Scientific Élan Vital: Entropy Deficit or Inhomogeneity as a Unified Concept of Driving Forces of Life in Hierarchical Biosphere Driven by Photosynthesis
Abstract
:1. Introduction
2. Definitions
2.1. Thermodynamic Entropy
2.2. Entropy in Statistical Mechanics
2.3. Entropy in Information Theory
2.4. Order and Inhomogeneity
3. Generalized Entropy Deficit or Inhomogeneity in Biochemical Reactions
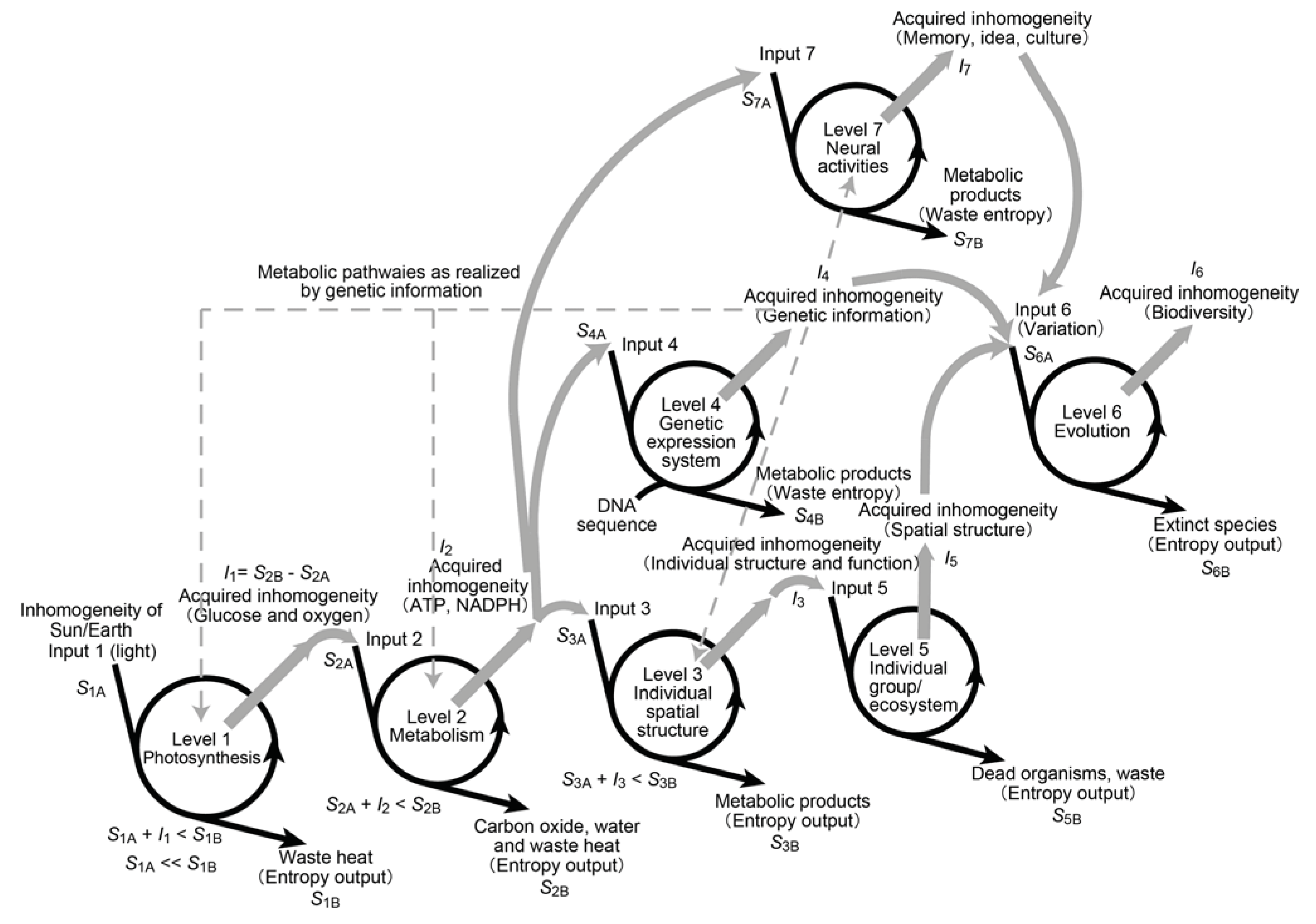
4. Forms of Inhomogeneity in Macromolecules, Cells, Organisms and the Whole Bioshere
4.1. Inhomogeneity in Genetic Information
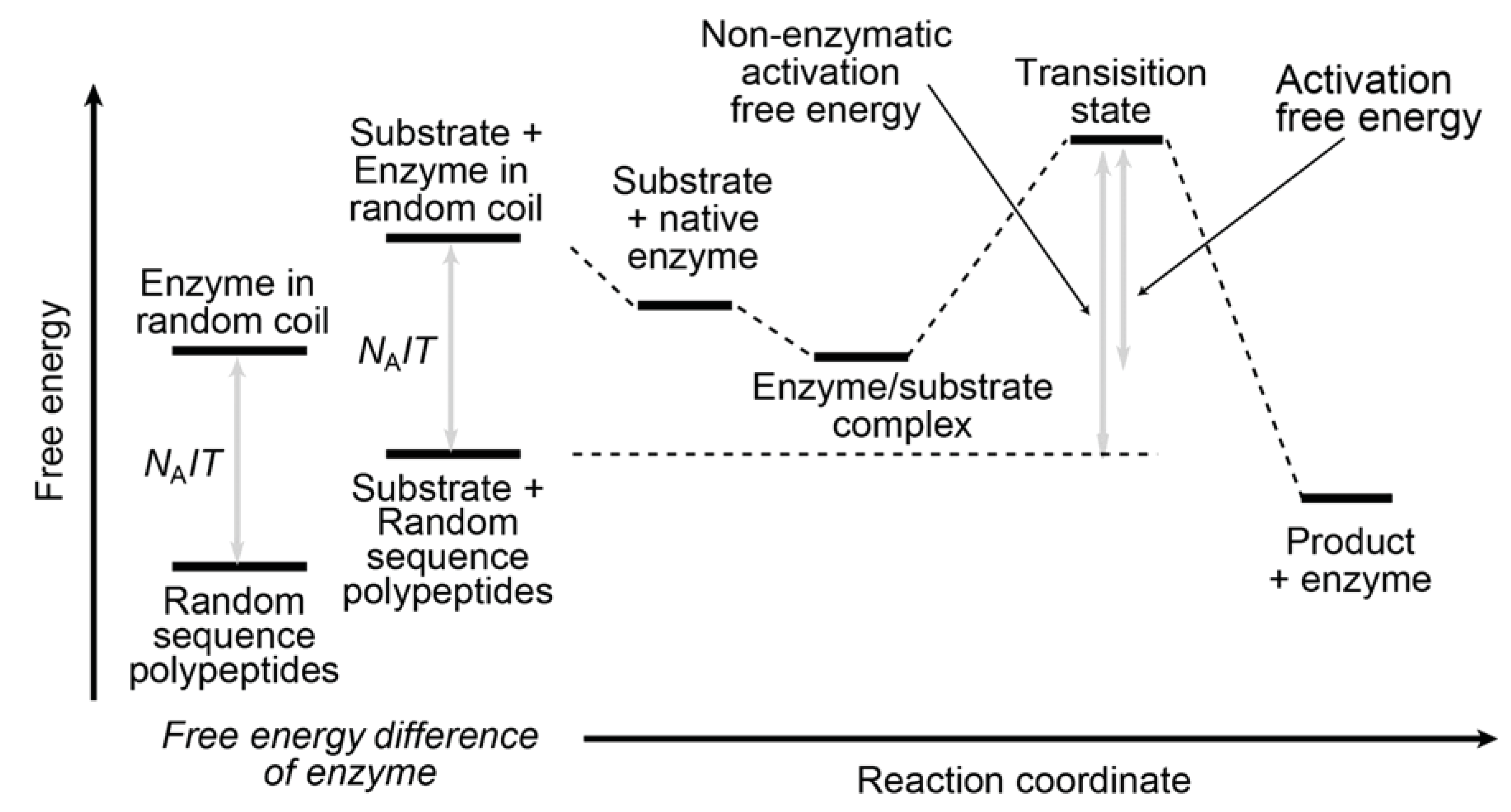
4.2. Inhomogeneity of an Enzyme
4.3. Inhomogeneity of Spatial Distribution
4.4. Inhomogeneity of Motion
4.5. Inhomogeneity of Evolution
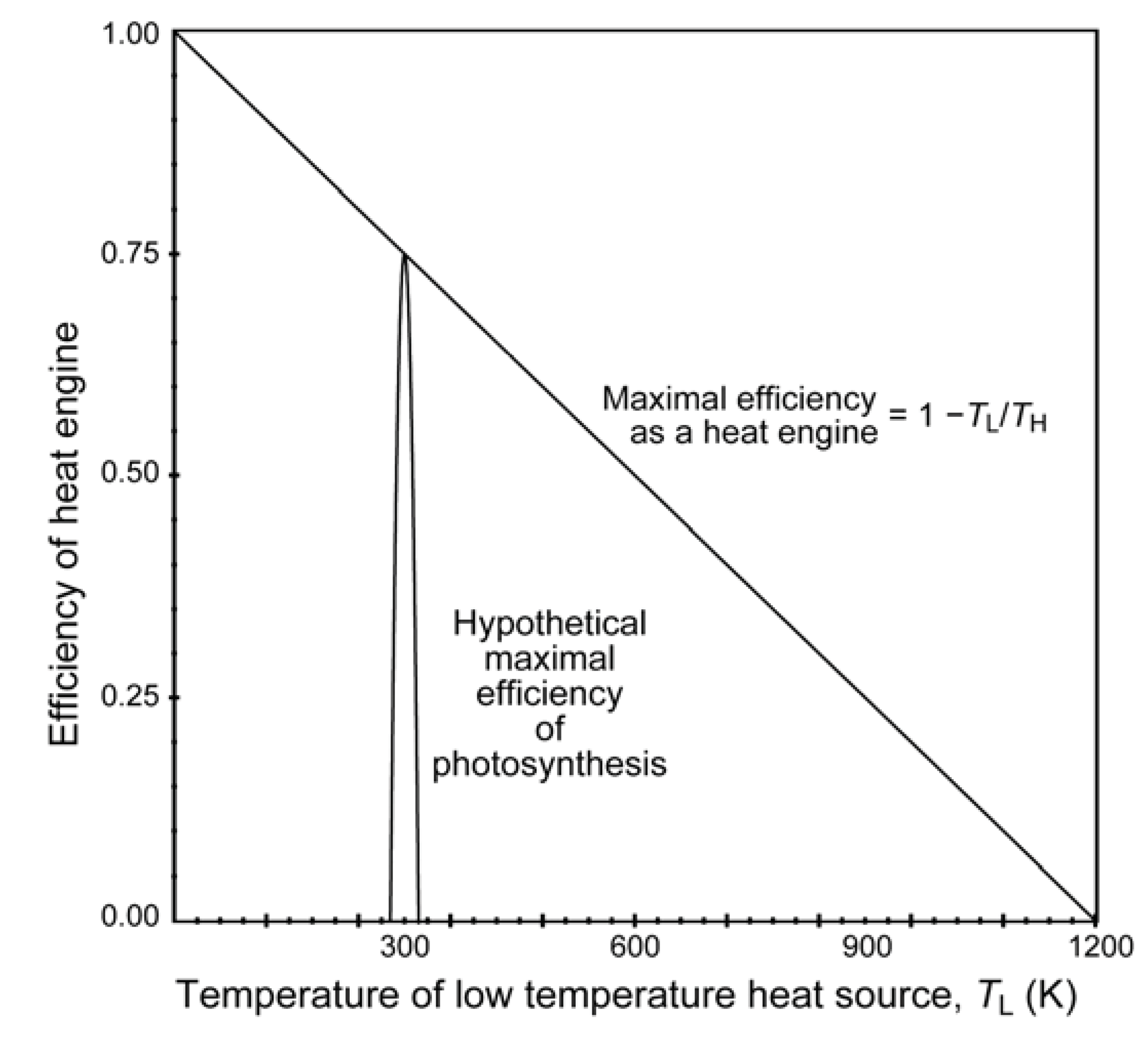
5. Photosynthesis: What Is Its Driving Force?
6CO2 + 6H2O → C6H12O6 + 6O2
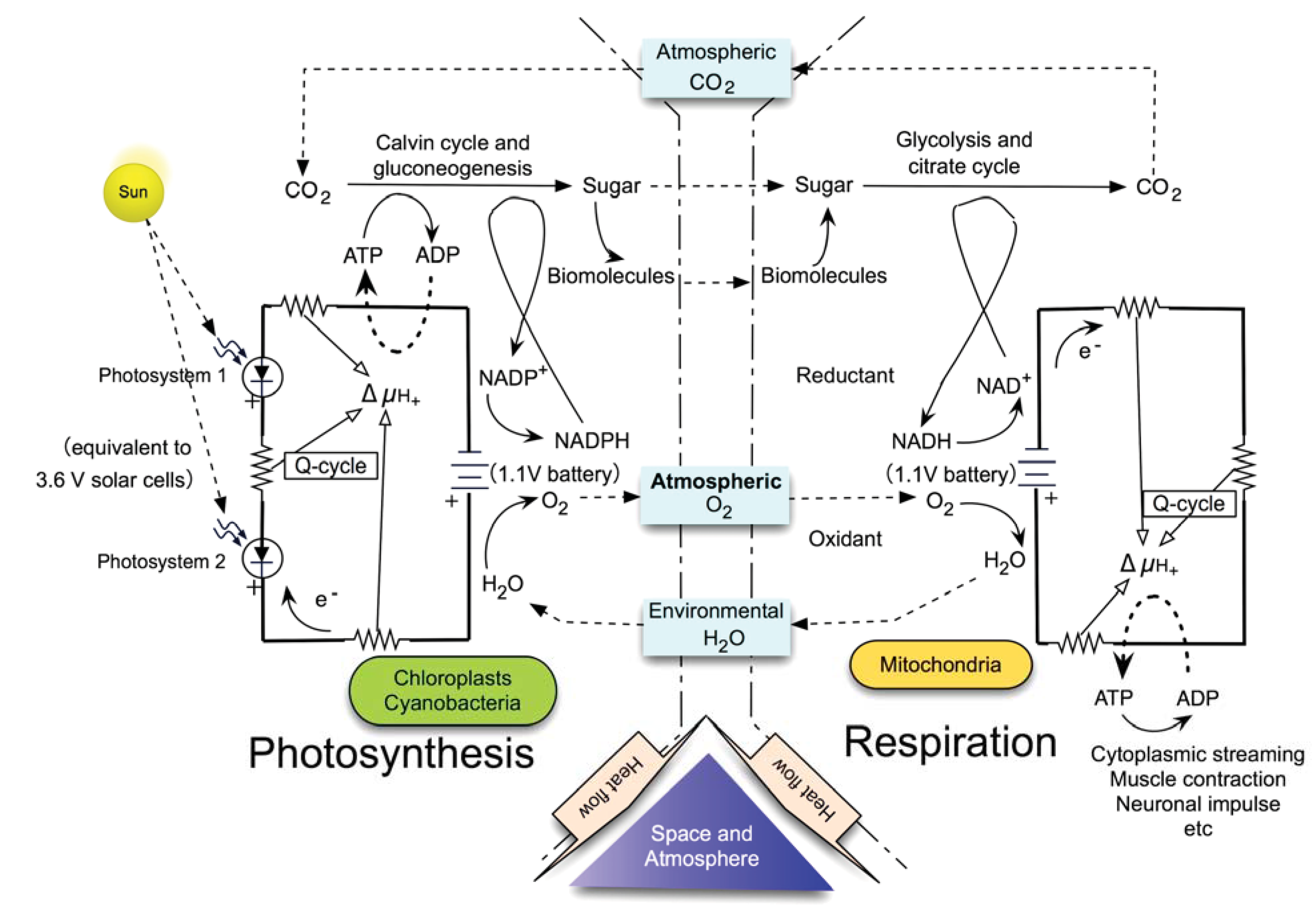
6. The Sole and Ultimate Source of Entropy Deficit/Inhomogeneity in Photosynthesis
7. Transpiration Is Not a Driving Force of Photosynthesis
8. Initial Entropy Deficit in Photosynthesis
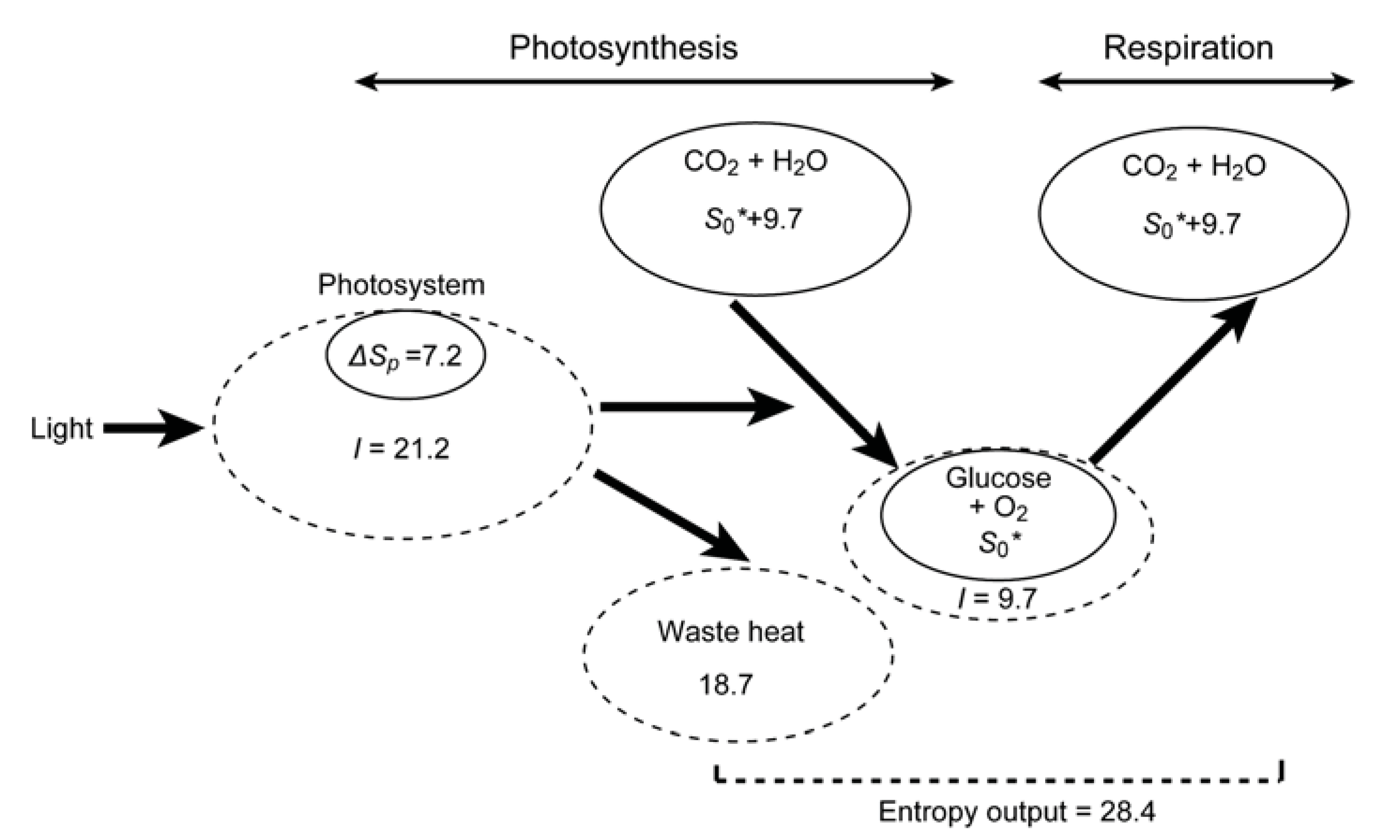
9. Entropy Deficit Inherent in ATP and NAD(P)H
10. Recognition of Photosynthesis as the Primary Driving Force of Whole Biosphere
11. Inhomogeneity, a Scientific Equivalent of Élan Vital
Acknowledgments
References
- Schrödinger, E. What is Life? The Physical Aspect of the Living Cell; Cambridge University Press: Cambridge, UK, 1944. [Google Scholar]
- Brillouin, L. Science and Information Theory, 2nd ed.; Academic Press: New York, NY, USA, 1962. [Google Scholar]
- Tsuchida, A. Entropy and Ecology (in Japanese); Diamond Inc.: Tokyo, Japan, 1986. [Google Scholar]
- Jennings, R.C.; Engelmann, E.; Garlaschi, F.; Casazza, A.P.; Zucchelli, G. Photosynthesis and negative entropy production. Biochim. Biophys. Acta 2005, 1709, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Lavergne, J. Commentary on: ‘Photosynthesis and negative entropy production by Jennings and coworkers’. Biochim. Biophys. Acta 2006, 1757, 1453–1439. [Google Scholar] [CrossRef] [PubMed]
- Knox, R.S.; Parson, W.W. Entropy production and the second law in photosynthesis. Biochim. Biophys. Acta 2007, 1767, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Voet, D.; Voet, J.G. Biochemistry, 3rd ed.; Wiley: Hoboken, NJ, USA, 2004. [Google Scholar]
- Ksenzhek, O.S.; Volkov, A.G. Plant Energetics; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Albarrán-Zavala, E.; Angulo-Brown, F. A simple thermodynamic analysis of photosynthesis. Entropy 2007, 9, 152–168. [Google Scholar] [CrossRef]
- Hall, D.O.; Rao, K.K. Photosynthesis, 6th ed.; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Cheetham, N.W.H. Introducing Biological Energetics; Oxford University Press: New York, NY, USA, 2011. [Google Scholar]
- Sato, N. Re-thinking entropy of photosynthesis. News Letter of the Japanese Society of Photosynthesis Research 2011, 21, 70–80. [Google Scholar]
- Prigogine, I.; Condepudi, D. Thermodynamique; Editions Odile Jacob: Paris, France, 1999. [Google Scholar]
- Atkins, P.W.; de Paula, J. Elements of Physical Chemistry, 4th ed.; Oxford University Press: Oxford, UK, 2005. [Google Scholar]
- Haynie, D.T. Biological Thermodynamics; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Garrod, C. Statistical Mechanics and Thermodynamics; Oxford University Press: New York, NY, USA, 1995. [Google Scholar]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423, 623–656. [Google Scholar] [CrossRef]
- Jaynes, E. T. Information theory and statistical mechanics. Phys. Rev. 1957, 106, 620–630. [Google Scholar] [CrossRef]
- Jaynes, E. T. Information theory and statistical mechanics. II. Phys. Rev. 1957, 108, 171–190. [Google Scholar] [CrossRef]
- Landsberg, P.T. Can entropy and “order” increase together? Phys. Lett. 1984, 102A, 171–173. [Google Scholar] [CrossRef]
- Sato, N. Gclust: Trans-kingdom classification of proteins using automatic individual threshold setting. Bioinformatics 2009, 25, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Mount, D.W. Bioinformatics; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- Strait, B.J.; Dewey, T.G. The Shannon information entropy of protein sequences. Biophys. J. 1996, 71, 148–155. [Google Scholar] [CrossRef]
- Allison, L.; Stern, L.; Edgoose, T.; Dix, T.I. Sequence complexity for biological sequence analysis. Comput. Chem. 2000, 24, 43–55. [Google Scholar] [CrossRef]
- Dewey, T.G.; Donne, M.D. Non-equilibrium thermodynamics of molecular evolution. J. Theor. Biol. 1998, 193, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Dewey, T.G. Algorithmic complexity and thermodynamics of sequence-structure relationships in proteins. Phys. Rev. E 1997, 56, 4545–4552. [Google Scholar] [CrossRef]
- Wagensberg, J.; Valls, J. The [extended] maximum entropy formalism and the statistical structure of ecosystem. Bull. Math. Biol. 1987, 49, 531–538. [Google Scholar] [CrossRef]
- Srienc, F.; Unrean, P. A statistical thermodynamical interpretation of metabolism. Entropy 2010, 12, 1921–1935. [Google Scholar] [CrossRef]
- Vallino, J.J. Ecosystem biogeochemistry considered as a distributed metabolic network ordered by maximum entropy production. Phil. Trans. Roy. Soc. B 2010, 365, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Wang, J.-C.; Wang, J.-F.; Hu, Y.-H. Motion entropy feature and its applications to event-based segmentation of sports video. EURASIP J. Adv. Signal Process. 2008. ID 460913. [Google Scholar] [CrossRef]
- Kimura, M. The Neutral Theory of Molecular Evolution; Cambridge University Press: Cambridge, UK, 1983. [Google Scholar]
- Demetrius, L. Thermodynamics and evolution. J. Theor. Biol. 2000, 206, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Annila, A.; Kuismanen, E. Natural hierarchy emerges from energy dispersal. BioSystems 2009, 95, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Crofts, A.R. Life, information, entropy, and time. Complexity 2007, 13, 14–50. [Google Scholar] [CrossRef] [PubMed]
- Abiko, S. Photosynthesis as a mechanism of entropy decrease (in Japanese). Science Journal Kagaku 1984, 54, 285–293. [Google Scholar]
- Ross, R.T. Thermodynamic limitations on the conversion of radiant energy into work. J. Chem. Phys. 1966, 45, 1–7. [Google Scholar] [CrossRef]
- Gebhardt, W. Photosynthetic efficiency. Radiat. Environ. Biophys. 1986, 25, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Terashima, I.; Fujita, T.; Inoue, T.; Chow, W.S.; Oguchi, R. Green light drives leaf photosynthesis more efficiently than red light in strong white light: Revisiting the enigmatic question of why leaves are green. Plant Cell Physiol. 2009, 50, 684–697. [Google Scholar] [CrossRef] [PubMed]
- Pänke, O.; Rumberg, B. Energy and entropy balance of ATP synthesis. Biochim. Biophys. Acta 1997, 1322, 183–194. [Google Scholar] [CrossRef]
- Miller, S.L.; Smith-Magowan, D. The thermodynamics of the Krebs cycle and related compounds. J. Phys. Chem. Ref. Data 1990, 19, 1049–1073. [Google Scholar] [CrossRef]
- Bergson, H. L’Évolution Créatrice; Presses Universitaires de France: Paris, France, 1907. [Google Scholar]
- Monod, J. Le Hasard et la Nécessité. Essai sur la philosophie naturelle de la biologie moderne; Seuil: Paris, France, 1970. [Google Scholar]
- Nicolis, G.; Prigogine, I. Self-Organization in Nonequilibrium Systems: From Dissipative Structures to Order through Fluctuations; Wiley International: New York, NY, USA, 1977. [Google Scholar]
- Stuart, Stuart. A. The Origins of Order. Self-Organization and Selection in Evolution; Oxford University Press: New York, NY, USA, 1993. [Google Scholar]
- Wagner, A. The Origins of Evolutionary Innovations; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sato, N. Scientific Élan Vital: Entropy Deficit or Inhomogeneity as a Unified Concept of Driving Forces of Life in Hierarchical Biosphere Driven by Photosynthesis. Entropy 2012, 14, 233-251. https://doi.org/10.3390/e14020233
Sato N. Scientific Élan Vital: Entropy Deficit or Inhomogeneity as a Unified Concept of Driving Forces of Life in Hierarchical Biosphere Driven by Photosynthesis. Entropy. 2012; 14(2):233-251. https://doi.org/10.3390/e14020233
Chicago/Turabian StyleSato, Naoki. 2012. "Scientific Élan Vital: Entropy Deficit or Inhomogeneity as a Unified Concept of Driving Forces of Life in Hierarchical Biosphere Driven by Photosynthesis" Entropy 14, no. 2: 233-251. https://doi.org/10.3390/e14020233
APA StyleSato, N. (2012). Scientific Élan Vital: Entropy Deficit or Inhomogeneity as a Unified Concept of Driving Forces of Life in Hierarchical Biosphere Driven by Photosynthesis. Entropy, 14(2), 233-251. https://doi.org/10.3390/e14020233