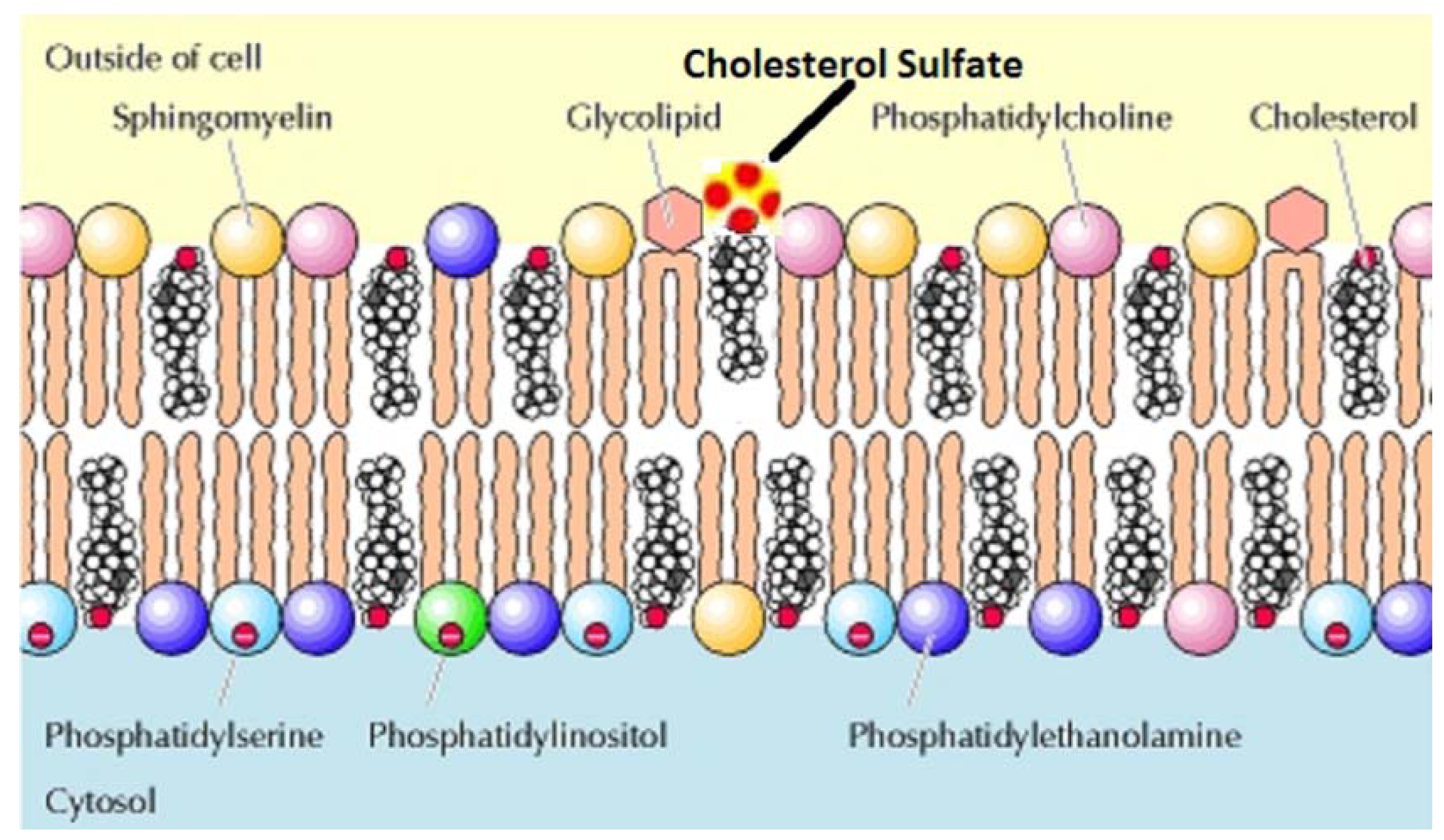
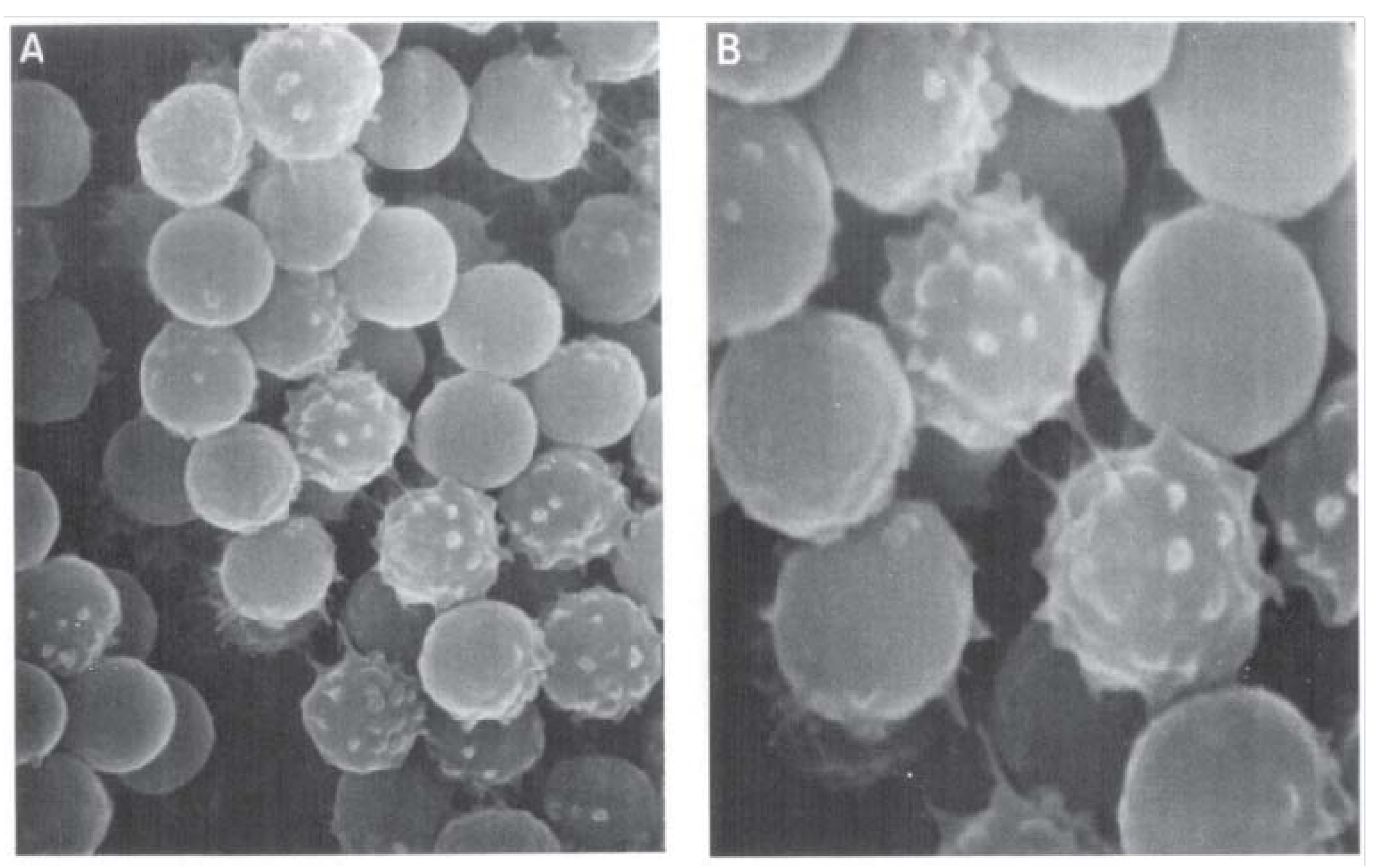
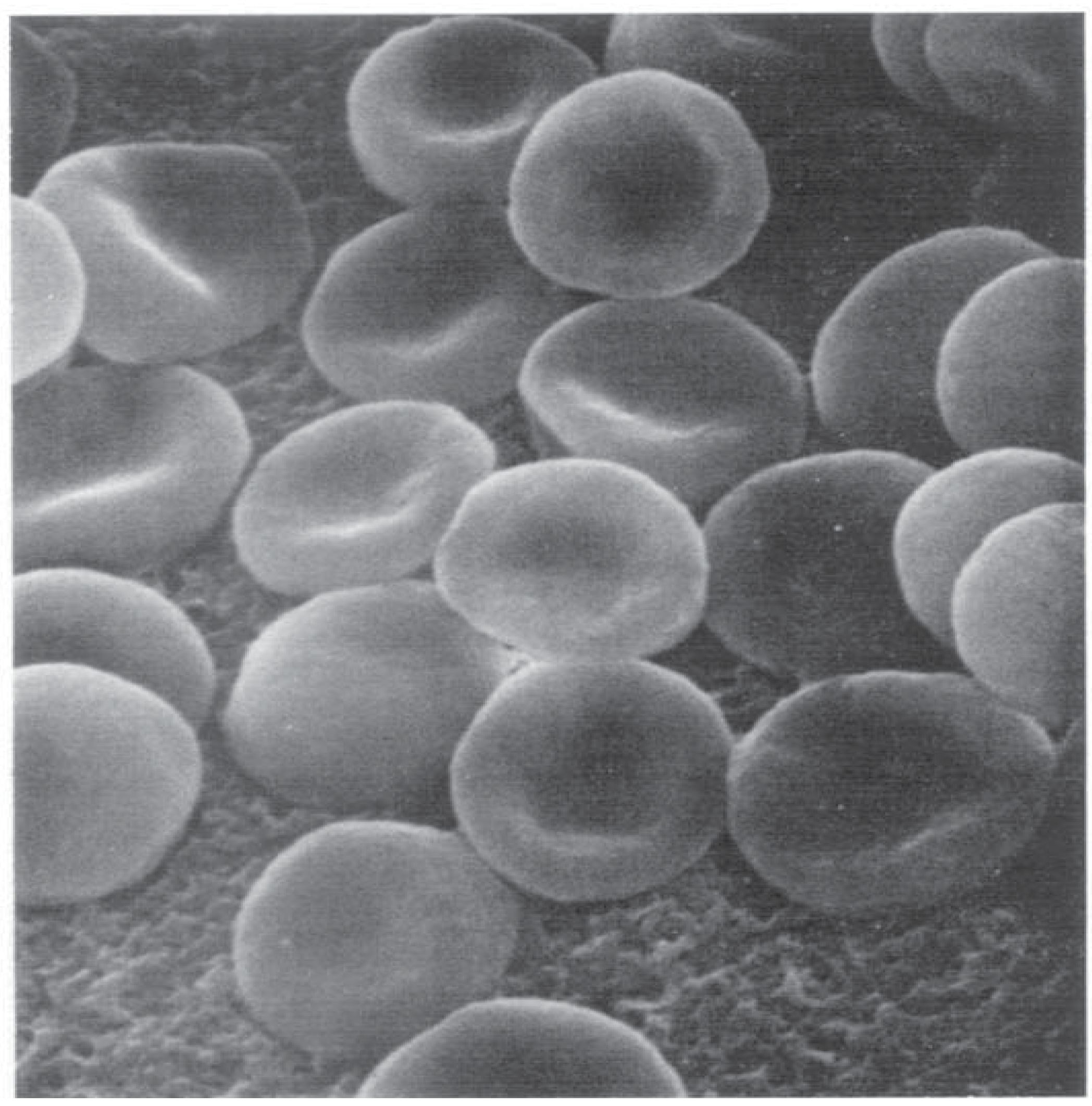
2.8. The Major Determinants of RBC Deformability
2.9. Acute Shock and Role of Endothelial NOS-derived NO in SDS
3. Discussion
4. Conclusions
Acknowledgments
Glossary of Terms
| Anaphylaxis | a severe, rapidly progressing, life-threatening, generalized allergic reaction. |
| Biological equipoise | a stable, non-equilibrium, dissipative system synonymous with life. |
| Cholesterol sulfate (Ch-S) | quantitatively the most important known sterol sulfate in human plasma where it regulates the activity of the serine proteases, in cell membranes where it has a stabilizing role, and in platelet membranes where it supports platelet adhesion. |
| Coherence domain (CD) | a water CD is a collection of liquid water molecules which oscillate in unison in tune with a self-trapped electromagnetic field at a well-defined frequency. The coherent oscillations produce an ensemble of quasi-free electrons, able to collect noise energy from the environment and transform it into high-grade coherent energy in the form of electron vortices. This high-grade energy may then activate biomolecules resonating with the water CD. |
| Colloidal instability | a property attributed to a colloidal suspension that develops when stabilizing repulsive steric and electrostatic forces between colliding particles are insufficient to prevent their natural tendency to aggregate into masses large enough to precipitate. |
| Colloidal suspension | a colloid that has a continuous liquid phase in which a solid is suspended in a liquid, e.g., our flowing blood. |
| Exclusion zone (EZ) | a glass-like, gel phase consisting of water CDs resonating in-phase, adjacent to hydrophilic surfaces, several hundred micrometers wide which excludes colloidal particles and various solutes as a consequence of water molecules re-orienting to produce a more ordered structure, which then excludes the particles. |
| Exogenous interfacial water stress (EIWS) | a property of interfacial water—interfacial tension—which destabilizes enzymes, protein structure, and cell membranes. |
| Glycosaminoglycans | a group of high molecular weight linear polysaccharides constructed with various disaccharide repeating units usually occurring in proteoglycans, including the chondroitin sulfates, dermatan sulfates, heparan sulfate and heparin, keratan sulfates, and hyaluronic acid, with the primary configurations containing an amino sugar and a uronic acid. |
| Hofmeister series | the Hofmeister series or lyotropic series is a classification of ions in order of their ability to change water structure. A scale can be established wherein: kosmotropic ions or nonionic kosmotropes stabilize proteins and hydrophobic aggregates in solution and reduce the solubility of hydrophobes, and chaotropic ions or nonionic chaotropes unfold proteins, destabilize hydrophobic aggregates and increase the solubility of hydrophobes. |
| Interfacial tension | a measure of the cohesive (excess) energy present at an interface arising from the imbalance of forces between molecules at an interface (gas/liquid, liquid/liquid, gas/solid, liquid/solid). The excess energy is called surface free energy and can be quantified as a measurement of energy/area, i.e., the energy required to increase the surface area of the interface by a unit amount. |
| Jones-Ray effect | the observation of a minimum in the surface tension at very low ionic concentrations (<1 mM). |
| Kinetic Terahertz Absorption (KITA) | KITA monitors the changing THz electric field pulse shape on the picosecond time scale Δt, as a chemical reaction proceeds on a longer time scale t, and has been applied to measure the changing protein-hydration-water dynamics. |
| Shwartzman reaction | or Shwartzman phenomenon occurs when a “preparatory”, i.e., injection of bacterial filtrates, is followed after a proper time interval by intravenous “provocation” with the same or some similar material. |
| Surface tension | the cohesive forces among liquid molecules responsible for the surface free energy at a gas liquid interface is produced by the attraction between the molecules being directed away from the surface as surface molecules are more attracted to the molecules within the liquid than they are to molecules of the gas at the surface. |
| Surfactant | in principle, anything can be called a surfactant that affects the surface tension of a liquid, the interfacial tension between two liquids, or that between a liquid and a solid. |
| Thrombohemorrhagic phenomenon (THP) | a change characterized by thrombosis and hemorrhage. |
| Zeta potential (ZP) | a measure of the net charge density of a particle. |
References
- Bechamp, A. The Blood and Its Third Element; Metropolis Inc: New York, NY, USA, 2002. [Google Scholar]
- Selye, H. In Vivo: The Case for Supramolecular Biology; Liveright Publishing Corporation: New York, NY, USA, 1967. [Google Scholar]
- Selye, H. Thrombohemorrhagic Phenomena; Charles, C., Ed.; Thomas: Springfield, IL, USA, 1966. [Google Scholar]
- Filiano, J.J.; Kinney, H.C. A perspective on neuropathologic findings in victims of the sudden infant death syndrome: The triple-risk model. Biol. Neonate 1994, 65, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Vatta, M.; Dumaine, R.; Varghese, G.; Richard, T.A.; Shimizu, W.; Aihara, N.; Nademanee, K.; Brugada, R.; Brugada, J.; Veerakul, G.; et al. Genetic and biophysical basis of sudden unexplained nocturnal death syndrome (SUNDS), a disease allelic to Brugada syndrome. Hum. Mol. Genet. 2002, 11, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Kinney, H.C. Brainstem mechanisms underlying the sudden infant death syndrome: Evidence from human pathologic studies. Dev. Psychobiol. 2009, 51, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Krous, H.F.; Beckwith, J.B.; Byard, R.W.; Rognum, T.O.; Bajanowski, T.; Corey, T.; Cutz, E.; Hanzlick, R.; Keens, T.G.; Mitchell, E.A. Sudden infant death syndrome and unclassified sudden infant deaths: A definitional and diagnostic approach. Pediatrics 2004, 114, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Blood-Siegfried, J.; Bowers, M.T.; Lorimer, M. Is shock a key element in the pathology of sudden infant death syndrome (SIDS)? Biol. Res. Nurs. 2009, 11, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Buttram, H. Shaken baby syndrome or vaccine-induced encephalitis? Med. Sentin. 2001, 6, 83–89. [Google Scholar]
- Mage, D.T.; Donner, M. A unifying theory for SIDS. Int. J. Pediatr. 2009. [Google Scholar] [CrossRef] [PubMed]
- Scheibner, V. Adverse effects of adjuvants in vaccines. Nexus 2000, 8, 37–40. [Google Scholar]
- Scheibner, V. Vaccination: One Hundred Years of Orthodox Research Shows That Vaccines Represent a Medical Assault on the Immune System; New Atlantean Press: Santa Fe, NM, USA, 1993. [Google Scholar]
- Scheibner, V. Shaken baby syndrome: The vaccination link. Nexus Magazine 1998, 5, 35–38, 41–44, continued on page 87. [Google Scholar]
- Scheibner, V. Adverse effects of adjuvants in vaccines. Nexus Magazine 2001, 8, 37–40, 41–44, continued on page 84. [Google Scholar]
- Scheibner, V. Dynamics of critical days as part of the dynamics of non-specific stress syndrome discovered during monitoring with Cotwatch Breathing Monitor. J. Australas. Coll. Nutr. Environ. Med. 2004, 23, 1–5. [Google Scholar]
- Hofmeister, F. Naunyn-Schmiedebergs Zur Lehre von der Wirkung der Salze. (in German). Arch. Pharmacol. 1888, 24, 247–260. [Google Scholar] [CrossRef]
- Setschenow, J. Über die Konstitution der Salzlösungen auf Grund ihres Verhaltens zu Kohlensäure. (in German). Z. Phys. Chem. 1889, 4, 117–125. [Google Scholar]
- Heydweiller, A. Über physikalische Eigenschaften von Lösungen in ihrem Zusammenhang. II. Oberflächenspannung und elektrisches Leitvermögen wässeriger Salzlösungen. (in German). Annalen der Physik 1910, 4, 145–185. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. II. Liquids. J. Am. Chem. Soc. 1917, 39, 1848–1906. [Google Scholar] [CrossRef]
- Dér, A.; Kelemen, L.; Fabian, L.; Taneva, S.G.; Fodor, E.; Pali, T.; Cupane, A.; Cacace, M.G.; Ramsden, J.J. Interfacial water structure controls protein conformation. J. Phys. Chem. B 2007, 111, 5344–5350. [Google Scholar] [CrossRef] [PubMed]
- Dér, A. Salts, Interfacial water and protein conformation. Biotechnol. Biotechnol. Equip. 2008, 22, 629–633. [Google Scholar] [CrossRef]
- Callen, H.B.; Welton, T.A. Irreversibility and generalized noise. Phys. Rev. 1951, 83, 34–40. [Google Scholar] [CrossRef]
- Grassia, P. Dissipation, fluctuations, and conservation laws. Am. J. Phys. 2000, 69, 113–119. [Google Scholar] [CrossRef]
- Neagu, A.; Neagu, M.; Dér, A. Fluctuations and the Hofmeister effect. Biophys. J. 2001, 81, 1285–1294. [Google Scholar] [CrossRef]
- Dér, A.; Ramsden, J.J. Evidence for loosening of a protein mechanism. Naturwissenschaften 1998, 85, 353–355. [Google Scholar]
- Rosina, J.; Kvasnak, E.; Suta, D.; Kolarova, H.; Malek, J.; Krajci, L. Temperature dependence of blood surface tension. Physiol. Res./Acad. Sci. Bohemoslov. 2007, 56, S93–S98. [Google Scholar]
- Coates, E.L.; Li, A.; Nattie, E.E. Widespread sites of brain stem ventilatory chemoreceptors. J. App. Physiol. 1993, 75, 5–14. [Google Scholar]
- Kato, H.; Shibano, M.; Saito, T.; Yamaguchi, J.; Yoshihara, S.; Goto, N. Relationship between hemolytic activity and adsorption capacity of aluminum hydroxide and calcium phosphate as immunological adjuvants for biologicals. Microbiol. Immunol. 1994, 38, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Lupidi, G.; Angeletti, M.; Eleuteri, A.M.; Fioretti, E.; Marini, S.; Gioia, M.; Coletta, M. Aluminum modulation of proteolytic activities. Coordin. Chem. Rev. 2002, 228, 263–269. [Google Scholar] [CrossRef]
- Peeters, B.W.; Cheung, K.S.; Vossen, J.M.; Coenen, A.M. Some solvents for antiepileptics have proepileptic potencies in the WAG/Rij rat model for absence epilepsy. Brain Res. Bull. 1992, 29, 515–517. [Google Scholar] [CrossRef]
- Platt, B.; Busselberg, D. Actions of aluminum on voltage-activated calcium channel currents. Cell. Mol. Neurobiol. 1994, 14, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Preté, P.S.C.; Gomes, K.; Malheiros, S.V.P.; Meirelles, N.C.; de Paula, E. Solubilization of human erythrocyte membranes by non-ionic surfactants of the polyoxyethylene alkyl ethers series. Biophys. Chem. 2002, 97, 45–54. [Google Scholar] [CrossRef]
- Preté, P.S.C.; Malheiros, S.V.P.; Meirelles, N.C.; de Paula, E. Quantitative assessment of human erythrocyte membrane solubilization by Triton X-100. Biophys. Chem. 2002, 97, 1–5. [Google Scholar] [CrossRef]
- Shafer, T.J.; Mundy, W.R.; Tilson, H.A. Aluminum decreases muscarinic, adrenergic, and metabotropic receptor-stimulated phosphoinositide hydrolysis in hippocampal and cortical slices from rat brain. Brain Res. 1993, 629, 133–140. [Google Scholar] [CrossRef]
- Shaw, C.A.; Petrik, M.S. Aluminum hydroxide injections lead to motor deficits and motor neuron degeneration. J. Inorg. Biochem. 2009, 103, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Strupp, W.; Weidinger, G.; Scheller, C.; Ehret, R.; Ohnimus, H.; Girschick, H.; Tas, P.; Flory, E.; Heinkelein, M.; Jassoy, C. Treatment of cells with detergent activates caspases and induces apoptotic cell death. J. Membr. Biol. 2000, 175, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Weis, C.; Haug, A. Aluminum-induced conformational changes in calmodulin alter the dynamics of interaction with melittin. Arch. Biochem. Biophys. 1987, 254, 304–312. [Google Scholar] [CrossRef]
- Joshi, M.; Pathak, S.; Sharma, S.; Patravale, V. Design and in vivo pharmacodynamic evaluation of nanostructured lipid carriers for parenteral delivery of artemether: Nanoject. Int. J. Pharm. 2008, 364, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Goto, N.; Kato, H.; Maeyama, J.; Eto, K.; Yoshihara, S. Studies on the toxicities of aluminium hydroxide and calcium phosphate as immunological adjuvants for vaccines. Vaccine 1993, 11, 914–918. [Google Scholar] [CrossRef]
- Collins, K.D. Ions from the Hofmeister series and osmolytes: Effects on proteins in solution and in the crystallization process. Methods 2004, 34, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, V.; Nielsen, S.O.; Klein, M.L.; Discher, D.E. Unfolding a linker between helical repeats. J. Mol. Biol. 2005, 349, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Sah, H.; Bahl, Y. Effects of aqueous phase composition upon protein destabilization at water/organic solvent interface. J. Control. Release 2005, 106, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Washabaugh, M.W.; Collins, K.D. The systematic characterization by aqueous column chromatography of solutes which affect protein stability. J. Biol. Chem. 1986, 261, 12477–12485. [Google Scholar] [PubMed]
- Gallez, D.; Coakley, W.T. Interfacial instability at cell membranes. Prog. Biophys. Mol. Biol. 1986, 48, 155–199. [Google Scholar] [CrossRef]
- Absolom, D.R. Measurement of Surface Properties of Phagocytes, Bacteria, and Other Particles. In Methods in Enzymology; di Sabato, G., Everse, J., Eds.; Academic Press: Waltham, MA, USA, 1986; Volume 132, pp. 16–95. [Google Scholar]
- Absolom, D.R.; Zingg, W.; Neumann, A.W. Measurement of contact angles on biological and other highly hydrated surfaces. J. Colloid Interface Sci. 1986, 112, 599–601. [Google Scholar] [CrossRef]
- Nevo, A.; de Vries, A.; Katchalsky, A. Interaction of basic polyamino acids with the red blood cell. I. Combination of polylysine with single cells. Biochim. Biophys. Acta 1955, 17, 536–547. [Google Scholar] [CrossRef]
- Karchalsky, A.; Danon, D.; Nevo, A.; de Vries, A. Interaction of basic polyelectrolytes with the red blood cell II. Agglutination of red blood cells by polymeric bases. Biochim. Biophys. Acta 1959, 33, 120–138. [Google Scholar] [CrossRef]
- Coakley, W.T.; Hewison, L.A.; Tilley, D. Interfacial instability and the agglutination of erythrocytes by polylysine. Eur. Biophys. J. 1985, 13, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Kratochvil, A.; Hrncir, E. Correlation between the blood surface tension and the activity of some enzymes. Phys. Res./Acad. Sci. Bohemoslov. 2001, 50, 433–437. [Google Scholar]
- Kratochvil, A.; Hrncir, E. Correlations between the cerebrospinal fluid surface tension value and 1. Concentration of total proteins 2. Number of cell elements. Gen. Physiol. Biophys. 2002, 21, 47–53. [Google Scholar] [PubMed]
- Krishnan, A.; Cha, P.; Liu, Y.H.; Allara, D.; Vogler, E.A. Interfacial energetics of blood plasma and serum adsorption to a hydrophobic self-assembled monolayer surface. Biomaterials 2006, 27, 3187–3194. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.; Wilson, A.; Sturgeon, J.; Siedlecki, C.A.; Vogler, E.A. Liquid-vapor interfacial tension of blood plasma, serum and purified protein constituents thereof. Biomaterials 2005, 26, 3445–3453. [Google Scholar] [CrossRef] [PubMed]
- Esitashvili, T.; Msuknishvili, M. Increase of blood surface tension during acute myocardial infarction. In Proceedings of 8th World Congress on Heart Failure, Internation Academy of Cardiology, Washington, DC, USA, 16 July 2002.
- Surface tensiometry in rheumatology. In Studies in Interface Science; Kazakov, V.N.; Sinyachenko, O.V.; Fainerman, V.B.; Pison, U.; Miller, R. (Eds.) Elsevier: Waltham, MA, USA, 2000; Volume 8, Chapter 5; pp. 191–244.
- Beccerica, E.; Piergiacomi, G.; Curatola, G.; Ferretti, G. Changes of lymphocyte membrane fluidity in rheumatoid arthritis: A fluorescence polarisation study. Ann. Rheum. Dis. 1988, 47, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Kazakov, V.N.; Vozianov, A.F.; Sinyachenko, O.V.; Trukhin, D.V.; Kovalchuk, V.I.; Pison, U. Studies on the application of dynamic surface tensiometry of serum and cerebrospinal liquid for diagnostics and monitoring of treatment in patients who have rheumatic, neurological or oncological diseases. Adv. Colloid Interface 2000, 86, 1–38. [Google Scholar] [CrossRef]
- Weinstein, M.B.; Joensuu, O.I.; Duffy, P.; Bennett, B. Technical difficulties in labeling erythrocytes with 99mTc: Identification of agglutinating substance. J. Nucl. Med. 1971, 12, 183–185. [Google Scholar] [PubMed]
- Lin, M.S.; MacGregor, R.D., Jr.; Yano, Y. Ionic aluminum (3) in generator eluate as an erythrocyte-agglutinating agent. J. Nucl. Med. 1971, 12, 297–299. [Google Scholar] [PubMed]
- Jandl, J.H.; Simmons, R.L. The agglutination and sensitization of red cells by metallic cations: Interactions between multivalent metals and the red-cell membrane. Br. J. Haematol. 1957, 3, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.-P.; Kuo, Y.-C.; Chang, Y.-I. Electrostatic interaction between a charge-regulated particle and a solid surface in electrolyte solution: Effect of cationic electrolytes. Colloid Polym. Sci. 1994, 272, 946–954. [Google Scholar] [CrossRef]
- Chang, Y.-I.; Hsieh, C.-Y. The effect of cationic electrolytes on the electrophoretic properties of bacterial cells. Colloid Surface 1991, 53, 21–31. [Google Scholar] [CrossRef]
- Chang, Y.-I. The effect of cationic electrolytes on the adhesion of cells. Biotechnol. Adv. 1993, 11, 711–724. [Google Scholar] [CrossRef]
- Chang, Y.-I.; Hsu, J.-P. Effect of multivalent cations on the adhesion rate of cellular surfaces bearing ionizable groups. Colloid Surface A 1995, 96, 155–163. [Google Scholar] [CrossRef]
- Hong, Y.; Brown, D.G. Electrostatic behavior of the charge-regulated bacterial cell surface. Langmuir 2008, 24, 5003–5009. [Google Scholar] [CrossRef] [PubMed]
- Hartvig, R.A.; van de Weert, M.; Østergaard, J.; Jorgensen, L.; Jensen, H. Protein adsorption at charged surfaces: The role of electrostatic interactions and interfacial charge regulation. Langmuir 2011, 27, 2634–2643. [Google Scholar] [CrossRef] [PubMed]
- Hartvig, R.A.; van de Weert, M.; Østergaard, J.; Jorgensen, L.; Jensen, H. Formation of dielectric layers and charge regulation in protein adsorption at biomimetic interfaces. Langmuir 2011, 28, 1804–1815. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.E.; Coakley, W.T.; Akay, G. The lateral separation of contacts on erythrocytes agglutinated by polylysine. Cell Biophys. 1992, 20, 125–147. [Google Scholar] [CrossRef] [PubMed]
- Coakley, W.T.; Gallez, D.; de Souza, E.R.; Gauci, H. Ionic strength dependence of localized contact formation between membranes: Nonlinear theory and experiment. Biophys. J. 1999, 77, 817–828. [Google Scholar] [CrossRef]
- Thomas, N.E.; Coakley, W.T. Localized contact formation by erythrocyte membranes: Electrostatic effects. Biophys. J. 1995, 69, 1387–1401. [Google Scholar] [CrossRef]
- Yoshisuke, E. The studies on the effects of gelatin and its related substances. Available online: http://www.ps-corp.co.jp/column/thesis/n001_e.html (accessed on 24 July 2012).
- Kratochvil, A.; Hrncir, E. Surface tension of body fluids in pathophysiology and diagnosis. (in Czech). Prakt. Lek. 1999, 79, 258–259. [Google Scholar]
- Damm, E.M.; Pelkmans, L.; Kartenbeck, J.; Mezzacasa, A.; Kurzchalia, T.; Helenius, A. Clathrin- and caveolin-1-independent endocytosis: Entry of simian virus 40 into cells devoid of caveolae. J. Cell Biol. 2005, 168, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.M.; Sarkar, M. Membrane fusion induced by small molecules and ions. J. Lipids 2011. [Google Scholar] [CrossRef]
- Korte, S.; Wiesinger, A.; Straeter, A.S.; Peters, W.; Oberleithner, H.; Kusche-Vihrog, K. Firewall function of the endothelial glycocalyx in the regulation of sodium homeostasis. Pflug. Arch. Eur. J. Phy. 2012, 463, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Tamasawa, N.; Tamasawa, A.; Takebe, K. Higher levels of plasma cholesterol sulfate in patients with liver cirrhosis and hypercholesterolemia. Lipids 1993, 28, 833–836. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Yamazaki, M. Membrane fusion of giant unilamellar vesicles of neutral phospholipid membranes induced by La3+. Langmuir 2004, 20, 5160–5164. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lee, C.-C.; Huang, H.W. Adhesion and merging of lipid bilayers: A method for measuring the free energy of Adhesion and Hemifusion. Biophys. J. 2011, 100, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, I. The role of bacteriolysis in the pathophysiology of inflammation, infection and post-infectious sequelae. APMIS 2002, 110, 753–770. [Google Scholar] [CrossRef] [PubMed]
- Jinendra, B.; Tamaki, K.; Kuroki, S.; Vassileva, M.; Yoshida, S.; Tsenkova, R. Near infrared spectroscopy and aquaphotomics: Novel approach for rapid in vivo diagnosis of virus infected soybean. Biochem. Biophys. Res. Commun. 2010, 397, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Perera, R.; Riley, C.; Isaac, G.; Hopf-Jannasch, A.S.; Moore, R.J.; Weitz, K.W.; Pasa-Tolic, L.; Metz, T.O.; Adamec, J.; Kuhn, R.J. Dengue virus infection perturbs lipid homeostasis in infected mosquito cells. PLoS Pathog. 2012, 8, e1002584. [Google Scholar] [CrossRef] [PubMed]
- Fiszer-Kierzkowska, A.; Vydra, N.; Wysocka-Wycisk, A.; Kronekova, Z.; Jarzab, M.; Lisowska, K.; Krawczyk, Z. Liposome-based DNA carriers may induce cellular stress response and change gene expression pattern in transfected cells. BMC Mol. Biol. 2011, 12. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, D.; Cortassa, S.; O’Rourke, B.; Aon, M.A. What yeast and cardiomyocytes share: Ultradian oscillatory redox mechanisms of cellular coherence and survival. Integr. Biol. (Camb) 2012, 4, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-W.; Wu, C.-A.; Morrow, W.J.W. Cell death induced by vaccine adjuvants containing surfactants. Vaccine 2004, 22, 1524–1536. [Google Scholar] [CrossRef] [PubMed]
- Gilman, S.; Koller, M.; Black, R.S.; Jenkins, L.; Griffith, S.G.; Fox, N.C.; Eisner, L.; Kirby, L.; Rovira, M.B.; Forette, F.; et al. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology 2005, 64, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Turgut, S.; Bor-Kucukatay, M.; Emmungil, G.; Atsak, P.; Turgut, G. The effects of low dose aluminum on hemorheological and hematological parameters in rats. Arch. Toxicol. 2007, 81, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, M.; Kato-Negishi, M. Link between aluminum and the pathogenesis of alzheimer’s disease: The integration of the aluminum and amyloid cascade hypotheses. Int. J. Alzheimer’s Dis. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Shaw, C.A.; Petrik, M.S. Aluminum hydroxide injections lead to motor deficits and motor neuron degeneration. J. Inorg. Biochem. 2009, 103, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Tomljenovic, L.; Shaw, C.A. Do aluminum vaccine adjuvants contribute to the rising prevalence of autism? J. Inorg. Biochem. 2011, 105, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Lemire, J.; Appanna, V.D. Aluminum toxicity and astrocyte dysfunction: A metabolic link to neurological disorders. J. Inorg. Biochem. 2011, 105, 1513–1517. [Google Scholar] [CrossRef] [PubMed]
- Gardener, H.; Spiegelman, D.; Buka, S.L. Perinatal and neonatal risk factors for autism: A comprehensive meta-analysis. Pediatrics 2011, 128, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Oller, J.W.; Oller, S.D. Autism: The Diagnosis, Treatment,& Etiology of the Undeniable Epidemic; Jones & Bartlett Learning: Sudbury, MA, USA, 2010. [Google Scholar]
- Olmsted, D.; Blaxill, M. The Age of Autism: Mercury, Medicine, and a Man-Made Epidemic; St. Martin’s Press: New York, NY, USA, 2010. [Google Scholar]
- Botkin, S.P. Über die Wirkung der Salze auf die circulirenden rothen Blutkörperchen [On the action of salts on living blood-corpuscles]. (in German). Virchows Arch. Path. Anat. 1858, 15, 173–176. [Google Scholar] [CrossRef]
- Sanarelli, J. Etudes sur la fievre typhoide experimentale. Ann. Inst. Pasteur (Paris) 1894, 8, 193–230. [Google Scholar]
- Hjort, P.; Rapaport, S. The Shwartzman reaction: Pathogenetic mechanisms and clinical manifestations. Annu. Rev. Med. 1965, 16, 135–168. [Google Scholar] [CrossRef] [PubMed]
- Brozna, J.P. Shwartzman reaction. Semin. Thromb. Hemost. 1990, 16, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, B.; Jeljaszewicz, J. A hypothesis for the pathogenesis of the generalized Shwartzman reaction. J. Infect. Dis. 1969, 120, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Levy, G.A.; Schwartz, B.S.; Curtiss, L.K.; Edgington, T.S. Regulatory roles of T mu and T gamma cells in the collaborative cellular initiation of the extrinsic coagulation pathway by bacterial lipopolysaccharide. J. Clin. Invest. 1985, 76, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.J.; Kuhn, R.; Rajewsky, K.; Muller, W.; Menon, S.; Davidson, N.; Grunig, G.; Rennick, D. Interleukin-10 is a central regulator of the response to LPS in murine models of endotoxic shock and the Shwartzman reaction but not endotoxin tolerance. J. Clin. Invest. 1995, 96, 2339–2347. [Google Scholar] [CrossRef] [PubMed]
- Bauer, E. Theoretical Biology; VIEM Publishing House: Moscow-Leningrad, Russia, 1935. [Google Scholar]
- Voeikov, V.; del Giudice, E. Water Respiration-The Basis of the Living State. Water 2009, 1, 52–75. [Google Scholar]
- Shiga, J.; Mori, W. A light- and electron-microscopic study of the so-called uni-visceral Shwartzman reaction. Acta Pathol. Japon. 1980, 30, 705–712. [Google Scholar] [CrossRef]
- Watanabe, T.; Tanaka, K. Electron microscopic observations of the kidney in the generalized Shwartzman reaction. Virchows Arch. A 1977, 374, 183–196. [Google Scholar] [CrossRef]
- Selye, H. Confusion and controversy in the stress field. J. Hum. Stress 1975, 1, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Mori, W. The Shwartzman reaction: A review including clinical manifestations and proposal for a univisceral or single organ third type. Histopathology 1981, 5, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Morowitz, H.; Smith, E. Energy flow and the organization of life: Essays and commentaries. Complexity 2007, 13, 51–59. [Google Scholar] [CrossRef]
- Van der Kraan, P.M.; de Vries, B.J.; Vitters, E.L.; van den Berg, W.B.; van de Putte, L.B. The effect of low sulfate concentrations on the glycosaminoglycan synthesis in anatomically intact articular cartilage of the mouse. J. Orthop. Res. 1989, 7, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Nimni, M.E.; Han, B.; Cordoba, F. Are we getting enough sulfur in our diet? Nutr. Metab. 2007, 4. [Google Scholar] [CrossRef] [PubMed]
- Cole, D.E.; Evrovski, J. The clinical chemistry of inorganic sulfate. Crit. Rev. Clin. Lab. Sci. 2000, 37, 299–344. [Google Scholar] [CrossRef] [PubMed]
- Hoffer, L.J.; Hamadeh, M.J.; Robitaille, L.; Norwich, K.H. Human sulfate kinetics. Am. J. Physiol. 2005, 289, R1372–R1380. [Google Scholar] [CrossRef] [PubMed]
- Markovich, D. Physiological roles and regulation of mammalian sulfate transporters. Physiol. Rev. 2001, 81, 1499–1533. [Google Scholar] [PubMed]
- Bleau, G. Biochimie du Sulfate de Cholestérol; (in French). Université de Montréal: Montreal, QC, Canada, 1972. [Google Scholar]
- Seneff, S.; Davidson, R.; Mascitelli, L. Might cholesterol sulfate deficiency contribute to the development of autistic spectrum disorder? Med. Hypotheses 2011, 78, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Seneff, S.; Wainwright, G.; Mascitelli, L. Is the metabolic syndrome caused by a high fructose, and relatively low fat, low cholesterol diet? Arch. Med. Sci. 2010, 7, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Seneff, S.; Wainwright, G.; Mascitelli, L. Nutrition and Alzheimer’s disease: The detrimental role of a high carbohydrate diet. Eur. J. Int. Med. 2011, 2011, 22, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, G.; Mascitelli, L.; Goldstein, M. Cholesterol-lowering therapy and cell membranes. Stable plaque at the expense of unstable membranes? Arch. Med. Sci. 2009, 5, 289–295. [Google Scholar]
- Seneff, S. Could sulfur deficiency be a contributing factor in obesity, heart disease, alzheimer’s and chronic fatigue syndrome? 2010. Available online: http://people.csail.mit.edu/seneff/sulfur_obesity_alzheimers_muscle_wasting.html (accessed on 24 July 2012).
- Kim, S.J.; Born, B.; Havenith, M.; Gruebele, M. Real-time detection of protein-water dynamics upon protein folding by terahertz absorption spectroscopy. Angew. Chem. Int. Ed. 2008, 47, 6486–6489. [Google Scholar] [CrossRef] [PubMed]
- Heugen, U.; Schwaab, G.; Brundermann, E.; Heyden, M.; Yu, X.; Leitner, D.M.; Havenith, M. Solute-induced retardation of water dynamics probed directly by terahertz spectroscopy. Proc. Natl. Acad. Sci. USA 2006, 103, 12301–12306. [Google Scholar] [CrossRef] [PubMed]
- Heyden, M.; Ebbinghaus, S.; Havenith, M. Terahertz Spectroscopy as a Tool to Study Hydration Dynamics. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd: New York, NY, USA, 2006; pp. 1–19. [Google Scholar]
- Heyden, M.; Sun, J.; Funkner, S.; Mathias, G.; Forbert, H.; Havenith, M.; Marx, D. Dissecting the THz spectrum of liquid water from first principles via correlations in time and space. Proc. Natl. Acad. Sci. USA 2010, 107, 12068–12073. [Google Scholar] [CrossRef] [PubMed]
- Luong, T.Q.; Verma, P.K.; Mitra, R.K.; Havenith, M. Do hydration dynamics follow the structural perturbation during thermal denaturation of a protein: A terahertz absorption study. Biophys. J. 2011, 101, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Grossman, M.; Born, B.; Heyden, M.; Tworowski, D.; Fields, G.B.; Sagi, I.; Havenith, M. Correlated structural kinetics and retarded solvent dynamics at the metalloprotease active site. Nat. Struct. Mol. Biol. 2011, 18, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Ebbinghaus, S.; Meister, K.; Born, B.; DeVries, A.L.; Gruebele, M.; Havenith, M. Antifreeze glycoprotein activity correlates with long-range protein-water dynamics. J. Am. Chem. Soc. 2010, 132, 12210–12211. [Google Scholar] [CrossRef] [PubMed]
- Havenith, M. Watching the dance of water in the hydration shell of ions and biomolecules in the THz frequency range. In Keynote Lecture, 86th Am. Chem. Soc. Colloid & Surface Science Symposium, Johns-Hopkins University, 11 June 2012.
- Chen, L.Y.; Mehta, J.L. Evidence for the presence of L-arginine-nitric oxide pathway in human red blood cells: Relevance in the effects of red blood cells on platelet function. J. Cardiovasc. Pharm. 1998, 32, 57–61. [Google Scholar] [CrossRef]
- Kleinbongard, P.; Schulz, R.; Rassaf, T.; Lauer, T.; Dejam, A.; Jax, T.; Kumara, I.; Gharini, P.; Kabanova, S.; Ozuyaman, B.; et al. Red blood cells express a functional endothelial nitric oxide synthase. Blood 2006, 107, 2943–2951. [Google Scholar] [CrossRef] [PubMed]
- Revel, J.; Ito, S. The Surface Components of Cells; Prentice Hall: Englewood Cliffs, NJ, USA, 1967. [Google Scholar]
- Seaman, G.V. The surface chemistry of the erythrocyte and thrombocyte membrane. J. Supramol. Str. Cell 1973, 1, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Conrad, H.E. Inhibition of Heparan Sulfate Synthesis by Chlorate. Proteoglycan Protocols in Methods in Molecular Biology; Iozzo, R.V., Ed.; Humana Press Inc: Clifton, NJ, USA, 2001; Volume 171, pp. 325–328. [Google Scholar]
- Riddick, T. Control of Colloid Stability through Zeta Potential (with a Closing Chapter on Its Relationship to Cardiovascular Disease); Livingston Pub. Co.: Wynnewood, PA, USA, 1968. [Google Scholar]
- Carter, D.; Ho, J. Structure of serum albumin. In Advances in Protein Chemistry; Academic Press: San Diego, CA, USA, 1994; Volume 45, Chapter 4. [Google Scholar]
- Rezwan, K.; Meier, L.; Rezwan, M. UV-Vis Measurements. Langmuir 2004, 20, 10055–10061. [Google Scholar] [CrossRef] [PubMed]
- Zalups, R.K. Molecular interactions with mercury in the kidney. Pharmacol. Rev. 2000, 52, 113–143. [Google Scholar] [PubMed]
- Ritz, T.; Simon, E.; Trueba, A.F. Stress-induced respiratory pattern changes in asthma. Psychosom. Med. 2011, 73, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Guz, A.; Trenchard, D. The mechanism of tachypnoea in pulmonary microembolism and pulmonary inflammation. Acta Neurobiol. Exp. 1973, 33, 9–14. [Google Scholar]
- Abraham, E.; Shoemaker, W.C.; Bland, R.D.; Cobo, J.C. Sequential cardiorespiratory patterns in septic shock. Crit. Care Med. 1983, 11, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Okrent, D.G.; Abraham, E.; Winston, D. Cardiorespiratory patterns in viral septicemia. Am. J. Med. 1987, 83, 681–686. [Google Scholar] [CrossRef]
- Nikki, P.; Tahvanainen, J.; Rasanen, J.; Makelainen, A. Ventilatory pattern in respiratory failure arising from acute myocardial infarction. II. PtcO2 and PtcCO2 compared to Pao2 and PaCO2 during IMV4 vs. IPPV12 and PEEP0 vs. PEEP10. Crit. Care Med. 1982, 10, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.C.; Klassen, A.C.; Heaney, L.M.; Resch, J.A. Respiratory rate and pattern disturbances in acute brain stem infarction. Stroke 1976, 7, 382–385. [Google Scholar] [CrossRef] [PubMed]
- Connolly, G.; Razak, A.R.; Hayanga, A.; Russell, A.; McKenna, P.; McNicholas, W.T. Inspiratory flow limitation during sleep in pre-eclampsia: Comparison with normal pregnant and nonpregnant women. Eur. Respir. J. 2001, 18, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Tukur, J. The use of magnesium sulphate for the treatment of severe pre-eclampsia and eclampsia. Ann. Afr. Med. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Vetander, M.; Helander, D.; Lindquist, C.; Hedlin, G.; Alfvén, T.; Östblom, E.; Nilsson, C.; Lilja, G.; Wickman, M. Classification of anaphylaxis and utility of the EAACI Taskforce position paper on Anaphylaxis in Children. Pediatr. Allergy Immunol. 2011, 22, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Bloch, S.; Lemeignan, M.; Aguilera, N. Specific respiratory patterns distinguish among human basic emotions. Int. J. Psychophysiol. 1991, 11, 141–154. [Google Scholar] [CrossRef]
- Boiten, F.A.; Frijda, N.H.; Wientjes, C.J. Emotions and respiratory patterns: Review and critical analysis. Int. J. Psychophysiol. 1994, 17, 103–128. [Google Scholar] [CrossRef]
- Selye, H. The Stress of Life; McGill University Press: Montreal, QC, Canada, 1978. [Google Scholar]
- Kunz, W.; Lo Nostro, P.; Ninham, B.W. The present state of affairs with Hofmeister effects. Curr. Opin. Colloid Interface 2004, 9, 1–18. [Google Scholar] [CrossRef]
- Chaplin, M.F. Information Exchange Within Intracellular Water. In Water and the Cell; Pollack, G., Cameron, I., Wheatley, D., Eds.; Springer: Berlin, Germany, 2006; pp. 113–123. [Google Scholar]
- Chaplin, M.F. Water in biological recognition processes. In Wiley Encyclopedia of Chemical Biology; John Wiley & Sons: New York, NY, USA, 2008; pp. 1–8. [Google Scholar]
- Chaplin, M.F. A proposal for the structuring of water. Biophys. Chem. 2000, 83, 211–221. [Google Scholar] [CrossRef]
- Collins, K.D. Charge density-dependent strength of hydration and biological structure. Biophys. J. 1997, 72, 65–76. [Google Scholar] [CrossRef]
- Ho, M. The Rainbow and the Worm, the Physics of Organisms, 3rd ed.; World Scientific: Singapore, Singapore, 1993. [Google Scholar]
- Ho, M. Water forms massive exclusion zones. Sci. Soc. Ser. 2004, 23, 50–51. [Google Scholar]
- Ho, M. Cooperative and coherent water. Sci. Soc. Ser. 2010, 48, 6–9. [Google Scholar]
- Ho, M. Quantum coherent water and life. Sci. Soc. Ser. 2011, 51, 26–29. [Google Scholar]
- Ho, M. Quantum coherent water, non-thermal EMF effects, and homeopathy. Sci. Soc. Ser. 2011, 51, 30–33. [Google Scholar]
- Zhang, Y.; Cremer, P. The inverse and direct Hofmeister series for lysozyme. Proc. Natl. Acad. Sci. USA 2009, 106, 15249–15253. [Google Scholar] [CrossRef] [PubMed]
- Pollack, G. Cells, Gels and the Engines of Life: A New, Unifying Approach to Cell Function; Ebner & Sons: Seattle, WA, USA, 2001. [Google Scholar]
- Pollack, G.H.; Clegg, J. Unexpected Linkage Between Unstirred Layers, Exclusion Zones, and Water Phase Transitions in Cell Biology; Pollack, G.H., Chin, W.-C., Eds.; Springer: Berlin, Germany, 2008; pp. 143–152. [Google Scholar]
- Pollack, G.H.; Figueroa, X.; Zhao, Q. Molecules, water, and radiant energy: New clues for the origin of life. Int. J. Mol. Sci. 2009, 10, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Ling, G.N. Life at the Cell and Below-Cell Level: The Hidden History of a Functional Revolution in Biology; Pacific Press: Melville, NY, USA, 2001. [Google Scholar]
- Wiggins, P. Life depends upon two kinds of water. PLoS One 2008, 3, e1406. [Google Scholar] [CrossRef] [PubMed]
- Horan, F.E.; Hirsch, F.G.; Wood, L.A.; Wright, I.S. Surface effects on blood-clotting components as determined by Zeta-Potentials. J. Clin. Invest. 1950, 29, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Tigrek, S.; Barnes, F. Water structures and effects of electric and magnetic fields. In Non-Thermal Effects and Mechanisms of Interaction between Electromagnetic Fields and Living Matter: An ICEMS Monograph; Giuliani, L., Soffritti, M., Eds.; Fidenza: Bologna, Italy, 2010; pp. 25–50. [Google Scholar]
- Jones, G.; Ray, J. J. Am. Chem. Soc. 1937, 59, 187–198. [CrossRef]
- Petersen, P.B.; Johnson, J.C.; Knutsen, K.P.; Saykally, R.J. Direct experimental validation of the Jones–Ray effect. Chem. Phys. Lett. 2004, 397, 46–50. [Google Scholar] [CrossRef]
- Petersen, P.B.; Saykally, R.J. On the nature of ions at the liquid water surface. Annu. Rev. Phys. Chem. 2006, 57, 333–364. [Google Scholar] [CrossRef] [PubMed]
- Vander, A.; Sherman, J.; Luciano, D. Human Physiology: The Mechanisms of Body Function, 4th ed.; McGraw-Hill: New York, NY, USA, 1985. [Google Scholar]
- Kent, L.; Emerton, J.; Bhadravathi, V.; Weisblatt, E.; Pasco, G.; Willatt, L.R.; McMahon, R.; Yates, J.R. X-linked ichthyosis (steroid sulfatase deficiency) is associated with increased risk of attention deficit hyperactivity disorder, autism and social communication deficits. J. Med. Genet. 2008, 45, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.L.; Hughes-Fulford, M.; Elias, P.M. Inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity and sterol synthesis by cholesterol sulfate in cultured fibroblasts. Biochim. Biophys. Acta 1985, 845, 349–357. [Google Scholar] [CrossRef]
- Cheetham, J.J.; Epand, R.M.; Andrews, M.; Flanagan, T.D. Cholesterol sulfate inhibits the fusion of Sendai virus to biological and model membranes. J. Biol. Chem. 1990, 265, 12404–12409. [Google Scholar] [PubMed]
- Smondyrev, A.M.; Berkowitz, M.L. Molecular dynamics simulation of dipalmitoylphosphatidylcholine membrane with cholesterol sulfate. Biophys. J. 2000, 78, 1672–1680. [Google Scholar] [CrossRef]
- Von Hippel, P.; Schleich, T. Structure and Stability of Biological Macromolecules; Timashev, S., Fasman, G., Eds.; Marcel Dekker: New York, NY, USA, 1969. [Google Scholar]
- Dos Santos, A.P.; Diehl, A.; Levin, Y. Surface tensions, surface potentials, and the Hofmeister series of electrolyte solutions. Langmuir 2010, 26, 10778–10783. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, A.P.; Levin, Y. Ion specificity and the theory of stability of colloidal suspensions. Phys. Rev. Lett. 2011, 106. [Google Scholar] [CrossRef]
- Breslow, R.; Guo, T. Surface tension measurements show that chaotropic salting-in denaturants are not just water-structure breakers. Proc. Natl. Acad. Sci. USA 1990, 87, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Richardt, G.; Federolf, G.; Habermann, E. The interaction of aluminum and other metal ions with calcium-calmodulin-dependent phosphodiesterase. Arch. Toxicol. 1985, 57, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F. Free energy changes in denaturation of ribonuclease A by mixed denaturants. Effects of combinations of guanidine hydrochloride and one of the denaturants lithium bromide, lithium chloride, and sodium bromide. J. Biol. Chem. 1984, 259, 4183–4186. [Google Scholar] [PubMed]
- Timasheff, S.N. Water as ligand: Preferential binding and exclusion of denaturants in protein unfolding. Biochemistry-US 1992, 31, 9857–9864. [Google Scholar] [CrossRef]
- Vidanovic, D.; Milic Askrabic, J.; Stankovic, M.; Poprzen, V. Effects of nonionic surfactants on the physical stability of immunoglobulin G in aqueous solution during mechanical agitation. Pharmazie 2003, 58, 399–404. [Google Scholar] [PubMed]
- Klahn, M.; Lim, G.S.; Seduraman, A.; Wu, P. On the different roles of anions and cations in the solvation of enzymes in ionic liquids. Phys. Chem. Chem. Phys. 2011, 13, 1649–1662. [Google Scholar] [CrossRef] [PubMed]
- Klahn, M.; Lim, G.S.; Wu, P. How ion properties determine the stability of a lipase enzyme in ionic liquids: A molecular dynamics study. Phys. Chem. Chem. Phys. 2011, 13, 18647–18660. [Google Scholar] [CrossRef] [PubMed]
- Laage, D.; Hynes, J.T. Reorientional dynamics of water molecules in anionic hydration shells. Proc. Natl. Acad. Sci. USA 2007, 104, 11167–11172. [Google Scholar] [CrossRef] [PubMed]
- Ho, M. Positive electricity zaps through water chains. ISIS Report. [Online]. 2005. Available online: http://www.i-sis.org.uk/full/PEZTWCFull.php (accessed on 24 July 2012).
- Dill, K.A.; Truskett, T.M.; Vlachy, V.; Hribar-Lee, B. Modeling water, the hydrophobic effect, and ion solvation. Annu. Rev. Biophys. Biom. 2005, 34, 173–199. [Google Scholar] [CrossRef] [PubMed]
- Tielrooij, K.J.; Garcia-Araez, N.; Bonn, M.; Bakker, H.J. Cooperativity in ion hydration. Science 2010, 328, 1006–1009. [Google Scholar] [CrossRef] [PubMed]
- Petersen, P.B.; Saykally, R.J. Adsorption of ions to the surface of dilute electrolyte solutions: The Jones-Ray effect revisited. J. Am. Chem. Soc. 2005, 127, 15446–15452. [Google Scholar] [CrossRef] [PubMed]
- Weissenborn, P.K.; Pugh, R.J. Surface tension of aqueous solutions of electrolytes: Relationship with ion hydration, oxygen solubility, and bubble coalescence. J. Colloid Interface Sci. 1996, 184, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Lo Nostro, P.; Ninham, B.W.; Milani, S.; Lo Nostro, A.; Pesavento, G.; Baglioni, P. Hofmeister effects in supramolecular and biological systems. Biophys. Chem. 2006, 124, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Lo Nostro, P.; Ninham, B.W. Hofmeister phenomena: An update on ion specificity in biology. Chem. Rev. 2012, 112, 2286–2322. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Giese, R.F., Jr.; van Oss, C.J. Linkage between ζ-potential and electron donicity of charged polar surfaces 2. Repeptization of flocculation caused by plurivalent counterions by means of complexing agents. Colloid Surface A 1994, 89, 253–262. [Google Scholar] [CrossRef]
- Nakajima, Y. Shwartzman reaction in the brain induced by fractions of Fusobacterium necrophorum and Escherichia coli lipopolysaccharide in rabbits. Acta Pathol. Japon. 1988, 38, 541–547. [Google Scholar]
- Del Giudice, E.; Spinetti, P.R.; Tedeschi, A. Water dynamics at the root of metamorphosis in living organisms. Water 2010, 2, 566–586. [Google Scholar] [CrossRef]
- Del Giudice, E.; Fuchs, E.; Vitiello, G. Collective molecular dynamics of a floating water bridge. Water 2010, 2, 69–82. [Google Scholar]
- Del Giudice, E.; Giuliani, L. Coherence in water and the kT problem in living matter. In Non-Thermal Effects and Mechanisms of Interaction Between Electromagnetic Fields and Living Matter: An ICEMS Monograph; Giuliani, L., Soffritti, M., Eds.; Fidenza: Bologna, Italy, 2010; pp. 7–24. [Google Scholar]
- Burcik, E.J.; Vaughn, C.R. The effect of pH on the rate of surface tension lowering. J. Colloid Sci. 1951, 6, 522–527. [Google Scholar] [CrossRef]
- Yoon, R.-H.; Yordan, J.L. Zeta-potential measurements on microbubbles generated using various surfactants. J. Colloid Interface Sci. 1986, 113, 430–438. [Google Scholar] [CrossRef]
- Li, C.; Somasundaran, P. Reversal of bubble charge in multivalent inorganic salt solutions—Effect of aluminum. J. Colloid Interface Sci. 1992, 148, 587–591. [Google Scholar] [CrossRef]
- Jin, F.; Li, J.; Ye, X.; Wu, C. Effects of pH and ionic strength on the stability of nanobubbles in aqueous solutions of α-Cyclodextrin. J. Phys. Chem. B 2007, 111, 11745–11749. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Ye, X.; Wu, C. Observation of kinetic and structural scalings during slow coalescence of nanobubbles in an aqueous solution. J. Phys. Chem. B 2007, 111, 13143–13146. [Google Scholar] [CrossRef] [PubMed]
- Craig, V.S.J.; Ninham, B.W.; Pashley, R.M. Effect of electrolytes on bubble coalescence. Nature 1993, 364, 317–319. [Google Scholar] [CrossRef]
- Henry, C.L.; Craig, V.S. The link between ion specific bubble coalescence and Hofmeister effects is the partitioning of ions within the interface. Langmuir 2010, 26, 6478–6483. [Google Scholar] [CrossRef] [PubMed]
- Karakashev, S.I.; Nguyen, P.T.; Tsekov, R.; Hampton, M.A.; Nguyen, A.V. Anomalous ion effects on rupture and lifetime of aqueous foam films formed from monovalent salt solutions up to saturation concentration. Langmuir 2008, 24, 11587–11591. [Google Scholar] [CrossRef] [PubMed]
- Nickolov, Z.S.; Miller, J.D. Water structure in aqueous solutions of alkali halide salts: FTIR spectroscopy of the OD stretching band. J. Colloid Interface Sci. 2005, 287, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Verdel, N.; Jerman, I.; Bukovec, P. The “autothixotropic” phenomenon of water and its role in proton transfer. Int. J. Mol. Sci. 2011, 12, 7481–7494. [Google Scholar] [CrossRef] [PubMed]
- Green, K.; Otori, T. Direct measurements of membrane unstirred layers. J. Physiol. 1970, 207, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.-M.; Pollack, G.H. Long-range forces extending from polymer-gel surfaces. Phys. Rev. E 2003, 68, e031408. [Google Scholar] [CrossRef]
- Levy, G.; Edgington, T. Lymphocyte cooperation is required for amplification of macrophage procoagulant activity. J. Exp. Med. 1980, 151, 1232–1244. [Google Scholar] [CrossRef] [PubMed]
- Liuba, P.; Aburawi, E.H.; Pesonen, E.; Andersson, S.; Truedsson, L.; Yla-Herttuala, S.; Holmberg, L. Residual adverse changes in arterial endothelial function and LDL oxidation after a mild systemic inflammation induced by influenza vaccination. Ann. Med. 2007, 39, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Bhakdi, S.; Lackner, K.; Doerr, H.W. Possible hidden hazards of mass vaccination against new influenza A/H1N1: Have the cardiovascular risks been adequately weighed? Med. Microbiol. Immunol. 2009, 198, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.; Knutsen, S.F.; Blix, G.G.; Lee, J.W.; Fraser, G.E. Water, other fluids, and fatal coronary heart disease: The Adventist Health Study. Am. J. Epidemiol. 2002, 155, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Monsalvo, A.C.; Batalle, J.P.; Lopez, M.F.; Krause, J.C.; Klemenc, J.; Hernandez, J.Z.; Maskin, B.; Bugna, J.; Rubinstein, C.; Aguilar, L.; et al. Severe pandemic 2009 H1N1 influenza disease due to pathogenic immune complexes. Nat. Med. 2011, 17, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Reik, L., Jr. Disseminated vasculomyelinopathy: An immune complex disease. Ann. Neurol. 1980, 7, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Lucena, J.; Blanco, M.; Jurado, C.; Rico, A.; Salguero, M.; Vazquez, R.; Thiene, G.; Basso, C. Cocaine-related sudden death: A prospective investigation in south-west Spain. Eur. Heart J. 2010, 31, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Sherman, I.A. Interfacial tension effects in the microvasculature. Microvasc. Res. 1981, 22, 296–307. [Google Scholar] [CrossRef]
- Botker, H.; Sonne, H.; Sorensen, K. Frequency of systemic microvascular dysfunction in syndrome X and in variant angina. Am. J. Cardiol. 1996, 78, 182–186. [Google Scholar] [CrossRef]
- Gardener, H.; Spiegelman, D.; Buka, S.L. Perinatal and neonatal risk factors for autism: A comprehensive meta-analysis. Pediatrics 2011, 128, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Simon, E.N. Auditory system damage and anoxic birth. Arch. Pediatr. Adolesc. Med. 2007, 161, 1106–1107. [Google Scholar] [CrossRef] [PubMed]
- Al-Bayati, M. Analysis of causes that led to Baby Robert’s respiratory arrest and death in August of 2000. Med. Veritas 2004, 1, 179–200. [Google Scholar] [CrossRef]
- Al-Bayati, M. Analysis of causes that led to Evyn Vaugn’s respiratory arrest, intracranial and retinal bleeding, and death. Med. Veritas 2009, 6, 1937–1958. [Google Scholar]
- Al-Bayati, M. Analysis of causes that led to baby Ron James Douglas’ cardiopulmonary arrest, bleeding (intracranial, retinal, and pulmonary), and rib fracture. Med. Veritas 2009, 6, 1959–1976. [Google Scholar]
- Sakorafas, G.H.; Tsiotos, G.G.; Sarr, M.G. Ischemia/reperfusion-induced pancreatitis. Dig. Surg. 2000, 17, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Stys, P.K. Anoxic and ischemic injury of myelinated axons in CNS white matter: From mechanistic concepts to therapeutics. J. Cereb. Blood Flow Metab. 1998, 18, 2–25. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.G.; Chen, Y.D. Influencing factors of pancreatic microcirculatory impairment in acute panceatitis. World J. Gastroenterol. 2002, 8, 406–412. [Google Scholar] [PubMed]
- Al-Bayati, M. Shaken baby syndrome or medical malpractice? Med. Veritas 2004, 1, 117–129. [Google Scholar] [CrossRef]
- Ho, M. Membrane potential rules. ISIS Report. [Online]. 2011. Available online: http://www.i-sis.org.uk/Membrane_potential_rules.php (accessed online 24 July 2012).
- Busselberg, D.; Platt, B.; Haas, H.L.; Carpenter, D.O. Voltage gated calcium channel currents of rat dorsal root ganglion (DRG) cells are blocked by Al3+. Brain Res. 1993, 622, 163–168. [Google Scholar] [CrossRef]
- Cauwels, A.; Janssen, B.; Buys, E.; Sips, P.; Brouckaert, P. Anaphylactic shock depends on PI3K and eNOS-derived NO. J. Clin. Invest. 2006, 116, 2244–2251. [Google Scholar] [CrossRef] [PubMed]
- Duran, W.N.; Breslin, J.W.; Sanchez, F.A. The NO cascade, eNOS location, and microvascular permeability. Cardiovasc. Res. 2010, 87, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Mashhoodi, T.; Zahedi-Asl, S.; Sarkaki, A. Inhibitory effect of aluminum on kcl and phenylephrine induced contraction in isolated rat aorta. Acta Medica Iranica 2004, 42, 379–382. [Google Scholar]
- Burbacher, T.M.; Shen, D.D.; Liberato, N.; Grant, K.S.; Cernichiari, E.; Clarkson, T. Comparison of blood and brain mercury levels in infant monkeys exposed to methylmercury or vaccines containing thimerosal. Environ. Health Perspect. 2005, 113, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Horn, R.G. Evidence for participation of granulocytes in the pathogenesis of the generalized Shwartzman reaction: A review. J. Infect. Dis. 1973, 128, S134–S143. [Google Scholar] [CrossRef]
- Horn, R.G.; Spicer, S.S. Sulfated mucopolysaccharide and basic protein in certain granules of circulating heterophils of rabbits during endotoxin-induced leukocytosis. Am. J. Pathol. 1964, 44, 905–919. [Google Scholar] [PubMed]
- Horn, R.G.; Spicer, S.S. Sulfated mucopolysaccharide in fibrinoid glomerular occlusions of the generalized Shwartzman reaction. Am. J. Pathol. 1965, 46, 197–213. [Google Scholar] [PubMed]
- Berenson, G.S.; Dalferes, E.R. Metabolism of acid mucopolysaccharides in the Shwartzman phenomenon. J. Exp. Med. 1960, 112, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Portier, P.; Richet, C. De l’action anaphylactique de certains venins [The anaphylactic reaction to certain venoms]. (in French). C R Soc. Biol. 1902, 2, 170. [Google Scholar]
- Peavy, R.D.; Metcalfe, D.D. Understanding the mechanisms of anaphylaxis. Curr. Opin. Allergy Clin. 2008, 8, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.Q.; Kemp, S.F. Pathophysiology of anaphylaxis. Curr. Opin. Allergy Clin. 2011, 11, 319–325. [Google Scholar] [CrossRef] [PubMed]
- McKay, D.G. Progress in disseminated intravascular coagulation. Calif. Med. 1969, 111, 186–199. [Google Scholar] [PubMed]
- Letsky, E.A. Disseminated intravascular coagulation. Best Pract. Res. Clin. Obstet. 2001, 15, 623–644. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R.; Barron, B.; White, P.; Fraire, A. Diagnosis by radiocolloid imaging of postpartum hepatic necrosis in the syndrome of hemolysis, elevated liver enzymes, and low platelets. Clin. Nucl. Med. 1992, 17, 322–324. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R.; Dhekne, R.; Moore, W. Indium-111 WBC Scan in acute toxic centrilobular hepatic necrosis. Clin. Nucl. Med. 1989, 14, 877–880. [Google Scholar] [CrossRef] [PubMed]
- Weir, J.M. The vascular processes in the Shwartzman phenomenon as observed in pulmonary reactions. J. Immunol. 1938, 34, 75–90. [Google Scholar]
- Parmar, M.S. Pancreatic necrosis associated with preeclampsia-eclampsia. JOP: J. Pancreas 2004, 5, 101–104. [Google Scholar]
- McKay, D.G.; Merrill, S.J.; Weiner, A.E.; Hertig, A.T.; Reid, D.E. The pathologic anatomy of eclampsia, bilateral renal cortical necrosis, pituitary necrosis, and other acute fatal complications of pregnancy, and its possible relationship to the generalized Shwartzman phenomenon. Am. J. Obstet. Gynecol. 1953, 66, 507–539. [Google Scholar] [PubMed]
- Scott, J.S. Blood coagulation failure in obstetrics; effects of dextran and plasma. Br. Med. J. 1955, 2, 290–292. [Google Scholar] [CrossRef] [PubMed]
- Antonova, N.; Riha, P.; Ivanov, I. Experimental evaluation of mechanical and electrical properties of RBC suspensions under flow. Role of RBC aggregating agent. Clin. Hemorheol. Microcirc. 2010, 45, 253–261. [Google Scholar] [PubMed]
- Tarbell, J.M.; Pahakis, M.Y. Mechanotransduction and the glycocalyx. J. Intern. Med. 2006, 259, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Weinbaum, S.; Tarbell, J.M.; Damiano, E.R. The structure and function of the endothelial glycocalyx layer. Ann. Rev. Biomed. Eng. 2007, 9, 121–167. [Google Scholar] [CrossRef] [PubMed]
- Henrich, M.; Gruss, M.; Weigand, M.A. Sepsis-induced degradation of endothelial glycocalix. Sci. World J. 2010, 10, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Lennon, F.E.; Singleton, P.A. Hyaluronan regulation of vascular integrity. Am. J. Cardiovasc. Dis. 2011, 1, 200–213. [Google Scholar] [PubMed]
- Higashi, Y.; Fuda, H.; Yanai, H.; Lee, Y.; Fukushige, T.; Kanzaki, T.; Strott, C.A. Expression of cholesterol sulfotransferase (SULT2B1b) in human skin and primary cultures of human epidermal keratinocytes. J. Invest. Dermatol. 2004, 122, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Bortagary, V.; Aramburu, R.; Barrios, L.; Ojeda, P.; Puerto, G.; Rodriguez-Ithurralde, D. Embryotoxicity and teratogenesis in zebrafish embryos exposed in vitro to glyphosate-type herbicides. J. Dev. Toxicol. 2010. Available online: http://drithurralde.wordpress.com/2010/06/ (accessed on 24 July 2012).
- Yu, R.K.; Nakatani, Y.; Yanagisawa, M. The role of glycosphingolipid metabolism in the developing brain. J. Lipid Res. 2009, 50, S440–S445. [Google Scholar] [CrossRef] [PubMed]
- Brodland, G.W. The Differential Interfacial Tension Hypothesis (DITH): A comprehensive theory for the self-rearrangement of embryonic cells and tissues. J. Biomech. Eng. 2002, 124, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Schoetz, E. Dynamics and Mechanics of Zebrafish Embryonic Tissues; Der Fakultat fur Physik der Technischen Universitat Dresden: Dresden, Germany, 2007. [Google Scholar]
- Rodrigueza, W.V.; Wheeler, J.J.; Klimuk, S.K.; Kitson, C.N.; Hope, M.J. Transbilayer movement and net flux of cholesterol and cholesterol sulfate between liposomal membranes. Biochemistry 1995, 34, 6208–6217. [Google Scholar] [CrossRef] [PubMed]
- Strott, C.A.; Higashi, Y. Cholesterol sulfate in human physiology: What’s it all about? J. Lipid Res. 2003, 44, 1268–1278. [Google Scholar] [CrossRef] [PubMed]
- Langlais, J.; Zollinger, M.; Plante, L.; Chapdelaine, A.; Bleau, G.; Roberts, K.D. Localization of cholesteryl sulfate in human spermatozoa in support of a hypothesis for the mechanism of capacitation. Proc. Natl. Acad. Sci. USA 1981, 78, 7266–7270. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.D. Sterol sulfates in the epididymis; synthesis and possible function in the reproductive process. J. Steroid Biochem. 1987, 27, 337–341. [Google Scholar] [CrossRef]
- Gadella, B.M.; Tsai, P.S.; Boerke, A.; Brewis, I.A. Sperm head membrane reorganisation during capacitation. Int. J. Dev. Biol. 2008, 52, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Tulsiani, D.R.P.; Abou-Haila, A. Is sperm capacitation analogous to early phases of Ca2+-triggered membrane fusion in somatic cells and viruses? Bioessays 2004, 26, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Zitranski, N.; Borth, H.; Ackermann, F.; Meyer, D.; Viewig, L.; Breit, A.; Gudermann, T.; Boekhoff, I. The “acrosomal synapse”: Subcellular organization by lipid rafts and scaffolding proteins exhibits high similarities in neurons and mammalian spermatozoa. Commun. Integr. Biol. 2010, 3, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Mennerick, S.; Lamberta, M.; Shu, H.J.; Hogins, J.; Wang, C.; Covey, D.F.; Eisenman, L.N.; Zorumski, C.F. Effects on membrane capacitance of steroids with antagonist properties at GABAA receptors. Biophys. J. 2008, 95, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Chisari, M.; Wu, K.; Zorumski, C.F.; Mennerick, S. Hydrophobic anions potently and uncompetitively antagonize GABA(A) receptor function in the absence of a conventional binding site. Br. J. Pharmacol. 2011, 164, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Przybylska, M.; Faber, M.; Zaborowski, A.; Świe’toslawski, J.; Bryszewska, M. Morphological changes of human erythrocytes induced by cholesterol sulphate. Clin. Biochem. 1998, 31, 73–79. [Google Scholar] [CrossRef]
- Cignarelli, M.; Damato, A.; Cospite, M.R.; Guastamacchia, E.; Nardelli, G.M.; Giorgino, R. Erythrocyte cholesterol and red blood cells deformability in diabetes mellitus. Boll Soc. Ital. Biol. Sper. 1982, 58, 1115–1118. [Google Scholar] [PubMed]
- Lalumiere, G.; Longpre, J.; Trudel, J.; Chapdelaine, A.; Roberts, K.D. Cholesterol sulfate. II. Studies on its metabolism and possible function in canine blood. Biochim. Biophys. Acta 1975, 394, 120–128. [Google Scholar] [CrossRef]
- Bleau, G.; Bodley, F.H.; Longpre, J.; Chapdelaine, A.; Roberts, K.D. Cholesterol sulfate. I. Occurrence and possible biological function as an amphipathic lipid in the membrane of the human erythrocyte. Biochim. Biophys. Acta 1974, 352, 1–9. [Google Scholar] [CrossRef]
- Bleau, G.; Lalumiure, G.; Chapdelaine, A.; Roberts, K. Red cell surface structure. Stabilization by cholesterol sulfate as evidenced by scanning electron microscopy. Biochim. Biophys. Acta 1975, 375, 220–223. [Google Scholar] [CrossRef]
- Cooper, G.M.; Hausman, R.E. The Cell: A Molecular Approach; Sinauer Associates: Sunderland, MA, USA, 2009. [Google Scholar]
- Shin, S.; Ku, Y.; Babu, N.; Singh, M. Erythrocyte deformability and its variation in diabetes mellitus. Indian J. Exp. Biol. 2007, 45, 121–128. [Google Scholar] [PubMed]
- Dondorp, A.M.; Angus, B.J.; Chotivanich, K.; Silamut, K.; Ruangveerayuth, R.; Hardeman, M.R.; Kager, P.A.; Vreeken, J.; White, N.J. Red blood cell deformability as a predictor of anemia in severe falciparum malaria. Am. J. Trop. Med. Hyg. 1999, 60, 733–737. [Google Scholar] [PubMed]
- Babu, N. Influence of hypercholesterolemia on deformability and shape parameters of erythrocytes in hyperglycemic subjects. Clin. Hemorheol. Microcirc. 2009, 41, 169–177. [Google Scholar] [PubMed]
- Condon, M.; Senthil, M.; Xu, D.Z.; Mason, L.; Sheth, S.U.; Spolarics, Z.; Feketova, E.; Machiedo, G.W.; Deitch, E.A. Intravenous injection of mesenteric lymph produced during hemorrhagic shock decreases RBC deformability in the rat. J. Trauma 2011, 70, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Zaets, S.B.; Berezina, T.L.; Caruso, J.; Xu, D.Z.; Deitch, E.A.; Machiedo, G.W. Mesenteric lymph duct ligation prevents shock-induced RBC deformability and shape changes. J. Surg. Res. 2003, 109, 51–56. [Google Scholar] [CrossRef]
- Mohanty, J.G.; Eckley, D.M.; Williamson, J.D.; Launer, L.J.; Rifkind, J.M. Do red blood cell-beta-amyloid interactions alter oxygen delivery in Alzheimer’s disease? Adv. Exp. Med. Biol. 2008, 614, 29–35. [Google Scholar] [PubMed]
- Misiti, F.; Carelli-Alinovi, C.; Sampaolese, B.; Giardina, B. beta-amyloid decreases detectable endothelial nitric oxide synthase in human erythrocytes: A role for membrane acetylcholinesterase. Cell Biochem. Funct. 2012. [Google Scholar] [CrossRef]
- Walker, R.H.; Jung, H.H.; Dobson-Stone, C.; Rampoldi, L.; Sano, A.; Tison, F.; Danek, A. Neurologic phenotypes associated with acanthocytosis. Neurology 2012, 68, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.J.; Danek, A.; Walker, R.H. Neuroacanthocytosis syndromes. Orphanet J. Rare Dis. 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Brotherton, J.M.; Gold, M.S.; Kemp, A.S.; McIntyre, P.B.; Burgess, M.A.; Campbell-Lloyd, S. Anaphylaxis following quadrivalent human papillomavirus vaccination. Can. Med. Assoc. J. 2008, 179, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Singleton, J.R.; Smith, A.G.; Russell, J.W.; Feldman, E.L. Microvascular complications of impaired glucose tolerance. Diabetes 2003, 52, 2867–2873. [Google Scholar] [CrossRef] [PubMed]
- Nieuwdorp, M.; van Haeften, T.W.; Gouverneur, M.C.L.G.; Mooij, H.L.; van Lieshout, M.H.P.; Levi, M.; Meijers, J.C.M.; Holleman, F.; Hoekstra, J.B.L.; Vink, H.; et al. Loss of endothelial glycocalyx during acute hyperglycemia coincides with endothelial dysfunction and coagulation activation in vivo. Diabetes 2012, 55, 480–486. [Google Scholar] [CrossRef]
- McMillan, D. Insulin, diabetes, and the cell membrane: An hypothesis. Diabetologia 1983, 24, 308–310. [Google Scholar] [CrossRef] [PubMed]
- Maksimenko, A.V.; Turashev, A.D. No-reflow phenomenon and endothelial glycocalyx of microcirculation. Biochem. Res. Int. 2012. [Google Scholar] [CrossRef] [PubMed]
- Platts, S.H.; Linden, J.; Duling, B.R. Rapid modification of the glycocalyx caused by ischemia-reperfusion is inhibited by adenosine A2A receptor activation. Am. J. Physiol. Heart C. 2003, 284, H2360–H2367. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.S.; Vijayalakshmi, B.; Salimath, P.V. Effect of bitter gourd and spent turmeric on constituents of glycosaminoglycans in different tissues in streptozotocin induced diabetic rats. Mol. Cell. Biochem. 2006, 286, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.Y.; Yamanuha, J. Charles Robert Richet (1850–1935): Discoverer of anaphylaxis. Singap. Med. J. 2010, 51, 184–185. [Google Scholar]
- Huber, B. 100 years of allergy: Clemens von Pirquet—His idea of allergy and its immanent concept of disease. Wien. Klin. Wochenschr. 2006, 118, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, W. Strange Malady; Doubleday, Doran & Company, Inc: New York, NY, USA, 1941. [Google Scholar]
- Virchow, R. Thrombose und Embolie. Gefäßentzündung und septische Infektion. In Abhandlungen zur wissenschaftlichen Medicin; [Thrombosis and embolie: Possibility and septic infection, in Essays on the Science of Medicine] (in German). Matzdorff, A., Bell, W., Eds.; Von Meidinger & Sohn: Frankfurt am Main, Germany, 1856; pp. 219–732. [Google Scholar]
- Tachev, K.D.; Danov, K.D.; Kralchevsky, P.A. On the mechanism of stomatocyte–echinocyte transformations of red blood cells: Experiment and theoretical model. Colloid Surface B 2004, 34, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Rudenko, S.V. Erythrocyte morphological states, phases, transitions and trajectories. BBA-Biomembranes 2010, 1798, 1767–1778. [Google Scholar] [CrossRef] [PubMed]
- Betz, T.; Bakowsky, U.; Müller, M.R.; Lehr, C.-M.; Bernhardt, I. Conformational change of membrane proteins leads to shape changes of red blood cells. Bioelectrochemistry 2007, 70, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Iglič, A.; Kralj-Iglič, V.; Hägerstrand, H. Amphiphile induced echinocyte-spheroechinocyte transformation of red blood cell shape. Eur. Biophys. J. 1998, 27, 335–339. [Google Scholar] [PubMed]
- Sheetz, M.P. Cation effects on cell shape. Prog. Clin. Biol. Res. 1977, 17, 559–567. [Google Scholar] [PubMed]
- Muñoz, S.; Sebastián, J.L.; Sancho, M.; Martínez, G. Analysis of radiofrequency energy stored in the altered shapes: Stomatocyte-echinocyte of human erythrocytes. Bioelectrochemistry 2010, 77, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, S.; Sebastián, J.L.; Sancho, M.; Miranda, J.M. Transmembrane voltage induced on altered erythrocyte shapes exposed to RF fields. Bioelectromagnetics 2004, 25, 631–633. [Google Scholar] [CrossRef] [PubMed]
- Stasiuk, M.; Kijanka, G.; Kozubek, A. Transformations of erythrocytes shape and its regulation. Postepy Biochem. 2009, 55, 425–433. [Google Scholar] [PubMed]
- Brecher, G.; Bessis, M. Present status of spiculed red cells and their relationship to the discocyte-echinocyte transformation: A critical review. Blood 1972, 40, 333–344. [Google Scholar] [PubMed]
- Foglia, A. The acanthocyte-echinocyte differential: The example of chorea-acanthocytosis. Swiss Med. Wkly. 2010, 140. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.W.G.; Wortis, M.; Mukhopadhyay, R. Stomatocyte-discocyte-echinocyte sequence of the human red blood cell: Evidence for the bilayer-couple hypothesis from membrane mechanics. Proc. Natl. Acad. Sci. USA 2002, 99, 16766–16769. [Google Scholar] [CrossRef] [PubMed]
- Reinhart, W.; Chien, S. Red cell rheology in stomatocyte-echinocyte transformation: Roles of cell geometry and cell shape. Blood 1986, 67, 1110–1118. [Google Scholar] [PubMed]
- Chabanel, A.; Reinhart, W.; Chien, S. Increased resistance to membrane deformation of shape-transformed human red blood cells. Blood 1987, 69, 739–743. [Google Scholar] [PubMed]
- Hagerstrand, H.; Mrowczynska, L.; Salzer, U.; Prohaska, R.; Michelsen, K.A.; Kralj-Iglic, V.; Iglic, A. Curvature-dependent lateral distribution of raft markers in the human erythrocyte membrane. Mol. Membr. Biol. 2006, 23, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, E.; Vitiello, G. Influence of gravity on the collective molecular dynamics of liquid water: The case of the floating water bridge. Water 2011, 2, 133–141. [Google Scholar]
- Szent-Gyorgyi, A. Introduction to Supramolecular Biology; Academic Press: New York, NY, USA, 1960. [Google Scholar]
- Wayne Brodland, G.; Chen, H.H. The mechanics of cell sorting and envelopment. J. Biomech. 2000, 33, 845–851. [Google Scholar] [CrossRef]
- Knutton, S. The mechanism of virus-induced cell fusion. Micron (1969) 1978, 9, 133–154. [Google Scholar] [CrossRef]
- Väänänen, P.; Gahmberg, C.G.; Kääriäinen, L. Fusion of Semliki forest virus with red cell membranes. Virology 1981, 110, 366–374. [Google Scholar] [CrossRef]
- Noiri, E.; Lee, E.; Testa, J.; Quigley, J.; Colflesh, D.; Keese, C.R.; Giaever, I.; Goligorsky, M.S. Podokinesis in endothelial cell migration: Role of nitric oxide. Am. J. Physiol. 1998, 274, C236–C244. [Google Scholar] [PubMed]
- Stuehr, D.J.; Santolini, J.; Wang, Z.Q.; Wei, C.C.; Adak, S. Update on mechanism and catalytic regulation in the NO synthases. J. Biol. Chem. 2004, 279, 36167–36170. [Google Scholar] [CrossRef] [PubMed]
- Whited, C.A.; Warren, J.J.; Lavoie, K.D.; Weinert, E.E.; Agapie, T.; Winkler, J.R.; Gray, H.B. Gating NO release from nitric oxide synthase. J. Am. Chem. Soc. 2011, 134, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Gorren, A.C.; Mayer, B. Nitric-oxide synthase: A cytochrome P450 family foster child. Biochim. Biophys. Acta 2007, 1770, 432–445. [Google Scholar] [CrossRef] [PubMed]
- Carman, C.V. Mechanisms for transcellular diapedesis: Probing and pathfinding by invadosome-like protrusions’. J. Cell Sci. 2009, 122, 3025–3035. [Google Scholar] [CrossRef] [PubMed]
- McNeil, P.L. Mechanisms of nutritive endocytosis. I. Phagocytic versatility and cellular recognition in Chlorohydra digestive cells, a scanning electron microscope study. J. Cell Sci. 1981, 49, 311–339. [Google Scholar] [PubMed]
- Kruth, H.S. Receptor-independent fluid-phase pinocytosis mechanisms for induction of foam cell formation with native low-density lipoprotein particles. Curr. Opin. Lipidol. 2011, 22, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Plihtari, R.; Kovanen, P.T.; Öörni, K. Acidity increases the uptake of native LDL by human monocyte-derived macrophages. Atherosclerosis 2011, 217, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Gould, N.S.; Min, E.; Day, B.J. Macropinocytosis of extracellular glutathione ameliorates tumor necrosis factor alpha release in activated macrophages. PLoS One 2011, 6, e25704. [Google Scholar] [CrossRef] [PubMed]
- Gawarammana, I.; Mendis, S.; Jeganathan, K. Acute ischemic strokes due to bites by Daboia russelii in Sri Lanka-first authenticated case series. Toxicon 2009, 54, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, U.; Dissanayake, S. Neurological manifestations of snake bite in Sri Lanka. J. Postgrad. Med. 2002, 48, 275–278; discussion 278–279. [Google Scholar] [PubMed]
- Kuhnert, R.; Hecker, H.; Poethko-Muller, C.; Schlaud, M.; Vennemann, M.; Whitaker, H.J.; Farrington, C.P. A modified self-controlled case series method to examine association between multidose vaccinations and death. Stat. Med. 2011, 30, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, G.; Matturri, L.; Mingrone, R.; Lavezzi, A.M. Hypoplasia and neuronal immaturity of the hypoglossal nucleus in sudden infant death. J. Clin. Pathol. 2006, 59, 497–500. [Google Scholar] [CrossRef] [PubMed]
- D'Errico, S.; Neri, M.; Riezzo, I.; Rossi, G.; Pomara, C.; Turillazzi, E.; Fineschi, V. Beta-tryptase and quantitative mast-cell increase in a sudden infant death following hexavalent immunization. Forensic Sci. Int. 2008, 179, e25–e29. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, G.; Lavezzi, A.M.; Matturri, L. Sudden infant death syndrome (SIDS) shortly after hexavalent vaccination: Another pathology in suspected SIDS? Virchows Arch. 2006, 448, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, G.; Lavezzi, A.M.; Matturri, L. Fibromuscular hyperplasia of the pulmonary artery in sudden infant and perinatal unexpected death. Cardiovasc. Pathol. 2009, 18, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Jaster, J.H.; Ottaviani, G.; Matturri, L.; Lavezzi, A.M.; Zamecnik, J.; Smith, T.W. Sudden unexpected death related to medullary brain lesions. Am. J. Forensic Med. Pathol. 2008, 29, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, H.J.; Siegrist, C.A.; Salmaso, S.; Law, B.; Booy, R.; Zinka, B. Unexplained cases of sudden infant death shortly after hexavalent vaccination. Vaccine 2006, 24, 5781–5782; author reply 5785–5786. [Google Scholar] [CrossRef] [PubMed]
- Traversa, G.; Spila-Alegiani, S.; Bianchi, C.; Ciofi degli Atti, M.; Frova, L.; Massari, M.; Raschetti, R.; Salmaso, S.; Scalia Tomba, G. Sudden unexpected deaths and vaccinations during the first two years of life in Italy: A case series study. PLoS One 2011, 6, e16363. [Google Scholar] [CrossRef] [PubMed]
- von Kries, R.; Toschke, A.M.; Strassburger, K.; Kundi, M.; Kalies, H.; Nennstiel, U.; Jorch, G.; Rosenbauer, J.; Giani, G. Sudden and unexpected deaths after the administration of hexavalent vaccines (diphtheria, tetanus, pertussis, poliomyelitis, hepatitis B, Haemophilius influenzae type B): Is there a signal? Eur. J. Pediatr. 2005, 164, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Zinka, B.; Rauch, E.; Buettner, A.; Rueff, F.; Penning, R. Unexplained cases of sudden infant death shortly after hexavalent vaccination. Vaccine 2006, 24, 5779–5780. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.Z.; Goldman, G.S. Infant mortality rates regressed against number of vaccine doses routinely given: Is there a biochemical or synergistic toxicity? Hum. Exp. Toxicol. 2011, 30, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Eberlin, L.S.; Dill, A.L.; Costa, A.B.; Ifa, D.R.; Cheng, L.; Masterson, T.; Koch, M.; Ratliff, T.L.; Cooks, R.G. Cholesterol sulfate imaging in human prostate cancer tissue by desorption electrospray ionization mass spectrometry. Anal. Chem. 2010, 82, 3430–3434. [Google Scholar] [CrossRef] [PubMed]
- Israeli, E. Gulf War syndrome as a part of the autoimmune (autoinflammatory) syndrome induced by adjuvant (ASIA). Lupus 2012, 21, 190–194. [Google Scholar] [CrossRef] [PubMed]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Davidson, R.M.; Seneff, S. The Initial Common Pathway of Inflammation, Disease, and Sudden Death. Entropy 2012, 14, 1399-1442. https://doi.org/10.3390/e14081399
Davidson RM, Seneff S. The Initial Common Pathway of Inflammation, Disease, and Sudden Death. Entropy. 2012; 14(8):1399-1442. https://doi.org/10.3390/e14081399
Chicago/Turabian StyleDavidson, Robert M., and Stephanie Seneff. 2012. "The Initial Common Pathway of Inflammation, Disease, and Sudden Death" Entropy 14, no. 8: 1399-1442. https://doi.org/10.3390/e14081399
APA StyleDavidson, R. M., & Seneff, S. (2012). The Initial Common Pathway of Inflammation, Disease, and Sudden Death. Entropy, 14(8), 1399-1442. https://doi.org/10.3390/e14081399




