Is Cholesterol Sulfate Deficiency a Common Factor in Preeclampsia, Autism, and Pernicious Anemia? †
Abstract
:1. Introduction
2. A Crucial Role for Cholesterol Sulfate and Endothelial Nitric Oxide Synthase
3. A Relationship between Disseminated Intravascular Coagulation and Vaccines?
4. NO Synthesis and Gut Bacteria in Autism
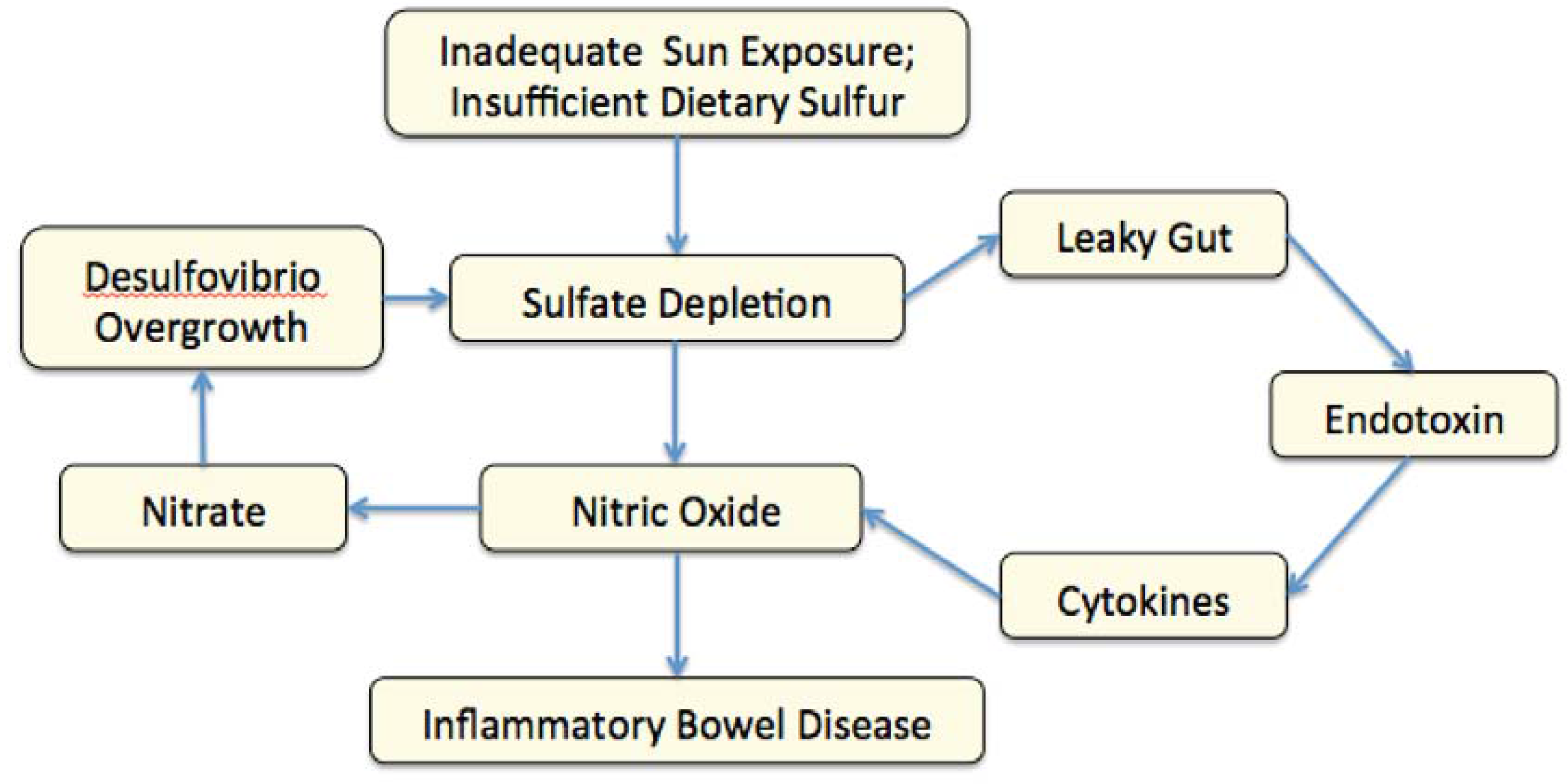
5. Autism, Nitric Oxide and Seizures
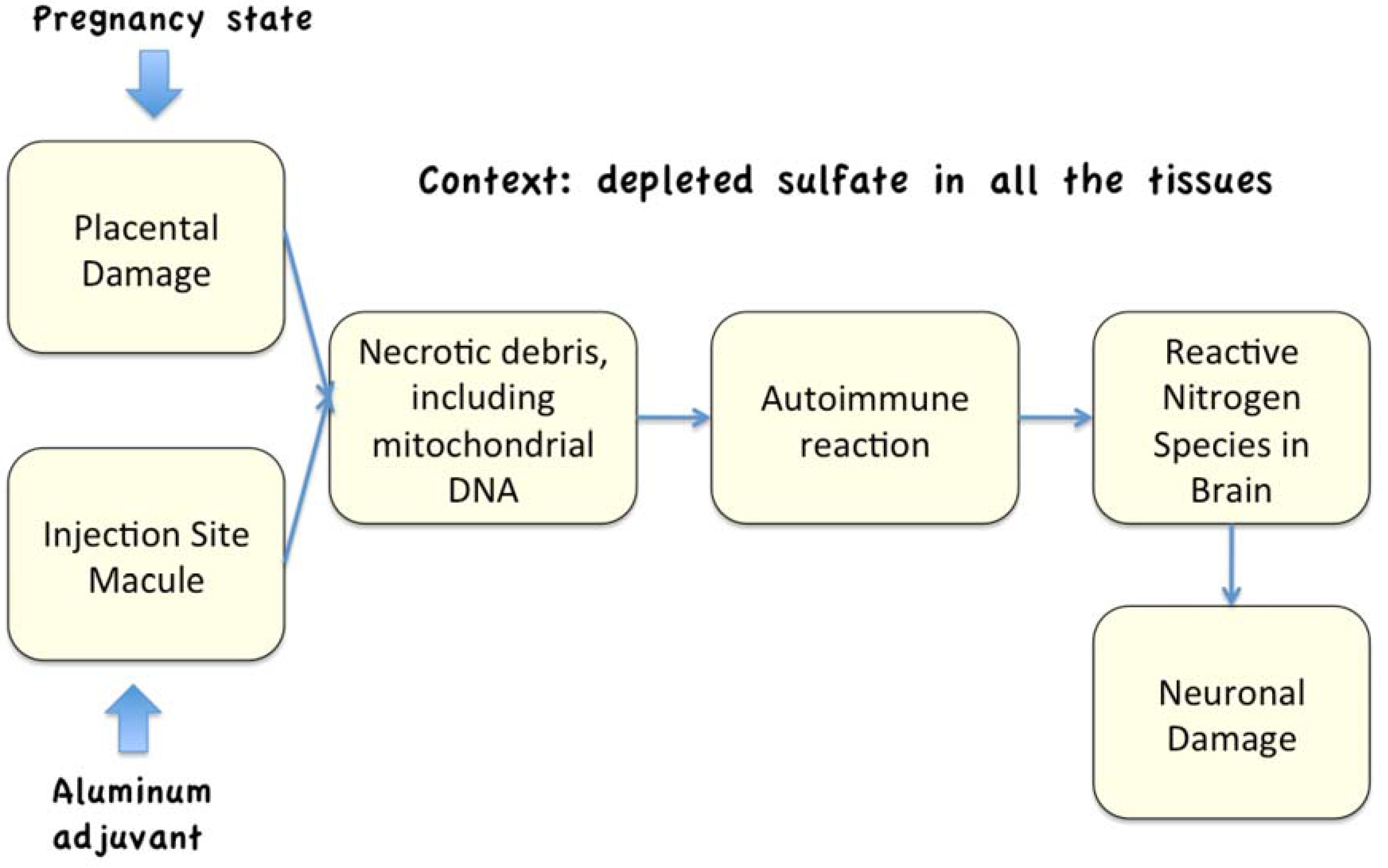
6. Necrosis and Autoimmune Reactions
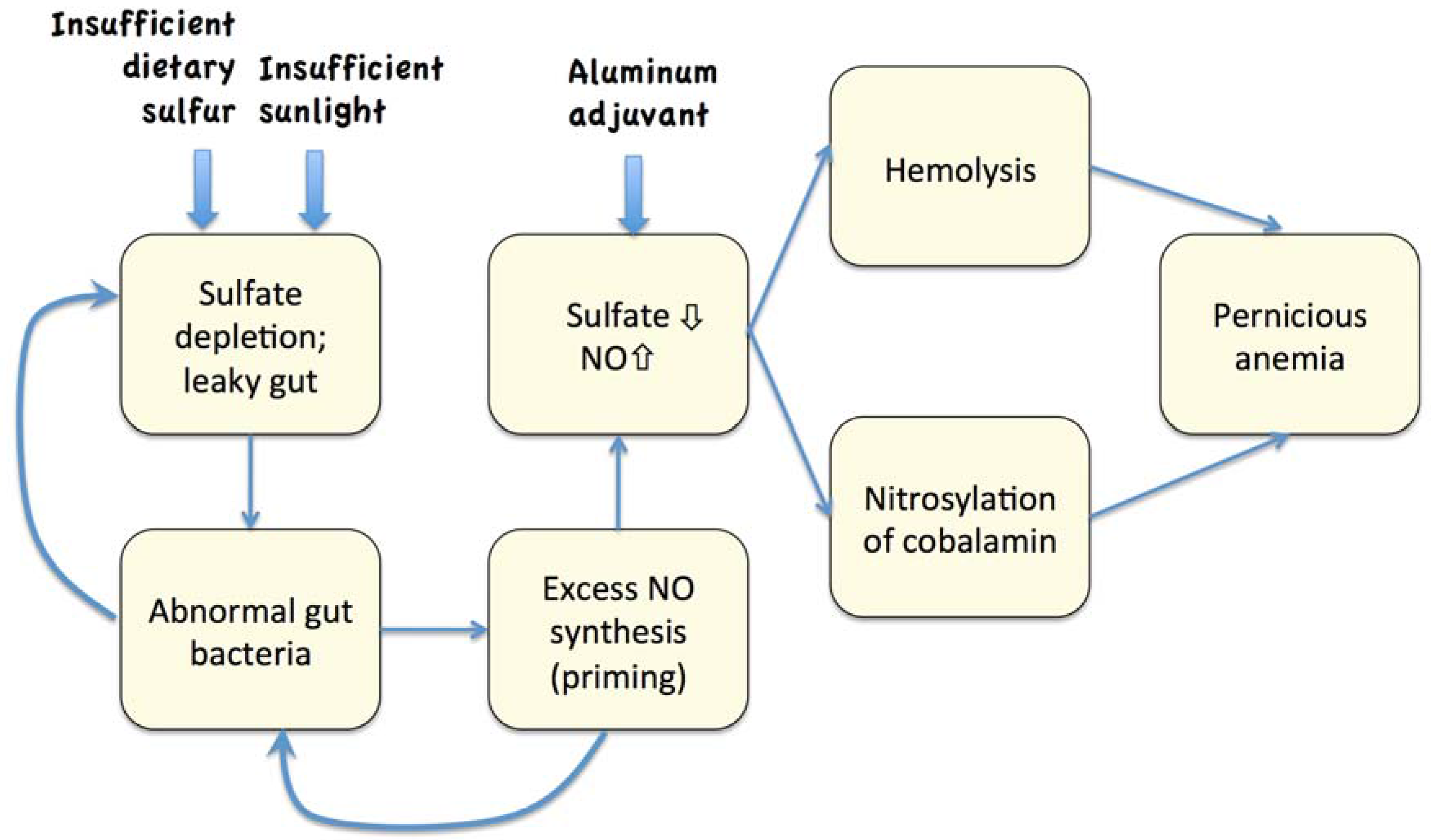
7. The Hemolysis/Nitric Oxide Synthesis Cascade
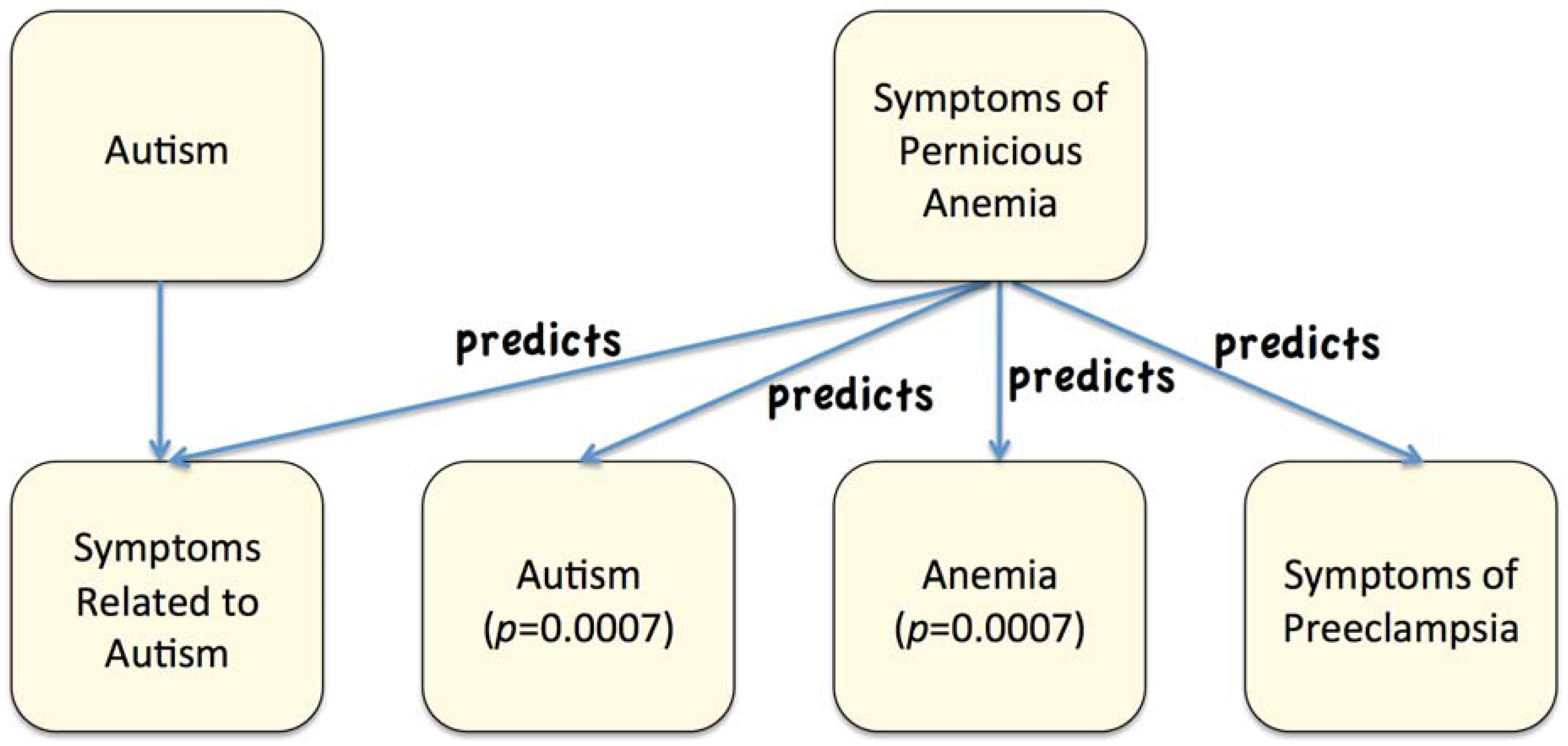
8. Experiments with VAERS
| Class | Alternates |
|---|---|
| diarrhoea | diarrhea |
| constipation | |
| fatigue | lethargy; lack of energy |
| light-headed | light headed; faint |
| appetite loss | loss of appetite; reduced appetite |
| pallor | pale skin |
| dyspnoea | dyspnea; hypoventilation; shortness of breath |
| swollen tongue | |
| depression | |
| loss of balance | gait disturbance |
| numbness | tingling; paresthesia; prickling |
8.1. Common Symptoms Found in Association with Autism, Preeclampsia, and Anemia
| Symptom | count: autism | count: control | p-value | count: anemia | count: control | p-value |
|---|---|---|---|---|---|---|
| anxiety | 49 | 2 | 0.01 | 1720 | 728 | 6.78E-06 |
| infection | 54 | 6 | 0.01 | 1015 | 354 | 2.20E-05 |
| autism | - | - | - | 206 | 28 | 0.00066 |
| ear infection | 32 | 3 | 0.03 | 117 | 24 | 0.0053 |
| eczema | 18 | 0 | 0.04 | 134 | 44 | 0.0096 |
| asthma | 24 | 3 | 0.05 | 682 | 307 | 0.00054 |
| premature | 20 | 1 | 0.05 | 75 | 24 | 0.024 |
| pneumonia | 19 | 1 | 0.05 | 613 | 350 | 0.0035 |
| Symptoms | count: anemia | count: control | p-value |
|---|---|---|---|
| nausea | 8817 | 3088 | 4.20E-14 |
| headache | 4495 | 1839 | 9.50E-10 |
| abdominal pain | 945 | 146 | 8.30E-07 |
| anxiety | 1720 | 728 | 6.70E-06 |
| pulmonary | 453 | 113 | 0.00016 |
| vision blurred | 420 | 129 | 0.00042 |
| visual impairment | 258 | 54 | 0.00069 |
| facial swelling | 288 | 162 | 0.015 |
| eye irritation | 119 | 50 | 0.022 |
| sensitivity to light | 70 | 11 | 0.011 |
| Symptom | count: anemia | count: control | p-value |
|---|---|---|---|
| Brain and Nervous System Problems | |||
| sleep disorder | 534 | 140 | 0.000097 |
| seizure | 1144 | 632 | 0.00047 |
| nerve injury | 69 | 0 | 0.0042 |
| disorientation | 112 | 32 | 0.01 |
| Heart Problems | |||
| Symptom | count: anemia | count: control | p-value |
| chest pain | 1366 | 278 | 2.00E-07 |
| heart rate irregular | 963 | 279 | 1.00E-05 |
| heart failure | 64 | 8 | 0.011 |
| Muscle Problems | |||
| Symptom | count: anemia | count: control | p-value |
| myalgia | 981 | 416 | 0.000096 |
| paralysis | 384 | 71 | 0.00013 |
| dysphagia | 353 | 96 | 0.0005 |
| Life Threatening Conditions | |||
| Symptom | count: anemia | count: control | p-value |
| loss of consciousness | 832 | 447 | 0.001 |
| death | 180 | 74 | 0.01 |
| Other | |||
| Symptom | count: anemia | count: control | p-value |
| swollen tongue | 1116 | 322 | 0.0000044 |
| pulmonary | 453 | 113 | 0.00016 |
| red blood cell | 361 | 109 | 0.00065 |
| drooling/foam | 123 | 32 | 0.007 |
| lymph node pain | 219 | 107 | 0.013 |
8.2. Effects of Aluminum in Vaccines
| Symptom | count: aluminum | count: not aluminum | p-value |
|---|---|---|---|
| sleep disorder | 224 | 86 | 0.0051 |
| fatigue | 2262 | 1817 | 0.006 |
| joint pain | 495 | 291 | 0.0066 |
| depression | 319 | 175 | 0.011 |
| pain | 2008 | 1658 | 0.013 |
| infection | 329 | 192 | 0.014 |
| seizure | 536 | 369 | 0.018 |
9. Discussion
10. Conclusions
References
- ACOG Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia, Number 33. Obstet. Gynecol. 2002, 99, 159–167. [Google Scholar]
- Roberts, J.M.; Taylor, R.N.; Musci, T.J.; Rodgers, G.M.; Hubel, C.A.; McLaughlin, M.K. Preeclampsia: An endothelial cell disorder. Am. J. Obstet. Gynecol. 1989, 161, 1200–1204. [Google Scholar] [CrossRef]
- Rayman, M.P.; Barlis, J.; Evans, R.W.; Redman, C.W.; King, L.J. Abnormal iron parameters in the pregnancy syndrome preeclampsia. Am. J. Obstet. Gynecol. 2002, 187, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.R.; McDermott, S.; Bao, H.; Hardin, J.; Gregg, A. Pre-eclampsia, birth weight, and autism spectrum disorders. J. Autism Dev. Disord. 2010, 40, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Seneff, S.; Davidson, R.; Mascitelli, L. Might cholesterol sulfate deficiency contribute to the development of autistic spectrum disorder? Med. Hypotheses 2012, 8, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Raats, C.J.I.; van Den Born, J.; Berden, J.H.M. Glomerular heparan sulfate alterations: Mechanisms and relevance for proteinuria. Kidney Int. 2000, 57, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Hasanzadeh, M.; Ayatollahi, H.; Farzadnia, M.; Ayati, S.; Khoob, M.K. Elevated plasma total homocysteine in preeclampsia. Saudi Med. J. 2008, 29, 875–878. [Google Scholar] [PubMed]
- Makedos, G.; Papanicolaou, A.; Hitoglou, A.; Kalogiannidis, I.; Makedos, A.; Vrazioti, V.; Goutzioulis, M. Homocysteine, folic acid and B12 serum levels in pregnancy complicated with preeclampsia. Arch. Gynecol. Obstet. 2007, 275, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Mujawar, S.A.; Patil, V.W.; Daver, R.G. Study of serum homocysteine, folic acid and vitamin B12 in patients with preeclampsia. Ind. J. Clin. Biochem. 2011, 26, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Olszewski, A.J.; McCully, K.S. Homocysteine metabolism and the oxidative modification of proteins and lipids. Free Radic. Biol. Med. 1993, 14, 683–693. [Google Scholar] [CrossRef]
- Toh, B.-H.; van Driel, I.R.; Gleeson, P.A. Pernicious Anemia. N. Engl. J. Med. 1997, 337, 1441–1448. [Google Scholar] [PubMed]
- Strott, C.A. Cholesterol Sulfate in human physiology: What’s it all about? J. Lipid Res. 2003, 44, 1268–1278. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Kubushiro, K.; Akiba, Y.; Cui, Y.; Tsukazaki, K.; Nozawa, S.; Iwamori, M. Alteration of acidic lipids in human sera during the course of pregnancy: Characteristic increase in the concentration of cholesterol sulfate. J. Chromatogr. B 1997, 704, 99–104. [Google Scholar] [CrossRef]
- Horan, F.E.; Hirsch, F.G.; Wood, L.A.; Wright, I.S. Surface effects on blood-clotting components as determined by zeta potentials. J. Clin. Invest. 1950, 29, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Cheetham, J.J.; Epand, R.M.; Andrews, M.; Flanagan, T.D. Cholesterol sulfate inhibits the fusion of Sendai virus to biological and model membranes. J. Biol. Chem. 1990, 265, 12404–12409. [Google Scholar] [PubMed]
- Bleau, G.; Bodley, F.H.; Longpré, J.; Chapdelaine, A.; Roberts, K.D. Cholesterol sulfate. I. Occurrence and possible biological function as an amphipathic lipid in the membrane of the human erythrocyte. Biochim. Biophys. Acta 1974, 352, 1–9. [Google Scholar] [CrossRef]
- Yanai, H.; Javitt, N.B.; Higashi, Y.; Fuda, H.; Strott, C.A. Expression of cholesterol sulfotransferase (SULT2B1b) in human platelets. Circulation 2004, 109, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.; Frame, F.; Weller, R.; McKenzie, R.C. Expression of nitric oxide synthase III (eNOS) mRNA by human skin cells: Melanocytes but not keratinocytes express eNOS mRNA. Arch. Dermatol. Res. 1998, 290, 350–352. [Google Scholar] [CrossRef] [PubMed]
- Hyde, R.R. Heparin inhibition of anaphylactic shock. J. Epidemiol. 1927, 7, 614–618. [Google Scholar]
- Michel, J.B.; Feron, O.; Sacks, D.; Michel, T. Reciprocal regulation of endothelial nitric-oxide synthase by Ca2+-calmodulin and caveolin. J. Biol. Chem. 1997, 272, 15583–15586. [Google Scholar] [CrossRef] [PubMed]
- Vallance, P.; Chan, N. Endothelial function and nitric oxide: Clinical relevance. Heart 2001, 85, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.S.; Ferguson, T.B., Jr.; Han, T.H.; Hyduke, D.R.; Liao, J.C.; Rassaf, T.; Bryan, N.; Feelisch, M.; Lancaster, J.R., Jr. Nitric oxide is consumed, rather than conserved, by reaction with oxyhemoglobin under physiological conditions. Proc. Natl. Acad. Sci. USA 2002, 99, 10341–10346. [Google Scholar] [CrossRef] [PubMed]
- Doherty, D.H.; Doyle, M.P.; Curry, S.R.; Vali, R.J.; Fattor, T.J.; Olson, J.S.; Lemon, D.D. Rate of reaction with nitric oxide determines the hypertensive effect of cell-free hemoglobin. Nat. Biotechnol. 1998, 16, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Palmer, R.M.J.; Ferrige, A.G.; Moncada, S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 1987, 327, S24–S26. [Google Scholar] [CrossRef] [PubMed]
- Margulis, A.; Sitaramayya, A. Rate of deactivation of nitric oxide-stimulated soluble guanylate cyclase: Influence of nitric oxide scavengers and calcium. Biochemistry 2000, 39, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Azarov, I.; Huang, K.T.; Basu, S.; Gladwin, M.T.; Hogg, N.; Kim-Shapiro, D.B. Nitric oxide scavenging by red blood cells as a function of hematocrit and oxygenation. J. Biol. Chem. 2005, 280, 39024–39032. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R.M.; Barron, B.J.; White, P.A.; Fraire, A.E. Diagnosis by radiocolloid imaging of postpartum hepatic necrosis in the syndrome of hemolysis, elevated liver enzymes, and low platelets. Clin. Nucl. Med. 1992, 17, 322–324. [Google Scholar] [CrossRef] [PubMed]
- McKay, D.G. Diseases of hypersensitivity: Disseminated intravascular coagulation. Arch. Intern. Med. 1965, 116, 83–94. [Google Scholar] [CrossRef] [PubMed]
- McKay, D.G. Progress in disseminated intravascular coagulation. Calif. Med. 1969, 111, 186–199. [Google Scholar] [PubMed]
- Gemsa, D.; Woo, C.H.; Fudenberg, H.H.; Schmidt, R. Stimulation of heme oxygenase in macrophages and liver by endotoxin. J. Clin. Invest. 1974, 53, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Roach, J.P.; Moore, E.E.; Partrick, D.A.; Damle, S.S.; Silliman, C.C.; McIntyre, R.C., Jr.; Banerjee, A. Heme oxygenase-1 induction in macrophages by a hemoglobin-based oxygen carrier reduces endotoxin-stimulated cytokine secretion. Shock 2009, 31, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Viaro, F.; Dalio, M.B.; Evora, P.R.B. Catastrophic cardiovascular adverse reactions to protamine are nitric oxide/cyclic guanosine monophosphate dependent and endothelium mediated: Should methylene blue be the treatment of choice? Chest 2002, 122, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Cauwels, A.; Janssen, B.; Buys, E.; Sips, P.; Brouckaert, P. Anaphylactic shock depends on PI3K and eNOS-derived NO. J. Clin. Invest. 2006, 116, 2244–2251. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R.M.; Seneff, S. The initial common pathway of inflammation, disease, and sudden death. Entropy 2012, 14, 1399–1442. [Google Scholar] [CrossRef]
- Zangi, R. Can salting-in/salting-out ions be classified as chaotropes/kosmotropes? J. Phys. Chem. B 2010, 114, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Kvech, S.; Edwards, M. Solubility controls on aluminum in drinking water at relatively low and high pH. Water Res. 2002, 36, 4356–4368. [Google Scholar] [CrossRef]
- Carpenter, E.; Fray, L.; Gormley, E. Antigen-specific lymphocytes enhance nitric oxide production in Mycobacterium bovis BCG-infected bovine macrophages. Immunol. Cell Biol. 1998, 76, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.N.; Peluffo, G.; Piacenza, L.; Radi, R. Intraphagosomal peroxynitrite as a macrophage-derived cytotoxin against internalized Trypanosoma cruzi: Consequences for oxidative killing and role of microbial peroxiredoxins in infectivity. J. Biol. Chem. 2011, 286, 6627–6640. [Google Scholar] [CrossRef] [PubMed]
- Kinross, J.M.; Darzi, A.W.; Nicholson, J.K. Gut microbiome-host interactions in health and disease. Genome Med. 2011, 3, 14. [Google Scholar] [CrossRef] [PubMed]
- Parracho, H.M.R.T.; Bingham, M.O.; Gibson, G.R.; McCartney, A.L. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J. Med. Microbiol. 2005, 54, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Oller, J.W., Jr.; Oller, S.D.; Oller, S.N. Autism: The Diagnosis, Treatment, & Etiology of the Undeniable Epidemic; Jones & Bartlett Learning: Sudbury, MA, USA, 2010. [Google Scholar]
- Molloy, C.A.; Manning-Courtney, P. Prevalence of chronic gastrointestinal symptoms in children with autism and autistic spectrum disorders. Autism 2003, 7, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Sandler, R.H.; Finegold, S.M.; Bolter, E.R.; Buchanan, C.P.; Maxwell, A.P.; Väisänen, M.L.; Nelson, M.N.; Wexler, H.M. Short-term benefit from oral vancomycin treatment of regressive-onset Autism. J. Child Neurol. 2000, 15, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Finegold, S.M. State of the art; Microbiology in health and disease. Intestinal bacterial flora in autism. Anaerobe 2011, 17, 367–368. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Cummings, H.; MacFarlane, G.T. Competition for hydrogen between sulphate-reducing bacteria and methanogenic bacteria from the human large intestine. J. Applied Bacteriol. 1988, 65, 241–247. [Google Scholar] [CrossRef]
- Gibson, G.R.; Cummings, J.H.; Macfarlane, G.T. Use of a three-stage continuous culture system to study the effect of mucin on dissimilatory sulfate reduction and methano-genesis by mixed populations of human gut bacteria. Appl. Environ. Microbiol. 1988, 54, 2750–2755. [Google Scholar] [PubMed]
- Levine, J.J.; Pettei, M.J.; Valderrama, E.; Gold, D.M.; Kessler, B.H.; Trachtman, H. Nitric oxide and inflammatory bowel disease: Evidence for local intestinal production in children with active colonic disease. J. Pediatr. Gastr. Nutr. 1998, 26, 34–38. [Google Scholar] [CrossRef]
- Gibson, G.R.; Cummings, J.H.; Macfarlane, G.T. Growth and activities of sulphate-reducing bacteria in gut contents of healthy subjects and patients with ulcerative colitis. FEMS Microbiol. Ecol. 1991, 86, 103–112. [Google Scholar] [CrossRef]
- Sweeten, T.L.; Posey, D.J.; Shankar, S.; McDougle, C.J. High nitric oxide production in autistic disorder: A possible role for interferon-γ. Biol. Psychiat. 2004, 55, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Sögüt, S.S.; Zoroglu, S.S.; Özyurt, H.; Ylmaz, H.R.; Ozugurlu, F.; Sivasli, E.; Yetkin, O.; Yanik, M.; Tutkun, H.; Savas, H.A.; et al. Changes in nitric oxide levels and antioxidant enzyme activities may have a role in the pathophysiological mechanisms involved in autism. Clin. Chim. Acta 2003, 331, 111–117. [Google Scholar] [CrossRef]
- Akyol, O.; Zoroglu, S.S.; Armutcu, F.; Sahin, S.; Gurel, A. Nitric Oxide as a Physiopathological Factor in Neuropsychiatric Disorders. In Vivo 2004, 18, 377–390. [Google Scholar] [PubMed]
- Zoroglu, S.S.; Yürekli, M.; Meram, I.; Sögüt, S.; Tutkun, H.; Yetkin, O.; Sivasli, E.; Savas, H.A.; Yanik, M.; Herken, H.; et al. Pathophysiological role of nitric oxide and adrenomedullin in autism. Cell Biochem. Funct. 2003, 21, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Murch, S.H.; Macdonald, T.T.; Walker-Smith, J.A.; Levin, M.; Lionetti, P.; Klein, N.J. Disruption of sulphated glycosaminoglycans in intestinal inflammation. The Lancet 1993, 341, 711–714. [Google Scholar] [CrossRef]
- Finegold, S.M.; Downes, J.; Summanen, P.H. Microbiology of regressive autism. Anaerobe 2012, 18, 260–262. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Doyle, R. Autism, gut–blood–brain barrier, and mast cells. J. Clin. Psychopharm. 2008, 2, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Emanuele, E.; Orsi, P.; Boso, M.; Broglia, D.; Brondino, N.; Barale, F.; di Nemi, S.U.; Politi, P. Low-grade endotoxemia in patients with severe autism. Neurosci. Lett. 2010, 471, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Karteris, E.; Vatish, M.; Hillhouse, E.W.; Grammatopoulos, D.K. Preeclampsia is associated with impaired regulation of the placental nitric oxide-cyclic guanosine monophosphate pathway by corticotropin-releasing hormone (CRH) and CRH-related peptides. J. Clin. Endocrin. Metab. 2005, 90, 3680–3687. [Google Scholar] [CrossRef] [PubMed]
- Debry, P.; Nash, E.A.; Neklason, D.W.; Metherall, J.E. Role of multidrug resistance P-glycoproteins in cholesterol esterification. J. Biol. Chem. 1997, 272, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Haines, T.H. Do sterols reduce proton and sodium leaks through lipid bilayers? Prog. Lipid Res. 2001, 40, 299–324. [Google Scholar] [CrossRef]
- Seino, S.; Miki, T. Physiological and pathophysiological roles of ATP-sensitive K+ channels. Prog. Biophys. Mol. Biol. 2003, 81, 133–176. [Google Scholar] [CrossRef]
- Noma, A. ATP-regulated K+ channels in cardiac muscle. Nature 1983, 305, 147–148. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.T.; Patlak, J.B.; Worley, J.F.; Standen, N.B. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am. J. Physiol. 1990, 259, C3–C18. [Google Scholar] [PubMed]
- Khorram, O.; Han, G. The influence of progesterone on endometrial nitric oxide synthase expression. Fertil. Steril. 2009, 91, 2157–2162. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.; Gibraeil, H.D.; Mayer, B. Lack of involvement of extracellular signal- regulated kinase (ERK) in the agonist-induced endothelial nitric oxide synthesis. Biochem. Pharmacol. 2002, 63, 1137–1142. [Google Scholar] [CrossRef]
- Hahn, S.; Holzgreve, W. Fetal cells and cell-free fetal DNA in maternal blood: New insights into pre-eclampsia. Hum. Reprod. 2002, 8, 501–508. [Google Scholar] [CrossRef]
- Zhong, X.H.; Laivuori, H.; Livingston, J.C.; Ylikorkala, O.; Sibai, B.M.; Holzgreve, W.; Hahn, S. Elevation of both maternal and fetal extracellular circulating deoxyribonucleic acid concentrations in the plasma of pregnant women with preeclampsia. Am. J. Obstet. Gynecol. 2001, 184, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Redman, C.W.; Sargent, I.L. Placental debris, oxidative stress and pre-eclampsia. Placenta 2000, 21, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Redman, C.W.; Sargent, I.L. The pathogenesis of pre-eclampsia. Gynecol. Obstet. Fertil. 2001, 29, 518–522. [Google Scholar] [CrossRef]
- Wolff, J.J.; Gu, H.; Gerig, G.; Elison, J.T.; Styner, M.; Gouttard, S.; Botteron, K.N.; Dager, S.R.; Dawson, G.; Estes, A.M.; et al. Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. Am. J. Psychiatry 2012, 169, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Garthwaite, G.; Goodwin, D.A.; Batchelor, A.M.; Leeming, K.; Garthwaite, J. Nitric oxide toxicity in CNS white matter: An in vitro study using rat optic nerve. Neuroscience 2002, 109, 145–155. [Google Scholar] [CrossRef]
- Garthwaite, G.; Goodwin, D.A.; Neale, S.; Riddall, D.; Garthwaite, J. Soluble guanylyl cyclase activator YC-1 protects white matter axons from nitric oxide toxicity and metabolic stress, probably through Na+ channel inhibition. Mol. Pharmacol. 2002, 61, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Volkmar, F.R.; Nelson, D.S. Seizure disorders in autism. J. Am. Acad. Child Adolesc. Psychiatry 1990, 29, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.C.; Dettbarn, W.D. Prevention of kainic acid seizures-induced changes in levels of nitric oxide and high-energy phosphates by 7-nitroindazole in rat brain regions. Brain Res. 2003, 981, 184–192. [Google Scholar] [CrossRef]
- Kato, N.; Sato, S.; Yokoyama, H.; Kayama, T.; Yoshimura, T. Sequential changes of nitric oxide levels in the temporal lobes of kainic acid-treated mice following application of nitric oxide synthase inhibitors and phenobarbital. Epilepsy Res. 2005, 65, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, R.; Rabanus, A.; Otahal, J.; Patzak, A.; Kardos, J.; Albus, K.; Heinemann, U.; Kann, O. Endogenous nitric oxide is a key promoting factor for initiation of seizure-like events in hippocampal and entorhinal cortex slices. J. Neurosci. 2009, 29, 8565–8577. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C. Regulation of mitochondrial respiration by nitric oxide inhibition of cy- tochrome c oxidase. Biochim. Biophys. Acta 2001, 1504, 46–57. [Google Scholar] [CrossRef]
- Brown, G.C.; Borutaite, V. Inhibition of mitochondrial respiratory complex I by nitric oxide, peroxynitrite and S-nitrosothiols. Biochim. Biophys. Acta 2004, 1658, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Kunz, W.S.; Kudin, A.P.; Vielhaber, S.; Blumcke, I.; Zuschratter, W.; Schramm, J.; Beck, H.; Elger, E.E. Mitochondrial complex I deficiency in the epileptic focus of patients with temporal lobe epilepsy. Ann. Neurol. 2000, 48, 766–773. [Google Scholar] [CrossRef]
- Kann, O.; Kovacs, R.; Njunting, M.; Behrens, C.J.; Otahal, J.; Lehmann, T.N.; Gabriel, S.; Heinemann, U. Metabolic dysfunction during neuronal activation in the ex vivo hippocampus from chronic epileptic rats and humans. Brain 2005, 128, 2396–2407. [Google Scholar] [CrossRef] [PubMed]
- Patel, M. Mitochondrial dysfunction and oxidative stress: Cause and consequence of epileptic seizures. Free Radic. Biol. Med. 2004, 37, 1951–1962. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, D.A.; Bradstreet, J.J. Evidence of mitochondrial dysfunction in autism and implications for treatment. Am. J. Biochem. Biotech. 2008, 4, 208–217. [Google Scholar] [CrossRef]
- Lindblad, E.B. Aluminium adjuvants-in retrospect and prospect. Vaccine 2004, 22, 3658–3668. [Google Scholar] [CrossRef] [PubMed]
- Tritto, E.; Mosca, F.; de Gregorio, E. Mechanism of action of licensed vaccine adjuvants. Vaccine 2009, 27, 3331–3334. [Google Scholar] [CrossRef] [PubMed]
- Al-Akl, N.S.; Chakhtoura, M.; Kazzi, N.F.; Usta, J.; Chamoun, C.A.; Abdelnoor, A.M. Uric acid: A possible mediator of the adjuvant effect of alum in mice immunized with ovalbumin. WJV 2011, 1, 148–155. [Google Scholar] [CrossRef]
- Kool, M.; Soullié, T.; van Nimwegen, M.; Willart, M.A.M.; Muskens, F.; Jung, S.; Hoogsteden, H.C.; Hammad, H.; Lambrecht, B.N. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J. Exp. Med. 2008, 205, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Evans, J.E.; Rock, K.L. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature 2003, 425, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Webb, R.; Jeffries, M.; Sawalha, A.H. Uric acid directly promotes human T-cell activation. Am. J. Med. Sci. 2009, 337, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Blaurock-Busch, E.; Amin, O.R.; Dessoki, H.H.; Rabah, T. Toxic metals and essential elements in hair and severity of symptoms among children with autism. Maedica 2012, 7, 38–48. [Google Scholar] [PubMed]
- Goulopoulou, S.; Matsumoto, T.; Bomfim, G.F.; Webb, R.C. Toll-like receptor 9 activation: a novel mechanism linking placenta-derived mitochondrial DNA and vascular dysfunction in pre-eclampsia. Clin. Sci. (Lond.) 2012, 123, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Takeda, K.; Kaisho, T. Toll-like receptors: Critical proteins linking innate and acquired immunity. Nature Immunol. 2001, 2, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Tomljenovic, L.; Shaw, C.A. Aluminum vaccine adjuvants: Are they safe? Curr. Med. Chem. 2011, 18, 2630–2637. [Google Scholar] [CrossRef] [PubMed]
- Exley, C.; Swarbrick, L.; Gherardi, R.K.; Authier, F.-J. A role for the body burden of aluminium in vaccine-associated macrophagic myofasciitis and chronic fatigue syndrome. Med. Hypotheses 2009, 72, 135–139. [Google Scholar] [CrossRef] [PubMed]
- M. Kawahara, M.; Kato-Negishi, M. Link between aluminum and the pathogenesis of Alzheimer’s disease: The integration of the aluminum and amyloid cascade hypotheses. Int. J. Alzheimers Dis. 2011, 276393. [Google Scholar] [CrossRef] [PubMed]
- Blaylock, R.L. Aluminum induced immunoexcitotoxicity in neurodevelopmental and neurode-generative disorders. Curr. Inorg. Chem. 2012, 2, 46–53. [Google Scholar] [CrossRef]
- Passeri, E.; Villa, C.; Couette, M.; Itti, E.; Brugieres, P.; Cesaro, P.; Gherardi, R.K.; Bachoud-Levi, A.-C.; Authier, F.-J. Long-term persistence of vaccine-derived aluminum hydroxide is associated with chronic cognitive dysfunction. J. Inorg. Biochem. 2009, 103, 1571–1578. [Google Scholar]
- Agmon-Levin, N.; Hughes, G.R.V.; Shoenfeld, Y. The spectrum of ASIA: ‘Autoimmune (Auto-inflammatory) Syndrome induced by Adjuvants’. Lupus 2012, 21, 118–120. [Google Scholar] [CrossRef] [PubMed]
- Passeri, E.; Villa, C.; Couette, M.; Itti, E.; Brugieres, P.; Cesar, P.; Gherardi, R.K.; Bachoud-Levi, A.-C.; Authier, F.-J. Long-term follow-up of cognitive dysfunction in patients with aluminum hydroxide-induced macrophagic myofasciitis (MMF). J. Inorg. Biochem. 2011, 105, 1457–1463. [Google Scholar] [CrossRef] [PubMed]
- Wills, M.R.; Savory, J. Water content of aluminum, dialysis dementia, and osteomalacia. Environ. Health Persp. 1985, 63, 141–147. [Google Scholar] [CrossRef]
- Llansola, M.; Miñana, M.-D.; Montoliu, C.; Saez, R.; Corbalán, R.; Manzo, L.; Felipo, V. Prenatal exposure to aluminum reduces expression of neuronal nitric oxide synthase and of soluble guanylate cyclase and impairs glutamatergic neurotransmission in rat cerebellum. J. Neurochem. 1999, 73, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Lemire, J.; Appanna, V.D. Aluminum toxicity and astrocyte dysfunction: A metabolic link to neurological disorders. Inorg. Biochem. 2011, 105, 1513–1517. [Google Scholar] [CrossRef] [PubMed]
- Lemire, J.; Mailloux, R.; Puiseux-Dao, S.; Appanna, V.D. Aluminum-induced defective mitochondrial metabolism perturbs cytoskeletal dynamics in human astrocytoma cells. J. Neurosci. Res. 2009, 87, 1474–1483. [Google Scholar] [CrossRef] [PubMed]
- Redhead, K.; Quinlan, G.J.; Das, R.G.; Gutteridge, J.M. Aluminium-adjuvanted vaccines transiently increase aluminium levels in murine brain tissue. Pharmacol. Toxicol. 1992, 70, 278–280. [Google Scholar] [CrossRef] [PubMed]
- Onore, C.; Careaga, M.; Ashwood, P. The role of immune dysfunction in the pathophysiology of autism. Brain Behav. Immun. 2012, 26, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cremer, P.S. Interactions between macromolecules and ions: The Hofmeister series. Curr. Opin. Chem. Biol. 2006, 10, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Rother, R.P.; Bell, L.; Hillmen, P.; Gladwin, M.T. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: A novel mechanism of human disease. JAMA 2005, 293, 1653–1662. [Google Scholar] [CrossRef] [PubMed]
- Maimburg, R.D.; Vaeth, M.; Schendel, D.E.; Bech, B.H.; Olsen, J.; Thorsen, P. Neonatal jaundice: A risk factor for infantile autism? Paediatr. Perinat. Ep. 2008, 22, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Maimburg, R.D.; Bech, B.H.; Vaeth, M.; Mller-Madsen, B.; Olsen, J. Neonatal jaundice, Autism, and other disorders of psychological development. Pediatrics 2010, 126, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, M.; Chamulitrat, W.; Ferruzzi, G.; Sauls, D.L.; Weinberg, J.B. Nitric oxide interactions with cobalamins: Biochemical and functional consequences. Blood 1996, 88, 1857–1864. [Google Scholar] [PubMed]
- Acharya, U.; Gau, J.-T.; Horvath, W.; Ventura, P.; Hsueh, C.-T.; Carlsen, W. Hemolysis and hyperhomocysteinemia caused by cobalamin deficiency: Three case reports and review of the literature. J. Hematol. Oncol. 2008, 1, 26. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, R.; Brasch, N.E. Kinetic studies on the reaction between cob(I)alamin and peroxynitrite: Rapid oxidation of cob(I)alamin to cob(II)alamin by peroxynitrous acid. Chem. Eur. J. 2011, 17, 11723–11727. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, A.; Seneff, S. Automatic drug side effect discovery from online patient-submitted reviews: Focus on statin drugs. In Proceedings of the first international conference on advances in information mining and management, Barcelona, Spain, October 2011.
- Dunning, T. Accurate methods for the statistics of surprise and coincidence. Comp. Ling. 1993, 19, 61–74. [Google Scholar]
- PubMed Health, A.D.A.M. Medical Encyclopedia, Preeclampsia. Available on: http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0001900/ (accessed on 7 November 2012).
- Neufeld, A.H. Nitric oxide: A potential mediator of retinal ganglion cell damage in glaucoma. Surv. Ophthalmol. 1999, 43, S129–S135. [Google Scholar] [CrossRef]
- Liu, B.; Neufeld, A.H. Expression of nitric oxide synthase-2 (NOS-2) in reactive astrocytes of the human glaucomatous optic nerve head. Glia 2000, 30, 178–186. [Google Scholar] [CrossRef]
- Maiese, K.; Boniece, I.; DeMeo, D.; Wagner, J.A. Peptide growth factors protect against ischemia in culture by preventing nitric oxide toxicity. J. Neurosci. 1993, 73, 3034–3040. [Google Scholar]
- Donahoe, W.E. Cyanosis in infants with nitrates in drinking water as cause. Pediatrics 1949, 3, 308–311. [Google Scholar] [PubMed]
- Chilcote, R.R.; Williams, B.; Wolff, L.J.; Baehner, R.L. Sudden death in an infant from methemoglobinemia after administration of ‘sweet spirits of nitre’. Pediatrics 1977, 59, 280–282. [Google Scholar] [PubMed]
- Souayah, N.; Michas-Martin, P.A.; Nasar, A.; Krivitskaya, N.; Yacoub, H.A.; Khan, H.; Qureshi, A.I. Guillain-Barré syndrome after Gardasil vaccination: data from Vaccine Adverse Event Reporting System 2006–2009. Vaccine 2011, 29, 886–889. [Google Scholar] [CrossRef] [PubMed]
- Katoulis, A.C.; Liakou, A.; Bozi, E.; Theodorakis, M.; Alevizou, A.; Zafeiraki, A.; Mistidou, M.; Stavrianeas, N.G. Erythema Multiforme following vaccination for human Papillomavirus. Dermatology 2010, 220, 60–62. [Google Scholar] [CrossRef] [PubMed]
- Faas, M.M.; Schuiling, G.A.; Baller, J.F.; Visscher, C.A.; Bakker, W.W. A new animal model for human preeclampsia: Ultra-low-dose endotoxin infusion in pregnant rats. Am. J. Obstet. Gynecol. 1994, 1171, 158–164. [Google Scholar] [CrossRef]
- Antony, A.C. Megaloblastic anemias. In Hematology: Basic Principles and Practice, 5th ed.; Hoffman, R., Benz, E.J., Shattil, S.S., Eds.; Elsevier Churchill Livingstone: Philadelphia, PA, USA, 2008; Chapter 39. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Seneff, S.; Davidson, R.M.; Liu, J. Is Cholesterol Sulfate Deficiency a Common Factor in Preeclampsia, Autism, and Pernicious Anemia? Entropy 2012, 14, 2265-2290. https://doi.org/10.3390/e14112265
Seneff S, Davidson RM, Liu J. Is Cholesterol Sulfate Deficiency a Common Factor in Preeclampsia, Autism, and Pernicious Anemia? Entropy. 2012; 14(11):2265-2290. https://doi.org/10.3390/e14112265
Chicago/Turabian StyleSeneff, Stephanie, Robert M. Davidson, and Jingjing Liu. 2012. "Is Cholesterol Sulfate Deficiency a Common Factor in Preeclampsia, Autism, and Pernicious Anemia?" Entropy 14, no. 11: 2265-2290. https://doi.org/10.3390/e14112265
APA StyleSeneff, S., Davidson, R. M., & Liu, J. (2012). Is Cholesterol Sulfate Deficiency a Common Factor in Preeclampsia, Autism, and Pernicious Anemia? Entropy, 14(11), 2265-2290. https://doi.org/10.3390/e14112265





