Pathogenesis of Keratinocyte Carcinomas and the Therapeutic Potential of Medicinal Plants and Phytochemicals
Abstract
:1. Introduction
2. Methodology
3. The Origin and History of Keratinocyte Carcinoma
3.1. Basal Cell Carcinoma
3.2. Squamous Cell Carcinoma
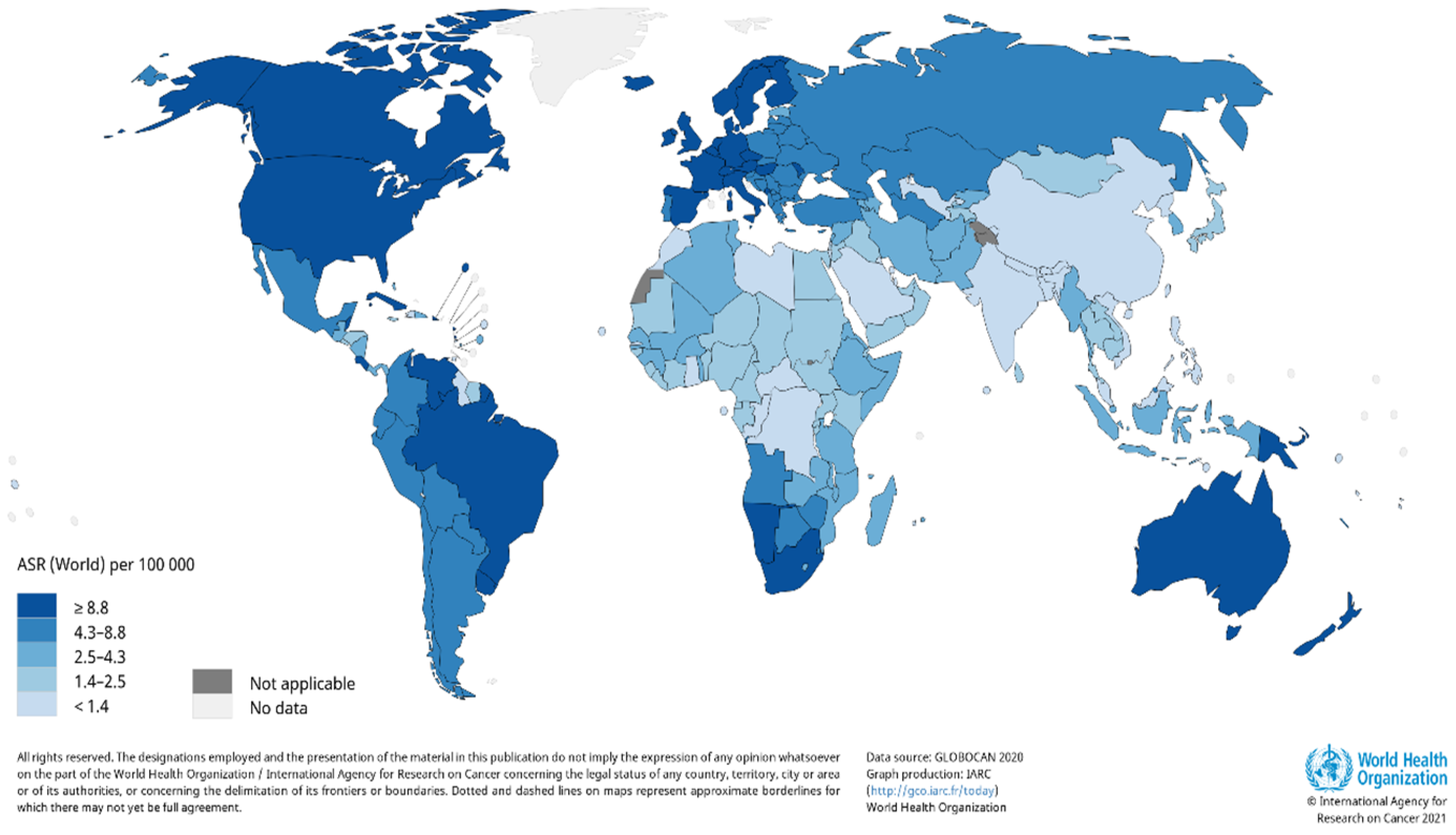
4. Incidence and Demographics of Keratinocyte Carcinoma
5. Diagnosis of Keratinocyte Carcinoma
6. Clinical Variants of BCC
7. Actinic Keratosis as Precursor Lesions of SCC
| Actinic Keratosis Subtype | Characteristics | Occurrence Percentage (%) | References |
|---|---|---|---|
| Pigmented actinic keratosis |
| 1.7 | [39,41,42] |
| Lichenoid actinic keratosis |
| - | [39,43] |
| Bowenoid actinic keratosis |
| 9.6 | [39,44] |
| Proliferative actinic keratosis |
| 29.6 | [39,44] |
| Hypertrophic actinic keratosis |
| 27 | [39,45] |
| Atrophic actinic keratosis |
| 8.7 | [39,46] |
| Acantholytic actinic keratosis |
| 18.3 | [39,47] |
| Actinic cheilitis/cheilosis (rare variant) |
| 3.5 | [48] |
| Cutaneous horn (uncommon variant) |
| 1.7 | [49] |
8. Squamous Cell Carcinoma
8.1. Squamous Cell Carcinoma In Situ
8.2. Invasive Squamous Cell Carcinoma (SCCI)
9. Recurrent and Metastatic KC
10. Risk Factors Associated with the Development of KC
10.1. External Risk Factors
10.1.1. Solar UV Radiation
10.1.2. Indoor Tanning
10.1.3. Ionizing Radiation
10.1.4. Arsenic Exposure
10.2. Internal Factors
10.2.1. Age
10.2.2. Skin Type
10.2.3. Immunosuppression
11. Topical Pharmacotherapies Currently Used for the Treatment of KC
12. Transdermal Delivery of Drugs
12.1. Targeted Delivery Through the Skin
12.2. Transdermal Drug Permeation Routes
12.3. Transdermal Delivery of Skin Cancer Drugs
13. Potential Therapeutic Effects of Phytochemical/Medicinal Plants against KC
14. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lomas, A.; Leonardi-Bee, J.; Bath-Hextall, F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br. J. Dermatol. 2012, 166, 1069–1080. [Google Scholar] [CrossRef]
- Simões, M.C.F.; Sousa, J.J.S.; Pais, A.A.C.C. Skin cancer and new treatment perspectives: A review. Cancer Lett. 2015, 357, 8–42. [Google Scholar] [CrossRef]
- Nehal, K.S.; Bichakjian, C.K. Update on Keratinocyte Carcinomas. N. Engl. J. Med. 2018, 379, 363–374. [Google Scholar] [CrossRef] [PubMed]
- ‘Skin Cancer Types|the Woodruff Institute’. Available online: https://www.thewoodruffinstitute.com/skin-cancer-types/ (accessed on 10 March 2021).
- Blanpain, C.; Fuchs, E. Epidermal stem cells of the skin. Annu. Rev. Cell Dev. Biol. 2006, 22, 339–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crouch, H.E. History of basal cell carcinoma and its treatment. J. R. Soc. Med. 1983, 76, 302–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lever, W.F. Pathogenesis of benign tumors of cutaneous appendages and of basal cell epithelioma: I. benign tumors of the cutaneous appendages. Arch. Derm. Syphilol. 1948, 57, 679–708. [Google Scholar] [CrossRef] [PubMed]
- Sellheyer, K. Basal cell carcinoma: Cell of origin, cancer stem cell hypothesis and stem cell markers. Br. J. Dermatol. 2011, 164, 696–711. [Google Scholar] [CrossRef]
- Ponten, F.; Ren, Z.; Nister, M.; Westermark, B.; Ponten, J. Epithelial-Stromal Interactions in Basal Cell Cancer: The PDGF System. J. Investig. Dermatol. 1994, 102. [Google Scholar] [CrossRef] [Green Version]
- Micke, P.; Kappert, K.; Ohshima, M.; Sundquist, C.; Scheidl, S.; Lindahl, P.; Heldin, C.H.; Botling, J.; Ponten, F.; Östman, A. In situ identification of genes regulated specifically in fibroblasts of human basal cell carcinoma. J. Investig. Dermatol. 2007, 127, 1516–1523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasper, M.; Jaks, V.; Hohl, D.; Toftgård, R. Basal cell carcinoma—Molecular biology and potential new therapies. J. Clin. Investig. 2012, 122, 455–463. [Google Scholar] [CrossRef]
- Tan, S.T.; Ghaznawie, M.; Heenan, P.J.; Dosan, R. Basal cell carcinoma arises from interfollicular layer of epidermis. J. Oncol. 2018, 2018. [Google Scholar] [CrossRef] [Green Version]
- Kipling, M.D.; Usherwood, R.; Varley, R. A monstrous growth: An historical note on carcinoma of the scrotum. Br. J. Ind. Med. 1970, 27, 382–384. [Google Scholar] [CrossRef] [Green Version]
- Azike, J.E. A Review of the History, Epidemiology and Treatment of Squamous Cell Carcinoma of the Scrotum. Rare Tumors 2009, 1, 47–49. [Google Scholar] [CrossRef]
- Johnson, T.M.; Rowe, D.E.; Nelson, B.R.; Swanson, N.A. Squamous cell carcinoma of the skin (excluding lip and oral mucosa). J. Am. Acad. Dermatol. 1992, 26, 467–484. [Google Scholar] [CrossRef]
- Centres for Disease Control and Prevention, ‘What Is Skin Cancer?|CDC’. Available online: https://www.cdc.gov/cancer/skin/basic_info/what-is-skin-cancer.htm (accessed on 11 November 2020).
- Yan, W.; Wistuba, I.I.; Emmert-Buck, M.R.; Erickson, H.S. Squamous Cell Carcinoma—Similarities and Differences among Anatomical Sites. Am. J. Cancer Res. 2011, 1, 275–300. [Google Scholar] [PubMed]
- World Health Organization, ‘Radiation: Ultraviolet (UV) Radiation and Skin Cancer. 2017. Available online: https://www.who.int/news-room/q-a-detail/ultraviolet-(uv)-radiation-and-skin-cancer (accessed on 11 November 2020).
- Choquet, H.; Ashrafzadeh, S.; Kim, Y.; Asgari, M.M.; Jorgenson, E. Genetic and environmental factors underlying keratinocyte carcinoma risk. JCI Insight 2020, 5. [Google Scholar] [CrossRef]
- Rogers, H.W.; Weinstock, M.A.; Feldman, S.R.; Coldiron, B.M. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the us population, 2012. JAMA Dermatol. 2015, 151, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization—International Agency for Research on Cancer, ‘Cancer Today’. Available online: https://gco.iarc.fr/today/online-analysis-map?v=2020&mode=population&mode_population=continents&population=900&populations=900&key=asr&sex=0&cancer=17&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&nb_items=10&group_cancer=1&include_nmsc=1&include_nmsc_other=1&projection=natural-earth&color_palette=default&map_scale=quantile&map_nb_colors=5&continent=0&show_ranking=0&rotate=%255B10%252C0%255D (accessed on 10 March 2021).
- Diepgen, T.L.; Mahler, V. The epidemiology of skin cancer. Br. J. Dermatol. 2002, 146, 1–6. [Google Scholar] [CrossRef]
- Ciaźyńska, M.; Narbutt, J.; Woźniacka, A.; Lesiak, A. Trends in basal cell carcinoma incidence rates: A 16-yearretrospective study of a population in central Poland. Postep. Dermatol. Alergol. 2018, 35, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Dessinioti, C.; Antoniou, C.; Katsambas, A.; Stratigos, A.J. Basal cell carcinoma: What’s new under the sun. Photochem. Photobiol. 2010, 86, 481–491. [Google Scholar] [CrossRef]
- Apalla, Z.; Lallas, A.; Sotiriou, E.; Lazaridou, E.; Ioannides, D. Epidemiological trends in skin cancer. Dermatol. Pract. Concept. 2017, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- South African National Health Laboratory Service, ‘National Cancer Registry’. 2016. Available online: https://cansa.org.za/files/2020/10/NCR_2016_Report_updated_14April2020.pdf (accessed on 11 November 2020).
- Hannuksela-Svahn, A.; Pukkala, E.; Karvonen, J. Basal cell skin carcinoma and other nonmelanoma skin cancers in Finland from 1956 through 1995. Arch. Dermatol. 1999, 135, 781–786. [Google Scholar] [CrossRef] [Green Version]
- Boi, S.; Cristofolini, M.; Micciolo, R.; Polla, E.; Palma, P.D. Epidemiology of Skin Tumors: Data from the Cutaneous Cancer Registry in Trentino, Italy. J. Cutan. Med. Surg. Inc. Med. Surg. Dermatol. 2020, 7, 300–305. Available online: https://www.academia.edu/5028523/Epidemiology_of_Skin_Tumors_Data_from_the_Cutaneous_Cancer_Registry_in_Trentino_Italy (accessed on 11 November 2020). [CrossRef]
- Smoller, B.R. Lever’s Histopathology of the Skin, 10th edition. J. Cutan. Pathol. 2009, 36, 605. [Google Scholar]
- Motaparthi, K.; Kapil, J.P.; Velazquez, E.F. Cutaneous Squamous Cell Carcinoma: Review of the Eighth Edition of the American Joint Committee on Cancer Staging Guidelines, Prognostic Factors, and Histopathologic Variants. Adv. Anat. Pathol. 2017, 24, 171–194. [Google Scholar] [CrossRef] [PubMed]
- Baheti, A.D.; Tirumani, S.H.; Giardino, A.; Rosenthal, M.H.; Tirumani, H.; Krajewski, K.; Ramaiya, N.H. Basal Cell Carcinoma: A Comprehensive Review for the Radiologist. Am. J. Roentgenol. 2015, 204, W132–W140. [Google Scholar] [CrossRef]
- Dębski, T.; Lembas, L.; Jethon, J. Basal Cell Carcinoma, Current Concepts in Plastic Surgery; Agullo, F., Ed.; InTech: London, UK, 2012; ISBN 978-953-51-0398-1. [Google Scholar]
- Edge, S.; Byrd, D.R.; Compton, C.C.; Fritz, A.G.; Greene, F.; Trotti, A. AJCC Cancer Staging Handbook—From the AJCC Cancer Staging Manual|Stephen Edge|Springer. 2010. Available online: https://www.springer.com/la/book/9780387884424 (accessed on 27 March 2019).
- Fahradyan, A.; Howell, A.; Wolfswinkel, E.; Tsuha, M.; Sheth, P.; Wong, A. Updates on the Management of Non-Melanoma Skin Cancer (NMSC). Healthcare 2017, 5, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dourmishev, L.; Rusinova, D.; Botev, I. Clinical variants, stages, and management of basal cell carcinoma. Indian Dermatol. Online J. 2013, 4, 12. [Google Scholar] [CrossRef]
- McDaniel, B.; Bermudez, R. Epitheliomas, Basal Cell. StatPearls 2018, 2, 161–168. [Google Scholar]
- Yanofsky, V.R.; Mercer, S.E.; Phelps, R.G. Histopathological Variants of Cutaneous Squamous Cell Carcinoma: A Review. J. Skin Cancer 2011, 2011, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Parekh, V.; Seykora, J.T. Cutaneous Squamous Cell Carcinoma. Clin. Lab. Med. 2017, 37, 503–525. [Google Scholar] [CrossRef]
- McKee, P.H.; Calonje, E.; Granter, S.R. Pathology of the Skin with Clinical Correlations, 3rd ed.; Mosby Ltd.: St. Louis, MO, USA, 2005. [Google Scholar]
- Şahin, N.; Bozdaǧ, Z.; Erkiliç, S.; Aydin, N.E.; Şener, S. Histopathological subtyping of actinic keratosis and it’s coexistence with nonmelanotic skin cancers in Gaziantep and Malatya regions. Turkderm Turk. Arch. Dematol. Venereol. 2016, 50, 103–108. [Google Scholar] [CrossRef]
- Abudu, B.; Calame, A.; Cohen, P.R. Pigmented Actinic Keratosis: Case Report and Review of an Uncommon Actinic Keratosis Variant that can Mimic Melanoma. Cureus 2019, 11. [Google Scholar] [CrossRef] [Green Version]
- Maor, D.; Ondhia, C.; Yu, L.L.; Chan, J.J. Lichenoid keratosis is frequently misdiagnosed as basal cell carcinoma. Clin. Exp. Dermatol. 2017, 42, 663–666. [Google Scholar] [CrossRef]
- Goldberg, L.H.; Joseph, A.K.; Tschen, J.A. Proliferative actinic keratosis. Int. J. Dermatol. 1994, 33, 341–345. [Google Scholar] [CrossRef]
- Billano, R.A.; Little, W.P. Hypertrophic actinic keratosis. J. Am. Acad. Dermatol. 1982, 7, 484–489. [Google Scholar] [CrossRef]
- Person, J.R. An actinic keratosis is neither malignant nor premalignant: It is an initiated tumor. J. Am. Acad. Dermatol. 2003, 48, 637–638. [Google Scholar] [CrossRef]
- Carapeto, F.J.; García-Pérez, A. Acantholytic Keratosis. Dermatology 1974, 148, 233–239. [Google Scholar] [CrossRef]
- Kim, S.M.; Myoung, H.; Eo, M.Y.; Cho, Y.J.; Lee, S.K. Proper management of suspicious actinic cheilitis. Maxillofac. Plast. Reconstr. Surg. 2019, 41, 15. [Google Scholar] [CrossRef]
- Copcu, E.; Sivrioglu, N.; Culhaci, N. Cutaneous horns: Are these lesions as innocent as they seem to be? World J. Surg. Oncol. 2004, 2, 18. [Google Scholar] [CrossRef] [Green Version]
- Ulrich, M.; Stockfleth, E.; Roewert-Huber, J.; Astner, S. Noninvasive diagnostic tools for nonmelanoma skin cancer. Br. J. Dermatol. 2007, 157, 56–58. [Google Scholar] [CrossRef] [PubMed]
- Hawrot, A.; Alam, M.; Ratner, D. Squamous cell carcinoma. Curr. Probl. Dermatol. 2003, 15, 91–133. [Google Scholar] [CrossRef]
- Ogawa, T.; Kiuru, M.; Konia, T.H.; Fung, M.A. Acantholytic squamous cell carcinoma is usually associated with hair follicles, not acantholytic actinic keratosis, and is not “high risk”: Diagnosis, management, and clinical outcomes in a series of 115 cases. J. Am. Acad. Dermatol. 2017, 76, 327–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nappi, O.; Pettinato, G.; Wick, M.R. Adenoid (acantholytic) squamous cell carcinoma of the skin. J. Cutan. Pathol. 1989, 16, 114–121. [Google Scholar] [CrossRef]
- Silvis, N.G.; Swanson, P.E.; Manivel, J.C.; Kaye, V.N.; Wick, M.R. Spindle-cell and pleomorphic neoplasms of the skin. A clinicopathologic and immunohistochemical study of 30 cases, with emphasis on “atypical fibroxanthomas”. Am. J. Dermatopathol. 1988, 10, 9–19. [Google Scholar] [CrossRef]
- Schwartz, R.A. Verrucous carcinoma of the skin and mucosa. J. Am. Acad. Dermatol. 1995, 32, 1–21. [Google Scholar] [CrossRef]
- Kuo, T. Clear cell carcinoma of the skin. A variant of the squamous cell carcinoma that simulates sebaceous carcinoma. Am. J. Surg. Pathol. 1980, 4, 573–583. [Google Scholar] [CrossRef]
- Ko, C.J.; McNiff, J.M.; Glusac, E.J. Squamous cell carcinomas with single cell infiltration: A potential diagnostic pitfall and the utility of MNF116 and p63. J. Cutan. Pathol. 2008, 35, 353–357. [Google Scholar] [CrossRef]
- Weber, F.; Bauer, J.W.; Sepp, N.; Högler, W.; Salmhofer, W.; Hintner, H.; Fritsch, P. Squamous cell carcinoma in junctional and dystrophic epidermolysis bullosa. Acta Derm. Venereol. 2001, 81, 189–192. [Google Scholar] [CrossRef]
- Sabin, S.R.; Goldstein, G.; Rosenthal, H.G.; Haynes, K.K. Aggressive Squamous Cell Carcinoma Originating as a Marjolin’s Ulcer. Dermatol. Surg. 2004, 30, 229–230. [Google Scholar] [CrossRef]
- Burton, K.A.; Ashack, K.A.; Khachemoune, A. Cutaneous Squamous Cell Carcinoma: A Review of High-Risk and Metastatic Disease. Am. J. Clin. Dermatol. 2016, 17, 491–508. [Google Scholar] [CrossRef]
- Clayman, G.L.; Lee, J.J.; Holsinger, F.C.; Zhou, X.; Duvic, M.; El-Naggar, A.K.; Prieto, V.G.; Altamirano, E.; Tucker, S.L.; Strom, S.S.; et al. Mortality risk from squamous cell skin cancer. J. Clin. Oncol. 2005, 23, 759–765. [Google Scholar] [CrossRef]
- Rowe, D.E.; Carroll, R.J.; Day, C.L. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip: Implications for treatment modality selection. J. Am. Acad. Dermatol. 1992, 26, 976–990. [Google Scholar] [CrossRef]
- Brunner, M.; Veness, M.J.; Ch’Ng, S.; Elliott, M.; Clark, J.R. Distant metastases from cutaneous squamous cell carcinoma-analysis of AJCC stage IV. Head Neck 2013, 35, 72–75. [Google Scholar] [CrossRef]
- Bonerandi, J.J.; Beauvillain, C.; Caquant, L.; Chassagne, J.F.; Chaussade, V.; Clavère, P.; Desouches, C.; Garnier, F.; Grolleau, J.L.; Grossin, M.; et al. Guidelines for the diagnosis and treatment of cutaneous squamous cell carcinoma and precursor lesions. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 1–51. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, H.M.; Herzig, R.H.; Bornstein, R.; Laipply, T.C. Metastatic squamous cell carcinoma of the skin. J. Natl. Med. Assoc. 1980, 72, 1196–1199. [Google Scholar] [PubMed]
- Piva De Freitas, P.; Senna, C.G.; Tabai, M.; Chone, C.T.; Altemani, A. Metastatic Basal Cell Carcinoma: A Rare Manifestation of a Common Disease. Case Rep. Med. 2017, 2017. [Google Scholar] [CrossRef] [Green Version]
- Vu, A.; Laub, D., Jr. Metastatic Basal Cell Carcinoma. In Basal Cell Carcinoma; InTech: London, UK, 2012. [Google Scholar]
- Gordon, R. Skin cancer: An overview of epidemiology and risk factors. Semin. Oncol. Nurs. 2013, 29, 160–169. [Google Scholar] [CrossRef]
- Xiang, F.; Lucas, R.; Hales, S.; Neale, R. Incidence of nonmelanoma skin cancer in relation to ambient UV radiation in white populations, 1978-2012 empirical relationships. JAMA Dermatol. 2014, 150, 1063–1071. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.Y.; He, Y.Y. Ultraviolet radiation-induced non-melanoma skin cancer: Regulation of DNA damage repair and inflammation. Genes Dis. 2014, 1, 188–198. [Google Scholar]
- Tran, T.N.T.; Schulman, J.; Fisher, D.E. UV and pigmentation: Molecular mechanisms and social controversies. Pigment. Cell Melanoma Res. 2008, 21, 509–516. [Google Scholar]
- Grossman, D. The Molecular Basis of Nonmelanoma Skin Cancer. Arch. Dermatol. 1997, 133, 1263. [Google Scholar]
- Ibrahim, S.F.; Brown, M.D. Tanning and Cutaneous Malignancy. Dermatol. Surg. 2008, 34, 460–474. [Google Scholar]
- Latonen, L.; Laiho, M. Cellular UV damage responses—Functions of tumor suppressor p53. Biochim. Biophys. Acta Rev. Cancer 2005, 1755, 71–89. [Google Scholar]
- Boukamp, P. Non-melanoma skin cancer: What drives tumor development and progression? Carcinogenesis 2005, 26, 1657–1667. [Google Scholar]
- Marks, R. The epidemiology of non-melanoma skin cancer: Who, why and what can we do about it. J. Dermatol. 1995, 22, 853–857. [Google Scholar] [CrossRef]
- Soehnge, H.; Ouhtit, A.; Ananthaswamy, O.N. Mechanisms of induction of skin cancer by UV radiation. Front. Biosci. 1997, 2. [Google Scholar] [CrossRef] [Green Version]
- Kooy, A.J.W.; Prens, E.P.; Van Heuklelum, A.; Vuzevski, V.D.; Van Joost, T.; Tank, B. Interferon-γ-induced ICAM-1 and CD40 expression, complete lack of HLA- DR and CD80 (B7.1), and inconsistent HLA-ABC expression in basal cell carcinoma: A possible role for interleukin-10? J. Pathol. 1999, 187, 351–357. [Google Scholar]
- El Ghissassi, F.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. Special Report: Policy A Review of Human Carcinogens-Part. D: Radiation. Lancet 2009, 10, 751–752. [Google Scholar] [CrossRef]
- Gerber, B.; Mathys, P.; Moser, M.; Bressoud, D.; Braun-Fahrländer, C. Ultraviolet Emission Spectra of Sunbeds. Photochem. Photobiol. 2002, 76, 664. [Google Scholar] [CrossRef]
- Wehner, M.R.; Shive, M.L.; Chren, M.M.; Han, J.; Qureshi, A.A.; Linos, E. Indoor tanning and non-melanoma skin cancer: Systematic review and meta-analysis. BMJ 2012, 345, e5909. [Google Scholar] [CrossRef] [Green Version]
- Karagas, M.R.; Nelson, H.H.; Zens, M.S.; Linet, M.; Stukel, T.A.; Spencer, S.; Applebaum, K.M.; Mott, L.; Mabuchi, K. Squamous cell and basal cell carcinoma of the skin in relation to radiation therapy and potential modification of risk by sun exposure. Epidemiology 2007, 18, 776–784. [Google Scholar] [CrossRef]
- Ryan, J.L. Ionizing radiation: The good, the bad, and the ugly. J. Investig. Dermatol. 2012, 132, 985–993. [Google Scholar] [CrossRef] [Green Version]
- Hunt, K.M.; Srivastava, R.K.; Athar, M. Cutaneous Toxicology of Arsenic. In Handbook of Arsenic Toxicology; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 301–314. ISBN 9780124199552. [Google Scholar]
- Leus, A.J.G.; Frie, M.; Haisma, M.S.; Terra, J.B.; Plaat, B.E.C.; Steenbakkers, R.J.H.M.; Halmos, G.B.; Rácz, E. Treatment of keratinocyte carcinoma in elderly patients—a review of the current literature. J. Eur. Acad. Dermatol. Venereol. 2020, jdv.16268. [Google Scholar] [CrossRef] [Green Version]
- Malaguarnera, G.; Giordano, M.; Cappellani, A.; Berretta, M.; Malaguarnera, M.; Perrotta, R. Skin Cancers in Elderly Patients. Anticancer Agents Med. Chem. 2013, 13, 1406–1411. [Google Scholar] [CrossRef]
- Perrotta, R.E.; Giordano, M.; Malaguarnera, M. Non-melanoma skin cancers in elderly patients. Crit. Rev. Oncol. Hematol. 2011, 80, 474–480. [Google Scholar] [CrossRef]
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef] [Green Version]
- Gloster, H.M.; Neal, K. Skin cancer in skin of color. J. Am. Acad. Dermatol. 2006, 55, 741–760. [Google Scholar] [CrossRef]
- Nagarajan, P.; Asgari, M.M.; Green, A.C.; Guhan, S.M.; Arron, S.T.; Proby, C.M.; Rollison, D.E.; Harwood, C.A.; Toland, A.E. Keratinocyte carcinomas: Current concepts and future research priorities. Clin. Cancer Res. 2019, 25, 2379–2391. [Google Scholar] [CrossRef] [Green Version]
- Micali, G.; Lacarrubba, F.; Nasca, M.R.; Ferraro, S.; Schwartz, R.A. Topical pharmacotherapy for skin cancer: Part II. Clinical applications. J. Am. Acad. Dermatol. 2014, 70, e1–e979. [Google Scholar] [CrossRef]
- Lanoue, J.; Goldenberg, G. Basal cell carcinoma: A comprehensive review of existing and emerging nonsurgical therapies. J. Clin. Aesthet. Dermatol. 2016, 9, 26–36. [Google Scholar] [PubMed]
- Eaglstein, W.H.; Weinstein, G.D.; Frost, P. Fluorouracil: Mechanism of Action in Human Skin and Actinic Keratoses: I. Effect on DNA Synthesis in Vivo. Arch. Dermatol. 1970, 101, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef] [PubMed]
- How to Use Fluorouracil and Imiquimod for Non-Melanoma Skin Cancer in a General Practice Setting. 2017. Available online: https://bpac.org.nz/2017/skin-cancer.aspx (accessed on 28 July 2018).
- Bahner, J.D.; Bordeaux, J.S. Non-melanoma skin cancers: Photodynamic therapy, cryotherapy, 5-fluorouracil, imiquimod, diclofenac, or what? Facts and controversies. Clin. Dermatol. 2013, 31, 792–798. [Google Scholar] [CrossRef]
- Metterle, L.; Nelson, C.; Patel, N. Intralesional 5-fluorouracil (FU) as a treatment for nonmelanoma skin cancer (NMSC): A review. J. Am. Acad. Dermatol. 2016, 74, 552–557. [Google Scholar] [CrossRef]
- Chosidow, O.; Dummer, R. Imiquimod: Mode of action and therapeutic potential. Acta Derm. Venereol. Suppl. (Stockh.) 2003, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.H.; Gupta, A.K.; Tyring, S.K. Dual mechanism of action of ingenol mebutate gel for topical treatment of actinic keratoses: Rapid lesion necrosis followed by lesion-specific immune response. J. Am. Acad. Dermatol. 2012, 66, 486–493. [Google Scholar] [CrossRef]
- Doan, H.Q.; Gulati, N.; Levis, W.R. Ingenol mebutate: Potential for further development of cancer immunotherapy. J. Drugs Dermatol. 2012, 11, 1156–1157. [Google Scholar]
- Lebwohl, M.; Sohn, A. Ingenol mebutate (ingenol 3-angelate, PEP005): Focus on its uses in the treatment of nonmelanoma skin cancer. Expert Rev. Dermatol. 2012, 7, 121–128. [Google Scholar] [CrossRef]
- Cozzi, S.J.; Le, T.T.; Ogbourne, S.M.; James, C.; Suhrbier, A. Effective treatment of squamous cell carcinomas with ingenol mebutate gel in immunologically intact SKH1 mice. Arch. Dermatol. Res. 2013, 305, 79–83. [Google Scholar] [CrossRef] [Green Version]
- Moreno Romero, J.A.; Campoy, A.; Perez, N.; Garcia, F.; Grimalt, R. Rapidly-growing squamous cell carcinoma shortly after treatment with ingenol mebutate for actinic keratoses: Report of two cases. Br. J. Dermatol. 2015, 173, 1514–1517. [Google Scholar] [CrossRef]
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Vrouenraets, M.B.; Visser, G.W.M.; Snow, G.B.; Van Dongen, G.A.M.S. Basic principles, applications in oncology and improved selectivity of photodynamic therapy. Anticancer Res. 2003, 23, 505–522. [Google Scholar]
- Cohen, D.K.; Lee, P.K. Photodynamic therapy for non-melanoma skin cancers. Cancers 2016, 8, 90. [Google Scholar] [CrossRef] [Green Version]
- Matei, C.; Tampa, M.; Poteca, T.; Panea-Paunica, G.; Georgescu, S.R.; Ion, R.M.; Popescu, S.M.; Giurcaneanu, C. Photodynamic therapy in the treatment of basal cell carcinoma. J. Med. Life 2013, 6, 50–54. [Google Scholar]
- Kang, Z.; Zhang, J.; Zhou, J.; Qi, Q.; Du, G.; Chen, J. Recent advances in microbial production of δ-aminolevulinic acid and vitamin B12. Biotechnol. Adv. 2012, 30, 1533–1542. [Google Scholar] [CrossRef]
- Cullen, J.K.; Simmons, J.L.; Parsons, P.G.; Boyle, G.M. Topical treatments for skin cancer. Adv. Drug Deliv. Rev. 2020, 153, 54–64. [Google Scholar] [CrossRef]
- Christensen, E.; Mørk, C.; Skogvoll, E. High and sustained efficacy after two sessions of topical 5-aminolaevulinic acid photodynamic therapy for basal cell carcinoma: A prospective, clinical and histological 10-year follow-up study. Br. J. Dermatol. 2012, 166. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, S.; McCarron, P.A.; Donnelly, R.F.; Woolfson, A.D.; McKenna, K. Evaluation of the penetration of 5-aminolevulinic acid through basal cell carcinoma: A pilot study. Exp. Dermatol. 2004, 13. [Google Scholar] [CrossRef]
- Jansen, M.H.E.; Mosterd, K.; Arits, A.H.M.M.; Roozeboom, M.H.; Sommer, A.; Essers, B.A.B.; van Pelt, H.P.A.; Quaedvlieg, P.J.F.; Steijlen, P.M.; Nelemans, P.J.; et al. Five-Year Results of a Randomized Controlled Trial Comparing Effectiveness of Photodynamic Therapy, Topical Imiquimod, and Topical 5-Fluorouracil in Patients with Superficial Basal Cell Carcinoma. J. Investig. Dermatol. 2018, 138, 527–533. [Google Scholar] [CrossRef] [Green Version]
- Tschen, E.H.; Wong, D.S.; Pariser, D.M.; Dunlap, F.E.; Houlihan, A.; Ferdon, M.B. Photodynamic therapy using aminolaevulinic acid for patients with nonhyperkeratotic actinic keratoses of the face and scalp: Phase IV multicentre clinical trial with 12-month follow up. Br. J. Dermatol. 2006, 155. [Google Scholar] [CrossRef] [PubMed]
- Peris, K.; Fargnoli, M.C.; Chimenti, S. Preliminary Observations on the Use of Topical Tazarotene to Treat Basal-Cell Carcinoma. N. Engl. J. Med. 1999, 341, 1767–1768. [Google Scholar] [CrossRef]
- Bardazzi, F.; Bianchi, F.; Parente, G.; Guareschi, E.; Landi, C. A pilot study on the use of topical tazarotene to treat squamous cell carcinoma in situ. J. Am. Acad. Dermatol. 2005, 52, 1102–1104. [Google Scholar] [CrossRef]
- Bianchi, L.; Orlandi, A.; Campione, E.; Angeloni, C.; Costanzo, A.; Spagnoli, L.G.; Chimenti, S. Topical treatment of basal cell carcinoma with tazarotene: A clinicopathological study on a large series of cases. Br. J. Dermatol. 2004, 151, 148–156. [Google Scholar] [CrossRef]
- Ramadon, D.; McCrudden, M.T.C.; Courtenay, A.J.; Donnelly, R.F. Enhancement strategies for transdermal drug delivery systems: Current trends and applications. Drug Deliv. Transl. Res. 2021, 1–34. [Google Scholar] [CrossRef]
- Zeb, A.; Arif, S.T.; Malik, M.; Shah, F.A.; Din, F.U.; Qureshi, O.S.; Lee, E.S.; Lee, G.Y.; Kim, J.K. Potential of nanoparticulate carriers for improved drug delivery via skin. J. Pharm. Investig. 2019, 49, 485–517. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, N.; Singh, V.; Yusuf, M.; Khan, R.A.; Khan, R.A. Non-invasive drug delivery technology: Development and current status of transdermal drug delivery devices, techniques and biomedical applications. Biomed. Tech. 2020, 65, 243–272. [Google Scholar] [CrossRef] [Green Version]
- Yavuz, H.; Çetin, K.; Akgönüllü, S.; Battal, D.; Denizli, A. Therapeutic protein and drug imprinted nanostructures as controlled delivery tools. In Design and Development of New Nanocarriers; Elsevier: Amsterdam, The Netherlands, 2018; pp. 439–473. [Google Scholar]
- Benson, H.A.E.; Grice, J.E.; Mohammed, Y.; Namjoshi, S.; Roberts, M.S. Topical and Transdermal Drug Delivery: From Simple Potions to Smart Technologies. Curr. Drug Deliv. 2019, 16, 444–460. [Google Scholar] [CrossRef]
- Dianzani, C.; Zara, G.P.; Maina, G.; Pettazzoni, P.; Pizzimenti, S.; Rossi, F.; Gigliotti, C.L.; Ciamporcero, E.S.; Daga, M.; Barrera, G. Drug delivery nanoparticles in skin cancers. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Alkilani, A.Z.; McCrudden, M.T.C.; Donnelly, R.F. Transdermal drug delivery: Innovative pharmaceutical developments based on disruption of the barrier properties of the stratum corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef] [Green Version]
- Tanwar, H.; Sachdeva, R. Transdermal Drug Delivery System: A Review. Int. J. Pharm. Sci. Res. 2016, 7, 2274–2290. [Google Scholar]
- Prow, T.W.; Grice, J.E.; Lin, L.L.; Faye, R.; Butler, M.; Becker, W.; Wurm, E.M.T.; Yoong, C.; Robertson, T.A.; Soyer, H.P.; et al. Nanoparticles and microparticles for skin drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 470–491. [Google Scholar] [CrossRef]
- Delouise, L.A. Applications of nanotechnology in dermatology. J. Investig. Dermatol. 2012, 132, 964–975. [Google Scholar] [CrossRef] [Green Version]
- Jiang, T.; Xu, G.; Chen, G.; Zheng, Y.; He, B.; Gu, Z. Progress in transdermal drug delivery systems for cancer therapy. Nano Res. 2020, 13, 1810–1824. [Google Scholar] [CrossRef]
- Roy, N.; Agrawal, M.; Chaudhary, S.; Tirkey, V.; Dhwaj, A.; Mishra, N. Review article on permeation enhancers: A major breakthrough in drug delivery technology. Int. J. Pharm. Sci. Res. 2017, 8, 1001. [Google Scholar]
- Fleury, S.; Vianna Lopez, R.F. Topical Administration of Anticancer Drugs for Skin Cancer Treatment. In Skin Cancers—Risk Factors, Prevention and Therapy; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Moses, M.A.; Brem, H.; Langer, R. Advancing the field of drug delivery: Taking aim at cancer. Cancer Cell 2003, 4, 337–341. [Google Scholar] [CrossRef] [Green Version]
- Hussain, A.; Samad, A.; Ramzan, M.; Ahsan, M.N.; Ur Rehman, Z.; Ahmad, F.J. Elastic liposome-based gel for topical delivery of 5-fluorouracil: In vitro and in vivo investigation. Drug Deliv. 2016, 23, 1115–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.H.; Al-Suwayeh, S.A.; Hung, C.F.; Chen, C.C.; Fang, J.Y. Camptothecin-loaded liposomes with α-melanocyte-stimulating hormone enhance cytotoxicity toward and cellular uptake by melanomas: An application of nanomedicine on natural product. J. Tradit. Complement. Med. 2013, 3, 102–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jose, A.; Labala, S.; Venuganti, V.V.K. Co-delivery of curcumin and STAT3 siRNA using deformable cationic liposomes to treat skin cancer. J. Drug Target. 2017, 25, 330–341. [Google Scholar] [CrossRef]
- Tupal, A.; Sabzichi, M.; Ramezani, F.; Kouhsoltani, M.; Hamishehkar, H. Dermal delivery of doxorubicin-loaded solid lipid nanoparticles for the treatment of skin cancer. J. Microencapsul. 2016, 33, 372–380. [Google Scholar] [CrossRef]
- Khallaf, R.A.; Salem, H.F.; Abdelbary, A. 5-Fluorouracil shell-enriched solid lipid nanoparticles (SLN) for effective skin carcinoma treatment. Drug Deliv. 2016, 23, 3452–3460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabri, A.; Ogilvie, J.; McKenna, J.; Segal, J.; Scurr, D.; Marlow, M. Intradermal Delivery of an Immunomodulator for Basal Cell Carcinoma; Expanding the Mechanistic Insight into Solid Microneedle-Enhanced Delivery of Hydrophobic Molecules. Mol. Pharm. 2020, 17, 2925–2937. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.W.; Kim, D.Y.; Kwon, D.Y.; Kwon, J.S.; Jin, L.M.; Lee, B.; Kim, J.H.; Min, B.H.; Kim, M.S. Injectable intratumoral hydrogel as 5-fluorouracil drug depot. Biomaterials 2013, 34. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wang, X.; Wu, J.; Zhang, D.; Zhang, L.; Song, X.; Hong, H.; He, C.; Mo, X.; Wu, S.; et al. Polyethylenimine and sodium cholate-modified ethosomes complex as multidrug carriers for the treatment of melanoma through transdermal delivery. Nanomedicine 2019, 14, 2395–2408. [Google Scholar] [CrossRef]
- Ijaz, S.; Akhtar, N.; Khan, M.S.; Hameed, A.; Irfan, M.; Arshad, M.A.; Ali, S.; Asrar, M. Plant derived anticancer agents: A green approach towards skin cancers. Biomed. Pharmacother. 2018, 103, 1643–1651. [Google Scholar] [CrossRef]
- Khazir, J.; Mir, B.A.; Pilcher, L.; Riley, D.L. Role of plants in anticancer drug discovery. Phytochem. Lett. 2014, 7, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Anastyuk, S.D.; Shevchenko, N.M.; Usoltseva (Menshova), R.V.; Silchenko, A.S.; Zadorozhny, P.A.; Dmitrenok, P.S.; Ermakova, S.P. Structural features and anticancer activity in vitro of fucoidan derivatives from brown alga Saccharina cichorioides. Carbohydr. Polym. 2017, 157, 1503–1510. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [Green Version]
- Penta, D.; Somashekar, B.S.; Meeran, S.M. Epigenetics of skin cancer: Interventions by selected bioactive phytochemicals. Photodermatol. Photoimmunol. Photomed. 2018, 34, 42–49. [Google Scholar] [CrossRef]
- Iqbal, J.; Abbasi, B.A.; Ahmad, R.; Batool, R.; Mahmood, T.; Ali, B.; Khalil, A.T.; Kanwal, S.; Afzal Shah, S.; Alam, M.M.; et al. Potential phytochemicals in the fight against skin cancer: Current landscape and future perspectives. Biomed. Pharmacother. 2019, 109, 1381–1393. [Google Scholar] [CrossRef]
- Fox, F.E.; Niu, Z.; Tobia, A.; Rook, A.H. Photoactivated Hypericin is an Anti-Proliferative Agent that Induces a High Rate of Apoptotic Death of Normal, Transformed, and Malignant T Lymphocytes: Implications for the Treatment of Cutaneous Lymphoproliferative and Inflammatory Disorders. J. Investig. Dermatol. 1998, 111, 327–332. [Google Scholar] [CrossRef] [Green Version]
- Alecu, M.; Ursaciuc, C.; Hãlãlãu, F.; Coman, G.; Merlevede, W.; Waelkens, E.; De Witte, P. Photodynamic treatment of basal cell carcinoma and squamous cell carcinoma with hypericin. Anticancer Res. 1998, 18, 4651–4654. [Google Scholar]
- Kacerovská, D.; Pizinger, K.; Majer, F.; Šmíd, F. Photodynamic therapy of nonmelanoma skin cancer with topical Hypericum perforatum extract—A pilot study. Photochem. Photobiol. 2008, 84, 779–785. [Google Scholar] [CrossRef]
- Head, C.S.; Luu, Q.; Sercarz, J.; Saxton, R. Photodynamic therapy and tumor imaging of hypericin-treated squamous cell carcinoma. World J. Surg. Oncol. 2006, 4, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eastman, K.L.; McFarland, L.V.; Raugi, G.J. Buyer beware: A black salve caution. J. Am. Acad. Dermatol. 2011, 65, e154–e155. [Google Scholar] [CrossRef]
- Saltzberg, F.; Barron, G.; Fenske, N. Deforming Self-Treatment with Herbal “Black Salve”. Dermatol. Surg. 2009, 35, 1152–1154. [Google Scholar] [CrossRef]
- Osswald, S.S.; Elston, D.M.; Farley, M.F.; Alberti, J.G.; Cordero, S.C.; Kalasinsky, V.F. Self-treatment of a basal cell carcinoma with “black and yellow salve”. J. Am. Acad. Dermatol. 2005, 53, 508–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Affleck, A.G.; Varma, S. A case of do-it-yourself Mohs’ surgery using bloodroot obtained from the internet. Br. J. Dermatol. 2007, 157, 1078–1079. [Google Scholar] [CrossRef]
- McDaniel, S.; Goldman, G.D. Consequences of using escharotic agents as primary treatment for nonmelanoma skin cancer. Arch. Dermatol. 2002, 138, 1593–1596. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.W.; Goldstein, G.D.; Birkby, C.S. Auto-Mohs.com. Dermatol. Surg. 2001, 27, 975–978. [Google Scholar]
- Es-saady, D.; Simon, A.; Ollier, M.; Maurizis, J.C.; Chulia, A.J.; Delage, C. Inhibitory effect of ursolic acid on B16 proliferation through cell cycle arrest. Cancer Lett. 1996, 106, 193–197. [Google Scholar] [CrossRef]
- Junco, J.J.; Cho, J.; Mancha, A.; Malik, G.; Wei, S.J.; Kim, D.J.; Liang, H.; DiGiovanni, J.; Slaga, T.J. Role of AMPK and PPARα in the anti-skin cancer effects of ursolic acid. Mol. Carcinog. 2018, 57, 1698–1706. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Liu, Y.; Zhang, L.; Yan, L.; Fan, F.; Shen, C.; Wang, A.; Zheng, S.; Wang, S.; Lu, Y. Luteolin reduces the invasive potential of malignant melanoma cells by targeting β3 integrin and the epithelial-mesenchymal transition. Acta Pharmacol. Sin. 2012, 33, 1325–1331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwashita, K.; Kobori, M.; Yamaki, K.; Tsushida, T. Flavonoids inhibit cell growth and induce apoptosis in b16 melanoma 4A5 cells. Biosci. Biotechnol. Biochem. 2000, 64, 1813–1820. [Google Scholar] [CrossRef]
- Matsuda, H.; Nakashima, S.; Oda, Y.; Nakamura, S.; Yoshikawa, M. Melanogenesis inhibitors from the rhizomes of Alpinia officinarum in B16 melanoma cells. Bioorg. Med. Chem. 2009, 17. [Google Scholar] [CrossRef]
- Verschooten, L.; Smaers, K.; Van Kelst, S.; Proby, C.; Maes, D.; Declercq, L.; Agostinis, P.; Garmyn, M. The flavonoid luteolin increases the resistance of normal, but not malignant keratinocytes, against UVB-induced apoptosis. J. Investig. Dermatol. 2010, 130, 2277–2285. [Google Scholar] [CrossRef] [Green Version]
- Afaq, F.; Adhami, V.M.; Ahmad, N. Prevention of short-term ultraviolet B radiation-mediated damages by resveratrol in SKH-1 hairless mice. Toxicol. Appl. Pharmacol. 2003, 186, 28–37. [Google Scholar] [CrossRef]
- Ahmad, N.; Adhami, V.M.; Afaq, F.; Feyes, D.K.; Mukhtar, H. Resveratrol causes WAF-1/p21-mediated G1-phase arrest of cell cycle and induction of apoptosis in human epidermoid carcinoma A431 cells. Clin. Cancer Res. 2001, 7, 1466–1473. [Google Scholar] [PubMed]
- Hail, N.; Lotan, R. Examining the role of mitochondrial respiration in vanilloid-induced apoptosis. J. Natl. Cancer Inst. 2002, 94, 1281–1292. [Google Scholar] [CrossRef]
- US20100166895A1—Capsicum Extract for Treatment of Skin Cancer—Google Patents’. Available online: https://patents.google.com/patent/US20100166895 (accessed on 15 November 2020).
- Rademan, S.; Anantharaju, P.G.; Madhunapantula, S.V.; Lall, N. The anti-proliferative and antioxidant activity of four indigenous South African plants. Afr. J. Tradit. Complement. Altern. Med. 2019, 16, 13–23. [Google Scholar] [CrossRef]
- Rezadoost, M.H.; Kumleh, H.H.; Ghasempour, A. Cytotoxicity and apoptosis induction in breast cancer, skin cancer and glioblastoma cells by plant extracts. Mol. Biol. Rep. 2019, 46, 5131–5142. [Google Scholar] [CrossRef]
- Twilley, D.; Kishore, N.; Meyer, D.; Kumar, V. Lall The Effect of Helichrysum odoratissimum (L.) Sweet on Cancer Cell Proliferation and Cytokine Production. Int. J. Pharmacogn. Phytochem. Res. 2017, 9. [Google Scholar] [CrossRef]
- Twilley, D.; Langhansová, L.; Palaniswamy, D.; Lall, N. Evaluation of traditionally used medicinal plants for anticancer, antioxidant, anti-inflammatory and anti-viral (HPV-1) activity. S. Afr. J. Bot. 2017, 112, 494–500. [Google Scholar] [CrossRef]
- Vijaybabu, K.; Punnagai, K. In-vitro anti-proliferative effects of ethanolic extract of vanilla planifolia leaf extract against A431 human epidermoid carcinoma cells. Biomed. Pharmacol. J. 2019, 12, 1141–1146. [Google Scholar]
- Iliescu, I.A.; Peter, S.; Albert, I.; Skalicka-Woźniak, K.; Miron, A.; Luca, S.V.; Wolfram, E. Verbascum nigrum: Cytotoxicity Evaluation in A431 Epidermoid Carcinoma Cells and Untargeted LC-HR-MS/MS Metabolite Profiling. Chem. Biodivers. 2020, 17, e2000644. [Google Scholar] [CrossRef] [PubMed]
- Kessels, J.; Voeten, L.; Nelemans, P.; Cleutjens, J.; Hillen, L.M.; Mosterd, K.; Kelleners-Smeets, N.W.J. Topical sinecatechins, 10%, ointment for superficial basal cell carcinoma: A randomized clinical trial. JAMA Dermatol. 2017, 153, 1061–1063. [Google Scholar] [CrossRef]
- Kuttan, R.; Sudheeran, P.C.; Josph, C.D. Turmeric and curcumin as topical agents in cancer therapy. Tumori 1987, 73, 29–31. [Google Scholar] [CrossRef]
- Sonavane, K.; Phillips, J.; Ekshyyan, O.; Moore-Medlin, T.; Roberts Gill, J.; Rong, X.; Lakshmaiah, R.R.; Abreo, F.; Boudreaux, D.; Clifford, J.L.; et al. Topical Curcumin-Based Cream Is Equivalent to Dietary Curcumin in a Skin Cancer Model. J. Skin Cancer 2012, 2012, 1–9. [Google Scholar] [CrossRef]
- Lu, Y.P.; Lou, Y.R.; Xie, J.G.; Peng, Q.Y.; Liao, J.; Yang, C.S.; Huang, M.T.; Conney, A.H. Topical applications of caffeine or (-)-epigallocatechin gallate (EGCG) inhibit carcinogenesis and selectively increase apoptosis in UVB-induced skin tumors in mice. Proc. Natl. Acad. Sci. USA 2002, 99, 12455–12460. [Google Scholar] [CrossRef] [Green Version]
- Pflugfelder, A.; Andonov, E.; Weide, B.; Dirschka, T.; Schempp, C.; Stockfleth, E.; Stratigos, A.; Krüger-Krasagakis, S.; Bauer, J.; Garbe, C.; et al. Lack of activity of betulin-based Oleogel-S10 in the treatment of actinic keratoses: A randomized, multicentre, placebo-controlled double-blind phase II trial. Br. J. Dermatol. 2015, 172, 926–932. [Google Scholar] [PubMed]
- Lim, A. Black salve treatment of skin cancer: A review. J. Dermatolog. Treat. 2018, 29, 388–392. [Google Scholar] [PubMed]
- Croaker, A.; King, G.J.; Pyne, J.H.; Anoopkumar-Dukie, S.; Simanek, V.; Liu, L. Carcinogenic potential of sanguinarine, a phytochemical used in ‘therapeutic’ black salve and mouthwash. Mutat. Res. Rev. Mutat. Res. 2017, 774, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Huyke, C.; Reuter, J.; Rodig, M.; Kersten, A.; Laszczyk, M.; Scheffler, A.; Nashan, D.; Schempp, C. Treatment of actinic keratoses with a novel betulin-based oleogel. A prospective, randomized, comparative pilot study. J. Dtsch. Dermatol. Ges. 2009, 7, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Camptothecin|C20H16N2O4—PubChem’. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Camptothecine (accessed on 17 November 2020).

| Stage | Description |
|---|---|
| 0 | In situ carcinoma, cancer has developed but has not spread or grown into surrounding tissue |
| 1 | Tumor ≤2 cm in size, with less than two high-risk features |
| 2 | Tumor >2 cm in size, with two or more high-risk features |
| 3 | Tumor with invasion of the maxilla, mandible, orbit, or temporal bone or the tumor has spread to nearby lymph nodes (<3 cm in size) |
| 4 | Tumor with invasion of skeleton or perineural invasion of skull base or the tumor has spread to lymph nodes (>3 cm in size) or an internal organ |
| Basal Cell Carcinoma Variant | Characteristics | References |
|---|---|---|
| Nodular BCC |
| [38,39,40,41] |
| Pigmented BCC |
| [39,40,42] |
| Superficial BCC |
| [39] |
| Morphoeic/sclerosing BCC |
| [39] |
| Squamous Cell Carcinoma Subtype | Characteristics | References |
|---|---|---|
| Generic/simplex SCC |
| [51] |
| Acantholytic SCC (adenoid/lobular SCC) |
| [52,53] |
| Spindle cell SCC (sarcomatoid SCC) |
| [38,54] |
| Verrucous SCC |
| [38,55] |
| Clear-cell SCC (hydropic SCC) |
| [56] |
| Single cell infiltrates |
| [57] |
| De Novo SCC |
| [58,59] |
| Site of Metastases | Occurrence Percentage (%) |
|---|---|
| Squamous Cell Carcinoma | |
| Lymph node | 4.3 |
| Lung | 0.2 |
| Liver | 1.1 |
| Bone | 0.2 |
| Subcutaneous tissue | 0.2 |
| Brain | 0.2 |
| Generalized | 0.1 |
| Site unspecified | 1.6 |
| Basal Cell Carcinoma | |
| Lymph nodes | 56 |
| Lung | 36 |
| Paotid gland | 20 |
| Bone | 16 |
| Submandibular gland | 12 |
| Thyroid | 4 |
| Skin | 0 |
| Liver | 0 |
| Pharmacotherapy | Efficacy | Mechanism | Disadvantages/Adverse Side Effects | References |
|---|---|---|---|---|
| 5-Fluorouracil (5-FU) | 5% FU: 80% and 54–86% efficacy for superficial BCC and SCC in situ, respectively 30 mg/mL (one-thrice weekly) intralesional 5-FU: 90–100% efficacy for small superficial/modular tumors (0.6–1.5 cm) 50 mg/mL (biweekly) intralesional 5-FU: 67% efficacy for large tumors (2.4 cm) | Disrupts DNA synthesis and repair by inhibiting thymidylate synthase; causes DNA damage, DNA strand breaks and cell death Misincorporation of 5-FU in RNA; inhibits conversion of pre-rRNA to mature rRNA and disrupts post-transcriptional modification of tRNA | High rate of tumor recurrence; optimal for small-sized tumors Erythema, scaling, blisters, necrosis, ulceration, erosions, pruritus, burning, headaches, fever, diarrhea, nausea, and mouth ulcers | [92,93,94,95,96,97] |
| Imiquimod (IMQ) | 5% IMQ: 43–94% for superficial BCC 5% IMQ: 50–65% for nodular BCC 5% IMQ: 71% for invasive SCC 5% IMQ: 57–80% for Bowen’s disease | Induces pro-inflammatory cytokines secretion, interferon gamma (IFN-γ), tumor necrosis factor alpha (TNF-α), interferon alpha (IFN-α), interleukin (IL)-6, IL-1α, IL-1β, IL-8, and IL-12, thereby activation acquired and natural immune response and antitumor activity | Extensive recurrence rate after first 12–24 months; optimal for tumors <2 cm in size Erythema, discomfort, erosion, scaling, blisters, necrosis, ulcerations, erosions, pruritus, burning, flu-like symptoms, dizziness, and headaches | [95,96,98] |
| Ingenol mebutate (IM) Active compound in Euphorbia peplus L. (milkweed) sap | 0.05% IM: 63% efficacy for superficial BCC (increased efficacy directly proportional to higher dosage) 0.25% IM gel: 70 and 30% efficacy for SCC growth in female and male mice respectively | Induces cell necrosis through loss of mitochondrial membrane potential and induction of mitochondrial membrane polarization Necrosis induced pro-inflammatory cytokines resulting in neutrophil-mediated antibody-dependent cellular cytotoxicity | Thicker skin in male mice resulted in poorer drug penetration and decreased efficacy Crusting, flaking, erythema, erosion/ulceration, swelling, and blistering | [99,100,101,102,103] |
| Photodynamic therapy (PDT) | 72–100% efficacy on superficial BCC | Increased uptake of PDT by cancerous cells; once PDT has exited normal cells, tumor cells (with PDT) are exposed to light at a specific wavelength, resulting in release of reactive oxygen species, thereby inducing cell death | Nodular BCC and BCC tumors >2 mm are less responsive to PDT (inadequate penetration); BCC tumors between 1–2 mm in thickness, SCC an AK lesions have a high recurrence rate; less effective on superficial BCC than IMQ and 5-FU Burning, prickling, erythema, edema, hypo-and hyper-pigmentation, allergic contact dermatitis (rare) and pain, which often leads to incomplete treatments | [104,105,106,107,108,109,110,111,112,113] |
| Retinoids Class of compounds derived from vitamin A | 0.1% tazarotene gel (daily for eight months): cleared 11 of 13 superficial BCC and 5 of 17 nodular BCC; a 24 week trial recorded 70.8% of BCC with >50% regression and 30.5% healed with no recurrence after 3 years 0.1% tazarotene gel (daily): efficacy of 46.6% of SCC in situ (0.5–4 cm); complete clearance from month 3–5 (no recurrence after 3 month follow-up) | Antiproliferative activity and induction of apoptosis in basaliomatous cells | Effective against undifferentiated BCC tumors, however not effective against keratotic BCCs (overexpression of p53 and cellular retinol binding protein-1) Mild erythema, edema, and local skin irritation | [114,115,116] |
| Nano-Carrier | Study | Outcome | Reference |
|---|---|---|---|
| Liposomes | Synthesized elastic liposomes loaded with 5-fluorouracil (5-FU) investigated (in vitro and in vivo) for drug permeation enhancement across the stratum corneum of the skin. |
| [131] |
| Uptake of α-melanocyte-stimulating hormone (α-MSH)-conjugated liposomes in melanoma cells (B16-F10) |
| [132] | |
| Cytotoxicity of co-delivered curcumin encapsulated cationic liposomes complexed with STAT3 siRNA against SCC cells |
| [133] | |
| Solid lipid nanoparticles (SLNs) | Cytotoxicity of doxorubicin-loaded solid lipid nanoparticles against B16-F10 cells and melanoma-induced Balb/C mice |
| [134] |
| 5-FU loaded SLNs for the treatment of skin carcinoma in vivo |
| [135] | |
| Microneedles | Treatment of BCC using the intradermal delivery of an immunomodulator (5% w/v imiquimod cream), using an oscillating microneedle device (Dermapen). Dermal permeation analysis was performed on the cross-sections of porcine skin |
| [136] |
| Hydrogel | Investigation of injectable intra-tumoral 5-FU hydrogel to enhance efficacy and decrease systemic toxicity associated with 5-FU observed in cancer patients |
| [137] |
| Ethosomes | A complex of CUR-Eth-PEI/DOX-Eth-SC (cytotoxic drug and a chemosensitizer) was evaluated (in vitro and in vivo) for potential anticancer activity on B16-F10 cells. Two modified ethosomes were synthesized, namely polyethyleneimine (PEI)-modified ethosomes (Eth-PEI) and sodium cholate (SC)-modified ethosomes (Eth-SC). These modified ethosomes functioned as carriers for doxorubicin (DOX) and curcumin (CUR) |
| [138] |
| Phytochemical | Source/Origin | Treatment | Outcome | References |
|---|---|---|---|---|
| Hypericin | Hypericum perforatum L. (St John’s Wort) | Hypericin directly injected into affected tissue, 3–5 times weekly (SCC (40–100 μg) and BCC (40–200 μg)), showed no necrosis of surrounding tissue and was successful as a targeted delivery system | Combination of hypericin and PDT resulted in pain and burning | [145,146] |
| Effect of 0.07% hypericin on BCC, AK, and Bowen’s disease, followed by irradiation (weekly, for 6 weeks) | All patients experiences pain and burning after irradiation, 50% complete clinical remission of AKs, 11% histological clearance of sBCC, and 80% histological clearance of Bowen’s disease | [147] | ||
| Mice injected with SCC cells to develop tumors (3–15 mm diameter) were injected with 10 µL of DMSO containing 10 µg hypericin per gram of tumor and irradiated after 24 h | Hypericin retained in tumors for a prolonged period of time was observed to be more effective in small sized tumors (<400 mm3), whereas larger tumor displayed partial ablation followed by recurrence | [148] | ||
| Black salve (escharotic agent) Sanguinaria canadensis L. (bloodroot) | Ointment containing 300 mg bloodroot, galangal, sheep sorrel, and red clover resolved suspected melanoma neoplasm of the left naris (63 year old male) | Complete loss of the left naris and severe tissue damage | [149] | |
| Application on BCC located on the nasal cavity (83 year old male) | Complete loss of nasal ala | [150] | ||
| Application of black and “yellow” salve on micronodular BCC located on right nasal sidewall (65 year old female) | Patient discontinued use due to pain and tenderness, formation of 12 mm ulceration with eschar formation. Secondary intention healing treated the wound | [151] | ||
| Application on 5 mm BCC lesion (51 year old male) | Agonizing pain and formation of large eschar and formation of scar. Biopsy after 12-months showed no presence of BCC | [152] | ||
| Application on BCC located in the right-hand side of the neck (49 year old male) | Development of triangular keloidal scar that had to be surgically removed and repaired. After reconstruction, no tumor was identified | [153] | ||
| Application to SCC on the right lower leg (55 year old woman) | Formation of a thick escharotic plaque, which dislodged revealing normal granulation tissue. Histological examination revealed no residual SCC | [154] | ||
| Application of black salve (containing zinc chloride) on BCC on the left cheek of the face | Formation of a thick escharotic plaque, which dislodged revealing normal granulation tissue. Histological examination of the plaque revealed acute and subacute inflammation, necrosis and BCC in the dermis; however, scar did not have any BCC | [154] | ||
| Ursolic acid- UA (pentacyclic triterpene) | Several plant species such as Ocimum basilicum L. (basil), Salvia Rosmarinus Schleid. (rosemary), apple peels (Malus pumila Mill.), and berries | Effect against B16 mouse melanoma cells after 24 h exposure | UA showed a fifty percent inhibitory concentration of 7.7 μΜ. Cytotoxicity attributed to potential inhibition of lipoxygenase and cyclooxygenase and cytostatic activity. | [155] |
| Effect on Ca3/7 (mouse SCC) and MT1/2 (mouse skin papilloma) skin cancer cells | Induced cell death in both cell lines through activation of AMP-activated protein kinase (AMPK) and peroxisome proliferator activated receptor-α (PPAR-α) | [156] | ||
| Luteolin (flavonoid) | Several plant species such as Daucus carota L. (carrots), Capsicum annuum L. (peppers), Petroselinum crispum (Mill.) Fuss (parsley), and Brassica oleraea L. (broccoli) | Effect on B16F10 murine melanoma cells | Inhibited tumor progression by inhibiting hypoxia-induced epithelial-mesenchymal transition in melanoma cells through upregulation of β3 integrin | [157] |
| Effect on B16 murine melanoma cells | Induced apoptosis in melanoma cells through ERK1/2 signaling attenuation, upregulation of Bax, and down-regulation of Bcl-2 | [158,159] | ||
| Effect in normal human keratinocytes (NHK) after exposure to UVB radiation | Enhanced survival rate of NHK through inhibition of the mitochondrial intrinsic apoptotic pathway and inhibition of inflammatory mediators IL-1α and prostaglandin-E2. However, did not inhibit malignant keratinocytes | [160] | ||
| Resveratrol-RV (polyphenol) | Commonly found in Vitis vinifera L. (grapes), Morus spp (mulberries), and Arachis hypogaeae L. (peanuts) | Photo-chemopreventive activity of RV (25 μmol/0.2 mL acetone per mouse) in hairless mice induced with UVB radiation Topical application in hairless mice induced with UVB radiation | Inhibition of skin thickness growth and ear punch weight Topical application inhibited increased ornithine decarboxylase (ODC) enzyme activity and protein expression. Increased levels of ODC activity are linked to an increase in neoplastic growth | [161] |
| Effect of RV (1–50 μΜ for 24 h) on human epidermoid carcinoma (A431) cells | Inhibited cell growth, induced apoptosis, and cell cycle arrest at the G1 phase through the activation of the cyclin-dependent kinase inhibitor 1 (WAF1/p21), which in turn induced cyclin D1/D2-cdk6, cyclin D1/D2-cdk4, and cyclin E-cdk2 complex inhibition | [162] | ||
| Capsaicin (capsaicinoid) | Major compound present in plants belonging to the Capsicum genus | Effect on parental SCC cells | Induced apoptosis due to mitochondrial respiration suppression, antiproliferative activity potentially due to production of hydroperoxide and/or the inhibition of enzymatic processes within the electron transport chain | [163] |
| Topical application of alcoholic Capsicum extract (containing capsaicin) on BCC and SCC lesions | Topical application reduced the size of the lesion and the lesions disappeared after a certain period of time | [164] | ||
| Ethanolic fruit extract of Combretum molle | Effect of extract against A431 cells | Antiproliferative activity with IC50 value of 23.2 ± 0.8 μg/mL | [165] | |
| Methanolic leaf extract of Calystegia sepium | Antiproliferative activity with IC50 value of 24.71 μg/mL; induced cell cycle arrest at G0/G1 stage and induced the expression of nuclear factor kappa B1 (NF-κβ) and apoptotic peptidase activating factor 1 (APAF1) | [166] | ||
| Ethanolic aerial part extract of Euclea crispa subsp. crispa | Antiproliferative activity with IC50 value of 41.8 ± 0.4 μg/mL | [165] | ||
| Ethanolic leaf and stem extract of Helichrysum odoratissimum | Antiproliferative activity with IC50 value of 15.5 ± 0.2 μg/mL; induced apoptosis and increased IL-12 and inhibited IL-8 levels in U937 cells | [167] | ||
| Ethanolic aerial part extract of Sideroxylon inerme | Antiproliferative activity with IC50 value of 46.8 ± 2.0 μg/mL | [165] | ||
| Ethanolic leaf extract of Syzygium jambos | Antiproliferative activity with IC50 value of 54.70 ± 0.60 μg/mL; inhibited cyclooxygenase-2 enzyme with IC50 value 3.79 ± 0.90 μg/mL | [168] | ||
| Ethanolic leaf extract of Vanilla planifolia | Antiproliferative activity with IC50 value of 31.2 μg/mL; induced DNA fragmentation | [169] | ||
| Methanolic aerial part extract of Verbascum nigrum | Antiproliferative activity with IC50 value of 81.92 μg/mL; however, fraction VNF4 (consisting of ilwensisaponins A and C, songarosaponins A and B) showed an IC50 of 12.27 μg/mL | [170] | ||
| Name/Bioative Ingredient | Source/Origin | Treatment | Outcome | References |
|---|---|---|---|---|
| BCC | ||||
| 10% Sinecatechins ointment (Veregen®)/Epigallocatechin-gallate (EGCG) | Camellia sinensis L. leaf extract containing catechins (>85%) | Application to sBCC (39 patients) |
| [171] |
| SCC | ||||
| 0.5% Curcumin of 50% ethanolic turmeric extract in vaseline ointment | Polyphenolic present in rhizomes of Curcuma longa L. | Application to ulcerated tumor (62 patients) |
| [172,173] |
| EGCG (6.5 µmol) once daily for 5 days a week (18 weeks in total) | Major catechin found in Camellia sinensis L. | Application to SCC tumors developed in female hairless SKH-1 mice irradiated with UVB for 20 weeks (twice weekly) |
| [174] |
| Caffeine (6.2 µmol) once daily for 5 days a week (18 weeks in total) | Methylxanthine alkaloid found in coffee |
| [174] | |
| Betulin-based Oleogel-S10 | Pentacyclic triterpenes isolated from Betula pubescens Ehrh. | Application to patients with actinic keratosis (157 patients) |
| [175] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Josiah, A.J.; Twilley, D.; Pillai, S.K.; Ray, S.S.; Lall, N. Pathogenesis of Keratinocyte Carcinomas and the Therapeutic Potential of Medicinal Plants and Phytochemicals. Molecules 2021, 26, 1979. https://doi.org/10.3390/molecules26071979
Josiah AJ, Twilley D, Pillai SK, Ray SS, Lall N. Pathogenesis of Keratinocyte Carcinomas and the Therapeutic Potential of Medicinal Plants and Phytochemicals. Molecules. 2021; 26(7):1979. https://doi.org/10.3390/molecules26071979
Chicago/Turabian StyleJosiah, Andrea Jess, Danielle Twilley, Sreejarani Kesavan Pillai, Suprakas Sinha Ray, and Namrita Lall. 2021. "Pathogenesis of Keratinocyte Carcinomas and the Therapeutic Potential of Medicinal Plants and Phytochemicals" Molecules 26, no. 7: 1979. https://doi.org/10.3390/molecules26071979







