Recovery of Gold from Ore with Potassium Ferrocyanide Solution under UV Light
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Reagents
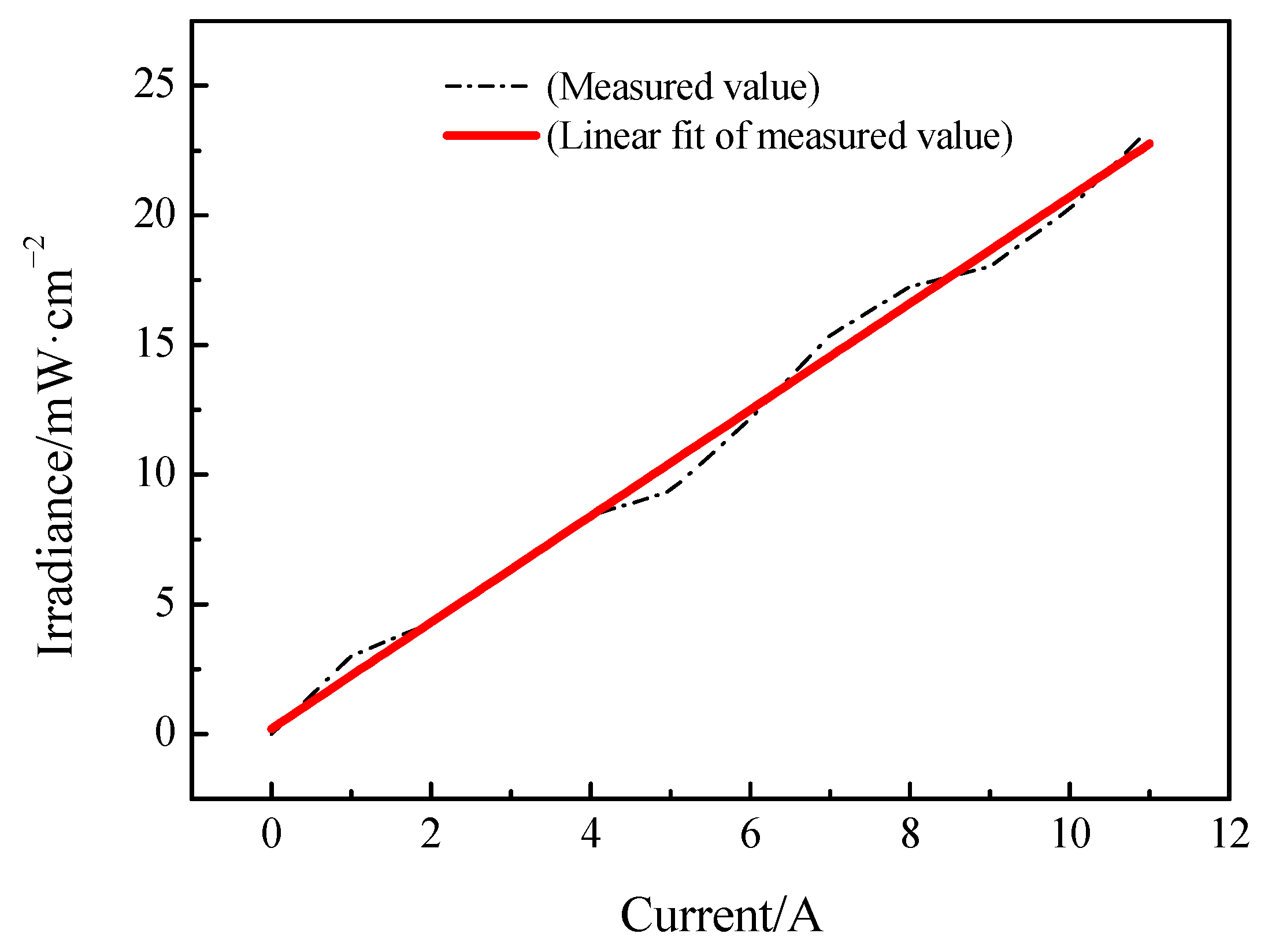
2.2. Light Source
2.3. Gold Ore Leaching Experimental Design
2.4. Quartz Crystal Microbalance with Dissipation (QCM-D)
3. Results and Discussion
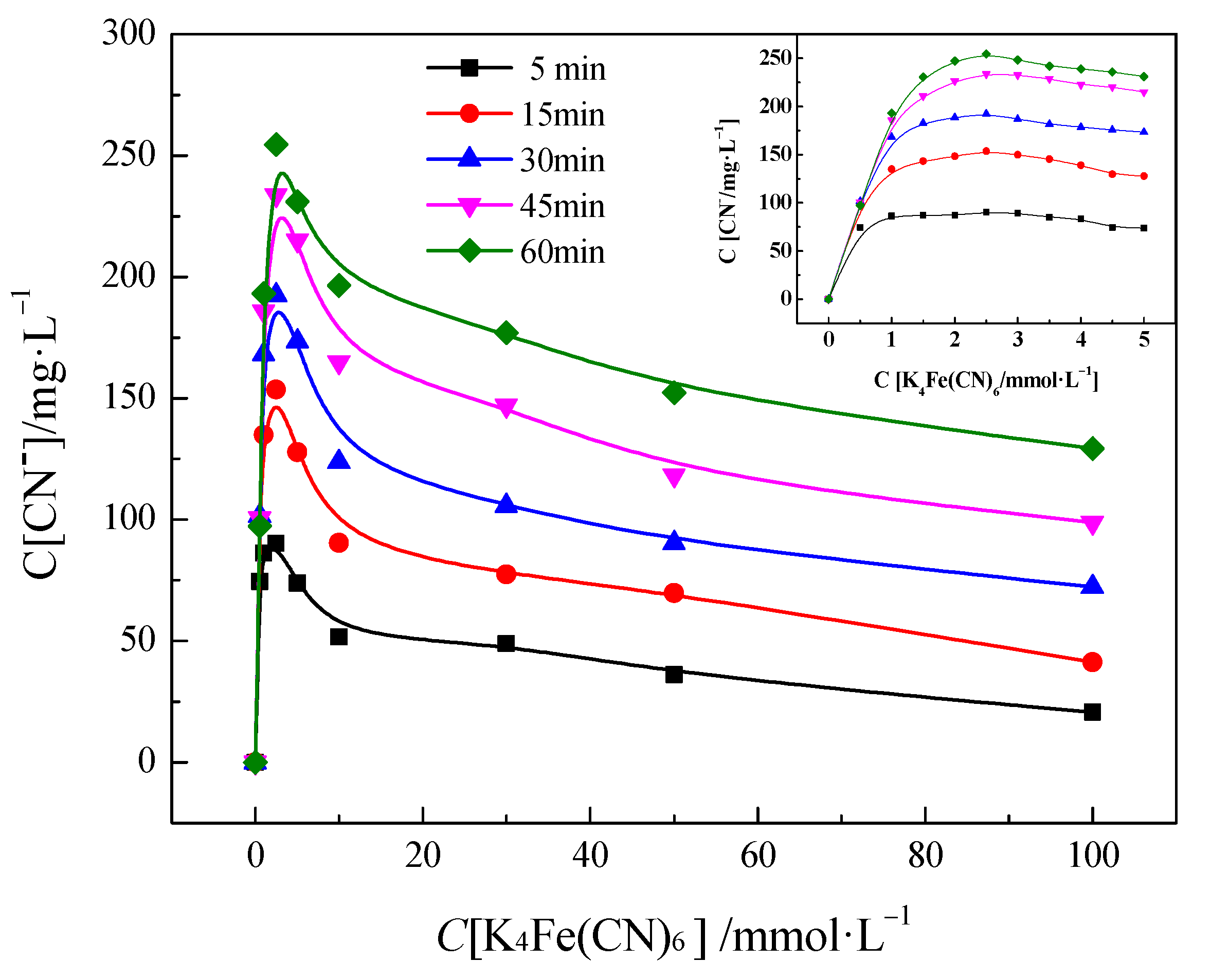
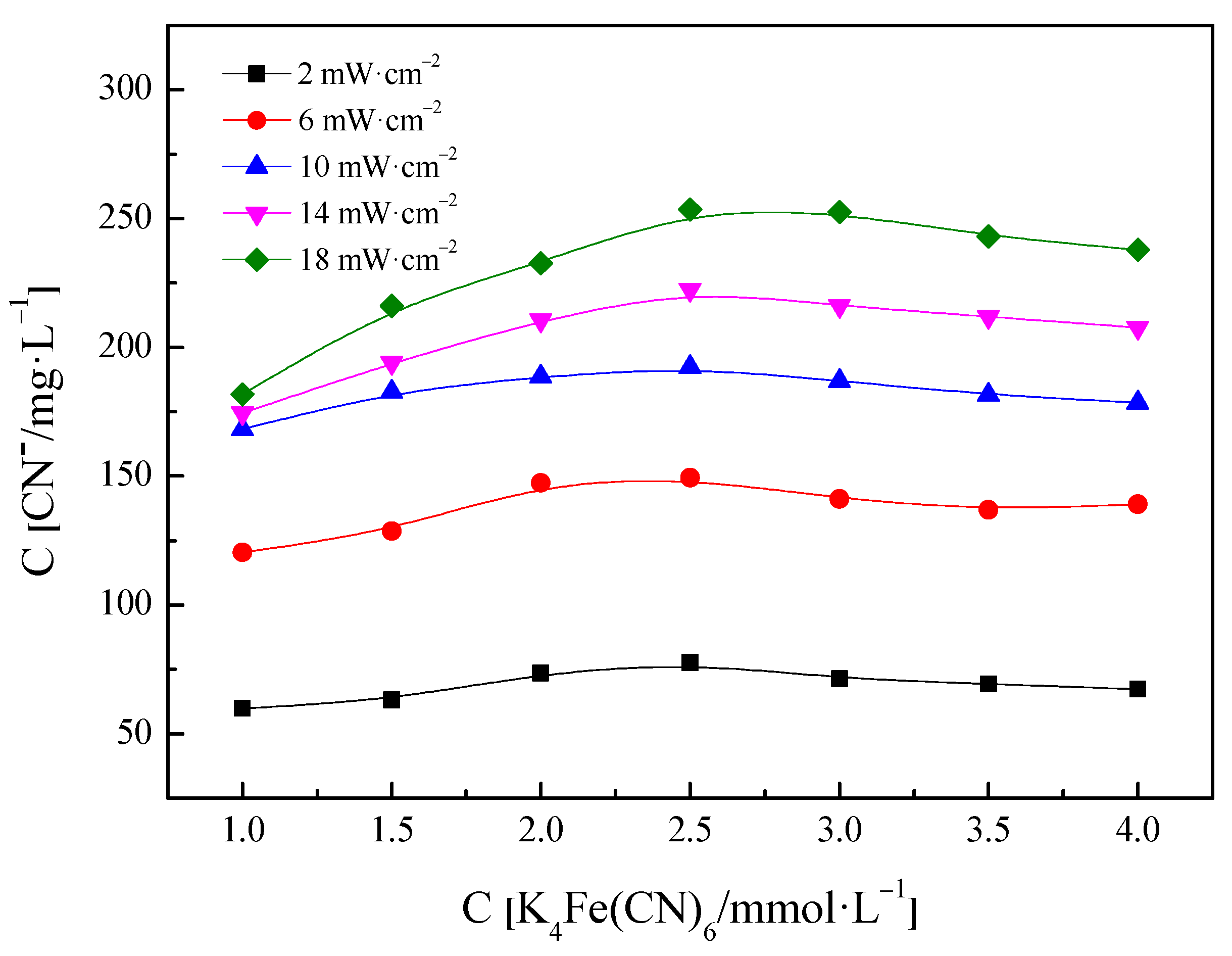
3.1. Decomposition of PF Solution under UV Light
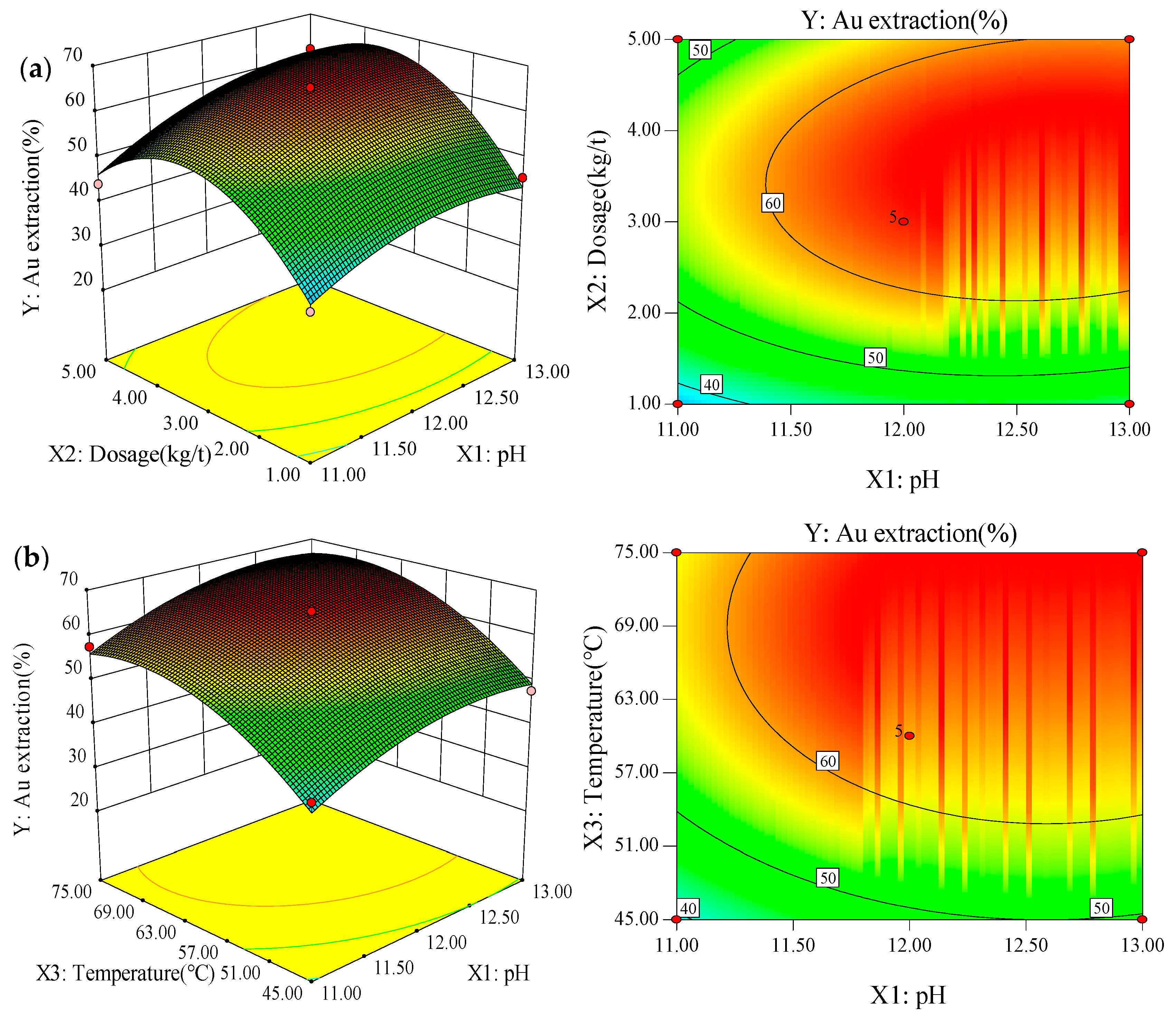
3.2. Box-Behnken Design and Response Surface Methodology
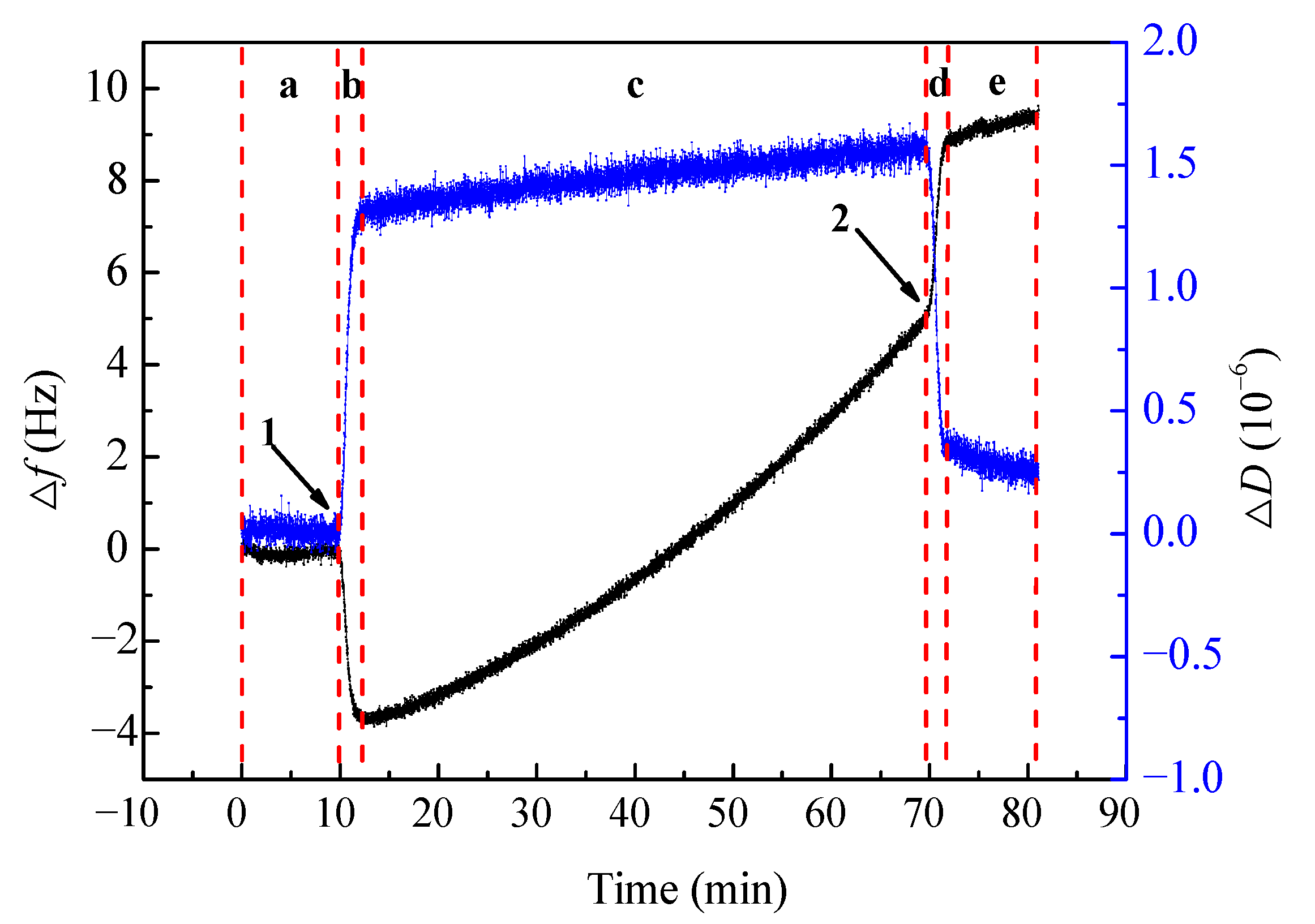
3.3. Kinetic Process of Gold Leaching on Au Sensor with PF by QCM-D
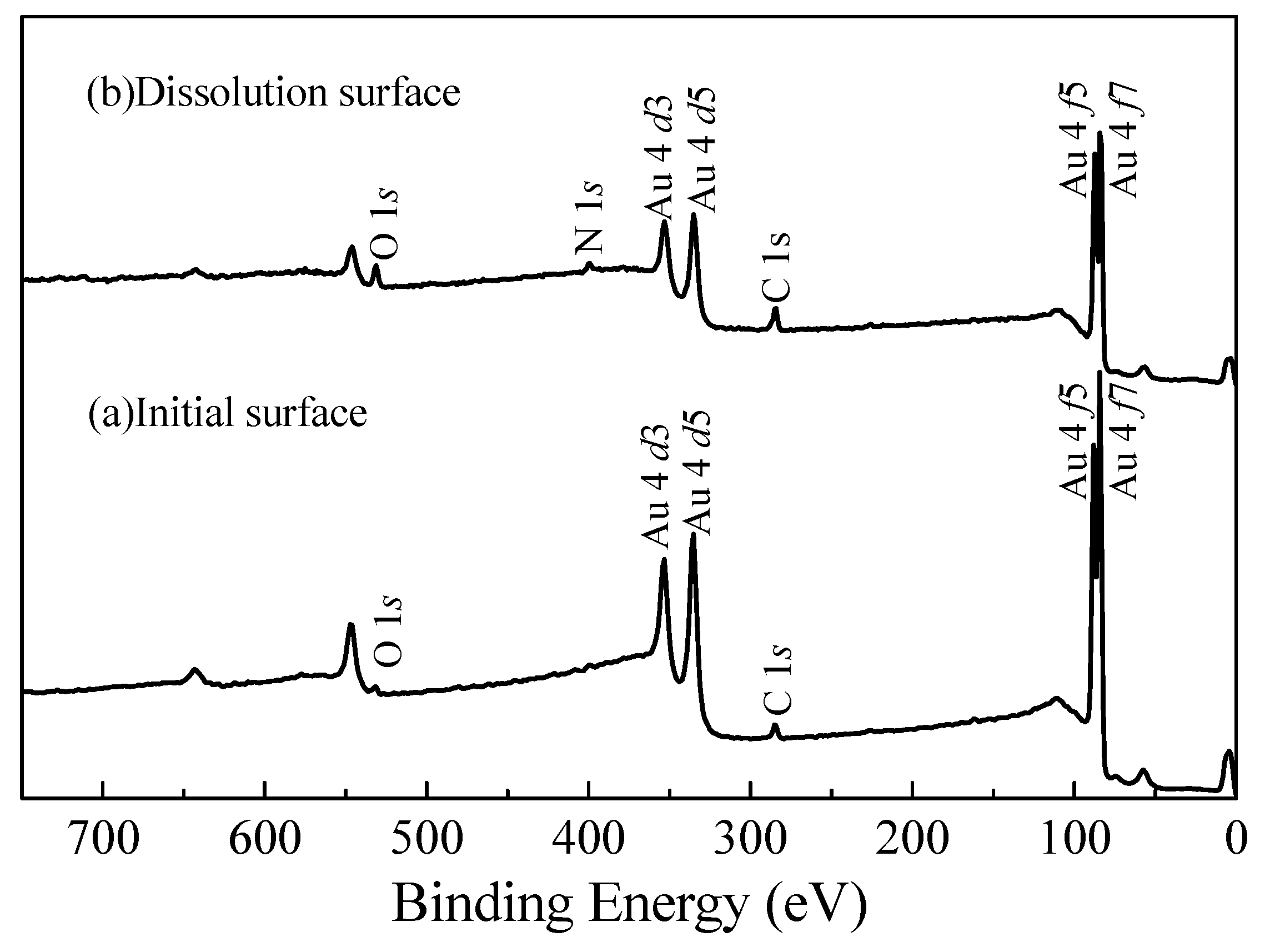
3.4. Surface Product Composition Analysis by XPS
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vargas, C.; Navarro, P.; Espinoza, D.; Manríquez, J.; Mejía, E. Dissolution Behavior of Gold in Alkaline Media Using Thiourea. Int. J. Nonferrous Metall. 2018, 8, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Feng, Y.; Huang, W.; Li, H.; Liao, S. The role of glycine in the ammonium thiocyanate leaching of gold. Hydrometallurgy 2019, 185, 111–116. [Google Scholar] [CrossRef]
- Liang, C.; Li, J. Recovery of gold in iodine-iodide system—A review. Sep. Sci. Technol. 2019, 54, 1055–1066. [Google Scholar] [CrossRef]
- Dong, Z.; Jiang, T.; Xu, B.; Yang, Y.; Li, Q. An eco-friendly and efficient process of low potential thiosulfate leaching-resin adsorption recovery for extracting gold from a roasted gold concentrate. J. Clean. Prod. 2019, 229, 387–398. [Google Scholar] [CrossRef]
- Jeffrey, M.I.; Breuer, P.L. The cyanide leaching of gold in solutions containing sulfide. Miner. Eng. 2000, 13, 1097–1106. [Google Scholar] [CrossRef]
- Oraby, E.; Eksteen, J.; Karrech, A.; Attar, M. Gold extraction from paleochannel ores using an aerated alkaline glycine lixiviant for consideration in heap and in-situ leaching applications. Miner. Eng. 2019, 138, 112–118. [Google Scholar] [CrossRef]
- Hilson, G.; Monhemius, A. Alternatives to cyanide in the gold mining industry: What prospects for the future? J. Clean. Prod. 2006, 14, 1158–1167. [Google Scholar] [CrossRef]
- Azizitorghabeh, A.; Wang, J.; Ramsay, J.A.; Ghahreman, A. A review of thiocyanate gold leaching—Chemistry, thermodynamics, kinetics and processing. Miner. Eng. 2021, 160, 106689. [Google Scholar] [CrossRef]
- Oraby, E.; Eksteen, J.; O’Connor, G. Gold leaching from oxide ores in alkaline glycine solutions in the presence of permanganate. Hydrometallurgy 2020, 198, 105527. [Google Scholar] [CrossRef]
- Tran, Q.B.; Lohitnavy, M.; Phenrat, T. Assessing potential hydrogen cyanide exposure from cyanide-contaminated mine tailing management practices in Thailand’s gold mining. J. Environ. Manag. 2019, 249, 109357. [Google Scholar] [CrossRef]
- Meeussen, J.C.; Keizer, M.G.; De Haan, F.A. Chemical stability and decomposition rate of iron cyanide complexes in soil solutions. Environ. Sci. Technol. 1992, 26, 511–516. [Google Scholar] [CrossRef]
- Lim, H.S.; Hwang, J.Y.; Choi, E.; Lee, G.; Yun, S.S.; Kim, M. Assessment of ferrocyanide intake from food-grade salt in the Korean population. LWT Food Sci. Technol. 2018, 93, 620–627. [Google Scholar] [CrossRef]
- Van Nguyen, M.; Thorarinsdottir, K.A.; Thorkelsson, G.; Gudmundsdottir, A.; Arason, S. Influences of potassium ferrocyanide on lipid oxidation of salted cod (Gadus morhua) during processing, storage and rehydration. Food Chem. 2012, 131, 1322–1331. [Google Scholar] [CrossRef]
- Ašpergěr, S. Kinetics of the decomposition of potassium ferrocyanide in ultra-violet light. Trans. Faraday Soc. 1952, 48, 617–624. [Google Scholar] [CrossRef]
- Pommeret, S.; Naskrecki, R.; van der Meulen, P.; Ménard, M.; Vigneron, G.; Gustavsson, T. Ultrafast events in the electron photodetachment from the hexacyanoferrate (II) complex in solution. Chem. Phys. Lett. 1998, 288, 833–840. [Google Scholar] [CrossRef]
- Betancourt-Buitrago, L.; Vásquez, C.; Veitia, L.; Ossa-Echeverry, O.; Rodriguez-Vallejo, J.; Barraza-Burgos, J.; Marriaga-Cabrales, N.; Machuca-Martínez, F. An approach to utilize the artificial high power LED UV-A radiation in photoreactors for the degradation of methylene blue. Photochem. Photobiol. Sci. 2017, 16, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Hanela, S.; Durán, J.; Jacobo, S. Removal of iron–cyanide complexes from wastewaters by combined UV–ozone and modified zeolite treatment. J. Environ. Chem. Eng. 2015, 3, 1794–1801. [Google Scholar] [CrossRef]
- Chen, W.D.; Kang, S.-K.; Stark, W.J.; Rogers, J.A.; Grass, R.N. The light triggered dissolution of gold wires using potassium ferrocyanide solutions enables cumulative illumination sensing. Sens. Actuators B 2019, 282, 52–59. [Google Scholar] [CrossRef]
- Chemical Book Database. Available online: https://www.chemicalbook.com/ProductChemicalPropertiesCB3329047.htm (accessed on 26 March 2021).
- Jeffrey, M.I.; Woods, R. Frequency changes observed with the EQCM resulting from hydrophilic/hydrophobic transitions. Colloids Surf. 2007, 302, 488–493. [Google Scholar] [CrossRef]
- Kou, J.; Xu, S. In situ kinetics and conformation studies of dodecylamine adsorption onto zinc sulfide using a quartz crystal microbalance with dissipation (QCM-D). Colloids Surf. 2016, 490, 110–120. [Google Scholar] [CrossRef]
- Hieda, M.; Garcia, R.; Dixon, M.; Daniel, T.; Allara, D.; Chan, M. Ultrasensitive quartz crystal microbalance with porous gold electrodes. Appl. Phys. Lett. 2004, 84, 628–630. [Google Scholar] [CrossRef]
- Sauerbrey, G. Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung. Z. Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Swiatek, S.; Komorek, P.; Jachimska, B. Adsorption of β-lactoglobulin A on gold surface determined in situ by QCM-D measurements. Food Hydrocoll. 2019, 91, 48–56. [Google Scholar] [CrossRef]
- Jeffrey, M.I.; Linda, L.; Breuer, P.L.; Chu, C.K. A kinetic and electrochemical study of the ammonia cyanide process for leaching gold in solutions containing copper. Miner. Eng. 2002, 15, 1173–1180. [Google Scholar] [CrossRef]
- Josefsson, P.; Henriksson, G.; Wågberg, L. The physical action of cellulases revealed by a quartz crystal microbalance study using ultrathin cellulose films and pure cellulases. Biomacromolecules 2008, 9, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Gutig, C.; Grady, B.P.; Striolo, A. Experimental studies on the adsorption of two surfactants on solid—Aqueous interfaces: Adsorption isotherms and kinetics. Langmuir 2008, 24, 4806–4816. [Google Scholar] [CrossRef]
- Liu, J.-Z.; Weng, L.-P.; Zhang, Q.-L.; Xu, H.; Ji, L.-N. Optimization of glucose oxidase production by Aspergillus niger in a benchtop bioreactor using response surface methodology. World J. Microbiol. Biotechnol. 2003, 19, 317–323. [Google Scholar] [CrossRef]
- Johnson, C.A. The fate of cyanide in leach wastes at gold mines: An environmental perspective. Appl. Geochem. 2015, 57, 194–205. [Google Scholar] [CrossRef]
- Chastain, J.; King, R.C., Jr. Handbook of X-ray photoelectron spectroscopy. Perkin Elmer Corp. 1992, 40, 221. [Google Scholar]
- Xu, S.; Kou, J.; Sun, T.; Jong, K. A study of adsorption mechanism of dodecylamine on sphalerite. Colloids Surf. 2015, 486, 145–152. [Google Scholar] [CrossRef]
- Ping, Z.; Xiaolin, W.; Jiangrong, Y.; Xiaoguo, F.; Lizhu, L. Effect of Vacuum Heat Treatment on oxidation of Uranium Surfaces. Rare Met. Mater. Eng. 2008, 1, 94–97. [Google Scholar]
- Xu, Y.; Sun, B.-L.; Ma, C.; Zhang, P.; Cai, M.-L.; Wu, X.-M. XPS and SEM spectroscopy study of hyperdispersant on atrazine surface. Spectrosc. Spectral Anal. 2011, 31, 2569–2573. [Google Scholar]
- Abbott, A.P.; Frisch, G.; Gurman, S.J.; Hillman, A.R.; Hartley, J.; Holyoak, F.; Ryder, K.S. Ionometallurgy: Designer redox properties for metal processing. Chem. Commun. (Camb.) 2011, 47, 10031–10033. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Feng, Y. Investigation on the electrocatalytic characteristics of SnO2 electrodes with nanocoating prepared by electrodeposition method. Sci. China Ser. E Technol. Sci. 2009, 52, 1799–1803. [Google Scholar] [CrossRef]
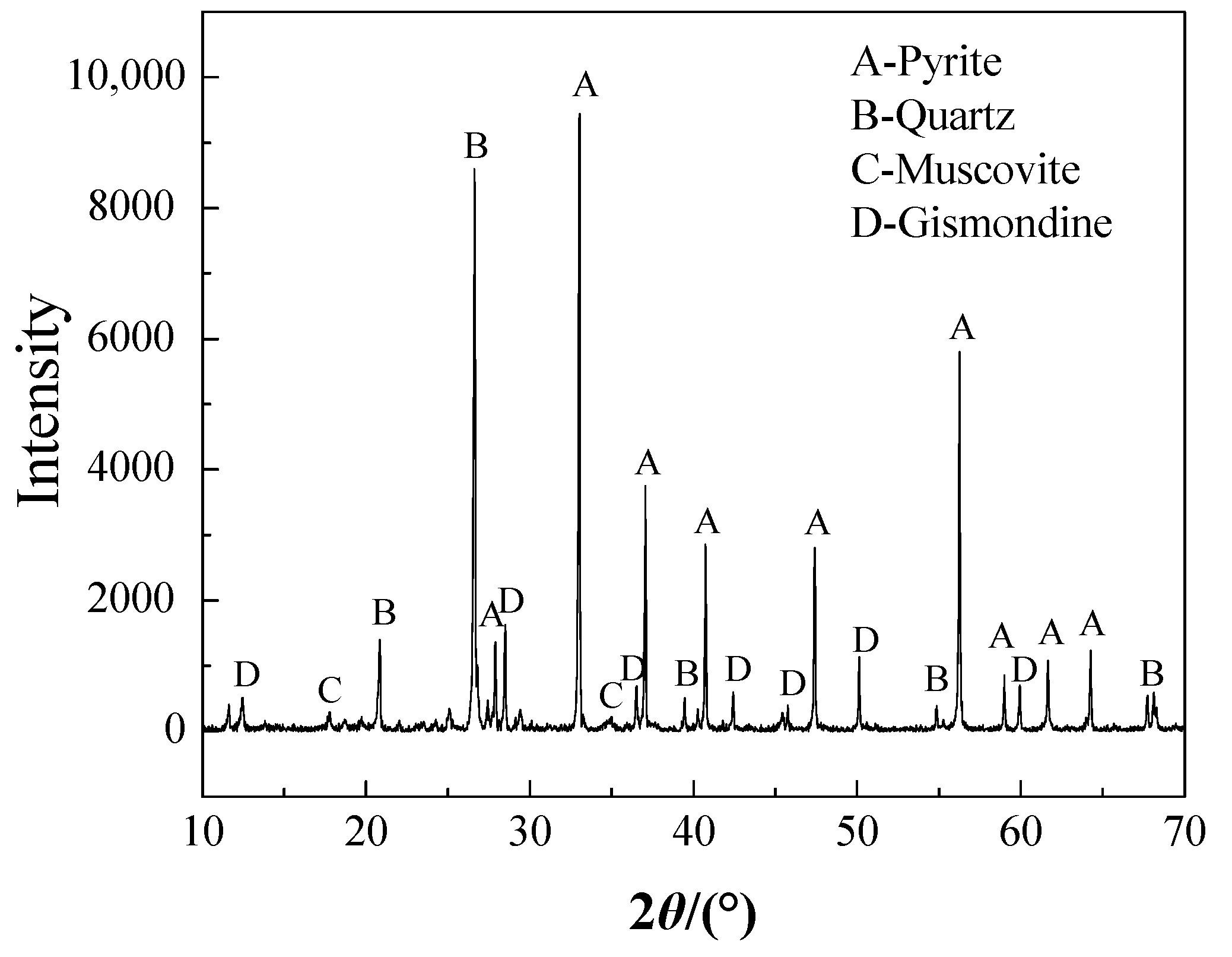
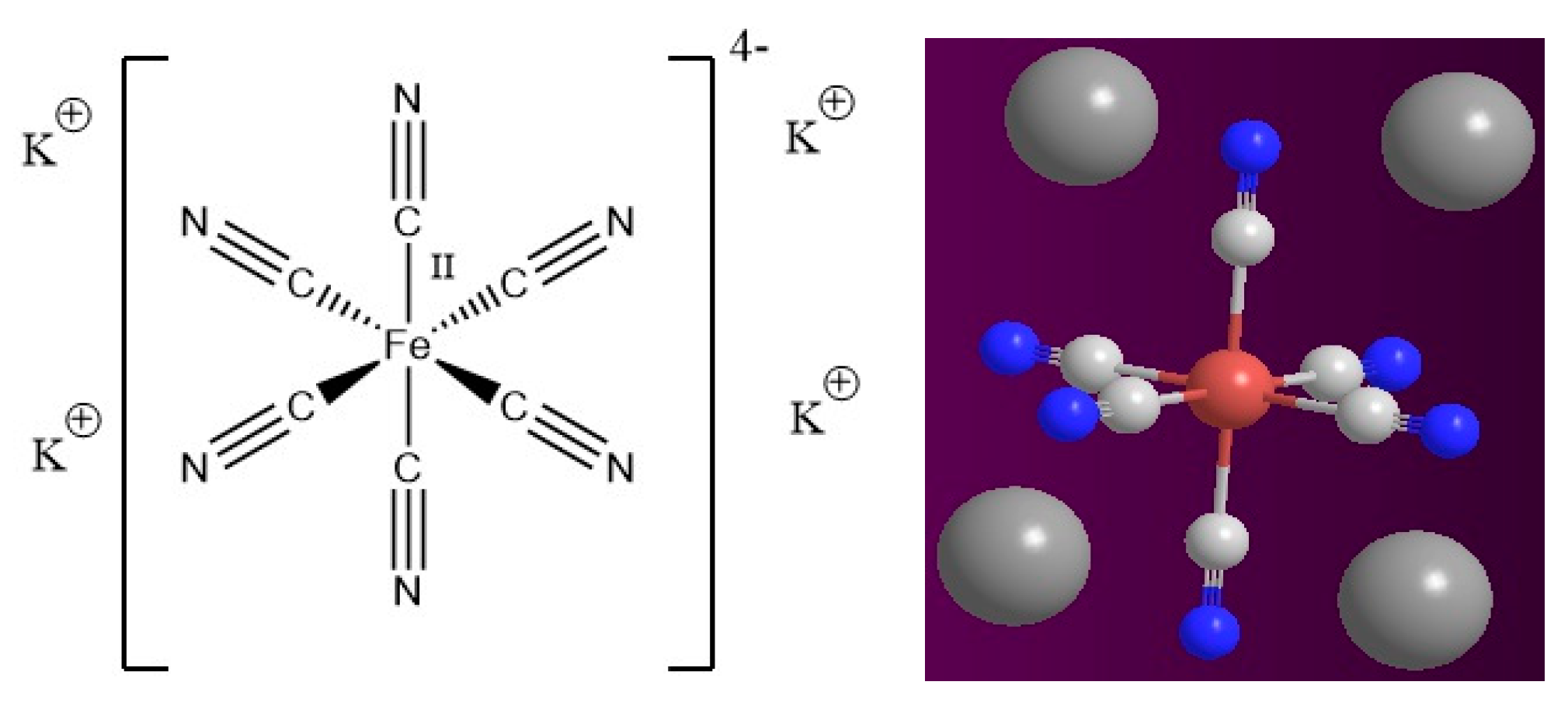
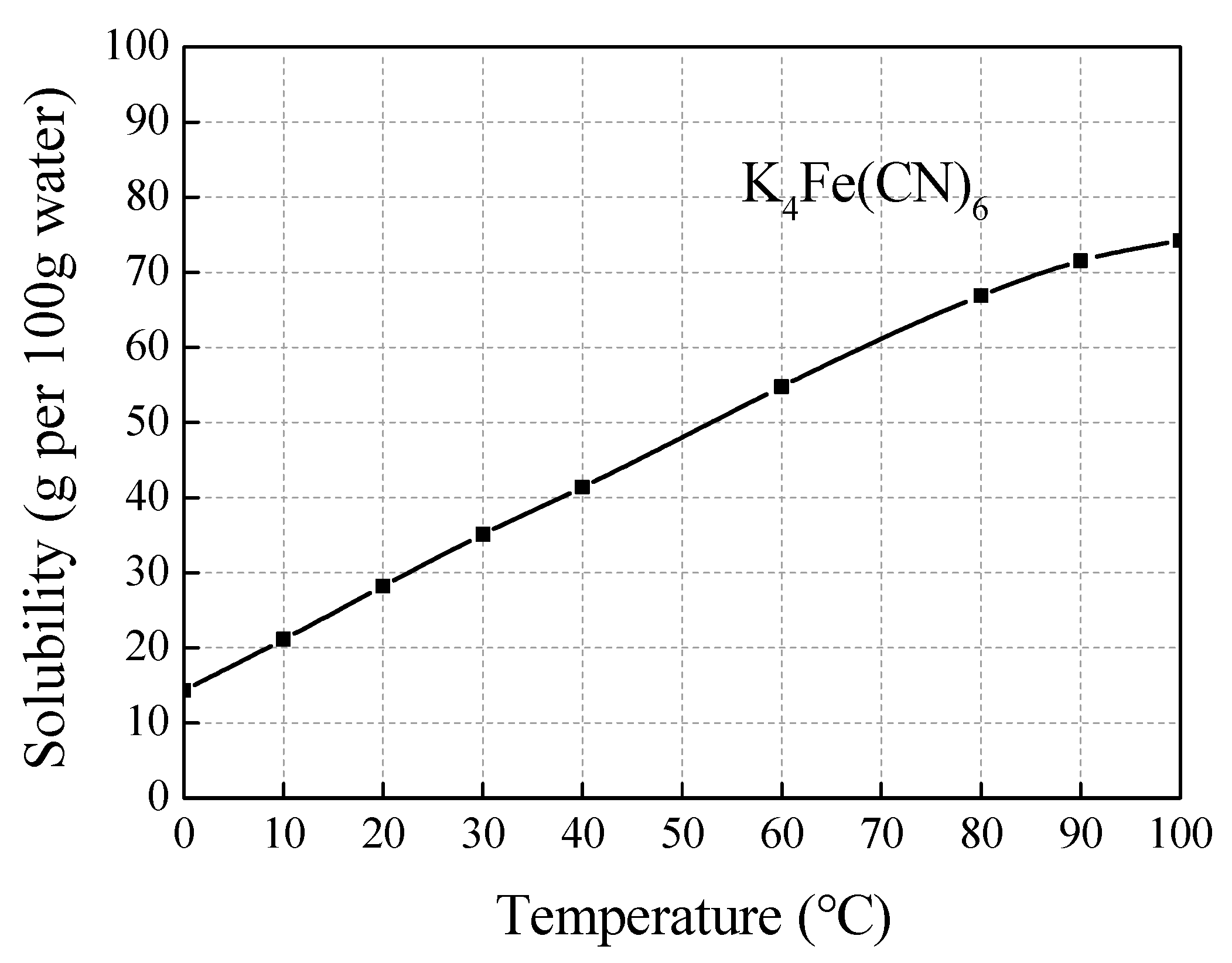
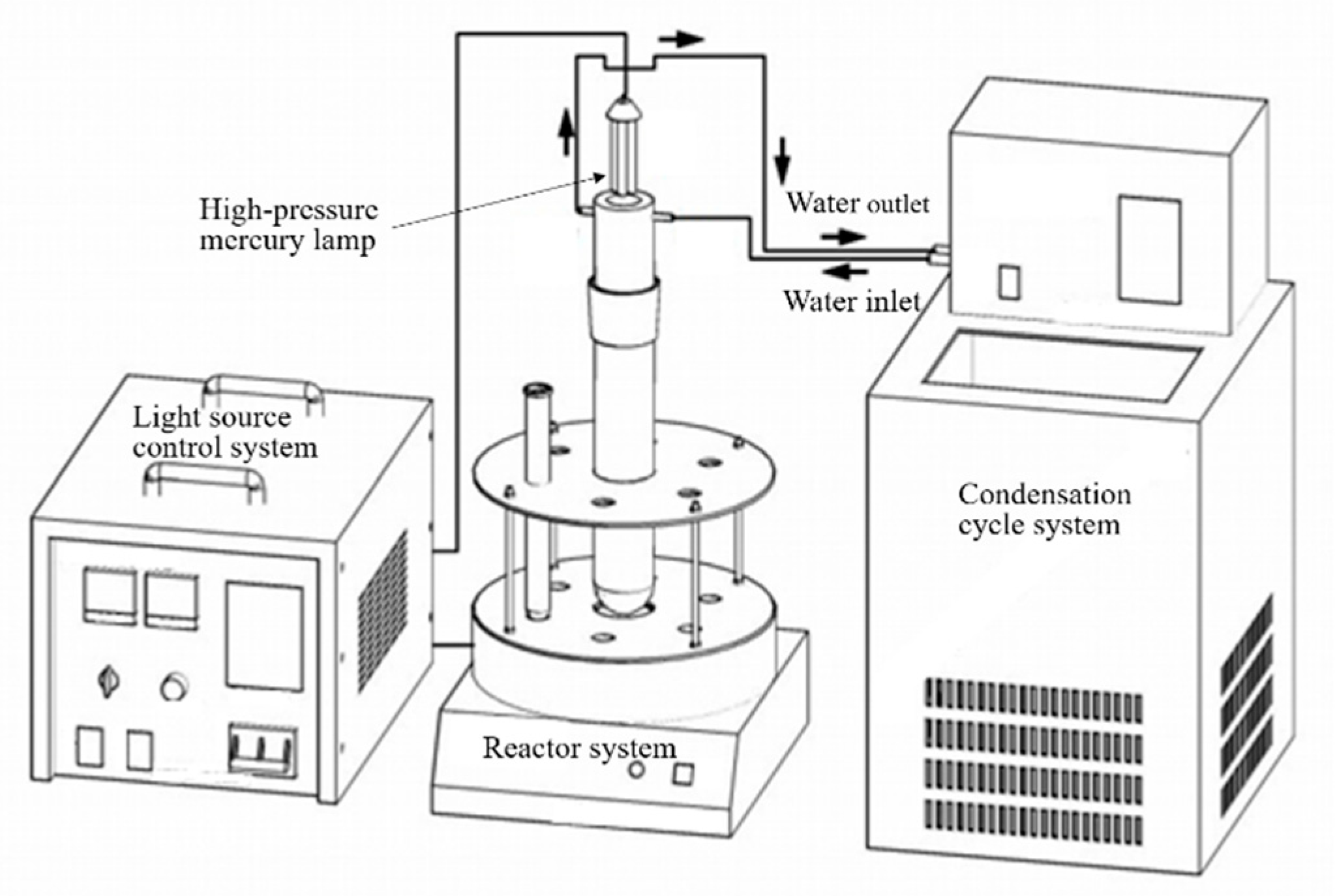



| Au * | Ag * | Fe | Cu | As | Zn |
| 56.78 | 38.8 | 26.03 | 0.11 | 0.082 | 0.044 |
| Pb | S | CaO | MgO | Al2O3 | SiO2 |
| 0.10 | 26.64 | 1.27 | 0.95 | 6.69 | 33.51 |
| Wavelength/nm | 250 | 313 | 365 | 400 | 510 | 620 | 720 |
| Relative intensity/% | 20 | 85 | 100 | 30 | 20 | 40 | 80 |
| Levels | X1: pH | X2: Dosage (kg/t) | X3: Temperature (°C) |
|---|---|---|---|
| −1 | 11 | 1 | 45 |
| 0 | 12 | 3 | 60 |
| 1 | 13 | 5 | 75 |
| Run | Factors | Response | ||
|---|---|---|---|---|
| X1: pH | X2: Dosage (kg/t) | X3: Temperature (°C) | Y: Au Extraction (%) | |
| 1 | 11 | 5 | 60 | 44.17 |
| 2 | 12 | 1 | 45 | 29.38 |
| 3 | 12 | 3 | 60 | 63.19 |
| 4 | 13 | 3 | 45 | 47.69 |
| 5 | 12 | 3 | 60 | 62.49 |
| 6 | 13 | 3 | 75 | 64.42 |
| 7 | 12 | 5 | 75 | 60.73 |
| 8 | 11 | 3 | 75 | 57.56 |
| 9 | 12 | 5 | 45 | 40.65 |
| 10 | 12 | 3 | 60 | 65.13 |
| 11 | 12 | 3 | 60 | 64.95 |
| 12 | 13 | 1 | 60 | 45.58 |
| 13 | 11 | 3 | 45 | 41.18 |
| 14 | 12 | 1 | 75 | 44.52 |
| 15 | 11 | 1 | 60 | 35.01 |
| 16 | 13 | 5 | 60 | 61.61 |
| 17 | 12 | 3 | 60 | 65.30 |
| Source | Sum of Squares | Degree of Freedom | Adjusted Mean Square | F Value | P Value Probability > F |
|---|---|---|---|---|---|
| Model | 2289.29 | 9 | 254.37 | 51.48 | <0.0001 |
| X1 | 214.04 | 1 | 214.04 | 43.32 | 0.0003 |
| X2 | 346.77 | 1 | 346.77 | 70.19 | <0.0001 |
| X3 | 583.62 | 1 | 583.62 | 118.13 | <0.0001 |
| X1X2 | 11.8 | 1 | 11.80 | 2.39 | 0.1662 |
| X1X3 | 0.031 | 1 | 0.031 | 0.0062 | 0.9394 |
| X2X3 | 6.1 | 1 | 6.10 | 1.23 | 0.3032 |
| X12 | 80.17 | 1 | 80.17 | 16.23 | 0.005 |
| X22 | 739.88 | 1 | 739.88 | 149.76 | <0.0001 |
| X32 | 214.41 | 1 | 214.41 | 43.40 | 0.0003 |
| Lack of Fit | 28 | 3 | 9.33 | 5.67 | 0.0634 |
| Reagent | pH | Dosage (kg/t) | Temperature (°C) | Cyanide Ion (mg/L) | Gold Extraction (Test Value %) |
|---|---|---|---|---|---|
| PF | 12.6 | 3.8 | 62 | 19.1 | 67.01 |
| PF | 12.6 | 3.8 | 62 | 17.9 | 67.47 |
| PF | 12.6 | 3.8 | 62 | 18.4 | 67.33 |
| Cyanide | 12.6 | 2 | 62 | 84.9 | 68.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Kou, J.; Xing, Y.; Sun, C. Recovery of Gold from Ore with Potassium Ferrocyanide Solution under UV Light. Minerals 2021, 11, 387. https://doi.org/10.3390/min11040387
Liu Z, Kou J, Xing Y, Sun C. Recovery of Gold from Ore with Potassium Ferrocyanide Solution under UV Light. Minerals. 2021; 11(4):387. https://doi.org/10.3390/min11040387
Chicago/Turabian StyleLiu, Ziyuan, Jue Kou, Yi Xing, and Chunbao Sun. 2021. "Recovery of Gold from Ore with Potassium Ferrocyanide Solution under UV Light" Minerals 11, no. 4: 387. https://doi.org/10.3390/min11040387
APA StyleLiu, Z., Kou, J., Xing, Y., & Sun, C. (2021). Recovery of Gold from Ore with Potassium Ferrocyanide Solution under UV Light. Minerals, 11(4), 387. https://doi.org/10.3390/min11040387







