Production of Nanocellulose by Enzymatic Treatment for Application in Polymer Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
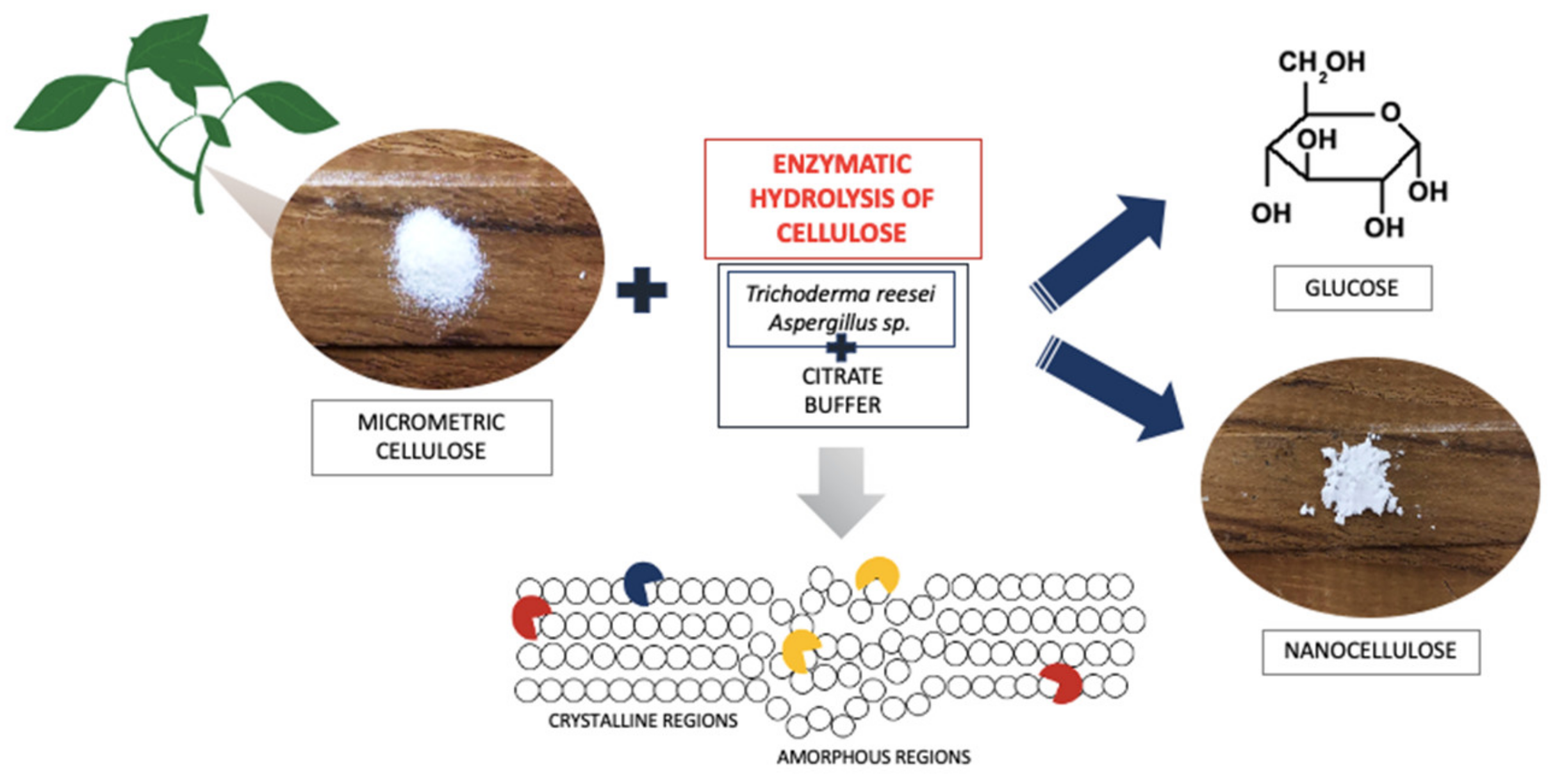
2.2. The Process of Enzymatic Treatment of Cellulose
2.3. Characteristics of Cellulose Fillers after the Enzymatic Treatment Process
2.3.1. Glucose Analysis by Liquid Chromatography
2.3.2. X-ray Diffraction (XRD)
2.3.3. Dynamic Light Scattering
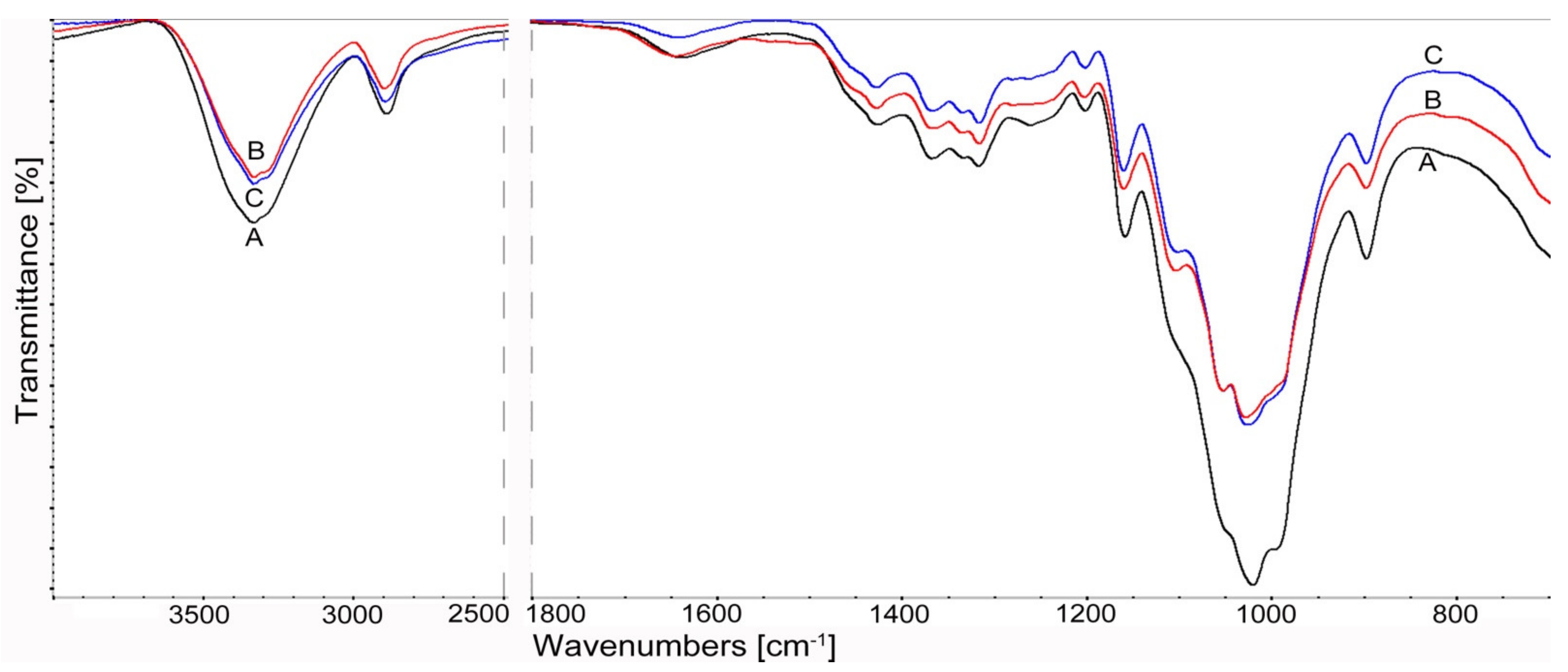
2.3.4. Fourier Transform Infrared Spectroscopy (FTIR) Analysis of Cellulose
2.4. Obtaining Polymer Nanocomposites
2.5. Characteristics of Composite Materials
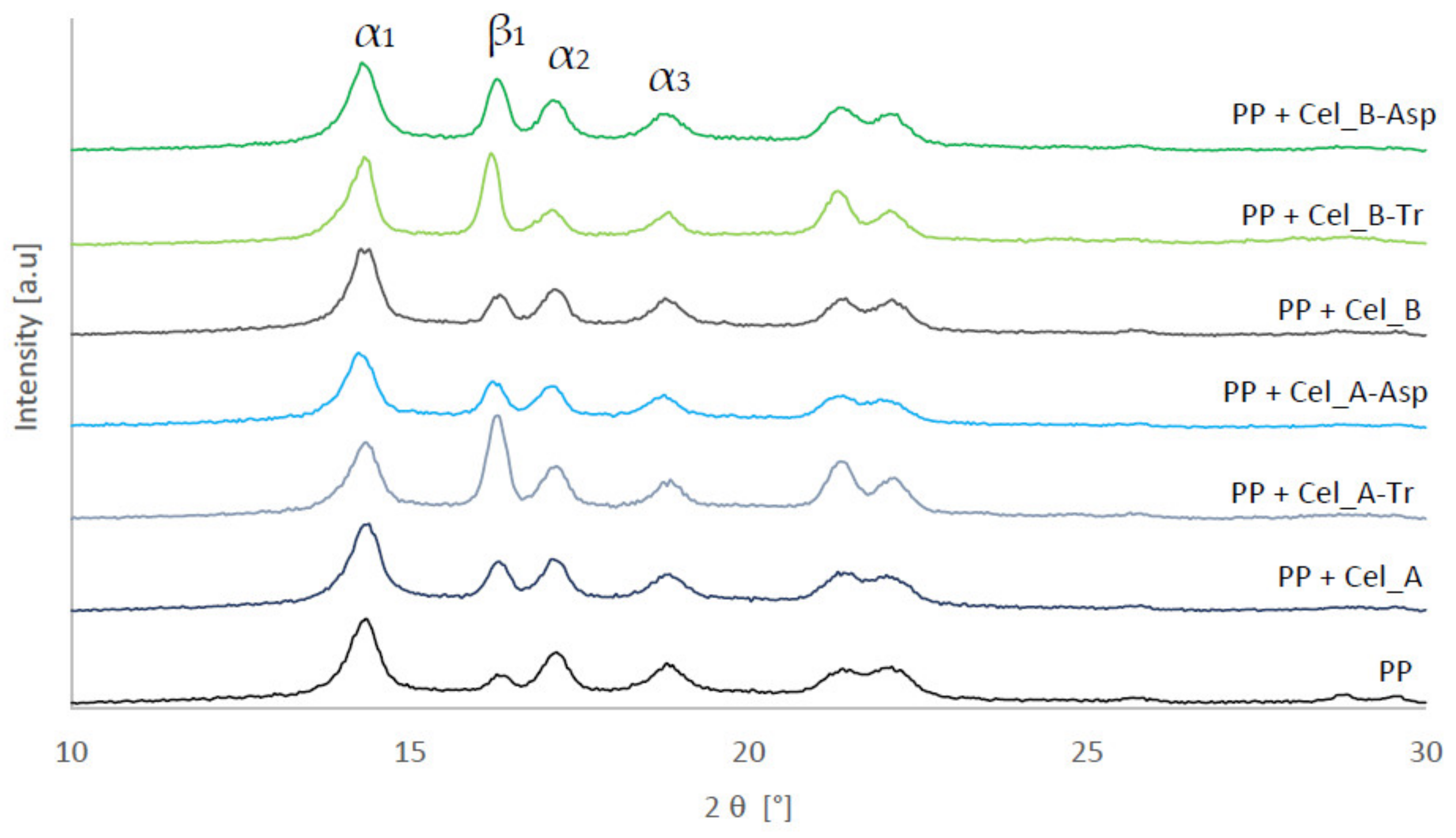
2.5.1. Structural Investigations (XRD)
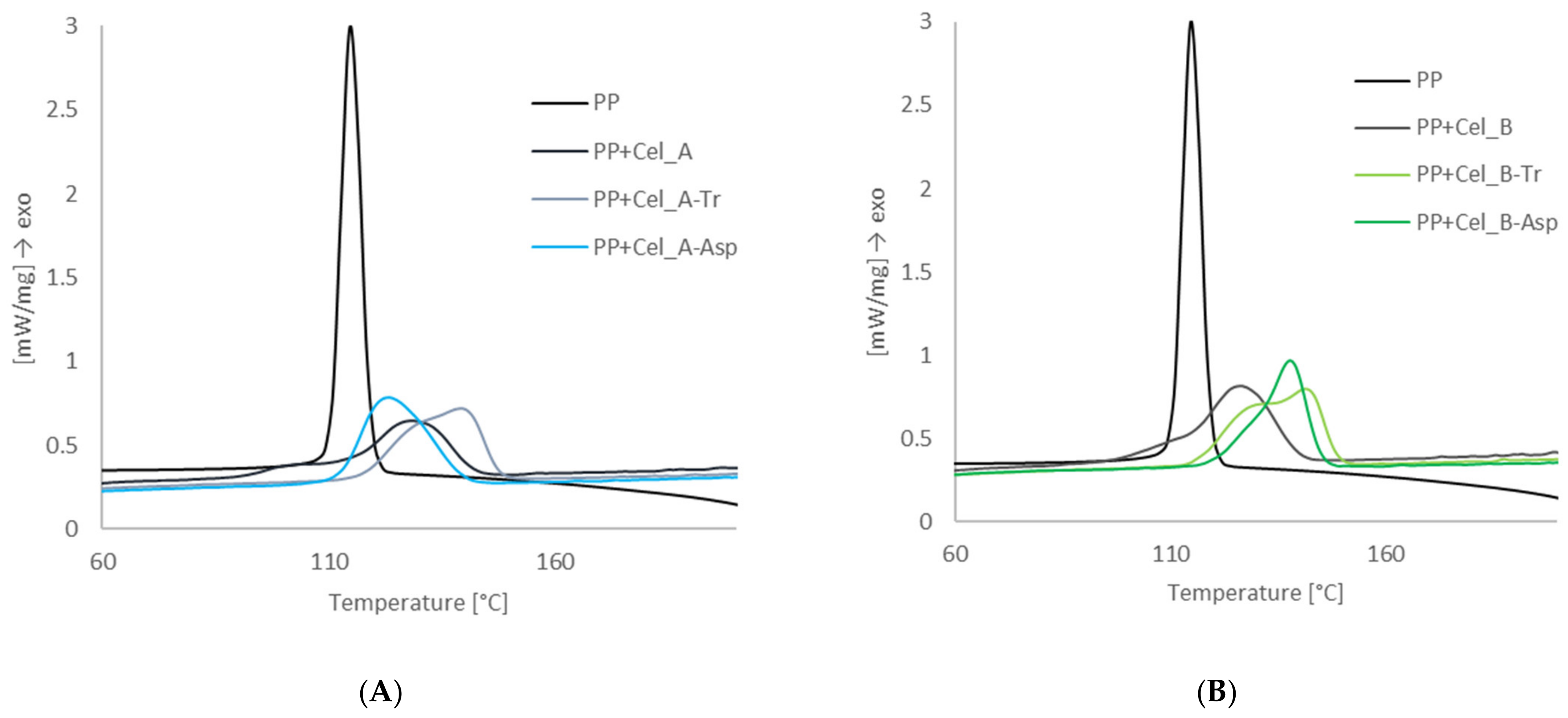
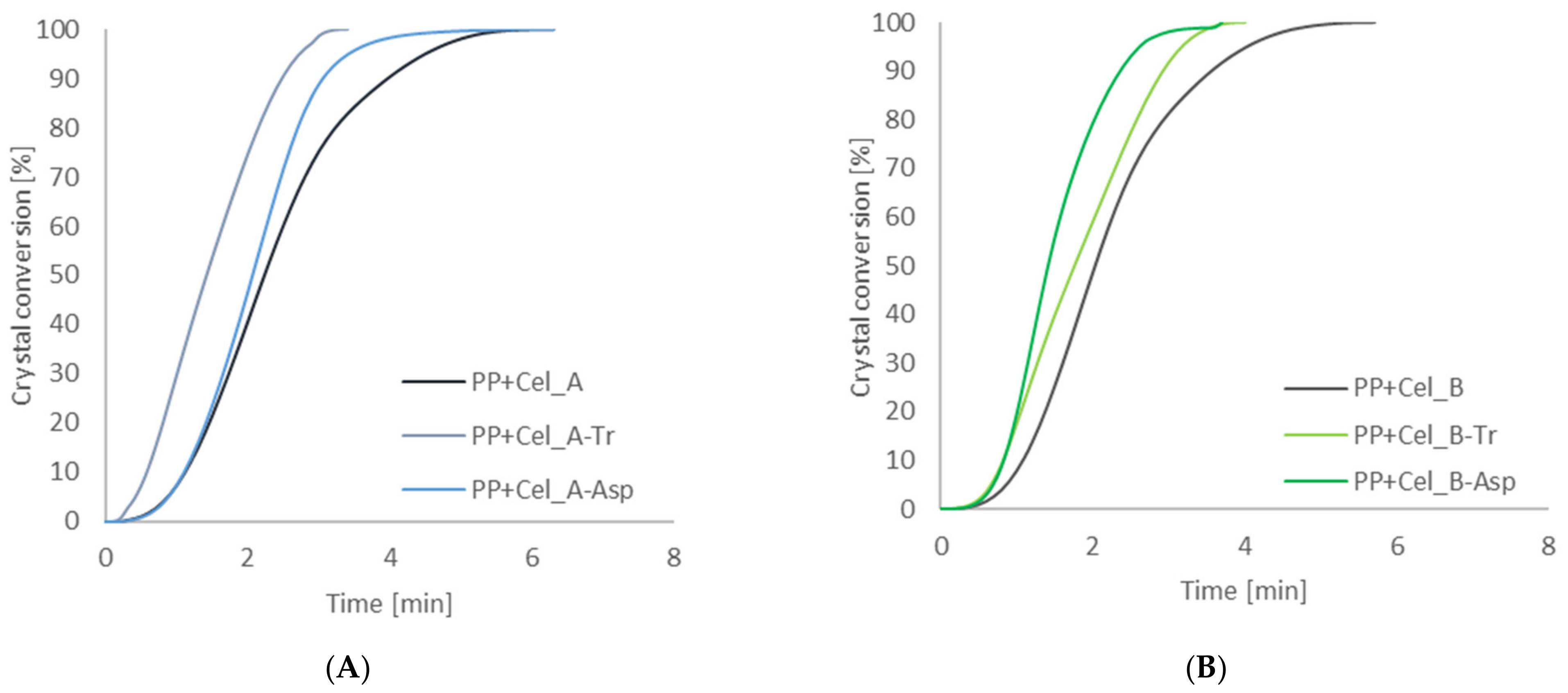
2.5.2. Differential Scanning Calorimetry
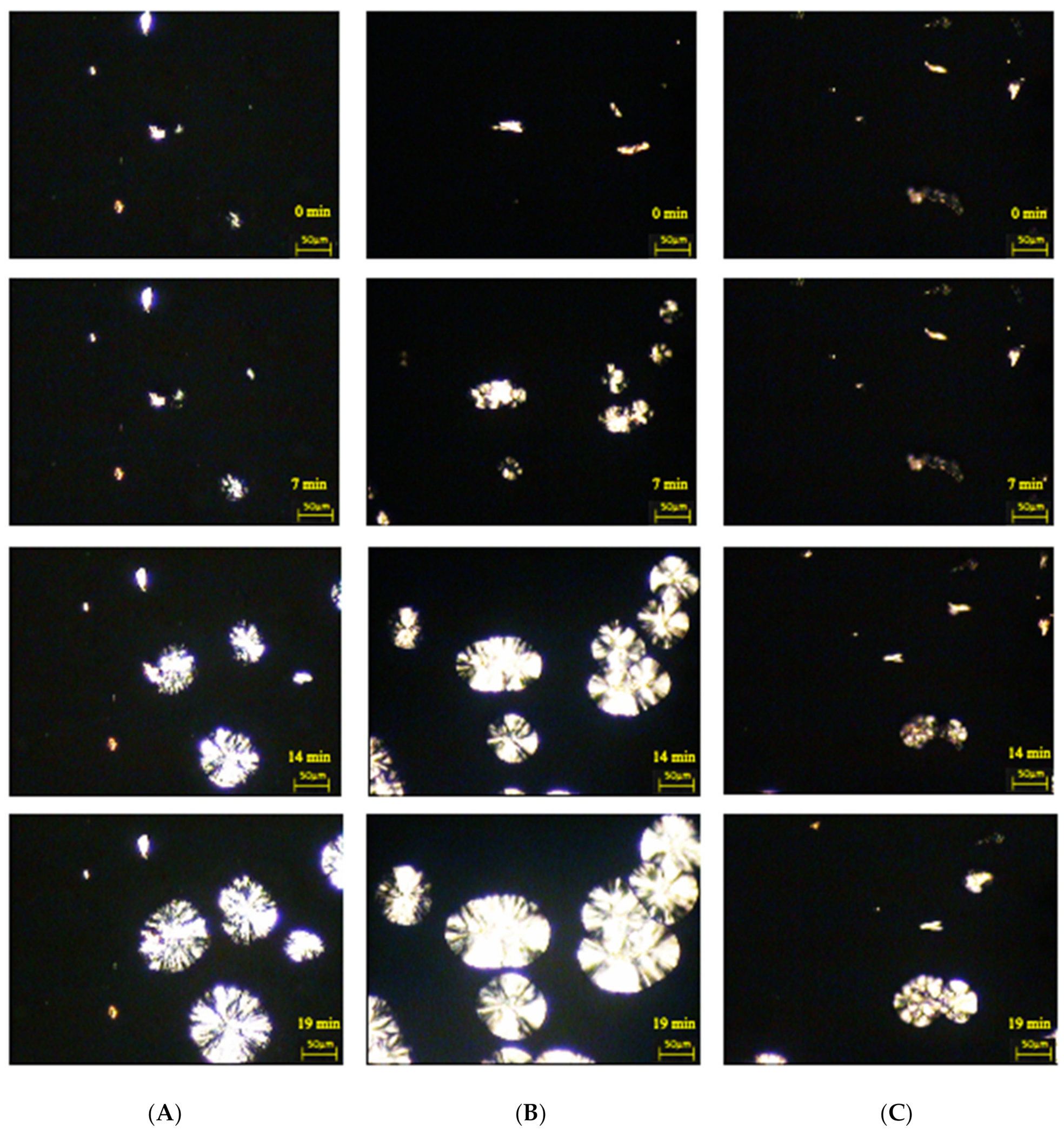
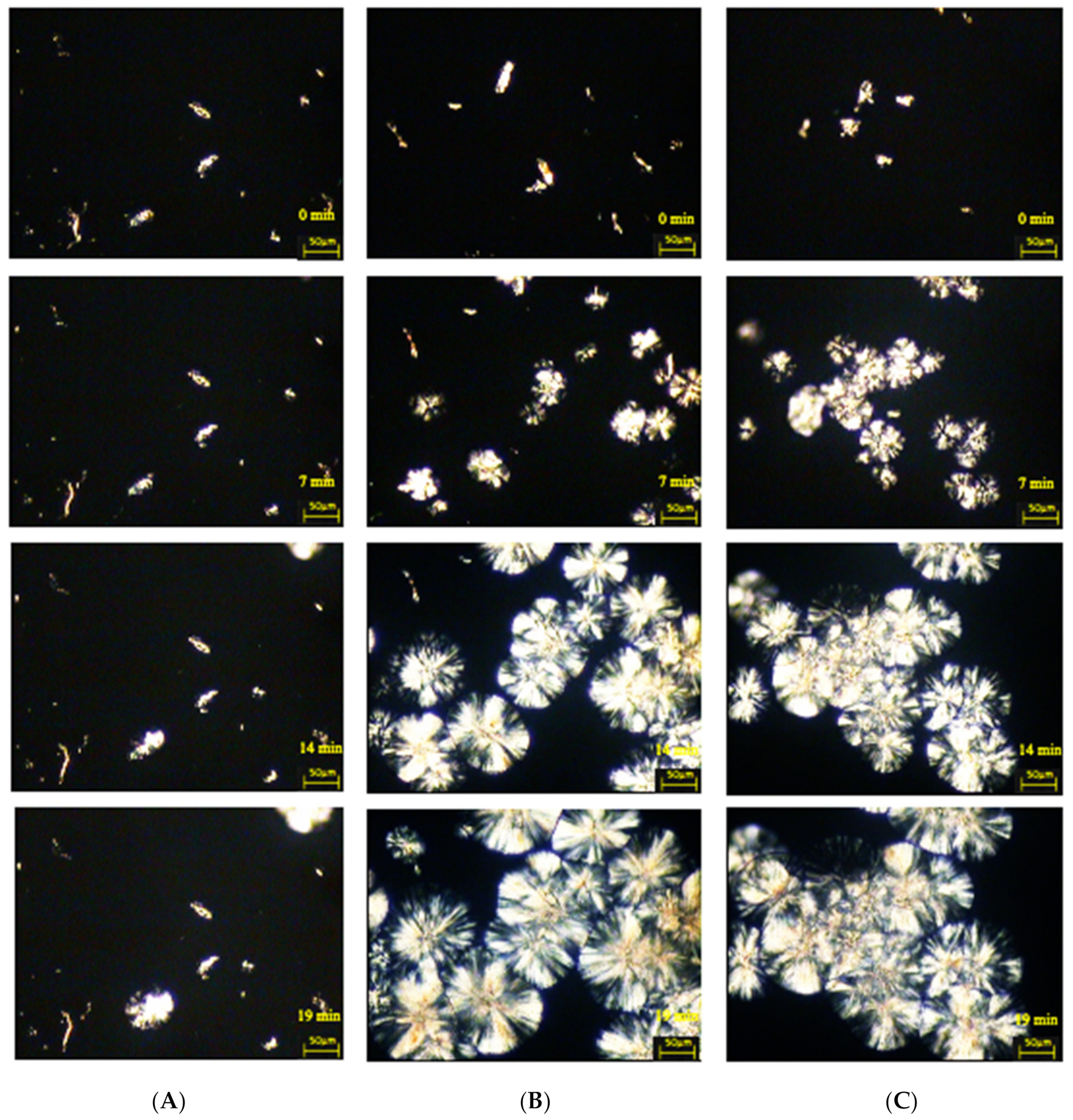
2.5.3. Hot-Stage Polarized Light Microscopy
2.5.4. Tensile Tests
3. Results and Discussion
3.1. Characterization of Cellulosic Fillers after Enzymatic Treatment
3.1.1. HPLC Analysis of Glucose
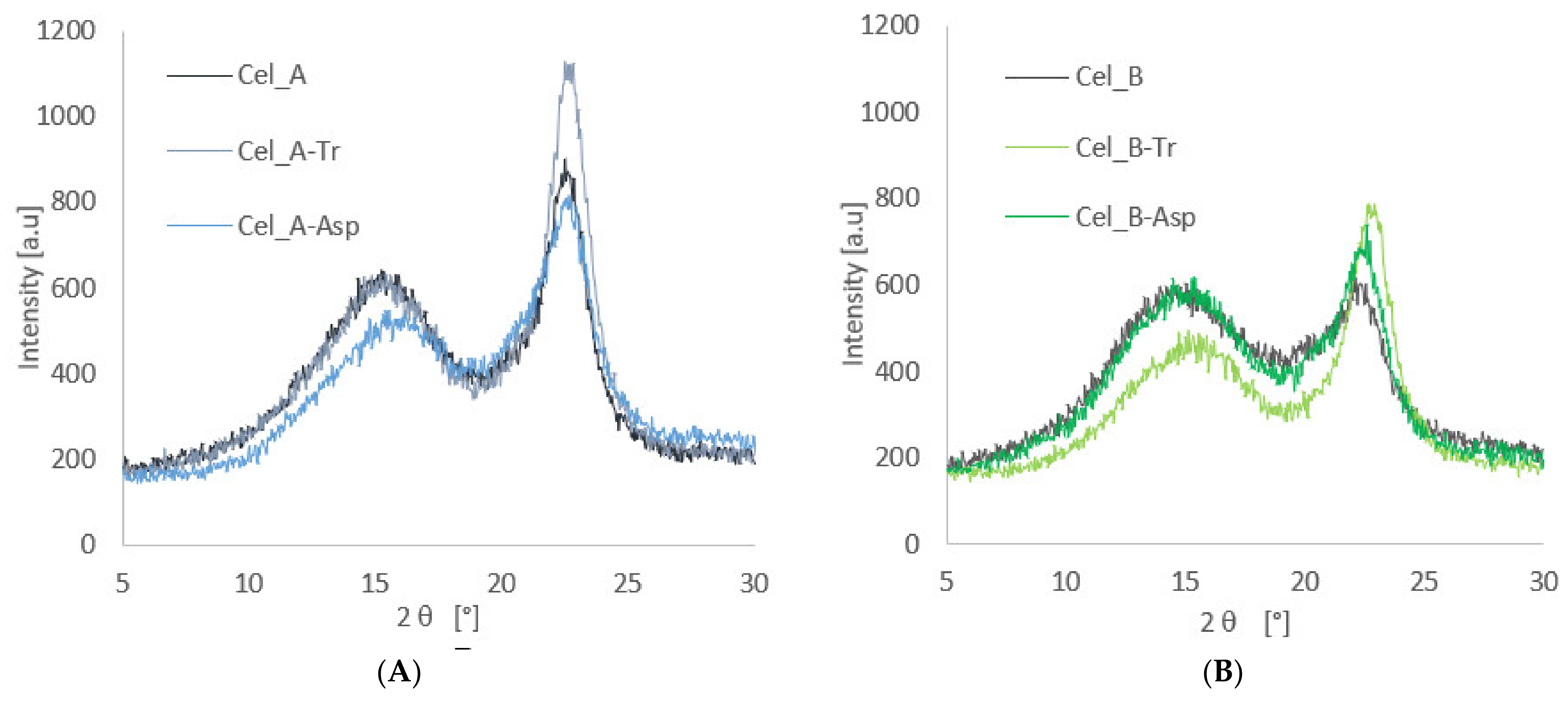
3.1.2. XRD Investigation of Cellulose after Enzymatic Treatment
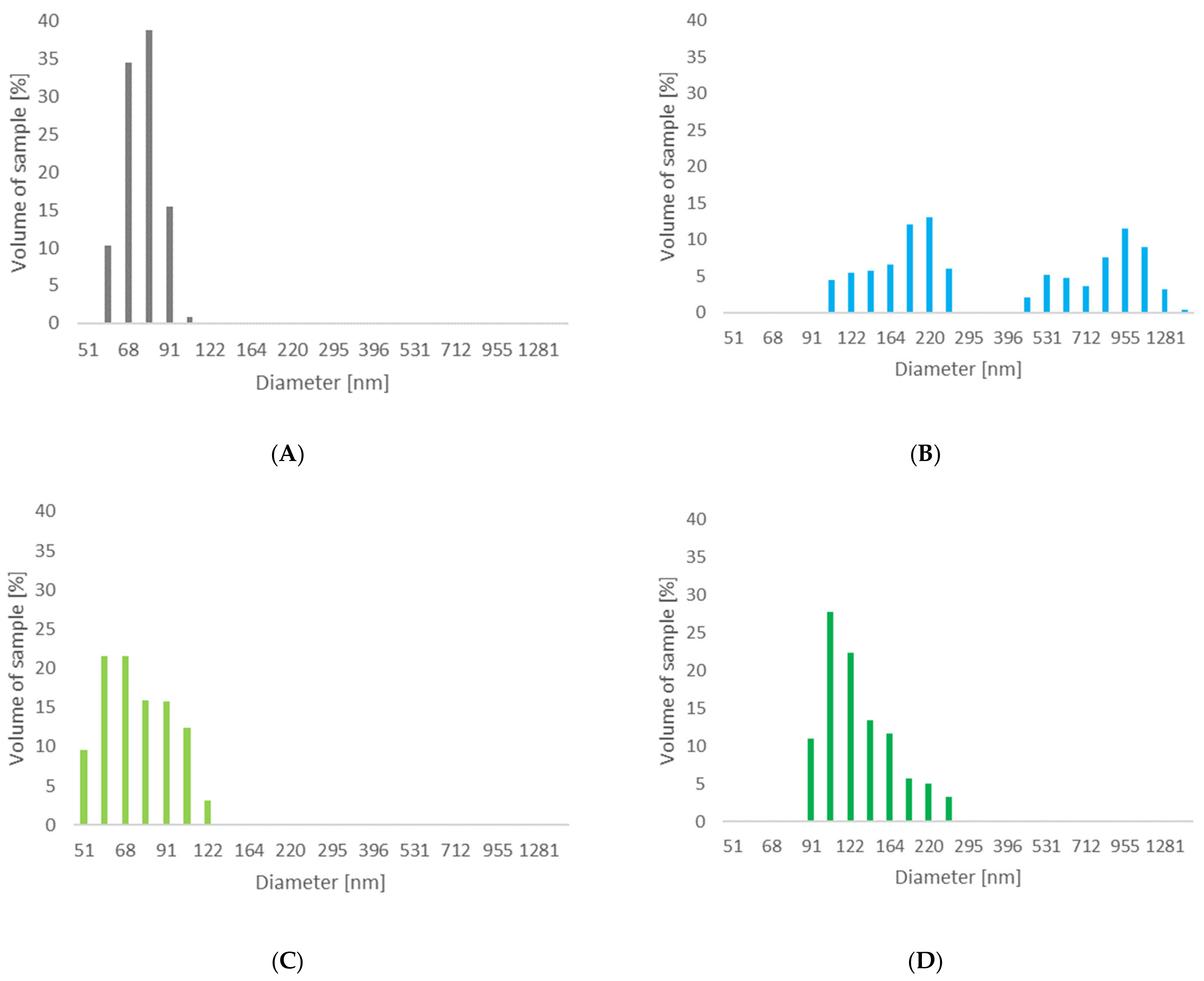
3.1.3. Determination of Particle Sizes for Treated Celluloses
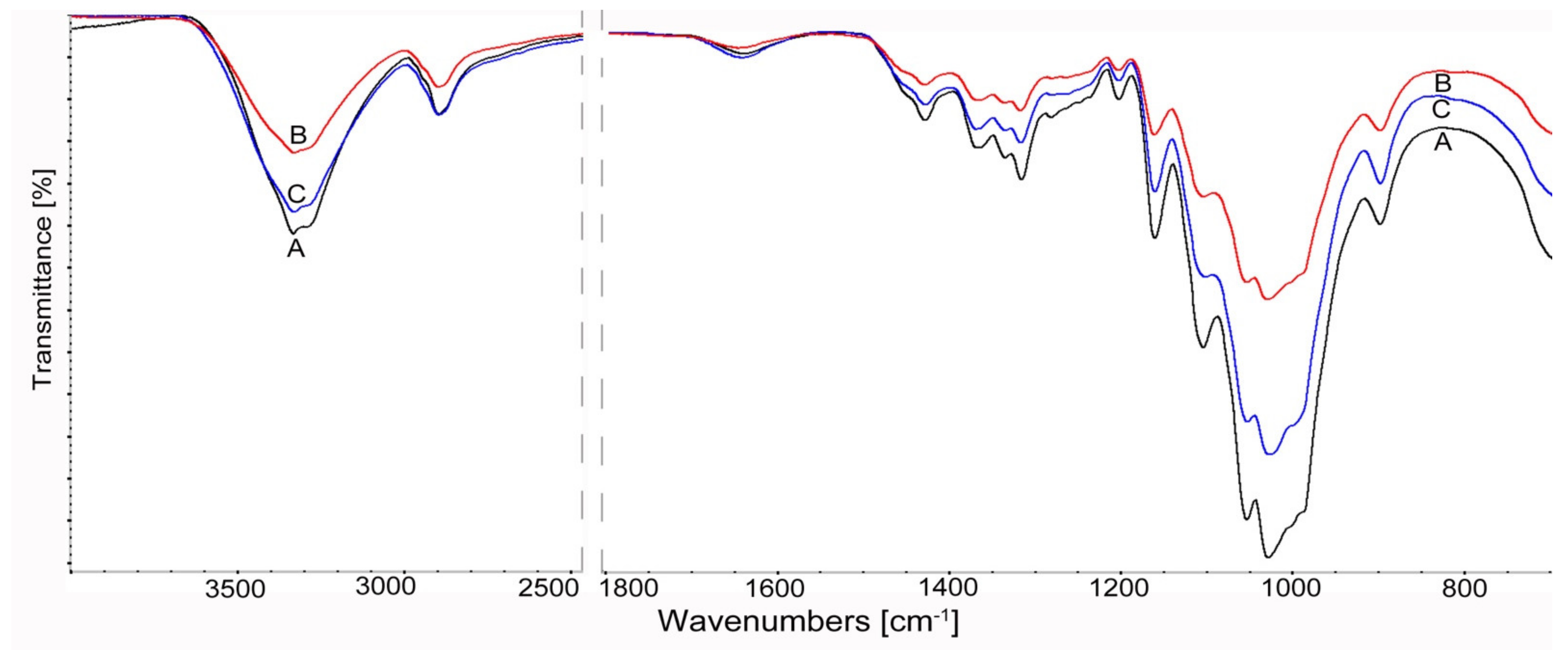
3.1.4. FTIR Spectroscopy
3.2. Characteristics of Composite Materials
3.2.1. Structural Studies of Polymer Composites
3.2.2. Investigation of the Nucleation Activity of Composite Materials by Differential Scanning Calorimetry
3.2.3. Examination of the Morphology of Composite Materials by Microscopy with a Heating Attachment
3.2.4. Tests of the Strength Properties of Composite Materials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teeri, T.T. Crystalline cellulose degradation: New insight into the function of cellobiohydrolases. Trends Biotechnol. 1997, 15, 160–167. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and its derivatives: Towards biomedical applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Phanthong, P.; Reubroycharoen, P.; Hao, X.; Xu, G.; Abudula, A.; Guan, G. Nanocellulose: Extraction and application. CRC 2018, 1, 32–43. [Google Scholar] [CrossRef]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef]
- Martelli-Tosi, M.; Masson, M.M.; Silva, N.C.; Esposto, B.S.; Barros, T.T.; Assis, O.B.G.; Tapia-Blácido, D.R. Soybean straw nanocellulose produced by enzymatic or acid treatment as a reinforcing filler in soy protein isolate films. Carbohydr. Polym. 2018, 198, 61–68. [Google Scholar] [CrossRef]
- Hao, W.; Wang, M.; Zhou, F.; Luo, H.; Xie, X.; Luo, F.; Cha, R. A review on nanocellulose as a lightweight filler of polyolefin composites. Carbohydr. Polym. 2020, 243, 116466. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A New Family of Nature-Based Materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Thakur, M.; Bhattacharya, M.; Mandal, T.; Goswami, S. Commercial Application of Cellulose Nano-composites—A review. Biotechnol. Rep. 2019, 21, e00316. [Google Scholar] [CrossRef] [PubMed]
- Filho, A.S.; Parveen, S.; Rana, S.; Vanderlei, R.; Fangueiro, R. Micro-structure and mechanical properties of microcrystalline cellulose-sisal fiber reinforced cementitious composites developed using cetyltrimethylammonium bromide as the dispersing agent. Cellulose 2021, 28, 1663–1686. [Google Scholar] [CrossRef]
- Omran, A.A.B.; Mohammed, A.A.B.A.; Sapuan, S.M.; Ilyas, R.A.; Asyraf, M.R.M.; Rahimian Koloor, S.S.; Petrů, M. Micro-and Nanocellulose in Polymer Composite Materials: A Review. Polymers 2021, 13, 231. [Google Scholar] [CrossRef] [PubMed]
- Lengowski, E.C.; Júnior, E.A.B.; Simon, L.; De Muñiz, G.I.B.; De Andrade, A.S.; Nisgoski, S.; Klock, U. Different degree of fibrillation: Strategy to reduce permeability in nanocellulose-starch films. Cellulose 2020, 27, 10855–10872. [Google Scholar] [CrossRef]
- ISO—International Organization for Standardization. ISO/TS 20477:2017 Nanotechnologies—Standard Terms and Their Definition for Cellulose Nanomaterial; ISO: Geneva, Switzerland, 2017. [Google Scholar]
- Mihaela, D.; Nicoleta, A.; Ghiurea, M.; Ilie, C.; Radovici, C.; Doina, M. Properties of Polymer Composites with Cellulose Microfibrils. Adv. Compos. Mater.—Ecodesign Anal. 2011, 5, 103–122. [Google Scholar] [CrossRef][Green Version]
- Wang, N.; Ding, E.; Cheng, R. Thermal degradation behaviors of spherical cellulose nanocrystals with sulfate groups. Polymer 2007, 48, 3486–3493. [Google Scholar] [CrossRef]
- Man, Z.; Muhammad, N.; Sarwono, A.; Azmi Bustam, M.; Vignesh Kumar, M.; Rafiq, S. Preparation of Cellulose Nanocrystals Using an Ionic Liquids. J. Polym. Environ. 2011, 19, 726–731. [Google Scholar] [CrossRef]
- Johar, N.; Ahmad, I.; Dufresne, A. Extraction, preparation and characterization of cellulose fibres and nanocrystals from rice husk. Ind. Crops Prod. 2012, 37, 93–99. [Google Scholar] [CrossRef]
- Maiti, S.; Jayaramudu, J.; Das, K.; Reddy, S.M.; Sadiku, R.; Ray, S.S.; Liu, D. Preparation and characterization of nano-cellulose with new shape from different precursor. Carbohydr. Polym. 2013, 98, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Lindman, B.; Karlström, G.; Stigsson, L. On the mechanism of dissolution of cellulose. J. Mol. Liq. 2010, 156, 76–81. [Google Scholar] [CrossRef]
- Lee, H.V.; Hamid, S.B.A.; Zain, S.K. Conversion of lignocellulosic biomass to nanocellulose: Structure and chemical process. Sci. World J. 2014, 2014, 631013. [Google Scholar] [CrossRef]
- El Seoud, O.A.; Koschella, A.; Fidale, L.C.; Dorn, S.; Heinze, T. Applications of ionic liquids in carbohydrate chemistry: A window of opportunities. Biomacromolecules 2007, 8, 2629–2647. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Bhat, A.H.; Ireana Yusra, A.F. Green composites from sustainable cellulose nanofibrils: A review. Carbohydr. Polym. 2012, 87, 963–979. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Davoudpour, Y.; Islam, M.N.; Mustapha, A.; Sudesh, K.; Dungani, R.; Jawaid, M. Production and modification of nanofibrillated cellulose using various mechanical processes: A review. Carbohydr. Polym. 2014, 99, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Siró, I.; Plackett, D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Yeh, A.I.; Huang, Y.-C.; Chen, S.H. Effect of particle size on the rate of enzymatic hydrolysis of cellulose. Carbohydr. Polym. 2010, 79, 192–199. [Google Scholar] [CrossRef]
- Chen, X.Q.; Deng, X.Y.; Shen, W.H.; Jia, M.Y. Preparation and characterization of the spherical nanosized cellulose by the enzymatic hydrolysis of pulp fibers. Carbohydr. Polym. 2018, 181, 879–884. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, S.; Li, Z.; Men, Y.; Wu, J. Impacts of Cellulase and Amylase on Enzymatic Hydrolysis and Methane Production in the Anaerobic Digestion of Corn Straw. Sustainability 2020, 12, 5453. [Google Scholar] [CrossRef]
- Salimi, S.; Sotudeh-Gharebagh, R.; Zarghami, R.; Chan, S.Y.; Yuen, K.H. Production of nanocellulose and its applications in drug delivery: A critical review. ACS Sustain. Chem. Eng. 2019, 7, 15800–15827. [Google Scholar] [CrossRef]
- Mokhena, T.C.; John, M.J. Cellulose nanomaterials: New generation materials for solving global issues. Cellulose 2020, 27, 1149–1194. [Google Scholar] [CrossRef]
- Tibolla, H.; Pelissari, F.M.; Rodrigues, M.I.; Menegalli, F.C. Cellulose nanofibers produced from banana peel by enzymatic treatment: Study of process conditions. Ind. Crops. Prod. 2017, 95, 664–674. [Google Scholar] [CrossRef]
- Hu, J.; Arantes, V.; Pribowo, A.; Saddler, J.N. The synergistic action of accessory enzymes enhances the hydrolytic potential of a “cellulase mixture” but is highly substrate specific. Biotechnol. Biofuels 2013, 6, 112. [Google Scholar] [CrossRef] [PubMed]
- Albornoz-Palma, G.; Ching, D.; Valerio, O.; Mendonça, R.T.; Pereira, M. Effect of lignin and hemicellulose on the properties of lignocellulose nanofibril suspensions. Cellulose 2020, 27, 10631–10647. [Google Scholar] [CrossRef]
- Arantes, V.; Dias, I.K.R.; Berto, G.L.; Pereira, B.; Marotti, B.S.; Nogueira, C.F.O. The current status of the enzyme-mediated isolation and functionalization of nanocelluloses: Production, properties, techno-economics, and opportunities. Cellulose 2020, 27, 10571–10630. [Google Scholar] [CrossRef]
- Roth, J.C.G.; Hoeltz, M.; Benitez, L.B. Current approaches and trends in the production of microbial cellulases using residual lignocellulosic biomass: A bibliometric analysis of the last 10 years. Arch. Microbiol. 2020, 202, 935–951. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Kumar, V.; Khakalo, A.; Lahtinen, P.; Solin, K.; Pere, J.; Toivakka, M. Rheological behavior of high consistency enzymatically fibrillated cellulose suspensions. Cellulose 2021, 28, 2087–2104. [Google Scholar] [CrossRef]
- Michelin, M.; Gomes, D.G.; Romaní, A.; Polizeli, M.d.L.T.M.; Teixeira, J.A. Nanocellulose Production: Exploring the Enzymatic Route and Residues of Pulp and Paper Industry. Molecules 2020, 25, 3411. [Google Scholar] [CrossRef]
- Chen, X.Q.; Pang, G.X.; Shen, W.H.; Tong, X.; Jia, M.Y. Preparation and characterization of the ribbon-like cellulose nanocrystals by the cellulase enzymolysis of cotton pulp fibers. Carbohydr. Polym. 2019, 207, 713–719. [Google Scholar] [CrossRef]
- Tong, X.; Shen, W.; Chen, X.; Jia, M.; Roux, J.C. Preparation and mechanism analysis of morphology-controlled cellulose nanocrystals via compound enzymatic hydrolysis of eucalyptus pulp. J. Appl. Polym. Sci. 2020, 137, 1–11. [Google Scholar] [CrossRef]
- Szentner, K.; Waśkiewicz, A.; Kaźmierczak, S.; Wojciechowicz, T.; Goliński, P.; Lewandowska, E.; Wasielewski, O. Enzymatic hydrolysis of cellulose using extracts from insects. Carbohydr. Res. 2019, 485, 107811. [Google Scholar] [CrossRef] [PubMed]
- Imai, M.; Horikawa, Y.; Kiyoto, S.; Imai, T.; Sugiyama, J. Structural changes in sugarcane bagasse cellulose caused by enzymatic hydrolysis. J. Wood Sci. 2020, 66, 11. [Google Scholar] [CrossRef]
- Borysiak, S. Influence of wood mercerization on the crystallization of polypropylene in wood/PP composites. J. Therm. Anal. Calorim. 2012, 109, 595–603. [Google Scholar] [CrossRef]
- Borysiak, S. Influence of cellulose polymorphs on the polypropylene crystallization. J. Therm. Anal. Calorim. 2013, 113, 281–289. [Google Scholar] [CrossRef][Green Version]
- Borysiak, S.; Grząbka-Zasadzińska, A. Influence of the polymorphism of cellulose on the formation of nanocrystals and their application in chitosan/nanocellulose composites. J. Appl. Polym. Sci. 2016, 133, 42864–42873. [Google Scholar] [CrossRef]
- Grząbka-Zasadzińska, A.; Smułek, W.; Kaczorek, E.; Borysiak, S. Chitosan biocomposites with enzymatically produced nanocrystalline cellulose. Polym. Compos. 2018, 39, 448–456. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Sabo, R.; Luo, X.L. Integrated Production of NanoFibrillated Cellulose and Cellulosic Biofuel (Ethanol) by Enzymatic Fractionation of Wood Fibers. Green Chem. 2011, 13, 1339–1344. [Google Scholar] [CrossRef]
- Yarbrough, J.M.; Zhang, R.; Mittal, A.; Vander Wall, T.; Bomble, Y.J.; Decker, S.R.; Ciesielski, P.N. Multifunctional Cellulolytic Enzymes Outperform Processive Fungal Cellulases for Coproduction of Nanocellulose and Biofuels. ACS Nano 2017, 11, 3101–3109. [Google Scholar] [CrossRef]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Ribeiro, R.S.A.; Pohlmann, B.C.; Calado, V.; Bojorge, N.; Pereira, N. Production of nanocellulose by enzymatic hydrolysis: Trends and challenges. Eng. Life Sci. 2019, 19, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Paukszta, D.; Borysiak, S. The Influence of Processing and the Polymorphism of Lignocellulosic Fillers on the Structure and Properties of Composite Materials—A Review. Materials 2013, 6, 2747–2767. [Google Scholar] [CrossRef] [PubMed]
- Naghmouchi, I.; Espinach, F.X.; Mutjé, P.; Boufi, S. Polypropylene composites based on lignocellulosic fillers: How the filler morphology affects the composite properties. Mater. Des. 2015, 65, 454–461. [Google Scholar] [CrossRef]
- Sozen, E.; Aydemir, D.; Zor, M. The Effects of Lignocellulosic Fillers on Mechanical, Morphological and Thermal Properties of Wood Polymer Composites. Drv. Ind. 2017, 68, 195–204. [Google Scholar] [CrossRef]
- Borysiak, S.; Grząbka-Zasadzińska, A.; Odalanowska, M.; Skrzypczak, A.; Ratajczak, I. The effect of chemical modification of wood in ionic liquids on the supermolecular structure and mechanical properties of wood/polypropylene composites. Cellulose 2018, 25, 4639–4652. [Google Scholar] [CrossRef]
- Erdogan, S.; Huner, U. Physical and Mechanical Properties of PP Composites based on Different Types of Lignocellulosic Fillers. J. Wuhan Univ. Technol.—Mater. Sci. Edit. 2018, 33, 1298–1307. [Google Scholar] [CrossRef]
- Merijs-Meri, R.; Zicans, J.; Ivanova, T.; Bochkov, I.; Varkale, M.; Franciszczak, P.; Bledzki, A.K.; Danilovas, P.P.; Gravitis, J.; Rubenis, K.; et al. Development and Characterization of Grain Husks Derived Lignocellulose Filler Containing Polypropylene Composites. Polym. Eng. Sci. 2019, 59, 2467–2473. [Google Scholar] [CrossRef]
- Kufel, A.; Kuciel, S. Hybrid Composites Based on Polypropylene with Basalt/Hazelnut Shell Fillers: The Influence of Temperature, Thermal Aging, and Water Absorption on Mechanical Properties. Polymers 2020, 12, 18. [Google Scholar] [CrossRef]
- Spoljaric, S.; Genovese, A.; Shanks, R.A. Polypropylene–microcrystalline cellulose composites with enhanced compatibility and properties. Compos. Part A Appl. Sci. Manuf. 2009, 40, 791–799. [Google Scholar] [CrossRef]
- Ratnakumar, A.; Rathnayake, W.S.M.; Karunanayake, L.; Samarasekara, A.M.P.B.; Amarasinghe, D.A.S. Microcrystalline Cellulose as Reinforcing Agents for Polypropylene Composites. Trop. Agric. Res. 2020, 31, 106–114. [Google Scholar] [CrossRef]
- Sergi, C.; Sbardella, F.; Lilli, M.; Tirillò, J.; Calzolari, A.; Sarasini, F. Hybrid Cellulose–Basalt Polypropylene Composites with Enhanced Compatibility: The Role of Coupling Agent. Molecules 2020, 25, 4384. [Google Scholar] [CrossRef]
- Liu, J.-C.; Martin, D.J.; Moon, R.J.; Youngblood, J.P. Enhanced thermal stability of biomedical thermoplastic polyurethane with the addition of cellulose nanocrystals. J. Appl. Polym. Sci. 2015, 132, 41970. [Google Scholar] [CrossRef]
- Ferreira, F.; Pinheiro, I.; de Souza, S.; Mei, L.; Lona, L. Polymer Composites Reinforced with Natural Fibers and Nanocellulose in the Automotive Industry: A Short Review. J. Compos. Sci. 2019, 3, 51. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, S.; Wang, X.; Pan, Y.; Zhang, F.; Huang, J. Mechanical and aging resistance properties of polypropylene (PP) reinforced with nanocellulose/attapulgite composites (NCC/AT). Compos. Interfaces 2019, 27, 73–85. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Jin, T.; He, H.; Liu, L. Preparation of nanocellulose and its potential in reinforced composites: A review. J. Biomater. Sci. Polym. Ed. 2019, 30, 919–946. [Google Scholar] [CrossRef]
- Nozaki, A.P.M.; Lona, L.M.F. Comparison between cellulose nanocrystal and microfibrillated cellulose as reinforcement of poly(vinyl acetate) composites obtained by either in situ emulsion polymerization or a simple mixing technique. Cellulose 2021, 28, 2273–2286. [Google Scholar] [CrossRef]
- Kurihara, T.; Isogai, A. Mechanism of TEMPO-oxidized cellulose nanofibril film reinforcement with poly(acrylamide). Cellulose 2015, 22, 2607–2617. [Google Scholar] [CrossRef]
- Tang, H.; Butchosa, N.; Zhou, Q. A transparent, hazy, and strong macroscopic ribbon of oriented cellulose nanofibrils bearing poly(ethylene glycol). Adv. Mater. 2015, 27, 2070–2076. [Google Scholar] [CrossRef]
- Larsson, P.A.; Berglund, L.A.; Wågberg, L. Ductile All-Cellulose Nanocomposite Films Fabricated from Core–Shell Structured Cellulose Nanofibrils. Biomacromolecules 2014, 15, 2218–2223. [Google Scholar] [CrossRef] [PubMed]
- Nasseri, R.; Mohammadi, N. Starch-based nanocomposites: A comparative performance study of cellulose whiskers and starch nanoparticles. Carbohydr. Polym. 2014, 106, 432–439. [Google Scholar] [CrossRef]
- Yano, H.; Sugiyama, J.; Nakagaito, A.; Nogi, M.; Matsuura, T.; Hikita, M.; Handa, K. Optically transparent composites reinforced with networks of bacterial nanofibers. Adv. Mater. 2005, 17, 153–155. [Google Scholar] [CrossRef]
- Song, Z.; Xiao, H.; Zhao, Y. Hydrophobic-modified nano-cellulose fiber/PLA biodegradable composites for lowering water vapor transmission rate (WVTR) of paper. Carbohydr. Polym. 2014, 111, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Almasi, H.; Ghanbarzadeh, B.; Dehghannya, J.; Entezami, A.A.; Asl, A.K. Novel nanocomposites based on fatty acid modified cellulose nanofibers/poly(lactic acid): Morphological and physical properties. Food Packag. Shelf Life 2015, 5, 21–31. [Google Scholar] [CrossRef]
- Kiziltas, E.E.; Kiziltas, A.; Bollin, S.C.; Gardner, D.J. Preparation and characterization of transparent PMMA-cellulose-based nanocomposites. Carbohydr. Polym. 2015, 27, 381–389. [Google Scholar] [CrossRef]
- Abitbol, T.; Rivkin, A.; Cao, Y.; Nevo, Y.; Abraham, E.; Ben-Shalom, T.; Lapidot, S.; Shoseyov, O. Nanocellulose, a tiny fiber with huge applications. Curr. Opin. Biotechnol. 2016, 39, 76–88. [Google Scholar] [CrossRef]
- Ljungberg, N.; Cavaillé, J.Y.; Heux, L. Nanocomposites of isotactic polypropylene reinforced with rod-like cellulose whiskers. Polymer 2006, 47, 6285–6292. [Google Scholar] [CrossRef]
- Yang, H.-S.; Gardner, D.J.; Nader, J.W. Characteristic impact resistance model analysis of cellulose nanofibril-filled polypropylene composites. Compos. Part A Appl. Sci. Manuf. 2011, 42, 2028–2035. [Google Scholar] [CrossRef]
- Agarwal, U.P.; Sabo, R.; Reiner, R.S.; Clemons, C.M.; Rudie, A.W. Spatially Resolved Characterization of Cellulose Nanocrystal–Polypropylene Composite by Confocal Raman Microscopy. Appl. Spectrosc. 2012, 66, 750–756. [Google Scholar] [CrossRef]
- Pandey, J.K.; Lee, H.T.; Takagi, H.; Ahn, S.H.; Saini, D.R.; Misra, M. Dispersion of Nanocellulose (NC) in Polypropylene (PP) and Polyethylene (PE) Matrix. In Handbook of Polymer Nanocomposites. Processing, Performance and Application; Springer: Berlin/Heidelberg, Germany, 2014; pp. 179–189. [Google Scholar]
- Sojoudiasli, H.; Heuzey, M.-C.; Carreau, P.J. Mechanical and morphological properties of cellulose nanocrystal-polypropylene composites. Polym. Compos. 2017, 39, 3605–3617. [Google Scholar] [CrossRef]
- Hindeleh, A.M.; Johnson, D.J. The resolution of multipeak data in fibre science. J. Phys. D Appl. Phys. 1971, 4, 259–263. [Google Scholar] [CrossRef]
- Rabiej, S. A comparison of two X-ray diffraction procedures for crystallinity determination. Eur. Polym. J. 1991, 27, 947–954. [Google Scholar] [CrossRef]
- Turner-Jones, A.; Aizlewood, A.; Beckett, D.R. Crystalline forms of isotactic polypropylene. Macromol. Chem. Phys. 1964, 75, 134–158. [Google Scholar] [CrossRef]
- Borysiak, S. Fundamental Studies on Lignocellulose/Polypropylene Composites: Effects of Wood Treatment on the Transcrystalline Morphology and Mechanical Properties. J. Appl. Polym. Sci. 2013, 127, 1309–1322. [Google Scholar] [CrossRef]
- Van Wyk, J.P.H.; Mohulatsi, M. Biodegradation of Waste Cellulose. J. Polym. Environ. 2003, 11, 23–28. [Google Scholar] [CrossRef]
- Taherzadeh, M.; Karimi, K. Pretreatment of Lignocellulosic Wastes to Improve Ethanol and Biogas Production: A Review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef] [PubMed]
- Cologna, N.M.D.; Gómez-Mendoza, D.P.; Zanoelo, F.F.; Giannesi, G.C.; Guimarães, N.C.A.; Moreira, L.R.S.; Filho, E.X.F.; Ricart, C.A.O. Exploring Trichoderma and Aspergillus secretomes: Proteomics approaches for the identification of enzymes of biotechnological interest. Enzyme Microb. Technol. 2018, 109, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hu, H. X-ray diffraction study of bamboo fibers treated with NaOH. Fibers Polym. 2008, 9, 735–739. [Google Scholar] [CrossRef]
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Dimarogona, M.; Topakas, E.; Christakopoulos, P. Cellulose degradation by oxidative enzymes. Comput. Struct. Biotechnol. J. 2012, 2, e201209015. [Google Scholar] [CrossRef] [PubMed]
- Dashtban, M.; Schrafth, H.; Qin, W. Fungal bioconversion of lignocellulosic residues; opportunities & perspectivies. Int. J. Biol. Sci. 2009, 5, 578–595. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Squina, F.; Segato, F.; Mort, A.; Lee, D.; Pappan, K.; Prade, R. High-temperature enzymatic breakdown of cellulose. Appl. Environ. Microbiol. 2011, 77, 5199–5206. [Google Scholar] [CrossRef]
- Langston, J.A.; Shaghasi, T.; Abbate, E.; Xu, F.; Vlasenko, E.; Sweeney, M.D. Oxidoreductive cellulose depolymerization by the enzymes cellobiose dehydrogenase and glycoside hydrolase 61. Appl. Environ. Microbiol. 2011, 77, 7007–7015. [Google Scholar] [CrossRef]
- Houfani, A.A.; Anders, N.; Spiess, A.C.; Baldrian, P.; Benallaoua, S. Insights from enzymatic degradation of cellulose and hemicellulose to fermentable sugars—A review. Biomass Bioenerg. 2020, 134, 105481. [Google Scholar] [CrossRef]
- Pirich, C.L.; Picheth, G.F.; Fontes, A.M.; Delgado-Aguilar, M.; Ramos, L.P. Disruptive enzyme-based strategies to isolate nanocelluloses: A review. Cellulose 2020, 27, 5457–5475. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? JCREEC 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.S.A.; Bojorge, N.; Junior, N.P. Statistical analysis of the crystallinity index of nanocellulose produced from Kraft pulp via controlled enzymatic hydrolysis. Biotechnol. Appl. Biochem. 2020, 67, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Beltramino, F.; Roncero, M.B.; Torres, A.L.; Vidal, T.; Valls, C. Optimization of sulfuric acid hydrolysis conditions for preparation of nanocrystalline cellulose from enzymatically pretreated fibers. Cellulose 2016, 23, 1777–1789. [Google Scholar] [CrossRef]
- Cao, Y.; Tan, H. Structural characterization of cellulose with enzymatic treatment. J. Mol. Struct. 2004, 705, 189–193. [Google Scholar] [CrossRef]
- Zhbankov, R.G.; Firsov, S.P.; Buslov, D.K.; Nikonenko, N.A.; Marchewka, M.K.; Ratajczak, H. Structural physico-chemistry of cellulose macromolecules. Vibrational spectra and structure of cellulose. J. Mol. Struct. 2002, 614, 117–125. [Google Scholar] [CrossRef]
- Mazzanti, V.; Mollica, F. A Review of Wood Polymer Composites Rheology and Its Implications for Processing. Polymers 2020, 12, 2304. [Google Scholar] [CrossRef]
- Santi, C.R.; Hage, E.; Vlachopoulos, J.; Correa, C.A. Rheology and processing of HDPE/wood flour composites. Int. Polym. Process. 2009, 24, 346–353. [Google Scholar] [CrossRef]
- Hristov, V.; Vlachopoulos, J. Effects of polymer molecular weight and filler particle size on flow behavior of wood polymer composites. Polym. Compos. 2008, 29, 831–839. [Google Scholar] [CrossRef]
- Bose, S.; Mahanwar, P.A. Effect of particle size of filler on properties of nylon-6. J. Miner. Mater. Char. Eng. 2004, 3, 23–31. [Google Scholar] [CrossRef]
- Zanjanijam, A.R.; Hakim, S.; Azizi, H. Morphological, dynamic mechanical, rheological and impact strength properties of the PP/PVB blends: The effect of waste PVB as a toughener. RSC Adv. 2016, 6, 44673–44686. [Google Scholar] [CrossRef]
- Shabani, A.; Babaei, A.; Zanjanijam, A.R.; Ramezani, M.; Haji Abdolrasouli, M. Investigating the mechanical, morphological, and thermal behavior of PA-6/SAN/MWCNT blends: Application of Taguchi experimental design. Polym. Compos. 2019, 40, 4753–4762. [Google Scholar] [CrossRef]
- Kose, R.; Kondo, T. Size effects of cellulose nanofibers for enhancing the crystallization of poly (lactic acid). J. Appl. Polym. Sci. 2013, 128, 1200–1205. [Google Scholar] [CrossRef]
- Xu, J.; Manepalli, P.H.; Zhu, L.; Narayan-Sarathy, S.; Alavi, S. Morphological, barrier and mechanical properties of films from poly (butylene succinate) reinforced with nanocrystalline cellulose and chitin whiskers using melt extrusion. J. Polym. Res. 2019, 26, 188. [Google Scholar] [CrossRef]
- Borysiak, S.; Klapiszewski, Ł.; Bula, K.; Jesionowski, T. Nucleation ability of advanced functional silica/lignin hybrid fillers in polypropylene composites. J. Therm. Anal. Calorim. 2016, 126, 251–262. [Google Scholar] [CrossRef]
- Lenes, M.; Gregersen, O.W. Effect of surface chemistry and topography of sulphite fibres on the transcrystallinity of polypropylene. Cellulose 2006, 13, 345–355. [Google Scholar] [CrossRef]
- Lee, B.G.; Lee, S.; Via, B.K. Influence of surface morphology of the kraft pulp fibers on the growth of the transcrystalline layer of polypropylene. J. Appl. Polym. Sci. 2010, 116, 1958–1966. [Google Scholar] [CrossRef]
- Odalanowska, M.; Woźniak, M.; Ratajczak, I.; Zielińska, D.; Cofta, G.; Borysiak, S. Propolis and Organosilanes as Innovative Hybrid Modifiers in Wood-Based Polymer Composites. Materials 2021, 14, 464. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Xu, Y.; Wei, Z.; Song, P.; Chen, G.; Zhang, W. Mechanical properties, crystallization and melting behaviors of carbon fiber-reinforced PA6 composites. J. Therm. Anal. Calorim. 2013, 115, 209–218. [Google Scholar] [CrossRef]
- Noorbakhsh-Soltani, S.M.; Zerafat, M.M.; Sabbaghi, S. A comparative study of gelatin and starch-based nano-composite films modified by nano-cellulose and chitosan for food packaging applications. Carbohydr. Polym. 2018, 189, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Chirayil, C.J.; Mathew, L.; Hassan, P.A.; Mozetic, M.; Thomas, S. Rheological behaviour of nanocellulose reinforced unsaturated polyester nanocomposites. Int. J. Biol. Macromol. 2014, 69, 274–281. [Google Scholar] [CrossRef] [PubMed]
- De Souza, A.G.; de Lima, G.F.; Colombo, R.; Rosa, D.S. A new approach for the use of anionic surfactants: Nanocellulose modification and development of biodegradable nanocomposites. Cellulose 2020, 27, 5707–5728. [Google Scholar] [CrossRef]
- Dehnad, D.; Emam-Djomeh, Z.; Mirzaei, H.; Jafari, S.M.; Dadashi, S. Optimization of physical and mechanical properties for chitosan-nanocellulose biocomposites. Carbohydr. Polym. 2014, 105, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Yoon, M.J.; Lee, E.S.; Lim, D.Y.; Kim, K.Y. Preparation and characterization of cellulose nanofibers (CNFs) from microcrystalline cellulose (MCC) and CNF/polyamide 6 composites. Macromol. Res. 2014, 22, 738–745. [Google Scholar] [CrossRef]
- El-Hadi, A.M. Increase the elongation at break of poly (lacticacid) composites for use in food packaging films. Sci. Rep. 2017, 7, 46767. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, K.; Dixit, S.; Pal, D.; Mishra, P.; Srivastava, P.; Srivastava, N.; Hashem, A.; Alqarawi, A.A.; Abd_Allah, E.F. Effect of nanocellulose on mechanical and barrier properties of PVA–banana pseudostem fiber composite films. Environ. Technol. Innov. 2021, 21, 101312. [Google Scholar] [CrossRef]
- Jacoby, P.; Bersted, B.H.; Kissel, W.J.; Smith, C.E. Studies on the β-crystalline form of isotactic polypropylene. J. Polym. Sci. Part B Polym. Phys. 1986, 24, 461–491. [Google Scholar] [CrossRef]
| Sample | Material |
|---|---|
| Cel_A | Sigmacell Type 20 |
| Cel_A-Tr | Sigmacell Type 20—Trichoderma reesei |
| Cel_A-Asp | Sigmacell Type 20—Aspergillus sp. |
| Cel_B | Sigmacell Type 101 |
| Cel_B-Tr | Sigmacell Type 101—Trichoderma reesei |
| Cel_B-Asp | Sigmacell Type 101—Aspergillus sp. |
| Sample | Glucose Concentration (mg/mL) | Recovery of Nanocellulose (%) |
|---|---|---|
| Cel_A | n.d. | n.d. |
| Cel_A-Tr | 27.31 | 18 |
| Cel_A-Asp | 3.66 | 89 |
| Cel_B | n.d | n.d |
| Cel_B-Tr | 17.10 | 49 |
| Cel_B-Asp | 4.30 | 87 |
| Sample | Degree of Crystallinity (%) |
|---|---|
| Cel_A | 49 |
| Cel_A-Tr | 56 |
| Cel_A-Asp | 43 |
| Cel_B | 29 |
| Cel_B-Tr | 51 |
| Cel_B-Asp | 32 |
| Sample | Z-Average (nm) | PDI |
|---|---|---|
| Cel_A-Tr | 75 | 0.105 |
| Cel_A-Asp | 505 | 0.277 |
| Cel_B-Tr | 76 | 0.181 |
| Cel_B-Asp | 135 | 0.217 |
| Sample | Content of the β-PP Form (%) |
|---|---|
| PP | 10 |
| PP + Cel_A | 20 |
| PP + Cel_A-Tr | 41 |
| PP + Cel_A-Asp | 22 |
| PP + Cel_B | 17 |
| PP + Cel_B-Tr | 40 |
| PP + Cel_B-Asp | 29 |
| Sample | Half-Time of Crystallization (min) | Temperature of Crystallization (°C) | Degree of Crystallinity (%) | Induction Time (min) | Transcrystalline Layer Growth Rate (μm/min) |
|---|---|---|---|---|---|
| PP | 2.7 | 113.3 | 39 | n.d. | n.d. |
| PP + Cel_A | 2.2 | 122.5 | 20 | 5 | 2.2 |
| PP + Cel_A-Tr | 1.4 | 128.2 | 20 | 3 | 2.8 |
| PP + Cel_A-Asp | 2.1 | 120.3 | 21 | 11 | 1.2 |
| PP + Cel_B | 2.0 | 122.1 | 22 | 13 | 1.5 |
| PP + Cel_B-Tr | 1.4 | 129.2 | 25 | 3 | 3.2 |
| PP + Cel_B-Asp | 1.8 | 127.6 | 20 | 3 | 2.4 |
| Sample | Tensile Strength (MPa) | Modulus (GPa) | Elongation at Break (%) | Impact Strength (kJ/m2) |
|---|---|---|---|---|
| PP | 30.2 (0.18) | 1.31 (0.08) | 342 (21.1) | 51.8 (0.89) |
| PP + Cel_A | 31.5 (0.37) | 1.75 (0.20) | 18.2 (3.8) | 18.4 (2.31) |
| PP + Cel_A-Tr | 35.8 (0.20) | 1.43 (0.13) | 72.5 (5.6) | 37.6 (1.24) |
| PP + Cel_A-Asp | 32.4 (0.33) | 1.70 (0.21) | 21.6 (3.1) | 20.4 (2.04) |
| PP + Cel_B | 31.1 (0.41) | 1.77 (0.19) | 15.7 (3.9) | 16.9 (2.75) |
| PP + Cel_B-Tr | 36.1 (0.26) | 1.46 (0.11) | 69.8 (4.3) | 38.1 (1.56) |
| PP + Cel_B-Asp | 34.4 (0.23) | 1.52 (0.14) | 34.7 (3.6) | 27.3 (1.94) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zielińska, D.; Szentner, K.; Waśkiewicz, A.; Borysiak, S. Production of Nanocellulose by Enzymatic Treatment for Application in Polymer Composites. Materials 2021, 14, 2124. https://doi.org/10.3390/ma14092124
Zielińska D, Szentner K, Waśkiewicz A, Borysiak S. Production of Nanocellulose by Enzymatic Treatment for Application in Polymer Composites. Materials. 2021; 14(9):2124. https://doi.org/10.3390/ma14092124
Chicago/Turabian StyleZielińska, Daria, Kinga Szentner, Agnieszka Waśkiewicz, and Sławomir Borysiak. 2021. "Production of Nanocellulose by Enzymatic Treatment for Application in Polymer Composites" Materials 14, no. 9: 2124. https://doi.org/10.3390/ma14092124
APA StyleZielińska, D., Szentner, K., Waśkiewicz, A., & Borysiak, S. (2021). Production of Nanocellulose by Enzymatic Treatment for Application in Polymer Composites. Materials, 14(9), 2124. https://doi.org/10.3390/ma14092124







