Magnetic Particles with Polymeric Shells Bearing Cholesterol Moieties Sensitize Breast Cancer Cells to Low Doses of Doxorubicin
Abstract
1. Introduction
2. Results
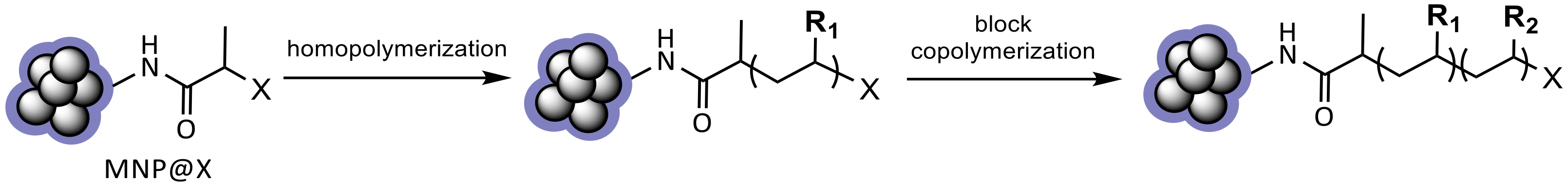
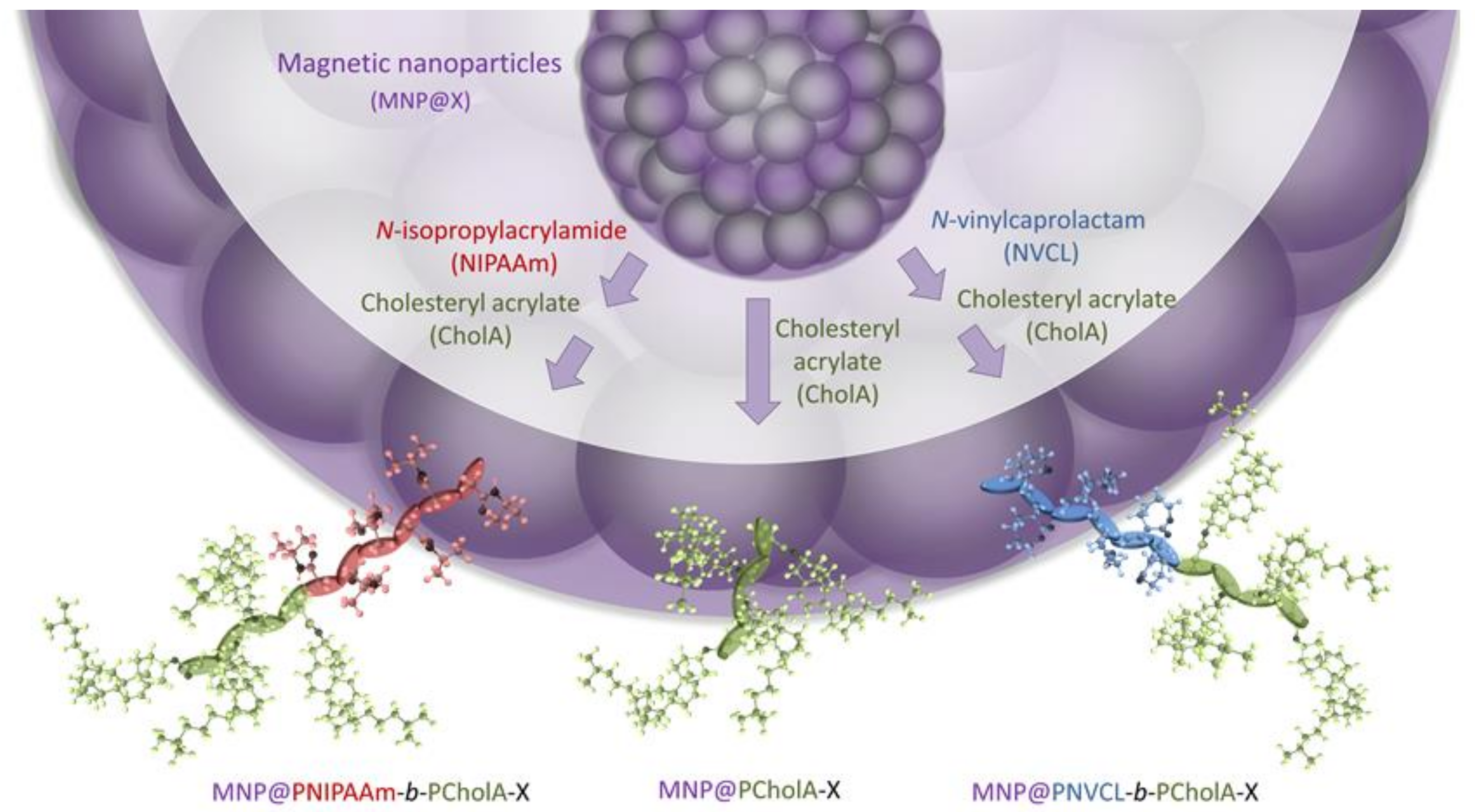
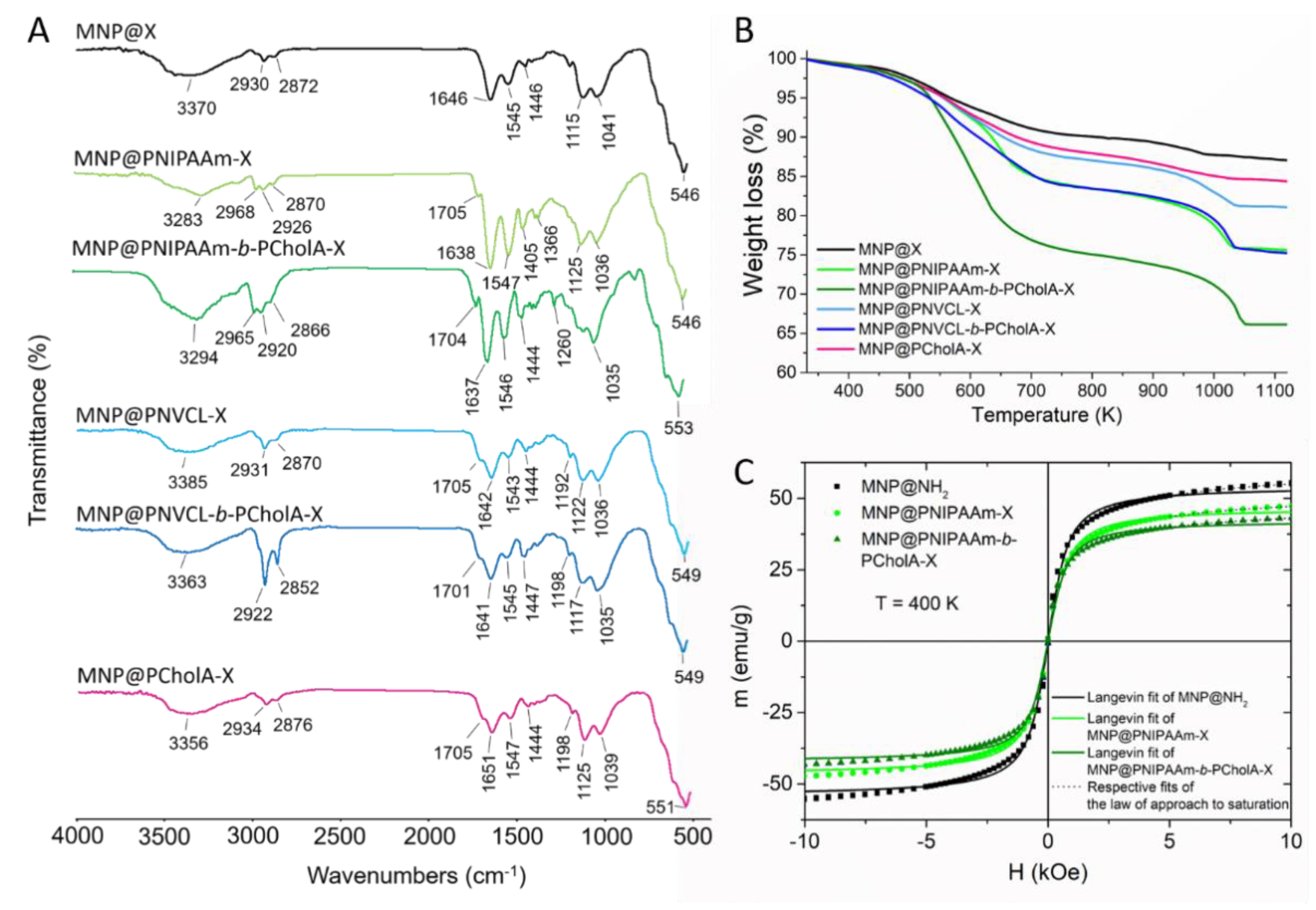
2.1. Formation and Characterization of Polymer-Coated Iron Oxide Particles
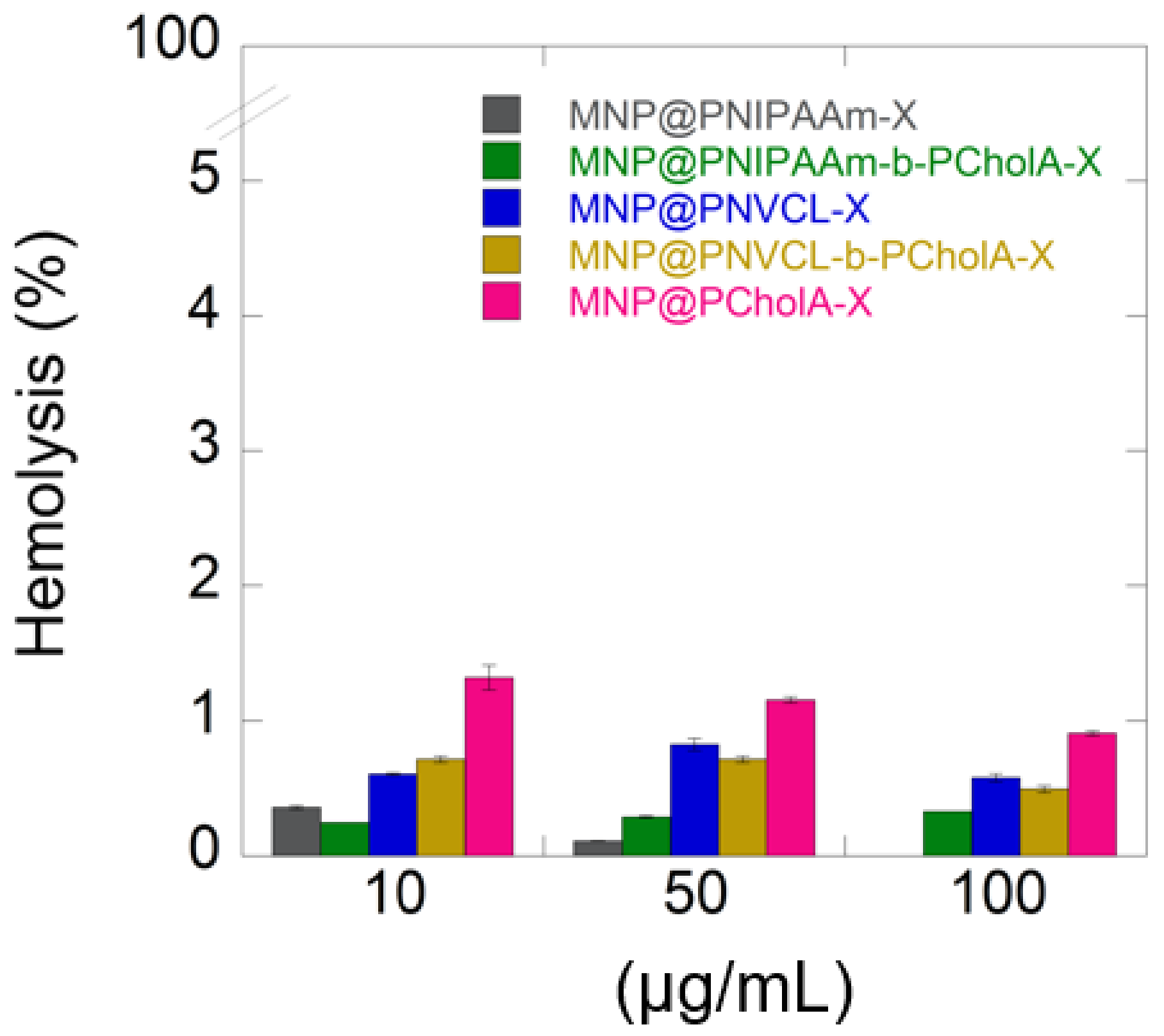
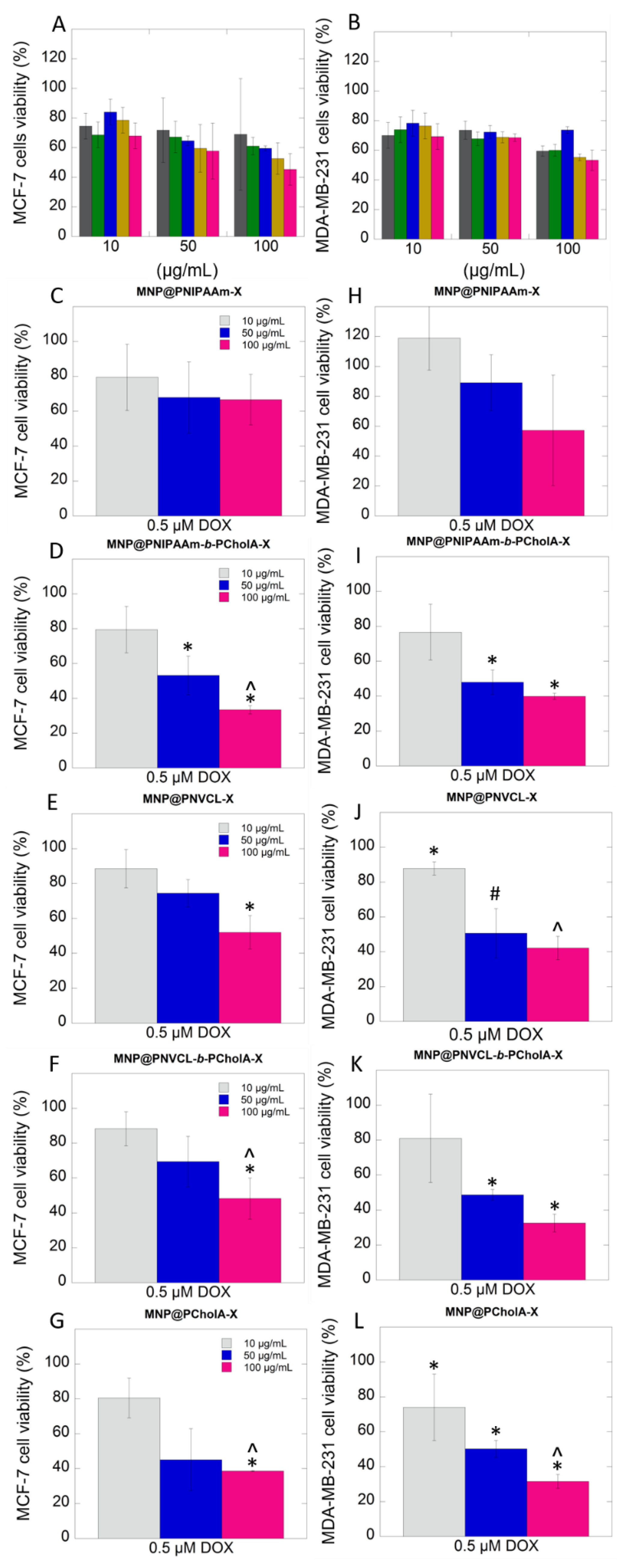
2.2. Biological Activity
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Physicochemical Characterization
4.2.2. Biological Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer Treatment and Survivorship Statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef] [PubMed]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug Resistance in Cancer: An Overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef]
- Hasan, S.; Taha, R.; Omri, H.E. Current Opinions on Chemoresistance: An Overview. Bioinformation 2018, 14, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-M.J.; Zhang, L. Nanoparticle-Based Combination Therapy toward Overcoming Drug Resistance in Cancer. Biochem. Pharmacol. 2012, 83, 1104–1111. [Google Scholar] [CrossRef]
- Xiao, B.; Ma, L.; Merlin, D. Nanoparticle-Mediated Co-Delivery of Chemotherapeutic Agent and SiRNA for Combination Cancer Therapy. Expert Opin. Drug Deliv. 2017, 14, 65–73. [Google Scholar] [CrossRef]
- Leary, M.; Heerboth, S.; Lapinska, K.; Sarkar, S. Sensitization of Drug Resistant Cancer Cells: A Matter of Combination Therapy. Cancers 2018, 10, 483. [Google Scholar] [CrossRef]
- Al-Lazikani, B.; Banerji, U.; Workman, P. Combinatorial Drug Therapy for Cancer in the Post-Genomic Era. Nat. Biotechnol. 2012, 30, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Pugazhendhi, A.; Edison, T.N.J.I.; Karuppusamy, I.; Kathirvel, B. Inorganic Nanoparticles: A Potential Cancer Therapy for Human Welfare. Int. J. Pharm. 2018, 539, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Sahoo, S.K. Magnetic Nanoparticles: A Novel Platform for Cancer Theranostics. Drug Discov. Today 2014, 19, 474–481. [Google Scholar] [CrossRef]
- Piktel, E.; Markiewicz, K.H.; Wilczewska, A.Z.; Daniluk, T.; Chmielewska, S.; Niemirowicz-Laskowska, K.; Mystkowska, J.; Paprocka, P.; Savage, P.B.; Bucki, R. Quantification of Synergistic Effects of Ceragenin CSA-131 Combined with Iron Oxide Magnetic Nanoparticles Against Cancer Cells. Int. J. Nanomed. 2020, 15, 4573–4589. [Google Scholar] [CrossRef]
- Misiak, P.; Niemirowicz-Laskowska, K.; Markiewicz, K.H.; Misztalewska-Turkowicz, I.; Wielgat, P.; Kurowska, I.; Siemiaszko, G.; Destarac, M.; Car, H.; Wilczewska, A.Z. Evaluation of Cytotoxic Effect of Cholesterol End-Capped Poly(N-Isopropylacrylamide)s on Selected Normal and Neoplastic Cells. Int. J. Nanomed. 2020, 15, 7263–7278. [Google Scholar] [CrossRef]
- Minotti, G.; Menna, P.; Salvatorelli, E.; Cairo, G.; Gianni, L. Anthracyclines: Molecular Advances and Pharmacologic Developments in Antitumor Activity and Cardiotoxicity. Pharmacol. Rev. 2004, 56, 185–229. [Google Scholar] [CrossRef]
- Carvalho, C.; Santos, R.; Cardoso, S.; Correia, S.; Oliveira, P.; Santos, M.; Moreira, P. Doxorubicin: The Good, the Bad and the Ugly Effect. Curr. Med. Chem. 2009, 16, 3267–3285. [Google Scholar] [CrossRef]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin Pathways: Pharmacodynamics and Adverse Effects. Pharmacogenet. Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, C.S.; Alam, S.; Aishwarya, R.; Miriyala, S.; Bhuiyan, M.A.N.; Panchatcharam, M.; Pattillo, C.B.; Orr, A.W.; Sadoshima, J.; Hill, J.A.; et al. Doxorubicin-Induced Cardiomyopathy Associated with Inhibition of Autophagic Degradation Process and Defects in Mitochondrial Respiration. Sci. Rep. 2019, 9, 2002. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Arbor, K.; Dubey, R. Doxorubicin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Wilczewska, A.Z.; Markiewicz, K.H. Surface-Initiated RAFT/MADIX Polymerization on Xanthate-Coated Iron Oxide Nanoparticles. Macromol. Chem. Phys. 2014, 215, 190–197. [Google Scholar] [CrossRef]
- Markiewicz, K.H.; Zembko, P.; Półtorak, K.; Misztalewska, I.; Wojtulewski, S.; Majcher, A.M.; Fornal, E.; Wilczewska, A.Z. Magnetic Nanoparticles with Chelating Shells Prepared by RAFT/MADIX Polymerization. New J. Chem 2016, 40, 9223–9231. [Google Scholar] [CrossRef]
- Misztalewska, I.; Wilczewska, A.Z.; Wojtasik, O.; Markiewicz, K.H.; Kuchlewski, P.; Majcher, A.M. New Acetylacetone-Polymer Modified Nanoparticles as Magnetically Separable Complexing Agents. RSC Adv. 2015, 5, 100281–100289. [Google Scholar] [CrossRef]
- Moskowitz, B.M.; Frankel, R.B.; Walton, S.A.; Dickson, D.P.E.; Wong, K.K.W.; Douglas, T.; Mann, S. Determination of the Preexponential Frequency Factor for Superparamagnetic Maghemite Particles in Magnetoferritin. J. Geophys. Res. Solid Earth 1997, 102, 22671–22680. [Google Scholar] [CrossRef]
- Kim, D.K.; Zhang, Y.; Voit, W.; Rao, K.V.; Muhammed, M. Synthesis and Characterization of Surfactant-Coated Superparamagnetic Monodispersed Iron Oxide Nanoparticles. J. Magn. Magn. Mater. 2001, 225, 30–36. [Google Scholar] [CrossRef]
- Abbas, M.; Takahashi, M.; Kim, C. Facile Sonochemical Synthesis of High-Moment Magnetite (Fe3O4) Nanocube. J. Nanoparticle Res. 2013, 15, 1354. [Google Scholar] [CrossRef]
- Guivar, J.A.R.; Martínez, A.I.; Anaya, A.O.; Valladares, L.D.L.S.; Félix, L.L.; Dominguez, A.B. Structural and Magnetic Properties of Monophasic Maghemite (γ-Fe2O3) Nanocrystalline Powder. Adv. Nanoparticles 2014, 3, 114–121. [Google Scholar] [CrossRef]
- Weber, M.; Steinle, H.; Golombek, S.; Hann, L.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Blood-Contacting Biomaterials: In Vitro Evaluation of the Hemocompatibility. Front. Bioeng. Biotechnol. 2018, 6, 99. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.B. The Anthracyclines: Will We Ever Find a Better Doxorubicin? Semin. Oncol. 1992, 19, 670–686. [Google Scholar] [PubMed]
- Popel, A.S.; Johnson, P.C. Microcirculation and Hemorheology. Annu. Rev. Fluid Mech. 2005, 37, 43–69. [Google Scholar] [CrossRef] [PubMed]
- Köhne, I. Haemolysis Induced by Mechanical Circulatory Support Devices: Unsolved Problems. Perfusion 2020, 35, 474–483. [Google Scholar] [CrossRef]
- Minasyan, H. Erythrocyte and Blood Antibacterial Defense. Eur. J. Microbiol. Immunol. 2014, 4, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Doll, D.C.; Weiss, R.B. Hemolytic Anemia Associated with Antineoplastic Agents. Cancer Treat. Rep. 1985, 69, 777–782. [Google Scholar]
- Bryer, E.; Henry, D. Chemotherapy-Induced Anemia: Etiology, Pathophysiology, and Implications for Contemporary Practice. Int. J. Clin. Transfus. Med. 2018, 6, 21–31. [Google Scholar] [CrossRef]
- Niemirowicz, K.; Markiewicz, K.; Wilczewska, A.; Car, H. Magnetic Nanoparticles as New Diagnostic Tools in Medicine. Adv. Med. Sci. 2012, 57, 196–207. [Google Scholar] [CrossRef]
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as Drug Delivery Systems. Pharmacol. Rep. 2012, 2012, 1020–1037. [Google Scholar] [CrossRef]
- Jackson, J.; Leung, D.; Burt, H. The Use of Ultrasound to Increase the Uptake and Cytotoxicity of Dual Taxane and P-Glycoprotein Inhibitor Loaded, Solid Core Nanoparticles in Drug Resistant Cells. Ultrasonics 2020, 101, 106033. [Google Scholar] [CrossRef]
- Xu, R.; Ma, J.; Sun, X.; Chen, Z.; Jiang, X.; Guo, Z.; Huang, L.; Li, Y.; Wang, M.; Wang, C.; et al. Ag Nanoparticles Sensitize IR-Induced Killing of Cancer Cells. Cell Res. 2009, 19, 1031–1034. [Google Scholar] [CrossRef] [PubMed]
- Tomankova, K.; Polakova, K.; Pizova, K.; Binder, S.; Kolarova, M.; Kriegova, E.; Zapletalova, J.; Malina, L.; Horakova, J.; Malohlava, J.; et al. In Vitro Cytotoxicity Analysis of Doxorubicin-Loaded/Superparamagnetic Iron Oxide Colloidal Nanoassemblies on MCF7 and NIH3T3 Cell Lines. Int. J. Nanomed. 2015, 949. [Google Scholar] [CrossRef] [PubMed]
- Aljarrah, K.; Mhaidat, N.M.; Al-Akhras, M.-A.H.; Aldaher, A.N.; Albiss, B.; Aledealat, K.; Alsheyab, F.M. Magnetic Nanoparticles Sensitize MCF-7 Breast Cancer Cells to Doxorubicin-Induced Apoptosis. World J. Surg. Oncol. 2012, 10, 62. [Google Scholar] [CrossRef]
- Lacava, Z.G.M.; Estevanato, L.L.C.; Da Silva, J.R.; Falqueiro, A.M.; Mosiniewicz-Szablewska, E.; Suchocki, P.; Tedesco, C.A.; Morais, P.C. Co-Nanoencapsulation of Magnetic Nanoparticles and Selol for Breast Tumor Treatment: In Vitro Evaluation of Cytotoxicity and Magnetohyperthermia Efficacy. Int. J. Nanomed. 2012, 5287. [Google Scholar] [CrossRef]
- Wang, Q.; Zhong, Y.; Liu, W.; Wang, Z.; Gu, L.; Li, X.; Zheng, J.; Du, H.; Zhong, Z.; Xie, F. Enhanced Chemotherapeutic Efficacy of the Low-Dose Doxorubicin in Breast Cancer via Nanoparticle Delivery System Crosslinked Hyaluronic Acid. Drug Deliv. 2019, 26, 12–22. [Google Scholar] [CrossRef]


| Nanohybrids | Type of Monomer | TG Weight Loss at 1120 K | |
|---|---|---|---|
| R1 | R2 | (%) | |
| MNP@PNIPAAm-X | 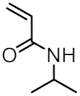 | - | 25 |
| MNP@PNIPAAm-b-PCholA-X | 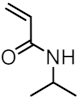 |  | 34 |
| MNP@PNVCL-X | 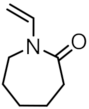 | - | 19 |
| MNP@PNVCL-b-PCholA-X | 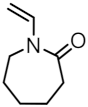 | 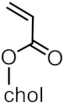 | 25 |
| MNP@PCholA-X | 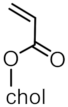 | - | 16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markiewicz, K.H.; Niemirowicz-Laskowska, K.; Szymczuk, D.; Makarewicz, K.; Misztalewska-Turkowicz, I.; Wielgat, P.; Majcher-Fitas, A.M.; Milewska, S.; Car, H.; Wilczewska, A.Z. Magnetic Particles with Polymeric Shells Bearing Cholesterol Moieties Sensitize Breast Cancer Cells to Low Doses of Doxorubicin. Int. J. Mol. Sci. 2021, 22, 4898. https://doi.org/10.3390/ijms22094898
Markiewicz KH, Niemirowicz-Laskowska K, Szymczuk D, Makarewicz K, Misztalewska-Turkowicz I, Wielgat P, Majcher-Fitas AM, Milewska S, Car H, Wilczewska AZ. Magnetic Particles with Polymeric Shells Bearing Cholesterol Moieties Sensitize Breast Cancer Cells to Low Doses of Doxorubicin. International Journal of Molecular Sciences. 2021; 22(9):4898. https://doi.org/10.3390/ijms22094898
Chicago/Turabian StyleMarkiewicz, Karolina H., Katarzyna Niemirowicz-Laskowska, Dawid Szymczuk, Kacper Makarewicz, Iwona Misztalewska-Turkowicz, Przemysław Wielgat, Anna M. Majcher-Fitas, Sylwia Milewska, Halina Car, and Agnieszka Z. Wilczewska. 2021. "Magnetic Particles with Polymeric Shells Bearing Cholesterol Moieties Sensitize Breast Cancer Cells to Low Doses of Doxorubicin" International Journal of Molecular Sciences 22, no. 9: 4898. https://doi.org/10.3390/ijms22094898
APA StyleMarkiewicz, K. H., Niemirowicz-Laskowska, K., Szymczuk, D., Makarewicz, K., Misztalewska-Turkowicz, I., Wielgat, P., Majcher-Fitas, A. M., Milewska, S., Car, H., & Wilczewska, A. Z. (2021). Magnetic Particles with Polymeric Shells Bearing Cholesterol Moieties Sensitize Breast Cancer Cells to Low Doses of Doxorubicin. International Journal of Molecular Sciences, 22(9), 4898. https://doi.org/10.3390/ijms22094898







