Anti-Photoaging and Potential Skin Health Benefits of Seaweeds
Abstract
1. Introduction
2. Seaweeds Extracts as Potential Anti-Photoaging Agents
3. Seaweed Compounds as Potential Sources of Anti-Photoaging Agents
3.1. Polysaccharides Rich Extract
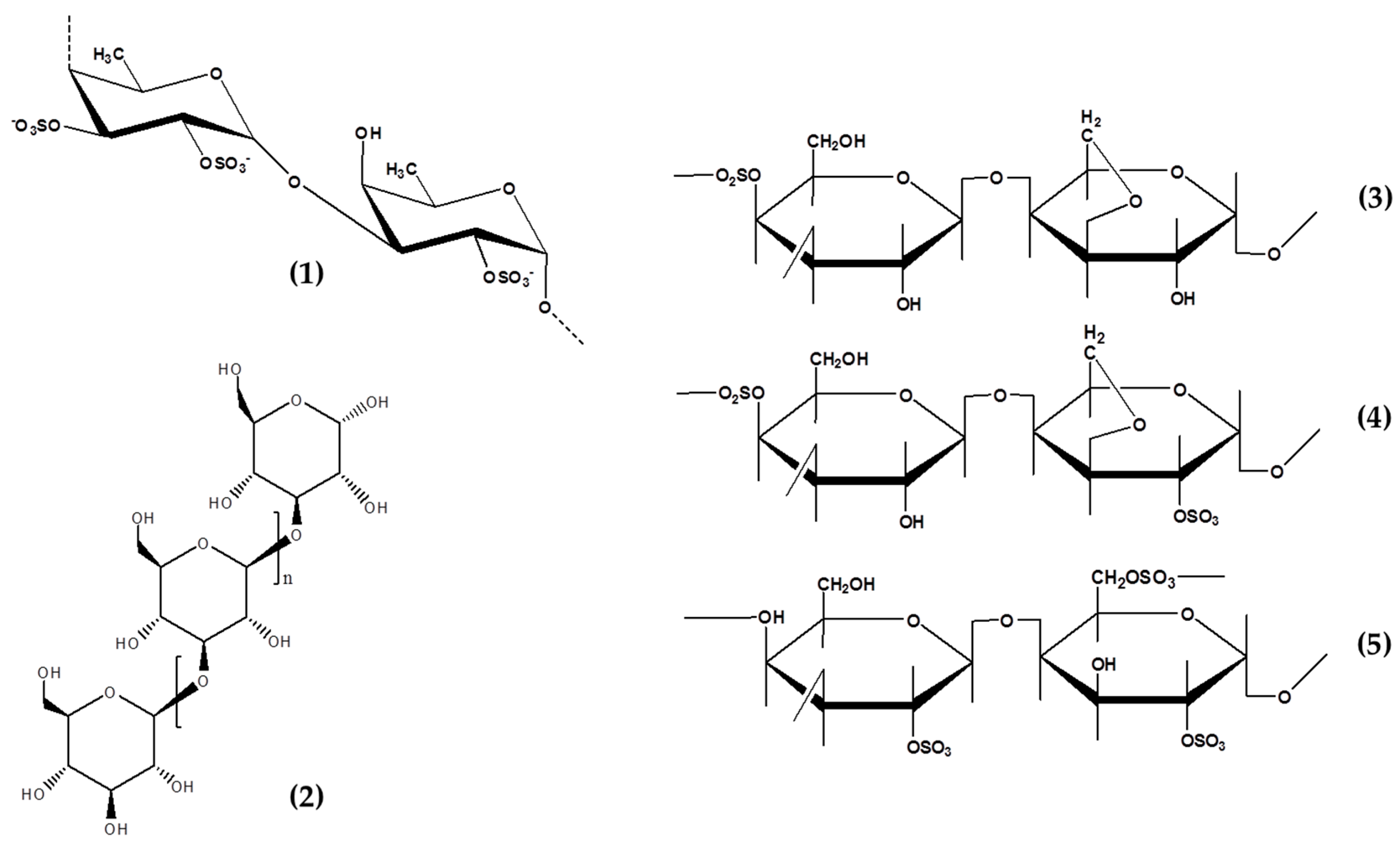
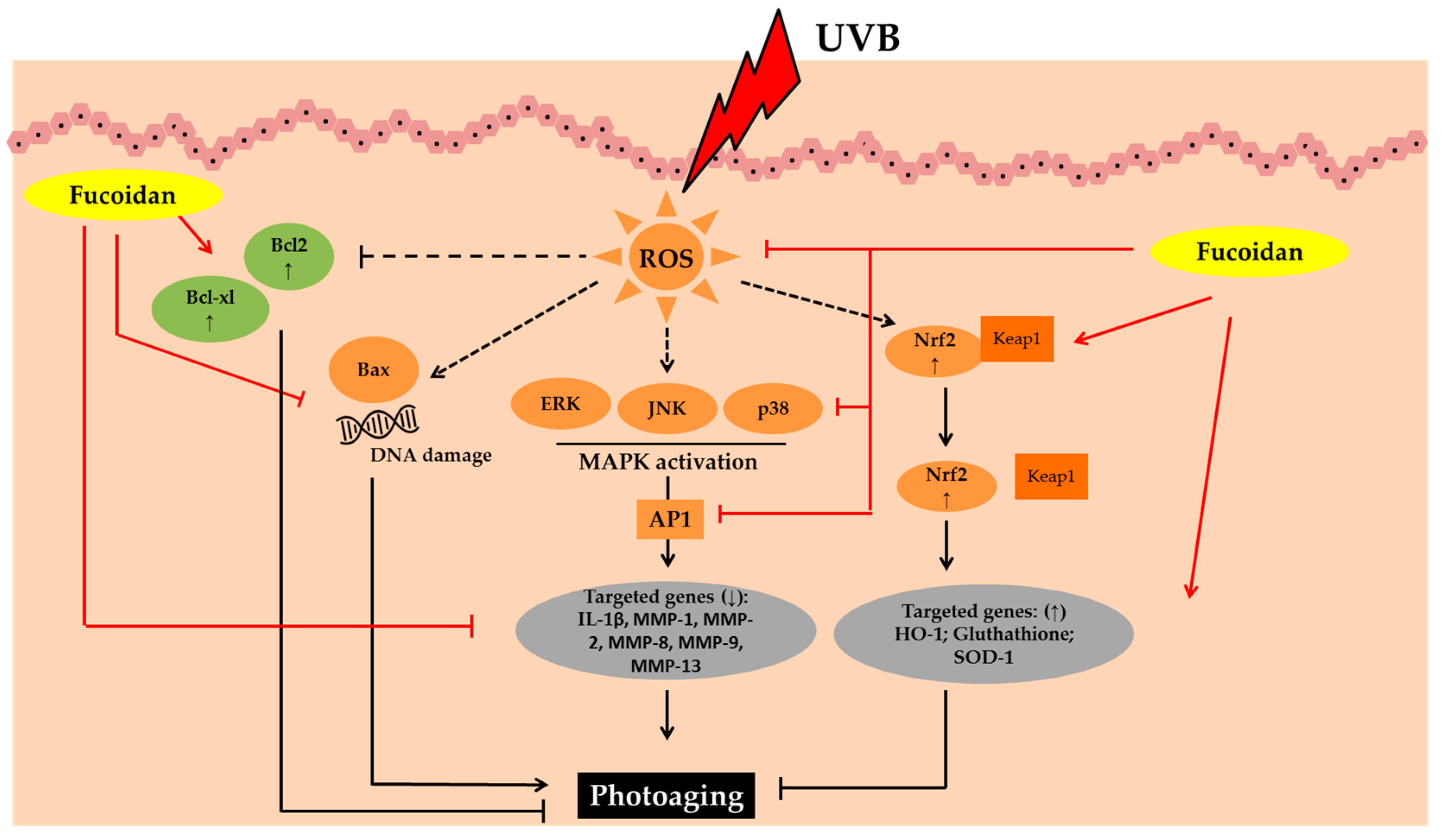
3.2. Fucoidans
3.3. Carrageenans
3.4. Laminarins
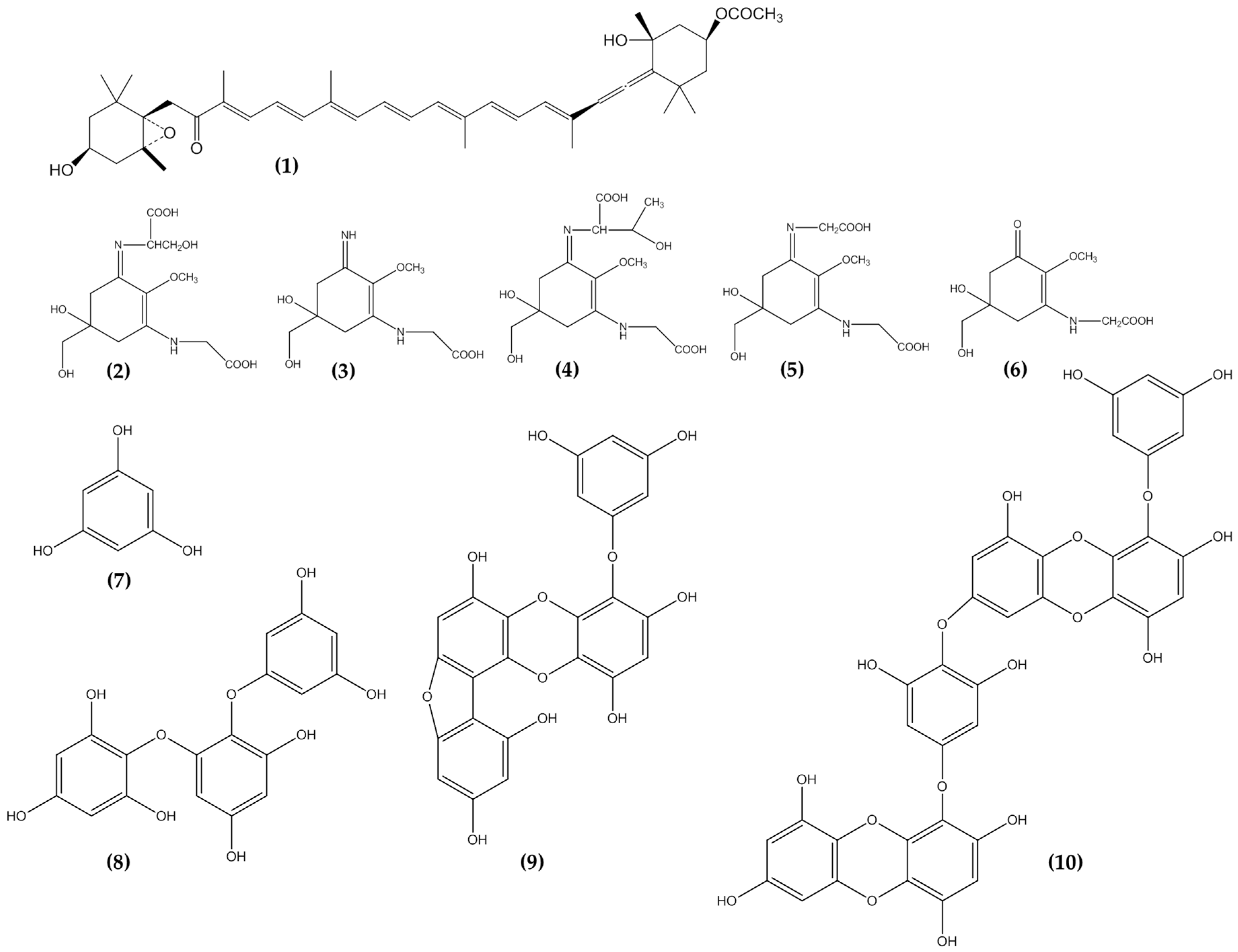
3.5. Phlorotannins
3.6. Mycosporine Like Amino Acids
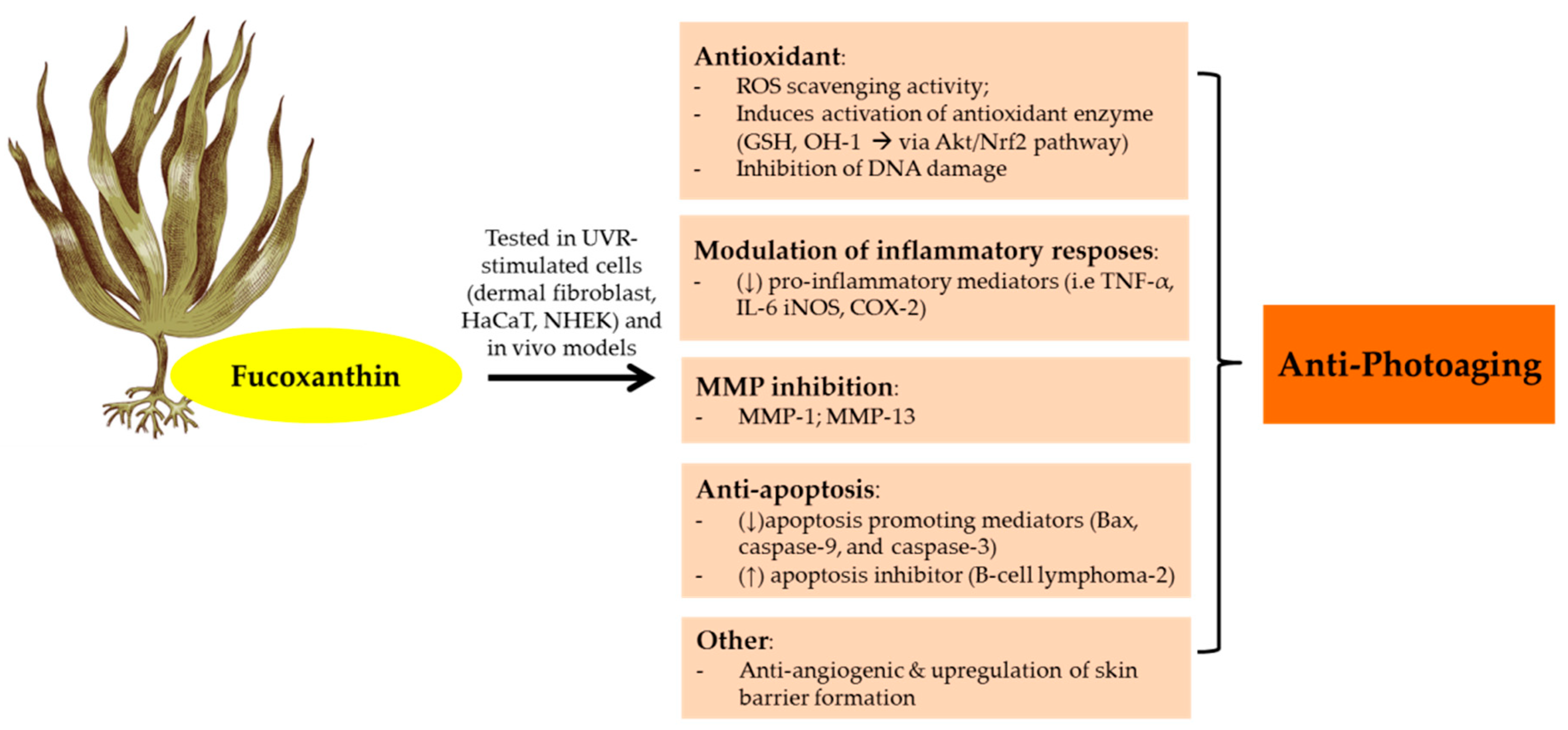
3.7. Carotenoids
4. Potential of Seaweeds in Anti-photoaging Products
4.1. Seaweed Diversity Opens Untapped Potential for Anti-Photoaging Products
4.2. Development of Sustainable Aquaculture to Support Seaweeds Potential in Skincare and Cosmetic Industries
4.3. Sustainable and Environmentally Friendly Extraction
4.4. Potential of Seaweeds-Derived Anti-Photoaging Products in the Market
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Abdallah, F.; Mijouin, L.; Pichon, C. Skin immune landscape: Inside and outside the organism. Mediat. Inflamm. 2017, 2017, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Solano, F. Photoprotection and skin pigmentation: Melanin-related molecules and some other new agents obtained from natural sources. Molecules 2020, 25, 1537. [Google Scholar] [CrossRef] [PubMed]
- Agar, N.; Young, A.R. Melanogenesis: A photoprotective response to DNA damage? Mutat. Res. Fundam. Mol. Mech. Mutagenesis 2005, 571, 121–132. [Google Scholar] [CrossRef]
- Solano, F. Photoprotection versus photodamage: Updating an old but still unsolved controversy about melanin. Polym. Int. 2016, 65, 1276–1287. [Google Scholar] [CrossRef]
- Xu, Y.; Fisher, G.J. Ultraviolet (UV) light irradiation induced signal transduction in skin photoaging. J. Dermatol. Sci. Suppl. 2005, 1, S1–S8. [Google Scholar] [CrossRef]
- Ichihashi, M.; Ando, H.; Yoshida, M.; Niki, Y.; Matsui, M. Photoaging of the skin. Anti Aging Med. 2009, 6, 46–59. [Google Scholar] [CrossRef]
- Panich, U.; Sittithumcharee, G.; Rathviboon, N.; Jirawatnotai, S. Ultraviolet Radiation-Induced Skin Aging: The Role of DNA Damage and Oxidative Stress in Epidermal Stem Cell Damage Mediated Skin Aging. Stem Cells Int. 2016, 2016, 7370642. [Google Scholar] [CrossRef] [PubMed]
- Pandel, R.; Poljšak, B.; Godic, A.; Dahmane, R. Skin photoaging and the role of antioxidants in its prevention. Int. Sch. Res. Not. 2013, 2013. [Google Scholar] [CrossRef]
- Wlaschek, M.; Tantcheva-Poór, I.; Naderi, L.; Ma, W.; Schneider, L.A.; Razi-Wolf, Z.; Schüller, J.; Scharffetter-Kochanek, K. Solar UV irradiation and dermal photoaging. J. Photochem. Photobiol. B: Biol. 2001, 63, 41–51. [Google Scholar] [CrossRef]
- Dahmane, R.; Pandel, R.; Trebse, P.; Poljsak, B. The Role of Sun Exposure in Skin Aging. In Sun Exposure: Risk Factors, Protection Practices and Health Effects; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2015; Volume 2015, pp. 1–40. [Google Scholar]
- Wang, M.; Charareh, P.; Lei, X.; Zhong, J.L. Autophagy: Multiple Mechanisms to Protect Skin from Ultraviolet Radiation-Driven Photoaging. Oxidative Med. Cell. Longev. 2019, 2019, 8135985. [Google Scholar] [CrossRef]
- Parvez, S.; Kang, M.; Chung, H.S.; Cho, C.; Hong, M.C.; Shin, M.K.; Bae, H. Survey and mechanism of skin depigmenting and lightening agents. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2006, 20, 921–934. [Google Scholar] [CrossRef] [PubMed]
- Pangestuti, R.; Siahaan, E.; Kim, S.-K. Photoprotective Substances Derived from Marine Algae. Mar. Drugs 2018, 16, 399. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Pangestuti, R. 15 Biological Properties of Cosmeceuticals Derived from Marine Algae. In Marine Cosmeceuticals: Trends Prospect; Kim, S.K., Ed.; CRC Press: Boca Raton, FL, USA, 2011; pp. 191–200. [Google Scholar]
- Pangestuti, R.; Getachew, A.T.; Siahaan, E.A.; Chun, B.-S. Characterization of functional materials derived from tropical red seaweed Hypnea musciformis produced by subcritical water extraction systems. J. Appl. Phycol. 2019, 31, 2517–2528. [Google Scholar] [CrossRef]
- Wijesekara, I.; Pangestuti, R.; Kim, S.K. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr. Polym. 2010, 84, 14–21. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S.-K. Biological activities of carrageenan. Mar. Carbohydr. Fundam. Appl. 2014, 72, 113–124. [Google Scholar]
- Zargarzadeh, M.; Amaral, A.J.R.; Custódio, C.A.; Mano, J.F. Biomedical applications of laminarin. Carbohydr. Polym. 2020, 232, 115774. [Google Scholar] [CrossRef]
- Ku, M.-J.; Jung, J.-W.; Lee, M.-S.; Cho, B.-K.; Lee, S.-R.; Lee, H.-S.; Vischuk, O.S.; Zvyagintseva, T.N.; Ermakova, S.P.; Lee, Y.-H. Effect of Fucus evanescens fucoidan on expression of matrix metalloproteinase-1 promoter, mRNA, protein and signal pathway. J. Life Sci. 2010, 20, 1603–1610. [Google Scholar] [CrossRef]
- Freitas, R.; Martins, A.; Silva, J.; Alves, C.; Pinteus, S.; Alves, J.; Teodoro, F.; Ribeiro, H.M.; Gonçalves, L.; Petrovski, Ž. Highlighting the Biological Potential of the Brown Seaweed Fucus spiralis for Skin Applications. Antioxidants 2020, 9, 611. [Google Scholar] [CrossRef] [PubMed]
- Jesumani, V.; Du, H.; Pei, P.; Aslam, M.; Huang, N. Comparative study on skin protection activity of polyphenol-rich extract and polysaccharide-rich extract from Sargassum vachellianum. PLoS ONE 2020, 15, e0227308. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S.-K. Biological activities and health benefit effects of natural pigments derived from marine algae. J. Funct. Foods 2011, 3, 255–266. [Google Scholar] [CrossRef]
- Sánchez-Lamar, Á.; González-Pumariega, M.; Fuentes-León, F.; Vernhes Tamayo, M.; Schuch, A.P.; Menck, C.F. Evaluation of Genotoxic and DNA Photo-Protective Activity of Bryothamnion triquetrum and Halimeda incrassata Seaweeds Extracts. Cosmetics 2017, 4, 23. [Google Scholar] [CrossRef]
- Wiraguna, A.A.G.P.; Pangkahila, W.; Astawa, I.N.M. Antioxidant properties of topical Caulerpa sp. extract on UVB-induced photoaging in mice. Dermatol. Rep. 2018, 10, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Guinea, M.; Franco, V.; Araujo-Bazán, L.; Rodríguez-Martín, I.; González, S. In vivo UVB-photoprotective activity of extracts from commercial marine macroalgae. Food Chem. Toxicol. 2012, 50, 1109–1117. [Google Scholar] [CrossRef]
- Kim, S.; You, D.H.; Han, T.; Choi, E.-M. Modulation of viability and apoptosis of UVB-exposed human keratinocyte HaCaT cells by aqueous methanol extract of laver (Porphyra yezoensis). J. Photochem. Photobiol. B Biol. 2014, 141, 301–307. [Google Scholar] [CrossRef]
- Rangel, K.C.; Villela, L.Z.; de Castro Pereira, K.; Colepicolo, P.; Debonsi, H.M.; Gaspar, L.R. Assessment of the photoprotective potential and toxicity of Antarctic red macroalgae extracts from Curdiea racovitzae and Iridaea cordata for cosmetic use. Algal Res. 2020, 50, 101984. [Google Scholar] [CrossRef]
- Mercurio, D.; Wagemaker, T.; Alves, V.; Benevenuto, C.; Gaspar, L.; Campos, P.M. In vivo photoprotective effects of cosmetic formulations containing UV filters, vitamins, Ginkgo biloba and red algae extracts. J. Photochem. Photobiol. B Biol. 2015, 153, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Boulho, R.; Le Roux, J.; Le Quémener, C.; Audo, G.; Bourgougnon, N.; Bedoux, G. Fractionation of UV-B absorbing molecules and of free radical scavenging compounds from Solieria chordalis by using centrifugal partition chromatography. Phytochem. Lett. 2017, 20, 410–414. [Google Scholar] [CrossRef]
- Kim, H.M.; Lee, D.E.; Park, S.D.; Kim, Y.T.; Kim, Y.J.; Jeong, J.W.; Lee, J.-H.; Jang, S.S.; Chung, D.K.; Sim, J.-H. Preventive effect of fermented Gelidium amansii and Cirsium japonicum extract mixture against UVB-induced skin photoaging in hairless mice. Food Sci. Biotechnol. 2014, 23, 623–631. [Google Scholar] [CrossRef]
- Hyun, Y.J.; Piao, M.J.; Kim, K.C.; Zheng, J.; Yao, C.W.; Cha, J.W.; Kang, H.K.; Yoo, E.S.; Koh, Y.S.; Lee, N.H. Photoprotective effect of a Polyopes affinis (Harvey) Kawaguchi and Wang (Halymeniaceae)-derived ethanol extract on human keratinocytes. Trop. J. Pharm. Res. 2014, 13, 863–871. [Google Scholar] [CrossRef]
- Bedoux, G.; Hardouin, K.; Marty, C.; Taupin, L.; Vandanjon, L.; Bourgougnon, N. Chemical characterization and photoprotective activity measurement of extracts from the red macroalga Solieria chordalis. Bot. Mar. 2014, 57, 291–301. [Google Scholar] [CrossRef]
- Piao, M.J.; Kang, H.K.; Yoo, E.S.; Koh, Y.S.; Kim, D.S.; Lee, N.H.; Hyun, J.W. Photo-protective effect of Polysiphonia morrowii Harvey against ultraviolet B radiation-induced keratinocyte damage. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 149–158. [Google Scholar] [CrossRef]
- Piao, M.J.; Hyun, Y.J.; Oh, T.-H.; Kang, H.K.; Yoo, E.S.; Koh, Y.S.; Lee, N.H.; Suh, I.S.; Hyun, J.W. Chondracanthus tenellus (Harvey) hommersand extract protects the human keratinocyte cell line by blocking free radicals and UVB radiation-induced cell damage. In Vitro Cell. Dev. Biol. Anim. 2012, 48, 666–674. [Google Scholar] [CrossRef]
- Piao, M.J.; Hyun, Y.J.; Cho, S.J.; Kang, H.K.; Yoo, E.S.; Koh, Y.S.; Lee, N.H.; Ko, M.H.; Hyun, J.W. An ethanol extract derived from Bonnemaisonia hamifera scavenges ultraviolet B (UVB) radiation-induced reactive oxygen species and attenuates UVB-induced cell damage in human keratinocytes. Mar. Drugs 2012, 10, 2826–2845. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.D.; Piao, M.J.; Hyun, Y.J.; Kang, H.K.; Suh, I.S.; Lee, N.H.; Hyun, J.W. Photo-protective properties of Lomentaria hakodatensis yendo against ultraviolet B radiation-induced keratinocyte damage. Biotechnol. Bioprocess Eng. 2012, 17, 1223–1231. [Google Scholar] [CrossRef]
- Piao, M.J.; Yoon, W.J.; Kang, H.K.; Yoo, E.S.; Koh, Y.S.; Kim, D.S.; Lee, N.H.; Hyun, J.W. Protective Effect of the Ethyl Acetate Fraction of Sargassum muticum Against Ultraviolet B–Irradiated Damage in Human Keratinocytes. Int. J. Mol. Sci. 2011, 12, 8146–8160. [Google Scholar] [CrossRef]
- Piao, M.J.; Kim, K.C.; Zheng, J.; Yao, C.W.; Cha, J.W.; Boo, S.J.; Yoon, W.J.; Kang, H.K.; Yoo, E.S.; Koh, Y.S. The ethyl acetate fraction of Sargassum muticum attenuates ultraviolet B radiation-induced apoptotic cell death via regulation of MAPK-and caspase-dependent signaling pathways in human HaCaT keratinocytes. Pharm. Biol. 2014, 52, 1110–1118. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Song, J.H.; Piao, M.J.; Han, X.; Kang, K.A.; Kang, H.K.; Yoon, W.J.; Ko, M.H.; Lee, N.H.; Lee, M.Y.; Chae, S. Anti-wrinkle effects of Sargassum muticum ethyl acetate fraction on ultraviolet B-irradiated hairless mouse skin and mechanistic evaluation in the human HaCaT keratinocyte cell line. Mol. Med. Rep. 2016, 14, 2937–2944. [Google Scholar] [CrossRef][Green Version]
- Li, Z.-y.; Yu, C.-H.; Lin, Y.-T.; Su, H.-L.; Kan, K.-W.; Liu, F.-C.; Chen, C.-T.; Lin, Y.-T.; Hsu, H.-F.; Lin, Y.-H. The potential application of spring Sargassum glaucescens extracts in the moisture-retention of keratinocytes and dermal fibroblast regeneration after UVA-irradiation. Cosmetics 2019, 6, 17. [Google Scholar] [CrossRef]
- Prasedya, E.S.; Syafitri, S.M.; Geraldine, B.A.; Hamdin, C.D.; Frediansyah, A.; Miyake, M.; Kobayashi, D.; Hazama, A.; Sunarpi, H. UVA photoprotective activity of Brown macroalgae Sargassum cristafolium. Biomedicines 2019, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Hyun, Y.J.; Piao, M.J.; Ko, M.H.; Lee, N.H.; Kang, H.K.; Yoo, E.S.; Koh, Y.S.; Hyun, J.W. Photoprotective effect of Undaria crenata against ultraviolet B-induced damage to keratinocytes. J. Biosci. Bioeng. 2013, 116, 256–264. [Google Scholar] [CrossRef]
- Zheng, J.; Hewage, S.M.; Piao, M.J.; Kang, K.A.; Han, X.; Kang, H.; Yoo, E.; Koh, Y.; Lee, N.; Ko, C. Photoprotective effect of carpomitra costata extract against ultraviolet B-induced oxidative damage in human keratinocytes. J. Environ. Pathol. Toxicol. Oncol. 2016, 35, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Jun, E.-S.; Kim, Y.J.; Kim, H.-H.; Park, S.Y. Gold Nanoparticles Using Ecklonia stolonifera Protect Human Dermal Fibroblasts from UVA-Induced Senescence through Inhibiting MMP-1 and MMP-3. Mar. Drugs 2020, 18, 433. [Google Scholar] [CrossRef] [PubMed]
- Ale, M.T.; Maruyama, H.; Tamauchi, H.; Mikkelsen, J.D.; Meyer, A.S. Fucose-containing sulfated polysaccharides from brown seaweeds inhibit proliferation of melanoma cells and induce apoptosis by activation of caspase-3 in vitro. Mar. Drugs 2011, 9, 2605–2621. [Google Scholar] [CrossRef]
- Wang, L.; Lee, W.; Oh, J.Y.; Cui, Y.R.; Ryu, B.; Jeon, Y.-J. Protective effect of sulfated polysaccharides from celluclast-assisted extract of Hizikia fusiforme against ultraviolet B-Induced skin damage by regulating NF-κB, AP-1, and MAPKs signaling pathways in vitro in human dermal fibroblasts. Mar. Drugs 2018, 16, 239. [Google Scholar] [CrossRef]
- Wang, L.; Oh, J.Y.; Yang, H.-W.; Kim, H.S.; Jeon, Y.-J. Protective effect of sulfated polysaccharides from a Celluclast-assisted extract of Hizikia fusiforme against ultraviolet B-induced photoaging in vitro in human keratinocytes and in vivo in zebrafish. Mar. Life Sci. Technol. 2019, 1, 104–111. [Google Scholar] [CrossRef]
- Ye, Y.; Ji, D.; You, L.; Zhou, L.; Zhao, Z.; Brennan, C. Structural properties and protective effect of Sargassum fusiforme polysaccharides against ultraviolet B radiation in hairless Kun Ming mice. J. Funct. Foods 2018, 43, 8–16. [Google Scholar] [CrossRef]
- Wang, L.; Jayawardena, T.U.; Yang, H.-W.; Lee, H.-G.; Jeon, Y.-J. The Potential of Sulfated Polysaccharides Isolated from the Brown Seaweed Ecklonia maxima in Cosmetics: Antioxidant, Anti-melanogenesis, and Photoprotective Activities. Antioxidants 2020, 9, 724. [Google Scholar] [CrossRef]
- Fitton, J.H.; Dell’Acqua, G.; Gardiner, V.-A.; Karpiniec, S.S.; Stringer, D.N.; Davis, E. Topical benefits of two fucoidan-rich extracts from marine macroalgae. Cosmetics 2015, 2, 66–81. [Google Scholar] [CrossRef]
- Gesztesi, J.-l.; Silva, L.V.N.; Robert, L.; Robert, A. Cosmetic composition of two polysaccharides based on fucose and rhamnose. Google Patents Patent number: S20060115443A1, 1 June 2006. [Google Scholar]
- Wang, J.; Zhang, Q.; Zhang, Z.; Song, H.; Li, P. Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2010, 46, 6–12. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Zhang, Z.; Zhang, J.; Li, P. Synthesized phosphorylated and aminated derivatives of fucoidan and their potential antioxidant activity in vitro. Int. J. Biol. Macromol. 2009, 44, 170–174. [Google Scholar] [CrossRef]
- Wang, J.; Liu, L.; Zhang, Q.; Zhang, Z.; Qi, H.; Li, P. Synthesized oversulphated, acetylated and benzoylated derivatives of fucoidan extracted from Laminaria japonica and their potential antioxidant activity in vitro. Food Chem. 2009, 114, 1285–1290. [Google Scholar] [CrossRef]
- Koh, H.S.A.; Lu, J.; Zhou, W. Structure characterization and antioxidant activity of fucoidan isolated from Undaria pinnatifida grown in New Zealand. Carbohydr. Polym. 2019, 212, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Khalafu, S.H.S.; Aida, W.M.W.; Lim, S.J.; Maskat, M.Y. Effects of deodorisation methods on volatile compounds, chemical properties and antioxidant activities of fucoidan isolated from brown seaweed (Sargassum sp.). Algal Res. 2017, 25, 507–515. [Google Scholar] [CrossRef]
- Lim, S.J.; Aida, W.M.W.; Maskat, M.Y.; Mamot, S.; Ropien, J.; Mohd, D.M. Isolation and antioxidant capacity of fucoidan from selected Malaysian seaweeds. Food Hydrocoll. 2014, 42, 280–288. [Google Scholar] [CrossRef]
- Moon, H.J.; Lee, S.R.; Shim, S.N.; Jeong, S.H.; Stonik, V.A.; Rasskazov, V.A.; Zvyagintseva, T.; Lee, Y.H. Fucoidan inhibits UVB-induced MMP-1 expression in human skin fibroblasts. Biol. Pharm. Bull. 2008, 31, 284–289. [Google Scholar] [CrossRef]
- Moon, H.J.; Park, K.S.; Ku, M.J.; Lee, M.S.; Jeong, S.H.; Imbs, T.I.; Zvyagintseva, T.N.; Ermakova, S.P.; Lee, Y.H. Effect of Costaria costata fucoidan on expression of matrix metalloproteinase-1 promoter, mRNA, and protein. J. Nat. Prod. 2009, 72, 1731–1734. [Google Scholar] [CrossRef]
- Moon, H.J.; Lee, S.H.; Ku, M.J.; Yu, B.C.; Jeon, M.J.; Jeong, S.H.; Stonik, V.A.; Zvyagintseva, T.N.; Ermakova, S.P.; Lee, Y.H. Fucoidan inhibits UVB-induced MMP-1 promoter expression and down regulation of type I procollagen synthesis in human skin fibroblasts. Eur. J. Dermatol. 2009, 19, 129–134. [Google Scholar] [CrossRef]
- Ku, M.-J.; Lee, M.-S.; Moon, H.-J.; Lee, Y.-H. Protective Effects of Fucoidan against UVB-Induced Oxidative Stress in Human Skin Fibroblasts. J. Life Sci. 2010, 20, 27–32. [Google Scholar] [CrossRef]
- Hwang, P.-A.; Yan, M.-D.; Kuo, K.-L.; Phan, N.N.; Lin, Y.-C. A mechanism of low molecular weight fucoidans degraded by enzymatic and acidic hydrolysis for the prevention of UVB damage. J. Appl. Phycol. 2017, 29, 521–529. [Google Scholar] [CrossRef]
- Kim, Y.-I.; Oh, W.-S.; Song, P.H.; Yun, S.; Kwon, Y.-S.; Lee, Y.J.; Ku, S.-K.; Song, C.-H.; Oh, T.-H. Anti-photoaging effects of low molecular-weight fucoidan on ultraviolet B-irradiated mice. Mar. Drugs 2018, 16, 286. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, M.J.; Jeon, Y.-J.; Lee, K.; Cheong, S.H.; Han, Y.S.; Park, S.R. Human Keratinocyte UVB-Protective Effects of a Low Molecular Weight Fucoidan from Sargassum horneri Purified by Step Gradient Ethanol Precipitation. Antioxidants 2020, 9, 340. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, M.J.; Jeon, Y.-J.; Ahn, G. Step gradient alcohol precipitation for the purification of low molecular weight fucoidan from Sargassum siliquastrum and its UVB protective effects. Int. J. Biol. Macromol. 2020, 163, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Wang, L.; Fu, X.; Ni, L.; Duan, D.; Xu, J.; Gao, X. Protective effect of a fucose-rich fucoidan isolated from Saccharina japonica against ultraviolet B-induced photodamage in vitro in human keratinocytes and in vivo in zebrafish. Mar. Drugs 2020, 18, 316. [Google Scholar] [CrossRef]
- Wang, L.; Oh, J.-Y.; Kim, Y.-S.; Lee, H.-G.; Lee, J.-S.; Jeon, Y.-J. Anti-Photoaging and Anti-Melanogenesis Effects of Fucoidan Isolated from Hizikia fusiforme and Its Underlying Mechanisms. Mar. Drugs 2020, 18, 427. [Google Scholar] [CrossRef]
- Wang, L.; Oh, J.-Y.; Lee, W.; Jeon, Y.-J. Fucoidan isolated from Hizikia fusiforme suppresses ultraviolet B-induced photodamage by down-regulating the expressions of matrix metalloproteinases and pro-inflammatory cytokines via inhibiting NF-κB, AP-1, and MAPK signaling pathways. Int. J. Biol. Macromol. 2021, 166, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Zayed, A.; El-Aasr, M.; Ibrahim, A.-R.S.; Ulber, R. Fucoidan Characterization: Determination of Purity and Physicochemical and Chemical Properties. Mar. Drugs 2020, 18, 571. [Google Scholar] [CrossRef] [PubMed]
- Mak, W.; Hamid, N.; Liu, T.; Lu, J.; White, W. Fucoidan from New Zealand Undaria pinnatifida: Monthly variations and determination of antioxidant activities. Carbohydr. Polym. 2013, 95, 606–614. [Google Scholar] [CrossRef]
- Ponce, N.M.A.; Stortz, C.A. A Comprehensive and Comparative Analysis of the Fucoidan Compositional Data across the Phaeophyceae. Front. Plant Sci. 2020, 11, 1844. [Google Scholar] [CrossRef] [PubMed]
- Morya, V.; Kim, J.; Kim, E.-K. Algal fucoidan: Structural and size-dependent bioactivities and their perspectives. Appl. Microbiol. Biotechnol. 2012, 93, 71–82. [Google Scholar] [CrossRef]
- García-Vaquero, M.; Rajauria, G.; O’doherty, J.; Sweeney, T. Polysaccharides from macroalgae: Recent advances, innovative technologies and challenges in extraction and purification. Food Res. Int. 2017, 99, 1011–1020. [Google Scholar] [CrossRef]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important determinants for fucoidan bioactivity: A critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef]
- Pozharitskaya, O.N.; Shikov, A.N.; Obluchinskaya, E.D.; Vuorela, H. The pharmacokinetics of fucoidan after topical application to rats. Mar. Drugs 2019, 17, 687. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, P.; Gupta, V.; Prakash, B. Application of nanotechnology to boost the functional and preservative properties of essential oils. In Functional and Preservative Properties of Phytochemicals; Elsevier: Amsterdam, The Netherlands, 2020; pp. 241–267. [Google Scholar]
- Pereira, L. Seaweeds as source of bioactive substances and skin care therapy—Cosmeceuticals, algotheraphy, and thalassotherapy. Cosmetics 2018, 5, 68. [Google Scholar] [CrossRef]
- de Souza, M.C.R.; Marques, C.T.; Dore, C.M.G.; da Silva, F.R.F.; Rocha, H.A.O.; Leite, E.L. Antioxidant activities of sulfated polysaccharides from brown and red seaweeds. J. Appl. Phycol. 2007, 19, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, E.; Barabanova, A.; Homenko, V.; Solov’eva, T.; Bogdanovich, R.; Yermak, I. In vitro and ex vivo studies of antioxidant activity of carrageenans, sulfated polysaccharides from red algae. Bull. Exp. Biol. Med. 2011, 150, 426–428. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Song, J.; Zhang, W.; Li, X.; Li, N.; Gao, X. Antioxidant activity and cytoprotective effect of κ-carrageenan oligosaccharides and their different derivatives. Bioorganic Med. Chem. Lett. 2006, 16, 1329–1334. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, B.; Wu, Y.; Liu, Y.; Gu, X.; Zhang, H.; Wang, C.; Cao, H.; Huang, L.; Wang, Z. Structural characterization and antioxidant activities of κ-carrageenan oligosaccharides degraded by different methods. Food Chem. 2015, 178, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Thevanayagam, H.; Mohamed, S.M.; Chu, W.-L. Assessment of UVB-photoprotective and antioxidative activities of carrageenan in keratinocytes. J. Appl. Phycol. 2014, 26, 1813–1821. [Google Scholar] [CrossRef]
- Shojaee-Aliabadi, S.; Hosseini, H.; Mohammadifar, M.A.; Mohammadi, A.; Ghasemlou, M.; Ojagh, S.M.; Hosseini, S.M.; Khaksar, R. Characterization of antioxidant-antimicrobial κ-carrageenan films containing Satureja hortensis essential oil. Int. J. Biol. Macromol. 2013, 52, 116–124. [Google Scholar] [CrossRef]
- Chen, L.; Hu, J.Y.; Wang, S.Q. The role of antioxidants in photoprotection: A critical review. J. Am. Acad. Dermatol. 2012, 67, 1013–1024. [Google Scholar] [CrossRef]
- Nantel, F.; Denis, D.; Gordon, R.; Northey, A.; Cirino, M.; Metters, K.M.; Chan, C.C. Distribution and regulation of cyclooxygenase 2 in carrageenan induced inflammation. Br. J. Pharmacol. 1999, 128, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Tripp, C.S.; Blomme, E.A.; Chinn, K.S.; Hardy, M.M.; LaCelle, P.; Pentland, A.P. Epidermal COX-2 induction following ultraviolet irradiation: Suggested mechanism for the role of COX-2 inhibition in photoprotection. J. Investig. Dermatol. 2003, 121, 853–861. [Google Scholar] [CrossRef]
- Purwaningsih, S.; Salamah, E.; Adnin, M.N. Efek fotoprotektif krim tabir surya dengan penambahan karaginan dan buah bakau hitam (Rhizopora mucronata Lamk.). J. Ilmu Dan Teknol. Kelaut. Trop. 2015, 7, 1–14. [Google Scholar]
- Abraham, R.E.; Su, P.; Puri, M.; Raston, C.L.; Zhang, W. Optimisation of biorefinery production of alginate, fucoidan and laminarin from brown seaweed Durvillaea potatorum. Algal Res. 2019, 38, 101389. [Google Scholar] [CrossRef]
- Ozanne, H.; Toumi, H.; Roubinet, B.; Landemarre, L.; Lespessailles, E.; Daniellou, R.; Cesaro, A. Laminarin Effects, a β-(1,3)-Glucan, on Skin Cell Inflammation and Oxidation. Cosmetics 2020, 7, 66. [Google Scholar] [CrossRef]
- Cuong, D.X. Laminarin (Beta-glucan) of Brown Algae Sargassum mcclurei: Extraction, Antioxidant Activity, Lipoxygenase Inhibition Activity, and Physicochemistry Properties. World J. Food Sci. Technol. 2020, 4, 31. [Google Scholar] [CrossRef]
- Sellimi, S.; Maalej, H.; Rekik, D.M.; Benslima, A.; Ksouda, G.; Hamdi, M.; Sahnoun, Z.; Li, S.; Nasri, M.; Hajji, M. Antioxidant, antibacterial and in vivo wound healing properties of laminaran purified from Cystoseira barbata seaweed. Int. J. Biol. Macromol. 2018, 119, 633–644. [Google Scholar] [CrossRef]
- Li, J.; Xie, L.; Qin, Y.; Liang, W.-H.; Mo, M.-Q.; Liu, S.-L.; Liang, F.; Wang, Y.; Tan, W.; Liang, Y. Effect of laminarin polysaccharide on activity of matrix metalloproteinase in photoaging skin. Zhongguo Zhong Yao Za Zhi 2013, 38, 2370–2373. [Google Scholar]
- Ahn, J.H.; Kim, D.W.; Park, C.W.; Kim, B.; Sim, H.; Kim, H.S.; Lee, T.-K.; Lee, J.-C.; Yang, G.E.; Her, Y. Laminarin Attenuates Ultraviolet-Induced Skin Damage by Reducing Superoxide Anion Levels and Increasing Endogenous Antioxidants in the Dorsal Skin of Mice. Mar. Drugs 2020, 18, 345. [Google Scholar] [CrossRef]
- Rajauria, G.; Ravindran, R.; Garcia-Vaquero, M.; Rai, D.K.; Sweeney, T.; O’Doherty, J. Molecular characteristics and antioxidant activity of laminarin extracted from the seaweed species Laminaria hyperborea, using hydrothermal-assisted extraction and a multi-step purification procedure. Food Hydrocoll. 2021, 112, 106332. [Google Scholar] [CrossRef]
- Choi, J.-I.; Kim, H.-J.; Lee, J.-W. Structural feature and antioxidant activity of low molecular weight laminarin degraded by gamma irradiation. Food Chem. 2011, 129, 520–523. [Google Scholar] [CrossRef]
- Choi, J.-I.; Kim, H.J.; Kim, J.H.; Lee, J.W. Enhanced biological activities of laminarin degraded by gamma-ray irradiation. J. Food Biochem. 2012, 36, 465–469. [Google Scholar] [CrossRef]
- Kadam, S.U.; O’Donnell, C.P.; Rai, D.K.; Hossain, M.B.; Burgess, C.M.; Walsh, D.; Tiwari, B.K. Laminarin from Irish Brown Seaweeds Ascophyllum nodosum and Laminaria hyperborea: Ultrasound Assisted Extraction, Characterization and Bioactivity. Mar. Drugs 2015, 13, 4270–4280. [Google Scholar] [CrossRef]
- Fernandes-Negreiros, M.M.; Batista, L.A.N.C.; Silva Viana, R.L.; Araujo Sabry, D.; Paiva, A.A.O.; Paiva, W.S.; Machado, R.I.A.; de Sousa Junior, F.L.; de Lima Pontes, D.; de Oliveira Vitoriano, J.; et al. Gallic Acid-Laminarin Conjugate Is a Better Antioxidant than Sulfated or Carboxylated Laminarin. Antioxidants 2020, 9, 1192. [Google Scholar] [CrossRef] [PubMed]
- Koivikko, R.; Loponen, J.; Honkanen, T.; Jormalainen, V. Contents of soluble, cell-wall-bound and exuded phlorotannins in the brown alga Fucus vesiculosus, with implications on their ecological functions. J. Chem. Ecol. 2005, 31, 195–212. [Google Scholar] [CrossRef] [PubMed]
- Lopes, G.; Sousa, C.; Silva, L.R.; Pinto, E.; Andrade, P.B.; Bernardo, J.; Mouga, T.; Valentão, P. Can phlorotannins purified extracts constitute a novel pharmacological alternative for microbial infections with associated inflammatory conditions? PLoS ONE 2012, 7, e31145. [Google Scholar] [CrossRef]
- Ko, S.-C.; Cha, S.-H.; Heo, S.-J.; Lee, S.-H.; Kang, S.-M.; Jeon, Y.-J. Protective effect of Ecklonia cava on UVB-induced oxidative stress: In vitro and in vivo zebrafish model. J. Appl. Phycol. 2011, 23, 697–708. [Google Scholar] [CrossRef]
- Milanović, Ž.; Tošović, J.; Marković, S.; Marković, Z. Comparison of the scavenging capacities of phloroglucinol and 2, 4, 6-trihydroxypyridine towards HO˙ radical: A computational study. RSC Adv. 2020, 10, 43262–43272. [Google Scholar] [CrossRef]
- Joe, M.-J.; Kim, S.-N.; Choi, H.-Y.; Shin, W.-S.; Park, G.-M.; Kang, D.-W.; Kim, Y.K. The inhibitory effects of eckol and dieckol from Ecklonia stolonifera on the expression of matrix metalloproteinase-1 in human dermal fibroblasts. Biol. Pharm. Bull. 2006, 29, 1735–1739. [Google Scholar] [CrossRef]
- Lee, J.-w.; Seok, J.K.; Boo, Y.C. Ecklonia cava extract and dieckol attenuate cellular lipid peroxidation in keratinocytes exposed to PM10. Evid. Based Complementary Altern. Med. 2018, 2018, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.W.; Song, H.; Hong, S.S.; Boo, Y.C. Marine alga ecklonia cava extract and dieckol attenuate prostaglandin E2 production in HaCaT keratinocytes exposed to airborne particulate matter. Antioxidants 2019, 8, 190. [Google Scholar] [CrossRef]
- Wang, L.; Lee, W.; Jayawardena, T.U.; Cha, S.-H.; Jeon, Y.-J. Dieckol, an algae-derived phenolic compound, suppresses airborne particulate matter-induced skin aging by inhibiting the expressions of pro-inflammatory cytokines and matrix metalloproteinases through regulating NF-κB, AP-1, and MAPKs signaling pathways. Food Chem. Toxicol. 2020, 146, 111823. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.C.; Piao, M.J.; Cho, S.J.; Lee, N.H.; Hyun, J.W. Phloroglucinol protects human keratinocytes from ultraviolet B radiation by attenuating oxidative stress. Photodermatol. Photoimmunol. Photomed. 2012, 28, 322–331. [Google Scholar] [CrossRef]
- Piao, M.J.; Ahn, M.J.; Kang, K.A.; Kim, K.C.; Zheng, J.; Yao, C.W.; Cha, J.W.; Hyun, C.L.; Kang, H.K.; Lee, N.H. Phloroglucinol inhibits ultraviolet B radiation-induced oxidative stress in the mouse skin. Int. J. Radiat. Biol. 2014, 90, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Im, A.R.; Nam, K.W.; Hyun, J.W.; Chae, S. Phloroglucinol reduces photodamage in hairless mice via matrix metalloproteinase activity through MAPK pathway. Photochem. Photobiol. 2016, 92, 173–179. [Google Scholar] [CrossRef]
- Piao, M.J.; Ahn, M.J.; Kang, K.A.; Kim, K.C.; Cha, J.W.; Lee, N.H.; Hyun, J.W. Phloroglucinol enhances the repair of UVB radiation-induced DNA damage via promotion of the nucleotide excision repair system in vitro and in vivo. DNA Repair 2015, 28, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Cha, H.-J.; Hong, S.H.; Kim, G.-Y.; Kim, S.; Kim, H.-S.; Kim, B.W.; Jeon, Y.-J.; Choi, Y.H. Protective effect of phloroglucinol on oxidative stress-induced DNA damage and apoptosis through activation of the Nrf2/HO-1 signaling pathway in HaCaT human keratinocytes. Mar. Drugs 2019, 17, 225. [Google Scholar] [CrossRef]
- Kang, K.A.; Zhang, R.; Piao, M.J.; Ko, D.O.; Wang, Z.H.; Lee, K.; Kim, B.J.; Shin, T.; Park, J.W.; Lee, N.H. Inhibitory effects of triphlorethol-A on MMP-1 induced by oxidative stress in human keratinocytes via ERK and AP-1 inhibition. J. Toxicol. Environ. HealthPart A 2008, 71, 992–999. [Google Scholar] [CrossRef] [PubMed]
- Piao, M.J.; Zhang, R.; Lee, N.H.; Hyun, J.W. Protective effect of triphlorethol-A against ultraviolet B-mediated damage of human keratinocytes. J. Photochem. Photobiol. B Biol. 2012, 106, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Piao, M.J.; Kumara, M.H.S.R.; Kim, K.C.; Kang, K.A.; Kang, H.K.; Lee, N.H.; Hyun, J.W. Diphlorethohydroxycarmalol suppresses ultraviolet B-induced matrix metalloproteinases via inhibition of JNK and ERK signaling in human keratinocytes. Biomol. Ther. 2015, 23, 557. [Google Scholar] [CrossRef]
- Wang, L.; Kim, H.S.; Je, J.-G.; Oh, J.Y.; Kim, Y.-S.; Cha, S.-H.; Jeon, Y.-J. Protective effect of diphlorethohydroxycarmalol isolated from Ishige okamurae against particulate matter-induced skin damage by regulation of NF-κB, AP-1, and MAPKs signaling pathways in vitro in human dermal fibroblasts. Molecules 2020, 25, 1055. [Google Scholar] [CrossRef]
- Wang, L.; Kim, H.S.; Oh, J.Y.; Je, J.G.; Jeon, Y.-J.; Ryu, B. Protective effect of diphlorethohydroxycarmalol isolated from Ishige okamurae against UVB-induced damage in vitro in human dermal fibroblasts and in vivo in zebrafish. Food Chem. Toxicol. 2020, 136, 110963. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.C.; Piao, M.J.; Zheng, J.; Yao, C.W.; Cha, J.W.; Kumara, M.H.S.R.; Han, X.; Kang, H.K.; Lee, N.H.; Hyun, J.W. Fucodiphlorethol G purified from Ecklonia cava suppresses ultraviolet B radiation-induced oxidative stress and cellular damage. Biomol. Ther. 2014, 22, 301. [Google Scholar] [CrossRef] [PubMed]
- Ham, Y.-M.; Baik, J.-S.; Hyun, J.-W.; Lee, N.-H. Isolation of a new phlorotannin, fucodiphlorethol G, from a brown alga Ecklonia cava. Bull. Korean Chem. Soc. 2007, 28, 1595–1597. [Google Scholar]
- Baxter, H.; Harborne, J.B.; Moss, G.P. Phytochemical Dictionary: A Handbook of Bioactive Compounds from Plants; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Miyamoto, K.T.; Komatsu, M.; Ikeda, H. Discovery of gene cluster for mycosporine-like amino acid biosynthesis from Actinomycetales microorganisms and production of a novel mycosporine-like amino acid by heterologous expression. Appl. Environ. Microbiol. 2014, 80, 5028–5036. [Google Scholar] [CrossRef] [PubMed]
- Wada, N.; Sakamoto, T.; Matsugo, S. Mycosporine-like amino acids and their derivatives as natural antioxidants. Antioxidants 2015, 4, 603–646. [Google Scholar] [CrossRef]
- Karsten, U.; Friedl, T.; Schumann, R.; Hoyer, K.; Lembcke, S. Mycosporine-like amino acids and phylogenies in green algae: Prasiola and its relatives from the trebouxiophyceae (chlorophyta) 1. J. Phycol. 2005, 41, 557–566. [Google Scholar] [CrossRef]
- Bhatia, S.; Garg, A.; Sharma, K.; Kumar, S.; Sharma, A.; Purohit, A.P. Mycosporine and mycosporine-like amino acids: A paramount tool against ultra violet irradiation. Pharm. Rev. 2011, 5, 138–146. [Google Scholar] [CrossRef]
- Singh, D.K.; Pathak, J.; Pandey, A.; Singh, V.; Ahmed, H.; Rajneesh; Kumar, D.; Sinha, R.P. Chapter 15—Ultraviolet-screening compound mycosporine-like amino acids in cyanobacteria: Biosynthesis, functions, and applications. In Advances in Cyanobacterial Biology; Singh, P.K., Kumar, A., Singh, V.K., Shrivastava, A.K., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 219–233. [Google Scholar]
- Kim, S.Y.; Cho, W.K.; Kim, H.-I.; Paek, S.H.; Jang, S.J.; Jo, Y.; Choi, H.; Lee, J.H.; Moh, S.H. Transcriptome Profiling of Human Follicle Dermal Papilla Cells in response to Porphyra-334 Treatment by RNA-Seq. Evid. Based Complementary Altern. Med. 2021, 2021, 6637513. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, N.; Zhou, J.; Dong, S.; Zhang, X.; Guo, L.; Guo, G. Distribution, contents, and types of mycosporine-like amino acids (MAAs) in marine macroalgae and a database for MAAs based on these characteristics. Mar. Drugs 2020, 18, 43. [Google Scholar] [CrossRef] [PubMed]
- De la Coba, F.; Aguilera, J.; Figueroa, F.L.; De Gálvez, M.; Herrera, E. Antioxidant activity of mycosporine-like amino acids isolated from three red macroalgae and one marine lichen. J. Appl. Phycol. 2009, 21, 161–169. [Google Scholar] [CrossRef]
- Hartmann, A.; Becker, K.; Karsten, U.; Remias, D.; Ganzera, M. Analysis of mycosporine-like amino acids in selected algae and cyanobacteria by hydrophilic interaction liquid chromatography and a novel MAA from the red alga Catenella repens. Mar. Drugs 2015, 13, 6291–6305. [Google Scholar] [CrossRef]
- Álvarez-Gómez, F.; Korbee, N.; Casas-Arrojo, V.; Abdala-Díaz, R.T.; Figueroa, F.L. UV photoprotection, cytotoxicity and immunology capacity of red algae extracts. Molecules 2019, 24, 341. [Google Scholar] [CrossRef]
- Torres, P.; Nagai, A.; Teixeira, D.I.A.; Marinho-Soriano, E.; Chow, F.; dos Santos, D.Y. Brazilian native species of Gracilaria (Gracilariales, Rhodophyta) as a source of valuable compounds and as nutritional supplements. J. Appl. Phycol. 2019, 31, 3163–3173. [Google Scholar] [CrossRef]
- Nishida, Y.; Kumagai, Y.; Michiba, S.; Yasui, H.; Kishimura, H. Efficient extraction and antioxidant capacity of mycosporine-like amino acids from red alga Dulse Palmaria palmata in Japan. Mar. Drugs 2020, 18, 502. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Park, S.-J.; Kim, I.-H.; Choi, Y.H.; Nam, T.-J. Protective effect of porphyra-334 on UVA-induced photoaging in human skin fibroblasts. Int. J. Mol. Med. 2014, 34, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Athukorala, Y.; Trang, S.; Kwok, C.; Yuan, Y.V. Antiproliferative and antioxidant activities and mycosporine-like amino acid profiles of wild-harvested and cultivated edible Canadian marine red macroalgae. Molecules 2016, 21, 119. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.-S.; Oh, S.K.; Lee, S.G.; Kim, I.-C.; Kim, S. Porphyra-334, a mycosporine-like amino acid, attenuates UV-induced apoptosis in HaCaT cells. Acta Pharm. 2017, 67, 257–264. [Google Scholar] [CrossRef]
- Gacesa, R.; Lawrence, K.P.; Georgakopoulos, N.D.; Yabe, K.; Dunlap, W.C.; Barlow, D.J.; Wells, G.; Young, A.R.; Long, P.F. The mycosporine-like amino acids porphyra-334 and shinorine are antioxidants and direct antagonists of Keap1-Nrf2 binding. Biochimie 2018, 154, 35–44. [Google Scholar] [CrossRef]
- Ying, R.; Zhang, Z.; Zhu, H.; Li, B.; Hou, H. The protective effect of mycosporine-like amino acids (MAAs) from Porphyra yezoensis in a mouse model of UV irradiation-induced photoaging. Mar. Drugs 2019, 17, 470. [Google Scholar] [CrossRef]
- Cho, M.J.; Jung, H.S.; Song, M.Y.; Seo, H.H.; Kulkarni, A.; Suh, S.S.; Lee, T.K.; Moh, S.H. Effect of sun screen utilizing Porphyra-334 derived from ocean algae for skin protection. J. Korea Acad. Ind. Coop. Soc. 2014, 15, 4272–4278. [Google Scholar]
- Pangestuti, R.; Bak, S.S.; Kim, S.K. Attenuation of pro-inflammatory mediators in LPS-stimulated BV2 microglia by chitooligosaccharides via the MAPK signaling pathway. Int. J. Biol. Macromol. 2011, 49, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Hartmann, A.; Ganzera, M.; Fuchs, D.; Gostner, J.M. Immunomodulatory effects of the mycosporine-like amino acids shinorine and porphyra-334. Mar. Drugs 2016, 14, 119. [Google Scholar] [CrossRef] [PubMed]
- Terazawa, S.; Nakano, M.; Yamamoto, A.; Imokawa, G. Mycosporine-like amino acids stimulate hyaluronan secretion by up-regulating hyaluronan synthase 2 via activation of the p38/MSK1/CREB/c-Fos/AP-1 axis: MAAs stimulate the secretion of HA via HAS2. J. Biol. Chem. 2020, 295, 7274–7288. [Google Scholar] [CrossRef]
- Rui, Y.; Zhaohui, Z.; Wenshan, S.; Bafang, L.; Hu, H. Protective effect of MAAs extracted from Porphyra tenera against UV irradiation-induced photoaging in mouse skin. J. Photochem. Photobiol. B Biol. 2019, 192, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Reilly, D.M.; Lozano, J. Skin collagen through the lifestages: Importance for skin health and beauty. Plast. Aesthetic Res. 2021, 8. [Google Scholar] [CrossRef]
- Orfanoudaki, M.; Hartmann, A.; Alilou, M.; Gelbrich, T.; Planchenault, P.; Derbré, S.; Schinkovitz, A.; Richomme, P.; Hensel, A.; Ganzera, M. Absolute configuration of mycosporine-like amino acids, their wound healing properties and in vitro anti-aging effects. Mar. Drugs 2020, 18, 35. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.-S.; Lee, S.G.; Youn, U.J.; Han, S.J.; Kim, I.-C.; Kim, S. Comprehensive expression profiling and functional network analysis of porphyra-334, one mycosporine-like amino acid (MAA), in human keratinocyte exposed with UV-radiation. Mar. Drugs 2017, 15, 196. [Google Scholar] [CrossRef]
- Daniel, S.; Cornelia, S.; Fred, Z. UV-A sunscreen from red algae for protection against premature skin aging. Cosmet Toilet. Manuf. Worldw. 2004, 2004, 139–143. [Google Scholar]
- Bhatia, S.; Sharma, K.; Namdeo, A.G.; Chaugule, B.; Kavale, M.; Nanda, S. Broad-spectrum sun-protective action of Porphyra-334 derived from Porphyra vietnamensis. Pharmacogn. Res. 2010, 2, 45. [Google Scholar] [CrossRef] [PubMed]
- de la Coba, F.; Aguilera, J.; Korbee, N.; de Gálvez, M.V.; Herrera-Ceballos, E.; Álvarez-Gómez, F.; Figueroa, F.L. UVA and UVB photoprotective capabilities of topical formulations containing mycosporine-like amino acids (MAAs) through different Biological Effective Protection Factors (BEPFs). Mar. Drugs 2019, 17, 55. [Google Scholar] [CrossRef] [PubMed]
- Proctor, M.S.; Pazderník, M.; Jackson, P.J.; Pilný, J.; Martin, E.C.; Dickman, M.J.; Canniffe, D.P.; Johnson, M.P.; Hunter, C.N.; Sobotka, R.; et al. Xanthophyll carotenoids stabilise the association of cyanobacterial chlorophyll synthase with the LHC-like protein HliD. Biochem. J. 2020, 477, 4021–4036. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef]
- Heo, S.-J.; Jeon, Y.-J. Protective effect of fucoxanthin isolated from Sargassum siliquastrum on UV-B induced cell damage. J. Photochem. Photobiol. B Biol. 2009, 95, 101–107. [Google Scholar] [CrossRef]
- Urikura, I.; Sugawara, T.; Hirata, T. Protective effect of fucoxanthin against UVB-induced skin photoaging in hairless mice. Biosci. Biotechnol. Biochem. 2011, 75, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Piao, M.J.; Keum, Y.S.; Kim, H.S.; Hyun, J.W. Fucoxanthin protects cultured human keratinocytes against oxidative stress by blocking free radicals and inhibiting apoptosis. Biomol. Ther. 2013, 21, 270. [Google Scholar] [CrossRef]
- Zheng, J.; Piao, M.J.; Kim, K.C.; Yao, C.W.; Cha, J.W.; Hyun, J.W. Fucoxanthin enhances the level of reduced glutathione via the Nrf2-mediated pathway in human keratinocytes. Mar. Drugs 2014, 12, 4214–4230. [Google Scholar] [CrossRef]
- Miyashita, K.; Beppu, F.; Hosokawa, M.; Liu, X.; Wang, S. Nutraceutical characteristics of the brown seaweed carotenoid fucoxanthin. Arch. Biochem. Biophys. 2020, 686, 108364. [Google Scholar] [CrossRef]
- Rodríguez-Luna, A.; Ávila-Román, J.; González-Rodríguez, M.L.; Cózar, M.J.; Rabasco, A.M.; Motilva, V.; Talero, E. Fucoxanthin-containing cream prevents epidermal hyperplasia and UVB-induced skin erythema in mice. Mar. Drugs 2018, 16, 378. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Luna, A.; Ávila-Román, J.; Oliveira, H.; Motilva, V.; Talero, E. Fucoxanthin and rosmarinic acid combination has anti-inflammatory effects through regulation of NLRP3 inflammasome in UVB-exposed HaCaT keratinocytes. Mar. Drugs 2019, 17, 451. [Google Scholar] [CrossRef]
- Wang, Z.; Man, M.-Q.; Li, T.; Elias, P.M.; Mauro, T.M. Aging-associated alterations in epidermal function and their clinical significance. Aging 2020, 12, 5551–5565. [Google Scholar] [CrossRef] [PubMed]
- Matsui, M.; Tanaka, K.; Higashiguchi, N.; Okawa, H.; Yamada, Y.; Tanaka, K.; Taira, S.; Aoyama, T.; Takanishi, M.; Natsume, C. Protective and therapeutic effects of fucoxanthin against sunburn caused by UV irradiation. J. Pharmacol. Sci. 2016, 132, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H.; Eun, H.C. Angiogenesis in skin aging and photoaging. J. Dermatol. 2007, 34, 593–600. [Google Scholar] [CrossRef]
- Chen, S.-J.; Lee, C.-J.; Lin, T.-B.; Liu, H.-J.; Huang, S.-Y.; Chen, J.-Z.; Tseng, K.-W. Inhibition of Ultraviolet B-Induced Expression of the Proinflammatory Cytokines TNF-α and VEGF in the Cornea by Fucoxanthin Treatment in a Rat Model. Mar. Drugs 2016, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Spagolla Napoleão Tavares, R.; Maria-Engler, S.S.; Colepicolo, P.; Debonsi, H.M.; Schäfer-Korting, M.; Marx, U.; Gaspar, L.R.; Zoschke, C. Skin irritation testing beyond tissue viability: Fucoxanthin effects on inflammation, homeostasis, and metabolism. Pharmaceutics 2020, 12, 136. [Google Scholar] [CrossRef]
- Gomez-Zavaglia, A.; Prieto Lage, M.A.; Jimenez-Lopez, C.; Mejuto, J.C.; Simal-Gandara, J. The Potential of Seaweeds as a Source of Functional Ingredients of Prebiotic and Antioxidant Value. Antioxidantsc 2019, 8, 406. [Google Scholar] [CrossRef]
- Pereira, R.; Yarish, C. Mass Production of Marine Macroalgae. In Encyclopedia of Ecology; Jørgensen, S.E., Fath, B.D., Eds.; Academic Press: Oxford, UK, 2008; pp. 2236–2247. [Google Scholar]
- García-Poza, S.; Leandro, A.; Cotas, C.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M. The Evolution Road of Seaweed Aquaculture: Cultivation Technologies and the Industry 4.0. Int. J. Environ. Res. Public Health 2020, 17, 6528. [Google Scholar] [CrossRef]
- Hayashi, L.; Cantarino, S.D.J.; Critchley, A.T. Challenges to the future domestication of seaweeds as cultivated species: Understanding their physiological processes for large-scale production. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2020; Volume 95, pp. 57–83. [Google Scholar]
- Chary, K.; Aubin, J.; Sadoul, B.; Fiandrino, A.; Covès, D.; Callier, M.D. Integrated multi-trophic aquaculture of red drum (Sciaenops ocellatus) and sea cucumber (Holothuria scabra): Assessing bioremediation and life-cycle impacts. Aquaculture 2020, 516, 734621. [Google Scholar] [CrossRef]
- Pliego-Cortés, H.; Bedoux, G.; Boulho, R.; Taupin, L.; Freile-Pelegrín, Y.; Bourgougnon, N.; Robledo, D. Stress tolerance and photoadaptation to solar radiation in Rhodymenia pseudopalmata (Rhodophyta) through mycosporine-like amino acids, phenolic compounds, and pigments in an Integrated Multi-Trophic Aquaculture system. Algal Res. 2019, 41, 101542. [Google Scholar] [CrossRef]
- Barceló-Villalobos, M.; Figueroa, F.L.; Korbee, N.; Álvarez-Gómez, F.; Abreu, M.H. Production of mycosporine-like amino acids from Gracilaria vermiculophylla (Rhodophyta) cultured through one year in an Integrated Multi-Trophic Aquaculture (IMTA) system. Mar. Biotechnol. 2017, 19, 246–254. [Google Scholar] [CrossRef]
- Noori, N.; Dukkipati, R.; Kovesdy, C.P.; Sim, J.J.; Feroze, U.; Murali, S.B.; Bross, R.; Benner, D.; Kopple, J.D.; Kalantar-Zadeh, K. Dietary Omega-3 Fatty Acid, Ratio of Omega-6 to Omega-3 Intake, Inflammation, and Survival in Long-term Hemodialysis Patients. Am. J. Kidney Dis. 2011, 58, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Lourenço-Lopes, C.; Garcia-Oliveira, P.; Carpena, M.; Fraga-Corral, M.; Jimenez-Lopez, C.; Pereira, A.G.; Prieto, M.A.; Simal-Gandara, J. Scientific approaches on extraction, purification and stability for the commercialization of fucoxanthin recovered from brown algae. Foods 2020, 9, 1113. [Google Scholar] [CrossRef]
- Gereniu, C.R.N.; Saravana, P.S.; Chun, B.-S. Recovery of carrageenan from Solomon Islands red seaweed using ionic liquid-assisted subcritical water extraction. Sep. Purif. Technol. 2018, 196, 309–317. [Google Scholar] [CrossRef]
- Saravana, P.S.; Tilahun, A.; Gerenew, C.; Tri, V.D.; Kim, N.H.; Kim, G.-D.; Woo, H.-C.; Chun, B.-S. Subcritical water extraction of fucoidan from Saccharina japonica: Optimization, characterization and biological studies. J. Appl. Phycol. 2018, 30, 579–590. [Google Scholar] [CrossRef]
- Fung, A.; Hamid, N.; Lu, J. Fucoxanthin content and antioxidant properties of Undaria pinnatifida. Food Chem. 2013, 136, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Roh, M.K.; Uddin, M.S.; Chun, B.S. Extraction of fucoxanthin and polyphenol from Undaria pinnatifida using supercritical carbon dioxide with co-solvent. Biotechnol. Bioprocess Eng. 2008, 13, 724–729. [Google Scholar] [CrossRef]
- Quitain, A.T.; Kai, T.; Sasaki, M.; Goto, M. Supercritical carbon dioxide extraction of fucoxanthin from Undaria pinnatifida. J. Agric. Food Chem. 2013, 61, 5792–5797. [Google Scholar] [CrossRef] [PubMed]
- Eom, S.J.; Kim, Y.E.; Kim, J.-E.; Park, J.; Kim, Y.H.; Song, K.-M.; Lee, N.H. Production of Undaria pinnatifida sporophyll extract using pilot-scale ultrasound-assisted extraction: Extract characteristics and antioxidant and anti-inflammatory activities. Algal Res. 2020, 51, 102039. [Google Scholar] [CrossRef]
- Xiao, X.; Si, X.; Yuan, Z.; Xu, X.; Li, G. Isolation of fucoxanthin from edible brown algae by microwave-assisted extraction coupled with high-speed countercurrent chromatography. J. Sep. Sci. 2012, 35, 2313–2317. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zeng, H.; Huang, J.; Jiang, L.; Chen, J.; Zeng, Q. Traditional Asian herbs in skin whitening: The current development and limitations. Front. Pharmacol. 2020, 11, 982. [Google Scholar] [CrossRef]
- Gelyma Helionori—Natural Sun Protection Thanks to Marine UVA Filters. Available online: http://www.gelyma.com/helionori.html (accessed on 7 February 2021).
- Mibellebiochemistry Helioguard™ 365 A Natural UV-Screening Active to Protect Against Photo-Aging. Available online: https://mibellebiochemistry.com/helioguardtm-365 (accessed on 7 February 2021).
- Laboratoires-biarritz Suncare Organic—Alga Maris®. Available online: https://www.laboratoires-biarritz.com/fr/ (accessed on 7 February 2021).
- Marinova Marinova Product Portofolio. Available online: https://www.marinova.com.au/product-portfolio/ (accessed on 7 February 2021).
| Class | Species | Origin | Extracts | Test | Functions | Mechanisms | Ref |
|---|---|---|---|---|---|---|---|
| Rhodophyceae | Solieria chordalis | France | MeOH extract/CPC fractionation n-heptane/EtOAc//MeOH/dW (19/1//19/1; v/v) | - | Photoprotective | UV absorption | [29] |
| Antioxidant | DPPH radical scavenging activity | ||||||
| Bryothamnion triquetrum | Cuba | Aqueous extract | UVC-irradiated plasmid DNA | Photoprotective | (↓) DNA dmage | [23] | |
| Porphyra umbilicalis | France | Cosmetic formula (5% extract) with Ginkgo biloba, vitamins | UVA/B-irradiated mice | Cell renewal | transepidermal water loss (TEWL) and erythema index. | [28] | |
| Anti-apoptosis | (↓) p53 and caspase-3 | ||||||
| Porphyra yezoensis | Korea | EtOH extract (80%)/Chl/MeOH/dW (2/1/0.9) | UVB irradiated HaCaT | Photoprotective | Absorb UVB rays | [26] | |
| Antioxidant | (↑) GSH/GSSG ratio | ||||||
| Gelidium amansii | Korea | Mix with Cirsium japonicum; MeOH extract and fermentation | UVB-irradiated HS 68 DF& SKH-1 hairless mice | Inhibit collagen degradation; wrinkle formation | (↑) type I pro-collagen; (↓) MMP-1; -2; -9; -13 | [30] | |
| Polyopes affinis | Korea | EtOH extract | UVB irradiated HaCaT | Antioxidant | (↓) intracellular ROS; (↓) superoxide radical (↓) hydroxyl radical; (↓) cellular damage | [31] | |
| Anti-apoptosis | NA | ||||||
| Photoprotective | Absorb UVB rays | ||||||
| Solieria chordalis | France | EtOAc; 2-OD and OE L-PCA extract | - | Photoprotective | Absorb UVB rays | [32] | |
| Protect synthetic chlorophyll sol. from UVB | |||||||
| Polysiphonia morrowii | Korea | 80% EtOH | UVB irradiated HaCaT | Antioxidant | (↓) intracellular ROS; (↑) antioxidant enzyme | [33] | |
| Anti-apoptosis | (↓) TUNEL-positive cells and DNA fragmentation | ||||||
| Chondracanthus tenellus | Korea | 80% EtOH | UVB irradiated HaCaT | Antioxidant | (↓) intracellular ROS; (↓) superoxide radical (↓) hydroxyl radical; (↓) cellular damage | [34] | |
| Anti-apoptosis | NA | ||||||
| Photoprotective | Absorb UVB rays | ||||||
| Bonnemaisonia hamifera | Korea | 80% EtOH | UVB irradiated HaCaT | Antioxidant | (↓) intracellular ROS; (↓) superoxide radical (↓) hydroxyl radical | [35] | |
| Anti-apoptosis | (↓) TUNEL-positive cells and DNA fragmentation | ||||||
| Photoprotective | Absorb UVB rays | ||||||
| Lomentaria hakodatensis | Korea | 80% EtOH | UVB irradiated HaCaT | Antioxidant | (↓) superoxide radical; (↓) hydroxyl radical | [36] | |
| Anti-apoptosis | (↓) DNA fragmentation (↓) apoptotic bodies | ||||||
| Photoprotective | Absorb UV rays | ||||||
| Macrocystis pyrifera | Argentina | Ace extract | UVB irradiated zebrafish embryo | Photoprotective | Survival of normal embryos (100%) | [25] | |
| Porphyra columbina | Argentina | Ace extract | Survival of normal embryos (100%) | ||||
| Sarcothalia radula | Spain | Ace extract | Survival of normal embryos (91.7%) | ||||
| Gigartina skottsbergii | Argentina | Ace extract | Survival of normal embryos (73.6%) | ||||
| Curdiea racovitzae | Antarctic | MeOH, aqueous extract | UVA irradiated fibroblast | Photoprotective | Absorb UVA and UVB rays | [27] | |
| (↑) cell proliferations | |||||||
| Antioxidant | (↓) DPPH radical; ROS;(↓) superoxide radical | ||||||
| Iridaea cordata | Antarctic | MeOH, aqueous extract | UVA irradiated fibroblast | Photoprotective | Absorb UVA and UVB rays | [27] | |
| (↑) cell proliferations | |||||||
| Antioxidant | (↓) DPPH radical; ROS; (↓) superoxide radical | ||||||
| Chlorophyceae | Halimeda incrassata | Cuba | Aqueous extract | UVC-irradiated plasmid DNA | Photoprotective | (↓) DNA damage | [23] |
| Caulerpa sp. | Indonesia | EtOH extract | UVB irradiated mice | Inhibit collagen degradation | (↓) MMP-1; | [24] | |
| Phaeophyceae | Sargassum muticum | Korea | 80% EtOH; EtOAc fraction | UVB irradiated HaCaT | Antioxidant | (↓) intracellular ROS; (↑) antioxidant enzyme | [37,38,39] |
| Anti-apoptosis | (↓) TUNEL-positive cells and DNA fragmentation; regulation of MAPK- and caspase-dependent signaling pathways; (↑) Bcl-2 and Mcl-1; (↓) Bax; (↓) caspase-9 and caspase-3 | ||||||
| Photoprotective | Absorb UVB rays | ||||||
| Inhibit collagen degradation | (↓) MMP-1; (↓) AP-1 | ||||||
| Sargassum glaucescens | Taiwan | Aqueous extract | UVA irradiated HaCaT | Antioxidant | (↓) intracellular ROS; (↑) antioxidant enzyme | [40] | |
| Sargassum cristafolium | Indonesia | EtOH extract | UVA irradiated HeLa; BALBL/c mice | Photoprotective | Absorb UVA rays; (↓) cellular damage | [41] | |
| Fucus spiralis | Portugal | EtOH;Cyclohex; EtOAc; Et2O; aqueous extract; | UVB irradiated HaCaT | Photoprotective | Absorb UVA; UVB; UVC rays | [20] | |
| Antioxidant | (↓) intracellular ROS; (↑) antioxidant enzyme | ||||||
| Mazzaella laminarioides | Chile | Ace extract | UVB irradiated zebrafish embryo | Survival of normal embryos (91.7%) | [25] | ||
| Undaria crenata | Korea | 80% EtOH | UVB irradiated HaCaT | Antioxidant | (↓) intracellular ROS; (↓) superoxide radical (↓) hydroxyl radical; | [42] | |
| Anti-apoptosis | (↓) apoptotic bodies and DNA fragmentation | ||||||
| Photoprotective | Absorb UVB rays | ||||||
| Carpomitra costata | Korea | 80% EtOH | UVB irradiated HaCaT | Antioxidant | (↓) intracellular ROS; (↓) superoxide radical (↓) hydroxyl radical; (↑) antioxidant enzyme | [43] | |
| Anti-apoptosis | (↑) Bcl-2; (↓) Bax(↓) caspase-9 and caspase-3 | ||||||
| Ecklonia stolonifera | Korea | 80% EtOH | UVA irradiated HDF | Antioxidant | (↓) intracellular ROS; | [44] | |
| Inhibit collagen degradation | (↓) MMP-1; -3 |
| Algae Source | Hizikia fusiforme | Sargassum fusiforme | Sargassum vachellianum | Ecklonia maxima |
|---|---|---|---|---|
| Carbohydrate (%) | NA | 58.10 | 53.51 | 69.37 |
| Sulfated polysaccharide (%) | 63.56 | NA | NA | NA |
| Sulfated group (%) | NA | 13.18 | 12.32 | 10.51 |
| Xylose (%) | 17.37 | 5.90 | 3.5 | NA |
| Galactose (%) | 23.15 | 18.40 | 9.3 | NA |
| Glucose (%) | NA | 1.50 | 2.20 | NA |
| Fucose (%) | 53.53 | 43.20 | 49.5 | NA |
| Rhamnose (%) | NA | 3.50 | NA | NA |
| Fructose (%) | NA | 18.50 | NA | NA |
| Mannose (%) | NA | 9 | 11.2 | NA |
| Glucuronic acid (%) | NA | 15.35 | 1.01 | NA |
| [46,47] | [48] | [21] | [49] |
| Algae Source | S. hemiphyllum | S. hemiphyllum | E. cava | S. horneri |
|---|---|---|---|---|
| Fucose | 208.2 ± 2.3 (μmol/g) | 210.9 ± 3.3 (μmol/g) | NA | 37.43% |
| Sulfate (%) | 40.1 ± 0.9 | 38.9 ± 0.4 | NA | 28.01 ± 0.50% |
| Average MW (kDa) | 270 | 0.8 | ~8 | 60 |
| Ref | [62] | [62] | [63] | [64] |
| Phlorotannins | Seaweeds | Origin | Anti-Photoaging | Ref |
|---|---|---|---|---|
| Eckol | Ecklonia stolonifera; Ecklonia cava | Korea | Inhibit NF-κB, AP-1, MMP-1 expression Protect UVB-induced cell damage; (↓) Pro-inflammatory mediators | [101,103,104] |
| Dieckol | Ecklonia stolonifera | Korea | Inhibit NF-κB, AP-1, MMP-1 expression Protect UVB-induced cell damage; (↓) Pro-inflammatory mediators | [103,104,105,106] |
| Phloroglucinol | Ecklonia cava | Korea | (↓) hydroxyl and superoxide radical, intracellular ROS; (↑) SOD, GSH; Activate Nrf2/HO-1 Inhibit NF-κB, MAPK; MMP-1 expression (↓) Bax; Caspase-3 (↓) Pro-inflammatory mediators | [101,107,108,109,110,111] |
| Triphlorethol-A | Ecklonia cava | Korea | Protect UVB-induced cell damage; (↓) intracellular ROS; Inhibit MAPK; MMP-1 expression (↓) Caspase-3 and -9 Strong absorption in UVB spectra | [101,112,113] |
| Eckstolonol | Ecklonia cava | Korea | Protect UVB-induced cell damage | [101] |
| Diphlorethohydroxycarmalol | Ishige okamurae | Korea | Inhibit MAPK; MMP-1; -2; -9 expression (↓) Pro-inflammatory mediators (↓) cellular damage | [114,115,116] |
| Fucodiphlorethol G | Ecklonia cava | Korea | (↓) DPPH, intracellular ROS; caspase-9 UVB absorption | [117,118] |
| Species | Origin | PI | AS | SH | PR | Myc-gly | Usu+PI | PL | CL | Total | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ahnfeltiopsis devoniensis (mg/g) | Spain | NA | NA | 0.55 | NA | NA | NA | NA | NA | NA | [127] |
| Curdiea racovitzae (μg/mg) | Antarctic | 111.49 | 36.51 | 2.17 | NA | NA | NA | NA | NA | 150.17 | [27] |
| Catenella repens (mg/g) | France | NA | NA | NA | NA | NA | NA | NA | 1.76 | NA | [128] |
| Catenella caespitosa (mg/g) | Puerto Rico | NA | NA | NA | NA | NA | NA | NA | 1.06 | NA | [128] |
| Gelidium corneum (mg/g) | Spain | 0.13 | 0.47 | 0.1 | NA | NA | NA | NA | NA | NA | [127] |
| Gracilariopsis longissima (mg/g) | NA | NA | NA | NA | NA | NA | NA | NA | NA | 1.6 | [129] |
| Gracilaria birdiae (mg/100 g) | Brazil | 14.67 | NA | 52.70 | 178.39 | NA | NA | NA | NA | 245.77 | [130] |
| Gracilaria caudate (mg/100g) | Brazil | 34.55 | NA | 32.20 | 48.15 | NA | NA | NA | NA | 114.90 | [130] |
| Gracilaria domingensis (mg/g) | Brazil | 10.41 | 1.25 | 7.56 | 28.82 | NA | NA | 1.54 | NA | 49.59 | [130] |
| Hydropuntia cornea (mg/g) | NA | NA | NA | NA | NA | NA | NA | NA | NA | 0.8 | [129] |
| Iridaea cordata (μg/mg) | Antarctic | 49.45 | 7.58 | 3.75 | NA | NA | NA | NA | NA | 60.78 | [27] |
| Palmaria palmata (µmol/g) | Japan | 2.964 | 0.078 | 0.155 | 1.900 | 0.276 | 0.276 | NA | NA | 5.372 | [131] |
| Palmaria palmata (mg/g) | UK | 9.94 | 0.08 | 0.63 | 0.56 | NA | NA | 0.11 | NA | NA | [128] |
| Porphyra rosengurttii (mg/g) | Spain | 0.17 | 0.14 | 0.38 | 3.84 | NA | NA | NA | NA | NA | [127] |
| Extraction Method | Solvent Extraction | Solvent Extraction | SFE | SFE | UAE | MAE |
|---|---|---|---|---|---|---|
| Solvent | MeOH (1:50 w/v) | MeOH (1:50 w/v) | CO2 and EtOH (3%, v/v) | CO2 | Deionized H2O (1:100 w/v) | EtOH (15:1 w/v) |
| Pretreatment | Wash, salted, boiled, blanched, cured | Avoid sunlight | Freeze dry | Milling and microwave assisted cell disruption | NA | NA |
| Extraction condition | 1 h, RT | 1 h, RT | 50 bar, 200 °C, 1 h | 40 bar, 400 °C, 3 h | 800 W, 80% amplitude, 20 kHz, 30 °C, 3 h. | ratios, 60 °C, 10 min, 300 W |
| Yield | 2.08 ± 0.04 mg/g | 4.96 ± 0.4 mg/g | 0.00753 μg/g | 38.5 mg/g | 0.031 mg/g | 2.12 mg/100 g |
| Notes | Processed | Fresh | Pressure and temperature affect yield | MW pretreatment increased fucoxanthin yield | Sporophyll; small pilot scale | No effect on microwave power |
| Ref | [173] | [173] | [174] | [175] | [176] | [177] |
| Algae Species | Trade Name | Company | Active Ingredients | Anti-Photoaging | Ref |
|---|---|---|---|---|---|
| Poprphyra umbilicalis | Helionori® | Gelyma, French | MAAs | Photoprotective (UV-A) DNA protection Prevention of sunburn | [179] |
| Poprphyra umbilicalis | Helioguard365 | Mibelle Biochemistry, Switzerland | Porphyra-334 and Shinorine | Photoprotective (UV-A) | [180] |
| Poprphyra umbilicalis | Algae gorria; Alga marris | Laboratoires de biarritz, French | NA | Photoprotective (UV-A) | [181] |
| Undaria pinnatifida | Fucorich | Marinova, Australia | Fucoidan | Anti-aging | [182] |
| Fucus vesiculosus | Maritech reverse | Marinova, Australia | Fucoidan | Anti-aging; antioxidant; anti-inflammation | [182] |
| Fucus vesiculosus | Maritech synergy | Marinova, Australia | Fucoidan and polyphenol complex | Anti-aging; antioxidant; anti-inflammation | [182] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pangestuti, R.; Shin, K.-H.; Kim, S.-K. Anti-Photoaging and Potential Skin Health Benefits of Seaweeds. Mar. Drugs 2021, 19, 172. https://doi.org/10.3390/md19030172
Pangestuti R, Shin K-H, Kim S-K. Anti-Photoaging and Potential Skin Health Benefits of Seaweeds. Marine Drugs. 2021; 19(3):172. https://doi.org/10.3390/md19030172
Chicago/Turabian StylePangestuti, Ratih, Kyung-Hoon Shin, and Se-Kwon Kim. 2021. "Anti-Photoaging and Potential Skin Health Benefits of Seaweeds" Marine Drugs 19, no. 3: 172. https://doi.org/10.3390/md19030172
APA StylePangestuti, R., Shin, K.-H., & Kim, S.-K. (2021). Anti-Photoaging and Potential Skin Health Benefits of Seaweeds. Marine Drugs, 19(3), 172. https://doi.org/10.3390/md19030172








