Definition
Porcelain enamel is an inorganic-type coating, which is applied to metals or glass for both decorative and functional purposes. This coating is a silica-based solidified glass mass obtained by high-temperature firing (temperature can range between 450 and 1200 °C depending on the substrate). Porcelain enamel coatings differ from ceramic coatings mainly by their glass structure and dilatation coefficient, and from organic paints mainly by the inorganic nature of the matrix and the chemical bond that exists between the coating and the substrate.
1. History
Porcelain enamel is one of the most popular industrial coatings for the protection of metal artifacts, as it gives the coated substrates high-quality physical, chemical, and aesthetical properties. This coating was developed in ancient times to decorate precious objects as if to imitate the shining of precious stones. Only with the advent of the First Industrial Revolution, in the 18th century, enamel began to arise interest as a low-cost coating for many functional applications; this way, technical enameling was rapidly developed. Porcelain enamel is a material with ancient origins, but it is still appreciated for high-duty applications where good corrosion protection, chemical, and external agents’ resistance is required. In addition to that, enamel can efficiently protect the covered substrate, maintaining its aesthetical properties unchanged in time.
1.1. Proto-Enamel
The first people to make extensive use of what can be considered the true precursor of enamel are the Egyptians. Many archaeological findings of amulets and jewels testify the incredible ability of Egyptians in creating beautiful “faience” objects [1]. The Egyptian faience is a no-clay and quartz-based ceramic displaying surface vitrification with colors varying from white to blue and green. All the faience-made objects were created by cast molding: the powdered quartz was mixed with alkali, calcite lime, and water; then, the object underwent a heating treatment that allowed the formation of an inner siliceous body covered by a vitreous surface layer of soda-lime [2,3]. This way, Egyptians were able to create objects and jewels with a shining appearance and beautiful colors. From Egypt, this art was transmitted to the people of the Eastern Mediterranean Sea thanks to the close commercial interactions that existed at that time.
1.2. Origins of Enamel
The cradle of enameling can almost certainly be identified in the Mediterranean Sea. The first real enameled objects have been found in a Mycenaean tomb in Cyprus and date back to the 13th century BC: the most remarkable examples of these archaeological findings are represented by the famous Kouklia gold rings and the Kourion golden royal scepter, both dating back to the Cyprus Golden Age period [4]. All the objects created in this historical period are decorated with colorful enamels arranged in small metallic cells, and they can be considered as the first examples of “cloisonné” enameling [4,5].
1.3. The Cloisonné and Champlevé Enameling Techniques
The art of enameling rapidly spread in the Eastern world, where the cloisonné technique was the only enameling method used. Other important cloisonné archaeological findings can be attributed to the Assyrian Empire around 650 BC, a period in which Assyria controlled territories, as Cyprus, where enameling was already developed [6]. From that moment onwards, the cloisonné enameling spread towards east, and toward the western Mediterranean Sea. The Scythians, an Iranian warrior people, brought the art of enameling to the Caucasian area and to Siberia as well [7]. On the other side, the diffusion of enameling toward Europe was favored by the presence of the Phoenicians, who established close commercial relationships with colonies in Spain and Magna Grecia. The enameling of metals spread in Europe only around 500 BC, as some Etruscan jewel findings can testify [8]. The diffusion of the cloisonné technique in Europe suffered a rapid decline with the success of the barbarian invasions, but it was reborn in Byzantium around the 600 AC for the decoration of icons [4]. Here, the cloisonné technique reached its maximum splendor, thanks also to the great experience of goldsmiths, who used to work with small metal artifacts and successfully applied this skill to the production of enameled artifacts. A remarakable example of the cloisonné technique is represented by the golden enameled altarpiece in the Basilica of San Marco [9]. The cloisonné technique was the most important enameling method until the end of 13th century, and its diffusion gave rise to several enameling schools in Spain, Italy, France, and Germany.
Differently to the cloisonné method, the champlevé technique consists in carving the metallic substrate, applying the enamel powder in the obtained carves, and then firing the whole object to obtain a smooth and glossy surface. This technique has been known since the 3rd century BC by the Celts, who used it to decorate small bronze objects by enameling [10,11]. The Romans played an important role in the diffusion of this technique, but the champlevé method flourished only at the end of the 11th century in Conques (France), where it was used for enameling of copper. In the following years, new artistic schools were born all over Europe, in Cologne (Germany), Silos (Spain), and Liege (Belgium), but the artistic school of Limoges, which flourished around 1130, was the only one that survived over centuries [12].
1.4. From the Renaissance Onwards
From the 13th century onwards, Limoges became the most important center for artistic enameling of all Europe. Enameled objects started to be produced as luxurious decorations objects, and they definitively lost the religious character that had been characterizing the enameled production over the centuries [13]. There, in Limoges, new techniques flourished around the 14th century: the “enamel-paint” and the “grisaille” techniques [14]. In the first case, matte enamels were used as if were colors on a canvas, whereas in the latter case, the enameler only used black and white enamels, firstly preparing a black background and then applying several layers of white enamel to create a wide range of gray shades and a relief effect. During the 15th and 16th century, the production in Limoges reached high-quality standards, and it was renowned all over Europe. In the next centuries, the production of enameled objects started to be relegated to clocks, ornaments, and small decoration objects only, thus decreeing the definitive decay of artistic enameling. Despite this, enameling would soon be reborn under a new form, to become commonly used in the everyday life of Western families. Many enameled decorative objects from different centuries and countries are conserved at the ARtCHIVIO Museum in Ponte San Pietro (BG), Italy, where the C.K.I Association (Creativ—Kreis—International) preserves and spreads the culture of artistic enameling. Figure 1 shows some examples of cloisonné and champlevé artworks.

Figure 1.
Examples of decorative enameled objects preserved and exhibited at the ARtCHIVIO museum in Ponte San Pietro (BG), Italy: (a) cloisonné enamel on gold icon in byzantine style, end of 20th century; (b) Champlevé enamel on bronze two-pieces statue, end of 19th century; (c) Enamel peint representing “Psyche’s toilet” by Jules Sarlandie, end of 19th century. Image courtesy of ARtCHIVIO museum, Ponte San Pietro (BG), Italy.
1.5. The Era of Technical Enameling
With the advent of the First Industrial Revolution, enamels started to be applied to substrates as iron and cast iron. The development of industrial enameling was so closely linked to the advances in metallurgy and chemistry of the late 18th century that the enameling industry was attracting the best chemists of the time. Although it is known that in the second half of the 1700s some industries were patenting the first enameling processes on steel sheets, it was only in 1851 that the first manual on technical enameling was published. At that time, iron sheets were obtained by the hammering of cast iron to produce the first enameled plates. Around 1870, the almost total enamel production was limited to cast iron hollow ware [15], but in the following years, it was possible to produce high-quality cast iron pans, which were white enameled both inside and outside [16].
In the second half of the 19th century, enameling faced different technical problems, such as the lack of pure raw materials and the development of new production methods for steel, but on the other side, many advancements were achieved, such as the discovery of new production methods for pigments. Probably, one of the most important discoveries in this field was represented, using clay to keep the powdered enamel in suspension in water: this way allows applying the enamel simply by painting, spraying, or the immersion method. This way, it was possible to produce more durable enamels at lower costs. Around the year 1900, Mr. John C. Reed introduced the machine molding of bath tubes, which boosted the sanitary enameling industry [17]. In the same years, the introduction of antimony compounds as opacifiers in dry coat enamels is considered an important achievement [17]. The enamel industry boomed some years after World War I, in the USA, and the manufacturing of refrigerators, stoves, sanitary ware, and household objects grew very rapidly, but it suddenly stopped with the advent of World War II, when enameling plants were converted to the treatment of war materials. In 1942, the development of titanium-based white enamels gave a great boost to the rebirth of the enameling industry, and new products, such as chimney pipes, dishwashers, cooking hobs, and water heaters started to be enameled [18]. In the following decades, the enamel industry continued to evolve, also thanks to the development of new deposition techniques, which made it possible to obtain better quality products in an increasingly efficient way. Nowadays, enamel is commonly applied to many everyday use objects, but it is also used for the covering of panels for architectural applications.
2. Substrates for Enameling and Surface Pretreatments
The quality and the chemical composition of the metal substrate have an important influence on the parameters of the glazing process. In addition to that, the properties of the metal determine the use of one or another deposition technique. For this reason, enamelers should know very well the main characteristics and properties of the used metal substrates.
2.1. Common Substrates for Enameling
From an industrial point of view, the most important substrates for enameling are cast iron, low-carbon steel, and aluminium alloys. Stainless steel is also suitable for enameling, while copper, silver, and gold are only enameled for artistic purposes. Despite this, it is important to remark that enamel coatings can be applied also on glass substrates and on high-temperature alloys [19,20]. Cast iron, steel, and sheet iron mainly differ for their content in carbon. Enameling steels commonly contain less than 0.20 wt % of carbon, as a higher percentage could lead to blistering phenomena [21]. Cast irons usually contain from 3.25 to 3.60 wt % of carbon.
2.1.1. Cast Iron for Enameling
Cast iron is a ferrous alloy with a carbon content higher than 2.06%. Its physical and mechanical properties depend on both its chemical composition and microstructure. The typical chemical elements present in a cast iron suitable for enameling are carbon, silicon, phosphorus, manganese, and sulfur. The metallographic structure of a cast iron is commonly constituted by graphite, ferrite, cementite, perlite, manganese sulfide and steatite (iron phosphide). The combined and uncombined carbon percentages play an important role in determining the suitability of cast iron substrates for enameling. In addition to that, the most used cast iron for enameling is gray cast iron, with a perlitic matrix and a graphitic structure [22,23].
2.1.2. Steel for Enameling
The “steel” term refers to a vast range of materials with very different mechanical and chemical characteristics. Conventional enameling on cold-rolled steel was developed in the 1960s thanks to the invention of open coil decarburized steel by the Bethlehem Steel Corporation in 1956. Enameling on hot-rolled substrates was developed in parallel, but it was mainly used for enameling of water heaters. The use of hot-rolled steel tends to cause fish scaling of enamel (e.g., formation of blisters on the enameled surface caused by oversaturation of hydrogen at the metal–enamel interface) [24,25]. For this reason, hot-rolled steels are only used for special applications where given strength requirements need to be addressed effectively, but the porcelain enameling process is usually limited to one side of the sheet to promote the removal of hydrogen from the unenameled side.
As regards cold rolled steels, the EN 10209:2013 standard [26] constitutes an important guide for the choice of the right steel quality for enameling. The main differentiation among the different steel grades regards the drawing process. The DC01EK steel is only suitable for light drawing, the DC04EK grade is also suitable for medium drawing, whereas the DC06EK, DC06ED, and DC04ED are suitable for deep drawing as well. The DC01EK, DC04EK, and DC04ED types are Al-killed steels, whereas the DC06EK and DC06ED qualities are IF-type (interstitial free) steels, decarburized in steel plants.
2.1.3. Aluminum Alloys for Enameling
Aluminum is a rather recently discovered material, and compared to steel, it shows some peculiar characteristics, which make it extremely interesting from a technological point of view. In fact, aluminum does not form rust, but it requires the use of low-melting enamels [27]. Aluminum alloys can be mainly divided in two groups: the heat-treatable alloys and the non-heat-treatable alloys, but another important classification is made on the alligant elements. The most suitable aluminum alloys for enameling are the 3003 and 4006 series alloys, but in general, a low content of Mg is required to avoid adherence problems between the substrate and the enamel layer [28].
2.2. Surface Pretreatments
The preparation of the metal support for the enameling process is a fundamental step. It guarantees the perfect cleaning of the surface from rolling oils and other surface contaminants and it gives the surface an adequate roughness, thus promoting adherence between the metal and the enamel coating. The surface pretreatment could involve only or both chemical and mechanical methods. Among the mechanical methods, grit blasting and sand blasting are the most common. Grit blasting is used in the pretreatment of heavy gauge pieces both made of steel or cast iron, such as hot water tanks and chemical vessels. On the other side, blasting is not common for the pretreatment of sheet iron or aluminum alloys-based pieces, as it could deform the material itself. Chemical pretreatments are commonly used on steel and aluminum substrates, for both cleaning and complete treatment operations of the metal surface.
2.2.1. Pretreatment of Cast Iron Substrate
The preparation of cast iron surfaces is mainly carried out by blasting. Blasting helps to completely clean the surface of the casted objects from production contaminants. Its principal effect is to guarantee the formation of a homogeneous surface in terms of roughness and surface defects in order to ease the adhesion of the enamel layer to the substrate [23]. In addition to that, the blasting treatment opens the residual subsurface porosities, which could cause important defects to the enamel layer [29,30]. The blasting process is sometimes followed by an annealing treatment at 800–850 °C. This thermal treatment is commonly used to obtain good results also on cast iron types with a high content of cementite.
2.2.2. Pretreatment of Steel Substrate
The pretreatment of steel is much more complicated, as it consists of many different steps. The first step to be considered is the chemical cleaning of the surface, which is also named as the “degreasing” step. During this step, the object to be enameled is properly cleaned with aqueous solutions of alkaline detergents in order to remove oils and greases. This cleaning step can be done by immersion or the spraying method. In both cases, the operating temperature ranges from 40 to 70 °C, for 5–10 min [29]. A good degreasing solution should be able to act as a wetting agent with a good saponifying and emulsifying action. Typical components of industrial cleaner solutions (pH = 10–13) are mainly constituted by sodium silicates (they are good saponifiers), sodium carbonate (it has good buffering action), and hydroxides (as a source of alkalinity), although other components can be added in limited concentrations [31]. Nowadays, the chemical cleaning is sometimes replaced by the electrolytic cleaning [31,32]. This procedure takes advantage of the flow of current through the bath, while the ware to be cleaned is made the cathode. The alkali content of the bath is about 40 g/L, and the pH of the bath is kept around 13. This cleaning procedure is very fast with respect to the still cleaning method, especially if automatic equipment is used. The cleaning procedure is always followed by a rinsing step made in running water.
The next step is called “pickling”, and it consists in the elimination of metal oxides from the substrate surface by using an acid solution [31]. The most common pickling solution is made of sulfuric acid in 5–10 wt % concentration commonly used at 65–75 °C. Pickling can be done both by spraying or by immersion; in the case of immersion, typical times are 8 min, whereas in the latter case, they are increased up to 30 min [29]. There are also acid pickling solutions based on phosphoric acid. These solutions have important advantages if used for the treatment of products that must undergo direct enameling, as the phosphoric acid attacks the metal product in a controlled manner, leaving a very homogeneous surface at the end of the treatment. The pickling step must be followed by a deep rinsing in water and by immersion in a neutralizing bath. The neutralizing bath, a hot solution of Na2O in water, is used to completely remove all the traces of acid. The last step of the pretreatment process is the drying step of the substrate.
2.2.3. Pretreatment of Aluminum Substrate
The successful application of enamel on aluminum is influenced both by the chosen aluminium alloy and by the effectiveness of the pretreatment. As a first thing, the aluminium substrate should be properly cleaned from greases and oils; after that, it should be degreased in an alkaline degreaser solution (20–40 g/L at 50 °C), leached in an alkaline bath (10 wt % NaOH at 70 °C for up to 5 min) to remove the natural oxide film, de-smudged in acid water (25 wt.% nitric acid solution) to remove adherent hydroxides, rinsed several times, and in some case pre-fired at about 400 °C to reform a uniform oxide layer [27,29,33].
3. Production of Enamels
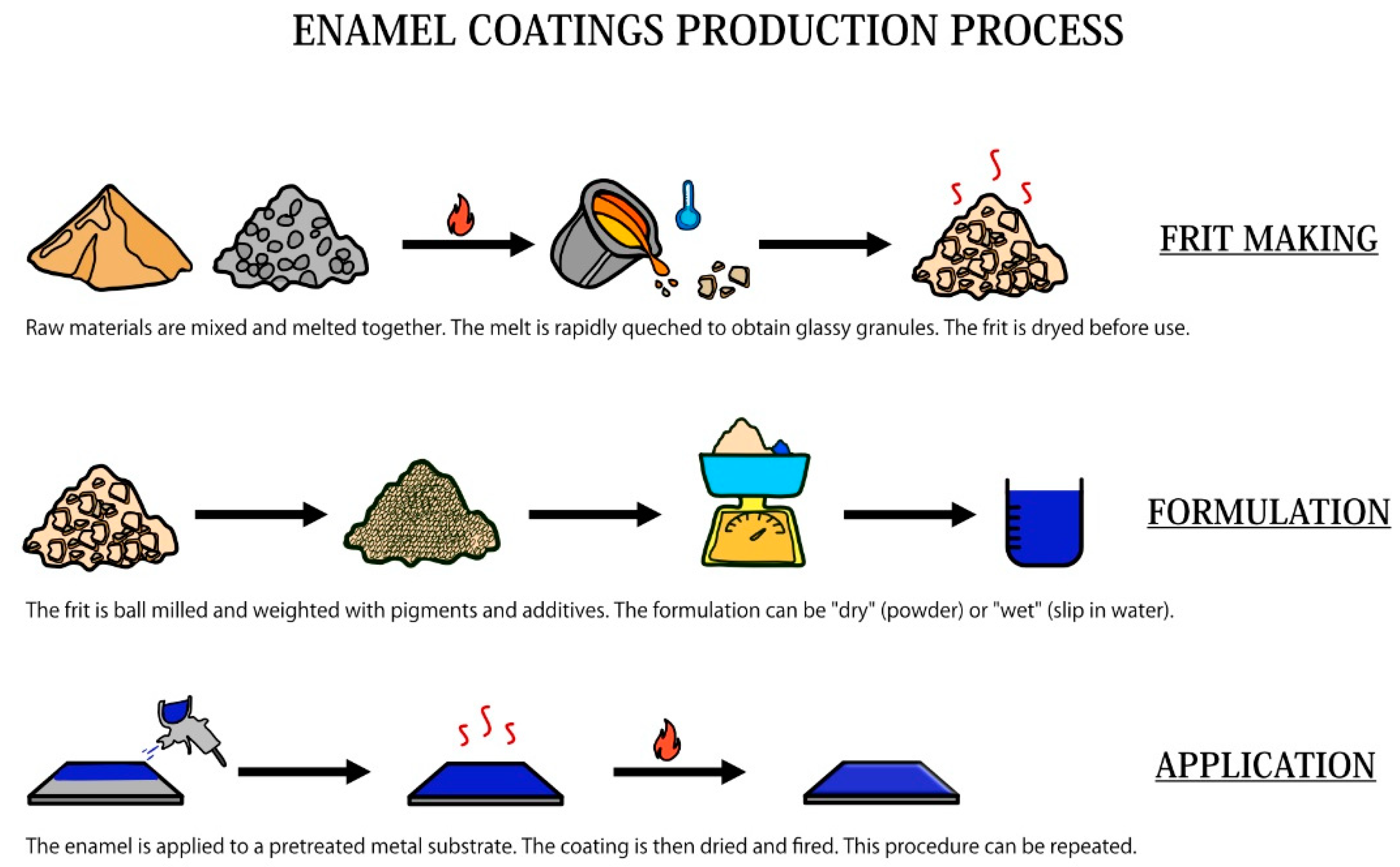
The production of enameled objects is an ancient tradition, and it can be somehow considered as form of science and art together. Figure 2 summarizes the main steps involved in the production of enamel coatings, starting from the production of the frit.
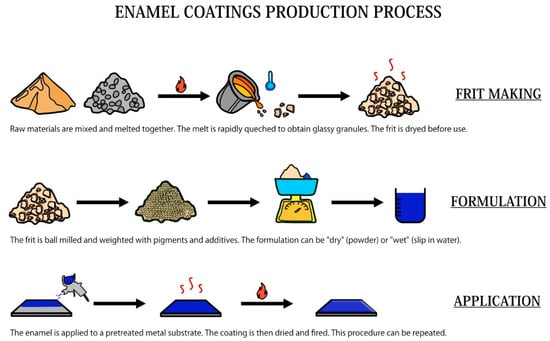
Figure 2.
Schematic representation of the enamel production process.
3.1. The Frit Making
The starting material of all enamels is the “frit”. The frit is the product of the process where simple oxides and salts are melted together at temperatures between 1000 and 1500 °C to form a mixture that is then cooled to obtain glassy granules or flakes, whose composition is specifically modified according to the chosen substrate and final application. The raw materials used in the frit making can be divided into four main groups: refractories, fluxes, opacifiers, and colors [29]. Refractories are acidic oxides that give body to the glassy enamel matrix, fluxes are alkaline oxides, which are mainly used to react with refractories to form the glass and to lower the melting temperature of the glass itself. Opacifiers, such as tin oxide and antimony compounds, are used to give the enamel their typical opaque appearance. Other important components of frits are adherence oxides. Table 1 shows some examples of common oxides forming the frits.

Table 1.
Common oxides used in frit making.
The starting materials to produce the frit are stored in silos, from which they are automatically taken and weighed. After that, these oxides are mixed and inserted into the furnace for the melting process. On leaving the oven, the frit can be cooled to obtain glassy flakes using rollers or small frit granules by rapid cooling. After cooling, the frit is appropriately dried before use.
3.2. Milling and Mill Additions
Before the formulation of enamel, the frit must be suitably grounded in ball or drum mills. There are mainly two different types of milling: the dry and wet methods [34]. In the first case, the grinding takes place in the absence of water, and the charge is usually composed only of the frit, any pigments, and additives for special applications. The main control that is carried out on the dry ground frit is exclusively a quick granulometric check. In the case of “wet” milling, the frit is mixed with water to obtain an aqueous suspension called “torbida” or “slip” [35]. The components that are added to the mill must ensure a perfect suspension of the frit particles in the liquid component. For this reason, it is essential to add floating agents and electrolytes, usually in a percentage that never exceeds 15% of the weight of the frit. The most common floating agent is clay, although bentonite, colloidal silica, and gums are used in some cases [29,35]. Electrolytes are important components of the slip as well, as they are soluble compounds (sodium aluminate, potassium carbonate) that are able to control the properties of the slip, as its consistency.
3.3. Application of Enamel on the Substrate
The proper application of enamels is extremely important, as the choice of the right application method has many consequences on the result. Enamels application techniques can be mainly divided into “dry” and “wet” methods, based on the type of milling previously performed. In addition to that, it is also important to distinguish between the application of ground and cover coat enamels. Ground enamels are formulated with the addition of cobalt oxides to promote adhesion with the substrate, whereas cover coat enamels are formulated to guarantee optimal aesthetical properties. Nowadays, the most common application sequence is addressed as “2C/2F” (two-coat, two-firing), but it is also possible to realize a “2C/1F” application [36]. The “2C/1F” application can be realized wet on wet, powder on powder, or powder on a wet dried layer. It is also possible to directly enamel the pieces to be covered, but it is necessary to effectively pretreat the decarburized steel substrate and use colored enamels (with a percentage of adherence oxides).
3.3.1. Enameling of Steel
Steel is currently the most widely used material in glazing, as it allows the widest choice of application methods. Table 2 summarizes the possible application methods for steel enameling.

Table 2.
Application techniques for enameling of steel.
Wet enameling can be carried out by immersion or by spraying. The immersion method is commonly used to apply ground enamels on big products, such as chimney pipes, washing machines baskets, and boilers, whereas the spraying method is used to apply cover coat layers as it guarantees good aesthetical features of the finished product.
The dipping method consists of immersing the product in a tank containing the enamel slip and extracting it with a controlled speed to freely drain away the enamel excess. The flow coating method uses a similar principle, but in this case, the enamel slip is poured from the above on the piece to be covered. This method is commonly used for the enameling of hollow tubular elements [29].
One of the most ancient application techniques for the enameling of steel is represented by the spraying technique. In this case, the slip is atomized in an air pressure gun, and it is applied to the piece to be covered. The evolution of the traditional spraying technique is represented by the electrostatic spraying method. In this case, the slurry particles are electrically charged by passing through a strong electric field and they are sprayed onto the substrate, which is grounded. In this way, the slip particles are able, following the electric field lines, to cover the whole object in a homogeneous way avoiding huge over sprayings. This application method, although very efficient, is influenced by many operating parameters, such as the spray distance and the application speed, which can have important implications on the result [37].
Among the dry enameling methods for steel, it is necessary to focus attention on the electrostatic dry powder method. The operating procedure is similar to that of electrostatic wet spraying, but in this case, enamel particles are encapsulated in organic compounds so that they can acquire an electric charge and be “sprayed” on the metal object. As a following step, the powder is deposited on the metal, and its electrical charge is passed to the metal. This application method hides many issues, which should be managed with extreme attention [38]. The most important parameters to be controlled are the humidity in the application chamber, which influences the adhesion of the powder onto the substrate (it modifies the resistivity of the enamel particles), and the granulometry of the enamel powder. This method is the most widespread worldwide, but it implies important investments and thus a big production to pay back costs.
Among the most innovative application methods, it is possible to mention the electrophoretic deposition technique. Similar to wet electrostatic deposition and electrostatic powder deposition, this method takes advantage of an electric field. This technology is based on the migration of negatively charged enamel particles toward the positively charged product to be coated. This migration occurs in the slip when it is subjected to an electric field [39]. This system is still not very widespread today, but it has some interesting advantages over other wet applications, such as controlled thicknesses, compact and automated production systems, and good edge coverage.
3.3.2. Enamelling of Cast Iron and Aluminum
Cast iron is usually enameled by wet spraying, but with respect to steel, the thickness of the enamel layer could be up to 400 microns to counteract the negative effect of the substrate roughness. As it regards aluminium alloys, they are usually covered by wet application methods, in particular wet spraying and flow coating.
3.4. Drying and Firing
The enamel, as it has been extensively described, can be applied by dry or wet methods. In the second case, the coating must necessarily be dried before the subsequent firing phase to remove the application vehicle. Water is the most common used vehicle, and it is removed by the drying process. The formation of a dried enamel layer, called “biscuit”, is of extreme importance; otherwise, trapped water could suddenly evaporate during the firing process, causing bubbling and enamel detachment and cracking [35].
The firing process is the last step involved in the production of enameled objects. All the furnaces are commonly powered by electricity or gaseous fuels; in the first case, operating costs are higher, but there is a quick response to temperature adjustments and less contamination of the enameled pieces. Regardless of the power supply, almost all industrial ovens are equipped with a conveyor.
The firing temperature mainly depends on the substrate type. The firing temperature of a steel product can vary between 750 and 890 °C, for times varying between 2 and 8 min, depending on the thickness of the sheet [29]. Cast iron pieces are usually fired at temperatures about 730–770 °C for times over 40 min. On the other hand, aluminum is fired at temperatures always below 600 °C, due to the low melting temperature of the substrate; this last operating condition represents a challenge in the development of low-melting glazes with high chemical and abrasion resistance.
4. Properties of Enamel Coatings and Common Applications
The glassy nature of the enamel matrix and the strong chemical/mechanical bond existing between the enamel layer and the substrate are the main reasons for the excellent thermal, chemical, and mechanical properties of enameled materials. Considering the cross-section of an enameled metal, we would observe a coating with a complex internal structure. The presence of a close porosity is a typical feature of all enamel coatings [29], as it is due to gas evolution during the firing treatment. The distribution and size of the bubbles can be tailored changing the rheological properties of the slip, modifying the chemical composition of the frit, or even adjusting the firing parameters. This feature has no effects on the corrosion resistance of the enamel coating, as the metal substrate and the corrosive environment are not in direct contact. Enamel coatings show excellent engineering properties, as they are limitedly affected by exposure to UV radiation and chemical agents, and they show good protection of the covered substrate against corrosion [40,41,42]. Certainly, enamels offer enormous advantages in terms of resistance over time compared to organic coatings, but at the same time, they entail some negative aspects that limit their application in some application fields. Enamel coatings are relatively hard but, in some cases, they show limited resistance to abrasion and mechanical shock, as the brittle nature of the matrix leads to the formation and propagation of cracks and to a consequent loss of protection properties.
4.1. Thermal Properties
Enamel coatings can easily withstand high temperatures, contact with direct flames, and thermal shock conditions, mainly thanks to the vitreous nature of their matrix. All enamels are almost resistant to temperatures up to 400 °C, and this is the main reason why they have been used for a long time to produce pans and kitchenware. The resistance to high temperatures and thermal shock are interesting properties that make enamel coatings also suitable to produce stoves. Another important aspect to consider is the fireproof property of enamels [43]. In case of fire, enamels do not release toxic gases in the environment and limit the propagation of fire; thus, enameled wall panels offer significant advantages in terms of fire safety when used for tunnel and galleries cladding in wall panels of cruise ships and metro stations.
4.2. Physical Properties
Enamel coatings represent an important solution to protect metal substrates, but one of the reasons why they have always aroused great interest is their ability to be both protective and aesthetically pleasing coatings, which are almost precious in appearance. The surface of enamels is very smooth and glossy, although it is also possible to produce matte coatings with the addition of opacifying agents. They can be colored in a great variety of shades thanks to the addition of inorganic pigments, which are protected by the glassy matrix over the years. Thus, time, aggressive atmospheres, and UV radiation have a negligible impact on the variation of enamel’s aesthetical properties [44,45].
The enamel surface is also free of open porosities; thus, the growth of bacteria and fungus is somehow hindered, and the accumulation of dirt is limited. In addition to that, enameled materials can withstand the frequent use of disinfectants, thus being an excellent solution for the cladding of surgery wards and similar applications.
The microstructure of enamel coatings is also important in determining the protective properties toward the covered substrates [46,47]. The strong adherence between the ceramic–glass layer and the metal, together with the inert nature of the matrix itself, play a fundamental role in protecting the covered metals against corrosion. Contrary to what occurs with organic paints, the damage process caused by corrosion processes does not advance in time and it is limited to the exposed metal.
4.3. Chemical Properties
Enamels commonly show a high resistance to chemical agents, but their degree of resistance largely depends on the application purpose for which they are formulated. In general, it is possible to state that enamels are resistant to most solvents, acid, and neutral solutions, while they are easily attacked by solutions containing fluorides and strong alkaline boiling solutions (pH > 12) [34]. Their resistance to solvents makes them suitable to be used in environments where cleaning with aggressive detergents is carried out frequently, such as in road signs and architectural panels in stations and subways.
4.4. Mechanical Properties
Enamel coatings show a high hardness (up to 450 HV) due to the glassy nature of the matrix, and for this reason, they can withstand the deterioration caused by mechanical actions. Their hardness can be greatly improved by the addition of additives [48] or hard particles [35,49]. The glassy nature of enamels plays both a positive and a negative role, as it is the main cause of the low resistance to mechanical shock. The formation of cracks after mechanical actions could be very detrimental for the conservation of the coating protection properties, as it could lead to the direct contact between the metal substrate and the aggressive environment.
Despite their low mechanical properties, enamel coatings are able to withstand a certain amount of bending without the formation of cracks or detachment of enamel flakes, as the strong chemical bonds at the interface between the coating and the metal guarantee the system to be unbreakable.
4.5. Common Application of Enamel Coatings
The reader should now be able to understand the potential application of enamel coatings. Figure 3 shows some examples of modern enameled samples.

Figure 3.
Modern examples of enamel application objects: (a) Enameled pyrolytic oven of a modern wood stove, image courtesy of Artecalore, Pergine Valsugana (TN), Italy; (b) Enameled cookware set, image courtesy TVS, Fermignano (PU), Italy; (c) Enameled bathroom washbasin, image courtesy of Kaldewei Italia, Conegliano (TV), Italy.
One of the most common application of enamels is kitchenware, but it is important to remark that these coatings are also suitable for industrial high-duty applications. The international standards are quite strict about the requirements that enameled objects must satisfy, but the testing conditions differ a lot from one application to another. Vitreous enameled cookers such as burner caps, hobs, and pan supports should resist to temperatures up to 400 °C and to thermal shocks up to 380 °C; kitchen sinks are tested to be resistant to boiling acids and detergents; small household appliances such as barbecues, hot plates, toasters, and enameled steel hollow ware, such as frying pan and pots, kettles, and saucepans should resist to high temperatures and not be attacked by acids and water vapours. The resistance to detergent solutions and boiling water is an important requirement that washing machines and sanitary ware must fulfil. Enameled pieces for space heating (water heaters and heat exchangers) are commonly tested for resistance to thermal shock, water vapours, and chemicals, but their quality is also checked by searching defects by high-voltage techniques. Enamel coatings can be also used for the production of architectural panels, advertising boards, and safety appliances: in these cases, good resistance to scratch, abrasion, and weathering is required, and a good stability of colors is desirable. From an industrial point of view, enameled pieces find many applications in the production of heat exchangers, industrial tanks, and chemical vessels, where good resistance to hot alkali solutions is needed.
5. Conclusions
Enameled materials, given their excellent characteristics of durability and resistance to corrosion and external agents, are the perfect candidates for applications where excellent technological characteristics must be coupled with long service life. Some successful examples of enamel coatings are outdoor architectural panels, applications where extreme hygiene, resistance to fire, or resistance to chemical products is required. Given the infinite possibilities of color and finishing, enamels are perfect coatings for design applications as well. Despite these positive characteristics, the application of enamels is somehow limited by their brittleness, low impact resistance, and a higher production cost with respect to organic coatings. Surely enamels have the potential to be applied on a large scale and guarantee excellent properties to the covered substrates, as long as academia and industry will bet on their growth and success.
Author Contributions
Conceptualization, S.R. and F.R.; Resources, S.R. and A.M.C.; Writing—original draft preparation, F.R.; Writing—review and editing, S.R. and A.M.C.; Supervision, S.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We would like to acknowledge Artecalore, Pergine Valsugana, TN, Italy; Kaldewei Italia, Conegliano, TV, Italy; TVS, Fermignano, PU, Itay, for having provided images that represent modern applications of enamel coatings. In addition to that, we would like to thank ARtCHIVIO, Museo dello Smalto, Ponte San Pietro (BG), Italy for having provided images of ancient enameled objects and C.K.I. Italia for useful discussion about the history of enamel.
Conflicts of Interest
The authors declare no conflict of interest.
Entry Link on the Encyclopedia Platform
References
- Kiefer, K.C.; Allibert, A. Pharonic blue ceramics: The process of self-glazing. Archaeology 1971, 24, 107–117. [Google Scholar]
- Carolyn, R.; Jennifer, M.; Jonathan, T. Egyptian faience inlay techniques: A process for obtaining detail and clarity by refiring. MRS Online Proc. Libr. 2001, 712, 107. [Google Scholar]
- Tite, M.S.; Bimson, M. Faience: An investigation of the microstructures associated with the different methods of glazing. Archaeometry 1986, 28, 69–78. [Google Scholar] [CrossRef]
- Tait, H. Enamelwork–History. Encyclopedia Britannica, 07/03/2016. Available online: https://www.britannica.com/art/enamelwork (accessed on 12 March 2021).
- The history of enamelling technique. Design 1957, 58, 158–162.
- Rogers, W.H. On the history of enamelling. J. Br. Archaeol. Assoc. 1848, 3, 280–296. [Google Scholar] [CrossRef]
- Curtis, J.E.; Kruszynski, M. Ancient caucasian and related material in the British Museum. In British Museum Occasional Paper 121; The British Museum: London, UK, 2002. [Google Scholar]
- Von Bothmer, D.; Picón, C.A.; Mertens, J.R.; Milleker, E.J.; Herrmann, A. Recent acquisitions: A selection 1994–1995. Bull. Metrop. Mus. Art 1995, 53, 11. [Google Scholar]
- Buckton, D.; Osborne, J. The enamel of Doge Ordelaffo Falier on the Pala d’Oro in Venice. Gesta 2000, 39, 43–49. [Google Scholar] [CrossRef]
- Hughes, M. A technical study of opaque red glass of the Iron Age in Britain. Proc. Prehist. Soc. 1972, 38, 98–107. [Google Scholar] [CrossRef]
- McIntosh, F. A study into Romano-Bristish enamelling–with a particular focus on brooches. Sch. Hist. Stud. Postgrad. Forum E-J. Ed. 2009, 7, 1–18. [Google Scholar]
- Rossi, S.; Scrinzi, E.; Compagnoni, A.M.; Gallucci, A.; Gjata, Y. Enamel and design. In The Potential of Enamelled Materials; Fausto Lupetti Editore: Bologna, Italy, 2011. [Google Scholar]
- De Chancel, B.; Drake Boehm, B.; Barrière, B.; Taburet, E.; Biron, I.; Becquet, J.; Gauthier, M.M.; Wypyski, M.T.; Pastoreau, M.; Dandrige, P. Enamels of Limoges 1100–1350; The Metropolitan Museum of Art: New York, NY, USA, 1996. [Google Scholar]
- Britannica, The Editors of Encyclopaedia. Limoges Painted Enamel. Encyclopedia Britannica, 17/03/2016. Available online: https://www.britannica.com/art/Limoges-painted-enamel (accessed on 12 March 2021).
- Dawes, E. Some early experiences in enamelling cast iron. J. Am. Ceram. Soc. 1923, 6, 234–237. [Google Scholar] [CrossRef]
- Vollrath, C.A.W. Early history of enamelling in the Vollrath company. J. Am. Ceram. Soc. 1923, 6, 237–240. [Google Scholar] [CrossRef]
- Staley, H.F. Developments in enamelling technology during the past twenty-five years. J. Am. Ceram. Soc. 1923, 6, 240–244. [Google Scholar] [CrossRef]
- Glenn, A.H. Porcelain enamels: Past, present and future. Analyst 1955, 11, 85–87. [Google Scholar]
- Wu, M.; Chen, M.; Zhu, S.; Wang, F. Protection mechanism of enamel–alumina composite coatings on a Cr-rich nickel-based superalloy against high-temperature oxidation. Surf. Coat. Technol. 2016, 285, 57–67. [Google Scholar] [CrossRef]
- Chen, M.; Shen, M.; Wang, X.; Zhu, S.; Wang, F. Interfacial reaction between SiO2–Al2O3–ZnO–CaO based glass coatings and K38G superalloy substrates. Surf. Coat. Technol. 2013, 216, 145–151. [Google Scholar] [CrossRef]
- Poste, E.P. The blistering of cast iron enamel. J. Am. Ceram. Soc. 1933, 16, 277–292. [Google Scholar] [CrossRef]
- Zaitsev, A.A.; Mogil’chenko, V.S. Improving the enamelling properties and quality of cast iron equipment. Glass Ceram. 1968, 2, 20–23. [Google Scholar] [CrossRef]
- Song, D.; Tang, R.; Yang, F.; Qiao, Y.; Sun, J.; Jiang, J.; Ma, A. Development of high-performance enamel coating on grey iron by low-temperature sintering. Materials 2018, 11, 2183. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, Z.; Liu, X.; Zhao, Y.; Li, X. Variations of microstructure and resistance to fish-scaling of a hot rolled enamel steel before and after enamel firing. J. Mater. Res. Technol. 2021, 11, 466–473. [Google Scholar] [CrossRef]
- Gavrilovski, D.; Gavrilovski, M. Properties of hot rolled steels for enamelling. In Proceedings of the 3rd BMC, Ohrid, Macedonia, 24–27 September 2003. [Google Scholar]
- UNI EN 10209 Standard. UNI Ente Nazionale Italiano di Unificazione; UNI: Milano, Italy, 2013. [Google Scholar]
- Ashis Janah, A. Vitreous enamelling of aluminium—A review. Trans. Ind. Ceram. Soc. 1968, 27, 1–15. [Google Scholar] [CrossRef]
- Compagnoni, A.; Ferraro, A. Smaltatura dell’alluminio ad alto tenore di magnesio. In Proceedings of the 20th IEI Congress, Istanbul, Turkey, 15–19 May 2005. [Google Scholar]
- Pagliuca, S.; Faust, W.D. Porcelain Vitreous Enamels and Industrial Enamelling Processes, 3rd ed.; IEI: Milan, Italy, 2011. [Google Scholar]
- Detail Explanation of Cast Iron Pretreatment. Available online: https://www.nolifrit.com/news/detail-explanation-on-cast-iron-pretreatment-104 (accessed on 14 March 2021).
- Danielson, R.R. The cleaning of sheet steel and iron for enamelling. J. Am. Ceram. Soc. 1919, 2, 883–894. [Google Scholar] [CrossRef]
- Nicholson, J.E. Practical Aspects of Electrolytic Pretreatment. In Proceedings of the 43rd Porcelain Enamel Institute Technical Forum 1982, The Ohio State University, Columbus, OH, USA, 7–8 October 1981. [Google Scholar]
- Rossi, S.; Fedel, M.; Deflorian, F.; Parziani, N. Abrasion and chemical resistance of composite enamel coatings with hard particles. Surf. Interface Anal. 2015, 48, 827–837. [Google Scholar] [CrossRef]
- Rossi, S.; Russo, F.; Calovi, M. Durability of vitreous enamel coatings and their resistance to abrasion, chemicals, and corrosion: A review. J. Coat. Technol. Res. 2021, 18, 39–52. [Google Scholar] [CrossRef]
- Rossi, S.; Calovi, M.; Velez, D.; Rodriguez, I.; Del Rincón, M.; Munoz, J.M.; Grande, H.J. Microstructural analysis and surface modification of a vitreous enamel modified with corundum particles. Adv. Eng. Mater. 2019, 21, 1900231. [Google Scholar] [CrossRef]
- Thiele, H.J. 2C/1F Enamelling Process—A Growing Demand. In 69th Porcelain Enamel Institute Technical Forum: Ceramic Engineering and Science Proceedings 2007; Evele, H., Vodak, P., Faust, W.D., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2008; p. 10. [Google Scholar]
- Willis, J.B. Electrostatic spraying of porcelain enamels. J. Am. Ceram. Soc. 1945, 28, 121–133. [Google Scholar] [CrossRef]
- Evele, H.F. Proper Care of Porcelain Enamel Powder for Electrostatic Application. Ceram. Eng. Sci. Proc. 2000, 21, 125–126. [Google Scholar]
- Fleischmann, R.; Schaper, W.; Schünemann, R. Some aspects of the electrophoretic enamel deposition. Electrochim. Acta 1984, 29, 77–80. [Google Scholar] [CrossRef]
- Perez Garcıa, M.R.; Munoz, J.; Diez, J.A. Corrosion resistance of enamel vitreous coating obtained by electrophoretic deposition. Key Eng. Mater. 2015, 654, 127–131. [Google Scholar] [CrossRef]
- Fan, L.; Tang, F.; Chen, G.; Reis, S.T.; Koenigstein, M.L. Corrosion resistances of steel pipe coated with two types of enamel by two coating processes. J. Mater. Eng. 2018, 27, 5341–5349. [Google Scholar] [CrossRef]
- Tang, F.; Chen, G.; Volz, J.S.; Brow, R.K.; Koenigstein, M. Microstructure and corrosion resistance of enamel coatings applied to smooth reinforcing steel. Constr. Build. Mater. 2012, 35, 376–384. [Google Scholar] [CrossRef]
- Rossi, S.; Bergamo, L.; Fontanari, V. Fire resistance and mechanical properties of enamelled aluminium foam. Mater. Des. 2017, 132, 129–137. [Google Scholar] [CrossRef]
- Scrinzi, E.; Rossi, S. The aesthetic and functional properties of enamel coatings on steel. Mater. Des. 2010, 31, 4138–4146. [Google Scholar] [CrossRef]
- Goodwin, J.W.; Whitelock, K.E. The importance of colour and its stability in vitreous enamels. Mater. Des. 1985, 6, 172–176. [Google Scholar] [CrossRef]
- Samiee, L.; Sarpoolaky, H.; Mirhabibi, A. Microstructure and adherence of cobalt containing and cobalt free enamels to low carbon steel. Mater. Sci. Eng. A 2007, 458, 88–95. [Google Scholar] [CrossRef]
- Shieu, F.S.; Lin, K.C.; Wong, J.C. Microstructure and adherence of porcelain enamel to low carbon steel. Ceram. Int. 1999, 25, 27–34. [Google Scholar] [CrossRef]
- Rossi, S.; Zanella, C.; Sommerhuber, R. Influence of mill additives on vitreous enamel properties. Mater. Des. 2014, 55, 880–887. [Google Scholar] [CrossRef]
- Rossi, S.; Russo, F.; Calovi, M.; Del Rincòn, M.; Velez, D. The influence of the size of corundum particles on the properties of chemically resistant porcelain enamels. Ceram. Int. 2021, 47, 11618–11627. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).