Algal Toxic Compounds and Their Aeroterrestrial, Airborne and other Extremophilic Producers with Attention to Soil and Plant Contamination: A Review
Abstract
1. Introduction
2. Main Types of Toxic Algal Compounds
3. Phycotoxins—Origin and Possible Biological Role
4. Phycotoxins and Factors That Affect Their Production
5. Phycotoxins of Aero-Terrestrial, Airborne and Extremophilic Algae
5.1. Aeroterrestrial Algae
5.1.1. Phycotoxins of Aeroterrestrial Algae from Ambient Habitats and Contamination of Soils and Plants
Phycotoxins of Aeroterrestrial Algae from Ambient Habitats
Phycotoxins Contamination of Soils and Plants
5.1.2. Aeroterrestrial Endolithic Algae
5.1.3. Aeroterrestrial Algae from Deserts and Polar Regions
5.1.4. Aeroterrestrial algae of Hypersaline Environments
5.2. Airborne Toxigenic Algae and Their Toxins
5.3. Extremophilic Algae and their Phycotoxins
5.3.1. Toxigenic Cave Algae
5.3.2. Toxigenic Acidophilic and Toxigenic Peat-Bog Algae
5.3.3. Toxigenic Algae of Hypersaline Environments
Toxigenic Halophilic Algae
Toxigenic Algae of Saline-Alkaline Environments
5.4. Toxigenic Radioresistant Algae
5.5. Toxigenic Algae of Thermal Springs
5.6. Toxigenic Algae of Cold Habitats
6. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Graham, L.E.; Graham, J.M.; Wilcox, L.W. Algae, 2nd ed.; Pearson Education, Inc.: San Francisco, CA, USA, 2009. [Google Scholar]
- Evert, R.F.; Eichhorn, S.E. Raven Biology of Plants, 8th ed.; W. H. Freeman and Company: New York, NY, USA, 2012. [Google Scholar]
- Koonin, E.V. Carl Woese’s vision of cellular evolution and the domains of life. RNA Biol. 2014, 11, 197–204. [Google Scholar] [CrossRef]
- Round, F.E. The Ecology of Algae; Cambrige University Press: Cambrige, UK, 1981. [Google Scholar]
- Rothschild, L.; Mancinelli, R. Life in extreme environments. Nature 2001, 409, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Seckbach, J. (Ed.) Algae and Cyanobacteria in Extreme Environments; Springer: Dordrecht, The Netherlands, 2007. [Google Scholar]
- Horikoshi, K. (Ed.) Extremophiles Handbook; Springer: Tokyo, Japan, 2011. [Google Scholar]
- Rampelotto, P.H. Extremophiles and Extreme Environments. Life 2013, 3, 482–485. [Google Scholar] [CrossRef]
- Varshney, P.; Mikulic, P.; Vonshak, A.; Beardall, J.; Wangikar, P.P. Extremophilic micro-algae and their potential contribution in biotechnology. Bioresour. Technol. 2015, 184, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Seckbach, J.; Rampeloto, P.H. Polyextremophiles. In Microbial Evolution under Extreme Conditions; Bakermans, C., Ed.; De Gruyter Publishers: Berlin, Germany, 2015; pp. 153–170. [Google Scholar]
- Padisák, J.; Naselli-Flores, L. Phytoplankton in extreme environments: Importance and consequences of habitat permanency. Hydrobiologia 2021, 848, 157–176. [Google Scholar] [CrossRef]
- Macelroy, R.D. Some comments on the evolution of extremophiles. Biosystems 1974, 6, 74–75. [Google Scholar] [CrossRef]
- Seckbach, J.; Oren, A.; Stan-Lotter, H. (Eds.) Polyextremophiles: Life under Multiple Forms of Stress; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar]
- Harrison, J.P.; Gheeraert, N.; Tsigelnitskiy, D.; Cockell, C.S. The limits for life under multiple extremes. Trends Microbiol. 2013, 21, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Ettl, H.; Gӓrtner, G. Syllabus der Boden-, Luft- und Flechtenalgen, 2nd ed.; Springer Spektrum: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Drobac-Čik, A.V.; Dulić, T.I.; Stojanović, D.B.; Svirčev, Z.B. The importance of extremophile cyanobacteria in the production of biologically active compounds. Proc. Nat. Sci. Matica Srp. Novi Sad 2007, 112, 57–66. [Google Scholar] [CrossRef]
- Mühlsteinová, R.; Hauer, T. Pilot survey of cyanobacterial diversity from the neighborhood of San Gerardo de Rivas, Costa Rica with a brief summary of current knowledge of terrestrial cyanobacteria in Central America. Braz. J. Bot. 2013, 36, 299–307. [Google Scholar] [CrossRef]
- Molish, H. Populare Biologischevortrag XIII Biol. Atmospharischen; Sfaubes: Jena, Germany, 1920; p. 280. [Google Scholar]
- Genitsaris, S.; Kormas, K.A.; Moustaka-Gouni, M. Airborne algae and cyanobacteria: Occurrence and related health effects. Front. Biosci. 2011, 3, 772–787. [Google Scholar]
- Wiśniewska, K.; Lewandowska, A.U.; Śliwińska-Wilczewska, S. The importance of cyanobacteria and microalgae present in aerosols to human health and the environment—Review study. Environ. Int. 2019, 131, 104964. [Google Scholar] [CrossRef] [PubMed]
- Seaman, P.; Buchardt, B. The columns of ikaite tufa in Ikka Fjord, Greenland. Monogr. Greenl. Geosci. 2006, 44, 1–39. [Google Scholar]
- Kristiansen, J.; Kristiansen, A. A new species of Chroomonas (Cryptophyceae) living inside the submarine ikaite columns in the Ikkafjord, Southwest Greenland, with remarks on its ultrastructure and ecology. Nord. J. Bot. 1999, 19, 747–758. [Google Scholar] [CrossRef]
- Trabelsi, L.; Mnari, A.; Abdel-Daim, M.M.; Abid-Essafi, S.; Aleya, L. Therapeutic properties in Tunisian hot springs: First evidence of phenolic compounds in the cyanobacterium Leptolyngbya sp. biomass, capsular polysaccharides and releasing polysaccharides. BMC Complement. Altern. Med. 2016, 16, 515. [Google Scholar] [CrossRef]
- Stoyneva-Gärtner, M.; Uzunov, B.; Gärtner, G. Enigmatic microalgae from aeroterrestrial and extreme habitats in cosmetics: The potential of the untapped natural sources. Cosmetics 2020, 7, 27. [Google Scholar] [CrossRef]
- Whitford, W.G.; Duval, B.D. Ecology of Desert Systems, 2nd ed.; Academic Press: London, UK, 2019. [Google Scholar] [CrossRef]
- Schirrmeister, B.E.; Gugger, M.; Donoghue, P.C.J. Cyanobacteria and the great oxidation event: Evidence from genes and fossils. Palaeontology 2015, 58, 935–936. [Google Scholar] [CrossRef]
- Leliaert, F.; Smith, D.R.; Moreau, H.; Herron, M.D.; Verbruggen, H.; Delwiche, C.F.; De Clerck, O. Phylogeny and molecular evolution of the green algae. Crit. Rev. Plant Sci. 2012, 31, 1–46. [Google Scholar] [CrossRef]
- Curtis, B.A.; Tanifuji, G.; Burki, F.; Gruber, A.; Irimia, M.; Maruyama, S.; Arias, M.C.; Ball, S.G.; Gile, G.H.; Hirakawa, Y.; et al. Algal genomes reveal evolutionary mosaicism and the fate of nucleomorphs. Nature 2012, 492, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Elbert, W.; Weber, B.; Burrows, S.; Steinkamp, J.; Büdel, B.; Andreae, M.O.; Pöschl, U. Contribution of cryptogamic covers to the global cycles of carbon and nitrogen. Nat. Geosci. 2012, 5, 459–462. [Google Scholar] [CrossRef]
- Mergelov, N.; Mueller, C.W.; Prater, I.; Shorkunov, I.; Dolgikh, A.; Zazovskaya, E.; Shishkov, V.; Krupskaya, V.; Abrosimov, K.; Cherkinsky, A.; et al. Alteration of rocks by endolithic organisms is one of the pathways for the beginning of soils on Earth. Sci. Rep. 2018, 8, 3367. [Google Scholar] [CrossRef]
- Evangelista, V.; Barsanti, L.; Frassanito, A.M.; Passarelli, V.; Gualtieri, P. (Eds.) Algal Toxins: Nature, Occurrence, Effect and Detection; NATO Advanced Study Institute on Sensor Systems for Biological Threats: The Algal Toxins Case, Pisa, Italy, 30 September–11 October 2007; Springer: Dordrecht, The Netherlands, 2007. [Google Scholar]
- Meriluoto, J.; Spoof, L.; Codd, J. (Eds.) Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; John Wiley & Sons, Ltd.: Chichester, UK, 2017. [Google Scholar] [CrossRef]
- Collins, M. Algal toxins. Microbiol. Mol. Biol. Rev. 1978, 42, 725–746. [Google Scholar] [CrossRef]
- Lee, S.; Jiang, X.; Manubolu, M.; Riedl, K.; Ludsin, S.A.; Martin, J.F.; Lee, J. Fresh produce and their soils accumulate cyanotoxins from irrigation water: Implications for public health and food security. Food Res. Int. 2017, 102, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Andersen, P. Design and Implementation of Some Harmful Algal Monitoring Systems; Ioc Technical Series; UNESCO: Paris, France, 1996. [Google Scholar]
- Stewart, C.E. Weapons of Mass Casualties and Terrorism Response Handbook; American Academy of Orthopaedic Surgeons Monograph; Jones & Bartlett Learning: Burlington, VT, USA, 2005. [Google Scholar]
- Wheelis, M.; Rózsa, L.; Dando, M. (Eds.) Deadly Cultures: Biological Weapons Since 1945; Harvard University Press: Cambridge, MA, USA, 2006. [Google Scholar]
- Stoyneva-Gärtner, M.; Uzunov, B.; Dimitrova, P.; Pavlova, V. Algal toxins—New risk factors for national security in Bulgaria. In Proceedings of the Actual Problems of the Security, Veliko Turnovo, Bulgaria, 26–27 October 2017; Publishing House Complex of NVU “Vasil Levski”: Veliko Turnovo, Bulgaria, 2017; pp. 271–281, (In Bulgarian, English). [Google Scholar]
- Singh, R.; Parihar, P.; Singh, M.; Bajguz, A.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Uncovering potential applications of cyanobacteria and algal metabolites in biology, agriculture and medicine: Current status and future prospects. Front. Microbiol. 2017, 8, 515. [Google Scholar] [CrossRef]
- Valdiglesias, V.; Prego-Faraldo, M.V.; Pasaro, E.; Mendez, J.; Laffon, B. Okadaic acid: More than a diarrheic toxin. Mar. Drugs 2013, 11, 4328–4349. [Google Scholar] [CrossRef] [PubMed]
- Biondi, N.; Piccardi, R.; Margheri, M.C.; Rodolfi, L.; Smith, G.D.; Tredicci, M.R. Evaluation of Nostoc strain ATCC 53789 as a potential source of natural pesticides. Appl. Environ. Microbiol. 2004, 70, 3313–3320. [Google Scholar] [CrossRef] [PubMed]
- Kamat, P.K.; Rai, S.; Swarnkar, S.; Shukla, R.; Nath, C. Molecular and cellular mechanism of okadaic acid (oka)-induced neurotoxicity: A novel tool for alzheimer’s disease therapeutic application. Mol. Neurobiol. 2014, 50, 852–865. [Google Scholar] [CrossRef]
- Kaminski, A.; Bober, B.; Chrapusta, E.; Bialczyk, J. Phytoremediation of anatoxin-a by aquatic macrophyte Lemna trisulca L. Chemosphere 2014, 112, 305–310. [Google Scholar] [CrossRef]
- Rojas, V.; Rivas, L.; Cárdenas, C.; Guzmán, F. Cyanobacteria and eukaryotic microalgae as emerging sources of antibacterial peptides. Molecules 2020, 25, 5804. [Google Scholar] [CrossRef] [PubMed]
- Mazard, S.; Penesyan, A.; Ostrowski, M.; Paulsen, I.T.; Egan, S. Tiny microbes with a big impact: The role of cyanobacteria and their metabolites in shaping our future. Mar. Drugs 2016, 14, 97. [Google Scholar] [CrossRef] [PubMed]
- Dittmann, E.; Fewer, D.P.; Neilan, B.A. Cyanobacterial toxins: Biosynthetic routes and evolutionary roots. FEMS Microbiol. Rev. 2013, 37, 23–43. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.A.; Mountfort, D.; Selwood, A.I.; Holland, P.T.; Puddick, J.; Cary, S.C. Widespread distribution and identification of eight novel microcystins in Antarctic cyanobacterial mats. Appl. Environ. Microbiol. 2008, 74, 7243–7251. [Google Scholar] [CrossRef]
- Martins, A.; Vasconcelos, V. Use of qPCR for the study of hepatotoxic cyanobacteria population dynamics. Arch. Microbiol. 2011, 193, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Cirés, S.; Casero, M.C.; Quesada, A. Toxicity at the edge of life: A review on cyanobacterial toxins from extreme environments. Mar. Drugs 2017, 15, 233. [Google Scholar] [CrossRef] [PubMed]
- Cantoral Uriza, E.A.; Asencio, A.D.; Aboal, M. Are we underestimating benthic cyanotoxins? Extensive sampling results from Spain. Toxins 2017, 9, 385. [Google Scholar] [CrossRef]
- Babica, P.; Bláha, L.; Maršálek, B. Exploring the natural role of microcystins—a review of effects on photoautotrophic organisms. J. Phycol. 2006, 42, 9–20. [Google Scholar] [CrossRef]
- Rangel, M.; Brunetti, R.L.; Garcia, A.N.; Cambui, C.C.N.; Conserva, G.A.A.; Neves, A.C.; Sant’Anna, C.L.; Carvalho, L.R. Acute effects of three Geitlerinema spp. (Cyanobacteria) extracts administrated in mice: Symptoms and histopathological aspects. Phytochem. Rev. 2013, 12, 543–553. [Google Scholar] [CrossRef]
- Rangel, M.; Martins, J.C.G.; Garcia, A.N.; Conserva, G.A.A.; Costa-Neves, A.; Sant’Anna, C.L.; De Carvalho, L.R. Analysis of the toxicity and histopathology induced by the oral administration of Pseudanabaena galeata and Geitlerinema splendidum (Cyanobacteria) extracts to mice. Mar. Drugs 2014, 12, 508–524. [Google Scholar] [CrossRef]
- Tartar, A.; Boucias, D.G.; Adams, B.J.; Becnel, J.J. Phylogenetic analysis identifies the invertebrate pathogen Helicosporidium sp. as a green alga (Chlorophyta). Int. J. Syst. Evol. Microbiol. 2002, 52, 273–279. [Google Scholar] [CrossRef]
- Leimann, B.C.Q.; Monteiro, P.C.F.; Lazéra, M.; Candanoza, E.R.U.; Wanke, B. Protothecosis. Med. Mycol. 2004, 42, 95–106. [Google Scholar]
- Hosaka, S.; Hosaka, M. A case report of canine protothecosis. J. Vet. Med. Sci. 2004, 66, 593–597. [Google Scholar] [CrossRef]
- Juttner, F. Liberation of 5,8,11,14,17-eicosapentaenoic acid and other polyunsaturated fatty acids from lipids as a grazer defence reaction in epilithic diatom biofilms. J. Phycol. 2001, 37, 744–755. [Google Scholar] [CrossRef]
- Fiore, M.F.; de Lima, S.T.; Carmichael, W.W.; McKinnie, S.M.K.; Chekan, J.R.; Moore, B.S. Guanitoxin, re-naming a cyanobacterial organophosphate toxin. Harmful Algae 2020, 92, 101737. [Google Scholar] [CrossRef] [PubMed]
- Fidor, A.; Konkel, R.; Mazur-Marzec, H. Bioactive peptides produced by cyanobacteria of the genus Nostoc: A review. Mar. Drugs 2019, 17, 561. [Google Scholar] [CrossRef] [PubMed]
- Kust, A.; Řeháková, K.; Vrba, J.; Maicher, V.; Mareš, J.; Hrouzek, P.; Chiriac, M.-C.; Benedová, Z.; Tesařová, B.; Saurav, K. Insight into unprecedented diversity of cyanopeptides in eutrophic ponds using an ms/ms networking approach. Toxins 2020, 12, 561. [Google Scholar] [CrossRef] [PubMed]
- Sano, T.; Kaya, K. Oscillatorin, a chymotrypsin inhibitor from toxic Oscillatoria agardhii. Tetrahedron Lett. 1996, 37, 6873–6876. [Google Scholar] [CrossRef]
- Churro, C.; Semedo-Aguiar, A.P.; Silva, A.D.; Pereira-Leal, J.B.; Leite, R.B. A novel cyanobacterial geosmin producer, revising GeoA distribution and dispersion patterns in Bacteria. Sci. Rep. 2020, 10, 8679. [Google Scholar] [CrossRef]
- Jakubowska, N.; Szeląg-Wasielewska, E. Toxic picoplanktonic cyanobacteria-review. Mar. Drugs 2015, 13, 1497–1518. [Google Scholar] [CrossRef]
- Barsanti, L.; Gualtieri, P. Algae: Anatomy, Biochemistry, and Biotechnology; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2006. [Google Scholar]
- Funari, E.; Testai, E. Human health risk assessment related to cyanotoxins exposure. Crit. Rev. Toxicol. 2008, 38, 97–125. [Google Scholar] [CrossRef]
- Prinsep, M.R.; Caplan, F.R.; Moore, R.E.; Patterson, G.M.L.; Honkanen, R.E.; Boynton, A.L. Microcystin LA from a blue-green alga belonging to the Stigonematales. Phytochemistry 1992, 31, 1247–1248. [Google Scholar] [CrossRef]
- Ishida, K.; Matsuda, H.; Murakami, M.; Yamaguchi, K. Kawaguchipeptin B, an antibacterial cyclic undecapeptide from the cyanobacterium Microcystis aeruginosa. J. Nat. Prod. 1997, 60, 724–726. [Google Scholar] [CrossRef] [PubMed]
- Higa, T.; Kuniyoshi, M. Toxins associated with medicinal and edible seaweeds. J. Toxicol. Toxin Rev. 2000, 19, 119–137. [Google Scholar] [CrossRef]
- Codd, G.A.; Azevedo, S.M.F.O.; Bagchi, S.N.; Burch, M.D.; Carmichael, W.W.; Harding, W.R.; Kaya, K.; Utkilen, H.C. CYANONET: A Global Network for Cyanobacterial Bloom and Toxin Risk Management. Initial Situation Assessment and Recommendations; IHP-VI Technical Document in Hydrology N 76. UNESCO Working Series SC-2005/WS/55; International Hydrological Programme (IHP) of the United Nations Educational, Scientific and Cultural Organization (UNESCO): Paris, France, 2005. [Google Scholar]
- Anas, A.R.J.; Kisugi, T.; Umezawa, T.; Matsuda, F.; Campitelli, M.R.; Quinn, R.J.; Okino, T. Thrombin inhibitors from the freshwater cyanobacterium Anabaena compacta. J. Nat. Prod. 2012, 75, 1546–1552. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Dörr, F.A.; Pinto, E. First report of spumigin production by the toxic Sphaerospermopsis torques-reginae cyanobacterium. Toxicon 2015, 108, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Bláha, L.; Babica, P.; Maršálek, B. Toxins produced in cyanobacterial water blooms-toxicity and risks. Interdiscip. Toxicol. 2009, 2, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Barboza, G.F.O.; Gorlach-Lira, K.; Sassi, C.F.C.; Sassi, R. Microcystins production and antibacterial activity of cyanobacterial strains of Synechocystis, Synechococcus and Romeria from water and coral reef organisms (Brazil). Rev. Biol. Trop. 2017, 65, 890–899. [Google Scholar] [CrossRef][Green Version]
- Motuhi, S.-E.; Feizbakhsh, O.; Foll-Josselin, B.; Baratte, B.; Delehouzé, C.; Cousseau, A.; Fant, X.; Bulinski, J.C.; Payri, C.E.; Ruchaud, S.; et al. Neurymenolide A, a novel mitotic spindle poison from the new caledonian Rhodophyta Phacelocarpus neurymenioides. Mar. Drugs 2019, 17, 93. [Google Scholar] [CrossRef] [PubMed]
- Maeno, Y.; Kotaki, Y.; Terada, R.; Cho, Y.; Konoki, K.; Yotsu-Yamashita, M. Six domoic acid related compounds from the red alga, Chondria armata, and domoic acid biosynthesis by the diatom, Pseudo-nitzschia multiseries. Sci. Rep. 2018, 8, 356. [Google Scholar] [CrossRef] [PubMed]
- Paz-Yepes, J.; Brahamsha, B.; Palenik, B. Role of a Microcin-C-like biosynthetic gene cluster in allelopathic interactions in marine Synechococcus. Proc. Natl. Acad. Sci. USA 2013, 110, 12030–12035. [Google Scholar] [CrossRef]
- Heck, K.; Alvarenga, D.O.; Shishido, T.K.; Varani, A.M.; Dörr, F.A.; Pinto, E.; Rouhiainen, L.; Jokela, J.; Sivonen, K.; Fiore, M.F. Biosynthesis of microcystin hepatotoxins in the cyanobacterial genus Fischerella. Toxicon 2018, 141, 43–50. [Google Scholar] [CrossRef]
- Sivonen, K.; Leikoski, N.; Fewer, D.P.; Jokela, J. Cyanobactins—ribosomal cyclic peptides produced by cyanobacteria. Appl. Microbiol. Biotechnol. 2010, 86, 1213–1225. [Google Scholar] [CrossRef]
- Massey, I.Y.; Yang, F. A mini review on microcystins and bacterial degradation. Toxins 2020, 12, 268. [Google Scholar] [CrossRef] [PubMed]
- Moffitt, M.C.; Neilan, B.A. Characterization of the nodularin synthetase gene cluster and proposed theory of the evolution of cyanobacterial hepatotoxins. Appl. Environ. Microbiol. 2004, 70, 6353–6362. [Google Scholar] [CrossRef]
- Rantala, A.; Fewer, D.P.; Hisbergues, M.; Rouhiainen, L.; Vaitomaa, J.; Börner, T.; Sivonen, K. Phylogenetic evidence for the early evolution of microcystin synthesis. Proc. Natl. Acad. Sci. USA 2004, 101, 568–573. [Google Scholar] [CrossRef]
- Murray, S.A.; Mihali, T.K.; Neilan, B.A. Extraordinary conservation, gene loss, and positive selection in the evolution of an ancient neurotoxin. Mol. Biol. Evol. 2011, 28, 1173–1182. [Google Scholar] [CrossRef]
- Holland, A.; Kinnear, S. Interpreting the possible ecological role(s) of cyanotoxins: Compounds for competitive advantage and/or physiological aide? Mar. Drugs 2013, 11, 2239–2258. [Google Scholar] [CrossRef] [PubMed]
- Mihali, T.K.; Kellmann, R.; Neilan, B.A. Characterisation of the paralytic shellfish toxin biosynthesis gene clusters in Anabaena circinalis AWQC131C and Aphanizomenon sp. NH-5. BMC Biochem. 2009, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Kurmayer, R.; Christiansen, G.; Fastner, J.; Börner, T. Abundance of active and inactive microcystin genotypes in populations of the toxic cyanobacterium Planktothrix spp. Environ. Microbiol. 2004, 6, 831–841. [Google Scholar] [CrossRef]
- Christiansen, G.; Molitor, C.; Philmus, B.; Kurmayer, R. Nontoxic strains of cyanobacteria are the result of major gene deletion events induced by a transposable element. Mol. Biol. Evol. 2008, 25, 1695–1704. [Google Scholar] [CrossRef] [PubMed]
- Fewer, D.P.; Halinen, K.; Sipari, H.; Bernardova, K.; Manttari, M.; Eronen, E.; Sivonen, K. Non-autonomous transposable elements associated with inactivation of microcystin gene clusters in strains of the genus Anabaena isolated from the Baltic Sea. Environ. Microbiol. Rep. 2011, 3, 189–194. [Google Scholar] [CrossRef]
- Omidi, A.; Esterhuizen-Londt, M.; Pflugmacher, S. Still challenging: The ecological function of the cyanobacterial toxin microcystin—what we know so far. Toxin Rev. 2018, 37, 87–105. [Google Scholar] [CrossRef]
- Schwabe, W.; Weihe, A.; Börner, T.; Henning, M.; Kohl, J.G. Plasmids in toxic and nontoxic strains of the cyanobacterium Microcystis aeruginosa. Curr. Microbiol. 1988, 17, 133–137. [Google Scholar] [CrossRef]
- Bolch, C.J.S.; Orr, P.T.; Jones, G.J.; Blackburn, S.I. Genetic, morphological, and toxicological variation among globally dis-tributed strains of Nodularia (Cyanobacteria). J. Phycol. 1999, 35, 339–355. [Google Scholar] [CrossRef]
- Tooming-Klunderud, A.; Fewer, D.P.; Rohrlack, T.; Jokela, J.; Rouhiainen, L.; Sivonen, K.; Kristensen, T.; Jakobsen, K.S. Evidence for positive selection acting on microcystin synthetase adenylation domains in three cyanobacterial genera. BMC Evol. Biol. 2008, 8, 256. [Google Scholar] [CrossRef]
- Tanabe, Y.; Kaya, K.; Watanabe, M.M. Evidence for recombination in the microcystin synthetase (mcy) genes of toxic cyanobacteria Microcystis spp. J. Mol. Evol. 2004, 58, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, Y.; Sano, T.; Kasai, F.; Watanabe, M.M. Recombination, cryptic clades and neutral molecular divergence of the microcystin synthetase (mcy) genes of toxic cyanobacterium Microcystis aeruginosa. BMC Evol. Biol. 2009, 9, 115. [Google Scholar] [CrossRef] [PubMed]
- Kellmann, R.; Mihali, T.K.; Neilan, B.A. Identification of a saxitoxin biosynthesis gene with a history of frequent horizontal gene transfers. J. Mol. Evol. 2008, 67, 526–538. [Google Scholar] [CrossRef] [PubMed]
- Gehringer, M.M.; Wannicke, N. Climate change and regulation of hepatotoxin production in Cyanobacteria. FEMS Microbiol. Ecol. 2014, 88, 1–25. [Google Scholar] [CrossRef]
- Ouellette, A.J.A.; Wilhelm, S.W. Toxic cyanobacteria: The evolving molecular toolbox. Front. Ecol. Environ. 2003, 1, 359–366. [Google Scholar] [CrossRef]
- Kaplan, A.; Harel, M.; Kaplan-Levy, R.N.; Hadas, O.; Sukenik, A.; Dittmann, E. The languages spoken in the water body (or the biological role of cyanobacterial toxins). Front. Microbiol. 2012, 3, 138. [Google Scholar] [CrossRef]
- Heckman, D.S.; Geiser, D.M.; Eidell, B.R.; Stauffer, R.L.; Kardos, N.L.; Hedges, S.B. Molecular evidence for the early colonization of land by fungi and plants. Science 2001, 293, 1129–1133. [Google Scholar] [CrossRef]
- Henao, E.; Rzymski, P.; Waters, M.N. A Review on the study of cyanotoxins in paleolimnological research: Current knowledge and future needs. Toxins 2020, 12, 6. [Google Scholar] [CrossRef]
- Rzymski, P.; Klimaszyk, P.; Jurczak, T.; Poniedziałek, B. Oxidative stress, programmed cell death and microcystin release in Microcystis aeruginosa in response to Daphnia grazers. Front. Microbiol. 2020, 11, 1201. [Google Scholar] [CrossRef]
- Lukač, M.; Aegerter, R. Influence of trace metals on growth and toxin production of Microcystis aeruginosa. Toxicon 1993, 31, 293–305. [Google Scholar] [CrossRef]
- Alexova, R.; Fujii, M.; Birch, D.; Cheng, J.; Waite, T.D.; Ferrari, B.C.; Neilan, B.A. Iron uptake and toxin synthesis in the bloom-forming Microcystis aeruginosa under iron limitation. Environ. Microbiol. 2011, 13, 1064–1077. [Google Scholar] [CrossRef] [PubMed]
- Dehm, D.; Krumbholz, J.; Baunach, M.; Wiebach, V.; Hinrichs, K.; Guljamow, A.; Tabuchi, T.; Jenke-Kodama, H.; Süssmuth, R.; Dittmann, E. Unlocking the spatial control of secondary metabolism uncovers hidden natural product diversity in Nostoc punctiforme. ACS Chem. Biol. 2019, 14, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Morgan-Kiss, R.M.; Priscu, J.C.; Pocock, T.; Gudynaite-Savitch, L.; Huner, N.P.A. Adaptation and acclimation of photosynthetic microorganisms to permanently cold environments. Microbiol. Mol. Biol. Rev. 2006, 70, 222–252. [Google Scholar] [CrossRef] [PubMed]
- Hitzfeld, B.; Lampert, C.S.; Spaeth, N.; Mountfort, D.; Kaspar, H.; Dietrich, D.R. Toxin production in cyanobacterial mats from ponds on the McMurdo Ice Shelf, Antarctica. Toxicon 2000, 38, 1731–1748. [Google Scholar] [CrossRef]
- Wood, S.A.; Stirling, D.J.; Briggs, L.R.; Sprosen, J.; Holland, P.T.; Ruck, J.G.; Wear, R.G. Survey of cyanotoxins in New Zealand waterbodies between 2001 and 2004. N. Z. J. Mar. Freshw. Res. 2006, 40, 585–595. [Google Scholar] [CrossRef]
- Guljamow, A.; Kreische, M.; Ishida, K.; Liaimer, A.; Altermark, B.; Bähr, L.; Hertweck, C.; Ehwald, R.; Dittmann, E. High-density cultivation of terrestrial Nostoc strains leads to reprogramming of secondary metabolome. Appl. Environ. Microbiol. 2017, 83, e01510-17. [Google Scholar] [CrossRef]
- Banack, S.A.; Metcalf, J.S.; Jiang, L.; Craighead, D.; Ilag, L.L.; Cox, P.A. Cyanobacteria produce n-(2-aminoethyl)glycine, a backbone for peptide nucleic acids which may have been the first genetic molecules for life on earth. PLoS ONE 2012, 7, e49043. [Google Scholar] [CrossRef]
- Metcalf, J.; Banack, S.; Richer, R.; Cox, P. Neurotoxic amino acids and their isomers in desert environments. J. Arid Environ. 2015, 112, 140–144. [Google Scholar] [CrossRef]
- Dittmann, E.; Erhard, M.; Kaebernick, M.; Scheler, C.; Neilan, B.A.; von Döhren, H.; Börner, T. Altered expression of two light-dependent genes in a microcystin-lacking mutant of Microcystis aeruginosa PCC 7806. Microbiology 2001, 147, 3113–3119. [Google Scholar] [CrossRef] [PubMed]
- Kaebernick, M.; Neilan, B.A. Ecological and molecular investigations of cyanotoxin production. FEMS Microbiol. Ecol. 2001, 35, 1–9. [Google Scholar] [CrossRef]
- Schatz, D.; Keren, Y.; Vardi, A.; Sukenik, A.; Carmeli, S.; Börner, T.; Dittmann, E.; Kaplan, A. Towards clarification of the biological role of microcystins, a family of cyanobacterial toxins. Environ. Microbiol. 2007, 9, 965–970. [Google Scholar] [CrossRef]
- Liaimer, A.; Helfrich, E.J.N.; Hinrichs, K.; Guljamow, A.; Ishidab, K.; Hertweck, C.; Dittmann, E. Nostopeptolide plays a governing role during cellular differentiation of the symbiotic cyanobacterium Nostoc punctiforme. Proc. Natl. Acad. Sci. USA 2015, 112, 61862–61867. [Google Scholar] [CrossRef]
- Kurmayer, R.; Christiansen, G.; Chorus, I. The abundance of microcystin-producing genotypes correlates positively with colony size in Microcystis sp. and determines its microcystin net production in lake wannsee. Appl. Environ. Microbiol. 2003, 69, 787–795. [Google Scholar] [CrossRef]
- Bouaïcha, N.; Corbel, S. Cyanobacterial toxins emerging contaminants in soils: A review of sources, fate and impacts on ecosystems, plants and animal and human health. In Soil Contamination—Current Consequences and Further Solutions; Larramendy, M.L., Soloneski, S., Eds.; InTechOpen: Rijeka, Croatia, 2016. [Google Scholar] [CrossRef]
- Hu, C.; Rzymski, P. Programmed cell death-like and accompanying release of microcystin in freshwater bloom-forming cyanobacterium microcystis: From identification to ecological relevance. Toxins 2019, 11, 706. [Google Scholar] [CrossRef] [PubMed]
- Jokela, J.; Herfindal, L.; Wahlsten, M.; Permi, P.; Selheim, F.; Vasconçelos, V.; Døskeland, S.O.; Sivonen, K. A novel cyanobacterial nostocyclopeptide is a potent antitoxin against microcystins. ChemBioChem 2010, 11, 1594–1599. [Google Scholar] [CrossRef]
- Kardinaal, W.E.A.; Visser, P.M. Dynamics of cyanobacterial toxins: Sources of variability in microcystin concentrations. In Harmful Cyanobacteria; Huisman, J., Matthijs, H.C.P., Visser, P.M., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 41–63. [Google Scholar]
- Neilan, B.A.; Pearson, L.A.; Muenchoff, J.; Moffitt, M.C.; Dittmann, E. Environmental conditions that influence toxin biosynthesis in cyanobacteria. Environ. Microbiol. 2013, 15, 1239–1253. [Google Scholar] [CrossRef]
- do Amaral, S.C.; Monteiro, P.R.; Neto, J.D.S.P.; Serra, G.M.; Gonçalves, E.C.; Xavier, L.P.; Santos, A.V. Current knowledge on microviridin from cyanobacteria. Mar. Drugs 2021, 19, 17. [Google Scholar] [CrossRef]
- Savadova-Ratkus, K.; Mazur-Marzec, H.; Karosienė, J.; Kasperovičienė, J.; Paškauskas, R.; Vitonytė, I.; Koreivienė, J. Interplay of nutrients, temperature, and competition of native and alien cyanobacteria species growth and cyanotoxin production in temperate lakes. Toxins 2021, 13, 23. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Madamwar, D.; Incharoensakdi, A. Bloom dynamics of cyanobacteria and their toxins: Environmental health impacts and mitigation strategies. Front. Microbiol. 2015, 6, 1254. [Google Scholar] [CrossRef]
- Kardinaal, W.E.A.; Tonk, L.; Janse, I.; Hol, S.; Slot, P.; Huisman, J.; Visser, P.M. Competition for light between toxic and nontoxic strains of the harmful cyanobacterium Microcystis. Appl. Environ. Microbiol. 2007, 73, 2939–2946. [Google Scholar] [CrossRef]
- Brandenburg, K.; Siebers, L.; Keuskamp, J.; Jephcott, T.G.; Van de Waal, D.B. Effects of nutrient limitation on the synthesis of n-rich phytoplankton toxins: A meta-analysis. Toxins 2020, 12, 221. [Google Scholar] [CrossRef] [PubMed]
- Utkilen, H.; Gjølme, N. Iron-stimulated toxin production in Microcystis aeruginosa. Appl. Environ. Microbiol. 1995, 61, 797–800. [Google Scholar] [CrossRef] [PubMed]
- Cox, P.A.; Banack, S.A.; Murch, S.J.; Rasmussen, U.; Tien, G.; Bidigare, R.R.; Metcalf, J.S.; Morrison, L.F.; Codd, G.A.; Bergman, B. Diverse taxa of cyanobacteria produce β-N-methylamino-l-alanine, a neurotoxic amino acid. Proc. Natl. Acad. Sci. USA 2005, 102, 5074–5078. [Google Scholar] [CrossRef] [PubMed]
- Pancrace, C.; Barny, M.A.; Ueoka, R.; Calteau, A.; Scalvenzi, T.; Pédron, J.; Barbe, V.; Piel, J.; Humbert, J.-F.; Gugger, M. Insights into the Planktothrix genus: Genomic and metabolic comparison of benthic and planktic strains. Sci. Rep. 2017, 7, 41181. [Google Scholar] [CrossRef] [PubMed]
- Rolland, J.L.; Stien, D.; Sanchez-Ferandin, S.; Lami, R. Quorum sensing and quorum quenching in the phycosphere of phytoplankton: A case of chemical interactions in ecology. J. Chem. Ecol. 2016, 42, 1201–1211. [Google Scholar] [CrossRef]
- Hofbauer, W.K.; Gӓrtner, G. Microbial Life on Façades; Springer Spectrum: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Harder, R. Ernährungsphysiologische Untersuchungen an Cyanophyceen, hauptsächlich dem endophytischen Nostoc punctiforme. Z. Bot. 1917, 9, 145–242. [Google Scholar]
- Gärtner, G.; Stoyneva, M.P.; Mancheva, A.D.; Uzunov, B.A. A new method in collection and cultivation of aerophytic andendolithic algae. Ber. Nat. Med. Ver. Innsbr. 2010, 96, 27–34. [Google Scholar]
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press, Inc: Orlando, USA, 1984. [Google Scholar]
- Barchi, J.J., Jr.; Norton, T.R.; Furusawa, E.; Patterson, G.M.L.; Moore, R.E. Identification of a cytotoxin from Tolypothrix byssoidea as tubercidin. Phytochemistry 1983, 22, 2851–2852. [Google Scholar] [CrossRef]
- Anazi, K.; Nakamura, D.; Suzuki, S. A new antibiotic, tubercidin. J. Antibiot. Ser. A 1957, 10, 201–204. [Google Scholar]
- Biabani, M.F.; Gunasekera, S.P.; Longley, R.E.; Wright, A.E.; Pomponi, S.A. Tubercidin, a cytotoxic agent from the marine sponge Caulospongia biflabellata. Pharm. Biol. 2002, 40, 302–303. [Google Scholar] [CrossRef]
- Frankmölle, W.P.; Larsen, L.K.; Caplan, F.R.; Patterson, G.M.L.; Knübel, G.; Levine, I.A.; Moore, R.E. Antifungal cyclic peptides from the terrestrial blue-green alga Anabaena laxa. I. Isolation and biological properties. J. Antibiot. 1992, 45, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Pergament, I.; Carmeli, S. Schizotrin A; a novel antimicrobial cyclic peptide from a cyanobacterium. Tetrahedron Lett. 1994, 35, 8473–8476. [Google Scholar] [CrossRef]
- Bonjouklian, R.; Smitka, T.A.; Hunt, A.H.; Occolowitz, J.L.; Perun, T.J., Jr.; Doolin, L.; Stevenson, S.; Knauss, L.; Wijayaratne, R.; Szewczyk, S.; et al. A90720A, a serine protease inhibitor isolated from a terrestrial blue-green alga Microchaete loktakensis. Tetrahedron 1996, 52, 395–404. [Google Scholar] [CrossRef]
- Todorova, A.K.; Juettner, F.; Linden, A.; Pluess, T.; von Philipsborn, W. Nostocyclamide: A new macrocyclic, thiazole-containing allelochemical from Nostoc sp. 31 (cyanobacteria). J. Org. Chem. 1995, 60, 7891–7895. [Google Scholar] [CrossRef]
- Todorova, A.; Jüttner, F. Ecotoxicological analysis of nostocyclamide, a modified cyclic hexapeptide from Nostoc. Phycologia 1996, 35, 183–188. [Google Scholar] [CrossRef]
- Jüttner, F.; Todorova, A.K.; Walch, N.; Philipsborn, W. Nostocyclamide M: A cyanobacterial cyclic peptide with allelopathic activity from Nostoc 31. Phytochemistry 2001, 57, 613–619. [Google Scholar] [CrossRef]
- Vishwakarma, R.; Rai, A.K. Separation of bioactive metabolites from aphanothece halophytica through hplc and characterization of the analytes through ESI-MS and NMR. Nat. Prod. J. 2013, 3. [Google Scholar] [CrossRef]
- Golakoti, T.; Ogino, J.; Heltzel, C.E.; Le Husebo, T.; Jensen, C.M.; Larsen, L.K.; Patterson, G.M.L.; Moore, R.E.; Mooberry, S.L.; Corbett, T.H.; et al. Structure determination, conformational analysis, chemical stability studies, and antitumor evaluation of the cryptophycins. Isolation of new 18 analogs from Nostoc sp. strain GSV 224. J. Am. Chem. Soc. 1995, 117, 12030–12049. [Google Scholar] [CrossRef]
- Golakoti, T.; Yoshida, W.Y.; Chaganty, S.; Moore, R.E. Isolation and structure determination of nostocyclopeptides A1 and A2 from the terrestrial cyanobacterium Nostoc sp. ATCC53789. J. Nat. Prod. 2001, 64, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.E.; Hirsch, C.F.; Sesin, D.F.; Flor, J.E.; Chartrain, M.; Fromtling, R.E.; Harris, G.H.; Salvatore, M.J.; Liesch, J.M.; Yudin, K. Pharmaceuticals from cultured algae. J. Ind. Microbiol. 1990, 5, 113–124. [Google Scholar] [CrossRef]
- Smith, C.D.; Zhang, X.; Mooberry, S.L.; Patterson, G.M.; Moore, R.E. Cryptophycin: A new antimicrotubule agent active against drug-resistant cells. Cancer Res. 1994, 54, 3779–3784. [Google Scholar]
- Barrow, R.A.; Hemscheidt, T.; Liang, J.; Paik, S.; Moore, R.E.; Tius, R.A. Total synthesis of cryptophycins. Revision of the structure of cryptophycins A and C. J. Am. Chem. Soc. 1995, 117, 2479–2490. [Google Scholar] [CrossRef]
- Magarvey, N.A.; Beck, Z.Q.; Golakoti, T.; Ding, Y.; Huber, U.; Hemscheidt, T.K.; Abelson, D.; Moore, R.A.; Sherman, D.H. Biosynthetic characterization and chemoenzymatic assembly of the cryptophycins. Potent anticancer agents from Nostoc cyanobionts. ACS Chem. Biol. 2006, 1, 766–779. [Google Scholar] [CrossRef]
- Kobayashi, M.; Aoki, S.; Ohyabu, N.; Kurosu, M.; Wang, W.; Kitagawa, I. Arenastatin A, a potent cytotoxic depsipeptide from the okinawan marine sponge Dysidea arenaria. Tetrahedron Lett. 1994, 35, 7969–7972. [Google Scholar] [CrossRef]
- White, J.D.; Hong, J.; Robarge, L.A. Total synthesis of Cryptophycins-1, -3, -4, -24 (Arenastatin A), and -29, cytotoxic depsipeptides from cyanobacteria of the Nostocaceae. J. Org. Chem. 1999, 64, 6206–6216. [Google Scholar] [CrossRef]
- Nowruzi, B.; Khavari-Nejad, R.; Sivonen, K.; Kazemi, B.; Najafi, F.; Nejadsattari, T. Indentification and toxigenic potential of Nostoc sp. Algae 2012, 27, 303–313. [Google Scholar] [CrossRef]
- Nowruzi, B.; Blanco, S.; Nejadsattari, T. Chemical and molecular evidences for the poisoning of a duck by anatoxin-a, nodularin and cryptophycin at the coast of lake Shoormast (Mazandaran province, Iran). Int. J. Algae 2018, 20, 359–376. [Google Scholar] [CrossRef]
- Weiss, C.; Figueras, E.; Borbely, A.N.; Sewald, N. Cryptophycins: Cytotoxic cyclodepsipeptides with potential for tumor targeting. J. Pept. Sci. 2017, 23, 514–531. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.E. Cyclic peptides and depsipeptides from cyanobacteria: A review. J. Ind. Microbiol. 1996, 16, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.; Corbett, T.; Patterson, G.M.L.; Valeriote, F.A. The search for new antitumor drugs from blue-green algae. Curr. Pharm. Des. 1996, 2, 317–330. [Google Scholar]
- Panda, D.; Himes, R.H.; Moore, R.E.; Wilson, L.; Jordan, M.A. Mechanism of action of the unusually potent microtubule inhibitor cryptophycin 1. Biochemistry 1997, 36, 12948–12953. [Google Scholar] [CrossRef]
- Harada, K. Production of secondary metabolites by freshwater cyanobacteria. Chem. Pharm. Bull. 2004, 52, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, A.; Kajiyama, S.; Inawaka, K.; Kanzaki, H.; Kawazu, K. Nostodione A, a novel mitotic spindle poison from a blue-green alga Nostoc commune. Z. Nat. C 1994, 49, 464–470. [Google Scholar] [CrossRef]
- Proteau, P.J.; Gerwick, W.H.; Garcia-Pichel, F.; Castenholz, R. The structure of scytonemin, an ultraviolet sunscreen pigment from the sheaths of cyanobacteria. Experientia 1993, 49, 825–829. [Google Scholar] [CrossRef]
- Ekebergh, A.; Börje, A.; Mårtensson, J. Total synthesis of Nostodione A, a cyanobacterial metabolite. Org. Lett. 2012, 14, 6274–6277. [Google Scholar] [CrossRef]
- Stout, E.P.; Hasemeyer, A.P.; Lane, A.L.; Davenport, T.M.; Engel, S.; Hay, M.E.; Fairchild, C.R.; Prudhomme, J.; Roch, K.L.; Aalbersberg, W.; et al. Antibacterial neurymenolides from the Fijian red alga Neurymenia fraxinifolia. Org. Lett. 2009, 11, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Nagatsu, A.; Kajitani, H.; Sakakibara, J. Muscoride A: A new oxazole peptide alkaloid from freshwater cyanobacterium Nostoc muscorum. Tetrahedron Lett. 1995, 36, 4097–4100. [Google Scholar] [CrossRef]
- Fujii, K.; Sivonen, K.; Kashiwagi, T.; Hirayama, K.; Harada, K.-I. Nostophycin, a novel cyclic peptide from the toxic cyanobacterium Nostoc sp. 152. J. Org. Chem. 1999, 64, 5777–5782. [Google Scholar] [CrossRef]
- Liu, L.; Jokela, J.; Herfindal, L.; Wahlsten, M.; Sinkkonen, J.; Permi, P.; Fewer, D.P.; Døskeland, S.O.; Sivonen, K. 4-methylproline guided natural product discovery: Co-occurrence of 4-hydroxy and 4-methylprolines in nostoweipeptins and nostopeptolides. ACS Chem. Biol. 2014, 9, 2646–2655. [Google Scholar] [CrossRef]
- Becker, J.E.; Moore, R.E.; Moore, B.S. Cloning, sequencing, and biochemical characterization of the nostocyclopeptide biosynthetic gene cluster: Molecular basis for imine macrocyclization. Gene 2004, 325, 35–42. [Google Scholar] [CrossRef]
- Herfindal, L.; Myhren, L.; Kleppe, R.; Krakstad, C.; Selheim, F.; Jokela, J.; Sivonen, K.; Døskeland, S.O. Nostocyclopeptide-M1: A potent, nontoxic inhibitor of the hepatocyte drug transporters OATP1B3 and OATP1B1. Mol. Pharm. 2011, 8, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Fidor, A.; Grabski, M.; Gawor, J.; Gromadka, R.; Węgrzyn, G.; Mazur-Marzec, H. Nostoc edaphicum CCNP1411 from the Baltic Sea—a new producer of nostocyclopeptides. Mar. Drugs 2020, 18, 442. [Google Scholar] [CrossRef] [PubMed]
- Golakoti, T.; Yoshida, W.Y.; Chaganty, S.; Moore, R.E. Isolation and structures of nostopeptolides A1, A2 and A3 from the cyanobacterium Nostoc sp. GSV 224. Tetrahedron 2000, 56, 9093–9102. [Google Scholar] [CrossRef]
- Hoffmann, D.; Hevel, J.M.; Moore, R.E.; Moore, B.S. Sequence analysis and biochemical characterization of the nostopeptolide A biosynthetic gene cluster from Nostoc sp. GSV 224. Gene 2003, 311, 171–180. [Google Scholar] [CrossRef]
- Boyd, M.R.; Gustafson, K.R.; McMahon, J.B.; Shoemaker, R.H.; O’keefe, B.R.; Mori, T.; Gulakowski, R.J.; Wu, L.; Rivera, M.I.; Laurencot, C.M.; et al. Discovery of Cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: Potential applications to microbicide development. Antimicrob. Agents Chemother. 1997, 41, 1521–1530. [Google Scholar] [CrossRef]
- Dey, B.; Lerner, D.L.; Lusso, P.; Boyd, M.R.; Elder, J.H.; Berger, E.A. Multiple antiviral activities of cyanovirin-n: Blocking of human immunodeficiency virus type 1 gp120 interaction with CD4 and coreceptor and inhibition of diverse enveloped viruses. J. Virol. 2000, 74, 4562–4569. [Google Scholar] [CrossRef] [PubMed]
- Botos, I.; O’Keefe, B.R.; Shenoy, S.R.; Cartner, L.K.; Ratner, D.M.; Seeberger, P.H.; Boyd, M.R.; Wlodawer, A. Structures of the complexes of a potent anti-hiv protein Cyanovirin-N and high mannose oligosaccharides. J. Biol. Chem. 2002, 277, 34336–34342. [Google Scholar] [CrossRef]
- Zappe, H.; Snell, M.E.; Bossard, M.J. PEGylation of cyanovirin-N, an entry inhibitor of HIV. Adv. Drug Deliv. Rev. 2008, 60, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Jokela, J.; Wahlsten, M.; Nowruzi, B.; Permi, P.; Zhang, Y.Z.; Xhaard, H.; Fewer, D.P.; Sivonen, K. Nostosins, trypsin inhibitors isolated from the terrestrial cyanobacterium Nostoc sp. strain FSN. J. Nat. Prod. 2014, 77, 1784–1790. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Feng, J.; Xu, Z.; Ye, T.; Meng, Y.; Zhang, Z. Total synthesis and stereochemical assignment of Nostosin, B. Mar. Drugs 2017, 15, 58. [Google Scholar] [CrossRef]
- Kapuścik, A.; Hrouzek, P.; Kuzma, M.; Bártová, S.; Novák, P.; Jokela, J.; Pflüger, M.; Eger, A.; Hundsberger, H.; Kopecký, J. Novel aeruginosin-865 from Nostoc sp. as a potent anti-inflammatory agent. ChemBioChem 2013, 14, 2329–2337. [Google Scholar] [CrossRef]
- Ploutno, A.; Carmeli, S. Modified peptides from a water bloom of the cyanobacterium Nostoc sp. Tetrahedron 2002, 58, 9949–9957. [Google Scholar] [CrossRef]
- Riba, M.; Kiss-Szikszai, A.; Gonda, S.; Parizsa, P.; Deák, B.; Török, P.; Valkó, O.; Felföldi, T.; Vasas, G. Chemotyping of terrestrial Nostoc-like isolates from alkali grassland areas by non-targeted peptide analysis. Algal Res. 2020, 46, 101798. [Google Scholar] [CrossRef]
- Mazur-Marzec, H.; Fidor, A.; Cegłowska, M.; Wieczerzak, E.; Kropidłowska, M.; Goua, M.; Macaskill, J.; Edwards, C. Cyanopeptolins with trypsin and chymotrypsin inhibitory activity from the cyanobacterium Nostoc edaphicum CCNP 1411. Mar. Drugs 2018, 16, 220. [Google Scholar] [CrossRef]
- Yamaki, H.; Sitachitta, N.; Sano, T.; Kaya, K. Two new chymotrypsin inhibitors isolated from the cyanobacterium Microcystis aeruginosa NIES-88. J. Nat. Prod. 2005, 68, 14–18. [Google Scholar] [CrossRef]
- Ishitsuka, M.O.; Kusumi, T.; Kakisawa, H.; Kunimitsu, K.; Watanabe, M.M. Microviridin. A novel tricyclic depsipeptide from the toxic cyanobacterium Microsystis viridis. J. Am. Chem. Soc. 1990, 112, 8180–8182. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, K.; Yang, G.; McBride, J.L.; Bruner, S.D.; Ding, Y. A distributive peptide cyclase processes multiple microviridin core peptides within a single polypeptide substrate. Nat. Commun. 2018, 9, 1780. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Park, Y.; Kim, S. Enzymatic cross-linking of side chains generates a modified peptide with four hairpin-like bicyclic repeats. Biochemistry 2017, 56, 4927–4930. [Google Scholar] [CrossRef] [PubMed]
- Okino, T.; Matsuda, H.; Murakami, M.; Yamaguchi, K. New microviridins, elastase inhibitors from the blue-green alga Microcystis aeruginosa. Tetrahedron 1995, 51, 10679–10686. [Google Scholar] [CrossRef]
- Shin, H.J.; Murakami, M.; Matsuda, H.; Yamaguchi, K. Microviridins D-F, serine protease inhibitors from the cyanobacterium Oscillatoria agardhii (NIES-204). Tetrahedron 1996, 52, 8159–8168. [Google Scholar] [CrossRef]
- Rohrlack, T.; Christoffersen, K.; Hansen, P.E.; Zhang, W.; Czarnecki, O.; Henning, M.; Fastner, J.; Erhard, M.; Neilan, B.A.; Kaebernick, M. Isolation, characterization, and quantitative analysis of microviridin J, a new Microcystis metabolite toxic to Daphnia. J. Chem. Ecol. 2003, 29, 1757–1770. [Google Scholar] [CrossRef]
- Rohrlack, T.; Christoffersen, K.; Kaebernick, M.; Neilan, B.A. Cyanobacterial protease inhibitor microviridin J causes a lethal molting disruption in Daphnia pulicaria. Appl. Environ. Microbiol. 2004, 70, 5047–5050. [Google Scholar] [CrossRef] [PubMed]
- Reshef, V.; Carmeli, S. New microviridins from a water bloom of the cyanobacterium Microcystis aeruginosa. Tetrahedron 2006, 62, 7361–7369. [Google Scholar] [CrossRef]
- Vegman, M.; Carmeli, S. Three aeruginosins and a microviridin from a bloom assembly of Microcystis spp. collected from a fishpond near Kibbutz Lehavot HaBashan, Israel. Tetrahedron 2014, 70, 6817–6824. [Google Scholar] [CrossRef]
- Philmus, B.; Christiansen, G.; Yoshida, W.Y.; Hemscheidt, T.K. Post-translational modification in microviridin biosynthesis. ChemBioChem 2008, 9, 3066–3073. [Google Scholar] [CrossRef]
- Murakami, M.; Sun, Q.; Ishida, K.; Matsuda, H.; Okino, T.; Yamaguchi, K. Microvirdins, elastase inhibitors from the cyanobacterium Nostoc minutum (NIES-26). Phytochemistry 1997, 45, 1197–1202. [Google Scholar] [CrossRef]
- Gehringer, M.M.; Adler, L.; Roberts, A.A.; Moffitt, M.C.; Mihali, T.K.; Mills, T.J.T.; Fieker, C.; Neilan, B.A. Nodularin, a cyanobacterial toxin, is synthesized in planta by symbiotic Nostoc sp. ISME J. 2012, 6, 1834–1847. [Google Scholar] [CrossRef]
- Jokela, J.; Heinilä, L.M.P.; Shishido, T.K.; Wahlsten, M.; Fewer, D.P.; Fiore, M.F.; Wang, H.; Haapaniemi, E.; Permi, P.; Sivonen, K. Production of high amounts of hepatotoxin nodularin and new protease inhibitors pseudospumigins by the Brazilian benthic Nostoc sp. CENA543. Front. Microbiol. 2017, 8, 1963. [Google Scholar] [CrossRef]
- Cox, P.A.; Banack, S.A.; Murch, S.J. Biomagnification of cyanobacterial neurotoxins and neurodegenerative disease among the Chamorro people of Guam. Proc. Natl. Acad. Sci. USA 2003, 100, 13380–13383. [Google Scholar] [CrossRef]
- Murch, S.J.; Cox, P.A.; Banack, S.A. A mechanism for slow release of biomagnified cyanobacterial neurotoxins and neurodegenerative disease in Guam. Proc. Natl. Acad. Sci. USA 2004, 101, 12228–12231. [Google Scholar] [CrossRef] [PubMed]
- Banack, S.A.; Johnson, H.E.; Cheng, R.; Cox, P.A. Production of the neurotoxin BMAA by a marine cyanobacterium. Mar. Drugs 2007, 5, 180–196. [Google Scholar] [CrossRef]
- Sivonen, K.; Carmichael, W.; Namikoshi, M.; Rinehart, K.; Dahlem, A.; Niemela, S. Isolation and characterization of hepatotoxic microcystin homologs from the filamentous freshwater cyanobacterium Nostoc sp. strain 152. Appl. Environ. Microbiol. 1990, 56, 2650–2657. [Google Scholar] [CrossRef]
- Beattie, K.; Kaya, K.; Sano, T.; Codd, G. Three dehydrobotyrine-containing microcystins from Nostoc. Phytochemistry 1998, 47, 1289–1292. [Google Scholar] [CrossRef]
- Amer, R.; Shehawy, R.; El-Dien, S.; Serie, M.; Shaker, K. Isolation and characterization of cyanobacterial community including a microcystin-producing Nostoc sp. strain in the Nile River, Egypt. Adv. Microbiol. 2013, 3, 38–46. [Google Scholar] [CrossRef]
- Teneva, I.; Stoyanov, P.; Belkinova, D.; Dimitrova-Dyulgerova, I.; Mladenov, R.; Dzhambazov, B. Production of cyanobacterial toxins from two Nostoc species (Nostocales) and evaluation of their cytotoxicity in vitro. J. Biosci. Biotechnol. 2012, 1, 33–43. [Google Scholar]
- Batsalova, T.; Moten, D.; Basheva, D.; Teneva, I.; Dzhambazov, B. In vitro cytotoxicity and antioxidative potential of Nostoc microscopicum (Nostocales, Cyanobacteria). Toxicol. Forensic Med. Open J. 2016, 1, 9–17. [Google Scholar] [CrossRef]
- Mohamed, Z.A.; El-Sharouny, H.M.; Ali, W.S.M. Microcystin production in benthic mats of cyanobacteria in the Nile River and irrigation canals, Egypt. Toxicon 2006, 47, 584–590. [Google Scholar] [CrossRef]
- Genuario, D.B.; Silva-Stenico, M.E.; Welker, M.; Moraes, L.A.B.; Fiore, M.F. Characterization of a microcystin and detection of microcystin synthetase genes from a Brazilian isolate of Nostoc. Toxicon 2010, 55, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, R.; Sharma, N.K.; Lawton, L.A.; Edwards, C.; Rai, A.K. Microcystin producing cyanobacterium Nostoc sp. BHU001 from a pond in India. Toxicon 2009, 53, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Oudra, B.; Andaloussi, M.D.E.; Vasconcelos, V.M. Identification and quantification of microcystins from a Nostoc muscorum bloom occurring in Ouka Meden River (High-Atlas Mountains of Marrakech, Morocco). Environ. Monit. Assess. 2009, 149, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Namikoshi, M.; Rinehart, K.L.; Sakai, R.; Sivonen, K.; Carmichael, W.W. Structures of three new cyclic heptapeptide hepatotoxins produced by the cyanobacterium (blue-green alga) Nostoc sp. strain 152. J. Org. Chem. 1990, 55, 6135–6139. [Google Scholar] [CrossRef]
- Kurmayer, R. The toxic cyanobacterium Nostoc sp. strain 152 produces highest amounts of microcystin and nostophycin under stress conditions. J. Phycol. 2010, 47, 200–207. [Google Scholar] [CrossRef]
- Puddick, J.; Prinsep, M.R.; Wood, S.A.; Cary, S.C.; Hamilton, D.P.; Holland, P.T. Further characterization of glycine-containing microcystins from the mcmurdo dry valleys of Antarctica. Toxins 2015, 7, 493–515. [Google Scholar] [CrossRef]
- Oksanen, I.; Jokela, J.; Fewer, D.P.; Wahlsten, M.; Rikkinen, J.; Sivonen, K. Discovery of rare and highly toxic microcystins from lichen-associated cyanobacterium Nostoc sp. strain IO-102-I. Appl. Environ. Microbiol. 2004, 70, 5756–5763. [Google Scholar] [CrossRef]
- Kaasalainen, U.; Fewer, D.P.; Jokela, J.; Wahlsten, M.; Sivonen, K.; Rikkinen, J. Cyanobacteria produce a high variety of hepatotoxic peptides in lichen symbiosis. Proc. Natl. Acad. Sci. USA 2012, 109, 5886–5891. [Google Scholar] [CrossRef]
- Takenaka, H.; Yamaguchi, Y.; Sakaki, S.; Watarai, K.; Tanaka, N.; Hori, M.; Seki, H.; Tsuchida, M.; Yamada, A.; Nishimori, T.; et al. Safety evaluation of Nostoc flagelliforme (Nostocales, Cyanophyceae) as a potential food. Food Chem. Toxicol. 1998, 36, 1073–1077. [Google Scholar] [CrossRef]
- Kabirnataj, S.; Nematzadeh, G.A.; Talebi, A.F.; Tabatabaei, M.; Singh, P. Neowestiellopsis gen. nov, a new genus of true branched cyanobacteria with the description of Neowestiellopsis persica sp. nov. and Neowestiellopsis bilateralis sp. nov., isolated from Iran. Plant. Syst. Evol. 2018, 304, 501–510. [Google Scholar] [CrossRef]
- Abed, I.J.; Abdulhasan, G.A.; Moushib, L.I. Molecular and immunological methods to confirm toxiginicity (microcystin production) of westiellopsis prolifica isolated from Tigris River—Iraq. Baghdad Sci. J. 2019, 16, 2019978. [Google Scholar] [CrossRef]
- Prakash, S.; Nikhil, K. Algae as a soil conditioner. Int. J. Eng. Tech. Res. 2014, 2, 68–70. [Google Scholar]
- MacKintosh, C.; Beattie, K.A.; Klumpp, S.; Cohen, P.; Codd, G.A. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett. 1990, 264, 187–192. [Google Scholar] [CrossRef]
- Kurki-Helasmo, K.; Meriluoto, J. Microcystin uptake inhibits growth and protein phosphatase activity in mustard (Sinapis alba L.) seedlings. Toxicon 1998, 36, 1921–1926. [Google Scholar] [CrossRef]
- Corbel, S.; Mougin, C.; Bouaïcha, N. Cyanobacterial toxins: Modes of actions, fate in aquatic and soil ecosystems, phytotoxicity and bioaccumulation in agricultural crops—A review. Chemosphere 2014, 96, 1–15. [Google Scholar] [CrossRef]
- Morris, R.J.; Williams, D.E.; Luu, H.A.; Holmes, C.F.B.; Andersen, R.J.; Calvert, S.E. The adsorption of microcystin-LR by natural clay particles. Toxicon 2000, 38, 303–308. [Google Scholar] [CrossRef]
- Miller, M.A.; Kudela, R.M.; Mekebri, A.; Crane, D.; Oates, S.C.; Tinker, M.T.; Staedler, M.; Miller, W.A.; Toy-Choutka, S.; Dominik, C.; et al. Evidence for a novel marine harmful algal bloom: Cyanotoxin (microcystin) transfer from Land to Sea Otters. PLoS ONE 2010, 5, e12576. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Song, L.; Gan, N.; Li, L. Sorption, degradation and mobility of microcystins in Chinese agriculture soils: Risk assessment for groundwater protection. Environ. Pollut. 2006, 144, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Bibo, L.; Yan, G.; Bangding, X.; Jiantong, L.; Yongding, L. A laboratory study on risk assessment of microcystin-RR in cropland. J. Environ. Manag. 2008, 86, 566–574. [Google Scholar] [CrossRef]
- Crush, J.R.; Briggs, L.R.; Sprosen, J.M.; Nichols, S.N. Effect of irrigation with lake water containing microcystins on microcystin content and growth of ryegrass, clover, rape, and lettuce. Environ. Toxicol. 2008, 23, 246–252. [Google Scholar] [CrossRef]
- Saqrane, S.; Oudra, B. CyanoHAB occurrence and water irrigation cyanotoxin contamination: Ecological impacts and potential health risks. Toxins 2009, 1, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Sathishkumar, M.; Pavagadhi, S.; Vijayaraghavan, K.; Balasubramanian, R.; Ong, S.L. Concomitant uptake of microcystin-LR and -RR by peat under various environmental conditions. Chem. Eng. J. 2011, 172, 754–762. [Google Scholar] [CrossRef]
- Codd, G.A.; Metcalf, J.S.; Beattie, K.A. Retention of Microcystis aeruginosa and microcystin by salad lettuce (Lactuca sativa) after spray irrigation with water containing cyanobacteria. Toxicon 1999, 37, 1181–1185. [Google Scholar] [CrossRef]
- Miller, A.; Russell, C. Food crops irrigated with cyanobacteria-contaminated water: An emerging public health issue in Canada. Environ. Health Rev. 2017, 60, 58–63. [Google Scholar] [CrossRef]
- Redouane, E.M.; El Amrani Zerrifi, S.; El Khalloufi, F.; Oufdou, K.; Oudra, B.; Lahrouni, M.; Campos, A.; Vasconcelos, V. Mode of action and fate of microcystins in the complex soil-plant ecosystems. Chemosphere 2019, 225, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Llana-Ruiz-Cabello, M.; Jos, A.; Cameán, A.; Oliveira, F.; Barreiro, A.; Machado, J.; Azevedo, J.; Pinto, E.; Almeida, A.; Campos, A.; et al. Analysis of the use of cylindrospermopsin and/or microcystin-contaminated water in the growth, mineral content, and contamination of Spinacia oleracea and Lactuca sativa. Toxins 2019, 11, 624. [Google Scholar] [CrossRef]
- Pflugmacher, S.; Wiegand, C.; Beattie, K.A.; Codd, G.A.; Steinberg, C. Uptake of the cyanobacterial hepatotoxin microcystin-LR by aquatic macrophytes. J. Appl. Bot. 1998, 72, 228–232. [Google Scholar]
- Pflugmacher, S.; Codd, G.A.; Steinberg, C.E.W. Effects of the cyanobacterial toxin microcystin-LR on detoxication enzymes in aquatic plants. Environ. Toxicol. 1999, 14, 111–115. [Google Scholar] [CrossRef]
- Pflugmacher, S.; Wiegand, C.; Beattie, K.A.; Krause, E.; Steinberg, C.E.W.; Codd, G.A. Uptake, effects, and metabolism of cyanobacterial toxins in the emergent reed plant Phragmites australis (Cav.) Trin. ex Steud. Environ. Toxicol. 2001, 20, 846–852. [Google Scholar] [CrossRef]
- Cordeiro-Araújo, M.K.; Chia, M.A.; de Arruda-Neto, J.D.T.; Tornisielo, V.L.; Vilca, F.Z.; do Carmo Bittencourt-Oliveira, M. Microcystin-LR bioaccumulation and depuration kinetics in lettuce and arugula: Human health risk assessment. Sci. Total Environ. 2016, 566, 1379–1386. [Google Scholar] [CrossRef]
- Pereira, A.L.; Azevedo, J.; Vasconcelos, V. Assessment of uptake and phytotoxicity of cyanobacterial extracts containing microcystins or cylindrospermopsin on parsley (Petroselinum crispum L.) and coriander (Coriandrum sativum L.). Environ. Sci. Pollut. Res. 2017, 24, 1999–2009. [Google Scholar] [CrossRef]
- Levizou, E.; Statiris, G.; Papadimitriou, T.; Laspidou, C.S.; Kormas, K.A. Lettuce facing microcystin-rich irrigation water at different developmental stages: Effects on plant performance and microcystins bioaccumulation. Ecotoxicol. Environ. Saf. 2017, 143, 193–200. [Google Scholar] [CrossRef]
- Cao, Q.; Steinman, A.D.; Wan, X.; Xie, L. Bioaccumulation of microcystin congeners in soil-plant system and human health risk assessment: A field study from Lake Taihu region of China. Environ. Pollut. 2018, 240, 44–50. [Google Scholar] [CrossRef]
- Spoof, L.; Jaakkola, S.; Važić, T.; Häggqvist, K.; Kirkkala, T.; Ventelä, A.-M.; Kirkkala, T.; Svirčev, Z.; Meriluoto, J. Elimination of cyanobacteria and microcystins in irrigation water-effects of hydrogen peroxide treatment. Environ. Sci. Pollut. Res. 2020, 27, 8638–8652. [Google Scholar] [CrossRef] [PubMed]
- Kinnear, S.H.W.; Fabbro, L.D.; Duivenvoorden, L.J. Variable growth responses of water thyme (Hydrilla verticillata) to whole-cell extracts of Cylindrospermopsis raciborskii. Arch. Environ. Contam. Toxicol. 2008, 54, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Guillén, R.; Campos, A.; Machado, J.; Freitas, M.; Azevedo, J.; Pinto, E.; Almeida, A.; Cameán, A.M.; Vasconcelos, V. Effects of Chrysosporum (Aphanizomenon) ovalisporum extracts containing cylindrospermopsin on growth, photosynthetic capacity, and mineral content of carrots (Daucus carota). Ecotoxicology 2017, 26, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Praena, D.; Campos, A.; Azevedo, J.; Neves, J.; Freitas, M.; Guzmán-Guillén, R.; Cameán, A.M.; Renaut, J.; Vasconcelos, V. Exposure of Lycopersicon esculentum to microcystin-LR: Effects in the leaf proteome and toxin translocation from water to leaves and fruits. Toxins 2014, 6, 1837–1854. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.; Azevedo, J.; Campos, A.; Vasconcelos, V.; Pereira, A.L. Biochemical and growth performance of the aquatic macrophyte Azolla filiculoides to sub-chronic exposure to cylindrospermopsin. Ecotoxicology 2015, 24, 1848–1857. [Google Scholar] [CrossRef]
- Peuthert, A.; Chakrabati, S.; Pflugmacher, S. Uptake of microcystins-LR and -LF (cyanobacterial toxins) in seedlings of several important agricultural plant species and the correlation with cellular damage (lipid peroxidation). Environ. Toxicol. 2007, 22, 436–442. [Google Scholar] [CrossRef]
- Freitas, M.; Campos, A.; Azevedo, J.; Barreiro, A.; Planchon, S.; Renaut, J.; Vasconcelos, V. Lettuce (Lactuca sativa L.) leaf-proteome profiles after exposure to cylindrospermopsin and a microcystin-LR/cylindrospermopsin mixture: A concentration-dependent response. Phytochemistry 2015, 110, 91–103. [Google Scholar] [CrossRef]
- Cordeiro-Araújo, M.K.; Chia, M.A.; do Carmo Bittencourt-Oliveira, M. Potential human health risk assessment of cylindrospermopsin accumulation and depuration in lettuce and arugula. Harmful Algae 2017, 68, 217–223. [Google Scholar] [CrossRef]
- Machado, J.; Campos, A.; Vasconcelos, V.; Freitas, M. Effects of microcystin-LR and cylindrospermopsin on plant-soil systems: A review of their relevance for agricultural plant quality and public health. Environ. Res. 2017, 153, 191–204. [Google Scholar] [CrossRef]
- Pflugmacher, S.; Aulhorn, M.; Grimm, B. Influence of a cyanobacterial crude extract containing microcystin-LR on the physiology and antioxidative defence systems of different spinach variants. New Phytol. 2007, 175, 482–489. [Google Scholar] [CrossRef]
- Redouane, E.M.; Lahrouni, M.; Martins, J.C.; El Amrani Zerrifi, S.; Benidire, L.; Douma, M.; Aziz, F.; Oufdou, K.; Mandi, L.; Campos, A.; et al. Protective role of native rhizospheric soil microbiota against the exposure to microcystins introduced into soil-plant system via contaminated irrigation water and health risk assessment. Toxins 2021, 13, 118. [Google Scholar] [CrossRef] [PubMed]
- Machado, J.; Azevedo, J.; Freitas, M.; Pinto, E.; Almeida, A.; Vasconcelos, V.; Campos, A. Analysis of the use of microcystin-contaminated water in the growth and nutritional quality of the root-vegetable, Daucus carota. Environ. Sci. Pollut. Res. 2017, 24, 752–764. [Google Scholar] [CrossRef]
- Zhu, J.; Ren, X.; Liu, H.; Liang, C. Effect of irrigation with microcystins-contaminated water on growth and fruit quality of Cucumis sativus L. and the health risk. Agric. Water Manag. 2018, 204, 91–99. [Google Scholar] [CrossRef]
- Lahrouni, M.; Oufdou, K.; El Khalloufi, F.; Baz, M.; Lafuente, A.; Dary, M.; Pajuelo, E.; Oudra, B. Physiological and biochemical defense reactions of Vicia faba L.-Rhizobium symbiosis face to chronic exposure to cyanobacterial bloom extract containing microcystins. Environ. Sci. Pollut. Res. 2013, 20, 5405–5415. [Google Scholar] [CrossRef] [PubMed]
- Bittencourt-Oliveira, M.C.; Cordeiro-Araújo, M.K.; Chia, M.A.; de Arruda-Neto, J.D.T.; de Oliveira, Ê.T.; dos Santos, F. Lettuce irrigated with contaminated water: Photosynthetic effects, antioxidative response and bioaccumulation of microcystin congeners. Ecotoxicol. Environ. Saf. 2016, 128, 83–90. [Google Scholar] [CrossRef]
- Kittler, K.; Schreiner, M.; Krumbein, A.; Manzei, S.; Koch, M.; Rohn, S.; Maul, R. Uptake of the cyanobacterial toxin cylindrospermopsin in Brassica vegetables. Food Chem. 2012, 133, 875–879. [Google Scholar] [CrossRef]
- Vasas, G.; Gáspár, A.; Surányi, G.; Batta, G.; Gyémánt, G.; M-Hamvas, M.; Máthé, C.; Grigorszky, I.; Molnár, E.; Borbély, G. Capillary electrophoretic assay and purification of cylindrospermopsin, a cyanobacterial toxin from Aphanizomenon ovalisporum, by plant test (Blue-Green Sinapis Test). Anal. Biochem. 2002, 302, 95–103. [Google Scholar] [CrossRef]
- Prieto, A.; Campos, A.; Cameán, A.; Vasconcelos, V. Effects on growth and oxidative stress status of rice plants (Oryza sativa) exposed to two extracts of toxin-producing cyanobacteria (Aphanizomenon ovalisporum and Microcystis aeruginosa). Ecotoxicol. Environ. Saf. 2011, 74, 1973–1980. [Google Scholar] [CrossRef] [PubMed]
- Sheen, J. Protein phosphatase activity is required for light-inducible gene expression in maize. EMBO J. 1993, 12, 3497–3505. [Google Scholar] [CrossRef]
- Smith, R.D.; Wilson, J.E.; Walker, J.C.; Baskin, T.I. Protein-phosphatase inhibitors block root hair growth and alter cortical cell shape of Arabidopsis roots. Planta 1994, 194, 516–524. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Codd, G.A. Microwave oven and boiling waterbath extraction of hepatotoxins from cyanobacterial cells. FEMS Microbiol. Lett. 2000, 184, 241–246. [Google Scholar] [CrossRef]
- Tsuji, K.; Naito, F.; Kondo, F.; Ishikawa, N.; Watanabe, M.F.; Suzuki, M.; Ken-ichi, H. Stability of microcystins from cyanobacteria. Effect of light on decomposition and isomerization. Environ. Sci. Technol. 1994, 28, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Song, L.; Peng, L.; Wan, N.; Zhang, X.; Gan, N. Reduction in microcystin concentrations in large and shallow lakes: Water and sediment-interface contributions. Water Res. 2008, 42, 763–773. [Google Scholar] [CrossRef]
- Miller, M.J.; Fallowfield, H.J. Degradation of cyanobacterial hepatotoxins in batch experiments. Water Sci. Technol. 2001, 43, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, H.-Q.; Hu, L.-B.; Shi, Z.-Q. Microcystin-LR-induced phytotoxicity in rice crown root is associated with the cross-talk between auxin and nitric oxide. Chemosphere 2013, 93, 283–293. [Google Scholar] [CrossRef]
- Edwards, C.; Graham, D.; Fowler, N.; Lawton, L.A. Biodegradation of microcystins and nodularin in freshwaters. Chemosphere 2008, 73, 1315–1321. [Google Scholar] [CrossRef]
- Miller, M.; Critchley, M.; Hutson, J.; Fallowfield, H. The adsorption of cyanobacterial hepatotoxins from water onto soil during batch experiments. Water Res. 2001, 35, 1461–1468. [Google Scholar] [CrossRef]
- Manage, P.M.; Edwards, C.; Singh, B.K.; Lawton, L.A. Isolation and identification of novel microcystin-degrading bacteria. Appl. Environ. Microbiol. 2009, 75, 6924–6928. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Pan, G. Simultaneous removal of harmful algal blooms and microcystins using microorganism- and chitosan-modified local soil. Environ. Sci. Technol. 2015, 49, 6249–6256. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Zhang, M.-M.; Chen, H.; Zou, H.; Yan, H. Removal of cyanobacterial blooms in Taihu Lake using local soils. I. Equilibrium and kinetic screening on the flocculation of Microcystis aeruginosa using commercially available clays and minerals. Environ. Pollut. 2006, 141, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Miao, X.; Bi, L.; Zhang, H.; Wang, L.; Wang, L.; Wang, Z.; Chen, J.; Ali, J.; Pan, M.; et al. Modified local soil (MLS) technology for harmful algal bloom control, sediment remediation, and ecological restoration. Water 2019, 11, 1123. [Google Scholar] [CrossRef]
- Mackay, E.B.; Maberly, S.C.; Pan, G.; Reitzel, K.; Bruere, A.; Corker, N.; Douglas, G.; Egemose, S.; Hamilton, D.; Hatton-Ellis, T.; et al. Geoengineering in lakes: Welcome attraction or fatal distraction? Inland Waters 2014, 4, 349–356. [Google Scholar] [CrossRef]
- Spears, B.M.; Maberly, S.C.; Pan, G.; Mackay, E.; Bruere, A.; Corker, N.; Douglas, G.; Egemose, S.; Hamilton, D.; Hatton-Ellis, T.; et al. Geo-engineering in lakes: A crisis of confidence? Environ. Sci. Technol. 2014, 48, 9977–9979. [Google Scholar] [CrossRef]
- Gu, Y.; Liang, C. Responses of antioxidative enzymes and gene expression in Oryza sativa L. and Cucumis sativus L. seedlings to microcystins stress. Ecotoxicol. Environ. Saf. 2020, 193, 110351. [Google Scholar] [CrossRef]
- Corbel, S.; Mougin, C.; Nélieu, S.; Delarue, G.; Bouaïcha, N. Evaluation of the transfer and the accumulation of microcystins in tomato (Solanum lycopersicum cultivar MicroTom) tissues using a cyanobacterial extract containing microcystins and the radiolabeled microcystin-LR (14C-MC-LR). Sci. Total Environ. 2016, 541, 1052–1058. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Cyanobacterial Toxins: Microcystins; Background Document for Development of WHO Guidelines for Drinking-Water Quality and Guidelines for Safe Recreational Water Environments; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- World Health Organization (WHO). Cyanobacterial Toxins: Anatoxin-a and Analogues; Background Document for Development of WHO Guidelines for Drinking-Water Quality and Guidelines for safe Recreational Water Environments; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- World Health Organization (WHO). Cyanobacterial Toxins: Cylindrospermopsins; Background Document for Development of WHO Guidelines for Drinking-Water Quality and Guidelines for Safe Recreational Water Environments; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- World Health Organization (WHO). Cyanobacterial Toxins: Saxitoxins; Background Document for Development of WHO Guidelines for Drinking-Water Quality and Guidelines for Safe Recreational Water Environments; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- McElhiney, J.; Lawton, L.A.; Leifert, C. Investigations into the inhibitory effects of microcystins on plant growth, and the toxicity of plant tissues following exposure. Toxicon 2001, 39, 1411–1420. [Google Scholar] [CrossRef]
- Friedmann, E.I.; Ocampo, R. Endolithic blue-green algae in the dry valleys: Primary producers in the Antarctic desert ecosystem. Science 1976, 193, 1247–1249. [Google Scholar] [CrossRef]
- Friedmann, E.I. Endolithic microbial life in hot and cold deserts. Orig. Life Evol. Biosph. 1980, 10, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Friedmann, E.I. Endolithic microorganisms in the Antarctic cold desert. Science 1982, 215, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Nienow, J.A.; Friedmann, E.I. Terrestrial lithophytic rock communities. In Antarctic Microbiology; Friedmann, E.I., Ed.; Wiley-Liss: New York, NY, USA, 1993; pp. 343–412. [Google Scholar]
- Walker, J.J.; Pace, N.R. Phylogenetic composition of Rocky Mountain endolithic microbial ecosystems. Appl. Environ. Microbiol. 2007, 73, 3497–3504. [Google Scholar] [CrossRef] [PubMed]
- Wierzchos, J.; de los Ríos, A.; Ascaso, C. Microorganisms in desert rocks: The edge of life on Earth. Int. Microbiol. 2012, 15, 171–181. [Google Scholar] [CrossRef]
- Wierzchos, J.; Ascaso, C.; McKay, C. Endolithic cyanobacteria in halite rocks from the hyperarid core of the Atacama Desert. Astrobiology 2006, 6, 415–422. [Google Scholar] [CrossRef]
- Billi, D.; Friedmann, E.I.; Hofer, K.G.; Caiola, M.G.; Ocampo-Friedmann, R. Ionizing-radiation resistance in the desiccation-tolerant cyanobacterium Chroococcidiopsis. Appl. Environ. Microbiol. 2000, 66, 1489–1492. [Google Scholar] [CrossRef]
- Ziolkowski, L.A.; Mykytczuk, N.C.S.; Omelon, C.R.; Johnson, H.; Whyte, L.G.; Slater, G.F. Arctic gypsum endoliths: A biogeochemical characterization of a viable and active microbial community. Biogeosciences 2013, 10, 7661–7675. [Google Scholar] [CrossRef]
- Casero, M.C.; Meslier, V.; DiRuggiero, J.; Quesada, A.; Ascaso, C.; Artieda, O.; Kowaluk, T.; Wierzchos, J. The composition of endolithic communities in gypcrete is determined by the specific microhabitat architecture. Biogeosciences 2021, 18, 993–1007. [Google Scholar] [CrossRef]
- Casero, M.C.; Ascaso, C.; Quesada, A.; Mazur-Marzec, H.; Wierzchos, J. Response of endolithic Chroococcidiopsis strains from the polyextreme Atacama Desert to light radiation. Front. Microbiol. 2021, 11, 614875. [Google Scholar] [CrossRef]
- Qu, E.; Omelon, C.R.; Oren, A.; Meslier, V.; Cowan, D.A.; Maggs-Kölling, G.; diRuggiero, J. Trophic selective pressures organize the composition of endolithic microbial communities from global deserts. Front. Microbiol. 2020, 10, 2952. [Google Scholar] [CrossRef]
- Smith, M.C.; Bowman, J.P.; Scott, F.J.; Line, M.A. Sublithic bacteria associated with Antarctic quartz stones. Antarct. Sci. 2000, 12, 177–184. [Google Scholar] [CrossRef]
- Büdel, B.; Weber, B.; Kühl, M.; Pfanz, H.; Sültemeyer, D.; Wessels, D. Reshaping of sandstone surfaces by cryptoendolithic cyanobacteria: Bioalkalization causes chemical weathering in arid landscapes. Geobiology 2004, 2, 261–268. [Google Scholar] [CrossRef]
- Stoyneva, M.; Mancheva, A.; Gärtner, G.; Uzunov, B. Are the algae from the uncommon Belogradchik rocks common ones? In Proceedings of the VII National Conference in Botany, Sofia, Bulgaria, 29–30 September 2011; Petrova, A., Ed.; Bulgarian Botanical Society: nSofia, Bulgaria, 2012; pp. 265–269. [Google Scholar]
- Herrera, A.; Cockell, C.S.; Self, S.; Blaxter, M.; Reitner, J.; Thorsteinsson, T.; Arp, G.; Dröse, W.; Tindle, A.G. A cryptoendolithic community in volcanic glass. Astrobiology 2009, 9, 369–381. [Google Scholar] [CrossRef] [PubMed]
- de la Torre, J.R.; Goebel, B.M.; Friedmann, E.I.; Pace, N.R. Microbial diversity of cryptoendolithic communities from the McMurdo Dry Valleys, Antarctica. Appl. Environ. Microbiol. 2003, 69, 3858–3867. [Google Scholar] [CrossRef]
- Gaylarde, P.M.; Jungblut, A.; Gaylarde, C.C.; Neilan, B.A. Endolithic phototrophs from an active geothermal region in New Zealand. Geomicrobiol. J. 2006, 23, 579–587. [Google Scholar] [CrossRef]
- de Los Ríos, A.; Wierzchos, J.; Ascaso, C. The lithic microbial ecosystems of Antarctica’s McMurdo Dry Valleys. Antarct. Sci. 2014, 26, 459–477. [Google Scholar] [CrossRef][Green Version]
- Vincent, W.F. Microbial Ecosystems of Antarctica; Cambridge University Press: Cambridge, UK, 1988. [Google Scholar]
- Crits-Christoph, A.; Robinson, C.K.; Ma, B.; Ravel, J.; Wierzchos, J.; Ascaso, C.; Artieda, O.; Souza-Egipsy, V.; Casero, M.C.; DiRuggiero, J. Phylogenetic and functional substrate specificity for endolithic microbial communities in hyper-arid environments. Front. Microbiol. 2016, 7, 301. [Google Scholar] [CrossRef]
- Vinogradova, O.N.; Kovalenko, O.V.; Levanets, A.A.; Nevo, E.; Wasser, S.P. Epilithic algal communities of dry rocks of the Negev desert, Israel. Ukr. Botan. J. 2004, 61, 7–20. [Google Scholar]
- Dadheech, P.K.; Abed, R.M.M.; Mahmoud, H.; Mohan, M.K.; Krienitz, L. Polyphasic characterization of cyanobacteria isolated from desert crusts, and the description of Desertifilum tharense gen. et sp. nov. (Oscillatoriales). Phycologia 2012, 51, 260–270. [Google Scholar] [CrossRef]
- Mazor, G.; Kidron, G.J.; Vonshak, A.; Abeliovich, A. The role of cyanobacterial exopolysaccharides in structuring desert microbial crusts. FEMS Microbiol. Ecol. 1996, 21, 121–130. [Google Scholar] [CrossRef]
- Warren, S.D.; Clair, L.L.S.; Stark, L.R.; Lewis, L.A.; Pombubpa, N.; Kurbessoian, T.; Stajich, J.E.; Aanderud, Z.T. Reproduction and dispersal of biological soil crust organisms. Front. Ecol. Evol. 2019, 7, 344. [Google Scholar] [CrossRef]
- Yeager, C.M.; Kornosky, J.L.; Housman, D.C.; Grote, E.E.; Belnap, J.; Kuske, C.R. Diazotrophic community structure and function in two successional stages of biological soil crusts from the Colorado Plateau and Chihuahuan Desert. Appl. Environ. Microbiol. 2004, 70, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Siegesmund, M.A.; Johansen, J.R.; Karsten, U.; Friedl, T. Coleofasciculus gen. nov. (Cyanobacteria): Morphological and molecular criteria for revision of the genus Microcoleus Gomont. J. Phycol. 2008, 44, 1572–1585. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Kaštánek, P.; Adhikary, S.P. Diversity of cyanobacteria in biological crusts on arid soils in the Eastern region of India and their molecular phylogeny. Curr. Sci. 2016, 110, 1999–2004. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Richer, R.; Cox, P.A.; Codd, G.A. Cyanotoxins in desert environments may present a risk to human health. Sci. Total Environ. 2012, 421–422, 118–123. [Google Scholar] [CrossRef]
- Cox, P.A.; Richer, R.; Metcalf, J.S.; Banack, S.A.; Codd, G.A.; Bradley, W.G. Cyanobacteria and BMAA exposure from desert dust—a possible link to sporadic ALS among Gulf War veterans. Amyotroph. Lateral Scler. 2009, 10, 109–117. [Google Scholar] [CrossRef]
- Chatziefthimiou, A.D.; Richer, R.; Rowles, H.; Powell, J.T.; Metcalf, J.S. Cyanotoxins as a potential cause of dog poisonings in desert environments. Vet. Rec. 2014, 174, 484–485. [Google Scholar] [CrossRef]
- Carson, J.L.; Brown, R.M., Jr. Studies of hawaiian freshwater and soil algae II. Algal colonization and succession on a dated volcanic substrate. J. Phycol. 1978, 14, 171–178. [Google Scholar] [CrossRef]
- Fermani, P.; Mataloni, G.; Van de Vijver, B. Soil microalgal communities on an antarctic active volcano (Deception Island, South Shetlands). Polar Biol. 2007, 30, 1381–1393. [Google Scholar] [CrossRef]
- Ibekwe, A.M.; Kennedy, A.C.; Halvorson, J.J.; Yang, C.H. Characterization of developing microbial communities in Mount St. Helens pyroclastic substrate. Soil Biol. Biochem. 2007, 39, 2496–2507. [Google Scholar] [CrossRef]
- Gomez-Alvarez, V.; King, G.M.; Nüsslein, K. Comparative bacterial in recent Hawaiian volcanic deposits of different ages. FEMS Microbiol. Ecol. 2007, 60, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.C.; Clarke, K.J. The spatial distribution of microalgae in Antarctic fellfield soils. Antarct. Sci. 1991, 3, 257–263. [Google Scholar] [CrossRef]
- Cowan, D.A.; Ah Tow, L. Endangered Antarctic environments. Ann. Rev. Microbiol. 2004, 58, 649–690. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.A.; Reuckert, A.; Cowan, D.A.; Cary, S.C. Sources of edaphic cyanobacterial diversity in the Dry Valleys of Eastern Antarctica. ISME J. 2008, 2, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Pointing, S.B.; Chan, Y.; Lacap, D.C.; Lau, M.C.Y.; Jurgens, J.A.; Farrel, R.L. Highly specialized microbial diversity in hyper-arid polar desert. Proc. Natl. Acad. Sci. USA 2009, 106, 19964–19969. [Google Scholar] [CrossRef] [PubMed]
- Chrapusta, E.; Węgrzyn, M.; Zabaglo, K.; Kaminski, A.; Adamski, M.; Wietrzyk, P.; Bialczyk, J. Microcystins and anatoxin-a in Arctic biocrust cyanobacterial communities. Toxicon 2015, 101, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Kleinteich, J.; Wood, S.A.; Puddick, J.; Schleheck, D.; Küppers, F.C.; Dietrich, D.R. Potent toxins in Arctic environments: Presence of saxitoxins and an unusual microcystin variant in Arctic freshwater ecosystems. Chem. Biol. Interact. 2013, 206, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Rippin, M.; Lange, S.; Sausen, N.; Becker, B. Biodiversity of biological soil crusts from the Polar Regions revealed by metabarcoding. FEMS Microbiol. Ecol. 2018, 94, fiy036. [Google Scholar] [CrossRef]
- Major, K.M.; Kirkwood, A.E.; Major, C.S.; McCreadie, J.W.; Henley, W.J. In situ studies of algal biomass in relation to physicochemical characteristics of the Salt Plains National Wildlife Refuge, Oklahoma, USA. Saline Syst. 2005, 1, 11. [Google Scholar] [CrossRef][Green Version]
- Kirkwood, A.E.; Henley, W.J. Algal community dynamics and halotolerance in a terrestrial, hypersaline environment. J. Phycol. 2006, 42, 537–547. [Google Scholar] [CrossRef]
- Vinogradova, O.M.; Darienko, T.M. Terrestrial algae of hypersaline environments of the Central Syvash islands (Kherson Region, Ukraine). Biologia 2008, 63, 813–823. [Google Scholar] [CrossRef]
- Kirkwood, A.E.; Buchheim, J.A.; Buchheim, M.A.; Henley, W.J. Cyanobacterial diversity and halotolerance in a variable hypersaline environment. Microb. Ecol. 2008, 55, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Stolz, J.F. Distribution of phototrophic microbes in the flat, laminated microbial mat at Laguna Figueroa, Baja California, Mexico. BioSystems 1990, 23, 345–357. [Google Scholar] [CrossRef]
- Demergasso, C.; Chong, G. Microbial mats from the Llamará salt flat, northern Chile. Rev. Chil. Hist. Nat. 2003, 76, 485–499. [Google Scholar]
- Sørensen, K.B.; Canfield, D.E.; Teske, A.P.; Oren, A. Community composition of a hypersaline endoevaporitic microbial mat. Appl. Environ. Microbiol. 2005, 71, 7352–7365. [Google Scholar] [CrossRef]
- Siegel, B.Z. Life in the calcium chloride environment of the Don Juan Pond, Antarctica. Nature 1979, 280, 828–829. [Google Scholar] [CrossRef]
- Siegel, B.Z.; Siegel, S.M.; Spetel, T.; Waber, J.; Stoecker, R. Brine organisms and the question of habitat-specific adaptation. Orig. Life Evol. Biosph. 1984, 14, 757–770. [Google Scholar] [CrossRef]
- Sharma, N.K.; Rai, A.K.; Singh, S.; Brown, R.M. Airborne algae: Their present status and relevance. J. Phycol. 2007, 43, 615–627. [Google Scholar] [CrossRef]
- Chu, W.-L.; Tneh, S.-Y.; Ambu, S. A survey of airborne algae and cyanobacteria within the indoor environment of an office building in Kuala Lumpur, Malaysia. Grana 2013, 52, 207–220. [Google Scholar] [CrossRef]
- Griffin, D.W. Atmospheric movement of microorganisms in clouds of desert dust and implications for human health. Clin. Microbiol. Rev. 2007, 20, 459–477. [Google Scholar] [CrossRef]
- Schlichting, H.E., Jr. The importance of airborne algae and protozoa. Air Pollut. Cont. Assoc. J. 1969, 19, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.K.; Rai, A.K.; Singh, S. Metereological factors affecting the diversity of airborne algae in an urban atmosphere. Ecography 2006, 29, 766–772. [Google Scholar] [CrossRef]
- Lewandowska, A.U.; Śliwińska-Wilczewska, S.; Wozniczka, D. Identification of cyanobacteria and microalgae in aerosols of various sizes in the air over the southern Baltic Sea. Mar. Pollut. Bull. 2017, 125, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Woodcock, A.H. Note concerning human respiratory irritation associated with high concentration of plankton and mass mortality of marine organisms. J. Mar. Res. 1948, 7, 56. [Google Scholar]
- Stetzenbach, L.D. Introduction to aerobiology. In Manual of Environmental Microbiology, 2nd ed.; Hurst, C.J., Crawford, R.L., Knudsen, G.R., McInerney, M.J., Stetzenbach, L.D., Eds.; ASM Press: Washington, DC, USA, 2002. [Google Scholar]
- Vareli, K.; Zarali, E.; Zacharioudakis, G.S.; Vagenas, G.; Varelis, V.; Pilidis, G.; Briasoulis, E.; Ioannis, S. Microcystin producing cyanobacterial communities in Amvrakikos Gulf (Mediterranean Sea, NW Greece) and toxin accumulation in mussels (Mytilus galloprovincialis). Harmful Algae 2012, 15, 109–118. [Google Scholar] [CrossRef]
- Wiśniewska, K.A.; Śliwińska-Wilczewska, S.; Lewandowska, A.U. The first characterization of airborne cyanobacteria and microalgae in the Adriatic Sea region. PLoS ONE 2020, 15, e0238808. [Google Scholar] [CrossRef]
- Ehrenberg, G.G. Bericht über die zur Bekanntmachung geeigneten Verhandlungen der Konigl. Ber. Acad. Wiss. 1844, 9, 194–197. [Google Scholar]
- Kristiansen, J. 16. Dispersal of freshwater algae—A review. Hydrobiologia 1996, 336, 151–157. [Google Scholar] [CrossRef]
- Sharma, N.K.; Rai, A.K. Allergenicity of airborne cyanobacteria Phormidium fragile and Nostoc muscorum. Ecotoxicol. Environ. Safe 2008, 69, 158–162. [Google Scholar] [CrossRef]
- Chrisostomou, A.; Moustaka-Gouni, M.; Sgardelis, S.; Lanaras, T. Air-dispersed phytoplankton in a Mediterranean riverreservoir system (Aliakmon-Polyphytos, Greece). J. Plankton Res. 2009, 31, 877–884. [Google Scholar] [CrossRef]
- Schlichting, H.E., Jr. Meteorological conditions affecting the dispersal of airborne algae and protozoa. Lloydia 1964, 27, 64–78. [Google Scholar]
- Pierce, R.H.; Henry, M.S.; Blum, P.C.; Lyons, J.; Cheng, Y.S.; Yazzie, D.; Zhou, Y. Brevetoxin concentrations in marine aerosol: Human exposure levels during a Karenia brevis harmful algal bloom. Bull. Environ. Contam. Toxicol. 2003, 70, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, B.; Fleming, L.E.; Squicciarini, D.; Backer, L.C.; Clark, R.; Abraham, W.; Benson, J.; Cheng, Y.S.; Johnson, D.; Pierce, R.; et al. Literature review of Florida red tide: Implications for human health effects. Harmful Algae 2004, 3, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, B.; Fleming, L.E.; Backer, L.C.; Bean, J.A.; Tamer, R.; Kirkpatrick, G.; Kane, T.; Wanner, A.; Dalpra, D.; Reich, A.; et al. Environmental exposures to Florida red tides: Effects on emergency room respiratory diagnoses admissions. Harmful Algae 2006, 5, 526–533. [Google Scholar] [CrossRef]
- Cheng, Y.S.; Villareal, T.A.; Zhou, Y.; Gao, J.; Pierce, R.H.; Wetzel, D.; Naar, J.; Baden, D.G. Characterization of red tide aerosol on the Texas coast. Harmful Algae 2005, 4, 87–94. [Google Scholar] [CrossRef]
- Flewelling, L.J.; Narr, J.P.; Abbott, J.P.; Baden, G.D.; Barros, N.B.; Bossart, G.D.; Bottei, M.-Y.D.; Hammond, D.G.; Haubold, E.M.; Heil, C.A.; et al. Red tides and marine mammal mortalities. Nature 2005, 435, 755–756. [Google Scholar] [CrossRef]
- Kirkpatrick, B.; Pierce, R.; Cheng, Y.S.; Henry, M.S.; Blum, P.; Osborn, S.; Nierenberg, K.; Pederson, B.A.; Fleming, L.E.; Reich, A.; et al. Inland transport of aerosolized Florida red tide toxins. Harmful Algae 2010, 9, 186–189. [Google Scholar] [CrossRef]
- Kirkpatrick, B.; Kohler, K.; Byrne, M.M.; Studts, J. Florida red tide knowledge and risk perception: Is there a need for tailored messaging? Harmful Algae 2014, 32, 27–32. [Google Scholar] [CrossRef]
- Ciminiello, P.; Dell’Aversano, C.; Fattorusso, E.; Forino, M. Palytoxins: A still haunting Hawaiian curse. Phytochem. Rev. 2010, 9, 491–500. [Google Scholar] [CrossRef]
- Deeds, J.R.; Schwartz, M.D. Human risk associated with palytoxin exposure. Toxicon 2010, 56, 150–162. [Google Scholar] [CrossRef]
- Turner, P.C.; Gammie, A.J.; Hollinrake, K.; Codd, G.A. Pneumonia associated with contact with cyanobacteria. Br. Med. J. 1990, 300, 1440–1441. [Google Scholar] [CrossRef] [PubMed]
- Backer, L.C.; Carmichael, W.; Kirkpatrick, B.; Williams, C.; Irvin, M.; Zhou, Y.; Johnson, T.B.; Nierenberg, K.; Hill, V.R.; Kieszak, S.M.; et al. Recreational exposure to low concentrations of microcystins during an algal bloom in a small lake. Mar. Drugs 2008, 6, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Backer, L.C.; McNeel, S.V.; Barber, T.; Kirkpatrick, B.; Williams, C.; Irvin, M.; Zhou, Y.; Johnson, T.B.; Nierenberg, K.; Aubel, M.; et al. Recreational exposure to microcystins during algal blooms in two California lakes. Toxicon 2010, 55, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Caller, T.A.; Doolin, J.W.; Haney, J.F.; Murby, A.J.; West, K.G.; Farrar, H.E.; Ball, A.; Harris, B.T.; Stommel, E.W. A cluster of amyotrophic lateral sclerosis in New Hampshire: A possible role for toxic cyanobacteria blooms. Amyotroph. Lateral Scler. 2009, 10, 101–108. [Google Scholar] [CrossRef]
- Creasia, D.A. Acute inhalation toxicity of microcystin-LR with mice. Toxicon 1990, 28, 605. [Google Scholar]
- Fitzgeorge, R.B.; Clark, S.A.; Keevil, C.W. Routes of intoxication. In Detection Methods for Cyanobacterial Toxins, Proceedings of the First International Symposium on Detection Methods for cyanobacterial (Blue-Green Algal) Toxins, Bath, UK, 27–29 September 1993; Codd, G.A., Jeffries, T.M., Keevil, C.W., Potter, E., Eds.; Royal Society of Chemistry: Cambridge, UK, 1994; pp. 69–74. [Google Scholar]
- Benson, J.M.; Hutt, J.A.; Rein, K.; Boggs, S.E.; Barr, E.B.; Fleming, L.E. The toxicity of microcystin-LR in mice following 7 days of inhalation exposure. Toxicon 2005, 45, 691–698. [Google Scholar] [CrossRef]
- Wood, S.A.; Dietrich, D.R. Quantitative assessment of aerosolized cyanobacterial toxins at two New Zealand lakes. J. Environ. Monit. 2011, 13, 1617–1624. [Google Scholar] [CrossRef]
- Tiberg, E.; Bergmann, B.; Wictorin, B.; Willen, T. Occurrence of microalgae in indoor and outdoor environment environments in Sweden. In Nordic Aerobiology, Proceedings of the Fifth Nordic Symposium on Aerobiology, Abisko, Sweden, 24–26 August 1983; Nilsson, S., Raj, B., Eds.; Almqvist and Wiksell International: Stockholm, Sweden, 1984; pp. 24–29. [Google Scholar]
- Bernstein, I.L.; Safferman, R.S. Viable algae in house dust. Nature 1970, 227, 851–852. [Google Scholar] [CrossRef]
- Mittal, A.; Agarwal, M.K.; Shivpuri, D.N. Studies on allergenic algae of Delhi area: Botanical aspects. Ann. Allergy 1979, 42, 248–252. [Google Scholar]
- Ng, E.H.P.; Chu, W.L.; Ambu, S. Occurrence of airborne algae within the township of Bukit Jalil in Kuala Lumpur, Malaysia. Grana 2011, 50, 217–227. [Google Scholar] [CrossRef]
- World Health Organisation (WHO). Indoor Air Pollution, Exposure and Health Effects Assessment; Euro-Report and Studies 78, Working Group Report; WHO Regional Office for Europe: Copenhagen, Denmark, 1983. [Google Scholar]
- Schlichting, H.E., Jr. Periodicity and seasonality of airborne algae and protozoa. In Phenology and Seasonality Modelling; Leith, H., Ed.; Springer: Berlin, Germany, 1974; pp. 407–413. [Google Scholar]
- Draganov, S. Taxonomic structure of cave algal flora. In Proceedings of the 7th International Speleological Congress, Sheffield, UK, September 1977; pp. 155–156. [Google Scholar]
- Couté, A.; Chauveau, O. Algae. In Encyclopaedia Biospeologica. Vol. 1. Soc. Biospeleol. Moulis; Juberthie, C., Decu, V., Eds.; Société de Biospéologie: Bucharest, Romania, 1994; pp. 371–380. [Google Scholar]
- Uzunov, B. Speleophyton. In Speleological Studies of Caves in Godech Municipality, Part 1; Toshkova, V., Tachev, I., Eds.; Association of Speleoclubs in Sofia: Sofia, Bulgaria, 2016; pp. 19–22. [Google Scholar]
- Mulec, J. Phototrophs in Caves. In Cave Ecology. Ecological Studies (Analysis and Synthesis); Moldovan, O., Kováč, Ľ., Halse, S., Eds.; Springer: Cham, Switzerland, 2018; Volume 235, pp. 91–106. [Google Scholar] [CrossRef]
- Vinogradova, O.N.; Mikhailyuk, T.I. Algal flora of the caves and grottoes of the National Nature Park “Podilsky Tovtry” (Ukraine). Int. J. Algae 2009, 11, 289–304. [Google Scholar] [CrossRef]
- Falasco, E.; Ector, L.; Isaia, M.; Wetzel, C.E.; Hoffmann, L.; Bona, F. Diatom flora in subterranean ecosystems: A review. Int. J. Speleol. 2014, 43, 231–251. [Google Scholar] [CrossRef]
- Smith, T.; Olson, R. A taxonomic survey of lamp flora (algae and cyanobacteria) in electrically lit passages within Mammoth Cave National Park, Kentucky. Int. J. Speleol. 2007, 36, 105–114. [Google Scholar] [CrossRef]
- Stoyneva, M.P.; Ganeva, A.S.; Valchanova, M.P. Mass algal and moss development in the humid urbanized cave “Ledenika” (North-western Bulgaria). Ann. Univ. Sofia 2002, 90, 39–42. [Google Scholar]
- Toplin, J.A.; Norris, T.B.; Lehr, C.R.; McDermott, T.R.; Castenholz, R.W. Biogeographic and phylogenetic diversity of thermoacidophilic Cyanidiales in Yellowstone National Park, Japan, and New Zealand. Appl. Environ. Microbiol. 2008, 74, 2822–2833. [Google Scholar] [CrossRef]
- Moser, M.; Weisse, T. Combined stress effect of pH and temperature narrows the niche width of flagellates in acid mining lakes. J. Plankton Res. 2011, 33, 1023–1032. [Google Scholar] [CrossRef]
- Wheeler, W. The commercial uses of peat. Nature 1901, 63, 590–591. [Google Scholar] [CrossRef]
- Amri, S.; Branes, Z.; Oudra, B. Inventaire des cyanobacteries potentiellement toxiques dans la tourbiere du lac noir «Parc National D’el-Kala» (Algerie). Rev. Microbiol. Ind. San Environn. 2010, 4, 49–68. [Google Scholar]
- Stoyneva, M.; Valchanova, M. Pilot studies on annual alteration of various dominant life strategists in the phytoplankton of the peat-bog Tschokljovo (South-western Bulgaria). Ann. Univ. Sofia 1997, 89, 23–33. [Google Scholar]
- Oren, A. Halophilic Microorganisms and Their Environments; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002. [Google Scholar]
- Bauld, J. Occurrence of benthic microbial mats in saline lakes. Hydrobiologia 1981, 81, 87–111. [Google Scholar] [CrossRef]
- Oren, A. Life at high salt concentrations. In Subsurface Microbiology and Biogeochemistry; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.-H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2000; pp. 263–282. Available online: http://link.Springer-ny.com/link/service/books/10125/ (accessed on 22 April 2021).
- Michev, T.; Stoyneva, M. (Eds.) Inventory of Bulgarian Wetlands and Their Biodiversity; Elsi-M: Sofia, Bulgaria, 2007. [Google Scholar]
- Descy, J.-P.; Stoyneva-Gärtner, M.P.; Uzunov, B.A.; Dimitrova, P.H.; Pavlova, V.T.; Gärtner, G. Studies on cyanoprokaryotes of the water bodies along the Bulgarian Black Sea Coast (1890–2017): A review, with special reference to new, rare and harmful taxa. Acta Zool. Bulgar. Suppl. 2018, 11, 43–52. [Google Scholar]
- Peckol, P.; Putnam, A.B. Differential toxic effects of Ulva lactuca (Chlorophyta) on the herbivorous gastropods, Littorina littorea and L. obtusata (Mollusca). J. Phycol. 2017, 53, 361–367. [Google Scholar] [CrossRef]
- Grant, W.D.; Mwatha, W.E.; Jones, B.E. Alkaliphiles, ecology, diversity and applications. FEMS Microbiol. Rev. 1990, 75, 255–270. [Google Scholar] [CrossRef]
- Krienitz, L.; Ballot, A.; Kotut, K.; Wiegand, C.; Pütz, S.; Metcalf, J.S.; Codd, G.A.; Pflugmacher, S. Contribution of hot spring cyanobacteria to the mysterious deaths of Lesser Flamingos at Lake Bogoria, Kenya. FEMS Microbiol. Ecol. 2003, 43, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Krienitz, L.; Ballot, A.; Casper, P.; Kotut, K.; Wiegand, C.; Pflugmacher, S. Cyanobacteria in hot springs of East Africa and their potential toxicity. Algol. Stud. 2005, 117, 297–306. [Google Scholar] [CrossRef]
- Ballot, A.; Pflugmacher, S.; Wiegand, C.; Kotut, K.; Krienitz, L. Cyanobacterialtoxins in Lake Baringo, Kenya. Limnologica 2003, 33, 2–9. [Google Scholar] [CrossRef]
- Ballot, A.; Krienitz, L.; Kotut, K.; Wiegand, C.; Metcalf, J.S.; Codd, G.A.; Pflugmacher, S. Cyanobacteria and cyanobacterial toxins in three alkaline lakes of Kenya—Lakes Bogoria, Nakuru and Elmenteita. J. Plankt. Res. 2004, 26, 925–935. [Google Scholar] [CrossRef]
- Ballot, A.; Pflugmacher, S.; Wiegand, C.; Kotut, K.; Krienitz, L. Cyanobacteria and cyanobacterial toxins in the alkaline crater lakes Sonachi and Simbi. Harmful Algae 2005, 4, 139–150. [Google Scholar] [CrossRef]
- Kotut, K.; Ballot, A.; Krienitz, L. Toxic cyanobacteria and their toxins in standing waters of Kenya: Implications for water resource use. J. Water Health 2006, 4, 233–245. [Google Scholar] [CrossRef]
- Lugomela, C.; Pratap, H.B.; Mgaya, Y.D. Cyanobacteria blooms—A possiblecause of mass mortality of lesser flamingos in Lake Manyara and Lake BigMomela, Tanzania. Harmful Algae 2006, 5, 534–541. [Google Scholar] [CrossRef]
- Anderson, G.C. Some limnological features of a shallow saline meromictic lake. Limnol. Oceanogr. 1958, 3, 250–270. [Google Scholar] [CrossRef]
- AlgaeBase. Available online: http://www.algaebase.org/ (accessed on 26 March 2021).
- Rejmánková, E.; Komárek, J.; Komárková, J. Cyanobacteria—A neglected component of biodiversity: Patterns of species diversity of inland marshes of northern Belize (Central America). Divers. Distrib. 2004, 10, 189–199. [Google Scholar] [CrossRef]
- Komárek, J.; Komárková–Legnerová, J. Taxonomic evaluation of cyanobacterial microflora from alkaline marshes of northern Belize. 1. Phenotypic diversity of coccoid morphotypes. Nova Hedwig. 2007, 84, 65–111. [Google Scholar] [CrossRef]
- Turicchia, S.; Ventura, S.; Komárková, J.; Komárek, J. Taxonomic evaluation of cyanobacterial microflora from alkaline marshes of northern Belize. 2. Diversity of oscillatorialean genera. Nova Hedwig. 2009, 89, 165–200. [Google Scholar] [CrossRef]
- Komárek, J.; Sirová, D.; Komárková, J.; Rejmánková, E. Structure and function of cyanobacterial mats in wetlands of belize. In Microbiology of the Everglades Ecosystem; Entry, J.A., Gottlieb, A.D., Jayachandran, K., Ogram, A., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 194–217. [Google Scholar]
- Komárek, J.; Komárková, J.; Ventura, S.; Kozlíková-Zapomělová, E.; Rejmánková, E. Taxonomic evaluation of cyanobacterial microflora from alkaline marshes of northern Belize. 3. Diversity of heterocytous genera. Nova Hedwig. 2017, 105, 445–486. [Google Scholar] [CrossRef]
- Mareš, J. Anabaena fuscovaginata (Nostocales), a new cyanobacterial species from periphyton of the freshwater alkaline marsh of Everglades, South Florida, USA. Fottea 2010, 10, 235–243. [Google Scholar] [CrossRef]
- Schiller, J. Die Mikroflora der Roten Tümpel auf den koralleninseln Los Aves in karibischen Meer. Ergeb. Dtsch. Limnol. Venez. Exped. 1956, 1, 197–216. [Google Scholar]
- Akcaalan, R.; Mazur-Marzec, H.; Zalewska, A.; Albay, M. Phenotypic and toxicological characterization of toxic Nodularia spumigena from a freshwater lake in Turkey. Harmful Algae 2009, 8, 273–278. [Google Scholar] [CrossRef]
- McGregor, G.B.; Sendall, B.C. Iningainema Pulvinus gen. nov., sp. nov. (Cyanobacteria, Scytonemataceae) a new nodularin producer from Edgbaston Reserve, north-eastern Australia. Harmful Algae 2017, 62, 10–19. [Google Scholar] [CrossRef]
- Papke, R.T.; Ramsing, N.B.; Bateson, M.M.; Ward, D.M. Geographical isolation in hot spring cyanobacteria. Environ. Microbiol. 2003, 5, 650–659. [Google Scholar] [CrossRef]
- McGregor, G.B.; Rasmussen, J.P. Cyanobacterial composition of microbial mats from an Australian thermal spring: A polyphasic evaluation. FEMS Microbiol. Ecol. 2008, 63, 23–35. [Google Scholar] [CrossRef]
- Roeselers, G.; Norris, T.B.; Castenholz, R.W.; Rysgaard, S.; Glud, R.N.; Kühl, M.; Muyzer, G. Diversity of phototrophic bacteria in microbial mats from Arctic hot springs (Greenland). Environ. Microbiol. 2007, 9, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Sompong, U.; Hawkins, P.R.; Besley, C.; Peerapornpisal, Y. The distribution of cyanobacteria across physical and chemical gradients in hot springs in northern Thailand. FEMS Microbiol. Ecol. 2005, 52, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Van Gemerden, H. Microbial mats: A joint venture. Mar. Geol. 1993, 113, 3–25. [Google Scholar] [CrossRef]
- Stoyneva, M.P. Survey on green algae of Bulgarian thermal springs. Biol. Bratisl. 2003, 58, 563–574. [Google Scholar]
- Stoyneva, M.P.; Gärtner, G. Taxonomic and ecological notes to the list of green algal species from Bulgarian thermomineral waters. Ber. Nat. Med. Ver. Innsbr. 2004, 91, 67–89. [Google Scholar]
- Sala, S.E.; Sar, E.A.; Ferrario, M.E. Review of materials reported as containing Amphora coffeaeformis (Agardh) Kützing in Argentina. Diatom Res. 1998, 13, 323–336. [Google Scholar] [CrossRef]
- Dhar, B.C.; Cimarelli, L.; Singh, K.S.; Brandi, L.; Brandi, A.; Puccinelli, C.; Marcheggiani, S.; Spurio, R. Molecular detection of a potentially toxic diatom species. Int. J. Environ. Res. Public Health 2015, 12, 4921–4941. [Google Scholar] [CrossRef]
- Stal, L.J. Physiological ecology of cyanobacteria in microbial mats and other communities. New Phytol. 1995, 131, 1–32. [Google Scholar] [CrossRef]
- Ward, D.M.; Ferris, M.J.; Nold, S.C.; Bateson, M.M. A natural view of microbial biodiversity within hot spring cyanobacterial mat communities. Microbiol. Mol. Biol. Rev. 1998, 62, 1353–1370. [Google Scholar] [CrossRef]
- Stoyneva-Gärtner, M.P.; Uzunov, B.A.; Gärtner, G. Checklist of algae from Bulgarian thermal waters. Annu. Sofia Univ. Kliment Ohridski Fac. Biol. Book 2 Bot. 2018, 102, 49–73. [Google Scholar]
- Kastovsky, J.; Johansen, J.R. Mastigocladus laminosus (Stigonematales, Cyanobacteria): Phylogenetic relationship of strains from thermal springs to soil-inhabiting genera of the order and taxonomic implications for the genus. Phycologia 2008, 47, 307–320. [Google Scholar] [CrossRef]
- Park, A.; Moore, R.E.; Patterson, G.M.L. Fischerindole L, a new isonitrile from the terrestrial blue-green alga Fischerella muscicola. Tetrahedron Lett. 1992, 33, 3257–3260. [Google Scholar] [CrossRef]
- Carmichael, W.W. The Cyanotoxins. Adv. Bot. Res. 1997, 27, 211–240. [Google Scholar] [CrossRef]
- Fiore, M.F.; Genuário, D.B.; Souza, C.; da Silva, P.; Shishido, T.K.; Moraes, L.A.B.; Neto, R.C.; Silva-Stenico, M.E. Microcystin production by a freshwater spring cyanobacterium of the genus Fischerella. Toxicon 2009, 53, 754–761. [Google Scholar] [CrossRef]
- Cirés, S.; Alvarez-Roa, C.; Wood, S.A.; Puddick, J.; Loza, V.; Heimann, K. First report of microcystin-producing Fischerella sp. (Stigonematales, Cyanobacteria) in tropical Australia. Toxicon 2014, 88, 62–66. [Google Scholar] [CrossRef]
- Cagide, E.; Becher, P.G.; Louzao, M.C.; Espiña, B.; Vieytes, M.R.; Jüttner, F.; Botana, L.M. Hapalindoles from the cyanobacterium fischerella: Potential sodium channel modulators. Chem. Res. Toxicol. 2014, 27, 1696–1706. [Google Scholar] [CrossRef]
- Pilotto, L.; Douglas, R.; Burch, M.; Cameron, S.; Beers, M.; Rouch, G.; Robinson, P.; Kirk, M.; Cowie, C.; Hardiman, S.; et al. Health effects of recreational exposure to cyanobacteria (blue-green algae) during recreational water-related activities. Aust. N. Z. J. Public Health 1997, 21, 562–566. [Google Scholar] [CrossRef]
- Beattiea, K.A.; Kaya, K.; Codd, G.A. The cyanobacterium Nodularia PCC 7804, of freshwater origin, produces [L-Har2]nodularin. Phytochemistry 2000, 54, 57–61. [Google Scholar] [CrossRef]
- Saito, K.; Konno, A.; Ishii, H.; Saito, H.; Nishida, F.; Abe, T.; Chen, C. Nodularin-Har: A new nodularin from Nodularia. J. Nat. Prod. 2001, 64, 139–141. [Google Scholar] [CrossRef]
- Moffitt, M.C.; Blackburn, S.I.; Neilan, B.A. rRNA sequences reflect the ecophysiology and define the toxic cyanobacteria of the genus Nodularia. Int. J. Syst. Evol. Microbiol. 2001, 51, 505–512. [Google Scholar] [CrossRef]
- Mohamed, Z.A. Toxic cyanobacteria and cyanotoxins in public hot springs in Saudi Arabia. Toxicon 2008, 51, 17–27. [Google Scholar] [CrossRef]
- Hindák, F. Thermal microorganisms from a hot spring on the coast of Lake Bogoria, Kenya. Nova Hedwig. Z. 2001, 123, 77–93. [Google Scholar]
- Moreira, C.; Martins, A.; Moreira, C.; Vasconcelos, V. Toxigenic cyanobacteria in volcanic lakes and hot springs of a North Atlantic island (S. Miguel, Azores, Portugal). Fresen. Environ. Bull. 2011, 20, 420–426. [Google Scholar]
- Stoyneva-Gärtner, M.P.; Uzunov, B.A. First application of a drone for studies of the biodiversity of Bulgarian extremophilic algae in the Marikostinovo thermal complex. Annu. Sofia Univ. Kliment Ohridski Fac. Biol. Book 2 Bot. 2019, 103, 5–37. [Google Scholar]
- Margesin, R. Psychrophilic microorganisms in alpine soils. In Plants in Alpine Regions; Lütz, C., Ed.; Springer: Vienna, Austria, 2012; pp. 187–198. [Google Scholar]
- Canganella, F.; Wiegel, J. Extremophiles: From abyssal to terrestrial ecosystems and possibly beyond. Naturwissenschaften 2011, 98, 253–279. [Google Scholar] [CrossRef] [PubMed]
- Leya, T.; Rahn, A.; Lütz, C.; Remias, D. Response of arctic snow and permafrost algae to high light and nitrogen stress by changes in pigment composition and applied aspects for biotechnology. FEMS Microbiol. Ecol. 2009, 67, 432–443. [Google Scholar] [CrossRef]
- Remias, D. Cell structure and physiology of alpine snow and ice algae. In Plants in Alpine Regions: Cell Physiology of Adaptation and Survival Strategies; Lütz, C., Ed.; Springer: Wien, Austria, 2012; pp. 175–185. [Google Scholar]
- Andersen, D.T.; Sumner, D.Y.; Hawes, I.; Webster-Brown, J.; McKay, C.P. Discovery of large conical stromatolites in Lake Untersee, Antarctica. Geobiology 2011, 9, 280–293. [Google Scholar] [CrossRef]
- Hodson, A.J.; Mumford, P.N.; Kohler, J.; Wynn, P.M. The high Arctic glacial ecosystem: New insights from nutrient budgets. Biogeochemistry 2005, 72, 233–256. [Google Scholar] [CrossRef]
- Anesio, A.M.; Hodson, A.J.; Fritz, A.; Psenner, R.; Sattler, B. High microbial activity on glaciers: Importance to the global carbon cycle. Glob. Chang. Biol. 2009, 15, 955–960. [Google Scholar] [CrossRef]
- Sattler, B.; Storrie-Lombardi, M.C.; Foreman, C.M.; Tilg, M.; Psenner, R. Laser-induced fluorescence emission (LIFE) from Lake Fryxell (Antarctica) cryoconites. Ann. Glaciol. 2010, 51, 145–152. [Google Scholar] [CrossRef][Green Version]
- Säwström, C.; Mumford, P.; Marshall, W.; Hodson, A.; Laybourn-Parry, J. The microbial communities and primary productivity of cryoconite holes in an Arctic glacier (Svalbard 79 N). Polar Biol. 2002, 25, 591–596. [Google Scholar] [CrossRef]
- Sehnal, L. Cryoconite holes on frozen lakes as source of interesting extremophilic and extremotolerant organisms. Czech Polar Rep. 2015, 5, 200–209. [Google Scholar] [CrossRef]
- Howard-Williams, C.; Pridmore, R.; Broady, P.; Vincent, W. Environmental and biological variability in the McMurdo Ice Shelf ecosystem. In Antarctic Ecosystems Ecological Change and Conservation; Kerry, K., Hempel, G., Eds.; Springer: Berlin, Germany, 1990; pp. 23–31. [Google Scholar]
- Jungblut, A.-D.; Hawes, I.; Mountfort, D.; Hitzfeld, B.; Dietrich, D.R.; Burns, B.P.; Neilan, B.A. Diversity within cyanobacterial mat communities in variable salinity meltwater ponds of McMurdo Ice Shelf, Antarctica. Environ. Microbiol. 2005, 7, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Jungblut, A.-D.; Hoeger, S.J.; Mountfort, D.; Hitzfeld, B.C.; Dietrich, D.R.; Neilan, B.A. Characterization of microcystin production in an Antarctic cyanobacterial mat community. Toxicon 2006, 47, 271–278. [Google Scholar] [CrossRef]
- Kleinteich, J.; Wood, S.A.; Küppers, F.C.; Camacho, A.; Quesada, A.; Frickey, T.; Dietrich, D.R. Temperature-related changes in polar cyanobacterial mat diversity and toxin production. Nat. Clim. Chang. 2012, 2, 356–360. [Google Scholar] [CrossRef]
- Jungblut, A.D.; Wilbraham, J.; Banack, S.A.; Metcalf, J.S.; Codd, G.A. Microcystins, BMAA and BMAA isomers in 100-year-old Antarctic cyanobacterial mats collected during Captain R.F. Scott’s Discovery Expedition. Eur. J. Phycol. 2018, 53, 115–121. [Google Scholar] [CrossRef]
- Zaki, S.; Mericana, F.; Muangmai, N.; Conveyc, P.; Broady, P. Discovery of microcystin-producing Anagnostidinema pseudacutissimum from cryopreserved Antarctic yanobacterial mats. Harmful Algae 2020, 93, 101800. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Beattie, K.A.; Purdie, E.L.; Bryant, J.A.; Irvine, L.M.; Codd, G.A. Analysis of microcystins and microcystin genes in 60–170-year-old dried herbarium specimens of cyanobacteria. Harmful Algae 2012, 15, 47–52. [Google Scholar] [CrossRef]
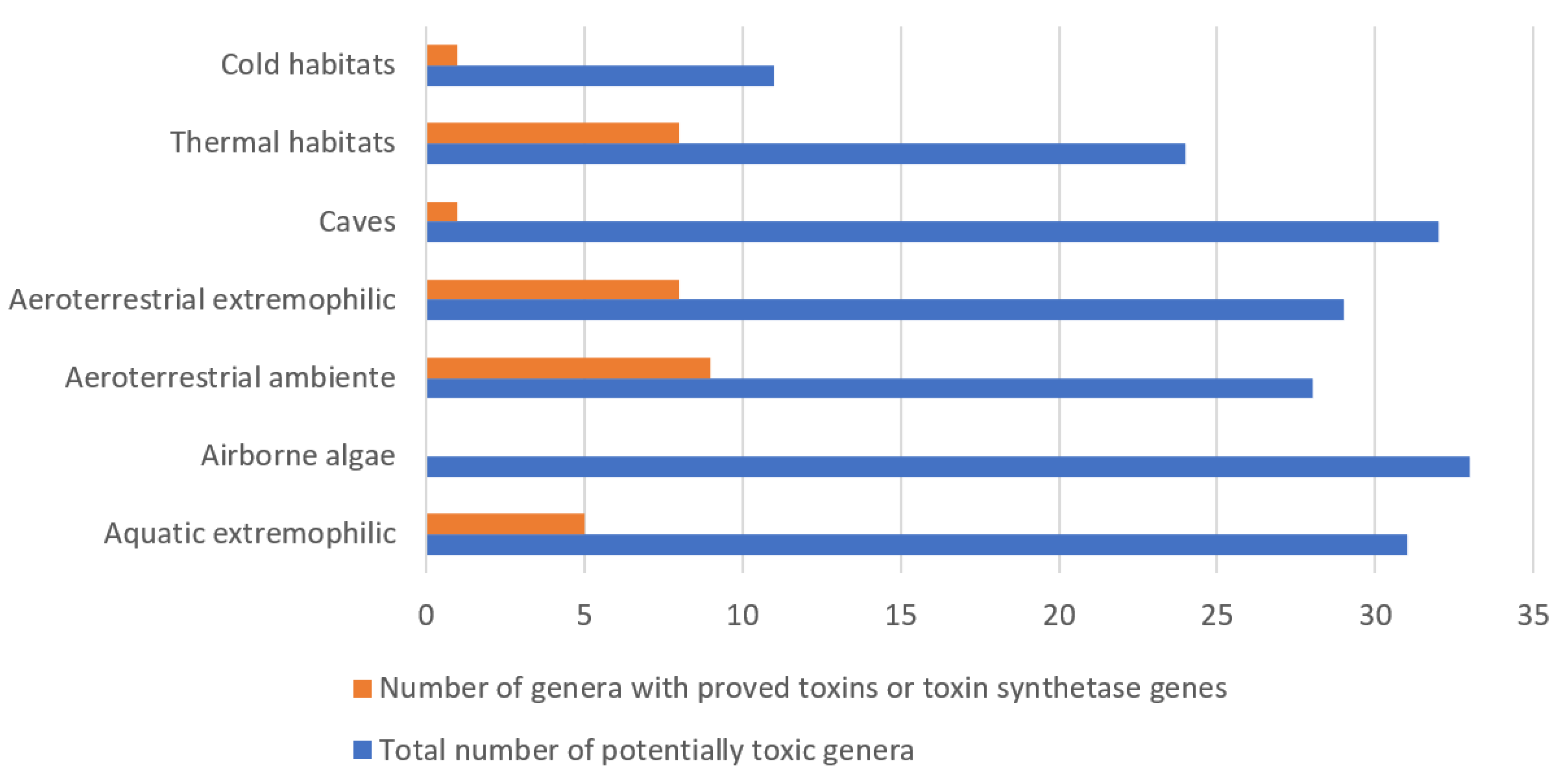
| Algal Phylum/Genus | Toxin/Toxins and TOCs | Aquatic Ambiente Envm | Aquatic Extreme Envm | Airborne | Aeroterr. Ambiente Envm | Aeroterr. Extreme Envm | Thermal Envm | Cold Envm | Caves | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T/G | OC | T/G | OC | T/G | OC | T/G | OC | T/G | OC | T/G | OC | T/G | OC | T/G | OC | ||
| Cyanopro- Karyota | |||||||||||||||||
| Anabaena s.l. | MC, CYL; BMAA, spumigin J, Laxaphysins A-E; Geo | x | x | x | x | x | x | x | x | X (?) | |||||||
| Anabaenopsis | MC | x | x | x | x | ||||||||||||
| Anagnostidinema | MC | x | x | ||||||||||||||
| Anacystis | MC (?) | x | x | x | x | X (?) | |||||||||||
| Aphanocapsa | MC-LR, NOD; MIB | x | x | x | x | x | x | x | |||||||||
| Aphanothece | nostocyclamide, NOD | x | x | x | x | x | x | x | x | x | |||||||
| Calothrix | BMAA, LPS; Geo | x | x | x | x | x | x | ||||||||||
| Chlorogloeopsis | BMAA | x | x | x | |||||||||||||
| Chroococcidiopsis | BMAA | x | x | x | x | ||||||||||||
| Chroococcus | MC (?) | x | x (?) | x | x | x | x (?) | x | x | x | x | ||||||
| Coleofasciculus | MC, BMAA, DAB, AEG, GTX | x | x | x (?) | x | x | |||||||||||
| Cyanobium | MC (mcyA gene), CYN (pks gene) | x | x | x | x | x | x | ||||||||||
| Cyanospira | MC | x | x | x | |||||||||||||
| Cylindrospermum | Geo | x | x | x | x | x | x | ||||||||||
| Fischerella | MCs (MC-LR, MC-LA, MCLF, MC-FR and demethyl-MC-LR), BMAA, fisherindole L, ambiguine isonitriles A–F, hapalindoles (G, H, L), fungicidal hapalindoletype alkaloids, ambigol A, B and indole alkaloid tjipanazole D, fischerellin A, LPS; Geo | x | x | x | x | x | x | x | x | x | |||||||
| Geitlerinema | MCs, SXTs; mitsoamide | x | x | x | x | x | x | ||||||||||
| Gloeocapsa | MC | x | x | x | x | x | x | x | |||||||||
| Gloeotrichia | MC-LF, MC-RR | x | x | x | |||||||||||||
| Gomphosphaeria | MCs | x | x | x | x | ||||||||||||
| Hapalosiphon | MCs (MC-LA), hapalosin | x | x | x | x | x | x | x | x | ||||||||
| Iningainema | NOD | x | x | ||||||||||||||
| Komvophoron | MCs, CYN (?) | x | x | x | x | ||||||||||||
| Leptolyngbya | MCs; Geo | x | x | x | x | x | x | x | |||||||||
| Limnospira | MC-YR; ATX | x | x | x | x | ||||||||||||
| Lyngbya | SXTs, CYN, deoxy-CYN, BMAA, LAs (A-C), AT, DAT | x | x | x | x | x | x | x | ? | ||||||||
| Merismopedia | MCs, NOD | x | x | x | x | ||||||||||||
| Microchaete | A90720A (protease inhibitor) | x | x | x | x | x | |||||||||||
| Microcoleus | MC (mcyA, mcyE genes), ANAs, BMAA (?), CYN (pks genes), LPS; GEO | x | x | x (?) | x | x (?) | x | x | x | x | x (?) | ||||||
| Microcystis | MCs, ATXs, BMAA, MV-J, kawaguchipeptin-B | x | x | x | x | x (?) | x (?) | ||||||||||
| Nodularia | NOD, [L-Har2] NOD, BMAA, spumigins | x | x | x | x | x | x | x | |||||||||
| Nostoc | MCs, NOD, NOD-R (desmethylNOD-R), ATX, SXT, BMAA, Aer-865, Aps, banyasides, CPs, Cr, CV-N, MVs, Ncps, Nos, Ns, nostocyclamide, Nd A, nostophycin, nostoweipeptins (W1-W7), nostoginins, nostopeptins, insulapeptolides, nostocyclins, microginins, muscaride A, Psp A-F; Geo | x | x | x | x | x | x | x | x | x | x | x | |||||
| Oscillatoria | MCs (-LR, -RR), ATX, HTX, CYN, 7-epi-CYN, DAT; Geo | x | x | x | x | x | x | x (?) | x | x | x | x | x | ||||
| Phormidium | MCs, ATX, HTX, BMAA | x | x | x | x | x (?) | x | x | x | x | |||||||
| Planktotohrix | MCs, ATXs, BMAA, oscillatorin (oscyllamide Y), oscillapeptin | x | x | ||||||||||||||
| Plectonema | MCs, BMAA | x | x | x | x | x | x | x | |||||||||
| Pseudanabaena | MC-LR, MC (mcyA gene), CYN (pks gene) | x | x | x | x | x | x | x | x | x (?) | |||||||
| Pseudocapsa | MC-RR, MC-YR | x | x | ||||||||||||||
| Schizothrix | DAT, LPS, schizothrin A | x | x | x | x | x | x | x | x | x | x | ||||||
| Scytonema | MC-LY, SXT, BMAA | x | x | x | x | x | x | x | |||||||||
| Snowella | MCs | x | x | x | |||||||||||||
| Spirulina | MCs, ATXs | x | x | x | x | ||||||||||||
| Symploca | BMAA | x | x | x | x | x | x | x | |||||||||
| Synechocystis | MC (incl. MC-LR), BMAA, LPS | x | x | x | x | x | x | ||||||||||
| Synechococcus | MC, microcin-C like, NOD, BMAA, LPS, Thionsulfolipid | x | x | x | x | x | x | x | x | ||||||||
| Tolypothrix | Tb | x | x | x | x | ||||||||||||
| Westiellopsis | MC, westiellamide | x | x | x | x | x | x (?) | ||||||||||
| Woronichinia | MCs | x | x | x | x | ||||||||||||
| Pyrrhophyta | |||||||||||||||||
| Gymnodinium | SXT, endotoxin; ichyotoxin | x | x | x | |||||||||||||
| Karenia | PbTx, gymnocin (GC) | x | x | x | |||||||||||||
| Ostreopsis | PLTXs (osteozin D, ovatoxin A, ostreotoxin-1 and 3, mascarenotoxin A and B) | x | x | x | |||||||||||||
| Peridinium s.l. | ichtyotoxins incl. alkaloid similar to 12-methoxyibogamine | x | x | x | x | ||||||||||||
| Prorocentrum | OA, DPX, prorocentrolides (PLC), borbotoxin | x | x | x | |||||||||||||
| Chlorophyta | |||||||||||||||||
| Ulva | hemolysins | x | x | x | |||||||||||||
| Ochrophyta | |||||||||||||||||
| Amphora | DA | x | x | x | x | x | x | ||||||||||
| Ochromonas | ichthyotoxic, hemolytic, and antispasmodic activities | x | x | x | x | ||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gӓrtner, G.; Stoyneva-Gӓrtner, M.; Uzunov, B. Algal Toxic Compounds and Their Aeroterrestrial, Airborne and other Extremophilic Producers with Attention to Soil and Plant Contamination: A Review. Toxins 2021, 13, 322. https://doi.org/10.3390/toxins13050322
Gӓrtner G, Stoyneva-Gӓrtner M, Uzunov B. Algal Toxic Compounds and Their Aeroterrestrial, Airborne and other Extremophilic Producers with Attention to Soil and Plant Contamination: A Review. Toxins. 2021; 13(5):322. https://doi.org/10.3390/toxins13050322
Chicago/Turabian StyleGӓrtner, Georg, Maya Stoyneva-Gӓrtner, and Blagoy Uzunov. 2021. "Algal Toxic Compounds and Their Aeroterrestrial, Airborne and other Extremophilic Producers with Attention to Soil and Plant Contamination: A Review" Toxins 13, no. 5: 322. https://doi.org/10.3390/toxins13050322
APA StyleGӓrtner, G., Stoyneva-Gӓrtner, M., & Uzunov, B. (2021). Algal Toxic Compounds and Their Aeroterrestrial, Airborne and other Extremophilic Producers with Attention to Soil and Plant Contamination: A Review. Toxins, 13(5), 322. https://doi.org/10.3390/toxins13050322






