Abstract
End-stage kidney disease (ESKD) is a main public health problem, the prevalence of which is continuously increasing worldwide. Due to adverse effects of renal replacement therapies, kidney transplantation seems to be the optimal form of therapy with significantly improved survival, quality of life and diminished overall costs compared with dialysis. However, post-transplant patients frequently suffer from post-transplant diabetes mellitus (PTDM) which an important risk factor for cardiovascular and cardiovascular-related deaths after transplantation. The management of post-transplant diabetes resembles that of diabetes in the general population as it is based on strict glycemic control as well as screening and treatment of common complications. Lifestyle interventions accompanied by the tailoring of immunosuppressive regimen may be of key importance to mitigate PTDM-associated complications in kidney transplant patients. More transplant-specific approach can include the exchange of tacrolimus with an alternative immunosuppressant (cyclosporine or mammalian target of rapamycin (mTOR) inhibitor), the decrease or cessation of corticosteroid therapy and caution in the prescribing of diuretics since they are independently connected with post-transplant diabetes. Early identification of high-risk patients for cardiovascular diseases enables timely introduction of appropriate therapeutic strategy and results in higher survival rates for patients with a transplanted kidney.
1. Introduction
End-stage kidney disease (ESKD) is a main public health problem, the prevalence of which is continuously increasing worldwide [1]. Due to the adverse effects of renal replacement therapies, kidney transplantation seems to be the optimal form of therapy with significantly improved survival, quality of life, and diminished overall costs compared with dialysis [2,3,4]. However, it does not mean that transplant patients are devoid of elevated mortality rates compared with the general population [5]. Such increased mortality is associated with a high rate of cardiovascular and cardiovascular-related deaths after the transplantation, which constitutes over 50% of all deaths in this group [6,7]. Numerous studies have also indicated that gender issues in transplantation are highly important as they not only exert an impact on graft functioning and the risk of rejections but also on the post-transplant diabetes mellitus (PTDM) development hazard. Nowadays, donors frequently cannot be matched by gender due to the not sufficient number of donors and high demand for transplantations. However, female-mismatch patients (male donor/female recipient) were found to have more episodes of acute rejection and more rehospitalizations [8,9]. Such finding could be ascribed not only to weight-mismatch or size-mismatch of an organ but also hormonal discrepancies. Several studies have demonstrated that male renal allografts may function better over time than grafts from female donors, while those from female donors show worse renal allograft survival [10,11,12,13]. According to studies, recipients of male kidneys are less likely to require antirejection therapies beyond their baseline immunosuppression and their renal function is significantly improved after transplantation [13]. In turn, female kidney recipients seem to be more likely to have acute rejection episodes (possibly as a result of sensitization after pregnancy), but less likely to develop chronic graft rejection [10,14]. It has also been suggested that benefits related to the transplantation of kidney from a male donor are associated with the fact that organs from males have greater number of nephrons or mass than female kidneys, however, this theory of “nephron under-dosing” has not been confirmed. There are also numerous pieces of evidence that the complex impact of androgens and estrogens on the functioning of many cells as well as immunologic differences between men and women may contribute to worse renal graft outcomes with female donors [10,13].
Post-transplant diabetes mellitus (PTDM) is an important risk factor for this enhanced risk. In the study of stable kidney transplant patients, 43% of participants had new impaired glucose metabolism developed within 6 months from the transplantation [15]. Numerous studies confirmed higher rates of cardiovascular disease occurrence, cardiovascular death and all-cause mortality in post-transplant patients [16,17]. Additionally, other forms of impaired glucose metabolism after transplantation seem to be associated with increased mortality [18].
The selection of articles for this literature review was based on a PubMed search (terms: “PTDM” + “risk factors” + “pathomechanism” + “cardiovascular risk” were applied). We tried to focus on those studies which were performed in large groups or which results were, in our opinion, particularly interesting.
2. Prediabetes and Post-Transplant Diabetes Mellitus (PTDM)—Diagnosis and Prevalence
Prediabetes and post-transplant diabetes are frequently occurring in renal transplant patients. According to the definition, prediabetes is an intermediate metabolic state between normoglycemia and diabetes [19]. The diagnosis of prediabetes is made on the basis of the presence of impaired fasting glucose (IFG), impaired glucose tolerance (IGT) in ordinary oral glucose tolerance test and glycated hemoglobin A1c (HbA1c) concentration between 5.7% and 6.4% [20]. This state does not fulfill the criteria of diabetes mellitus (DM), however, it is an independent risk factor for the progression to DM or post-transplantation diabetes mellitus (PTDM) [21]. The mechanisms of prediabetes pathogenesis remain not fully resolved. It has been suggested that impaired fasting glycemia may be associated with peripheral insulin resistance, while impaired glucose tolerance (IGT) with impaired β-cell function [22,23].
PTDM is defined as “newly diagnosed diabetes mellitus in the post-transplant setting (irrespective of timing or whether it was present but undetected prior to transplantation or not)” [16,24]. For the first time, post-transplantation diabetes mellitus was described in kidney transplant recipients in 1964 [25,26]. Through the years, the nomenclature of this disease has changed several times. In 2014, the International Expert Panel comprising transplant nephrologists, diabetologists, and clinical scientists recommended the replacement of NODAT (new-onset diabetes after transplantation) term with PTDM as a result of a high prevalence of undiagnosed pretransplant diabetes mellitus [16].
The diagnosis of post-transplant diabetes has been challenging for a long time. The first clear diagnostic criteria were introduced in 2003 by the American Diabetes Association and the World Health Organization (WHO) [17]. These guidelines have been updated in 2010 and according to them the following criteria must be met to diagnose post-transplant diabetes: “symptoms of diabetes and a non-fasting plasma glucose (PG) of > 200 mg/dL (11.1 mmol/L), fasting PG of > 126 mg/dL (7.0 mmol/L), PG > 200 mg/dL (11.1 mmol/L) 2 h following an oral glucose tolerance test and HbA1C > 6.5% (48 mmol/mol) [27].
According to estimations, prediabetes and post-transplant diabetes mellitus can affect about 10–30% of renal transplant patients [19,28,29]. Prospective studies utilizing contemporary consensus-based diagnostic criteria (e.g., repeated oral glucose tests) have confirmed the occurrence of PTDM in about 30% of renal transplant recipients and prediabetes in another 20% of this population [28]. The rates of PTDM may increase with time and reach 27% within 3 months and 30% after 36 months from the transplantation [28]. The highest rate of PTDM occurrence (83.7%) is observed within the first year [30]. However, due to the early reversibility rate of IFG or IGT, the diagnosis of prediabetes and PTDM in stable clinical conditions should be performed after the first 12 months from transplantation [19].
3. Prediabetes and Post-Transplant Diabetes Mellitus (PTDM)—Risk Factors and Pathophysiology
The pathomechanism of PTDM is not fully resolved. β-cell dysfunction is believed to be the vital mechanism involved in the development of PTDM due to the modification in insulin secretion [31]. Enhanced insulin resistance and predominantly decreased insulin secretion are considered to be the underlying causes of PTDM [15,32]. It remains unknown, whether the risk of progression to PTDM differs between patients with IFG and IGT, however, such knowledge would allow for further risk stratification [20]. The PTDM develops in the consequence of the presence of predisposing factors (which are similar to type 2 diabetes mellitus) as well as risk factors related to the transplantation, among which the most important are the following: metabolic adverse effects of immunosuppressive therapy with calcineurin inhibitors, mammalian target of rapamycin inhibitors (mTORi), and corticosteroids, infections with cytomegalovirus (CMV) and hepatitis C virus after the transplantation and hypomagnesemia [33,34,35,36]. According to studies, PTDM is nearly four times more frequent in anti-HCV-positive patients compared to uninfected individuals due to the fact that the hepatitis C virus stimulates the process of apoptosis-like death of pancreatic β -cells via the caspase 3-dependent pathway [37,38,39]. As CMV infection has been confirmed to be a risk factor for increasing incidence of PTDM, the prophylaxis against CMV infection after kidney transplantation is strongly recommended [40]. The impact of CMV on diminishing insulin secretion may involve direct β -cell damage by CMV and apoptosis or their destruction by infiltrative leukocytes or it is the result of the induction of proinflammatory cytokines. According to studies, allograft-associated factors, such as graft type have an impact on the development of PTDM [41]. Deceased donor allografts express higher levels of proinflammatory cytokines than allografts from living donors and it seems that the presence of such a proinflammatory state predisposes to the PTDM. Indeed, in the recipients of deceased donor grafts higher prevalence of PTDM is observed compared to living donor grafts (hazard ratio (HR) 3.3 (1.46–7.52), p = 0.004 risk) [42].
According to Cosio et al. [43], the increasing incidence of PTDM is related to the greatly improved bioavailability of calcineurin inhibitors (CNIs) and thus increased exposure to their diabetogenic properties as well as the change in recipient characteristics, particularly the increased body weight at the time of transplantation. Immunosuppressive drugs (calcineurin inhibitors, corticosteroids and mTORi) have been confirmed to exert adverse metabolic effects [33]. Calcineurin inhibitors (CNIs) have been shown to induce PTDM through multiple mechanisms, including the diminishing of insulin secretion and due to direct toxic effects on pancreatic β- cells [37]. The analysis of pancreatic histology sections confirmed that the administration of CNIs was associated with enhanced islet cell apoptosis and reduced β-cell mass [44,45]. This effect is related to the fact that calcineurin activates the transcription factors nuclear factor of activated T-cells (NFAT) which stimulates IRS2 transcription and cAMP response element-binding protein (CREB) mediating proliferative effects of glucagon-like peptide (GLP-1) and, therefore, it affects the survival of β-cells in the pancreas [41]. In turn, CNIs downregulate IRS2 expression via the inhibition of both NFAT and CREB [46]. Experimental studies confirmed that calcineurin inhibition decreased Akt phosphorylation in murine and human islets [46]. Some studies suggested that therapeutic levels of cyclosporine and tacrolimus could hinder glucose uptake into adipose cells via the stimulation of endocytosis of glucose transporters type 4 (GLUT 4) from the cell surface, while others failed to demonstrate strong impact of CNIs on insulin sensitivity [47,48].
The negative effects of therapy with corticosteroids involve the impairment of insulin secretion, the aggravation of insulin resistance related to higher rates of gluconeogenesis in the liver as well as indirect impact on weight gain, the rise in lipolysis-induced dyslipidemia, and the decrease in muscle mass, glycogen synthesis and glucose uptake in skeletal muscle cells [35,46,49]. However, the resignation from such treatment may improve insulin sensitivity but it also significantly enhances the risk of acute graft rejection, and worsens proteinuria and glomerulonephritis recurrence [50,51,52]. Tacrolimus-related adverse diabetogenic effects include the reduction in insulin gene transcription, β-cell apoptosis, and the reduction in insulin exocytosis [53]. Therefore, it seems that the adjustment of its dose may improve pancreatic β-cell function [54].
Additionally, mTOR inhibitors (sirolimus) have been found to be associated with a higher risk for PTDM as a result of sirolimus-induced hyperglycemia caused by compromised insulin-mediated suppression of hepatic glucose production, the accumulation of ectopic triglycerides and subsequent insulin resistance, as well as direct pancreatic β-cell toxicity [49]. mTOR inhibitors act on the insulin receptor-IRS-PI3K-Akt pathway; the activation of Akt and subsequent stimulation of protein synthesis require the activation of the mTOR-containing complex (mTORC1). The binding of sirolimus to mTOR results in the stimulation of the phosphorylation and the repression of IRS-1 leading to the inhibition of P13K/Akt signaling [55]. Sirolimus has been demonstrated to induce hyperinsulinemia, glucose intolerance, and hypertriglyceridemia as the result of enhanced hepatic gluconeogenesis and diminished stimulated glucose uptake in skeletal muscle [33,56,57]. The impact of everolimus on islet cell function is weaker compared to sirolimus, however, the rejection rates, in this case, are higher and some studies indicated that its administration brought no benefits in terms of the incidence of PTDM [58,59]. Moreover, similarly to CNIs, mTORi could promote apoptosis of pancreatic islet cells in vitro [60]. Besides, sirolimus has been found to impair pancreatic ductal proliferation and diminish ductal cell numbers in a culture which could translate into decreased glucose-stimulated insulin secretion [61]. It seems that the impact of mTOR inhibitors on insulin signaling is much more important than on insulin secretion. According to a large, retrospective study, treatment with sirolimus is an independent and vital risk factor for the development of PTDM, the impact of which is as strong as that of obesity or older age [62].
Additionally, the use of glucocorticoids was found to be associated with hyperglycemia resulting from the increase in glucose resistance, the reduction in insulin secretion, and the induction of β-cells apoptosis leading to reduced expression of glucose transporter 2 and glucokinase [63]. The effect is dose-dependent.
Among other modifiable risk factors of PTDM, there are the following: obesity, metabolic syndrome, and physical activity. While, the nonmodifiable include family history of DM, age, the presence of autosomal-dominant polycystic kidney disease (ADPKD), African American and Hispanic origin as well as some human leukocyte antigen (HLA) genotypes, including HLA-B27, HLA-DR3, and HLA-A3 [17,33,34,64,65]. Higher PTDM risk is observed in transplant patients of Hispanic and Caucasian and African American origin [66,67]. The results concerning the relationship between a family history of diabetes and PTDM are conflicting. Some studies failed to find any association [30,68], while other indicated that diabetes in first-degree relatives was an independent risk factor of glucose metabolism disorders or PTDM after kidney transplantation [69,70]. Gene polymorphisms within a leptin receptor and a cytochrome CYP24A1 have also been suggested as emerging risk factors for PTDM, however, further studies are required to assess their impact [71,72].
It has been suggested that genes participating in the regulation of lipid homeostasis and carbohydrate metabolism could be associated with the development of PTDM. Yang et al. [73] observed that in kidney transplant patients of Hispanic ethnicity 2 alleles of the HNF-4A gene encoding transcription factor (rs2144908 and rs1884614) were significantly associated with PTDM. Adiponectin and leptin have been demonstrated to be implicated in the regulation of insulin secretion, glucose and lipid metabolism. For example, LEP rs2167270 gene polymorphism was shown to be considerably associated with enhanced risk of PTDM [74]. Moreover, some studies suggested that mannose-binding lectin 2 (MBL2), which is a vital recognition molecule of the lectin pathway of complement activation, could be involved in the pathogenesis of PTDM since it could play an important role in noninfectious inflammatory damage following organ transplantation [75,76]. The results of the study conducted by Guad et al. [77] demonstrated that indeed AG heterozygous variant of the MBL2 gene (rs2232365) was associated with an elevated risk of PTDM compared to AA variant. It seems that genetic polymorphisms within this gene may affect insulin secretion from the pancreas. Other groups of genes assessed in their relation to PTDM are interleukins (ILs) and inflammation-related factors. Inflammatory chemokines and cytokines have been shown to be involved in peripheral insulin action and insulin secretion [78]. ILs and other molecules secreted by T cells mediate inflammation via the promotion of the production of inflammatory cytokines (TNF-α, IL-1B, and IL-6) [78]. G allele at position 2174 of the IL-6 gene promoter has been shown to be associated with the risk of PTDM among overweight subjects [79]. Additionally, variations of interleukin (IL)-7R, IL-17E, IL-17R, and IL-17RB were found to influence this risk, which may imply that the inflammation of islet β-cells might be of fundamental importance in the pathogenesis of PTDM in renal transplantation recipients [80]. Bamoulid et al. [79] found that in GG homozygotes (IL-6-174 SNP) the risk of PTDM was significantly higher, however, this effect was restricted mostly to overweight patients. Moreover, they demonstrated the link between G allele and serum IL-6 levels and lower insulin sensitivity in the GG carriers than in the CC carriers (2.15 ± 2 versus 1.32 ± 1.03; p = 0.03). In turn, Weng et al. [81] indicated a decreased risk of PTDM development in carriers of IL-6 G/G genotype. The analysis of the panel of 18 SNPs within 10 genes of IL or their receptors revealed that 11 of them (61.1%) were significantly associated with PTDM development after adjusting for sex, age and tacrolimus usage, which may confirm the thesis that inflammation of islet β cells is vital in the pathogenesis of PTDM in renal transplantation recipients [80]. These polymorphisms were in IL-1B (rs3136558), IL-2 (rs2069762), IL-4 (rs2243250, rs2070874), IL-7R (rs1494558, rs2172749), IL-17RE (rs1124053), IL-17R (rs2229151, rs4819554), and IL-17RB (rs1043261, rs1025689). The last four SNPs have been previously linked with type 1 diabetes mellitus [80].
Meta-analysis of genetic association studies found the relationship between interleukin-7 (IL7) (rs1494558), potassium voltage-gated channel subfamily Q member 1 (KCNQ1) (rs2237892), and transcription factor 7 like 2 (TCF7L2) (rs7903146) and PTDM development [82]. A genomic-wide association study (GWAS) indicates that TCF7L2, which belongs to T-cell transcription factor family controlling cell proliferation and differentiation as well as regulating pancreas development, maturation and islet function, is significantly associated with type 2 diabetes [83]. Some studies showed the relationship between the presence of T allele TCF7L2 rs7903146 and enhanced protein expression, compromised insulin secretion, impaired incretin effects and hepatic insulin resistance, however, other studies did not support these data [73,84,85,86,87]. Large meta-analysis confirmed that rs7903146 T variant increased the risk of PTDM in an allele dose-dependent manner [82].
Chen et al. [88] indicated that polymorphisms in the NFATc4 gene might confer certain protection or predisposition for PTDM. NFAT genes code transcription factor of the nuclear factor of activated T cells (NFAT) which regulates immune activation and insulin production. Calcineurin inhibitors (CNIs) have been shown to activate NFAT. According to the aforementioned authors, T allele (rs10141896) was associated with a lower cumulative incidence of PTDM (p = 0.02). They demonstrated that CNI-treated recipients with one of the five dominant NFATc4 haplotypes, T-T-T-T-G, had a reduced adjusted risk for PTDM (hazard ratio (HR): 0.45; 95% confidence interval (Cl): 0.19–1.01). In turn, in patients homozygous for the C-C-C-G-G haplotype enhanced risk (HR: 2.13; 95% Cl: 1.01–4.46) for PTDM was reported. Additionally, polymorphisms in C-C motif chemokine ligand 2 (CCL2) gene which codes an inflammatory or inducible chemokine and C-C motif chemokine ligand 5 (CCL5) gene which is a target of NF- B activity, have been suggested to alter the risk of post-transplant diabetes mellitus development. Dabrowska-Zamojcin et al. [89] found that the number of CCL2 rs1024611 G alleles (HR 1.65; 95%CI 1.08–2.53; p = 0.021) was significantly positively associated with the risk of PTDM onset in patients treated with tacrolimus or cyclosporine, irrespectively of organ recipients’ sex, age and BMI. In the Korean population polymorphisms within CCL5 gene (rs2107538, rs2280789 and rs3817655) (TCA haplotype) were found to be significantly associated with increased risk of PTDM [90].
In patients without diagnosed diabetes before renal transplantation, single nucleotide polymorphism (SNP) within angiotensinogen (AGT) (rs4762) seem to increase the risk of PTDM development in the dominant models (p = 0.03) after adjusting for age and tacrolimus usage, however, exact molecular mechanism of this relationship requires clarification [91]. In addition, polymorphism within a gene involved in oxidative stress (GPX1, SNP rs1050450) was associated with an increased risk of PTDM [92]. Functional polymorphisms in this gene have been previously demonstrated to increase the risk of cardiovascular and peripheral vascular diseases in type 2 diabetic patients [93,94]. Finally, the SNPs in genes involved in tacrolimus metabolism, including peroxisome proliferator-activated receptor α (PPARα) and P450 oxidoreductase (POR) have drawn attention. These two genes participate in the regulation of energy uptake, lipid and carbohydrate metabolism. Three polymorphisms, a coding POR variant (rs1057868) and two SNPs in PPARα (rs4823613 and rs4253728) were found to enhance the risk of PTDM [95]. Patients carrying multiple predisposing SNPs show a greater risk of PTDM [41]. The number of SNP in genes associated with PTDM is so large that it is impossible to mention all of them in this review.
Metabolic alterations occurring before the transplantation, including glucose metabolism impairment and increase in BMI (Body Mass Index) seem to play important role in the enhancement of PTDM risk [96]. Hypoglycemia before the transplantation, mirroring the presence of early insulin resistance or insulin secretion deficiency, eventually contributes to PTDM [97]. The presence of prediabetes (IGT or IFG), with fasting plasma glucose <7 mmol/L or fasting plasma glucose ≥6.1 and <7 mmol/L and 2-h plasma glucose after an oral glucose tolerance test (OGTT) ≥7.8 and <11.1 mmol/L are risk factors for the future development of diabetes, not only in post-transplant patients but also in general population [16]. According to studies, 15% of transplant patients with impaired glucose tolerance will develop PTDM after 1 year and another 27% of them over 6 years [98,99]. Additionally, perioperative hyperglycemia associated with a stress reaction to the surgery itself (the release of catecholamines), the administration of corticosteroids as well as the renal function, increases the risk of PTDM [37].
The age higher than 45 years old increases the risk of PTDM 2.2 times compared to transplant patients aged 18–44 years [66]. According to studies, higher age is related to diminished insulin secretion which mirrors the typical aging process and the apoptosis of β-cells. It has been indicated that among patients over the age of 48 years on a waiting list, the relative risk of prediabetes was 2.5-fold higher than in younger patients [20]. Additionally, higher BMI strongly influenced insulin sensitivity. Abdominal circumference of more than 94 cm at the time of renal transplantation was shown to be a vital independent PTDM risk factor in men, while in women BMI value at the time of renal transplantation was more important [100]. Obesity at transplantation has been confirmed to be an independent risk factor for PTDM [69,101,102]. A large study of renal transplant recipients showed that the risk of PTDM was enhanced in overweight patients (BMI > 25 kg/m2), and became obvious in obese subjects (BMI > 30 kg/m2) [102]. The relative risk for PTDM in obese patients was 1.73 (95% CI 1.57–1.90, p < 0.0001). Another study indicated that PTDM risk increased linearly for every 1 kg above 45 kg [103]. A greater PTDM risk in overweight patients may be the consequence of a chronic inflammatory state related to excessive fat which stimulates macrophage recruitment to adipocytes and the release of proinflammatory adipokines leading ultimately to the downregulation of insulin signaling [33,104,105]. Moreover, adipose tissue produces tumor necrosis factor-alpha (TNFα), the activation of which is associated with insulin resistance via the reduced expression of insulin-sensitive glucose transporters [106]. Rodrigo et al. [106] found that every 1 μg/mL decrease in adiponectin enhanced the risk of developing PTDM by 13%. In addition, proteinuria (low-grade proteinuria and very low-grade (<0.3 g/day)) which develops within 3–6 months from the transplantation was shown to be a strong independent risk factor for PTDM [107].
The disturbances in lipid profile, especially the rise in TG/HDL (triglyceride/high-density lipoprotein cholesterol) ratio (above 3.5) also increase the incidence of diabetes mellitus, as they are associated with the worsening of glucose homeostasis and poor glycemic control in women with type 2 DM [108]. According to de Lucena et al. [33], higher ratios of TG/HDL in post-transplant patients may be utilized as a surrogate marker of insulin resistance. Bayés et al. [109] demonstrated that low levels of adiponectin, increased levels of C-reactive protein and triglycerides, and high BMI before transplant were predictors of PTDM.
According to studies, also tacrolimus-related electrolyte abnormalities could raise the risk of PTDM [41]. Hypomagnesemia, which is frequently observed after tacrolimus therapy, has been demonstrated to be an independent risk factor for insulin resistance and hyperglycemia [110]. Van Laecke et al. [111] revealed that an Mg level <1.9 mg/dL compared to higher values was associated with the more accelerated development of PTDM in renal transplant recipients (log-rank p < 0.001). Finally, the hazard ratio for the development of PTDM is higher in patients with acute rejection after transplantation (HR 3.7), however, this finding may be associated also with antirejection therapy involving high-dose steroid and increased CNIs [111].
Experimental studies have shown that gender issues in transplantation are highly important as they not only exert an impact on the PTDM development hazard. Some risk factors are equally important in men and women, while others seem to be more relevant in one sex. The study of renal transplant patients revealed that age at the time of transplantation (KT) >60 years and hypovitaminosis D at the time of KT (<20 μg/L) were independent risk factors for PTDM in both sexes, while the strong relationship between a waist circumference at the time of KT > 94 cm, HOMA-IR > 2, C-peptide at the time of KT > 5 ng/mL, and triacylglycerols at the time of KT > 1.7 mmol/L and PTDM was observed only in men [100]. In turn, in women, BMI at the time of KT > 30 kg/m2 and menopause at the time of KT were dominant factors. Moreover, the authors found that women displayed pancreas β cell dysfunction, whereas insulin resistance and metabolic syndrome were principal in men [100].
Early identification of patients at risk of PTDM may enable the determination of appropriate strategies (e.g., lifestyle modification and modification of pharmacological treatment) to reduce its occurrence [112,113]. In contrast to PTDM, which is diagnosed and treated early, prediabetes is not looked for in renal transplant patients [19]. It seems strange since the identification and treatment of prediabetes may enable the reduction of the burden of diabetes and cardiovascular disease in this population.
Despite the fact that PTDM and diabetes mellitus type 2 share some predisposing factors and many characteristics, the prevention and treatment of these two disorders are often dissimilar [33].
Hyperglycemia undoubtedly exerts a serious adverse impact on post-transplant outcomes [114]. The development of PTDM appears to be an important risk factor for cardiovascular morbidity and mortality after transplantation. Abundant studies have indicated the relation between PTDM and enhanced risk of cardiovascular events [6,115]. Table 1 presents the summary of the results of studies concerning PTDM risk factors and involved pathomechanisms accompanied by our estimation of the strength of evidence.

Table 1.
The summary of post-transplant diabetes mellitus (PTDM) risk factors and involved pathomechanisms described in this manuscript.
4. Possible Biomarkers in PTDM
The review of available most recent publication has not revealed any predictive or prognostic biomarkers of PTDM showing high sensitivity and specificity. Some articles indicate the association between the inflammation of the pancreatic β-cells and PTDM as well as the link between enhanced innate immune system activity and development of PTDM [80,116,117]. Others suggest the involvement of tumor necrosis factor (TNF). Elevated levels of high sensitivity interleukin IL-6 were observed in PTDM patients by the team of Cieniawski and colleagues [118]. In turn, Heldal et al. [116] demonstrated a strong relationship between four inflammation-related parameters (sTNFR1, EPCR, PTX3, and MIF) and plasma glucose levels 2 h after an OGTT in patients, 10 weeks after kidney transplantation. This association remained significant after the adjustment for age, BMI, graft function, insulin levels, a calcineurin inhibitor, and prednisolone dose. Their finding may imply the role of an inflammatory state in the development of PTDM. As it has been mentioned in previous sections, decreased insulin secretion from the pancreatic islets and enhanced peripheral insulin resistance are observed in the course of PTDM [15,116,119]. The results of the Heldal et al. [116] study showing the association between markers of inflammation and impaired 2-h plasma glucose and the results of studies in which the presence of peripheral insulin resistance in T2DM was related to low-grade inflammation suggest similar mechanisms in both diseases. Therefore, it has been hypothesized that in renal transplant recipients, inflammatory microenvironment could promote the development of peripheral insulin resistance and may lead to impaired insulin secretion in pancreatic β-cells [80,117,120].
A large prospective cohort study of stable renal transplant recipients demonstrated a direct link between plasma malondialdehyde (MDA) and plasma glucose concentration [121]. Moreover, this study indicated that plasma MDA level inversely correlated with long-term risk of PTDM, even after the adjustment for potential confounders, such as BMI, baseline glucose concentration, and immunosuppressive therapy. This finding may suggest that also oxidative status plays an important role in glucose homeostasis.
Most studies examining genes related to PTDM focus on those previously associated with type 2 diabetes due to the fact that these two diseases share similar mechanisms controlling insulin production and maintenance of stable glucose levels, including insulin resistance and insulin hypo-secretion and analogous risk factors (advanced age, family history of diabetes and non-white ethnicity) [122]. One of analyses of genetic variants associated with PTDM in kidney transplant patients showed that TCF7L2 rs7903146, CDKAL1 rs10946398 and KCNQ1 rs2237892 were significantly associated with PTDM [82]. Polymorphisms within transcription factor 7-like-2 (TCF7L2) have been previously linked with DM type 2. The T allele was identified as a diabetes risk factor, however, the mechanism via which TCF7L2 influenced the risk of diabetes has not yet been completely resolved. It has been suggested to exert an impact on blood glucose homeostasis via altering levels of glucagon-like peptide 1 in the gut or to reduce insulin secretion via the pancreatic β, adipose, or liver cells [123]. In turn, cyclin-dependent kinase 5 regulatory subunit associated protein 1 like 1 (CDKAL1) has been found to be associated with impaired insulin secretion as it regulates CDK5 protein involved in the promotion of insulin production as well as the development of DM type 2 [124]. It was suggested that CDKAL1-related overexpression of CDK5 might increase the risk of DMT2 and NODAT by impairing insulin production [82]. Additionally, KCNQ1 was found to be a well-known risk factor for type 2 diabetes mellitus. The protein encoded by KCNQ1 is expressed in pancreatic islet cells (not only) and together with KCNE proteins they form voltage charged potassium channels found in the membranes. As pancreatic βcell survival rate is believed to be influenced by these potassium channels, their dysfunction could alter cell membrane potential and contribute to the development of T2D or NODAT [82]. Schultz et al. [125] suggested that homozygous carriers of C allele (rs2237892) showed impaired baseline insulin secretion.
MicroRNA (miRNA) have gained a lot of attention due to the fact that they are potential sensitive biomarkers in numerous human diseases and tissue injury [37]. They play an essential role in the modulation of gene expression as they bind to 3’ untranslated region of miRNA of protein-coding genes to downregulate their expression thus affecting almost every key cellular function [126]. miRNAs are short (19–23 nucleotides) non-coding RNA produced endogenously [37]. We found no available studies analyzing the role of miRNA in PTDM, however, the research of the repertoire of miRNAs involved in the development and progression of diabetes mellitus is better studied and it could be hypothesized that some alterations in the level of miRNA may be similar in DM and PTDM. Huang et al. [127] indicated that the level of miR-155 and miR-146a was over five times higher in diabetic samples compared to controls and they strongly correlated with serum creatinine levels. In a rat model, the induction of the aforementioned miRNA was found to increase gradually. According to some studies, also miR-126 can be utilized as a biomarker for prediabetes and DM as its levels were considerably diminished in patients with impaired fasting glucose, impaired glucose tolerance or with DM compared to healthy controls [128]. The pathogenesis of diabetic nephropathy and insulin resistance was found to be associated with the deregulation of miR-29. Patients with albuminuria demonstrated much higher levels of urinary miR-29a than those with normal albuminuria [129]. Celen et al. [130] suggested that miRNA biogenesis pathways might be affected by immunosuppressive treatment. They found that miRNA biogenesis components could serve as potential biomarkers indicative of graft outcome. Gene expression of these components was demonstrated to be considerably decreased after transplantation and probably even more reduced in patients undergoing chronic rejection [130]. In turn, Ulbing et al. [131] observed that the systemic expression of miR-223-3p and miR-93-5p was significantly diminished during later stages of CKD, however, kidney transplantation fully reversed this effect. These two miRNAs are strongly associated not only with CKD stages and kidney function but also with inflammatory state parameters and indices of glucose metabolism. Zampetaki et al. [132] revealed reduced plasma levels of miR-20b, miR-21, miR-24, miR-15a, miR-126, miR-191, miR-197, miR-223, miR-320, and miR-486 in prevalent DM. Moreover, they indicated that decreased miR-15a, miR-29b, miR-126, miR-223, and elevated miR-28-3p levels preceded the manifestation of diabetes.
5. Cardiovascular Risk and Morbidity of Renal Transplant Patients
Risk factors for the development of cardiovascular diseases in kidney transplant patients are more abundant compared to the general population and comprise traditional factors (diabetes mellitus, hypertension, hyperlipidemia), factors related to decreased glomerular filtration (anemia, hyperhomocysteinemia) as well transplantation-specific factors (immunosuppression or graft rejection). A large number of predisposing factors reflect the complex nature of risk observed in this group [133]. The enhanced risk of cardiovascular disease in patients with a transplanted kidney is associated with the accumulation of atherogenic risk factors before and after transplantation but also the use of immunosuppressive drugs. The presence of cardiovascular disease before the procedure is the predominant risk factor for the progression of this disease after transplantation. Before the transplantation, uremic milieu promotes the development of atherosclerosis and cardiovascular complications. In post-transplant patients, traditional cardiovascular risk factors are accompanied by risk factors linked with transplantation status and treatment (such as graft rejection, immunosuppressive agents, and cytomegalovirus infection) and those resulting from progressive regression in allograft function (oxidative stress, anemia, volume load, inflammation and proteinuria, hyperhomocysteinemia, secondary hyperparathyroidism) [133,134]. Patients with chronic kidney disease (CKD), and especially those undergoing dialysis exhibit increased cardiovascular (CV) risk compared to the general population [135,136]. Hemodialysis (HD) patients experience a 10–20 times higher risk of cardiovascular disease (CVD) mortality [137]. Transplantation has been shown to reduce cardiovascular risk compared to HD, however, this risk is still high [138]. The term “cardiovascular disease” covers coronary artery disease (CAD), congestive cardiac failure (CCF), peripheral vascular disease and cerebrovascular disease [136]. According to studies, the rate of cardiac death is estimated to be 10-times higher in renal transplant recipients compared with the general population, while the annual prevalence of fatal or nonfatal CV events is 50-times higher [139]. Cardiac disease is responsible for 17% of all deaths in renal transplant recipients, while cerebrovascular disease accounts for another 5% of all deaths. Cardiac arrest (45%) and then myocardial infarction (MI) (31%) and cardiac arrhythmia (13%) are the most frequent cardiac causes of death in this group of patients [140]. The incidence of myocardial infarction following transplantation is still high (6.5–11.1% at 36 months) and reaches its peak within the first 6 months from the procedure [141]. Aftab et al. [142] revealed that 86% of major adverse cardiac events occurred within the first 180 days. Moreover, it has been demonstrated that the development of post-transplant diabetes worsens graft function which in consequence leads to enhanced morbidity and mortality, especially from CV events [6]. Diabetes and PTDM partly contribute to the overall CV risk. Transplant recipients with post-transplant diabetes or impaired glucose tolerance have been shown to have an enhanced risk of developing CVD, but in those who suffered from diabetes pretransplantation, this risk is even greater [6,43]. In renal transplant, patients’ prediabetes is the main risk factor for PTDM, however, its impact on cardiovascular disease remains unclear [16,28,143]. The elusive mechanism of the relationship between prediabetes and cardiovascular disease may involve obesity, insulin resistance, hypertension, inflammation, and high triglyceride levels, all resulting in a pro-atherogenic state stimulating cardiovascular disease [144]. Porrini et al. [19] revealed that prediabetes (IFG and/or IGT) present at 12 months from transplantation posed an independent risk factor for cardiovascular events. In their study, the risk was two times higher in patients with prediabetes compared to those with normal glucose metabolism (HR 2.12, CI 1.14–3.93). The observed cardiovascular disease risk and also the survival curves for both complications were similar in prediabetes (HR 2.11, 95% CI 1.14–3.93) and PTDM (HR 2.24, 95% CI 1.11–4.52) [19]. Early glucose metabolism impairment, including altered fasting glucose observed within the first week after transplant, may also predict a higher risk of developing PTDM [145]. However, the early reversibility rate of IFG or IGT could be the result of the lack of association between prediabetes at 3 months with cardiovascular disease in some studies [19]. A systematic review and meta-analysis of 53 studies comprising 1,611,339 individuals indicated that prediabetes was related to a 15–30% enhanced risk for cardiovascular events [146]. In the study of 1410 consecutive transplant recipients who underwent repeated oral glucose tests (OGTTs) Valderhaug et al. [147] observed that IGT at 10 weeks after transplantation was associated with risk for total mortality but not with cardiovascular disease. However, Wauters et al. [115] found the correlation between impaired fasting glycemia (IFG) at 12 months and risk of major cardiovascular events (cardiac (hazard ratio (HR) = 1.113 (1.094–1.132), p < 0.0001), vascular (HR = 1.168 (1.140–1.197), p < 0.0001), and strokes (HR = 1.156 (1.123–1.191), p = 0.003), which was independent of other risk factors. Moreover, hyperglycemia also increased the risk of death (PTDM: HR = 2.410 (1.125–5.162), p = 0.024) in patients with major cardiovascular events after transplantation (n = 123, 11%). In another study, increasing fasting glucose levels at 1, 4, and/or 12 months from transplantation were significantly associated with CV events and these relationships were independent of other CV risk factors, including male gender, advanced age, CV events before transplantation and dyslipidemia [29]. Moreover, fasting glucose levels >100 mg/dL related to higher incidence of post-transplant cardiac (p = 0.001) and peripheral vascular disease events (p = 0.003).
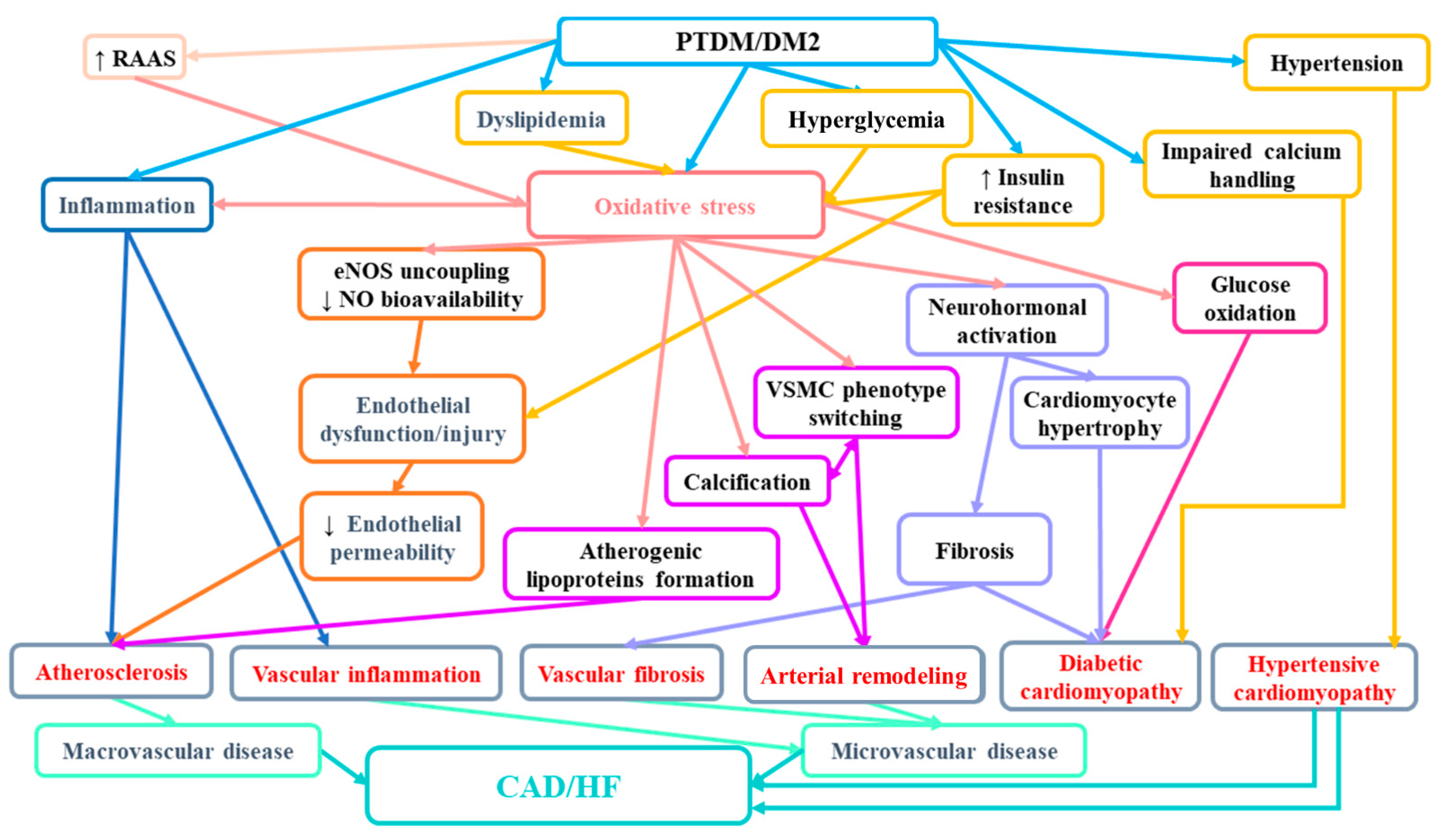
In the light of the evidence, it seems that not only PTDM but also prediabetes may play an important role in the burden of cardiovascular disease in the population of renal transplant patients. Therefore, the diagnosis of prediabetes with a simple tool, such as the OGTT, may enable the identification of patients at risk and help to avoid cardiovascular complications in this population. Figure 1 presents mechanisms involved in enhanced cardiovascular risk in patients with diabetes.
Figure 1.
The suggested mechanisms involved in increased CAD/HF risk in patients with PTDM/diabetes mellitus. RAAS—renin-angiotensin-aldosterone system; eNOS—endothelial nitric synthase; NO—nitric oxide; VSMC—vascular smooth muscle cells; CAD—cardiovascular disease; HF—heart failure.
6. The Assessment of PTDM Risk and Recommendations Concerning Its Diagnosis and Management
The assessment of the risk of PTDM should preferably be performed before kidney transplantation to enable early implementation of the appropriate intervention [97]. The results of studies indicated that the use San Antonio Diabetes Prediction Model and the Framingham Offspring Study-Diabetes Mellitus algorithm quite effectively identified patients at higher risk for PTDM beyond the first year [148]. In 2014, the International Expert Panel suggested that screening tests for PTDM should involve also postprandial glucose monitoring and HbA1C. However, HbA1C test should not be used early after transplantation (within 45 days from transplantation) due to potential confounding factors [16]. Normal value of HbA1C test does not exclude the diagnosis of PTDM in the presence of early post-transplant anemia and/or dynamic kidney allograft function [25]. In the opinion of Alnasrallah et al. [1] performing OGTTs after renal transplantation is recommended even in nondiabetic patients. According to other studies, homeostasis model assessment of insulin resistance and OGTT does not adequately reflect the altered carbohydrate metabolism of individuals with chronic kidney disease (CKD) or ESRD since in these patients, endogenous insulin concentrations may be increased due to impaired renal clearance of endogenous insulin [97].
The reduction of type 2 diabetes mellitus is related to the introduction of lifestyle interventions that promote decreased fat/energy consumption, moderate-intensity physical activity, and moderate weight loss [97]. According to two large studies, lifestyle intervention reduced the incidence of diabetes by 58% compared with placebo [149,150]. However, it is not obvious whether the aforementioned modification of diet and introducing physical activity can also prevent or delay the progression of PTDM. The results of some studies imply that intensive lifestyle modifications that are tailored to the requirements of patients with CKD or ESRD and which are delivered before and straightaway after transplantation might decrease the incidence of PTDM [97,151]. Due to the fact that higher BMI before the transplantation correlates with the development of insulin resistance after transplantation, it seems that obesity should be treated as the target for intervention to prevent PTDM. Current guidelines concerning the management of prediabetes or diabetes recommend lifestyle modifications enabling overweight or obese patients to lose weight [152]. Due to the fact that the maintenance of proper weight after its loss (arising from intensive lifestyle interventions) is difficult, guidelines suggest (in certain cases) the administration of pharmacological antidiabetic therapy immediately after the diagnosis of diabetes [152,153]. For example, Alnasrallah et al. [1] suggest introducing metformin therapy in transplant patients with IGT. Metformin has been demonstrated to exert favorable effects in this group of patients since it not only improves insulin sensitivity but also influences the cardiovascular system and weight gain [154,155]. In turn, Guthoff et al. [20] suggested that overweight patients with insulin resistance might benefit from steroid-free maintenance immunosuppression, due to the fact that corticosteroids negatively influence insulin sensitivity. They claim that the use of corticosteroid-free maintenance immunosuppression for standard immunological risk patients give outstanding long-term allograft survival. Due to the fact that calcineurin inhibitors directly impair β-cell function, a low-dose CNI or CNI-free regimen seems to be beneficial in patients with impaired insulin secretion and normal immunological risk.
After solid-organ transplantation, especially in the immediate post-transplant period, the incidence of “stress hyperglycemia” is high, however, there are no sound recommendations concerning the management strategies for post-transplant hyperglycemia and subsequent outcomes [156,157]. The rationale for hyperglycemia management is to limit stress on β-cells during the peri-transplant period in order to expand their long-term function [158]. Since compromised insulin secretion seems to be the major pathophysiological feature after kidney transplant, early therapeutic interventions aiming to preserve, maintain, or enhance β-cell function should be potentially undertaken in this population [15]. Several preventive strategies have been suggested to improve insulin secretion via the protection and amelioration of βcell function. βcell function seems to be protected when basal insulin is administered perioperatively. Hecking et al. [158] demonstrated that the treatment group showed significantly improved βcell function (assessed on the basis of insulinogenic index) but not insulin sensitivity, even 1 year after transplantation, despite the fact that insulin administration was ceased 3 months after the transplantation. The results of the randomized controlled trial of 50 renal transplant recipients revealed that early (<3 weeks) administration of basal insulin to treat post-transplant hyperglycemia considerably diminished the risk of developing PTDM within the first year by 73% [158]. However, there is no consensus regarding long-term glycemic targets for heart transplant recipients with PTDM [24].
Other studies recommended the use of incretins which have been found to exert a protective effect on βcells and improve clinical outcome in pancreatic islet cell transplantation, however, the efficacy and safety of incretin analogues and dipeptidyl peptidase (DPP)-4 inhibitors have not yet been established in solid organ transplantation [159,160,161].
Table 2 presents the results of studies described in this paragraph.

Table 2.
The summary of results of studies concerning the diagnosis as management of PTDM.
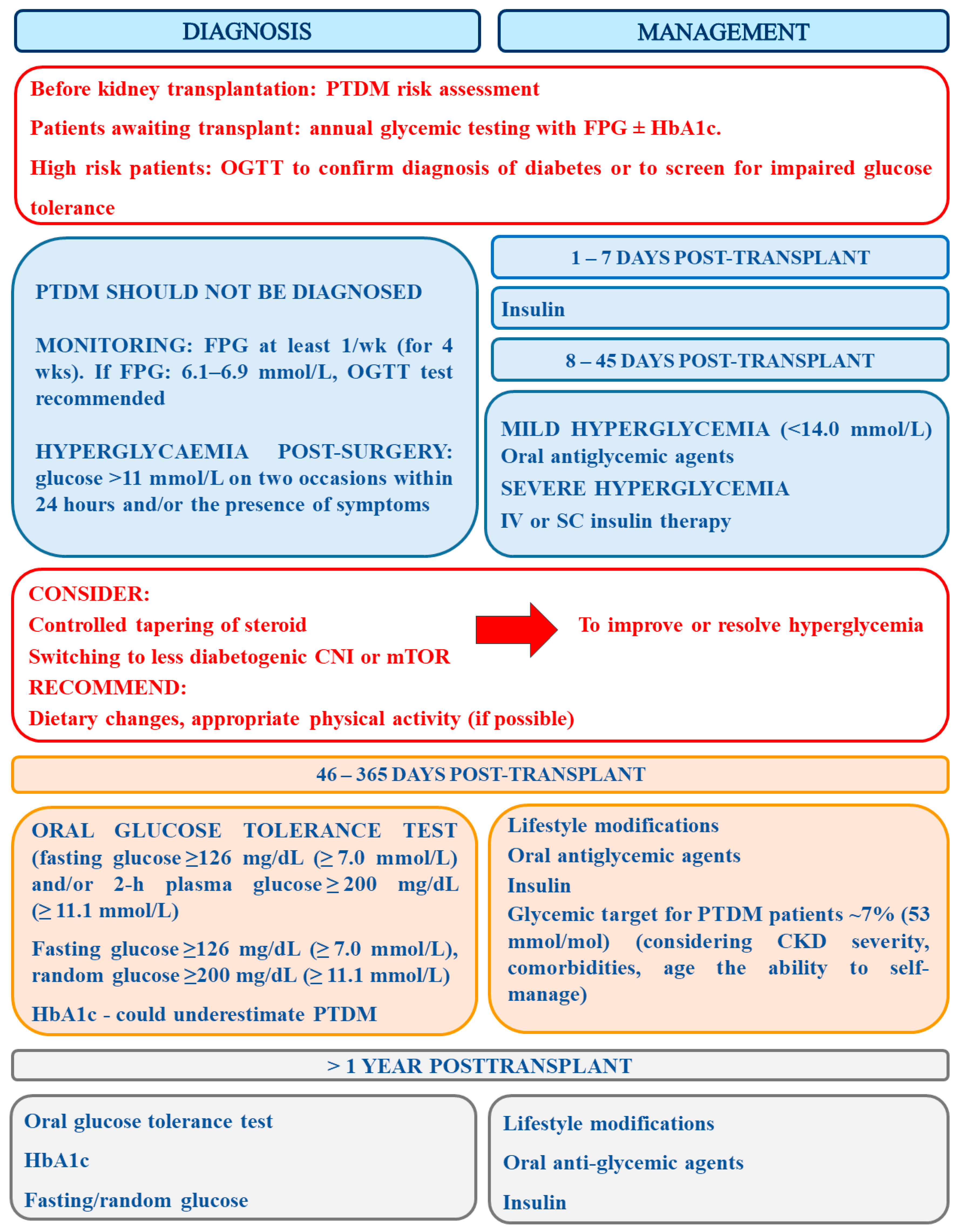
According to the prepared but not yet accepted Guidelines On The Detection And Management Of Diabetes Post Solid Organ Transplantation Detection of Association of British Clinical Diabetologist and Renal Association, the diagnosis of PTDM should be avoided in the immediate post-operative period when transient hyperglycemia is extremely common (Grade 1B) and thus a formal diagnosis can be made from 6-weeks post-transplantation (Grade 1B) [114]. The identification of patients with post-operative hyperglycemia should be made with the use of afternoon capillary blood glucose monitoring (AGM) and such patients ought to be closely monitored for PTDM (Grade 1B). Currently, oral glucose tolerance test is the gold standard for the diagnosis of PTDM, and HbA1c ≥6.5% (48 mmol/L) is a proper diagnostic test in clinically stable solid organ transplant recipients after the first 3 months post-transplantation (grade 1B). However, the results of the latter test should be used with caution since some factors may impair its accurate interpretation (Grade 1A). Results of abnormal fasting plasma glucose (FPG) ≥7 mmol/L and/or HbA1c >6.5% (48 mmol/mol) allows for the identification of the majority of PTDM cases in stable patients (Grade 2C). Patients awaiting transplant ought to receive annual glycemic testing with FPG ± HbA1c. Those at high risk patients should then undergo OGTT to confirm diagnosis of diabetes or to screen for impaired glucose tolerance (Grade 2C). The new guidelines do not recommend the use of novel diagnostic tools (e.g., fructosamine and glycated albumin) as clinical tools (Grade 2D). After transplantation, early postoperative hyperglycemia (glucose >11 mmol/L on two occasions within 24 h) should be straightaway actively monitored and treated with oral hyperglycemic therapy if it is mild (<14.0 mmol/L), or with early intravenous or subcutaneous insulin therapy in more severe cases (Grade 1C). Glycemic target for patients with PTDM should be established at c.a. 7% (53 mmol/mol), however, while setting the target the severity of CKD, comorbidities, age and the ability to self-manage ought to be taken into consideration (Grade 1B). Patients with a confirmed diagnosis of PTDM should be offered structured diabetes education as well as structured diabetes care, involving regular screening for complications (Grade 1B). Additionally, blood pressure should be controlled below 130/80 mmHg in all patients with PTDM (Grade 1B) [114].
Metformin should be considered first-line oral therapy for patients with confirmed PTDM and a stable eGFR > 30 mL/min/1.73 m2 and BMI > 25 kg/m2 (Grade 1C). Sulfonylureas, meglitinides, DPP-4 inhibitors, pioglitazone, and GLP-1 analogue can be safely used in patients with PTDM, however, caution should be exercised while using the first two drugs in those at risk of hypoglycemia (Grade 2C). In patients with stable eGFR and poor glycemic control, SGLT-2 inhibitors should be used with caution, after consultation with a nephrologist and a diabetologist (Grade 1C). In turn, insulin therapy should be considered in all patients with insufficient glucose control, or with symptomatic hyperglycemia (Grade 1C). Irrespective of cholesterol level, all patients suffering from PTDM ought to be provided with statin therapy (Grade 2D) [114].
Since immunosuppression is the main risk factor for PTDM, its modifications can be made to reduce this risk, however, the advantages must be balanced against the risk for allograft rejection (Grade 1B). The strategy to improve long-term transplant outcomes should involve the adjustment of immunosuppression on the basis of the recipient’s immunologic and glycemic risk (Grade 1C). For the time being, due to a lack of contradictory evidence, the selection of immunosuppressive therapy should be principally targeted at the prevention of rejection rather than preventing PTDM (Grade 1C) [114]. Finally, in patients before the transplantation, the risk for diabetes development should be assessed (Grade 1B) and such patients ought to be educated on the risk of developing PTDM as well as counseled about minimizing weight gain using lifestyle measures (Grade 1B). In patients awaiting the transplantation, the treatment of PTDM risk factors, including hepatitis C should be introduced (Grade 1C). Moreover, all patients who are at high risk for the development of PTDM should be screened yearly for diabetes whilst awaiting transplantation (Grade 1B) [114].
The summary of recommendations concerning PTDM diagnosis and management is presented in Figure 2.

Figure 2.
The summary of recommendations concerning PTDM diagnosis and management (prepared on the basis of [41,112,114].
Recently, the use of SGLT inhibitors has attracted great interest. The sodium-glucose cotransporters (SGLTs), located on the apical membrane of renal proximal tubule cells, participate in tubular reabsorption of the filtered glucose [163,164]. There are two types of these cotransporters—SGLT2 displaying low affinity but high capacity for glucose and reabsorbing ≈80–90% of the filtered glucose load under normal conditions and SGLT1 which has a high affinity but low capacity for glucose, and it is responsible for the reabsorption of the remaining 10–20% of the filtered glucose [165,166]. In poorly controlled diabetic subjects, the filtered glucose load can exceed maximum reabsorptive capacity for glucose, resulting in glucosuria. Greater reabsorption of glucose worsens hyperglycemia in patients with T2DM [162,167]. Recently, it has been suggested that the inhibition of renal glucose reabsorption is an efficient method of improving glycemic control, βcell function and insulin sensitivity in patients with type 2 diabetes mellitus (T2D) [163,168,169]. The results of studies of animal models of T2DM demonstrated enhanced mRNA expression of SGLT1 and SGLT2 in the kidney, its correlation with glycemia and HbA1c and attenuation following the administration of hypoglycemic agents [170,171]. Norton et al. [163] suggested that therapies hindering both SGLT1 and SGLT2 could be more efficient in the improving of glycemic control in patients with T2DM than it was previously hypothesized [172].
Sotagliflozin, which is a dual sodium-glucose cotransporter (SGLT)1/SGLT2 inhibitor, has been recently approved in Europe as an adjunct to insulin therapy in adults with type 1 diabetes (T1D) and a body mass index (BMI) ≥ 27 kg/m2 [173]. According to studies, concomitant use of 200 and 400 mg of Sotagliflozin and insulin decreased glycated hemoglobin level and lowered body weight and systolic blood pressure. The administration of this drug is associated with diminished occurrence of severe hypoglycemia and documented hypoglycemia ≤3.1 mmol/L events, but also with elevated incidence of diabetic ketoacidosis in patients with BMI ≥ 27 kg/m2. Additionally, in the phase 3, in TANDEM 1–3 trials, adjunctive use of oral sotagliflozin was associated with beneficial effects (better glycemic control, body mass reduction) which were maintained over 52 weeks of treatment. This trial demonstrated that this inhibitor was well tolerated and diminished the likelihood of hypoglycemia [174]. On the basis of its risk/benefit profile, sotagliflozin is indicated in the EU as an adjunct to insulin in adults with T1D with a BMI ≥ 27 kg/m2 who have failed to achieve adequate glycemic control despite optimal insulin therapy, thus expanding the currently limited adjunctive oral treatment options available for use in this population. The results of meta-analysis of 13 studies (7962 participants) involving the use of SGLT inhibitors demonstrated that they facilitate glycemic control with a decreased insulin dose [175]. Moreover, in comparison with a placebo, the effects of the use of a dual SGLT inhibitor (sotagliflozin) on type 1 diabetes are similar to those of SGLT2 inhibitors, however, this type of inhibitor did not rise the risk of genital infections. The meta-analysis indicated that SGLT inhibitor treatment enhanced the incidence of urinary tract and genital infections, diarrhea, and diabetic ketoacidosis [175]. The inhibition of SGLT1 is associated with the blockage of glucose in the intestine, followed by its breakage down into short-chain fatty acids by bacteria at the distal end of the small intestine, which stimulate the secretion and release of glucagon-like peptide-1 and peptide YY by L cells at the distal end of the intestine [176]. Due to the fact that SGLT inhibitors promote the excretion of a large amount of glucose through the urine rising glucose concentration in the genitourinary tract, they enhance the risk of bacterial and fungal infection in patients [175]. In turn, the risk of diabetic ketoacidosis observed in patients using SGLT inhibitors is probably associated with their impact on the secretion of insulin and glucagon, stimulation of the decomposition of adipose tissue and β-oxidation of fatty acids and consequent enhanced formation of ketones in the liver [177]. Moreover, these inhibitors promote the lipid mobilization and free fatty acid oxidation in vivo, raise the level of free fatty acid and 13-hydroxybutyric acid in plasma, diminish the removal of ketone bodies via the kidneys, and enhance the reabsorption of ketone bodies in the proximal convoluted tubules [175,178]. Therefore, such therapy should be used with caution in patients who suffer from recurrent urogenital infections, ketosis or acidosis [175].
Based on the risk/benefit profile, sotagliflozin is approved in the EU as an adjunct to insulin in adult patients with T1D with a BMI ≥ 27 kg/m2 who failed to accomplish adequate glycemic control despite optimal insulin therapy [174]. According to the present ABCD/Diabetes UK joint updated position statement, clinicians should help patients with type 1 diabetes using these drugs to mitigate this risk and other potential complications [179]. Especially patients who are at risk of diabetic ketoacidosis due to illnesses, low calorie diets, starvation, injuries, excessive exercise, reduced insulin administration, excessive alcohol consumption, and other factors increasing the risk for diabetic ketoacidosis should be treated with utmost caution.
To sum up, sotagliflozin therapy as an adjunct to optimized insulin treatment in overweight/obese patients with T1D was shown to address some unmet needs and to enable the achievement of optimal glycemic control [173]. However, there are no available publications concerning the use of these inhibitors in patients with PTDM.
7. Conclusions
Kidney transplantation seems to be the best therapy for ESRD, however, it is not deprived of drawbacks, including the development of PTDM which affects allograft and patient survival [97]. Management of post-transplant diabetes resembles that of diabetes in the general population as it is based on strict glycemic control as well as screening and treatment of common complications [136]. Lifestyle intervention accompanied by the tailoring of immunosuppressive regimen may be of key importance to mitigate PTDM-associated complications in kidney transplant patients [33,180,181]. A more transplant-specific approach can include the change of tacrolimus by an alternative immunosuppressant (cyclosporine or mTOR inhibitor), the decrease or cessation of corticosteroid therapy [69], and caution in the prescribing of diuretics since they are independently connected with post-transplant diabetes [182,183]. Moreover, early administration of basal insulin has been shown to considerably decrease the risk of PTDM, which might be related to insulin-mediated β-cell protection and “resting” [158].
Early identification of high-risk patients for cardiovascular diseases enables a timely introduction of appropriate therapeutic strategy and results in higher survival rates for patients with a transplanted kidney.
A lot is known about PTDM, however, still numerous topics require clarification. First of all, future large studies are necessary to reveal all risk factors associated with the development of PTDM. The good knowledge of genetic alterations that increase the risk of PTDM may enable, in the future, the development of personalized therapy of PTDM. The differences in risk factors between genders should be confirmed in large multicenter studies. It also seems interesting whether recipients of gender-mismatched organs are at higher risk of the development of PTDM. Moreover, the long-term outcomes of transplant patients with PTDM across different populations also need research. Do patients with PTDM suffer from the same complications and to the same extent as patients with standard type 2 diabetes mellitus? Additional prospective studies are necessary to understand the associations between immunosuppressant regimens and PTDM and to develop the strategy considerably lowering the hazard of PTDM. Better characterization of clinical grounds of PTDM will enable its early detection and the introduction of appropriate treatment as well as the prevention of devastating complications.
Finally, emerging evidence has indicated the relationship between the exposure of endocrine-disrupting chemicals (EDCs) and diabetes [184]. EDCs were suggested to play a vital role in the etiology of diabetes and metabolic disorders since these chemicals may disturb pancreatic endocrine system and glucose metabolism. Several epidemiological studies have revealed significant impact of EDCs and hyperglycemia, glucose intolerance, and insulin resistance [185,186,187]. The suggested course of action includes the interactions with the aryl hydrocarbon receptor (AhR) and nuclear hormone receptors (e.g., estrogen receptors), the alteration of ERK/Akt signaling pathways, the stimulation of oxidative and nitrosative stress, as well as pancreatitis and dysregulated hepatic metabolism [188]. Additionally, changes in the gut microbiota have been shown to contribute to the onset and maintenance of insulin resistance [189]. Alterations in intestinal ecosystem could stimulate inflammation, modify intestinal permeability, and modulate metabolism of bile acids, short-chain fatty acids and metabolites which synergistically exert impact on metabolic regulation systems thus promoting insulin resistance [189]. It has been suggested that interventions which restore the equilibrium in the gut may have beneficial effects and improve glycemic control. However, future research should be performed to reveal identify exact pathophysiological mechanisms of EDC and gut dysbiosis, discover new potential therapeutic targets and assess their impact on the strategies that reduce dysbiosis and improve glycemic control.
Author Contributions
All authors were involved in the preparation of this article and J.R. revised the final version. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alnasrallah, B.; Pilmore, H.; Manley, P. Protocol for a pilot randomised controlled trial of metformin in pre-diabetes after kidney transplantation: The Transplantation and Diabetes (Transdiab) study. BMJ Open 2017, 7, e016813. [Google Scholar] [CrossRef]
- Port, F.K.; Wolfe, R.A.; Mauger, E.A.; Berling, D.P.; Jiang, K. Comparison of survival probabilities for dialysis patients vs cadaveric renal transplant recipients. JAMA 1993, 270, 1339–1343. [Google Scholar] [CrossRef] [PubMed]
- Laupacis, A.; Keown, P.; Pus, N.; Krueger, H.; Ferguson, B.; Wong, C.; Muirhead, N. A study of the quality of life and cost-utility of renal transplantation. Kidney Int. 1996, 50, 235–242. [Google Scholar] [CrossRef]
- Howard, K.; Salkeld, G.; White, S.; McDonald, S.; Chadban, S.; Craig, J.C.; Cass, A. The cost-effectiveness of increasing kidney transplantation and home-based dialysis. Nephrology 2009, 14, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Van Walraven, C.; Manuel, D.G.; Knoll, G. Survival trends in ESRD patients compared with the general population in the United States. Am. J. Kidney Dis. 2014, 63, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Hjelmesaeth, J.; Hartmann, A.; Leivestad, T.; Holdaas, H.; Sagedal, S.; Olstad, M.; Jenssen, T. The impact of early-diagnosed new-onset post-transplantation diabetes mellitus on survival and major cardiac events. Kidney Int. 2006, 69, 588–595. [Google Scholar] [CrossRef]
- Pilmore, H.; Dent, H.; Chang, S.; McDonald, S.P.; Chadban, S.J. Reduction in cardiovascular death after kidney transplantation. Transplantation 2010, 89, 851–857. [Google Scholar] [CrossRef]
- Jalowiec, A.; Grady, K.L.; White-Williams, C. Mortality, rehospitalization, and post-transplant complications in gender-mismatched heart transplant recipients. Heart Lung 2017, 46, 265–272. [Google Scholar] [CrossRef]
- Weiss, E.S.; Allen, J.G.; Patel, N.D.; Russell, S.D.; Baumgartner, W.A.; Shah, A.S.; Conte, J.V. The impact of donor-recipient sex matching on survival after orthotopic heart transplantation of 18;000 transplants in the modern era. Circ. Heart Fail. 2009, 2, 401–408. [Google Scholar] [CrossRef]
- Csete, M. Gender Issues in Transplantation. Anesth. Analg. 2008, 107, 232–238. [Google Scholar] [CrossRef]
- Kouli, F.; Morrell, C.H.; Ratner, L.E.; Kraus, E.S. Impact of donor/recipient traits independent of rejection on long-term renal function. Am. J. Kidney Dis. 2001, 37, 356–365. [Google Scholar] [CrossRef][Green Version]
- Muller, V.; Szabo, A.; Viklicky, O.; Gaul, I.; Portl, S.; Philipp, T.; Heemann, U.W. Sex hormones and gender related differences: Their influence on chronic renal allograft rejection. Kidney Int. 1999, 55, 2011–2020. [Google Scholar] [CrossRef] [PubMed]
- Zeier, M.; Dohler, B.; Opelz, G.; Ritz, E. The effect of donor gender on graft survival. J. Am. Soc. Nephrol. 2002, 13, 2570–2576. [Google Scholar] [CrossRef]
- Sanfey, H. Gender-specific issues in liver and kidney failure and transplantation: A review. J. Womens Health 2005, 14, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Hecking, M.; Kainz, A.; Werzowa, J.; Haidinger, M.; Döller, D.; Tura, A.; Karaboyas, A.; Hörl, W.H.; Wolzt, M.; Sharif, A.; et al. Glucose Metabolism After Renal Transplantation. Diabetes Care 2013, 36, 2763–2771. [Google Scholar] [CrossRef] [PubMed]
- Sharif, A.; Hecking, M.; de Vries, A.P.J.; Porrini, E.; Hornum, M.; Rasoul-Rockenschaub, S.; Berlakovich, G.; Krebs, M.; Kautzky-Willer, A.; Schernthaner, G.; et al. Proceedings from an International Consensus Meeting on Posttransplantation Diabetes Mellitus: Recommendations and Future Directions. Am. J. Transplant. 2014, 14, 1992–2000. [Google Scholar] [CrossRef] [PubMed]
- Davidson, J.; Wilkinson, A.; Dantal, J.; Dotta, F.; Haller, H.; Hernández, D.; Kasiske, B.L.; Kiberd, B.; Krentz, A.; Legendre, C.; et al. New-onset diabetes after transplantation: 2003 International consensus guidelines. Transplantation 2003, 75, SS3–SS24. [Google Scholar] [CrossRef]
- Montori, V.M.; Basu, A.; Erwin, P.J.; Velosa, J.A.; Gabriel, S.E.; Kudva, Y.C. Posttransplantation diabetes: A systematic review of the literature. Diabetes Care 2002, 25, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Porrini, E.; Díaz, J.M.; Moreso, F.; Lauzurrica, R.; Ibernon, M.; Torres, I.S.; Ruiz, R.B.; Rodríguez Rodríguez, A.E.; Mallén, P.D.; Bayés-Genís, B.; et al. Prediabetes is a risk factor for cardiovascular disease following renal transplantation. Kidney Int. 2019, 96, 1374–1380. [Google Scholar] [CrossRef]
- Guthoff, M.; Vosseler, D.; Langanke, J.; Nadalin, S.; Königsrainer, A.; Häring, H.-U.; Fritsche, A.; Heyne, N. Diabetes Mellitus and Prediabetes on Kidney Transplant Waiting List- Prevalence, Metabolic Phenotyping and Risk Stratification Approach. PLoS ONE 2015, 10, e0134971. [Google Scholar] [CrossRef]
- Chakkera, H.A.; Weil, E.J.; Swanson, C.M.; Dueck, A.C.; Heilman, R.L.; Reddy, K.S.; Hamawi, K.; Khamash, H.; Moss, A.A.; Mulligan, D.C.; et al. Pretransplant risk score for new-onset diabetes after kidney transplantation. Diabetes Care 2011, 34, 2141–2145. [Google Scholar] [CrossRef] [PubMed]
- Bock, G.; Dalla Man, C.; Campioni, M.; Chittilapilly, E.; Basu, R.; Toffolo, G.; Cobelli, C.; Rizza, R. Pathogenesis of Pre-Diabetes: Pathogenesis of pre-diabetes: Mechanisms of fasting and postprandial hyperglycemia in people with impaired fasting glucose and/or impaired glucose tolerance. Diabetes 2006, 55, 3536–3549. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.A.; Jenkinson, C.P.; Richardson, D.K.; Tripathy, D.; DeFronzo, R.A. Insulin Secretion and Action in Subjects with Impaired Fasting Glucose and Impaired Glucose Tolerance. Results Veterans Adm. Genet. Epidemiol. Study 2006, 55, 1430–1435. [Google Scholar] [CrossRef]
- Cehic, M.G.; Nundall, N.; Greenfield, J.R.; Macdonald, P.S. Management Strategies for Posttransplant Diabetes Mellitus after Heart Transplantation: A Review. J. Transplant. 2018, 2018, 1025893. [Google Scholar] [CrossRef]
- Pham, P.T.; Sidhu, H.S.; Pham, P.M.; Pham, P.C. Diabetes Mellitus After Solid Organ Transplantation. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK378977/ (accessed on 2 September 2020).
- Starlz, T.E. Experience in Renal Transplantation; Saunders: Philadelphia, PA, USA, 1964. [Google Scholar]
- Standards of medical care in diabetes—2010. Diabetes Care 2010, 33 (Suppl. 1), S11–S61. [CrossRef] [PubMed]
- Porrini, E.L.; Díaz, J.M.; Moreso, F.; Delgado Mallén, P.I.; Silva Torres, I.; Ibernon, M.; Bayés-Genís, B.; Benitez-Ruiz, R.; Lampreabe, I.; Lauzurrica, R.; et al. Clinical evolution of post-transplant diabetes mellitus. Nephrol. Dial. Transplant. 2015, 31, 495–505. [Google Scholar] [CrossRef]
- Cosio, F.G.; Kudva, Y.; van der Velde, M.; Larson, T.S.; Textor, S.C.; Griffin, M.D.; Stegall, M.D. New onset hyperglycemia and diabetes are associated with increased cardiovascular risk after kidney transplantation. Kidney Int. 2005, 67, 2415–2421. [Google Scholar] [CrossRef]
- Copstein, L.A.; Zelmanovitz, T.; Gonçalves, L.F.; Manfro, R.C. Posttransplant diabetes mellitus in cyclosporine-treated renal allograft patients: A case-control study. Transplant. Proc. 2004, 36, 882–883. [Google Scholar] [CrossRef] [PubMed]
- Chiu, K.C.; Lee, N.P.; Cohan, P.; Chuang, L.M. Beta cell function declines with age in glucose tolerant Caucasians. Clin. Endocrinol. 2000, 53, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Zelle, D.M.; Corpeleijn, E.; Deinum, J.; Stolk, R.P.; Gans, R.O.; Navis, G.; Bakker, S.J. Pancreatic β-cell dysfunction and risk of new-onset diabetes after kidney transplantation. Diabetes Care 2013, 36, 1926–1932. [Google Scholar] [CrossRef] [PubMed]
- De Lucena, D.D.; de Sá, J.R.; Medina-Pestana, J.O.; Rangel, É.B. Modifiable Variables Are Major Risk Factors for Posttransplant Diabetes Mellitus in a Time-Dependent Manner in Kidney Transplant: An Observational Cohort Study. J. Diabetes Res. 2020, 2020, 1938703. [Google Scholar] [CrossRef]
- Kuypers, D.R.J.; Claes, K.; Bammens, B.; Evenepoel, P.; Vanrenterghem, Y. Early clinical assessment of glucose metabolism in renal allograft recipients: Diagnosis and prediction of post-transplant diabetes mellitus (PTDM). Nephrol. Dial. Transplant. 2008, 23, 2033–2042. [Google Scholar] [CrossRef] [PubMed]
- De Lucena, D.D.; Rangel, É.B. Glucocorticoids use in kidney transplant setting. Expert Opin. Drug Metab. Toxicol. 2018, 14, 1023–1041. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-Silva, G.; Ivani de Paula, M.; Rangel, É.B. mTOR inhibitors in pancreas transplant: Adverse effects and drug-drug interactions. Expert Opin. Drug Metab. Toxicol. 2017, 13, 367–385. [Google Scholar] [CrossRef]
- Goldmannova, D.; Karasek, D.; Krystynik, O.; Zadrazil, J. New-onset diabetes mellitus after renal transplantation. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech. Repub. 2016, 160, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Fabrizi, F.; Martin, P.; Dixit, V.; Bunnapradist, S.; Kanwal, F.; Dulai, G. Posttransplant diabetes mellitus and HCV seropositive status after renal transplantation: Meta-analysis of clinical studies. Am. J. Transpl. 2005, 5, 2433–2440. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, J.; Wang, Y.; Han, X.; Chen, X. Hepatitis C virus induced a novel apoptosis-like death of pancreatic beta cells through a caspase 3-dependent pathway. PLoS ONE 2012, 7, e38522. [Google Scholar] [CrossRef]
- Einollahi, B.; Motalebi, M.; Salesi, M.; Ebrahimi, M.; Taghipour, M. The impact of cytomegalovirus infection on new-onset diabetes mellitus after kidney transplantation: A review on current findings. J. Nephropathol. 2014, 3, 139–148. [Google Scholar] [CrossRef]
- Ahmed, S.H.; Biddle, K.; Augustine, T.; Shazli, A. Post-Transplantation Diabetes Mellitus. Diabetes Ther. 2020, 11, 779–801. [Google Scholar] [CrossRef] [PubMed]
- Gourishankar, S.; Jhangri, G.S.; Tonelli, M.; Wales, L.H.; Cockfield, S.M. Development of diabetes mellitus following kidney transplantation: A Canadian experience. Am. J. Transpl. 2004, 4, 1876–1882. [Google Scholar] [CrossRef]
- Cosio, F.G.; Pesavento, T.E.; Osei, K.; Henry, M.L.; Ferguson, R.M. Post-transplant diabetes mellitus: Increasing incidence in renal allograft recipients transplanted in recent years. Kidney Int. 2001, 59, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Shivaswamy, V.; Bennett, R.G.; Clure, C.C.; Ottemann, B.; Davis, J.S.; Larsen, J.L.; Hamel, F.G. Tacrolimus and sirolimus have distinct effects on insulin signaling in male and female rats. Transl. Res. 2014, 163, 221–231. [Google Scholar] [CrossRef]
- Drachenberg, C.B.; Klassen, D.K.; Weir, M.R.; Wiland, A.; Fink, J.C.; Bartlett, S.T.; Cangro, C.B.; Blahut, S.; Papadimitriou, J.C. Islet cell damage associated with tacrolimus and cyclosporine: Morphological features in pancreas allograft biopsies and clinical correlation. Transplantation 1999, 68, 396–402. [Google Scholar] [CrossRef]
- Soleimanpour, S.A.; Crutchlow, M.F.; Ferrari, A.M.; Raum, J.C.; Groff, D.N.; Rankin, M.M.; Liu, C.; De Leon, D.D.; Naji, A.; Kushner, J.A.; et al. Calcineurin signaling regulates human islet β-cell survival. J. Biol. Chem. 2010, 285, 40050–40059. [Google Scholar] [CrossRef]
- Asberg, A.; Midtvedt, K.; Voytovich, M.H.; Line, P.-D.; Narverud, J.; Reisaeter, A.V.; Mørkrid, L.; Jenssen, T.; Hartmann, A. Calcineurin inhibitor effects on glucose metabolism and endothelial function following renal transplantation. Clin. Transpl. 2009, 23, 511–518. [Google Scholar] [CrossRef]
- Rickels, M.R.; Naji, A.; Teff, K.L. Insulin sensitivity; glucose effectiveness; and free fatty acid dynamics after human islet transplantation for type 1 diabetes. J. Clin. Endocrinol. Metab. 2006, 91, 2138–2144. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chakkera, H.A.; Mandarino, L.J. Calcineurin inhibition and new-onset diabetes mellitus after transplantation. Transplantation 2013, 95, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Midtvedt, K.; Hjelmesæth, J.; Hartmann, A.; Lund, K.; Paulsen, D.; Egeland, T.; Jenssen, T. Insulin Resistance after Renal Transplantation: The Effect of Steroid Dose Reduction and Withdrawal. J. Am. Soc. Nephrol. 2004, 15, 3233–3239. [Google Scholar] [CrossRef]
- Leeaphorn, N.; Garg, N.; Khankin, E.V.; Cardarelli, F.; Pavlakis, M. Recurrence of IgA nephropathy after kidney transplantation in steroid continuation versus early steroid-withdrawal regimens: A retrospective analysis of the UNOS/OPTN database. Transpl. Int. 2018, 31, 175–186. [Google Scholar] [CrossRef]
- Pascual, J.; Zamora, J.; Galeano, C.; Royuela, A.; Quereda, C. Steroid avoidance or withdrawal for kidney transplant recipients. Cochrane Database Syst. Rev. 2009. [Google Scholar] [CrossRef]
- Rangel, E.B. Tacrolimus in pancreas transplant: A focus on toxicity, diabetogenic effect and drug–drug interactions. Expert Opin. Drug Metab. Toxicol. 2014, 10, 1585–1605. [Google Scholar] [CrossRef]
- Boots, J.M.; van Duijnhoven, E.M.; Christiaans, M.H.; Wolffenbuttel, B.H.; van Hooff, J.P. Glucose metabolism in renal transplant recipients on tacrolimus: The effect of steroid withdrawal and tacrolimus trough level reduction. J. Am. Soc. Nephrol. 2002, 13, 221–227. [Google Scholar]
- Shivaswamy, V.; Boerner, B.; Larsen, J. Post-transplant diabetes mellitus: Causes; treatment; and impact on outcomes. Endocr. Rev. 2016, 37, 37–61. [Google Scholar] [CrossRef]
- Morrisett, J.D.; Abdel-Fattah, G.; Hoogeveen, R.; Mitchell, E.; Ballantyne, C.M.; Pownall, H.J.; Opekun, A.R.; Jaffe, J.S.; Oppermann, S.; Kahan, B.D. Effects of sirolimus on plasma lipids, lipoprotein levels, and fatty acid metabolism in renal transplant patients. J. Lipid Res. 2002, 43, 1170–1180. [Google Scholar] [CrossRef]
- Teutonico, A.; Schena, P.F.; Di Paolo, S. Glucose Metabolism in Renal Transplant Recipients: Effect of Calcineurin Inhibitor Withdrawal and Conversion to Sirolimus. J. Am. Soc. Nephrol. 2005, 16, 3128–3135. [Google Scholar] [CrossRef] [PubMed]
- Kälble, F.; Seckinger, J.; Schaier, M.; Morath, C.; Schwenger, V.; Zeier, M.; Sommerer, C. Switch to an everolimus-facilitated cyclosporine A sparing immunosuppression improves glycemic control in selected kidney transplant recipients. Clin. Transplant. 2017, 31, e13024. [Google Scholar] [CrossRef]
- Sommerer, C.; Witzke, O.; Lehner, F.; Arns, W.; Reinke, P.; Eisenberger, U.; Vogt, B.; Heller, K.; Jacobi, J.; Guba, M.; et al. Onset and progression of diabetes in kidney transplant patients receiving everolimus or cyclosporine therapy: An analysis of two randomized, multicenter trials. BMC Nephrol. 2018, 19, 237. [Google Scholar] [CrossRef] [PubMed]
- Bell, E.; Cao, X.; Moibi, J.A.; Greene, S.R.; Young, R.; Trucco, M.; Gao, Z.; Matschinsky, F.M.; Deng, S.; Markman, J.F.; et al. Rapamycin has a deleterious effect on MIN-6 cells and rat and human islets. Diabetes 2003, 52, 2731–2739. [Google Scholar] [CrossRef]
- Bussiere, C.T.; Lakey, J.R.T.; Shapiro, A.M.J.; Korbutt, G.S. The impact of the mTOR inhibitor sirolimus on the proliferation and function of pancreatic islets and ductal cells. Diabetologia 2006, 49, 2341–2349. [Google Scholar] [CrossRef] [PubMed]
- Gyurus, E.; Kaposztas, Z.; Kahan, B.D. Sirolimus therapy predisposes to new-onset diabetes mellitus after renal transplantation: A long-term analysis of various treatment regimens. Transpl. Proc. 2011, 43, 1583–1592. [Google Scholar] [CrossRef] [PubMed]
- Penfornis, A.; Kury-Paulin, S. Immunosuppressive drug-induced diabetes. Diabetes Metab. 2006, 32, 539–546. [Google Scholar] [CrossRef]
- Hjelmesæth, J.; Hartmann, A.; Kofstad, J.; Stenstrøm, J.; Leivestad, T.; Egeland, T.; Fauchald, P. Glucose intolerance after renal transplantation depends upon prednisolone dose and recipient age. Transplantation 1997, 64, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Torres-Romero, L.F.; Santiago-Delpín, E.A.; de Echegaray, S.; Solis, D.R.; Rodriguez-Trinidad, A.T.; Gonzalez-Caraballo, Z.A.; Morales-Otero, L.A. HLA Is Not Predictive of Posttransplant Diabetes Mellitus. Transplant. Proc. 2006, 38, 914–915. [Google Scholar] [CrossRef] [PubMed]
- Kasiske, B.L.; Snyder, J.J.; Gilbertson, D.; Matas, A.J. Diabetes Mellitus after Kidney Transplantation in the United States. Am. J. Transplant. 2003, 3, 178–185. [Google Scholar] [CrossRef]
- Baron, P.W.; Infante, S.; Peters, R.; Tilahun, J.; Weissman, J.; Delgado, L.; Kore, A.H.; Beeson, W.L.; de Vera, M.E. Post-Transplant Diabetes Mellitus After Kidney Transplant in Hispanics and Caucasians Treated with Tacrolimus-Based Immunosuppression. Ann. Transpl. 2017, 22, 309–314. [Google Scholar] [CrossRef]
- Sulanc, E.; Lane, J.T.; Puumala, S.E.; Groggel, G.C.; Wrenshall, L.E.; Stevens, R.B. New-onset diabetes after kidney transplantation: An application of 2003 International Guidelines. Transplantation 2005, 80, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Brzezińska, B.; Junik, R.; Kamińska, A.; Włodarczyk, Z.; Adamowicz, A. Factors associated with glucose metabolism disorders after kidney transplantation. Endokrynol. Pol. 2013, 64, 21–25. [Google Scholar] [PubMed]
- Marin, M.; Renoult, E.; Bondor, C.I.; Kessler, M. Factors influencing the onset of diabetes mellitus after kidney transplantation: A single French center experience. Transpl. Proc. 2005, 37, 1851–1856. [Google Scholar] [CrossRef]
- Mota-Zamorano, S.; Luna, E.; Garcia-Pino, G.; González, L.M.; Gervasini, G. Variability in the leptin receptor gene and other risk factors for post-transplant diabetes mellitus in renal transplant recipients. Ann. Med. 2019, 51, 164–173. [Google Scholar] [CrossRef]
- Zhang, X.; Men, T.; Liu, H.; Li, X.; Wang, J.; Lv, J. Genetic risk factors for post-transplantation diabetes mellitus in Chinese Han renal allograft recipients treated with tacrolimus. Transpl. Immunol. 2018, 49, 39–42. [Google Scholar] [CrossRef]
- Yang, J.; Hutchinson, I.I.; Shah, T.; Min, D.I. Genetic and clinical risk factors of new-onset diabetes after transplantation in Hispanic kidney transplant recipients. Transplantation 2011, 91, 1114–1119. [Google Scholar] [CrossRef]
- Romanowski, M.; Dziedziejko, V.; Maciejewska-Karlowska, A.; Sawczuk, M.; Safranow, K.; Domanski, L.; Pawlik, A. Adiponectin and leptin gene polymorphisms in patients with post-transplant diabetes mellitus. Pharmacogenomics 2015, 16, 1243–1251. [Google Scholar] [CrossRef]
- Berger, S.P.; Roos, A.; Mallat, M.J.; Schaapherder, A.F.; Doxiadis, I.I.; van Kooten, C.; Dekker, F.W.; Daha, M.R.; de Fijter, J.W. Low pretransplantation mannose-binding lectin levels predict superior patient and graft survival after simultaneous pancreas-kidney transplantation. J. Am. Soc. Nephrol. 2007, 18, 2416–2422. [Google Scholar] [CrossRef]
- Boniotto, M.; Braida, L.; Baldas, V.; Not, T.; Ventura, A.; Vatta, S.; Radillo, O.; Tedesco, F.; Percopo, S.; Montico, M.; et al. Evidence of a correlation between mannose binding lectin and celiac disease: A model for other autoimmune diseases. J. Mol. Med. 2005, 83, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Guad, R.M.; Taylor-Robinson, A.W.; Wu, Y.S.; Gan, S.H.; Zaharan, N.L.; Basu, R.C.; Liew, C.; Wan Md Adnan, W. Clinical and genetic risk factors for new-onset diabetes mellitus after transplantation (NODAT) in major transplant centres in Malaysia. BMC Nephrol. 2020, 21, 388. [Google Scholar] [CrossRef] [PubMed]
- Tarnowski, M.; Słuczanowska-Głabowska, S.; Pawlik, A.; Mazurek-Mochol, M.; Dembowska, E. Genetic factors in pathogenesis of diabetes mellitus after kidney transplantation. Ther. Clin. Risk Manag. 2017, 13, 439–446. [Google Scholar] [CrossRef]
- Bamoulid, J.; Courivaud, C.; Deschamps, M.; Mercier, P.; Ferrand, C.; Penfornis, A.; Tiberghien, P.; Chalopin, J.M.; Saas, P.; Ducloux, D. IL-6 promoter polymorphism -174 is associated with new-onset diabetes after transplantation. J. Am. Soc. Nephrol. 2006, 17, 2333–2340. [Google Scholar] [CrossRef]
- Kim, Y.G.; Ihm, C.G.; Lee, T.W.; Lee, S.H.; Jeong, K.H.; Moon, J.Y.; Chung, J.H.; Kim, S.K.; Kim, Y.H. Association of genetic polymorphisms of interleukins with new-onset diabetes after transplantation in renal transplantation. Transplantation 2012, 93, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.C.; Shu, K.H.; Tarng, D.C.; Wu, M.J.; Chen, C.H.; Yu, T.M.; Chuang, Y.W.; Huang, S.T.; Cheng, C.H. Gene polymorphisms are associated with posttransplantation diabetes mellitus among Taiwanese renal transplant recipients. Transpl. Proc. 2012, 44, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Benson, K.A.; Maxwell, A.P.; McKnight, A.J. A HuGE review and meta-analyses of genetic associations in new onset diabetes after kidney transplantation. PLoS ONE 2016, 11, e0147323. [Google Scholar] [CrossRef]
- Voight, B.F.; Scott, L.J.; Steinthorsdottir, V.; Morris, A.P.; Dina, C.; Welch, R.P.; Zeggini, E.; Huth, C.; Aulchenko, Y.S.; Thorleifsson, G.; et al. MAGIC investigators; GIANT Consortium Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat. Genet. 2010, 42, 579–589. [Google Scholar] [CrossRef]
- Lyssenko, V.; Lupi, R.; Marchetti, P.; Del Guerra, S.; Orho-Melander, M.; Almgren, P.; Sjögren, M.; Ling, C.; Eriksson, K.F.; Lethagen, A.L.; et al. Mechanisms by which common variants in the TCF7L2 gene increase risk of type 2 diabetes. J. Clin. Investig. 2007, 117, 2155–2163. [Google Scholar] [CrossRef]
- Kurzawski, M.; Dziewanowski, K.; Kędzierska, K.; Wajda, A.; Lapczuk, J.; Droździk, M. Association of transcription factor 7-like 2 (TCF7L2) gene polymorphism with posttransplant diabetes mellitus in kidney transplant patients medicated with tacrolimus. Pharmacol. Rep. 2011, 63, 826–833. [Google Scholar] [CrossRef]
- Ghisdal, L.; Baron, C.; Le Meur, Y.; Lionet, A.; Halimi, J.M.; Rerolle, J.P.; Glowacki, F.; Lebranchu, Y.; Drouet, M.; Noël, C.; et al. TCF7L2 polymorphism associates with new-onset diabetes after transplantation. J. Am. Soc. Nephrol. 2009, 20, 2459–2467. [Google Scholar] [CrossRef] [PubMed]
- McCaughan, J.A.; McKnight, A.J.; Maxwell, A.P. Genetics of new-onset diabetes after transplantation. J. Am. Soc. Nephrol. 2014, 25, 1037–1049. [Google Scholar] [CrossRef]
- Chen, Y.; Sampaio, M.S.; Yang, J.W.; Min, D.; Hutchinson, I.V. Genetic polymorphisms of the transcription factor NFATc4 and development of new-onset diabetes after transplantation in Hispanic kidney transplant recipients. Transplantation 2012, 93, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Dabrowska-Zamojcin, E.; Romanowski, M.; Dziedziejko, V.; Maciejewska-Karlowska, A.; Sawczuk, M.; Safranow, K.; Domanski, L.; Pawlik, A. CCL2 gene polymorphism is associated with post-transplant diabetes mellitus. Int. Immunopharmacol. 2016, 32, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.H.; Moon, J.Y.; Chung, J.H.; Kim, Y.H.; Lee, T.W. Significant associations between CCL5 gene polymorphisms and post-transplantational diabetes mellitus in Korean renal allograft recipients. Am. J. Nephrol. 2010, 32, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Moon, J.Y.; Lee, S.H.; Ihm, C.G.; Lee, T.W.; Kim, S.K.; Chung, J.H.; Kang, S.W.; Kim, T.H.; Park, S.J.; et al. Angiotensinogen polymorphisms and post-transplantation diabetes mellitus in Korean renal transplant subjects. Kidney Blood Press Res. 2013, 37, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Dutkiewicz, G.; Domanski, L.; Pawlik, A.; Binczak-Kuleta, A.; Safranow, K.; Ciechanowicz, A.; Dziedziejko, V.; Pietrzak-Nowacka, M.; Ciechanowski, K. Polymorphisms of super-oxide dismutase; glutathione peroxidase and catalase genes in patients with post-transplant diabetes mellitus. Arch. Med. Res. 2010, 41, 350–355. [Google Scholar] [CrossRef]
- Forgione, M.A.; Weiss, N.; Heydrick, S.; Cap, A.; Klings, E.S.; Bierl, C.; Eberhardt, R.T.; Farber, H.W.; Loscalzo, J. Cellular glutathione peroxidase deficiency and endothelial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H1255–H1261. [Google Scholar] [CrossRef] [PubMed]
- Hamanishi, T.; Furuta, H.; Kato, H.; Doi, A.; Tamai, M.; Shimomura, H.; Sakagashira, S.; Nishi, M.; Sasaki, H.; Sanke, T.; et al. Functional variants in the glutathione peroxidase-1 (GPx-1) gene are associated with increased intima-media thickness of carotid arteries and risk of macrovascular diseases in Japanese type 2 diabetic patients. Diabetes 2004, 53, 2455–2460. [Google Scholar] [CrossRef]
- Elens, L.; Sombogaard, F.; Hesselink, D.A.; van Schaik, R.H.; van Gelder, T. Single-nucleotide polymorphisms in P450 oxidoreductase and peroxisome proliferator-activated receptor-α are associated with the development of new-onset diabetes after transplantation in kidney transplant recipients treated with tacrolimus. Pharm. Genom. 2013, 23, 649–657. [Google Scholar] [CrossRef]
- Hornum, M.; Lindahl, J.P.; von Zur-Mühlen, B.; Jenssen, T.; Feldt-Rasmussen, B. Diagnosis, management and treatment of glucometabolic disorders emerging after kidney transplantation. Transpl. Int. 2013, 26, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Chakkera, H.A.; Weil, E.J.; Pham, P.T.; Pomeroy, J.; Knowler, W.C. Can new-onset diabetes after kidney transplant be prevented? Diabetes Care 2013, 36, 1406–1412. [Google Scholar] [CrossRef] [PubMed]
- Stephen, J.; Anderson-Haag, T.L.; Gustafson, S.; Snyder, J.J.; Kasiske, B.L.; Israni, A.K. Metformin use in kidney transplant recipients in the United States: An observational study. Am. J. Nephrol. 2014, 40, 546–553. [Google Scholar] [CrossRef]
- Kurian, B.; Joshi, R.; Helmuth, A. Effectiveness and long-term safety of thiazolidinediones and metformin in renal transplant recipients. Endocr. Pract. 2008, 14, 979–984. [Google Scholar] [CrossRef]
- Dedinská, I.; Graňák, K.; Vnučák, M.; Skálová, P.; Kováčiková, L.; Laca, Ľ.; Miklušica, J.; Prídavková, D.; Galajda, P.; Mokáň, M. Role of sex in post-transplant diabetes mellitus development: Are men and women equal? J. Diabetes Complicat. 2019, 33, 315–322. [Google Scholar] [CrossRef]
- Madhav, D.; Ram, R.; Dakshinamurty, K.V. Posttransplant Diabetes Mellitus: Analysis of Risk Factors, Effects on Biochemical Parameters and Graft Function 5 Years after Renal Transplantation. Transplant. Proc. 2010, 42, 4069–4071. [Google Scholar] [CrossRef]
- González-Posada, J.M.; Hernández, D.; Bayés Genís, B.; García Perez, J.; Rivero Sanchez, M. Impact of diabetes mellitus on kidney transplant recipients in Spain. Nephrol. Dial. Transpl. 2004, 19 (Suppl. 3), iii57–iii61. [Google Scholar] [CrossRef][Green Version]
- Prasad, G.V.; Kim, S.J.; Huang, M.; Nash, M.M.; Zalzman, J.S.; Fenton, S.S.; Cattran, D.C.; Cole, E.H.; Cardella, C.J. Reduced incidence of new onset diabetes mellitus after renal transplantation with 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (statins). Am. J. Transpl. 2004, 4, 1897–1903. [Google Scholar] [CrossRef] [PubMed]
- Lê, K.-A.; Mahurkar, S.; Alderete, T.L.; Hasson, R.E.; Adam, T.C.; Kim, J.S.; Beale, E.; Xie, C.; Greenberg, A.S.; Allayee, H.; et al. Subcutaneous Adipose Tissue Macrophage Infiltration Is Associated with Hepatic and Visceral Fat Deposition, Hyperinsulinemia, and Stimulation of NF-κB Stress Pathway. Diabetes 2011, 60, 2802–2809. [Google Scholar] [CrossRef]
- Cai, R.; Wu, M.; Xing, Y. Pretransplant metabolic syndrome and its components predict post-transplantation diabetes mellitus in Chinese patients receiving a first renal transplant. Ther. Clin. Risk Manag. 2019, 15, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, E.; Fernández-Fresnedo, G.; Valero, R.; Ruiz, J.C.; Piñera, C.; Palomar, R.; González-Cotorruelo, J.; Gómez-Alamillo, C.; Arias, M. New-onset diabetes after kidney transplantation: Risk factors. J. Am. Soc. Nephrol. 2006, 17 (Suppl. 3), S291–S295. [Google Scholar] [CrossRef] [PubMed]
- Roland, M.; Gatault, P.; Al-Najjar, A.; Doute, C.; Barbet, C.; Chatelet, V.; Laouad, I.; Marlière, J.F.; Nivet, H.; Büchler, M.; et al. Early pulse pressure and low-grade proteinuria as independent long-term risk factors for new-onset diabetes mellitus after kidney transplantation. Am. J. Transpl. 2008, 8, 1719–1728. [Google Scholar] [CrossRef]
- Hermans, M.P.; Ahn, S.A.; Rousseau, M.F. The atherogenic dyslipidemia ratio [log(TG)/HDL-C] is associated with residual vascular risk, beta-cell function loss and microangiopathy in type 2 diabetes females. Lipids Health Dis. 2012, 11, 132. [Google Scholar] [CrossRef]
- Bayés, B.; Granada, M.L.; Pastor, M.C.; Lauzurica, R.; Salinas, I.; Sanmartí, A.; Espinal, A.; Serra, A.; Navarro, M.; Bonal, J.; et al. Obesity, adiponectin and inflammation as predictors of new-onset diabetes mellitus after kidney transplantation. Am. J. Transpl. 2007, 7, 416–422. [Google Scholar] [CrossRef]
- de Lourdes Lima, M.; Cruz, T.; Rodrigues, L.E.; Bomfim, O.; Melo, J.; Correia, R.; Porto, M.; Cedro, A.; Vicente, E. Serum and intracellular magnesium deficiency in patients with metabolic syndrome-Evidences for its relation to insulin resistance. Diabetes Res. Clin. Pract. 2009, 83, 257–262. [Google Scholar] [CrossRef]
- Schweer, T.; Gwinner, W.; Scheffner, I.; Schwarz, A.; Haller, H.; Blume, C. High impact of rejection therapy on the incidence of post-transplant diabetes mellitus after kidney transplantation. Clin. Transpl. 2014, 28, 512–519. [Google Scholar] [CrossRef]
- Alnasrallah, B.; Goh, T.L.; Chan, L.W.; Manley, P.; Pilmore, H. Transplantation and diabetes (Transdiab): A pilot randomised controlled trial of metformin in impaired glucose tolerance after kidney transplantation. BMC Nephrol. 2019, 20, 147. [Google Scholar] [CrossRef]
- Hjelmesæth, J.; Hartmann, A.; Midtvedt, K.; Aakhus, S.; Stenstrøm, J.; Mørkrid, L.; Egeland, T.; Tordarson, H.; Fauchald, P. Metabolic cardiovascular syndrome after renal transplantation. Nephrol. Dial. Transplant. 2001, 16, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, T.A.; Wahba, M.; Mallik, R.; Peracha, J.; Patel, D.; De, P.; Fogarty, D.; Frankel, A.; Karalliedde, J.; Mark, P.B.; et al. Association of British Clinical Diabetologists and Renal Association guidelines on the detection and management of diabetes post solid organ transplantation. Diabet. Med. 2021, e14523. [Google Scholar] [CrossRef]
- Wauters, R.P.; Cosio, F.G.; Suarez Fernandez, M.L.; Kudva, Y.; Shah, P.; Torres, V.E. Cardiovascular consequences of new-onset hyperglycemia after kidney transplantation. Transplantation 2012, 94, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Heldal, T.F.; Ueland, T.; Jenssen, T.; Hartmann, A.; Reisaeter, A.V.; Aukrust, P.; Michelsen, A.; Åsberg, A. Inflammatory and related biomarkers are associated with post-transplant diabetes mellitus in kidney recipients: A retrospective study. Transpl. Int. 2018, 31, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kim, S.K.; Park, J.Y.; Kim, Y.G.; Moon, J.Y.; Lee, S.H.; Ihm, C.G.; Lee, T.W.; Kim, S.K.; Chung, J.H.; et al. Significant association between toll-like receptor gene polymorphisms and posttransplantation diabetes mellitus. Nephron 2016, 133, 279. [Google Scholar] [CrossRef]
- Cieniawski, D.; Miarka, P.; Ignacak, E.; Bętkowska-Prokop, A.; Waluś-Miarka, M.; Idzior-Waluś, B.; Kuźniewski, M.; Sułowicz, W. ognostic value of proinflammatory markers in patients after kidney transplantation in relation to the presence of diabetes. Transpl. Proc. 2016, 48, 1604. [Google Scholar] [CrossRef]
- Hjelmesaeth, J.; Hagen, M.; Hartmann, A.; Midtvedt, K.; Egeland, T.; Jenssen, T. The impact of impaired insulin release and insulin resistance on glucose intolerance after renal transplantation. Clin. Transpl. 2002, 16, 389. [Google Scholar] [CrossRef]
- Hjelmesaeth, J.; Asberg, A.; Muller, F.; Hartmann, A.; Jenssen, T. New-onset posttransplantation diabetes mellitus: Insulin resistance or insulinopenia? Impact of immunosuppressive drugs, cytomegalovirus and hepatitis C virus infection. Curr. Diabetes Rev. 2005, 1, 1–10. [Google Scholar] [CrossRef]
- Yepes-Calderón, M.; Sotomayor, C.G.; Gomes-Neto, A.W.; Gans, R.O.B.; Berger, S.P.; Rimbach, G.; Esatbeyoglu, T.; Rodrigo, R.; Geleijnse, J.M.; Navis, G.J.; et al. Plasma Malondialdehyde and Risk of New-Onset Diabetes after Transplantation in Renal Transplant Recipients: A Prospective Cohort Study. J. Clin. Med. 2019, 8, 453. [Google Scholar] [CrossRef]
- Cattaneo, D.; Ruggenenti, P.; Baldelli, S.; Motterlini, N.; Gotti, E.; Sandrini, S.; Salvadori, M.; Segoloni, G.; Rigotti, P.; Donati, D.; et al. ABCB1 genotypes predict cyclosporine-related adverse events and kidney allograft outcome. J. Am. Soc. Nephrol. 2009, 20, 1404–1415. [Google Scholar] [CrossRef]
- Dormans, J.P.; Gunawardena, A.T.; Hakonarson, H.; Hecht, J.T. The type 2 diabetes associated rs7903146 T allele within TCF7L2 is significantly under-represented in hereditary multiple exostoses: Insights into pathogenesis. Bone 2015, 72, 123–127. [Google Scholar]
- Dehwah, M.S.; Wang, M.; Huang, Q.Y. CDKAL1 and type 2 diabetes: A global meta-analysis. Genet. Mol. Res. 2010, 9, 1109–1120. [Google Scholar] [CrossRef] [PubMed]
- Schultz, B.; Gallicio, G.; Cesaroni, M.; Lupey, L.; Engel, N. Enhancers compete with a long non-coding RNA for regulation of the Kcnq1 domain. Nucl. Acids Res. 2015, 43, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Valverde, S.L.; Taft, R.J.; Mattick, J.S. MicroRNAs in β-cell biology, insulin resistance, diabetes and its complications. Diabetes 2011, 60, 1825–1831. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, Y.; Li, L.; Su, B.; Yang, L.; Fan, W.; Yin, Q.; Chen, L.; Cui, T.; Zhang, J.; et al. Involvement of inflammation-related miR-155 and miR-146a in diabetic nephropathy: Implications for glomerular endothelial injury. BMC Nephrol. 2014, 15, 142. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, G.; Yang, C.; Zhou, K.; Shen, B.; Liang, H.; Jiang, X. The role of circulating microRNA-126 (miR-126): A novel biomarker for screening prediabetes and newly diagnosed type 2 diabetes mellitus. Int. J. Mol. Sci. 2014, 15, 10567–10577. [Google Scholar] [CrossRef]
- Peng, H.; Zhong, M.; Zhao, W.; Wang, C.; Zhang, J.; Liu, X.; Li, Y.; Paudel, S.D.; Wang, Q.; Lou, T. Urinary miR-29 correlates with albuminuria and carotid intima-media thickness in type 2 diabetes patients. PLoS ONE 2013, 8, e82607. [Google Scholar]
- Celen, E.; Ertosun, M.G.; Kocak, H.; Dinckan, A.; Yoldas, B. Expression Profile of MicroRNA Biogenesis Components in Renal Transplant Patients. Transplant. Proc. 2017, 49, 472–476. [Google Scholar] [CrossRef]
- Ulbing, M.; Kirsch, A.H.; Leber, B.; Lemesch, S.; Münzker, J.; Schweighofer, N.; Hofer, D.; Trummer, O.; Rosenkranz, A.R.; Müller, H.; et al. MicroRNAs 223-3p and 93-5p in patients with chronic kidney disease before and after renal transplantation. Bone 2017, 95, 115–123. [Google Scholar] [CrossRef]
- Zampetaki, A.; Kiechl, S.; Drozdov, I.; Willeit, P.; Mayr, U.; Prokopi, M.; Mayr, A.; Weger, S.; Oberhollenzer, F.; Bonora, E.; et al. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ. Res. 2010, 107, 810–817. [Google Scholar] [CrossRef]
- Sarnak, M.J.; Levey, A.S.; Schoolwerth, A.C.; Coresh, J.; Culleton, B.; Hamm, L.L.; McCullough, P.A.; Kasiske, B.L.; Kelepouris, E.; Klag, M.J.; et al. Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension 2003, 42, 1050–1065. [Google Scholar] [CrossRef]
- Boerner, B.P.; Shivaswamy, V.; Desouza, C.V.; Larsen, J.L. Diabetes and cardiovascular disease following kidney transplantation. Curr. Diabetes Rev. 2011, 7, 221–234. [Google Scholar] [CrossRef]
- Matsushita, K.; van der Velde, M.; Astor, B.C.; Woodward, M.; Levey, A.S.; de Jong, P.E.; Coresh, J.; Gansevoort, R.T. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 2010, 375, 2073–2081. [Google Scholar] [CrossRef] [PubMed]
- Neale, J.; Smith, A.C. Cardiovascular risk factors following renal transplant. World J. Transpl. 2015, 5, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Beto, J.A.; Coronado, B.E.; Eknoyan, G.; Foley, R.N.; Kasiske, B.L.; Klag, M.J.; Mailloux, L.U.; Manske, C.L.; Meyer, K.B.; et al. Controlling the epidemic of cardiovascular disease in chronic renal disease: What do we know? What do we need to learn? Where do we go from here? National Kidney Foundation Task Force on Cardiovascular Disease. Am. J. Kidney Dis. 1998, 32, 853–906. [Google Scholar] [CrossRef]
- Rao, P.S.; Merion, R.M.; Ashby, V.B.; Port, F.K.; Wolfe, R.A.; Kayler, L.K. Renal transplantation in elderly patients older than 70 years of age: Results from the Scientific Registry of Transplant Recipients. Transplantation 2007, 83, 1069–1074. [Google Scholar] [CrossRef]
- Liefeldt, L.; Budde, K. Risk factors for cardiovascular disease in renal transplant recipients and strategies to minimize risk. Transpl. Int. 2010, 23, 1191–1204. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kriesche, H.U.; Schold, J.D.; Srinivas, T.R.; Reed, A.; Kaplan, B. Kidney transplantation halts cardiovascular disease progression in patients with end-stage renal disease. Am. J. Transpl. 2004, 4, 1662–1668. [Google Scholar] [CrossRef]
- Lentine, K.L.; Brennan, D.C.; Schnitzler, M.A. Incidence and predictors of myocardial infarction after kidney transplantation. J. Am. Soc. Nephrol. 2005, 16, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Aftab, W.; Varadarajan, P.; Rasool, S.; Pai, R.G. Predictors and prognostic implications of major adverse cardiovascular events after renal transplant: 10 years outcomes in 321 patients. Int. J. Angiol. 2014, 23, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Yates, C.J.; Fourlanos, S.; Hjelmesæth, J.; Colman, P.G.; Cohney, S.J. New-Onset Diabetes After Kidney Transplantation—Changes and Challenges. Am. J. Transplant. 2012, 12, 820–828. [Google Scholar] [CrossRef]
- Kahn, R.; Buse, J.; Ferrannini, E.; Stern, M. The metabolic syndrome: Time for a critical appraisal. Diabetologia 2005, 48, 1684–1699. [Google Scholar] [CrossRef] [PubMed]
- Alshamsi, S. The risk factors and their relative strengths for new onset diabetes after transplantation (NODAT). Nephrol. Dial. Transplant. 2015, 30, iii653. [Google Scholar] [CrossRef][Green Version]
- Huang, Y.; Cai, X.; Mai, W.; Li, M.; Hu, Y. Association between prediabetes and risk of cardiovascular disease and all cause mortality: Systematic review and meta-analysis. BMJ 2016, 355, i5953. [Google Scholar] [CrossRef] [PubMed]
- Valderhaug, T.G.; Hjelmesæth, J.; Hartmann, A.; Røislien, J.; Bergrem, H.A.; Leivestad, T.; Line, P.D.; Jenssen, T. The association of early post-transplant glucose levels with long-term mortality. Diabetologia 2011, 54, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, E.; Santos, L.; Piñera, C.; Millán, J.C.; Quintela, M.E.; Toyos, C.; Allende, N.; Gómez-Alamillo, C.; Arias, M. Prediction at first year of incident new-onset diabetes after kidney transplantation by risk prediction models. Diabetes Care 2012, 35, 471–473. [Google Scholar] [CrossRef]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [CrossRef]
- Tuomilehto, J.; Lindström, J.; Eriksson, J.G.; Valle, T.T.; Hämäläinen, H.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Laakso, M.; Louheranta, A.; Rastas, M.; et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 2001, 344, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Cashion, A.K.; Sánchez, Z.V.; Cowan, P.A.; Hathaway, D.K.; Lo Costello, A.; Gaber, A.O. Changes in weight during the first year after kidney transplantation. Prog. Transpl. 2007, 17, 40–47. [Google Scholar] [CrossRef]
- Type 2 Diabetes in Adults: Management. NICE Guideline [NG28]. Available online: https://www.nice.org.uk/guidance/ng28 (accessed on 1 October 2020).
- Salpeter, S.R.; Greyber, E.; Pasternak, G.A.; Salpeter, E.E. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2010, 2010, Cd002967. [Google Scholar] [CrossRef]
- UK Prospective Diabetes Study (UKPDS) Group. Effect of Intensive Blood-Glucose Control with Metformin on Complications in Overweight Patients with Type 2 Diabetes (UKPDS 34). Lancet 1998, 352, 854–865. [Google Scholar] [CrossRef]
- Seifarth, C.; Schehler, B.; Schneider, H.J. Effectiveness of metformin on weight loss in non-diabetic individuals with obesity. Exp. Clin. Endocrinol. Diabetes 2013, 121, 27–31. [Google Scholar] [CrossRef]
- Chakkera, H.A.; Weil, E.J.; Castro, J.; Heilman, R.L.; Reddy, K.S.; Mazur, M.J.; Hamawi, K.; Mulligan, D.C.; Moss, A.A.; Mekeel, K.L.; et al. Hyperglycemia during the immediate period after kidney transplantation. Clin. J. Am. Soc. Nephrol. 2009, 4, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.C.; Mathew, T.H.; Russ, G.R.; Rao, M.M.; Moran, J. Early peri-operative glycaemic control and allograft rejection in patients with diabetes mellitus: A pilot study. Transplantation 2001, 72, 1321–1324. [Google Scholar] [CrossRef]
- Hecking, M.; Haidinger, M.; Döller, D.; Werzowa, J.; Tura, A.; Zhang, J.; Tekoglu, H.; Pleiner, J.; Wrba, T.; Rasoul-Rockenschaub, S.; et al. Early Basal Insulin Therapy Decreases New-Onset Diabetes after Renal Transplantation. J. Am. Soc. Nephrol. 2012, 23, 739–749. [Google Scholar] [CrossRef]
- Gallwitz, B.; Bretzel, R.G. How do we continue treatment in patients with type 2 diabetes when therapeutic goals are not reached with oral antidiabetes agents and lifestyle? Incretin versus insulin treatment. Diabetes Care 2013, 36 (Suppl. 2), S180–S189. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Jun, H.S. Anti-diabetic actions of glucagon-like peptide-1 on pancreatic beta-cells. Metabolism 2014, 63, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Padmasekar, M.; Lingwal, N.; Samikannu, B.; Chen, C.; Sauer, H.; Linn, T. Exendin-4 protects hypoxic islets from oxidative stress and improves islet transplantation outcome. Endocrinology 2013, 154, 1424–1433. [Google Scholar] [CrossRef]
- Farber, S.J.; Berger, E.Y.; Earle, D.P. Effect of diabetes and insulin of the maximum capacity of the renal tubules to reabsorb glucose. J. Clin. Investig. 1951, 30, 125–129. [Google Scholar] [CrossRef]
- Norton, L.; Shannon, C.E.; Fourcaudot, M.; Hu, C.; Wang, N.; Ren, W.; Song, J.; Abdul-Ghani, M.; DeFronzo, R.A.; Ren, J.; et al. Sodium-glucose co-transporter (SGLT) and glucose transporter (GLUT) expression in the kidney of type 2 diabetic subjects. Diabetes Obes. Metab. 2017, 19, 1322–1326. [Google Scholar] [CrossRef]
- Wright, E.M.; Turk, E. The sodium/glucose cotransport family SLC5. Pflug. Arch. 2004, 447, 510–518. [Google Scholar] [CrossRef]
- Wright, E.M. Renal Na(+)-glucose cotransporters. Am. J. Physiol. Ren. Physiol. 2001, 280, F10–F18. [Google Scholar] [CrossRef]
- Turk, E.; Martín, M.G.; Wright, E.M. Structure of the human Na+/glucose cotransporter gene SGLT1. J. Biol. Chem. 1994, 269, 15204–15209. [Google Scholar] [CrossRef]
- Mogensen, C.E. Maximum tubular reabsorption capacity for glucose and renal hemodynamcis during rapid hypertonic glucose infusion in normal and diabetic subjects. Scand. J. Clin. Lab. Investig. 1971, 28, 101–109. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Davidson, J.A.; Del Prato, S. The role of the kidneys in glucose homeostasis: A new path towards normalizing glycaemia. Diabetes Obes. Metab. 2012, 14, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, M.A.; Norton, L.; Defronzo, R.A. Role of sodium-glucose cotransporter 2 (SGLT 2) inhibitors in the treatment of type 2 diabetes. Endocr. Rev. 2011, 32, 515–531. [Google Scholar] [CrossRef]
- Freitas, H.S.; Anhê, G.F.; Melo, K.F.; Okamoto, M.M.; Oliveira-Souza, M.; Bordin, S.; Machado, U.F. Na(+) -glucose transporter-2 messenger ribonucleic acid expression in kidney of diabetic rats correlates with glycemic levels: Involvement of hepatocyte nuclear factor-1alpha expression and activity. Endocrinology 2008, 149, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Gallo, L.A.; Ward, M.S.; Fotheringham, A.K.; Zhuang, A.; Borg, D.J.; Flemming, N.B.; Harvie, B.M.; Kinneally, T.L.; Yeh, S.M.; McCarthy, D.A.; et al. Once daily administration of the SGLT2 inhibitor, empagliflozin, attenuates markers of renal fibrosis without improving albuminuria in diabetic db/db mice. Sci. Rep. 2016, 6, 26428. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, M.A.; DeFronzo, R.A.; Norton, L. Novel hypothesis to explain why SGLT2 inhibitors inhibit only 30-50% of filtered glucose load in humans. Diabetes 2013, 62, 3324–3328. [Google Scholar] [CrossRef]
- Danne, T.; Edelman, S.; Frias, J.P.; Ampudia-Blasco, F.J.; Banks, P.; Jiang, W.; Davies, M.J.; Sawhney, S. Efficacy and safety of adding sotagliflozin, a dual sodium-glucose co-transporter (SGLT)1 and SGLT2 inhibitor, to optimized insulin therapy in adults with type 1 diabetes and baseline body mass index ≥ 27 kg/m(2). Diabetes Obes. Metab. 2021, 23, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Deeks, E.D. Sotagliflozin: A Review in Type 1 Diabetes. Drugs 2019, 79, 1977–1987. [Google Scholar] [CrossRef]
- Zou, H.; Liu, L.; Guo, J.; Wang, H.; Liu, S.; Xing, Y.; Deng, C.; Xiao, Y.; Zhou, Z. Sodium-glucose cotransporter inhibitors as add-on therapy in addition to insulin for type 1 diabetes mellitus: A meta-analysis of randomized controlled trials. J. Diabetes Investig. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Martin, R.J.; Tulley, R.T.; Raggio, A.M.; McCutcheon, K.L.; Shen, L.; Danna, S.C.; Tripathy, S.; Hegsted, M.; Keenan, M.J. Dietary resistant starch upregulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodents. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1160–E1166. [Google Scholar] [CrossRef] [PubMed]
- Palmer, B.F.; Clegg, D.J.; Taylor, S.I.; Weir, M.R. Diabetic ketoacidosis, sodium glucose transporter-2 inhibitors and the kidney. J. Diabetes Complicat. 2016, 30, 1162–1166. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Novikov, A.; Vallon, V. Ketosis and diabetic ketoacidosis in response to SGLT2 inhibitors: Basic mechanisms and therapeutic perspectives. Diabetes/Metab. Res. Rev. 2017, 33, e2886. [Google Scholar] [CrossRef]
- Dashora, U.; Patel, D.C.; Gregory, R.; Winocour, P.; Dhatariya, K.; Rowles, S.; Macklin, A.; Rayman, G.; Nagi, D. Association of British Clinical Diabetologists (ABCD) and Diabetes UK joint position statement and recommendations on the use of sodium-glucose cotransporter inhibitors with insulin for treatment of type 1 diabetes (Updated October 2020). Diabet. Med. 2021, 38, e14458. [Google Scholar] [CrossRef] [PubMed]
- KDIGO clinical practice guideline for the care of kidney transplant recipients. Am. J. Transpl. 2009, 9 (Suppl. 3), S1–S155. [CrossRef]
- Jenssen, T.; Hartmann, A. Post-transplant diabetes mellitus in patients with solid organ transplants. Nat. Rev. Endocrinol. 2019, 15, 172–188. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.; Rodrigo, E.; Piñera, C.; Quintella, E.; Ruiz, J.C.; Fernández-Fresnedo, G.; Palomar, R.; Gómez-Alamillo, C.; de Francisco, A.; Arias, M. New-onset diabetes after transplantation: Drug-related risk factors. Transpl. Proc. 2012, 44, 2585–2587. [Google Scholar] [CrossRef]
- Palepu, S.; Prasad, G.V. New-onset diabetes mellitus after kidney transplantation: Current status and future directions. World J. Diabetes 2015, 6, 445–455. [Google Scholar] [CrossRef]
- Velmurugan, G.; Ramprasath, T.; Gilles, M.; Swaminathan, K.; Ramasamy, S. Gut Microbiota, Endocrine-Disrupting Chemicals, and the Diabetes Epidemic. Trends Endocrinol. Metab. 2017, 28, 612–625. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef] [PubMed]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, E1–E150. [Google Scholar] [CrossRef]
- Neel, B.A.; Sargis, R.M. The paradox of progress: Environmental disruption of metabolism and the diabetes epidemic. Diabetes 2011, 60, 1838–1848. [Google Scholar] [CrossRef] [PubMed]
- Yang, O.; Kim, H.L.; Weon, J.-I.; Seo, Y.R. Endocrine-disrupting Chemicals: Review of Toxicological Mechanisms Using Molecular Pathway Analysis. J. Cancer Prev. 2015, 20, 12–24. [Google Scholar] [CrossRef]
- Sircana, A.; Framarin, L.; Leone, N.; Berrutti, M.; Castellino, F.; Parente, R.; De Michieli, F.; Paschetta, E.; Musso, G. Altered Gut Microbiota in Type 2 Diabetes: Just a Coincidence? Curr. Diab. Rep. 2018, 18, 98. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).