Interaction of Lactoferrin with Unsaturated Fatty Acids: In Vitro and In Vivo Study of Human Lactoferrin/Oleic Acid Complex Cytotoxicity
Abstract
1. Introduction
1.1. Lipolytic Effect of LF
1.2. Anticancer Effect of LF
1.3. Complexes Formed by LF and Low-Molecular Substances
1.4. Complexes of Milk Proteins with Oleic Acid
Complex of LF with OA
1.5. Cytotoxicity of Oleic Acid
2. Materials and Methods
2.1. Reagents
2.2. Experimental Animals
2.3. Cell Cultures
2.4. Obtaining Complexes of LF with Fatty Acids. Assessing LF/FA Stoichiometry
2.5. Fluorescence and Circular Dichroism Spectra
2.5.1. Registering Circular Dichroism Spectra
2.5.2. Registering Fluorescence Spectra
2.6. Studying Hepatoma 22a Viability and Lysis of Erythrocytes Induced by LF and LF-8OA
2.6.1. Viability of Hepatoma 22a Cells
2.6.2. Isolation of Erythrocytes from Peripheral Blood and Studying the Hemolysis
2.7. Studying the Effect of LF and LF-8OA on Mortality of Mice with Hepatoma 22a
3. Results
3.1. Specificity of LF Interaction with Fatty Acids
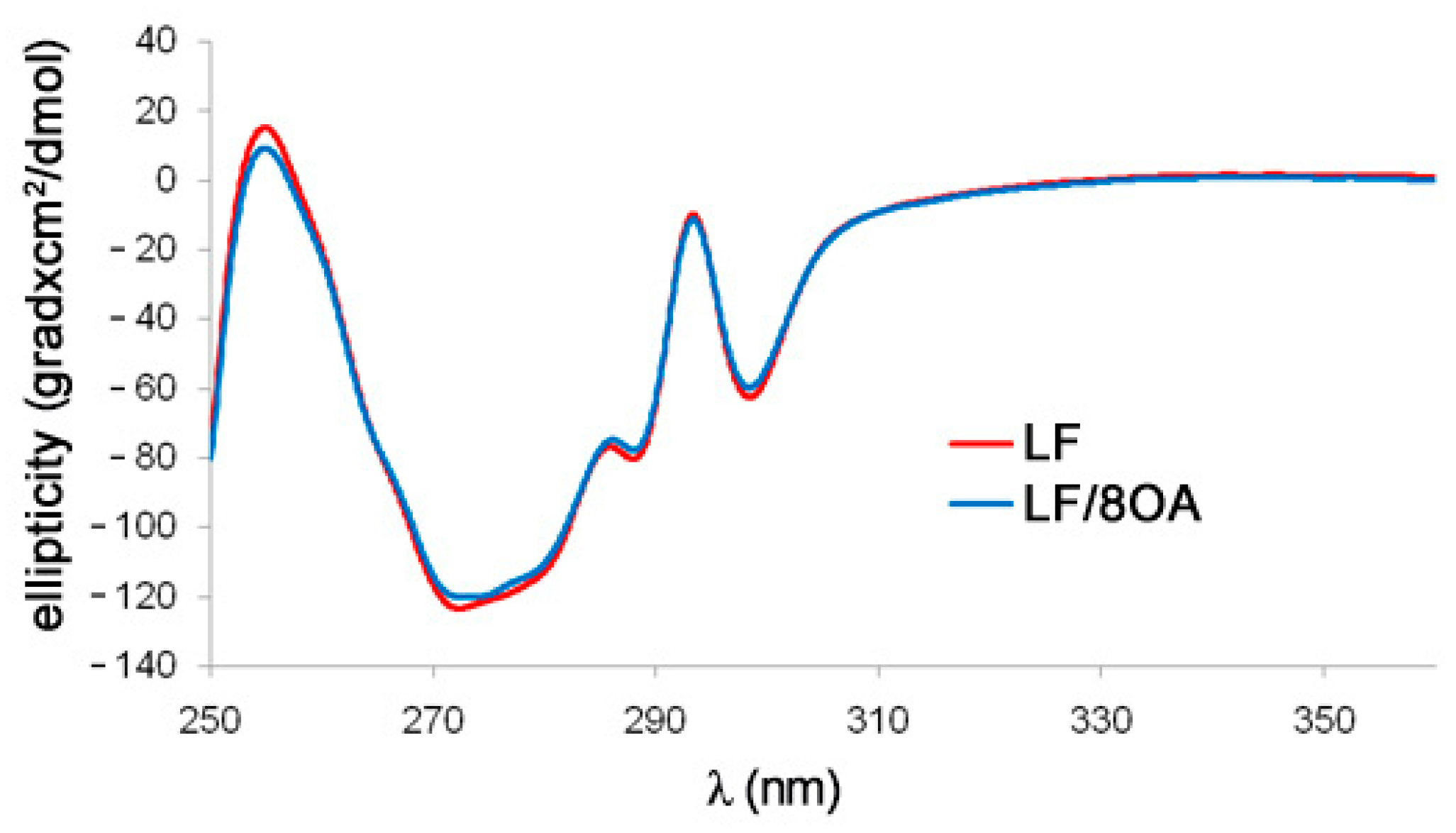
3.2. Tryptophan Fluorescence and CD Spectra of LF and LF-8OA
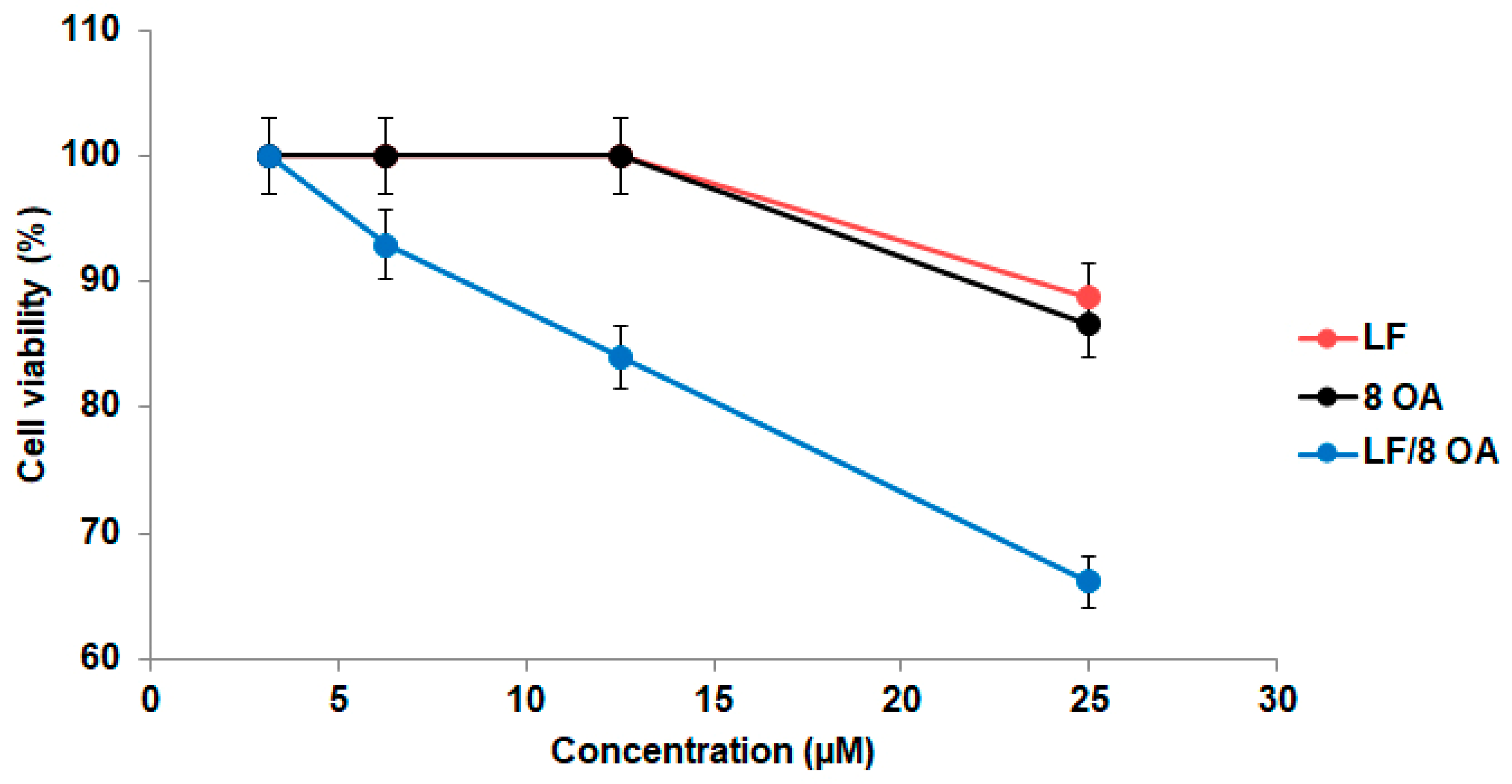
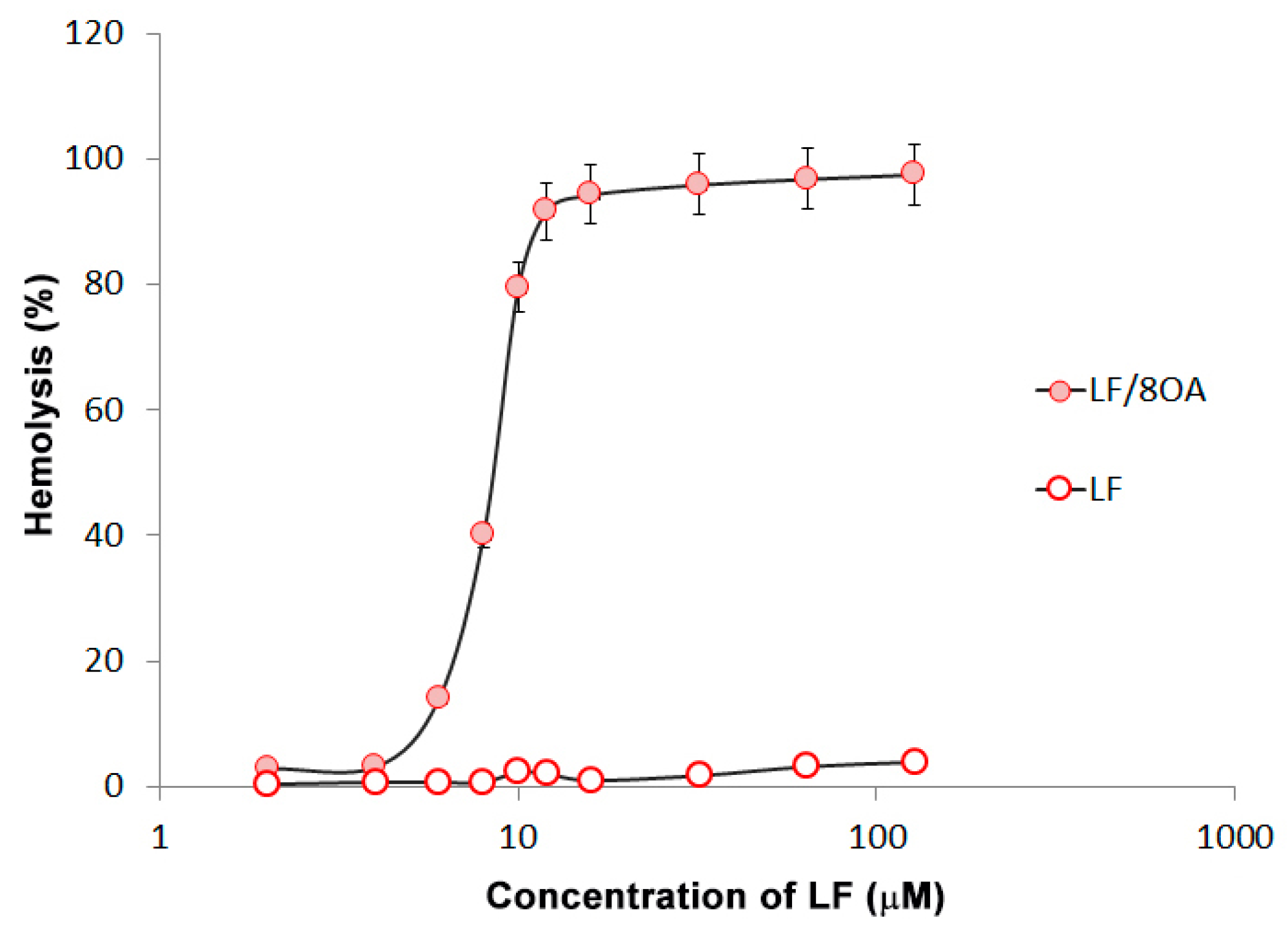
3.3. Effect of LF and LF-8OA on Hepatoma 22a Cells and on Stability of Erythrocytes
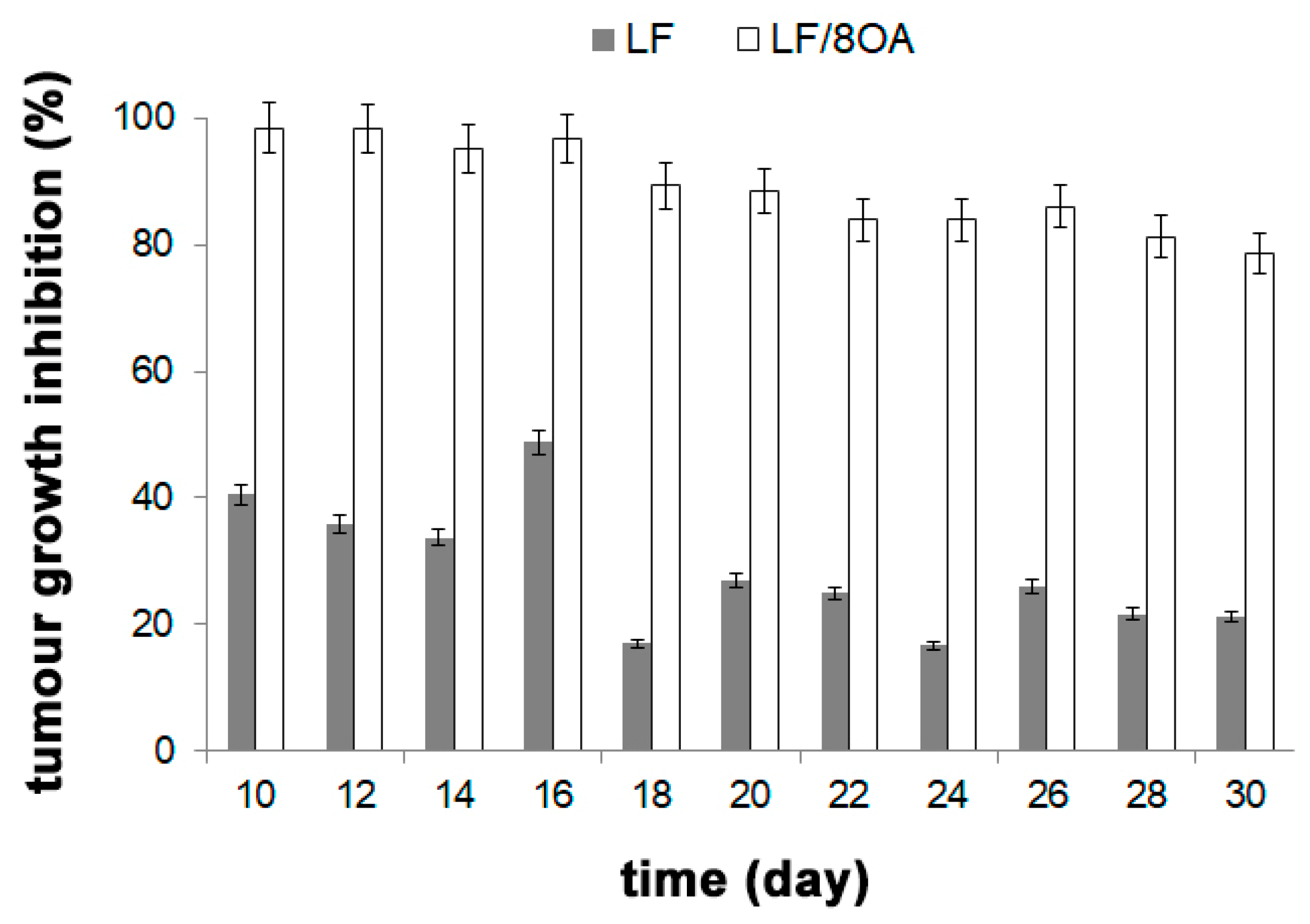
3.4. Effect of LF and LF-8OA on Mortality of Mice with Hepatoma 22a
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Gibbons, J.A.; Kanwar, R.K.; Kanwar, J.R. Lactoferrin and cancer in different cancer models. Front. Biosci. (Schol. Ed.) 2011, 3, 1080–1088. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.P.; Iigo, M.; Sato, J.; Sekine, K.; Adachi, I.; Tsuda, H. Activation of intestinal mucosal immunity in tumor-bearing mice by lactoferrin. Jpn. J. Cancer Res. 2000, 91, 1022–1027. [Google Scholar] [CrossRef] [PubMed]
- Iigo, M.; Shimamura, M.; Matsuda, E.; Fujita, K.; Nomoto, H.; Satoh, J.; Kojima, S.; Alexander, D.B.; Moore, M.A.; Tsuda, H. Orally administered bovine lactoferrin induces caspase-1 and interleukin-18 in the mouse intestinal mucosa: A possible explanation for inhibition of carcinogenesis and metastasis. Cytokine 2004, 25, 36–44. [Google Scholar] [CrossRef]
- Wolf, J.S.; Li, G.; Varadhachary, A.; Petrak, K.; Schneyer, M.; Li, D.; Ongkasuwan, J.; Zhang, X.; Taylor, R.J.; Strome, S.E.; et al. Oral lactoferrin results in T-cell dependent tumor inhibition of head and neck squamous cell carcinoma in vivo. Clin. Cancer Res. 2007, 13, 1601–1610. [Google Scholar] [CrossRef]
- Kanwar, J.R.; Palmano, K.P.; Sun, X.; Kanwar, R.K.; Gupta, R.; Haggarty, N.; Rowan, A.; Ram, S.; Krissansen, G.W. ‘Iron-saturated’ lactoferrin is a potent natural adjuvant for augmenting cancer chemotherapy. Immmunol. Cell. Biol. 2008, 86, 277–288. [Google Scholar] [CrossRef]
- Kimoto, Y.; Nishinohara, M.; Sugiyama, A.; Haruna, A.; Takeuchi, T. Protective effect of lactoferrin on Cisplatin-induced nephrotoxicity in rats. J. Vet. Med. Sci. 2013, 75, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Zhang, M.; Tian, M.; Jiang, L.; Guo, H.Y.; Ren, F.Z. Bovine lactoferrin binds oleic acid to form an anti-tumor complex similar to HAMLET. Biochim. Biophys. Acta. 2014, 1841, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, C.; Cavia, M.d.M.; Alonso-Torre, S.R. Antitumor effect of oleic acid; mechanisms of action: A review. Nutr. Hosp. 2012, 27, 1860–1865. [Google Scholar] [CrossRef]
- Ono, T.; Murakoshi, M.; Suzuki, N.; Iida, N.; Ohdera, M.; Iigo, M.; Yoshida, T.; Sugiyama, K.; Nishino, H. Potent anti-obesity effect of enteric-coated lactoferrin: Decrease in visceral fat accumulation in Japanese men and women with abdominal obesity after 8-week administration of enteric-coated lactoferrin tablets. Br. J. Nutr. 2010, 104, 1688–1695. [Google Scholar] [CrossRef]
- Ono, T.; Fujisaki, C.; Ishihara, Y.; Ikoma, K.; Morishita, S.; Murakoshi, M.; Sugiyama, K.; Kato, H.; Miyashita, K.; Yoshida, T.; et al. Potent lipolytic activity of lactoferrin in mature adipocytes. Biosci. Biotechnol. Biochem. 2013, 77, 566–571. [Google Scholar] [CrossRef]
- Campbell, T.; Skilton, R.A.; Coombes, R.C.; Shousha, S.; Graham, M.D.; Luqmani, Y.A. Isolation of a lactoferrin cDNA clone and its expression in human breast. Br. J. Cancer 1992, 65, 19–26. [Google Scholar] [CrossRef]
- Panella, T.J.; Liu, Y.H.; Huang, A.T.; Teng, C.T. Polymorphism and altered methylation of the lactoferrin gene in normal leukocytes, leukemic cells and breast cancer. Cancer Res. 1991, 51, 3037–3043. [Google Scholar]
- Penco, S.; Caligo, M.A.; Cipollini, G.; Bevilacqua, G.; Garré, C. Lactoferrin expression in human breast cancer. Cancer Biochem. Biophys. 1999, 17, 163–178. [Google Scholar] [PubMed]
- Teng, C.; Gladwell, W.; Raphiou, I.; Liu, E. Methylation and expression of the lactoferrin gene in human tissues and cancer cells. Biometals 2004, 17, 317–323. [Google Scholar] [CrossRef]
- Benaïssa, M.; Peyrat, J.P.; Hornez, L.; Mariller, C.; Mazurier, J.; Pierce, A. Expression and prognostic value of lactoferrin mRNA isoforms in human breast cancer. Int. J. Cancer 2005, 114, 299–306. [Google Scholar] [CrossRef]
- Siebert, P.D.; Huang, B.C. Identification of an alternative form of human lactoferrin mRNA that is expressed differentially in normal tissues and tumor-derived cell lines. Proc. Natl. Acad. Sci. USA. 1997, 94, 2198–2203. [Google Scholar] [CrossRef]
- Damiens, E.; El Yazidi, I.; Mazurier, J.; Duthille, I.; Spik, G.; Boilly-Marer, Y. Lactoferrin inhibits G1cyclin-dependent kinases during growth arrest of human breast carcinoma. J. Cell. Biochem. 1999, 74, 486–498. [Google Scholar] [CrossRef]
- Matsuda, Y.; Saoo, K.; Hosokawa, K.; Yamakawa, K.; Yokohira, M.; Zeng, Y.; Takeuchi, H.; Imaida, K. Post-initiation chemopreventive effects of dietary bovin lactoferrin on 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanoneinduced lung tumorigenesis in female A/J mice. Cancer Lett. 2007, 246, 41–46. [Google Scholar] [CrossRef]
- Xiao, Y.; Monitto, C.L.; Minhas, K.M.; Sidransky, D. Lactoferrin down-regulates G1 cyclin-dependent kinases during growth arrest of head and neck cancer cells. Clin. Cancer Res. 2004, 10, 8683–8686. [Google Scholar] [CrossRef]
- Iglesias-Figueroa, B.F.; Siqueiros-Cendón1, T.S.; Gutierrez, D.A.; Aguilera, R.J.; Espinoza-Sánchez, E.A.; Arévalo-Gallegos, S.; Varela-Ramirez, A.; Rascón-Cruz, Q. Recombinant human lactoferrin induces apoptosis, disruption of F-actin structure and cell cycle arrest with selective cytotoxicity on human triple negative breast cancer cells. Apoptosis 2019, 24, 562–577. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.M.; Pyo, C.W.; Kim, Y.; Choi, S.Y. Neutrophil lactoferrin upregulates the human p53 gene through induction of NF-kappaB activation cascade. Oncogene 2004, 23, 8282–8291. [Google Scholar] [CrossRef]
- Fujita, K.; Matsuda, E.; Sekine, K.; Iigo, M.; Tsuda, H. Lactoferrin enhances Fas expression and apoptosis in the colon mucosaof azoxymethane-treated rats. Carcinogenesis 2004, 25, 1961–1966. [Google Scholar] [CrossRef]
- Dinayadura, R. To Study the Apoptotic Pathways Activated by Selenium Saturated Bovine Lactoferrin (Se-bLF) Treatment for Colon Cancer. Ph.D Thesis, School of Life and Environmental Sciences, Deakin University, Melbourne, VIC, Australia, 2009; p. 93. [Google Scholar]
- Norrby, K.; Mattsby-Baltzer, I.; Innocenti, M.; Tuneberg, S. Orally administered bovine lactoferrin systemically inhibits VEGF(165)-mediated angiogenesis in the rat. Int. J. Cancer 2001, 91, 236–240. [Google Scholar] [CrossRef]
- Shimamura, M.; Yamamoto, Y.; Ashino, H.; Oikawa, T.; Hazato, T.; Tsuda, H.; Iigo, M. Bovine lactoferrin inhibits tumor-induced angiogenesis. Int. J. Cancer 2004, 111, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, H.; Sekine, K.; Nakamura, J.; Ushida, Y.; Kuhara, T.; Takasuka, N.; Kim, D.J.; Asamoto, M.; Baba-Toriyama, H.; Moore, M.A.; et al. Inhibition of azoxymethane initiated colon tumor and aberrant crypt foci development by bovine lactoferrin administration in F344 rats. Adv. Exp. Med. Biol. 1998, 443, 273–284. [Google Scholar] [CrossRef]
- Tsuda, H.; Ohshima, Y.; Nomoto, H.; Fujita, K.; Matsuda, E.; Iigo, M.; Takasuka, N.; Moore, M.A. Cancer prevention by natural compounds. Drug Metab. Pharmacokinet. 2004, 19, 245–263. [Google Scholar] [CrossRef]
- Ward, P.P.; Paz, E.; Conneely, O.M. Multifunctional roles of lactoferrin: A critical overview. Cell. Mol. Life Sci. 2005, 62, 2540–2548. [Google Scholar] [CrossRef]
- Fujita, K.; Ohnishi, T.; Sekine, K.; Iigo, M.; Tsuda, H. Down-regulation of 2-amino-3,8-dimethylimidazo(4,5) quinoxaline (MelQx)- induced CYP1A2 expression is associated with bovin lactoferrin inhibition of MelQx-induced liver and colon carcinogenesis in rats. Jpn. J. Cancer Res. 2002, 93, 616–625. [Google Scholar] [CrossRef]
- Kozu, T.; Iinuma, G.; Ohashi, Y.; Saito, Y.; Akasu, T.; Saito, D.; Alexander, D.B.; Iigo, M.; Kakizoe, T.; Tsuda, H. Effect of orally administered bovine lactoferrin on the growth of adenomatous colorectal polyps in a randomized, placebo-controlled clinical trial. Cancer Prev. Res. (Phila) 2009, 2, 975–983. [Google Scholar] [CrossRef]
- Iigo, M.; Kuhara, T.; Ushida, Y.; Sekine, K.; Moore, M.A.; Tsuda, H. Inhibitory effects of bovine lactoferrin on colon carcinoma 26 lung metastasis in mice. Clin. Exp. Metastasis 1999, 17, 35–40. [Google Scholar] [CrossRef]
- Bezault, J.; Bhimani, R.; Wiprovnick, J.; Furmanski, P. Human lactoferrin inhibits growth of solid tumors and development of experimental metastases in mice. Cancer Res. 1994, 54, 2310–2312. [Google Scholar]
- Fillebeen, C.; Descamps, L.; Dehouck, M.P.; Fenart, L.; Benaïssa, M.; Spik, G.; Cecchelli, R.; Pierce, A. Receptor-mediated transcytosis of lactoferrin through the blood-brain barrier. J. Biol. Chem. 1999, 274, 7011–7017. [Google Scholar] [CrossRef] [PubMed]
- Kamemori, N.; Takeuchi, T.; Sugiyama, A.; Miyabayashi, M.; Kitagawa, H.; Shimizu, H.; Ando, K.; Harada, E. Trans-endothelial and trans-epithelial transfer of lactoferrin into the brain through BBB and BCSFB in adult rats. J. Vet. Med. Sci. 2008, 70, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Ahsan, S.M.; Kumar, J.M.; Kondapi, A.K.; Rao, N.M. Overcoming blood brain barrier with a dual purpose Temozolomide loaded Lactoferrin nanoparticles for combating glioma (SERP-17-12433). Sci. Rep. 2017, 7, 6602. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Kondapi, A.K. Lactoferrin nanoparticle mediated targeted delivery of 5-fluorouracil for enhanced therapeutic efficacy. Int. J. Biol. Macromol. 2017, 95, 232–237. [Google Scholar] [CrossRef]
- Dosio, F.; Brusa, P.; Crosasso, P.; Arpicco, S.; Cattel, L. Preparation, characterization and properties in vitro and in vivo of a paclitaxel-albumin conjugate. J. Control. Release 1997, 47, 293–304. [Google Scholar] [CrossRef]
- Iyer, A.K.; Khaled, G.; Fang, J.; Maeda, H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov. Today 2006, 11, 812–818. [Google Scholar] [CrossRef]
- Koukourakis, G.V.; Kouloulias, V.; Zacharias, G.; Papadimitriou, C.; Pantelakos, P.; Maravelis, G.; Fotineas, A.; Beli, I.; Chaldeopoulos, D.; Kouvaris, J. Temozolomide with Radiation Therapy in Hig h Grade Brain Gliomas: Pharmaceuticals Considerations and Efficacy; A Review Article. Molecules 2009, 14, 1561–1577. [Google Scholar] [CrossRef]
- Yukalchuk, D.Y.; Ponomarenko, D.M.; Snetkov, Y.V.; Yukalchuk, T.N.; Shevchuk, A.V.; Demchenkova, M.V.; Shelekhov, A.V. Use of Parenteral Temodal® (Temozolomide) in Therapy of Glioblastoma. Effective Pharmacotherapy. Oncol. Hematol. Radiol. 2016, 8, 46–50. [Google Scholar]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Hakansson, A.; Zhivotovsky, B.; Orrenius, S.; Sabharwal, H.; Svanborg, C. Apoptosis induced by a human milk protein. Proc. Natl. Acad. Sci. USA 1995, 92, 8064–8068. [Google Scholar] [CrossRef]
- Svensson, M.; Hakansson, A.; Mossberg, A.K.; Linse, S.; Svanborg, C. Conversion of alpha-lactalbumin to a protein inducing apoptosis. Proc. Natl. Acad. Sci. USA 2000, 97, 4221–4226. [Google Scholar] [CrossRef]
- Hakansson, A.; Svensson, M.; Mossberg, A.K.; Sabharwal, H.; Linse, S.; Lazou, I.; Lonnerdal, B.; Svanborg, C. A folding variant of alpha-lactalbumin with bactericidal activity against Streptococcus pneumoniae. Mol Microbiol. 2000, 35, 589–600. [Google Scholar] [CrossRef]
- Fang, B.; Zhang, M.; Tian, M.; Ren, F.Z. Self-assembled β-lactoglobulin-oleic acid and β-lactoglobulin-linoleic acid complexes with antitumor activities. J. Dairy Sci. 2015, 98, 2898–2907. [Google Scholar] [CrossRef]
- Vukojević, V.; Bowen, A.M.; Wilhelm, K.; Ming, Y.; Ce, Z.; Schleucher, J.; Hore, P.J.; Terenius, L.; Morozova-Roche, L.A. Lipoprotein complex of equine lysozyme with oleic acid (ELOA) interactions with the plasma membrane of live cells. Langmuir 2010, 26, 14782–14787. [Google Scholar] [CrossRef]
- Wilhelm, K.; Darinskas, A.; Noppe, W.; Duchardt, E.; Mok, K.H.; Vukojević, V.; Schleucher, J.; Morozova-Roche, L.A. Protein oligomerization induced by oleic acid at the solid-liquid interface--equine lysozyme cytotoxic complexes. FEBS J. 2009, 276, 3975–3989. [Google Scholar] [CrossRef]
- Pettersson, J.; Mossberg, A.K.; Svanborg, C. α-Lactalbumin species variation, HAMLET formation, and tumor cell death. Biochem. Biophys. Res. Commun. 2006, 345, 260–270. [Google Scholar] [CrossRef]
- Mossberg, A.K.; Hun Mok, K.; Morozova-Roche, L.A.; Svanborg, C. Structure and function of human α-lactalbumin made lethal to tumor cells (HAMLET)-type complexes. FEBS J. 2010, 277, 4614–4625. [Google Scholar] [CrossRef]
- Spolaore, B.; Pinato, O.; Canton, M.; Zambonin, M.; Polverino de Laureto, P.; Fontana, A. α-Lactalbumin forms with oleic acid a high molecular weight complex displaying cytotoxic activity. Biochemistry 2010, 49, 8658–8667. [Google Scholar] [CrossRef]
- Liskova, K.; Kelly, A.L.; O’Brien, N.; Brodkorb, A. Effect of denaturation of alpha-lactalbumin on the formation of BAMLET (bovine alpha-lactalbumin made lethal to tumor cells). J. Agric. Food Chem. 2010, 58, 4421–4427. [Google Scholar] [CrossRef]
- Tolin, S.; De Franceschi, G.; Spolaore, B.; Frare, E.; Canton, M.; Polverino de Laureto, P.; Fontana, A. The oleic acid complexes of proteolytic fragments of alpha-lactalbumin display apoptotic activity. FEBS J. 2010, 277, 163–173. [Google Scholar] [CrossRef]
- Atri, M.S.; Saboury, A.A.; Moosavi-Movahedi, A.A.; Goliaei, B.; Sefidbakht, Y.; Alijanvand, H.H.; Sharifzadeh, A.; Niasari-Naslaji, A. Structure and stability analysis of cytotoxic complex of camel a-lactalbumin and unsaturated fatty acids produced at high temperature. J. Biomol. Struct. Dyn. 2011, 28, 919–928. [Google Scholar] [CrossRef]
- Brinkmann, C.R.; Heegaard, C.W.; Petersen, T.E.; Jensenius, J.C.; Thiel, S. The toxicity of bovine α-lactalbumin made lethal to tumor cells is highly dependent on oleic acid and induces killing in cancer cell lines and noncancer-derived primary cells. FEBS J. 2011, 278, 1955–1967. [Google Scholar] [CrossRef]
- Brinkmann, C.R.; Thiel, S.; Larsen, M.K.; Petersen, T.E.; Jensenius, J.C.; Heegaard, C.W. Preparation and comparison of cytotoxic complexes formed between oleic acid and either bovine or human a-lactalbumin. J. Dairy Res. 2011, 94, 2159–2170. [Google Scholar] [CrossRef]
- Lebedev, D.V.; Zabrodskaya, Y.A.; Pipich, V.; Kuklin, A.I.; Ramsay, E.; Sokolov, A.V.; Elizarova, A.Y.; Shaldzhyan, A.; Grudinina, N.A.; Pantina, R.A.; et al. Effect of alpha-lactalbumin and lactoferrin oleic acid complexes on chromatin structural organization. Biochem. Biophys. Res. Commun. 2019, 520, 136–139. [Google Scholar] [CrossRef]
- Martins Lima, T.; Cury-Boaventura, M.F.; Giannocco, G.; Nunes, M.T.; Curi, R. Comparative toxicity of fatty acids on a macrophage cell line (J774). Clin. Sci. (Lond.) 2006, 111, 307–317. [Google Scholar] [CrossRef]
- Andrade, L.N.; de Lima, T.M.; Curi, R.; Castrucci, A.M. Toxicity of fatty acids on murine and human melanoma cell lines. Toxicol. Vitro 2005, 19, 553–560. [Google Scholar] [CrossRef]
- Cury-Boaventura, M.F.; Gorjao, R.; de Lima, T.M.; Newsholme, P.; Curi, R. Comparative toxicity of oleic and linoleic acid on human lymphocytes. Life Sci. 2006, 78, 1448–1456. [Google Scholar] [CrossRef]
- Liu, J.; Shimizu, K.; Kondo, R. Anti-Androgenic Activity of Fatty Acids. Chem. Biodivers. 2009, 6, 503–512. [Google Scholar] [CrossRef]
- Moon, H.S.; Batirel, S.; Mantzoros, C.S. Alpha linolenic acid and oleic acid additively down-regulate malignant potential and positively cross-regulate AMPK/S6 axis in OE19 and OE33 esophageal cancer cells. Metabolism 2014, 63, 1447–1454. [Google Scholar] [CrossRef]
- Menendez, J.A.; Vellon, L.; Colomer, R.; Lupu, R. Oleic acid the main monounsaturated fatty acid of olive oil, suppresses Her-2/neu (erbB-2)expression and synergistically enhances the growth inhibitory effects of trastuzumab (HerceptinTM) in breast cancer cells. Ann. Oncol. 2005, 16, 359–371. [Google Scholar] [CrossRef]
- Laudadio, J.; Quigley, D.I.; Tubbs, R.; Wolff, D.J. HER2 testing: A review of detection methodologies and their clinical performance. Expert. Rev. Mol. Diagn. 2007, 7, 53–64. [Google Scholar] [CrossRef]
- Puertollano, M.A.; de Pablo, M.A.; Alvarez de Cienfuegos, G. Polyunsaturated fatty acids induce cell death in YAC-1 lymphoma by a caspase-3-independent mechanism. Anticancer Res. 2003, 23, 3905–3910. [Google Scholar]
- Piegari, M.; Soria, E.A.; Eynard, A.R.; Valentich, M.A. Delay of lung adenocarcinoma (LAC-1) development in mice by dietary oleic acid. Nutr. Cancer 2017, 69, 1069–1074. [Google Scholar] [CrossRef]
- Semak, I.; Budzevich, A.; Maliushkova, E.; Kuzniatsova, V.; Popkov, N.; Zalutsky, I.; Ivashkevich, O. Development of dairy herd of transgenic goats as biofactory for large-scale production of biologically active recombinant human lactoferrin. Transgenic Res. 2019, 28, 465–478. [Google Scholar] [CrossRef]
- Kelly, S.M.; Price, N.C. The Use of Circular Dichroism in the Investigation of Protein Structure and Function. Curr. Protein Pept. Sci. 2000, 1, 349–384. [Google Scholar] [CrossRef]
- Li, H.; Yao, Q.; Min, L.; Huang, S.; Wu, H.; Yang, H.; Fan, L.; Wang, J.; Zheng, N. The Combination of Two Bioactive Constituents, Lactoferrin and Linolenic Acid, Inhibits Mouse Xenograft Esophageal Tumor Growth by Downregulating Lithocholyltaurine and Inhibiting the JAK2/STAT3-Related Pathway. ACS Omega 2020, 5, 20755–20764. [Google Scholar] [CrossRef]




| Fatty Acid | FA/LF (Mole/Mole) |
|---|---|
| Linolenic | 4.7 |
| Arachidonic | 5.5 |
| Linoleic | 5.8 |
| Oleic | 8.4 |
| LF (mg) | FA/LF (Mole/Mole) | |
|---|---|---|
| Before loading | 4.0 ± 0.2 | 7.8 ± 0.2 |
| In eluted fraction | 3.8 ± 0.2 | 7.6 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elizarova, A.; Sokolov, A.; Kostevich, V.; Kisseleva, E.; Zelenskiy, E.; Zakharova, E.; Panasenko, O.; Budevich, A.; Semak, I.; Egorov, V.; et al. Interaction of Lactoferrin with Unsaturated Fatty Acids: In Vitro and In Vivo Study of Human Lactoferrin/Oleic Acid Complex Cytotoxicity. Materials 2021, 14, 1602. https://doi.org/10.3390/ma14071602
Elizarova A, Sokolov A, Kostevich V, Kisseleva E, Zelenskiy E, Zakharova E, Panasenko O, Budevich A, Semak I, Egorov V, et al. Interaction of Lactoferrin with Unsaturated Fatty Acids: In Vitro and In Vivo Study of Human Lactoferrin/Oleic Acid Complex Cytotoxicity. Materials. 2021; 14(7):1602. https://doi.org/10.3390/ma14071602
Chicago/Turabian StyleElizarova, Anna, Alexey Sokolov, Valeria Kostevich, Ekaterina Kisseleva, Evgeny Zelenskiy, Elena Zakharova, Oleg Panasenko, Alexander Budevich, Igor Semak, Vladimir Egorov, and et al. 2021. "Interaction of Lactoferrin with Unsaturated Fatty Acids: In Vitro and In Vivo Study of Human Lactoferrin/Oleic Acid Complex Cytotoxicity" Materials 14, no. 7: 1602. https://doi.org/10.3390/ma14071602
APA StyleElizarova, A., Sokolov, A., Kostevich, V., Kisseleva, E., Zelenskiy, E., Zakharova, E., Panasenko, O., Budevich, A., Semak, I., Egorov, V., Pontarollo, G., De Filippis, V., & Vasilyev, V. (2021). Interaction of Lactoferrin with Unsaturated Fatty Acids: In Vitro and In Vivo Study of Human Lactoferrin/Oleic Acid Complex Cytotoxicity. Materials, 14(7), 1602. https://doi.org/10.3390/ma14071602









