Poly(vinyl chloride) Composites with Raspberry Pomace Filler
Abstract
1. Introduction
2. Materials and Methods
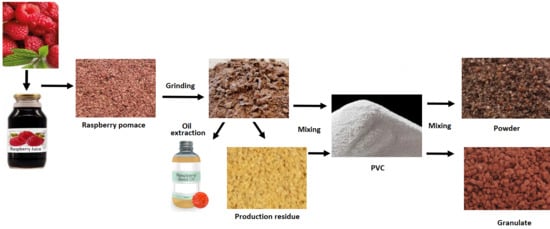
2.1. Raw Materials for Composite Materials Production
2.2. Preparation of Samples for Tests
2.3. Testing Method
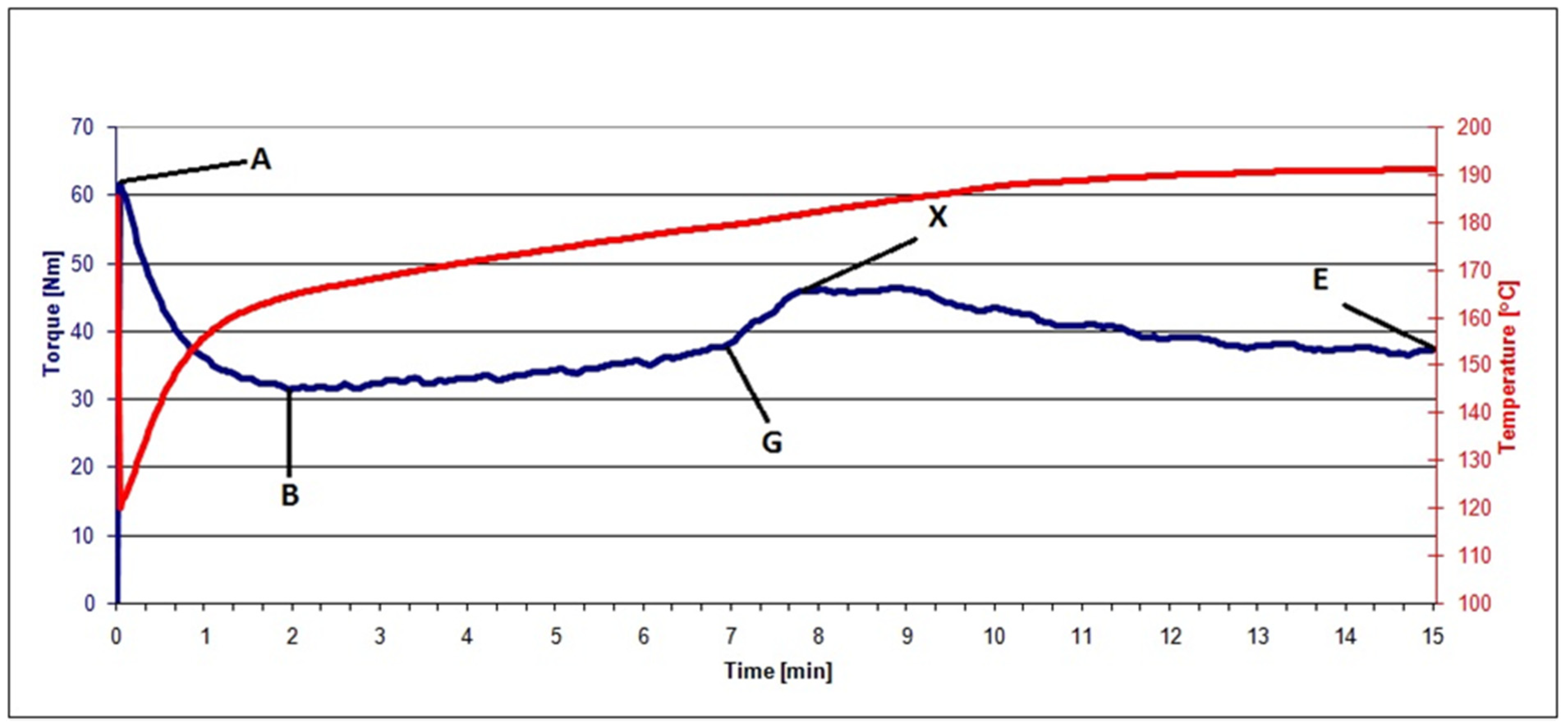
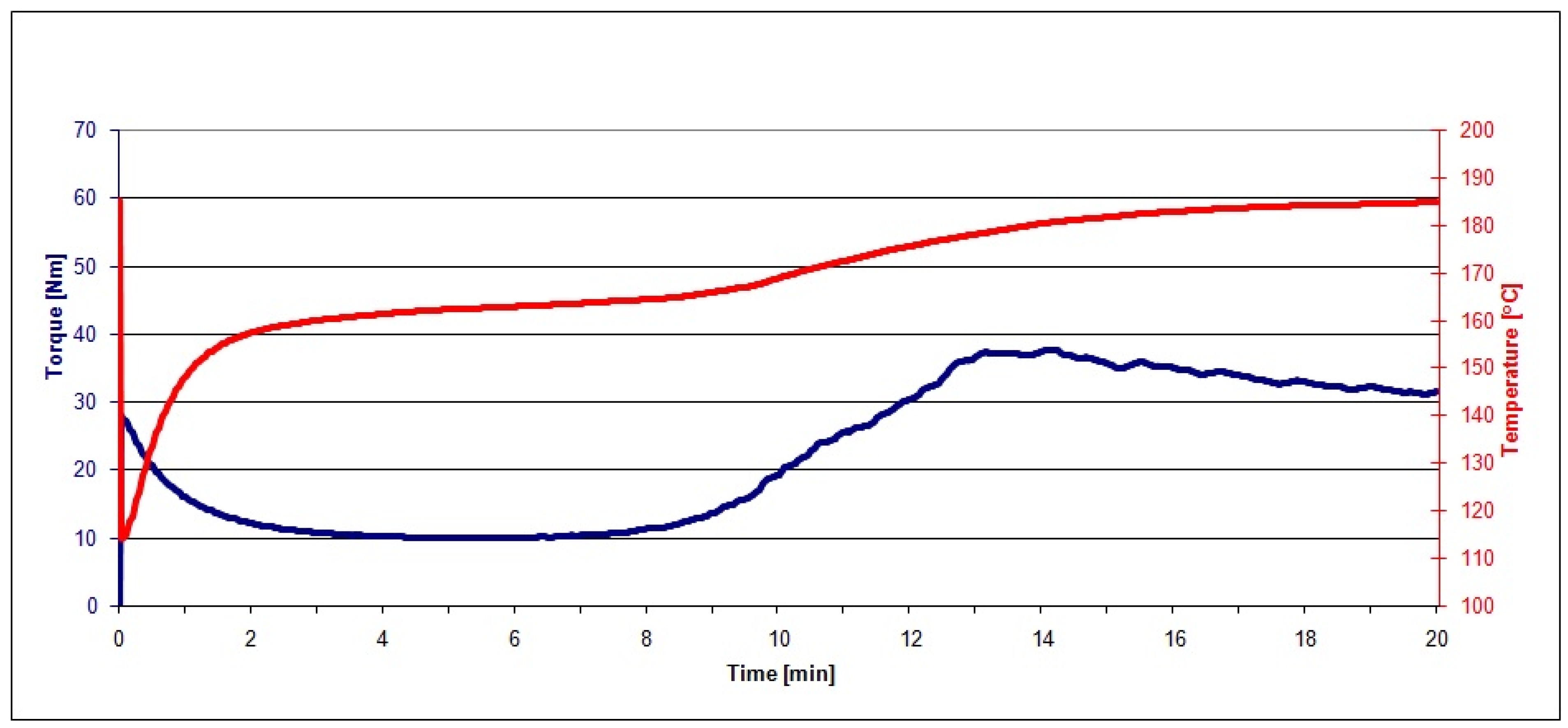
2.3.1. Plastograph Analysis
2.3.2. Thermal Stability
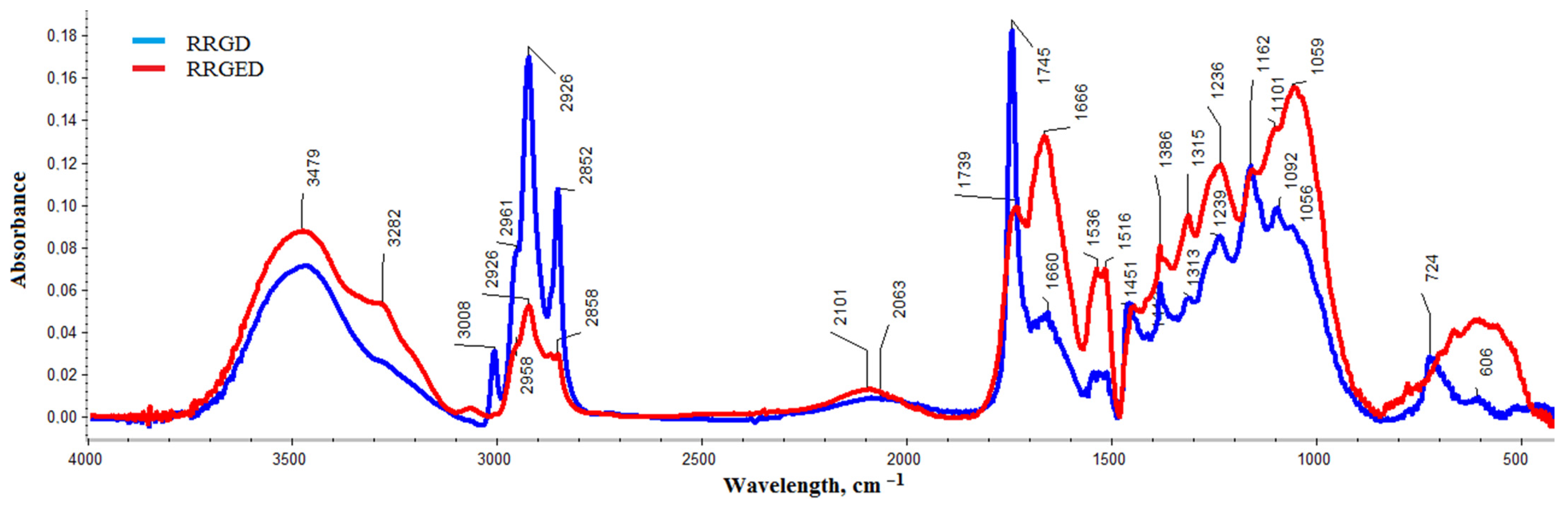
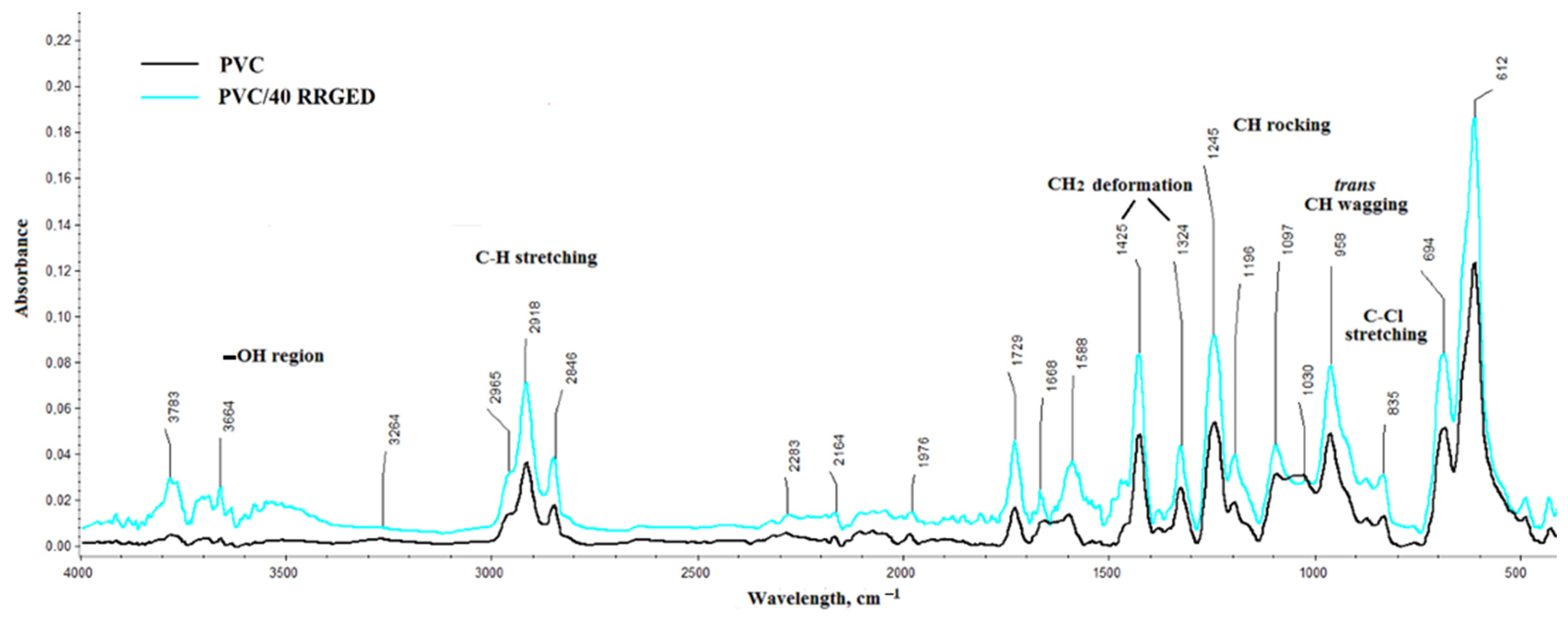
2.3.3. Chemical Structure
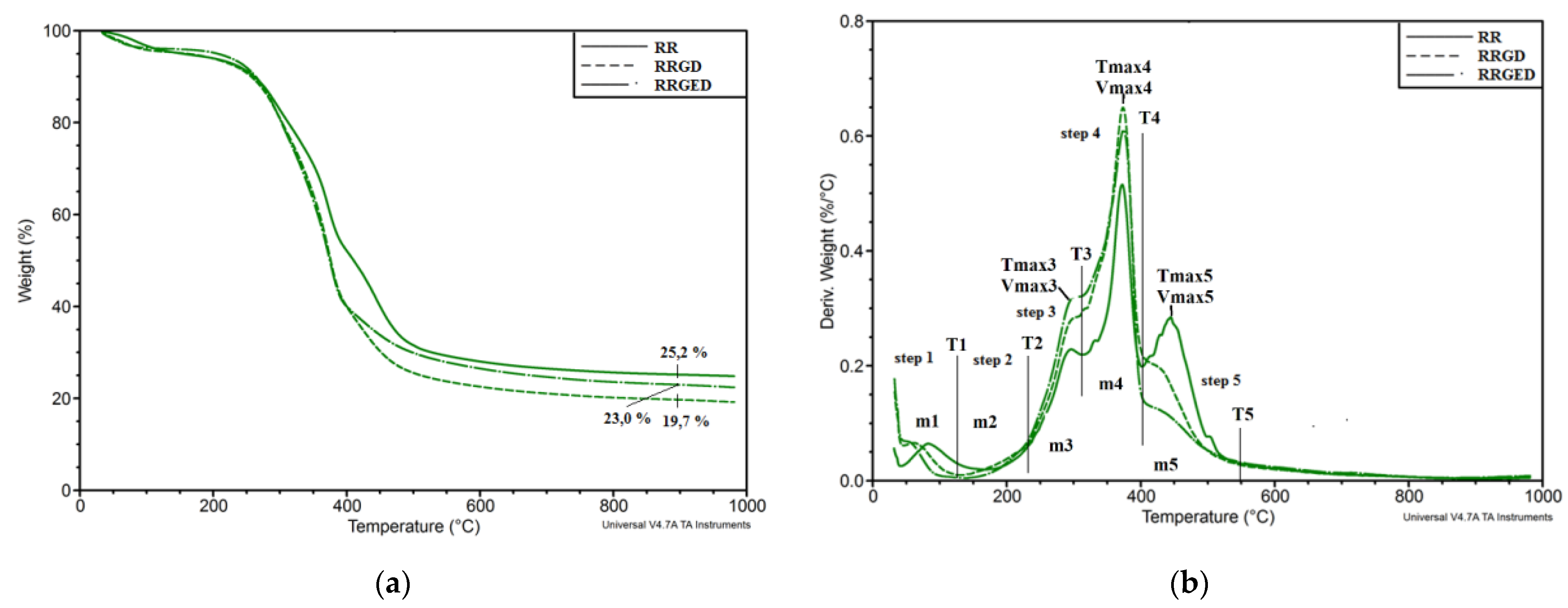
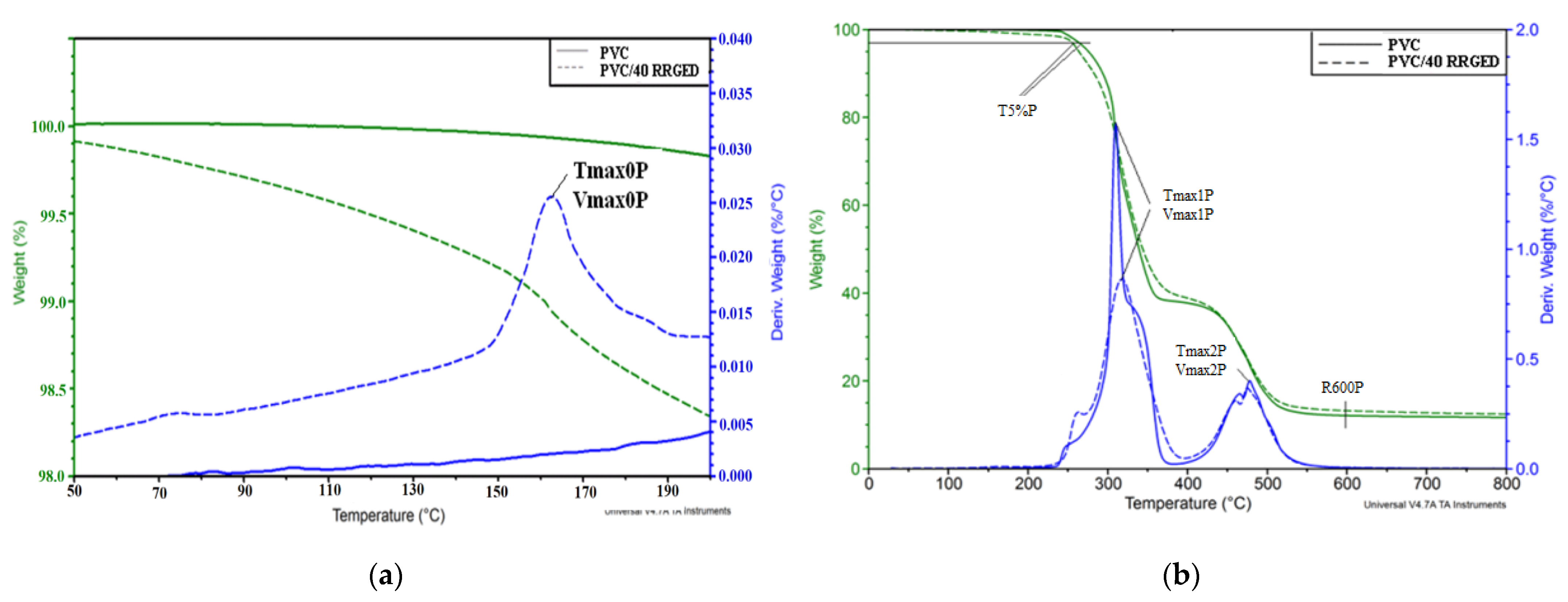
2.3.4. Thermal Analysis
2.3.5. Mass Loss
2.3.6. Density Determination
2.3.7. Mechanical Properties in Tensile Testing
2.3.8. Charpy Impact Strength
2.3.9. Shore Hardness
2.3.10. Oxygen Index Determination
2.3.11. Flammability—Test UL94
3. Results and Discussion
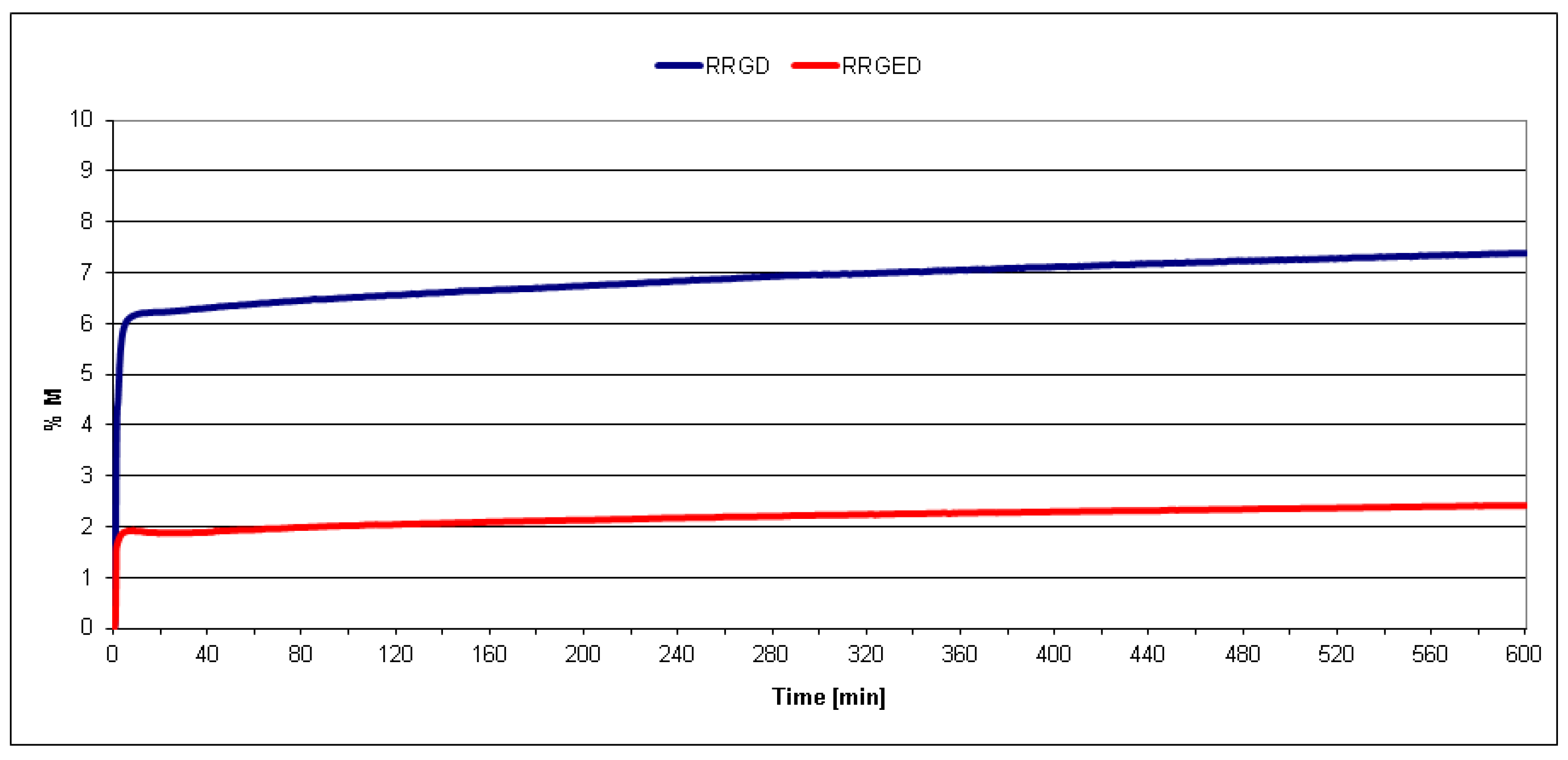
3.1. Filler Testing Results
3.2. Composite Testing Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sałasińska, K.; Ryszkowska, J. Natural Fiber Composites from Polyethylene Waste and Straw. In Proceedings of the 19th European Biomass Conference and Exhibition from Research to Industry and Markets, Berlin, Germany, 6–10 June 2011. [Google Scholar]
- Shah, D.U. Developing Plant Fibre Composites for Structural Applications by Optimising Composite Parameters: A Critical Review. J. Mater. Sci. 2013, 48, 6083–6107. [Google Scholar] [CrossRef]
- Sałasińska, K.; Osica, A.; Ryszkowska, J. The Use of Tree Leaves as Reinforcement in Composites with Recycled PE-HD Matrix. Polimery 2012, 57, 646–655. [Google Scholar] [CrossRef]
- Hammajam, A.; Nur, I.; Sapuan, S. Review of Agro Waste Plastic Composites Production. J. Miner. Mater. Charact. Eng. 2013, 1, 271–279. [Google Scholar] [CrossRef]
- Ibrahim, R.A. Tribological Performance of Polyester Composites Reinforced by Agricultural Wastes. Tribol. Int. 2015, 90, 463–466. [Google Scholar] [CrossRef]
- Antonic, B.; Jancikova, S.; Dordevic, D.; Tremlova, B. Apple Pomace as Food Fortification Ingredient: A Systematic Review and Meta-analysis. J. Food Sci. 2020, 85, 2977–2985. [Google Scholar] [CrossRef]
- Arbelaiz, A.; Fernández, B.; Ramos, J.A.; Retegi, A.; Llano-Ponte, R.; Mondragon, I. Mechanical Properties of Short Flax Fibre Bundle/Polypropylene Composites: Influence of Matrix/Fibre Modification, Fibre Content, Water Uptake and Recycling. Compos. Sci. Technol. 2005, 65, 1582–1592. [Google Scholar] [CrossRef]
- Klyosov, A.A. Wood-Plastic Composites; Wiley: Hobocen, NJ, USA, 2007; ISBN 9780470148914. [Google Scholar]
- Pirayesh, H.; Khazaeian, A. Using Almond (Prunus Amygdalus L.) Shell as a Bio-Waste Resource in Wood Based Composite. Compos. Part B Eng. 2012, 43, 1475–1479. [Google Scholar] [CrossRef]
- Bledzki, A.K.; Mamun, A.A.; Volk, J. Physical, Chemical and Surface Properties of Wheat Husk, Rye Husk and Soft Wood and Their Polypropylene Composites. Compos. Part A Appl. Sci. Manuf. 2010, 41, 480–488. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic Biomass: A Sustainable Platform for the Production of Bio-Based Chemicals and Polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Salasinska, K.; Ryszkowska, J. Natural Fiber Composites with Bioderivative and/or Degradable Polymers. In Handboock of Composites from Renewable Materials; Wiley: Hobocen, NJ, USA, 2017; Volume 1–8, ISBN 9781119441632. [Google Scholar]
- Salasinska, K.; Ryszkowska, J. The Effect of Filler Chemical Constitution and Morphological Properties on the Mechanical Properties of Natural Fiber Composites. Compos. Interfaces 2015, 22, 39–50. [Google Scholar] [CrossRef]
- Prithivirajan, R.; Jayabal, S.; Bharathiraja, G. Bio-Based Composites from Waste Agricultural Residues: Mechanical and Morphological Properties. Cellul. Chem. Technol. 2015, 49, 65–68. [Google Scholar]
- Reixach, R.; Del Rey, R.; Alba, J.; Arbat, G.; Espinach, F.X.; Mutjé, P. Acoustic Properties of Agroforestry Waste Orange Pruning Fibers Reinforced Polypropylene Composites as an Alternative to Laminated Gypsum Boards. Constr. Build. Mater. 2015, 77, 124–129. [Google Scholar] [CrossRef]
- Mäkinen, L.; Körkkö, M.; Ämmälä, A.; Haapala, A.; Suopajärvi, T.; Niinimäki, J. Recovering fibers from fine-prescreening reject at deinking mills. Tappi J. 2012, 11, 53–62. [Google Scholar] [CrossRef]
- Kotanen, L.; Körkkö, M.; Ämmälä, A.; Niinimäki, J. Resource efficiency in deinked pulp production—A review. Tappi J. 2014, 13, 37–43. [Google Scholar] [CrossRef]
- Smith, D. Chip Thickness Screening and Chip Quality in the Pulp Mill. Pap Ind. 2004, 21, 7–14. [Google Scholar]
- Chand, N.; Fahim, M.; Sharma, P.; Bapat, M.N. Influence of Foaming Agent on Wear and Mechanical Properties of Surface Modified Rice Husk Filled Polyvinylchloride. Wear 2012, 278–279, 83–86. [Google Scholar] [CrossRef]
- Ratanawilai, T.; Taneerat, K. Alternative Polymeric Matrices for Wood-Plastic Composites: Effects on Mechanical Properties and Resistance to Natural Weathering. Constr. Build. Mater. 2018, 172, 349–357. [Google Scholar] [CrossRef]
- Dan-asabe, B.; Yaro, S.A.; Yawas, D.S. Micro-Structural and Mechanical Characterization of Doum-Palm Leaves Particulate Reinforced PVC Composite as Piping Materials. Alex. Eng. J. 2018, 57, 2929–2937. [Google Scholar] [CrossRef]
- Allahbakhsh, A. PVC/Rice Straw/SDBS-Modified Graphene Oxide Sustainable Nanocomposites: Melt Mixing Process and Electrical Insulation Characteristics. Compos. Part A Appl. Sci. Manuf. 2020, 134, 105902. [Google Scholar] [CrossRef]
- Zajchowski, S.; Piszczek, K.; Tomaszewska, J. Gelation of rigid PVC during processing. Polimery 2001, 46, 232–243. [Google Scholar] [CrossRef]
- Tomaszewska, J.; Sterzyński, T.; Piszczek, K. Rigid Poly(Vinyl Chloride) (PVC) Gelation in the Brabender Measuring Mixer. I. Equilibrium State between Sliding, Breaking, and Gelation of PVC. J. Appl. Polym. Sci. 2004, 93, 966–971. [Google Scholar] [CrossRef]
- Tomaszewska, J.; Sterzyński, T.; Piszczek, K. Rigid Poly(Vinyl Chloride) (PVC) Gelation in the Brabender Measuring Mixer. II. Description of PVC Gelation in the Torque Inflection Point. J. Appl. Polym. Sci. 2007, 103, 3688–3693. [Google Scholar] [CrossRef]
- Tomaszewska, J.; Sterzyński, T.; Piszczek, K. Rigid Polyvinyl Chloride Gelation in a Brabender Measuring Mixer. III. Transformation in the Torque Maximum. J. Appl. Polym. Sci. 2007, 106, 3158–3164. [Google Scholar] [CrossRef]
- Hawkins, T. Evaluation of PVC Compound on the Brabender Torque Rheometer. J. Vinyl Technol. 1982, 4, 110–114. [Google Scholar] [CrossRef]
- Tomaszewska, J.; Sterzyński, T.; Zajchowski, S. Thermal and Structural Effects of Poly(Vinyl Chloride)/(Wood Flour) Compound Gelation in the Brabender Mixer. J. Vinyl Addit. Technol. 2011, 17, 239–244. [Google Scholar] [CrossRef]
- Bousmina, M.; Ait-Kadi, A.; Faisant, J.B. Determination of Shear Rate and Viscosity from Batch Mixer Data. J. Rheol. 1999, 43, 415–433. [Google Scholar] [CrossRef]
- Kemsley, E.K.K.; Holland, J.K.K.; Defernez, M.; Wilson, R.H.H. Detection of Adulteration of Raspberry Purees Using Infrared Spectroscopy and Chemometrics. J. Agric. Food Chem. 1996, 44, 3864–3870. [Google Scholar] [CrossRef]
- Miller, F.A. Chemical Applications of Infrared Spectroscopy; Rao, C.N.R., Ed.; Academic Press: New York, NJ, USA, 1963. [Google Scholar]
- Datta, R. Acidogenic Fermentation of Lignocellulose–Acid Yield and Conversion of Components. Biotechnol. Bioeng. 1981, 23, 2167–2170. [Google Scholar] [CrossRef]
- McKendry, P. Energy Production from Biomass (Part 1): Overview of Biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef]
- Mulligan, C.J.; Strezov, L.; Strezov, V. Thermal Decomposition of Wheat Straw and Mallee Residue under Pyrolysis Conditions. Energy Fuels 2010, 24, 46–52. [Google Scholar] [CrossRef]
- Strezov, V.; Popovic, E.; Filkoski, R.V.; Shah, P.; Evans, T. Assessment of the Thermal Processing Behavior of Tobacco Waste. Energy Fuels 2012, 26, 5930–5935. [Google Scholar] [CrossRef]
- Rahman, M.S.; Kasapis, S.; Al-Kharusi, N.S.Z.; Al-Marhubi, I.M.; Khan, A.J. Composition Characterisation and Thermal Transition of Date Pits Powders. J. Food Eng. 2007, 80, 1–10. [Google Scholar] [CrossRef]
- Disher, I.; Ali, M.; Alhattab, T. Extraction of Date Palm Seed Oil (Phoenix Dactylifera) by Soxhlet Apparatus. Int. J. Adv. Eng. Technol. 2015, 8, 261–271. [Google Scholar]
- Jadhav, A.J.; Holkar, C.R.; Goswami, A.D.; Pandit, A.B.; Pinjari, D.V. Acoustic Cavitation as a Novel Approach for Extraction of Oil from Waste Date Seeds. ACS Sustain. Chem. Eng. 2016, 4, 4256–4263. [Google Scholar] [CrossRef]
- Yao, F.; Wu, Q.; Lei, Y.; Guo, W.; Xu, Y. Thermal Decomposition Kinetics of Natural Fibers: Activation Energy with Dynamic Thermogravimetric Analysis. Polym. Degrad. Stab. 2008, 93, 90–98. [Google Scholar] [CrossRef]
- Dorez, G.; Taguet, A.; Ferry, L.; Lopez Cuesta, J.-M. Phosphorous Compounds as Flame Retardants for Polybutylene Succinate/Flax Biocomposite: Additive versus Reactive Route. Polym. Degrad. Stab. 2014, 102, 152–159. [Google Scholar] [CrossRef]
- Asim, M.; Jawaid, M.; Nasir, M.; Saba, N. Effect of Fiber Loadings and Treatment on Dynamic Mechanical, Thermal and Flammability Properties of Pineapple Leaf Fiber and Kenaf Phenolic Composites. J. Renew. Mater. 2018, 6, 383–393. [Google Scholar] [CrossRef]
- Essabir, H.; Bensalah, M.O.; Rodrigue, D.; Bouhfid, R.; Qaiss, A. Structural, Mechanical and Thermal Properties of Bio-Based Hybrid Composites from Waste Coir Residues: Fibers and Shell Particles. Mech. Mater. 2016, 93, 134–144. [Google Scholar] [CrossRef]
- Asim, M.; Paridah, M.T.; Chandrasekar, M.; Shahroze, R.M.; Jawaid, M.; Nasir, M.; Siakeng, R. Thermal Stability of Natural Fibers and Their Polymer Composites. Iran. Polym. J. Engl. Ed. 2020, 29, 625–648. [Google Scholar] [CrossRef]
- Methacanon, P.; Weerawatsophon, U.; Sumransin, N.; Prahsarn, C.; Bergado, D.T. Properties and Potential Application of the Selected Natural Fibers as Limited Life Geotextiles. Carbohydr. Polym. 2010, 82, 1090–1096. [Google Scholar] [CrossRef]
- Déniel, M.; Haarlemmer, G.; Roubaud, A.; Weiss-Hortala, E.; Fages, J. Bio-Oil Production from Food Processing Residues: Improving the Bio-Oil Yield and Quality by Aqueous Phase Recycle in Hydrothermal Liquefaction of Blackcurrant (Ribes nigrum L.) Pomace. Energy Fuels 2016, 30, 4895–4904. [Google Scholar] [CrossRef]
- Li, J.; Kazakov, A.; Dryer, F.L. Experimental and Numerical Studies of Ethanol Decomposition Reactions. J. Phys. Chem. A 2004, 108, 7671–7680. [Google Scholar] [CrossRef]
- Liang, G.; He, L.; Cheng, H.; Li, W.; Xiaoru, L.; Zhang, C.; Yu, Y.; Zhao, F. The Hydrogenation/Dehydrogenation Activity of Supported Ni Catalysts and Their Effect on Hexitols Selectivity in Hydrolytic Hydrogenation of Cellulose. J. Catal. 2014, 309, 468–476. [Google Scholar] [CrossRef]
- Opatokun, S.A.; Kan, T.; Al Shoaibi, A.; Srinivasakannan, C.; Strezov, V. Characterization of Food Waste and Its Digestate as Feedstock for Thermochemical Processing. Energy Fuels 2016, 30, 1589–1597. [Google Scholar] [CrossRef]
- Garcia-Perez, M.; Adams, T.T.; Goodrum, J.W.; Das, K.C.; Geller, D.P. DSC Studies to Evaluate the Impact of Bio-Oil on Cold Flow Properties and Oxidation Stability of Bio-Diesel. Bioresour. Technol. 2010, 101, 6219–6224. [Google Scholar] [CrossRef]
- Beltrán, M.; Marcilla, A. Fourier Transform Infrared Spectroscopy Applied to the Study of PVC Decomposition. Eur. Polym. J. 1997, 33, 1135–1142. [Google Scholar] [CrossRef]
- Khaleghi, M.; Didehban, K.; Shabanian, M. Simple and Fast Preparation of Graphene Oxide@ Melamine Terephthaldehyde and Its PVC Nanocomposite via Ultrasonic Irradiation: Chemical and Thermal Resistance Study. Ultrason. Sonochem. 2018, 43, 275–284. [Google Scholar] [CrossRef]
- Deshmukh, K.; Khatake, S.M.; Joshi, G.M. Surface Properties of Graphene Oxide Reinforced Polyvinyl Chloride Nanocomposites. J. Polym. Res. 2013, 20, 286. [Google Scholar] [CrossRef]
- Tian, C.; Wang, H.; Liu, X.; Ma, Z.; Guo, H.; Xu, J. Flame Retardant Flexible Poly(Vinyl Chloride) Compound for Cable Application. J. Appl. Polym. Sci. 2003, 89, 3137–3142. [Google Scholar] [CrossRef]
- Ning, Y.; Guo, S. Flame-Retardant and Smoke-Suppressant Properties of Zinc Borate and Aluminum Trihydrate-Filled Rigid PVC. J. Appl. Polym. Sci. 2000, 77, 3119–3127. [Google Scholar] [CrossRef]
- Gumargalieva, K.Z.; Ivanov, V.B.; Zaikov, G.E.; Moiseev, J.V.; Pokholok, T.V. Problems of Ageing and Stabilization of Poly(Vinyl Chloride). Polym. Degrad. Stab. 1996, 52, 73–79. [Google Scholar] [CrossRef]
- Gao, M.; Wan, M.; Chen, X. Synthesis of a Solid Superacid and Its Application in Flame-Retardant Poly(Vinyl Chloride) Material. ACS Omega 2019, 4, 7556–7564. [Google Scholar] [CrossRef] [PubMed]
- Elakesh, E.O.; Hull, T.R.; Price, D.; Carty, P. Thermal Decomposition of Chlorinated Poly(Vinyl Chloride) (CPVC). J. Vinyl Addit. Technol. 2003, 9, 116–126. [Google Scholar] [CrossRef]
- Tomaszewska, J.; Sterzyński, T.; Skórczewska, K. Effect of Polyhedral Oligomeric Silsesquioxanes Nanoparticles on Thermal and Mechanical Properties of Poly(Vinyl Chloride) Composite Materials. J. Vinyl Addit. Technol. 2019, 25, E48–E54. [Google Scholar] [CrossRef]
- Zajchowski, S.; Ryszkowska, J. Wood-Polymer Composites—General Characteristics and Their Preparation from Waste Materials. Polimery 2009, 54, 754–762. [Google Scholar] [CrossRef][Green Version]
- Tomaszewska, J.; Zajchowski, S. Study on the Mechanical Properties and Structure of the Compounds of PE/PVC Recyclates Filled with Wood Flour | Właściwości Mechaniczne i Struktura Mieszanin Recyklatów Polietylenu i Poli(Chlorku Winylu) Napełnionych Mączką Drzewną. Polimery 2013, 58, 106–113. [Google Scholar] [CrossRef]
- Sain, M.; Pervaiz, M. Mechanical Properties of Wood-Polymer Composites; University of Toronto: Toronto, ON, Canada, 2008; ISBN 9781845692728. [Google Scholar]
- Jia, P.; Hu, L.; Feng, G.; Bo, C.; Zhang, M.; Zhou, Y. PVC Materials without Migration Obtained by Chemical Modification of Azide-Functionalized PVC and Triethyl Citrate Plasticizer. Mater. Chem. Phys. 2017, 190, 25–30. [Google Scholar] [CrossRef]
- Crespo, J.E.; Sánchez, L.; García, D.; López, J. Study of the Mechanical and Morphological Properties of Plasticized PVC Composites Containing Rice Husk Fillers. J. Reinf. Plast. Compos. 2008, 27, 229–243. [Google Scholar] [CrossRef]
- Knümann, R.; Bockhorn, H. Investigation of the Kinetics of Pyrolysis of PVC by TG-MS-Analysis. Combust. Sci. Technol. 1994, 101, 285–299. [Google Scholar] [CrossRef]
- Pagacz, J.; Pielichowski, K. Preparation and Characterization of PVC/Montmorillonite Nanocomposites-A Review. J. Vinyl Addit. Technol. 2009, 15, 61–76. [Google Scholar] [CrossRef]



| Component | Trade Name | Concentration, phr | Function |
|---|---|---|---|
| Poly(vinyl chloride) | PVC Neralit 601 | 100 | Main component |
| Organotin | Patstab 2310 | 2 | Thermal stabilizer |
| Calcium stearate | Ceasit I | 1.2 | Thermal stabilizer |
| Fatty acid ester | Loxiol G-32 | 1.5 | External and internal lubricant |
| Acrylic-based polymer | Poraloid K-125 | 1 | Universal lubricant |
| Acrylic-based polymer | Poraloid K-175 | 1 | External lubricant |
| Paraffin | Naftolube FTP | 0.5 | External lubricant |
| Sample Type | Filler | Concentration, % mas |
|---|---|---|
| PVC | – | – |
| PVC/20 RRGD | RRGD | 20 |
| PVC/30 RRGD | RRGD | 30 |
| PVC/40 RRGD | RRGD | 40 |
| PVC/20 RRGED | RRGED | 20 |
| PVC/30 RRGED | RRGED | 30 |
| PVC/40 RRGED | RRGED | 40 |
| Bands | Wavelength of RRGD, cm−1 | Wavelength of RRGED, cm−1 | Intensity 1 | Wavelength of RR Oil, cm−1 [32] |
|---|---|---|---|---|
| O–H stretching vibrations of water, phenols, alcohols, or carboxylic acids | 3479 | 3479 |  | – |
| intermolecular bonded O–H stretching vibrations | 3282 | 3282 |  | – |
| CH2 deformation bands | 3008 | – |  | 3017 |
| asymmetric CH3 stretching | 2958 | 2961 |  | – |
| the stretching vibrations of aliphatic C–H in CH2 | 2926 | 2926 |  | 2929 |
| the stretching vibrations of aliphatic C–H in terminal CH3 groups | 2852 | 2858 |  | 2849 |
| C=O stretching vibration of carboxylic acids of the ester | 1745 | 1739 |  | 1745 |
| The aromatic ring stretch | 1660 | 1668 |  | – |
| The vibrations of the N–H in-plane bending of amides and amines group | 1536 | 1536 |  | – |
| C–C stretching, | 1516 | 1516 |  | – |
| CH2 scissoring | 1440 | 1451 | = | 1460 |
| The symmetric HCH bending | 1386 | 1386 |  | 1377 |
| CH2 wagging mode of cellulose | 1313 | 1315 |  | – |
| The aromatic −O, O−O−C, and C−N bonds | 1239 | 1236 |  | – |
| the C−O stretching alcohols groups C–C stretching | 1162 | 1162 |  | 1157 |
| polysaccharides region 900–1150 cm−1 | 1092 | 1100 |  | – |
| C–O–C asymmetric stretches for the mixed phenyl/methyl ether linkage | 1056 | 1059 |  | – |
| the aromatic compounds present in the oil | 724 | – |  | 721 |
 increase in intensity,
increase in intensity,  drop in intensity, = no change.
drop in intensity, = no change.| Parameter | RR | RRGD | RRGED |
|---|---|---|---|
| Step 1 | |||
| Stage 1 end temperature (T1), °C | 168 | 127 | 139 |
| Weight change (m1), % | 5.3 | 4.6 | 4.0 |
| Step 2 | |||
| Stage 2 end temperature (T2), °C | 233 | 235 | 231 |
| Weight change (m2), % | 1.9 | 3.2 | 2.3 |
| Step 3 | |||
| Stage 3 end temperature (T3), °C | 315 | 309 | 318 |
| Weight change (m3), % | 20.5 | 14.0 | 18.9 |
| Temperature at Vmax3 (Tmax3), °C | 294 | 301 | 300 |
| Maximum degradation rate (Vmax3), %/°C | 0.23 | 0.28 | 0.32 |
| Step 4 | |||
| Stage 4 end temperature (T4), °C | 398 | 399 | 400 |
| Weight change (m4), % | 16.9 | 37.7 | 34.4 |
| Temperature at Vmax4 (Tmax4), °C | 372 | 373 | 374 |
| Maximum degradation rate (Vmax4), %/°C | 0.52 | 0.65 | 0.61 |
| Step 5 | |||
| Stage 5 end temperature (T5), °C | 550 | 550 | 550 |
| Weight change (m5), % | 23.3 | 16.5 | 12.1 |
| Temperature at Vmax5 (Tmax5), °C | 440 | – | – |
| Maximum degradation rate (Vmax5), %/°C | 0.28 | – | – |
| Remainder at temperature 900 °C, % | 25.2 | 23.0 | 19.7 |
| Material | MX, Nm | TX, °C | tX, min | ME, Nm | TE, °C |
|---|---|---|---|---|---|
| PVC | 37.6 | 180.4 | 14.1 | 31.5 | 184.7 |
| PVC/20 RRGED | 38.8 | 181.3 | 8.7 | 34.1 | 185.8 |
| PVC/30 RRGED | 39.6 | 183.8 | 8.6 | 33.9 | 189.7 |
| PVC/40 RRGED | 46.3 | 184.5 | 8.8 | 37.2 | 191.0 |
| (a) Sample | S, min | Δm165 °C, % | Tmax0P, °C /Vmax0P, %/°C | Δm0P, % | ||
| PVC | 21.8 | 0.1 | - | 0.2 | ||
| PVC/20 RRGED | 29.5 | 0.7 | 163/0.011 | 1.0 | ||
| PVC/30 RRGED | 30.6 | 0.9 | 160/0.015 | 1.2 | ||
| PVC/40 RRGED | 31.4 | 1.1 | 165/0.026 | 1.7 | ||
| (b) Sample | T5%P, °C | Tmax1P, °C /Vmax1P, %/°C | Δm1P, % | Tmax2P, °C /Vmax2P, %/°C | Δm2P, % | R600P, % |
| PVC | 276 | 310/1.57 | 61.4 | 478/0.40 | 26.1 | 12.1 |
| PVC/20 RRGED | 264 | 318/0.86 | 59.5 | 475/0.37 | 25.7 | 13.3 |
| PVC/30 RRGED | 262 | 304/0.81 | 57.0 | 472/0.32 | 24.0 | 18.5 |
| PVC/40 RRGED | 257 | 296/0.91 | 54.9 | 473/0.31 | 23.1 | 20.0 |
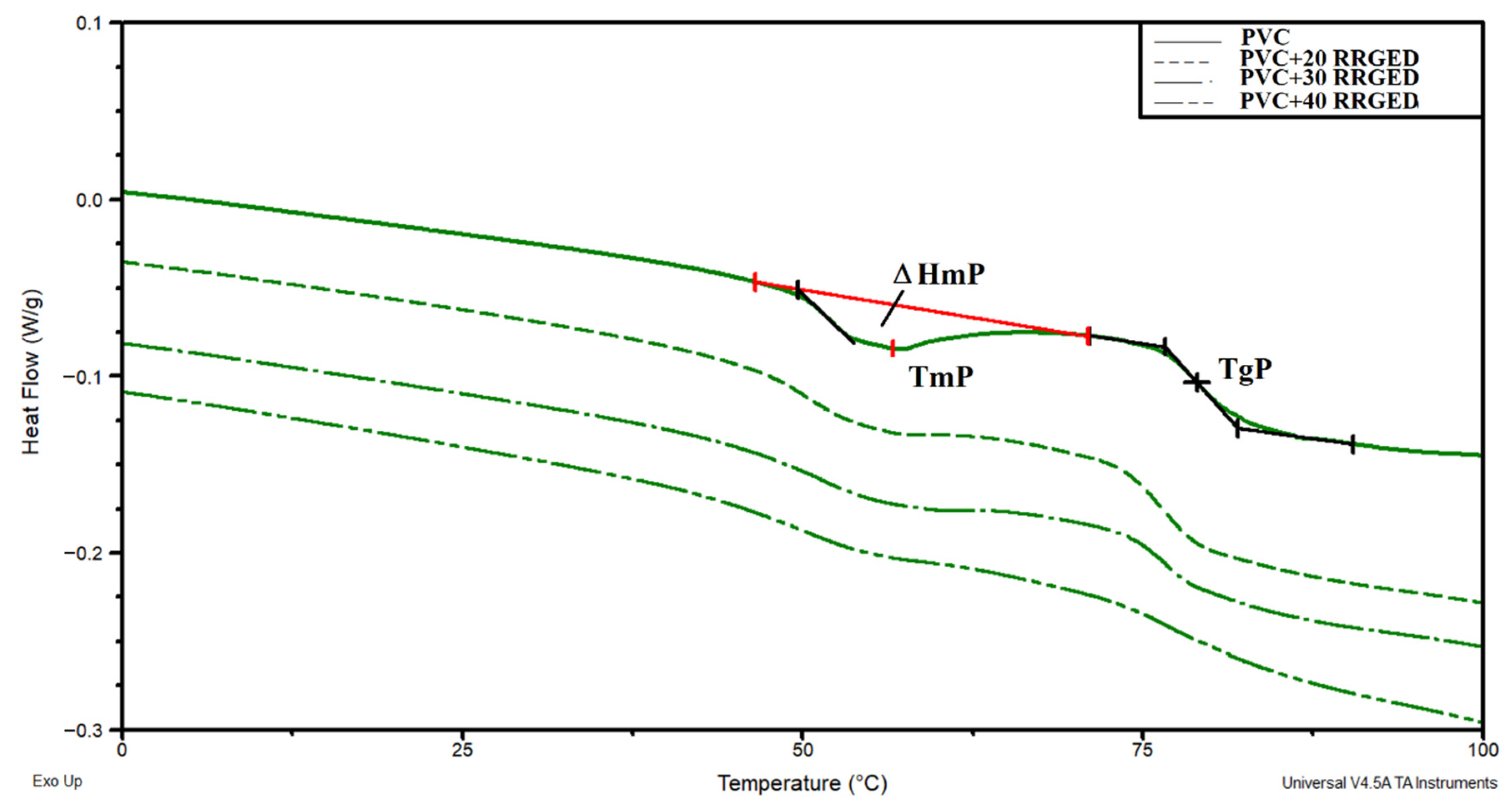
| Sample | TmP, °C | ΔHmP, J/g | TgP, °C | ΔCpP, J/g °C |
|---|---|---|---|---|
| PVC | 56.1 | 1.72 | 79.0 | 0.27 |
| PVC/20 RRGED | 55.3 | 1.04 | 76.0 | 0.27 |
| PVC/30 RRGED | 55.7 | 0.95 | 76.7 | 0.24 |
| PVC/40 RRGED | 54.5 | 0.53 | 76.3 | 0.21 |
| Material | D, g/cm3 | Et, MPa | σy, MPa | εy, % | σm, MPa | εm, % | σb, MPa | εb, % | H, °ShD | U, kJ/m2 |
|---|---|---|---|---|---|---|---|---|---|---|
| PVC | 1.37 ± 0.01 | 1230 ± 90 | 55.3 ± 4.5 | 4.1 ± 0.4 | 55.3 ± 4.5 | 4.1 ± 0.4 | 34.2 ± 1.0 | 11.3 ± 1.1 | 65 ± 1 | 58.7 ± 5.5 |
| PVC/20 RRGED | 1.36 ± 0.01 | 1290 ± 70 | – | – | 29.7 ± 0.1 | 3.5 ± 0.2 | 26.9 ± 0.1 | 4.1 ± 0.2 | 66 ± 2 | 5.5 ± 0.5 |
| PVC/30 RRGED | 1.34 ± 0.01 | 1520 ± 10 | – | – | 23.6 ± 0.5 | 2.1 ± 0.2 | 22.8 ± 0.2 | 2.5 ± 0.3 | 67 ± 2 | 3.3 ± 0.1 |
| PVC/40 RRGED | 1.33 ± 0.01 | 1630 ± 10 | – | – | 21.2 ± 0.2 | 1.7 ± 0.3 | 20.8 ± 0.1 | 1.9 ± 0.3 | 68 ± 2 | 3.2 ± 0.5 |
| Symbol | LOI, % | UL 94 |
|---|---|---|
| PVC | 37.4 ± 0.1 | V0 |
| PVC/20 RRGED | 34.0 ± 0.1 | V0 |
| PVC/30 RRGED | 32.4 ± 0.2 | V0 |
| PVC/40 RRGED | 32.0 ± 0.1 | V0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirowski, J.; Oliwa, R.; Oleksy, M.; Tomaszewska, J.; Ryszkowska, J.; Budzik, G. Poly(vinyl chloride) Composites with Raspberry Pomace Filler. Polymers 2021, 13, 1079. https://doi.org/10.3390/polym13071079
Mirowski J, Oliwa R, Oleksy M, Tomaszewska J, Ryszkowska J, Budzik G. Poly(vinyl chloride) Composites with Raspberry Pomace Filler. Polymers. 2021; 13(7):1079. https://doi.org/10.3390/polym13071079
Chicago/Turabian StyleMirowski, Jacek, Rafał Oliwa, Mariusz Oleksy, Jolanta Tomaszewska, Joanna Ryszkowska, and Grzegorz Budzik. 2021. "Poly(vinyl chloride) Composites with Raspberry Pomace Filler" Polymers 13, no. 7: 1079. https://doi.org/10.3390/polym13071079
APA StyleMirowski, J., Oliwa, R., Oleksy, M., Tomaszewska, J., Ryszkowska, J., & Budzik, G. (2021). Poly(vinyl chloride) Composites with Raspberry Pomace Filler. Polymers, 13(7), 1079. https://doi.org/10.3390/polym13071079