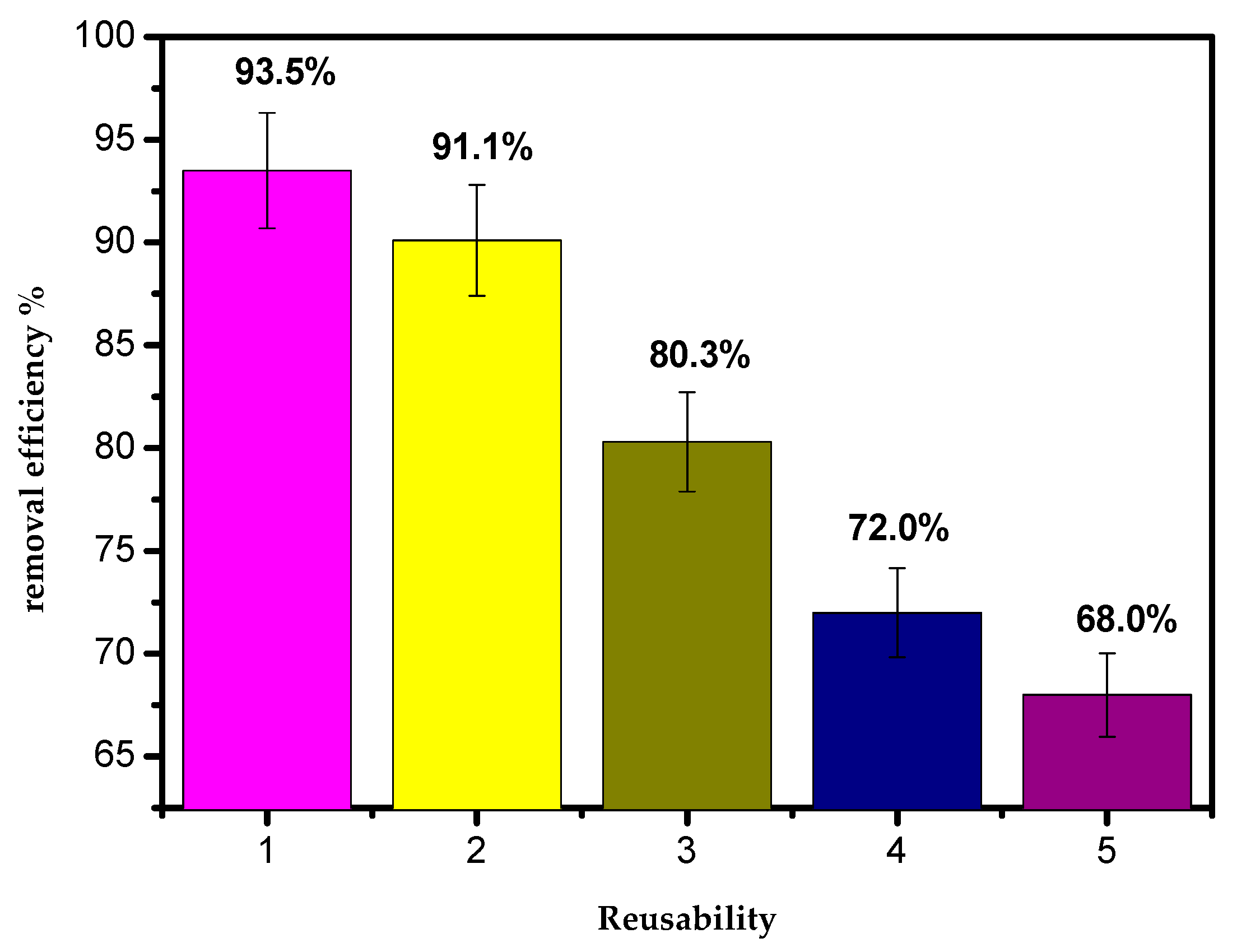Abstract
The efficiency of Azadirachta indica (neem leaves) on the removal of Pb(II) ions by adsorption from aqueous solution was investigated in this study. The efficiency of these leaves (without chemical or thermal treatment) for the adsorption of Pb(II) ions has not previously been reported. Batch experiments were performed to study the effect of the particle size, pH, adsorbent dose, contact time, initial Pb(II) ion concentration, and temperature. The maximum removal of 93.5% was achieved from an original Pb(II) ion solution concentration of 50 mg/L after 40 min, at pH 7, with 0.60 g of an adsorbent dose. The maximum adsorption capacity recorded was 39.7 mg/g. The adsorption process was also studied by examining Langmuir, Freundlich, Temkin isotherm, and Dubinin–Radushkevich (D-R) isotherm models. The results revealed that the adsorption system follows the pseudo-second-order model and fitted the Freundlich model. Several thermodynamic factors, namely, the standard free energy (∆G°), enthalpy (∆H°), and entropy (∆S°) changes, were also calculated. The results demonstrated that the adsorption is a spontaneous, physical, and exothermic process. The surface area, pore size, and volume of adsorbent particles were measured and presented using a surface area analyzer (BET); the morphology was scanned and presented with the scanning electron microscope technique (SEM); and the functional groups were investigated using μ-FTIR.
1. Introduction
Industrial activities have significantly contributed to environmental pollution. Toxic metals are one of the major pollutants causing serious damage to the environment, in addition to being a documented source of many diseases [1]. The heavy metal pollutants in wastewater are highly toxic, hazardous to plants and animals, and result in a shortage of clean water [2,3]. Lead is among these toxic elements, and has acute and chronic health effects on humans [4]. Lead pollution affected nearly 18–22 million people in 2010, motivating an investigation of water purification processes [5].
With respect to metal removal, wastewater purification includes various methods, such as biosorption, adsorption, electrochemical treatments, and oxidation [6,7]. Heavy metal biosorption is one of the favorable fields of remedy due to the low cost and easy accessibility of raw materials, as well as their eco-friendly nature [8]. Other benefits of biosorption are the selectivity for particular metals; low operational time; and most importantly, the absence of chemical waste generation [9]. Different types of cheap plant materials have been investigated as prospective biosorbents for heavy metals [10].
Biosorption is known to be more effective in removing lead metal contaminants from aqueous solutions, where the efficiency rate in batch adsorption tests is higher than 90% [11]. However, the success of biosorption in the removal of lead metal from wastewater depends on several experimental parameters, such as the temperature, pH, and original solution metal concentration [12,13]. The biosorption removal of Pb(II) ions using natural biosorbent from cactus cladodes revealed that the particle size, mass of biosorbent, and contact time are additional factors influencing the operation success [14]. The optimum biosorption of Pb(II) ions increases with an increase in the biosorbent particle size and dosage [14,15]. Various natural materials have been used for lead removal. These include pumpkin seeds, bamboo, cocoa shells, coconut shell, calotropis roots, peanut shells, Moringa bark, orange bark, cashew nut shells, and activated carbon (maize tassel, periwinkle shells, oil palm, and rice husk) [16,17]. Other natural materials have been reported to be efficient sorbents for various pollutants. Platanus orientalis leaf powder was used as an efficient sorbent for Cu(II) ion removal from aqueous solutions. The maximum adsorption capacity for this material was determined to be 49.94 mg/g [18]. Urtica has been reported as an effective sorbent for the removal of Methylene Blue dye from aqueous solutions, with a maximum adsorption capacity of 101.01 mg/g [19].
The chemical screening of neem leaves showed positive results for steroids, saponins, flavonoids, alkaloids, tannins, and amino acid [20]. Most of these constituents possess electron withdrawing groups which may enhance the adsorption capacity of the neem leaves.
This study aims to evaluate Azadirachta indica leaves’ powder (AILP) as an eco-friendly, efficient, and cheap biosorbent for Pb(II) ion removal.
2. Materials and Methods
2.1. Preparation and Adsorption Processes
Azadirachta indica (neem leaves) samples were collected from Jizan city, south of Saudi Arabia, and washed with deionized and distilled water. Then samples were dried at room temperature. The leaves were ground by a grinder into powder form, before being sieved. A stock solution of 50 mg/L was prepared using Pb(NO3)2 (Sigma-Aldrich, Saint Louis, MissourI, USA, ACS reagent ≥ 99%.)
2.2. Batch Adsorption Experiment
Batch experiments were performed using 250 mL stoppered bottles containing the desired mass at a pH of 5.5 with 50 mL of Pb(II) solution (50 mg/L). The mixture was shaken at 298 K (except when examining the temperature effect) by a temperature-controlled water bath shaker. At the end of the shaking time, the solid adsorbent was isolated from the solution by filtration, and the remaining concentration of Pb(II) ions was then determined using an atomic absorption spectrometer (AAS) (SpectrAA 220, Varian, UK). Parameters influencing the sorption efficiency were investigated by changing one factor and keeping the others constant. The adsorption extent was determined using Equation (1):
where V is the volume of the Pb(II) solution in the bottle, qe is the metal uptake capacity (mg/g), M is the mass of AILP (g), Co is the initial Pb(II) concentration, and Ce is the Pb(II) concentration at equilibrium. The total removal efficiency (R%) was calculated using Equation (2).
The investigated parameters, including the particle size, shaking time, mass of adsorbent, temperature, and solution concentration, were investigated.
2.3. Characterization of AILP
The surface area, pore-volume, and pore size were investigated using a Quanta Chrome NOVA 4200E Surface Area Analyzer. The morphology of the powder was examined using a Jeol 6360 (Japan) scanning electron microscope at an accelerating voltage of 20 kV. The functional groups of the powder before and after adsorption were investigated by μ-FT-IR (Cary 630 FTIR from Agilent, Santa Clara, USA) in the range of 400–4000 cm−1 at a spectral resolution of 8 cm−1.
2.4. Reusability of AILP
AILP was reactivated by vigorous shaking in an excess quantity of de-ionized water for 12 hrs. AILP was separated by filtration and then dried by heating it overnight at 105 °C. The optimal conditions were maintained throughout all reusability experiments.
3. Results
3.1. Characterization of AILP
3.1.1. Surface Area of AILP
The AILP sample exhibited a high surface area of 183 m2/g, enhancing its adsorption capabilities. The results revealed that the average pore diameter is 10.3 nm and the total pore volume is 0.355 cm3/g. Comparative data are given in Table 1. These data demonstrate that the AILP surface area is larger than that of the reported sorbents, indicting a high adsorption capacity.

Table 1.
The surface area, total pore volume, and average pore diameter for various adsorbents.
Table 1.
The surface area, total pore volume, and average pore diameter for various adsorbents.
| Adsorbent | Surface Area (m2/g) | Total Pore Volume (cm3/g) | Average Pore Diameter (nm) | Reference |
|---|---|---|---|---|
| AILP | 183 | 0.335 | 10.3 | This study |
| Date seeds | 124 | 0.469 | 9.8 | [21] |
| P(AN-AA)/AMP composite | 39 | 0.35 | 34.4 | [22] |
| Red clay | 35 | 0.57 | 6.5 | [23] |
| Modified sugarcane | 7 | 0.15 | Not reported | [24] |
3.1.2. IR Spectrum
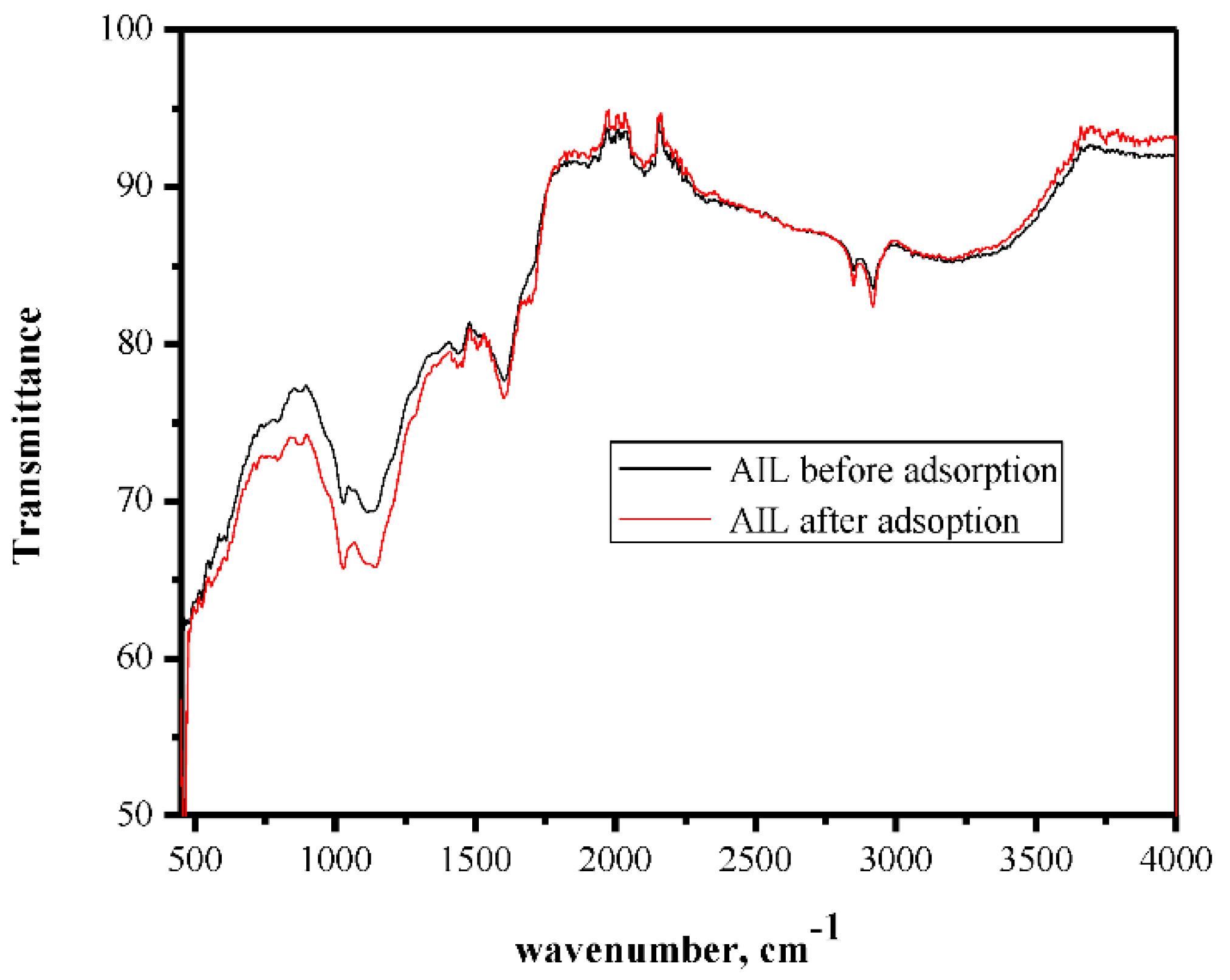
The μ-FT-IR spectrum is used to estimate the contribution of functional groups on the adsorbent surface in the adsorption process. The FT-IR analysis was performed before and after the adsorption process (Figure 1). The results revealed distinguished bands of Pb-O stretching vibrations that appeared at 462 cm−1 and OH stretching of the hydroxide groups. These bands may confirm the adsorption of Pb(II) ions on the adsorbent surface. Other bands appeared at 1298, 1350, and 1723 cm−1, and these bands are probably related to the stretching of -C-N-, -C=N-, and -C=C-, respectively, in polyheterocycles. However, the FT-IR spectra of the adsorbent before and after adsorption were very similar due to the physisorption process.
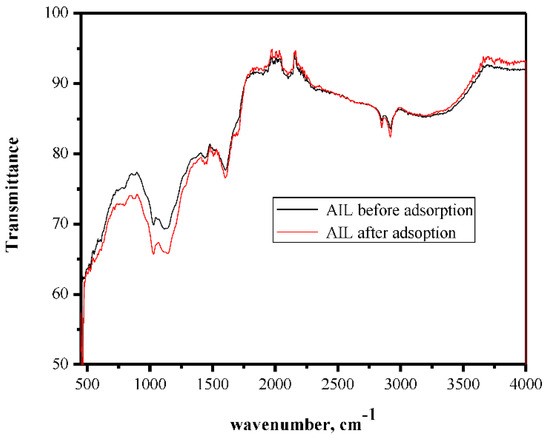
Figure 1.
FT-IR spectrum of Azadirachta indica leaves’ powder (AILP) before and after adsorption.
3.1.3. Scanning Electron Microscope (SEM)
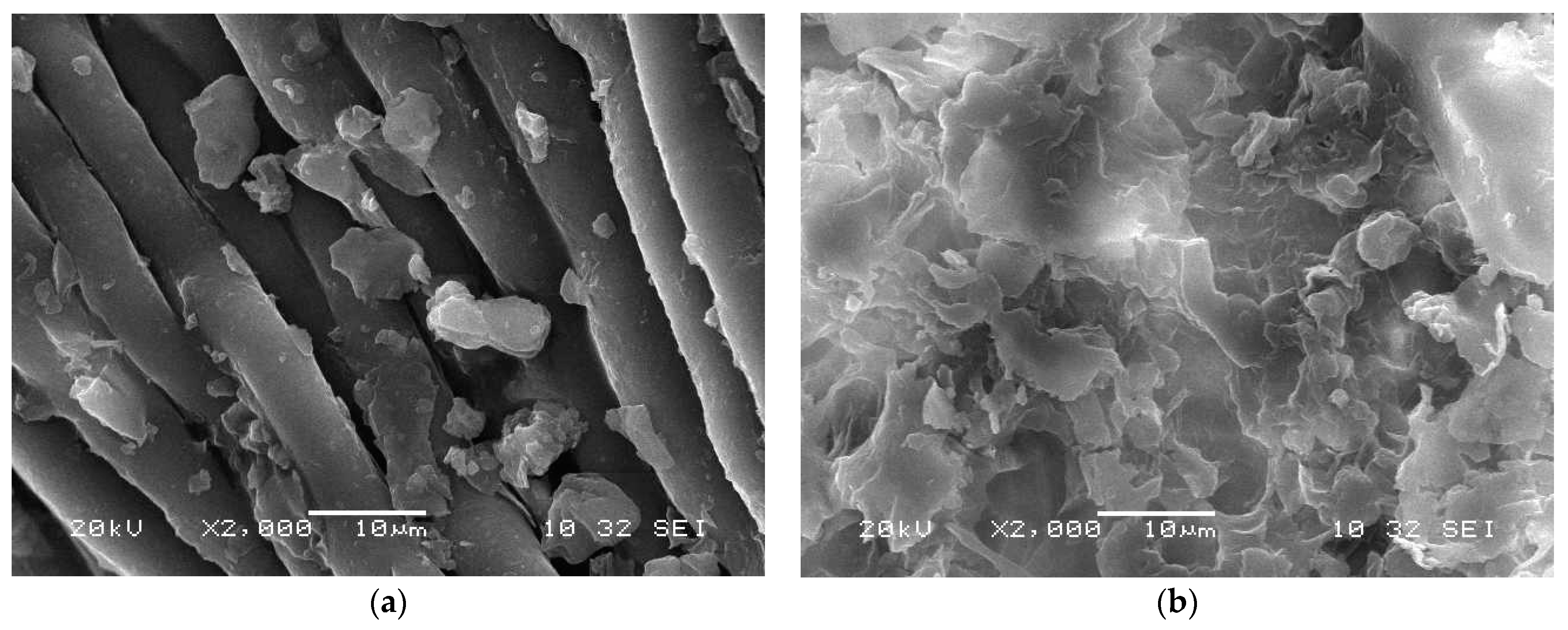
The SEM images of AILP before and after adsorption are presented in Figure 2. The SEM images illustrate differences in the surface morphologies of the AILP surfaces before and after adsorption. The surface of the AILP before adsorption showed micro flakes with an approximate size of 0.5 μm (Figure 2a). Additionally, the analysis of the AILP surface after adsorption revealed differences in the surface structure and the adsorption process led to the formation of many floccules and spots due to the adsorption of Pb(II) ions on the AILP surface, leading to a rough appearance.
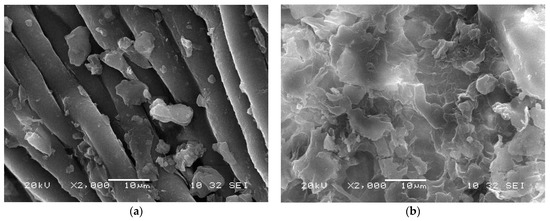
Figure 2.
Scanning Electron Microscope (SEM) images of the AILP surface (a) before and (b) after adsorption.
3.2. Effect of the Adsorbent Particle Size
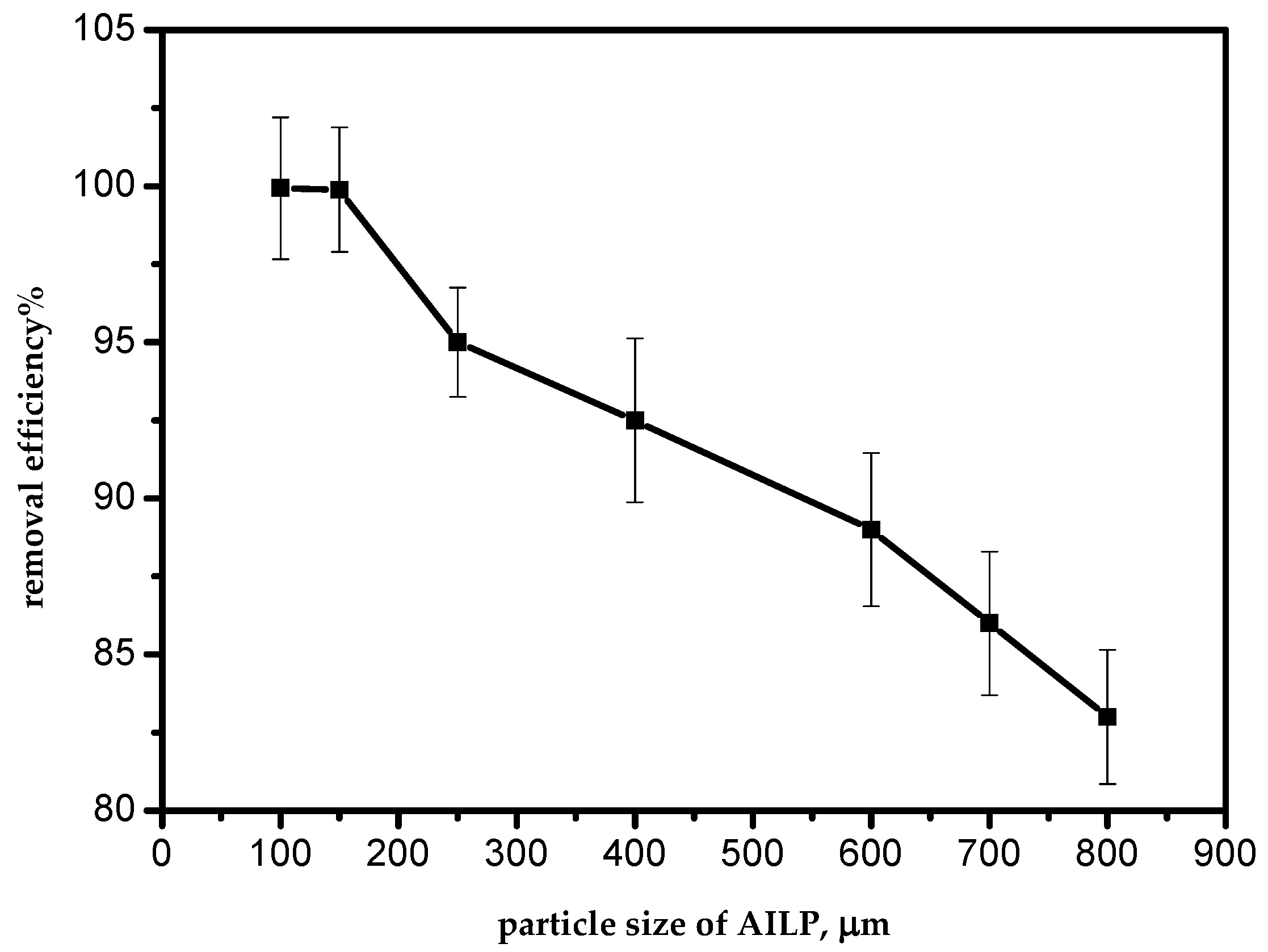
The removal efficiency of Pb(II) ions from aqueous solutions by the AILP increased from 82.9% to 99.1% as the AILP size decreased from 800 to 100 μm, at 298 K, for a 50 mg/L initial Pb(II) ions concentration (Figure 3). With a decrease in AILP particle sizes, the surface area of the AILP increased, so the number of active sites accessible on the AILP surface had a better exposure to the adsorbate. Similar findings have also been reported by other researchers [12].
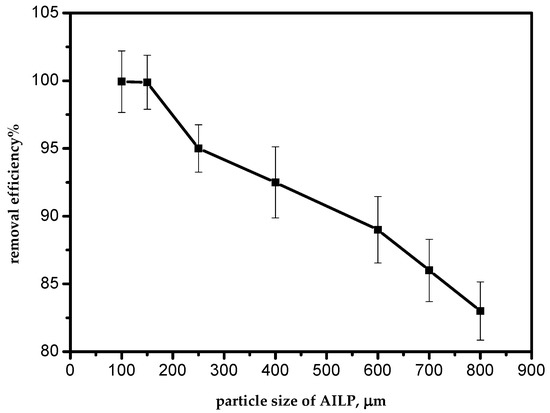
Figure 3.
Effect of the adsorbent particle size.
3.3. Effect of the pH
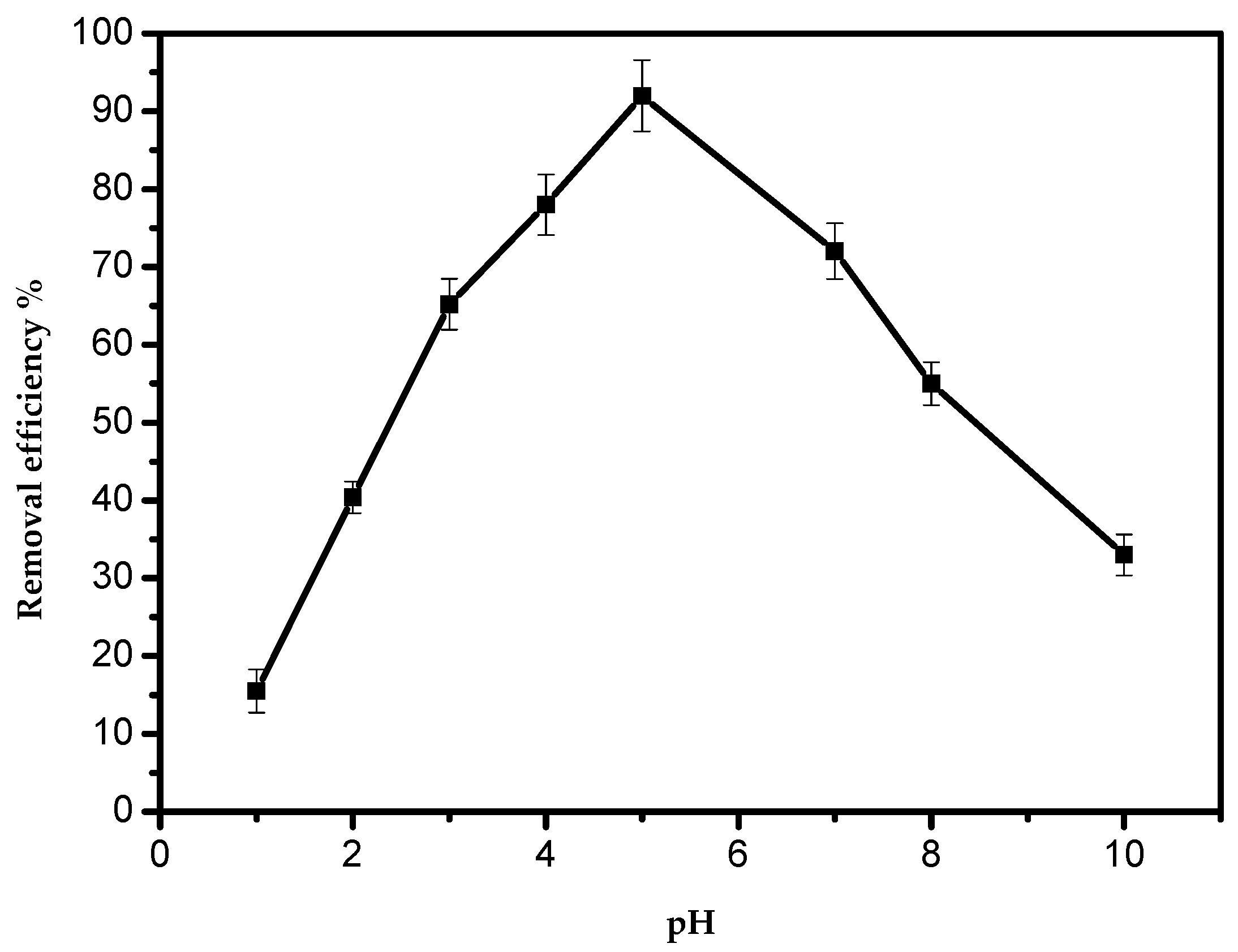
The maximum adsorption was achieved at pH 5.0 (Figure 4). It is presidentially noted that with a pH higher than 5.0, the adsorption activity decreased sharply. However, at pH values lower than 5.0, the adsorption process could be hindered by the existence of a high concentration of positive hydrogen ions, and the adsorption sites became positively charged, exerting a repelling effect on the Pb(II) cations. With an increase in the pH, the negative charge density on AILP increased as a result of deprotonation of the metal-binding sites, hence increasing the metal uptake; such findings are in agreement with previous studies. It is well-known that lead ions start to precipitate above pH 5.0–6.0 in the form of Pb(OH)2, suppressing the adsorption of lead ions [1].
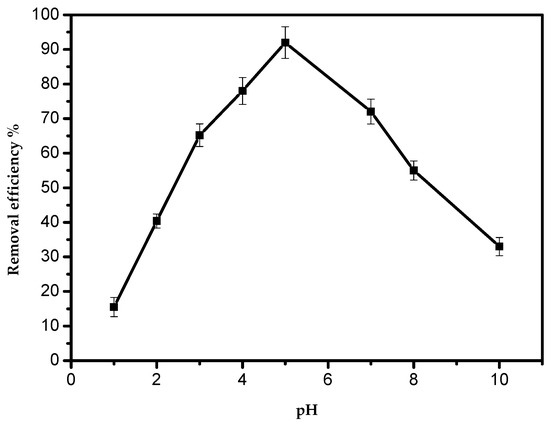
Figure 4.
Effect of the pH on the removal efficiency of Pb(II) ions by AILP.
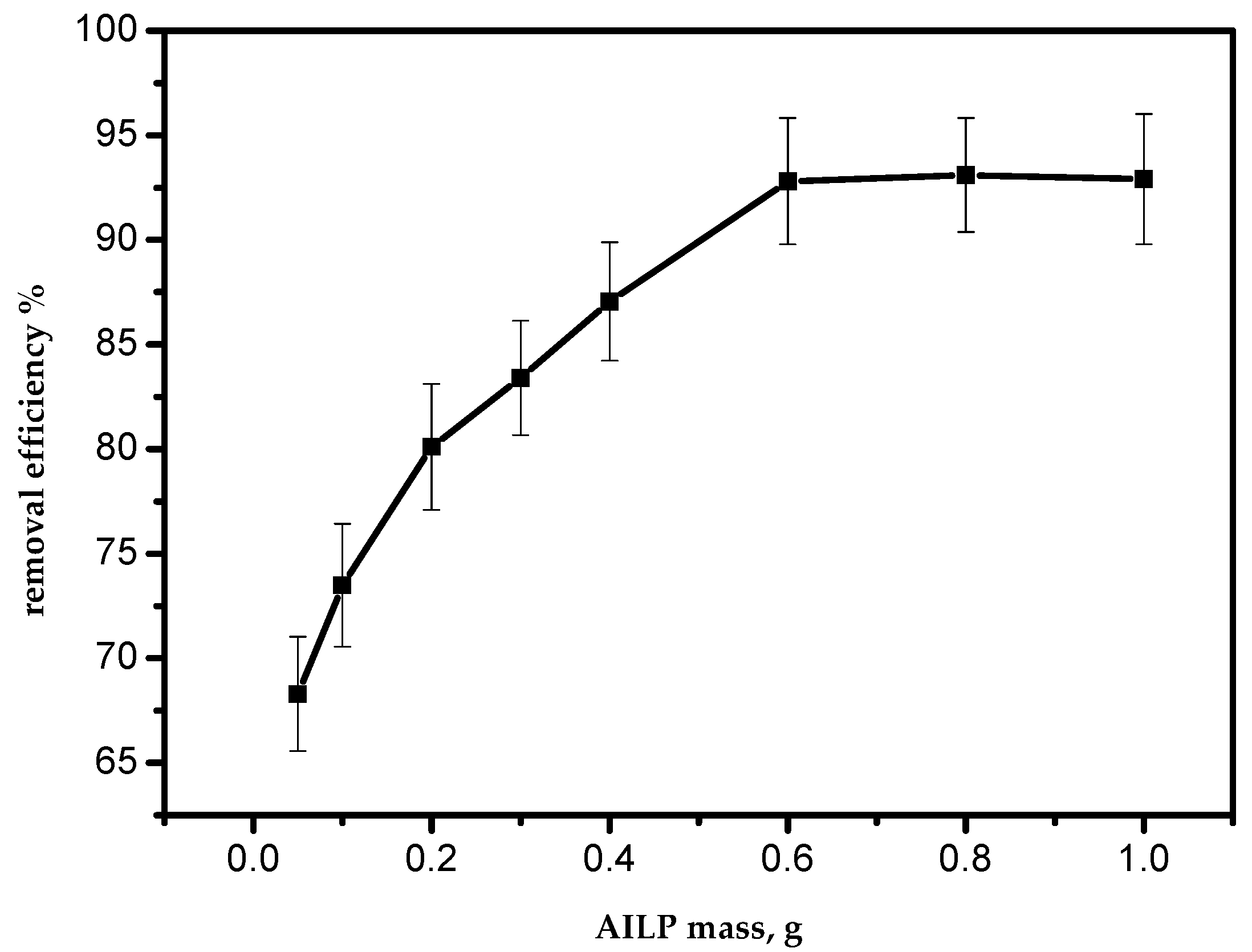
3.4. Effect of the Adsorbent Dosage
To investigate the effect of the sorbent mass on the efficiency of the adsorption process, a series of batch experiments were conducted with various sorbent masses of 0.05, 0.10, 0.20, 0.30, 0.40, 0.60, 0.80, and 1.0 g per 50 mL of aqueous solution of Pb(II) ions. The effect is presented in Figure 5. As the mass of the sorbent increased, the adsorption efficiency also increased. This can be attributed to an increase in the AILP surface area with its mass. The maximum adsorption efficiency for Pb(II) ions was achieved at 0.60 g/50 mL. From the results, it is clear that an increase in the adsorbent mass above 0.6 g had no effect on the adsorption efficiency of Pb(II) ions from the aqueous solution.
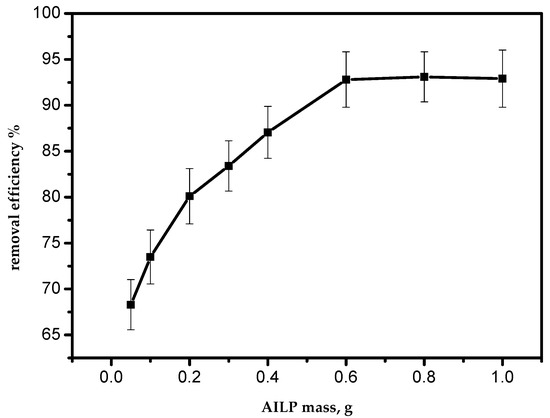
Figure 5.
Effect of the adsorbent mass on the removal efficiency of Pb(II) ions by AILP.
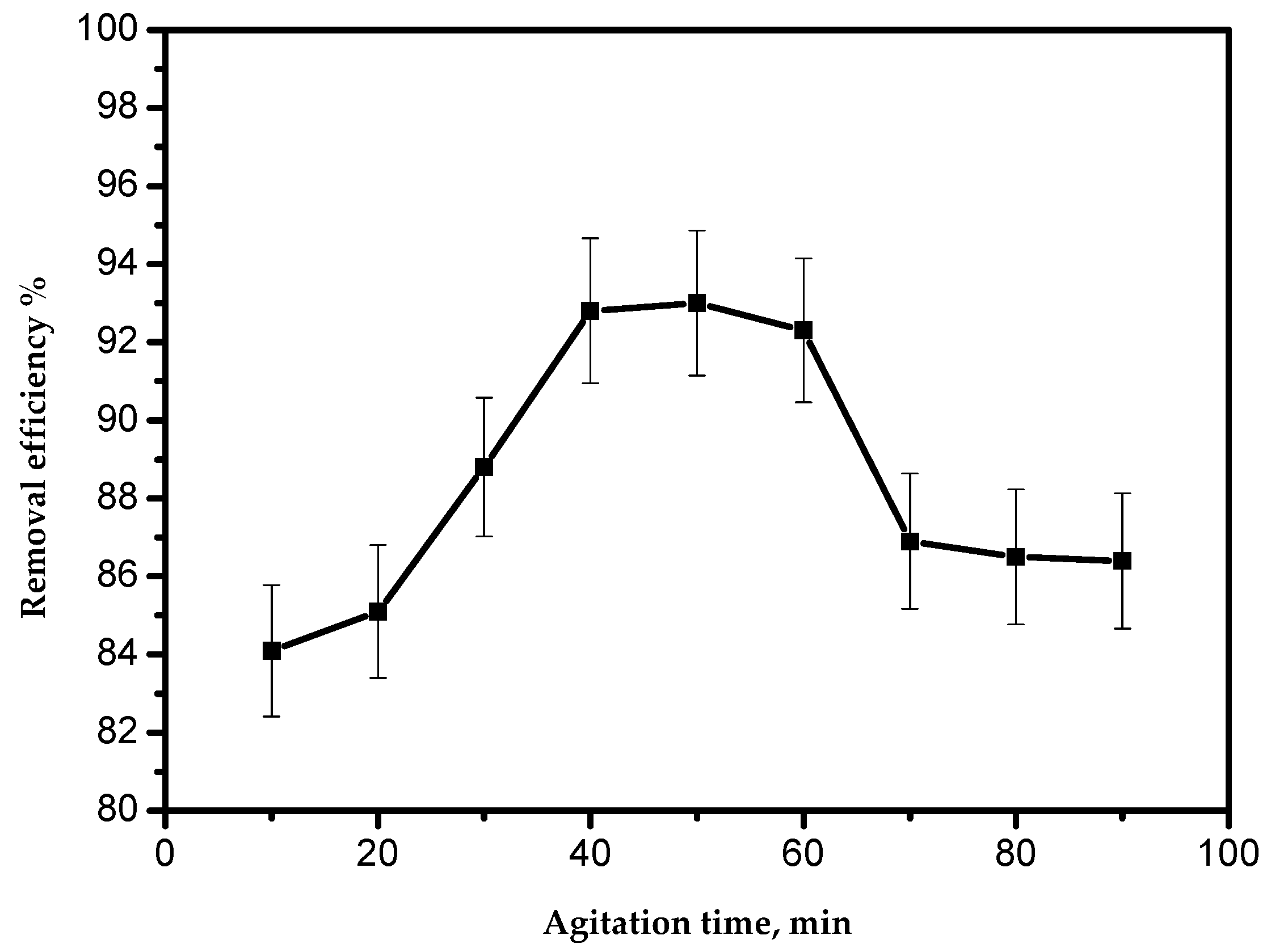
3.5. Effect of the Agitation Time
The effect of the agitation time on the adsorption of Pb(II) ions using the AILP is presented in Figure 6. The results showed that the time of 40 min was sufficient for achieving the optimum adsorption, without any influence from increasing the contact time. As demonstrated in Figure 6, the adsorption process could be divided into three phases. The first phase was significantly fast, representing about 88% removal within 30 min. The second phase showed slower adsorption. The first stage was caused by the large surface area of the AILP. With the progressive occupation of these sites, the process became slower in the second stage due to less adsorption sites being available for Pb(II) ions. The third phase was the desorption of Pb(II) ions from the AILP surface. Some of the adsorbed Pb(II) ions might have leached from the AILP into the solution and this is probably a result of the long agitation time.
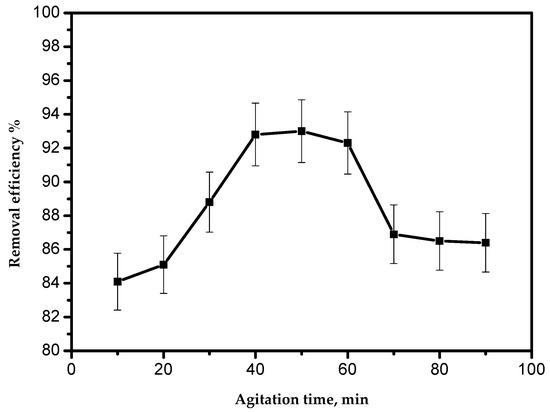
Figure 6.
Effect of the agitation time on the removal efficiency of Pb(II) ions by AILP.
3.6. Adsorption Kinetics
The aim of investigating the adsorption kinetics is to explore the mechanism of adsorption and evaluate the efficiency of the adsorbent. The pseudo-first-order, pseudo-second-order, and intra-particle diffusion kinetic models were used to investigate the obtained data on the adsorption of Pb(II) ions on the AILP.
3.6.1. Pseudo-First-Order Kinetic Model
The equation of this model is given by Equation (3):
where qe is the amount of solute (mg/g) removed at equilibrium, qt is the quantity of solute adsorbed at any time (t), and k is the rate constant. It was found that the data do not fit this model (R2 = 0.0006).
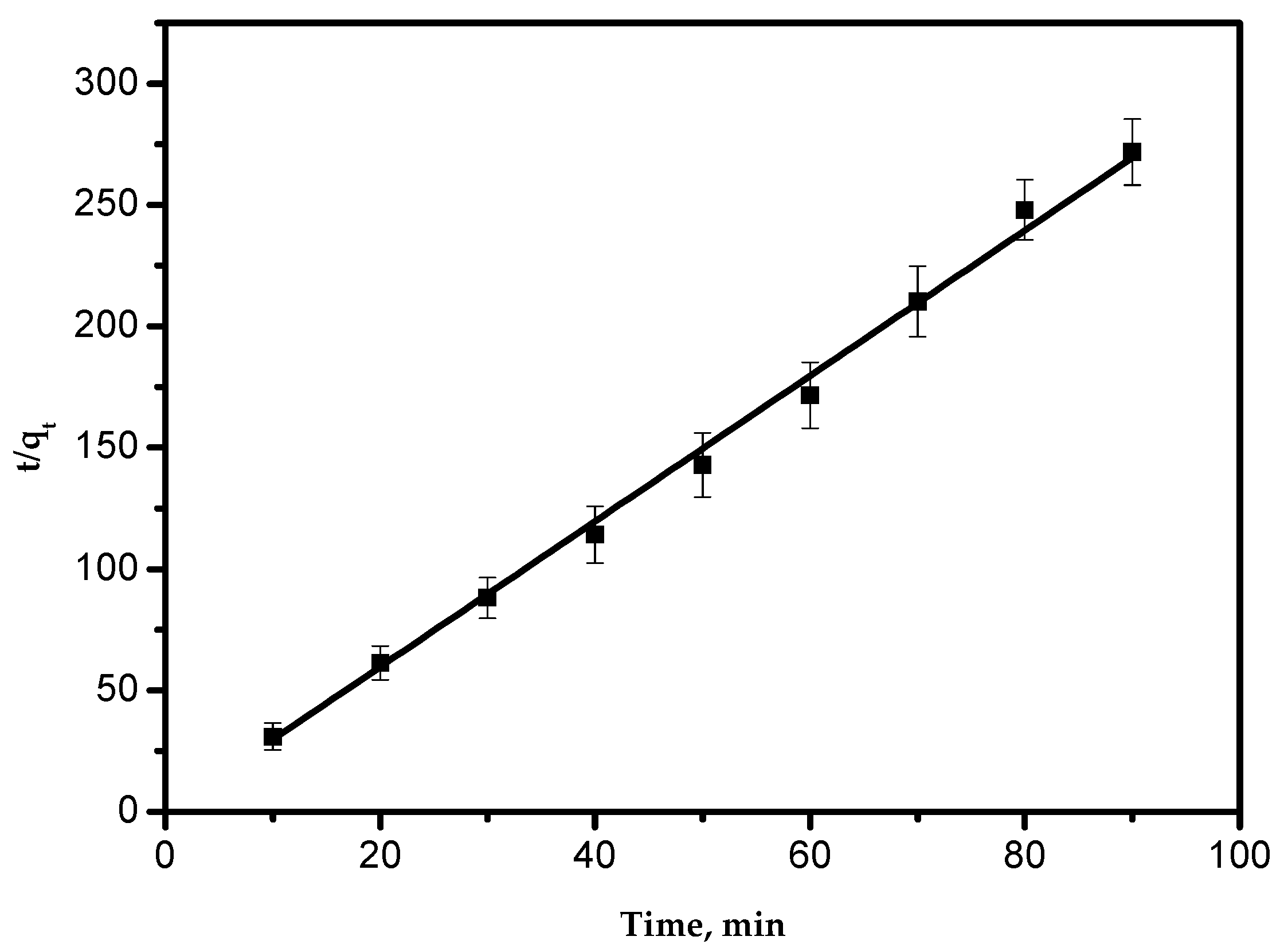
3.6.2. Pseudo-Second-Order Kinetic Model
The equation of this model is
A typical plot of the pseudo-second-order equation is shown in Figure 7. The straight line in the plot proves that the experimental data appropriately obey the pseudo-second-order model. Values of qe and k2 were determined from the intercept and slope of Figure 8. These parameters and the correlation coefficient are presented in Table 2. This agrees with previous results and explanations [1,25]. It can be concluded from previous studies that the adsorption kinetics model was governed by the adsorbent nature.
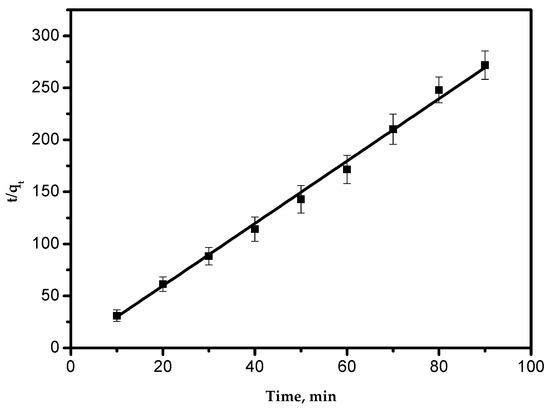
Figure 7.
Pseudo-second-order adsorption kinetics.
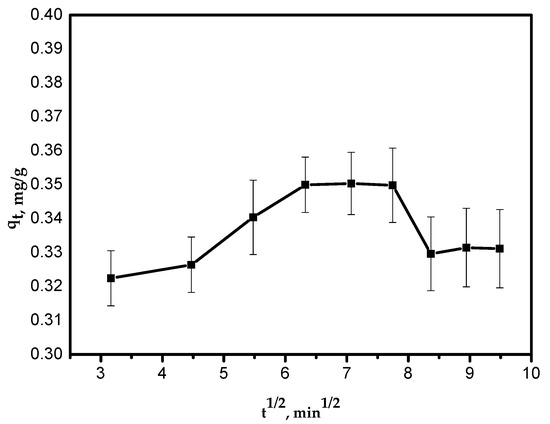
Figure 8.
Intra-particle diffusion kinetic model.

Table 2.
Kinetic parameters of the removal of Pb(II) ions from aqueous solutions by AILP.
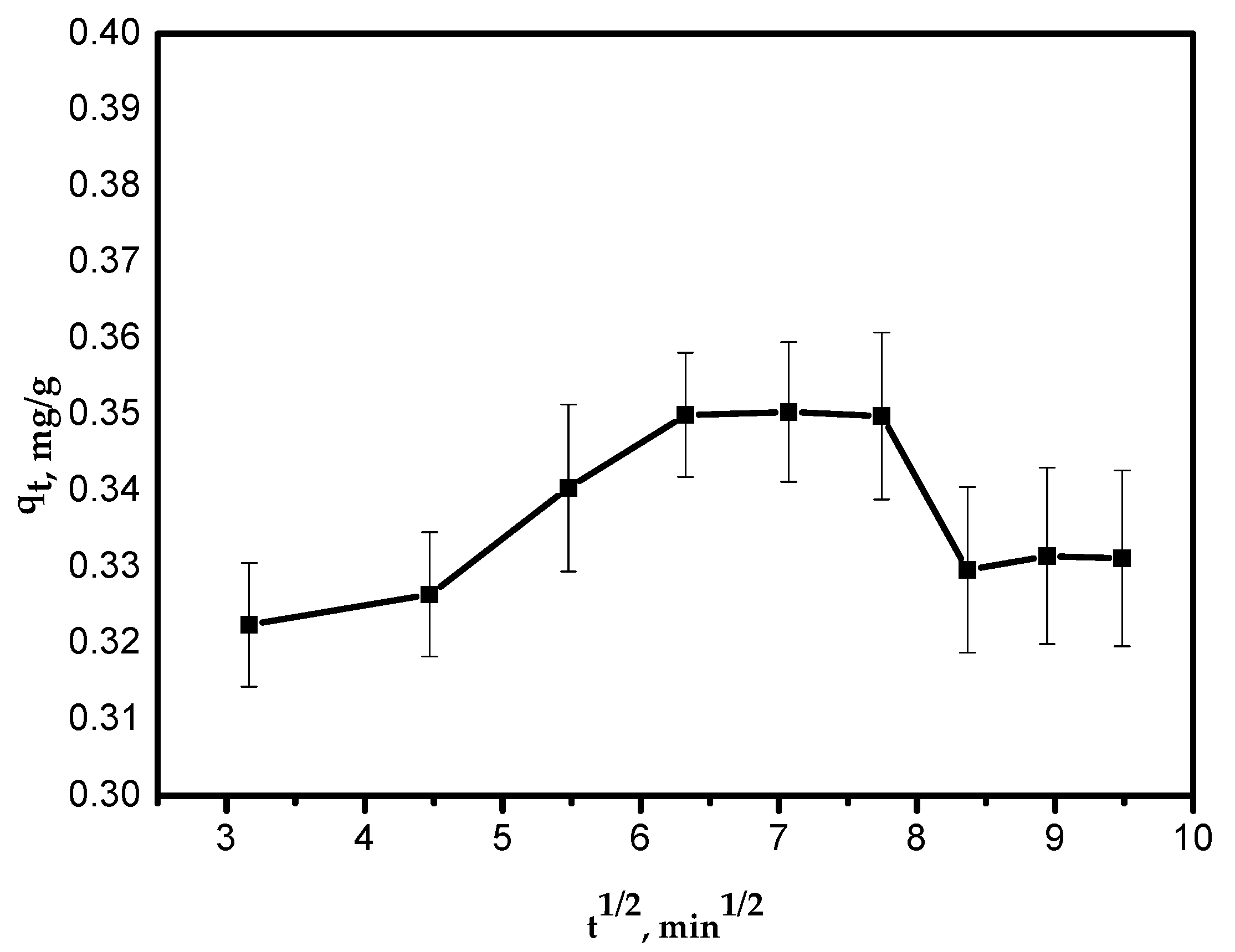
3.6.3. Intra-Particle Diffusion Kinetic Model
This model is shown by Equation (5):
where kid is the intra-particle diffusion rate constant, mg/g/min1/2. The value of (kid) was calculated from Equation (5) and is presented in Table 2. The relationship between qt and t1/2 was not linear over the entire time range. The first curved part of the plot can be attributed to the boundary layer diffusion effects and it represents intra-particle diffusion controlled by the rate constant kid (Figure 8).
3.7. Effect of the Initial Pb(II) Concentration
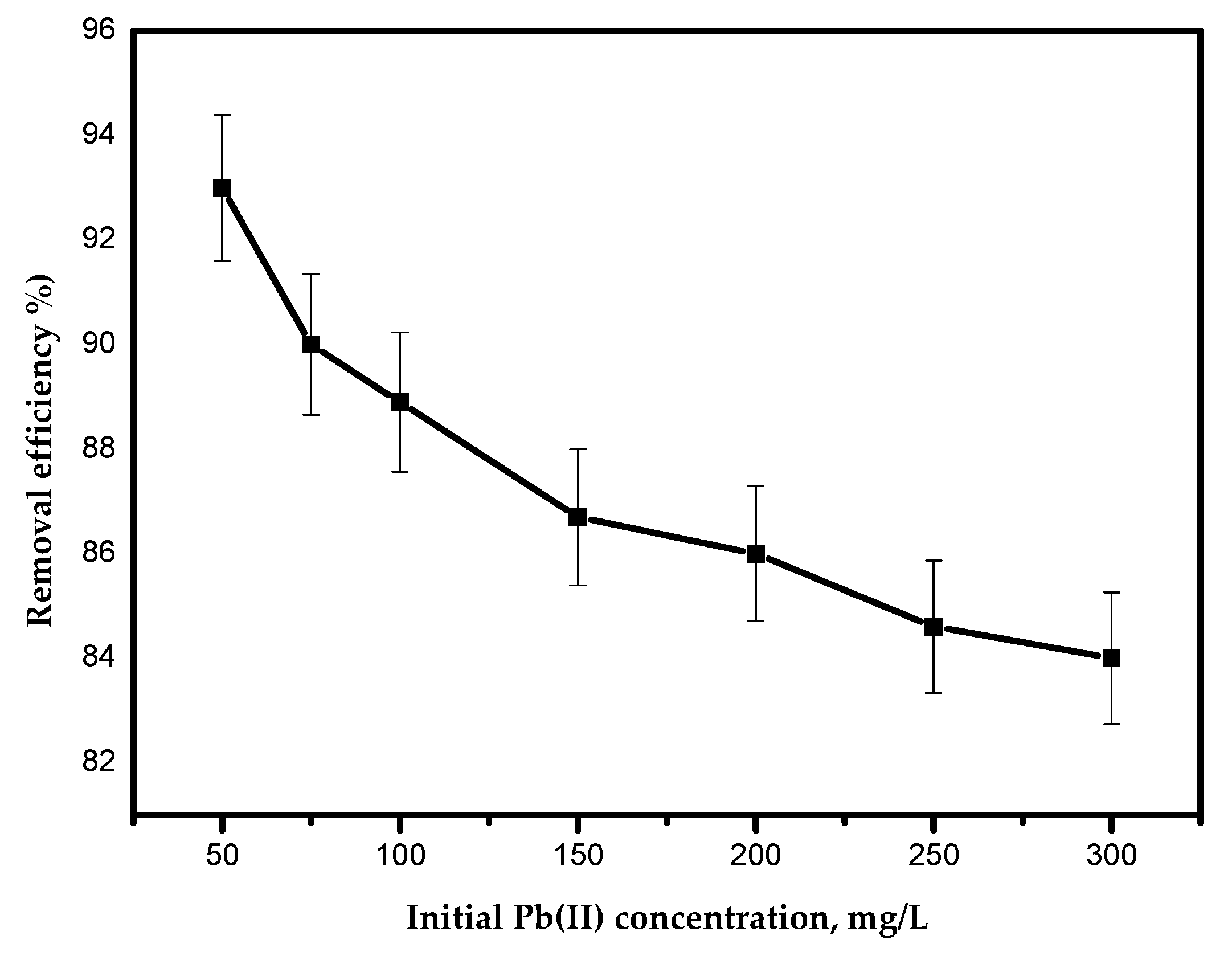
The effect of the initial concentration of Pb(II) ions on the adsorption efficiency is presented in Figure 9, where it is evident that the adsorption efficiency of Pb(II) ions decreased as the initial Pb(II) ion concentration increased. The decrease in adsorption efficiency can be attributed to the deficiency of sufficient adsorption sites for accumulating the large increase of metal ions available in the solution [21].
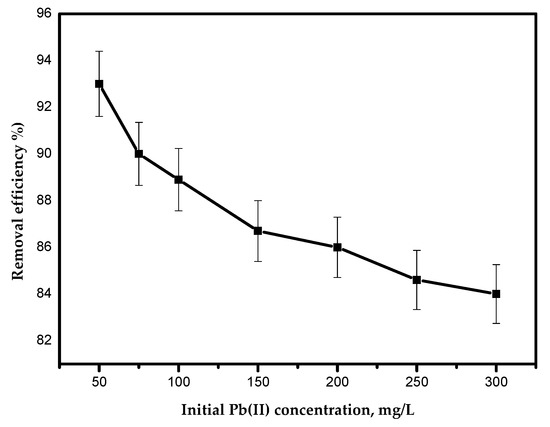
Figure 9.
Effect of the initial concentration of Pb(II) ions on the removal efficiency.
3.8. Adsorption Isotherm
The adsorption isotherm models were investigated to explore the interaction mechanism of Pb(II) ions on the adsorbent sites [1,21]. The adsorption isotherm models were analyzed by specific parameters, whose values express the surface characteristics and affinity of the adsorbent towards heavy metal ion adsorption [21]. Out of numerous isotherm models, four were examined for this study, namely, Freundlich, Langmuir, Temkin, and Dubinin–Radushkevich (D-R) models.
3.8.1. Langmuir Isotherm
Equation (6) presents the Langmuir linear equation:
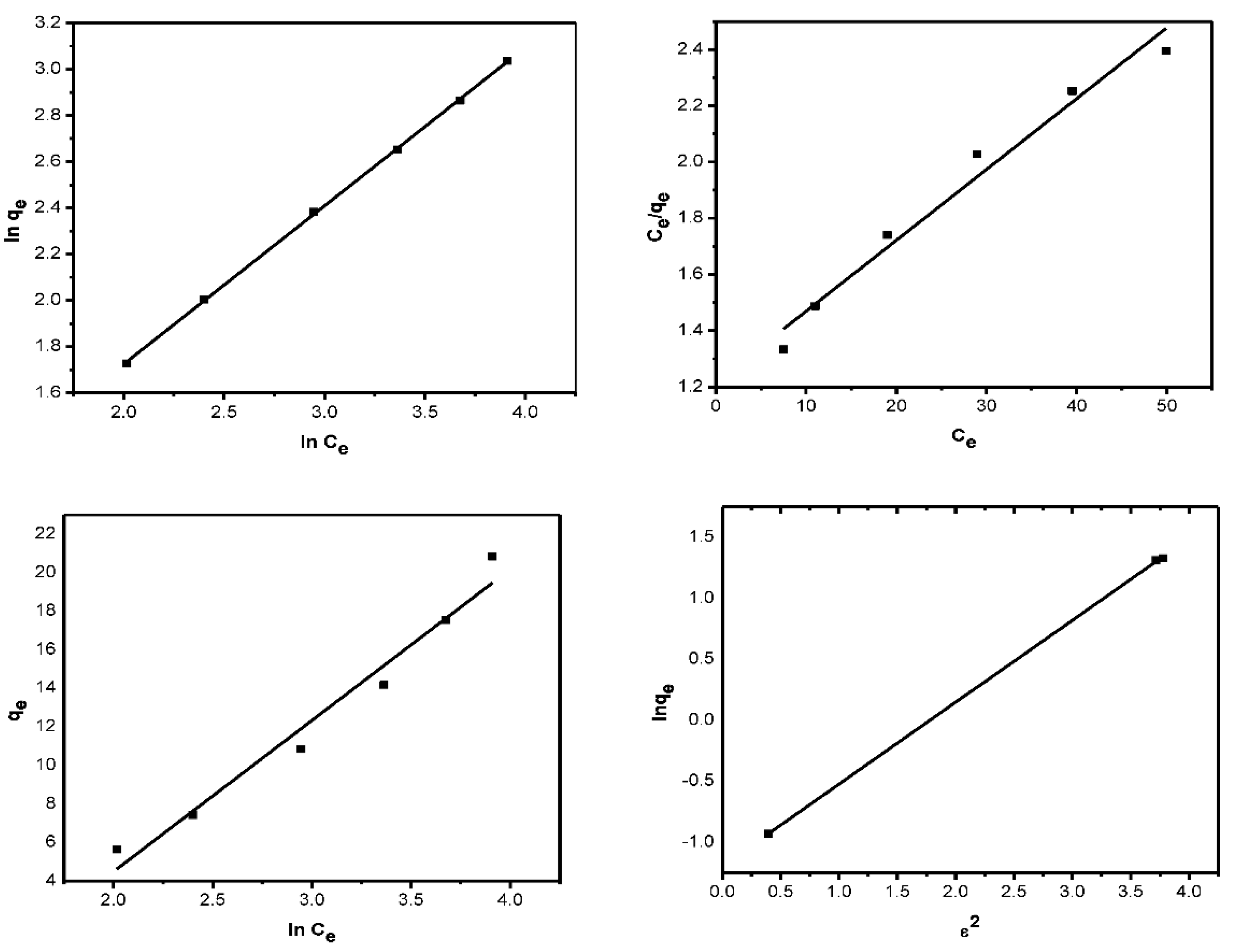
The results obtained in this study were fitted to the Langmuir isotherm model to determine the maximum adsorption capacity (qmax) of AILP on Pb(II) ions. The linear plot of Ce/qe vs. Ce (Figure 10a) obtained indicates the applicability of the Langmuir model. Values of b and qm were calculated graphically from the intercept and slope, respectively, and are presented in Table 3. From the Langmuir isotherm, the adsorption process can be classified as follows: when RL = 0, a favorable process occurs when 0 < RL < 1, linear process occurs when RL = 1, and unfavorable process occurs when RL > 1. The RL values for the initial AILP concentrations were between 0.13 and 0.49, which indicates that the adsorption of Pb(II) ions on AILP is a favorable process.
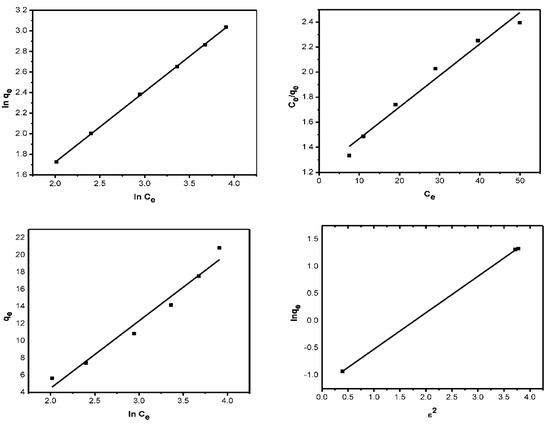
Figure 10.
Adsorption isotherm models: (a) Langmuir; (b) Freundlich; (c) Temkin; and (d) D-R.

Table 3.
Adsorption isotherm constants obtained from the four adsorption isotherm models.
3.8.2. Freundlich Isotherm
This is an experimental expression used for adsorption on heterogeneous surfaces with the interaction between adsorbed molecules. This model suggests an exponential drop in adsorption energy upon completion of the adsorption centers of the adsorbent. The linear equation of the Freundlich model is shown by Equation (7) [21]:
where Kf is the relative adsorption capacity of the adsorbent that is related to the bonding energy and n is the heterogeneity element stating the deviancy from the linearity of adsorption. A plot of ln qe versus ln Ce was used to confirm the Freundlich adsorption isotherm. Values of Kf and n were calculated from the intercept and slope (Figure 10b) and are tabulated in Table 3. An explanation of n, which controls the relationship between the solution concentration and adsorption, is as follows: If n < 1, adsorption is a physical process; if n = 1, adsorption is linear; and if n > 1, adsorption is a chemical process. The obtained n value was found to be 0.60, indicating physical processes.
3.8.3. Temkin Isotherm
In this model, the heat of adsorption AILP molecules in the layer is supposed to decrease linearly with the coverage of the adsorbent surface because of a reduction in the adsorbate–adsorbent interactions. The model is defined by Equation (8):
where B = (RT)/bt, R is the universal gas constant, and T is the temperature in Kelvin. The constant bt is associated with the heat of adsorption (J/mol). A is the equilibrium binding constant corresponding to the maximum binding energy.
3.8.4. Dubinin–Radushkevich (D-R) Isotherm
The D-R Isotherm model is commonly used to conclude the mean free energy of the adsorption process [1]. The formula of this model is described by Equation (9):
where ɛ is defined as
T denotes the temperature (K), R is the universal gas constant, and qmax and β are D-R isotherm constants. β and qmax were obtained from the slope and intercept of Equation (9) and Figure 10d and the values are presented in Table 3.
The mean free energy of adsorption process, Ef, is defined as the free energy change when one mole of ions is moved to the solid surface from infinity in solution and was determined from Equation (11).
It is well-established that the Ef value is crucial for predicting the adsorption type. For 8 < Ef < 16 kJ/mol, adsorption is considered a chemisorption process, and, when Ef < 8 kJ/mol, adsorption is classified as a physical process. In this study, the lower value of Ef (2.2 KJ/mol) indicates that the adsorption process was of a physical type.
3.9. Effect of the Temperature
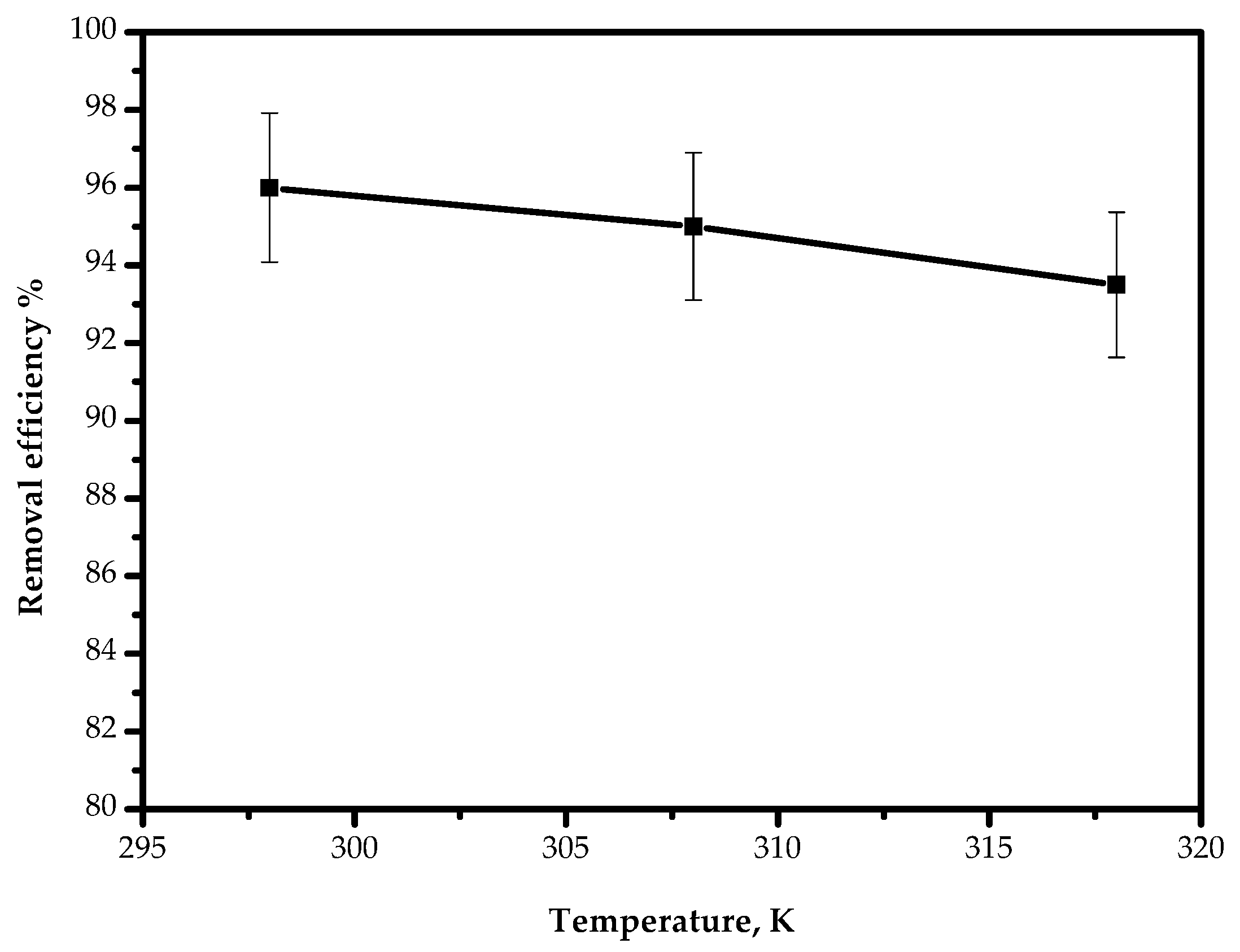
The temperature effect on the adsorption of Pb(II) ions on AILP was examined at a range from 298 to 318 K and is presented in Figure 11. The results revealed that the adsorption efficiency decreased slightly from 95.9% to 93.4% as the temperature increased from 298 to 318 K. This can be attributed to the possible damage of adsorption sites at elevated temperatures.
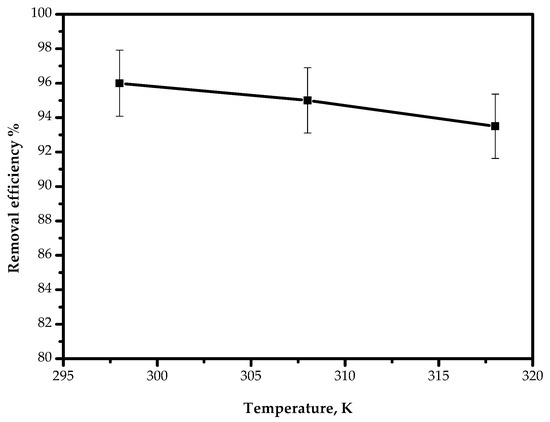
Figure 11.
Effect of the temperature on the removal of Pb(II) ions by AILP.
3.10. Thermodynamic Parameters
Enthalpy ∆H°, free energy ∆G°, and entropy ∆S° changes were determined using Equations (12)–(14):
where KD is the distribution coefficient and equivalent to the preliminary Pb(II) concentration (Co) divided by the final Pb(II) concentration (Ce).
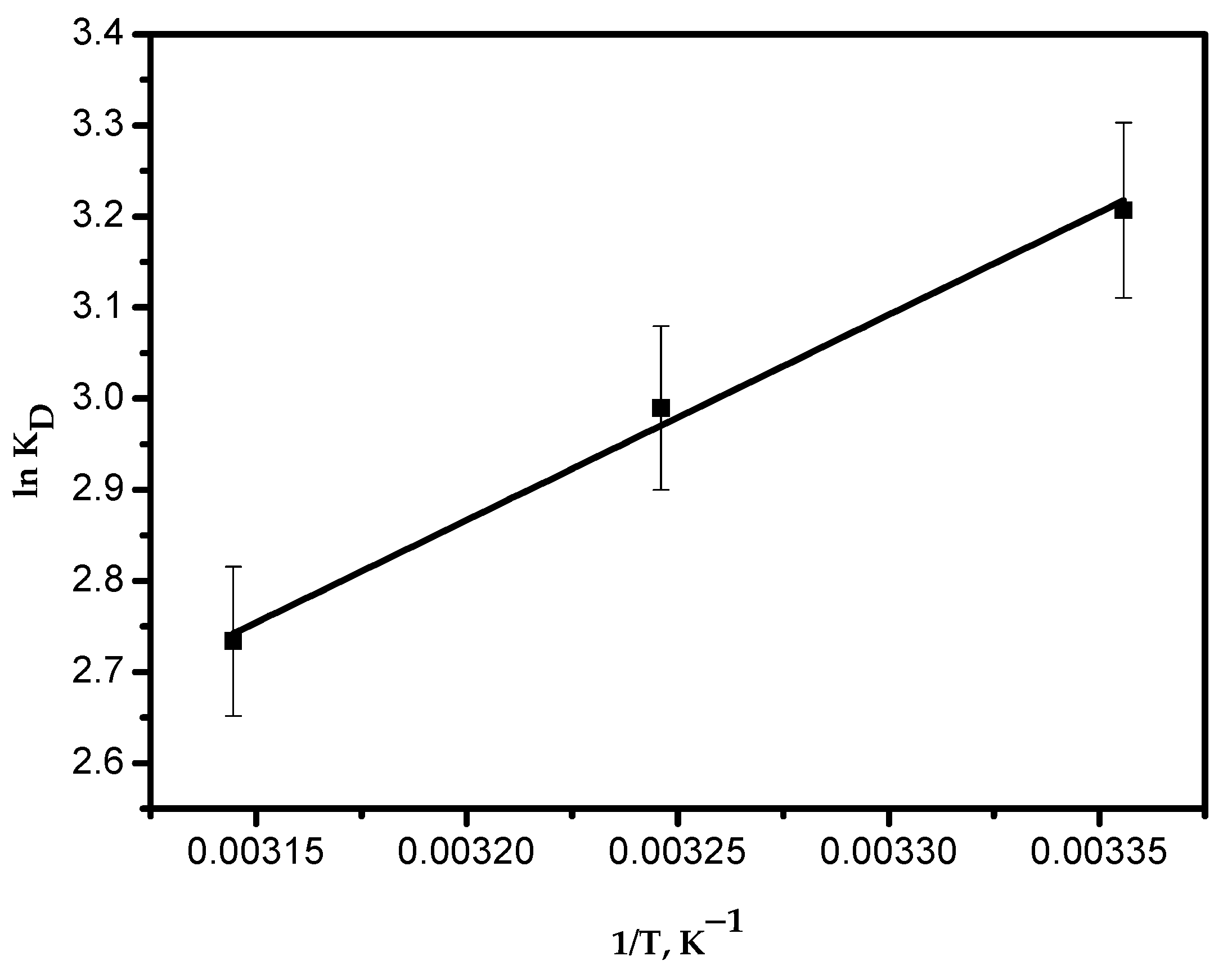
Figure 12 shows a linear plot of ln KD versus 1/T. The thermodynamic parameters were determined and are presented in Table 4. The negative value of ΔH° indicates the exothermic nature of the adsorption process. The positive value of entropy (ΔS°) proposes an increased randomness at the solid–solution interface. Negative values of Gibbs free energy (ΔG°) prove that the adsorption process is spontaneous, which becomes more positive with an increase in temperature. Therefore, this indicates that less adsorption occurs at elevated temperatures. This is in good agreement with the results obtained from Figure 10c.
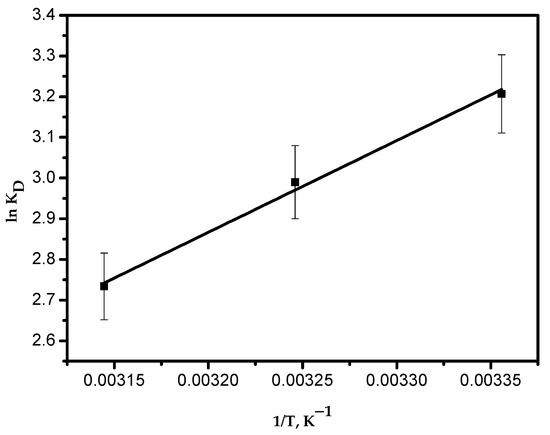
Figure 12.
Plot of ln KD versus 1/T.

Table 4.
Thermodynamic parameters of the adsorption of Pb(II) ions on AILP.
3.11. Reusability of AILP
The results of reusing the AILP are presented in Figure 13. It appeared that the adsorption effectiveness of the AILP decreased gradually with the reusability. The results confirm that the AILP can be efficiently recycled 2–3 times when removing Pb(II) ions from their aqueous solutions. The adsorption efficiency of the AILP decreased to 68.5% when it was reused for a fifth time.
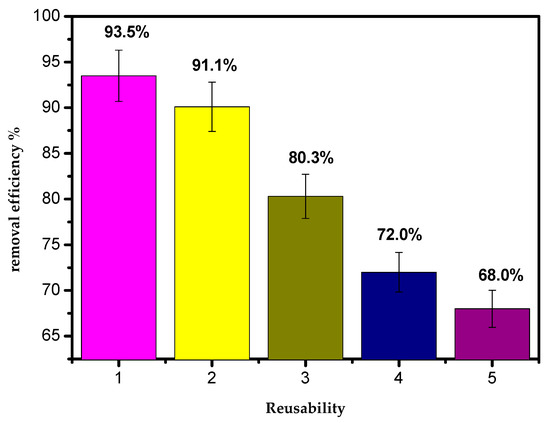
Figure 13.
The reusability of AILP.
3.12. Possible Adsorption Mechanism
The adsorption of Pb(II) ions on the AILP surface possibly jointly occurs by means of chemical interactions and physisorption. The ATR-FT-IR analysis proves that a Pb-O bond is formed after the adsorption process; on the other hand, values of ∆G° and bt suggest a physisorption process.
3.13. Comparison of AILP with Other Sorbents
An evaluation of qmax of the AILP according to the reported qmax values for other biosorbents is shown in Table 5. Variances in qmax can be attributed to the specific nature and properties of each adsorbent, surface area, and construction of the adsorbent. Making this comparison with other adsorbents confirms that AILP is a good and promising adsorbent.

Table 5.
Comparison of AILP and other biosorbents.
4. Conclusions
In this study, Azadirachta indica leaves’ powder (AILP), which is a natural adsorbent material, eco-friendly, and inexpensive, was investigated. AILP was used for the removal of Pb(II) ions from aqueous solution. The removal efficiency reached above 95%. The powder of the leaves spontaneously retained considerable amounts of Pb(II) ions. The maximum adsorption capacity for neem leaves was determined as being 39.7 mg/g. Experimental investigations defined the optimum conditions for the competent removal of 50 mg/L of Pb(II) ion to be 0.60 g, 40 min of contact time, and a pH of 7. These are significant characteristics for this tested adsorbent, making it an excellent choice for cleaning polluted water due to it (1) being a natural material and (2) cost-effective, as it requires no chemical treatment; (3) its availability, with this material being abundantly available in the region; and (4) its effectiveness, with it being capable of removing Pb(II) ions with a high efficiency. These positive characteristics would make this adsorbent a suitable candidate for removing other poisonous metal ions.
Author Contributions
Conceptualization A.E. and I.H.A.; methodology, A.E.; validation, E.I.B.; formal analysis, E.I.B.; investigation A.E.; resources, A.E.; writing—original draft preparation, A.E. and I.H.A.; writing—review and editing, E.I.B. and I.S.; project administration, I.H.A.; funding acquisition, A.B.E.; supervision, B.K. All authors have read and agreed to the published version of the manuscript.
Funding
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through a research groups program under grant number R.G.P.1/139/40.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ali, I.H.; Al Mesfer, M.K.; Khan, M.I.; Mohd, M.; Alghamdi, M.M. Exploring Adsorption Process of Lead (II) and Chromium (VI) Ions from Aqueous Solutions on Acid Activated Carbon Prepared from Juniperus procera Leaves. Processes 2019, 7, 217. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Morosanu, I.; Teodosiu, C.; Paduraru, C.; Ibanescu, D.; Tofan, L. Biosorption of lead ions from aqueous effluents by rapeseed biomass. New Biotechnol. 2017, 39, 110–124. [Google Scholar] [CrossRef]
- Gupta, V.K.; Rastogi, A. Biosorption of lead from aqueous solutions by green algae Spirogyra species: Kinetics and equilibrium studies. J. Hazard. Mater. 2008, 152, 407–414. [Google Scholar] [CrossRef]
- Amer, M.W.; Ahmad, R.A.; Awwad, A.M. Biosorption of Cu(II), Ni(II), Zn(II) and Pb(II) ions from aqueoussolution by Sophora japonica pods powder. Int. J. Ind. Chem. 2015, 6, 67–75. [Google Scholar] [CrossRef]
- Das, D.; Chakraborty, S.; Bhattacharjee, C.; Chowdhury, R. Biosorption of lead ions (Pb2+) from simulated wastewater using residual biomass of microalgae. Desalination Water Treat. 2016, 57, 4576–4586. [Google Scholar] [CrossRef]
- Papirio, S.; Frunzo, L.; Mattei, M.R.; Ferraro, A.; Race, M.; D’Acunto, B.; Esposito, G. Heavy metal removal from wastewaters by biosorption: Mechanisms and modeling. In Sustainable Heavy Metal Remediation, 1st ed.; Rene, E.R., Sahinkaya, E., Lewis, A., Lens, P.N.L., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; Volume 8, pp. 25–63. [Google Scholar]
- Xuan, Z. Study on the equilibrium, kinetics and isotherm of biosorption of lead ions onto pretreated chemically modified orange peel. Biochem. Eng. J. 2006, 31, 160–164. [Google Scholar] [CrossRef]
- Mungasavalli, P.D.; Viraraghavan, T.; Jin, Y. Biosorption of chromium from aqueous solutions by pr treated Aspergillus niger: Batch and column studies. Colloids Surf. A Physicochem. Eng. Asp. 2007, 301, 214–223. [Google Scholar] [CrossRef]
- Ucun, H.; Kemal, B.Y.; Kaya, Y.; Cakici, A.; Algur, O.F. Biosorption of lead (II) from aqueous solution by cone biomass of Pinus sylvestris. Desalination 2003, 154, 233–238. [Google Scholar] [CrossRef]
- Singanan, M. Removal of lead (II) and cadmium (II) ions from wastewater using activated biocarbon. Sci. Asia 2011, 37, 115–119. [Google Scholar] [CrossRef]
- Sulaymon, A.H.; Mohammed, A.A.; Al-Musawi, T.J. Competitive biosorption of lead, cadmium, copper, and arsenic ions using algae. Environ. Sci. Pollut. Res. 2013, 20, 3011–3023. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Kumar, S.; Kumar, S. Biosorption of lead ions from the aqueous solution by Sargassum filipendula: Equilibrium and kinetic studies. J. Environ. Chem. Eng. 2016, 4, 4587–4599. [Google Scholar] [CrossRef]
- Barka, N.; Abdennouri, M.; El Makhfouk, M.; Qourzal, S. Biosorption characteristics of cadmium and lead onto eco-friendly dried cactus (Opuntia ficus indica) cladodes. J. Environ. Chem. Eng. 2013, 1, 144–149. [Google Scholar] [CrossRef]
- Iram, S.; Shabbir, R.; Zafar, H.; Javaid, M. Biosorption and bioaccumulation of copper and lead by heavy metal-resistant fungal isolates. Arab. J. Sci. Eng. 2015, 40, 1867–1873. [Google Scholar] [CrossRef]
- Okoye, A.; Ejikeme, P.; Onukwuli, O. Lead removal from wastewater using fluted pumpkin seed shell activated carbon: Adsorption modeling and kinetics. Int. J. Environ. Sci. Technol. 2010, 7, 793–800. [Google Scholar] [CrossRef]
- Senthil, K.P. Adsorption of lead (II) ions from simulated wastewater using natural waste: A kinetic, thermodynamic and equilibrium study. Environ. Prog. Sustain. Energy 2014, 33, 55–64. [Google Scholar] [CrossRef]
- Abadian, S.; Rahbar-Kelishami, A.; Norouzbeigi, R.; Peydayesh, M. Cu(II) adsorption onto Platanus orientalis leaf powder: Kinetic, isotherm, and thermodynamic studies. Res. Chem. Intermed. 2015, 41, 7669–7681. [Google Scholar] [CrossRef]
- Peydayesh, M.; Isanejad, M.; Mohammadi, T.; Jafari, S.M.R.S. Assessment of Urtica as a low-cost adsorbent for methylene blue removal: Kinetic, equilibrium, and thermodynamic studies. Chem. Pap. 2015, 69, 930–937. [Google Scholar] [CrossRef]
- Al-Hashemi, Z.S.S.; Hossain, M.A. Biological activities of different neem leaf crude extracts used locally in Ayurvedic medicine. Pac. Sci. Rev. A Nat. Sci. Eng. 2016, 18, 128–131. [Google Scholar] [CrossRef]
- Elkhaleefa, A.; Ali, I.H.; Brima, E.I.; Elhag, A.B.; Karama, B. Efficient Removal of Ni(II) from Aqueous Solution by Date Seeds Powder Biosorbent: Adsorption Kinetics, Isotherm and Thermodynamics. Processes 2020, 8, 1001. [Google Scholar] [CrossRef]
- El-Zahhar, A.A.; Idris, A.M. Mercury(II) decontamination using a newly synthesized poly(acrylonitrile-acrylic acid)/ammonium molybdophosphate composite exchanger. Toxin Rev. 2020, 39, 1–13. [Google Scholar] [CrossRef]
- Khan, M.I.; Al Mesfer, M.K.; Danish, M.; Ali, I.H.; Shoukry, H.; Patel, R.; Gardy, J.; Rehan, M. Potential of Saudi natural clay as an effective adsorbent in heavy metals removal from wastewater. Desalination Water Treat. 2019, 15, 140–151. [Google Scholar] [CrossRef]
- Eleryan, A.; El Nemr, A.; Abubakr, M.; Idris, A.M.; Alghamdi, M.M.; El-Zahhar, A.A.; Tarek, O.; Said, T.O.; Sahlabji, T. Feasible and eco-friendly removal of hexavalent chromium toxicant from aqueous solutions using chemically modified sugarcane bagasse cellulose. Toxin Rev. 2020, 39, 1–12. [Google Scholar] [CrossRef]
- Kavitha, G.; Sridevi, V.; Venkateswarlu, P.; Babu, N.C. Biosorption of chromium from aqueous solution by Gracilaria corticata (Red Algae) and its statistical analysis using response surface methodology. Open Access Libr. J. 2016, 3, 1–32. [Google Scholar] [CrossRef]
- Taha, M.F.; Kiat, C.F.; Shaharun, M.S.; Ramli, A. Removal of Ni(II), Zn(II) and Pb(II) ions from single metal aqueous solution using activated carbon prepared from rice husk. Int. J. Environ. Ecol. Eng. 2011, 5, 855–860. [Google Scholar]
- Xiong, C.; Yao, C. Synthesis, characterization and application of triethylenetetraamine modified polystyrene resin in removal of mercury, cadmium and lead from aqueous solutions. Chem. Eng. J. 2009, 155, 844–850. [Google Scholar] [CrossRef]
- Xiong, C.; Yao, C. Preparation and application of acrylic acid grafted polytetraflouroethylene fiber as a weak acid cation exchanger for adsorption of Er(III). J. Hazard. Mater. 2009, 170, 1125–1132. [Google Scholar] [CrossRef]
- Abdulaziz Ali Alghamdi, A.A.; Al-Odayni, A.; Saeed, W.S.; Al-Kahtani, A.; Alharthi, F.A.; Aouak, T. Efficient Adsorption of Lead (II) from Aqueous Phase Solutions Using Polypyrrole-Based Activated Carbon. Materials 2019, 12, 1–16. [Google Scholar]
- Wang, L.; Zhang, J.; Zhao, R.; Li, Y.; Li, C.; Zhang, C. Adsorption of Pb (II) on activated carbon prepared from Polygonum orientale Linn.: Kinetics, isotherms, pH, and ionic strength studies. Bioresour. Technol. 2010, 101, 5808–5814. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).