Stem Cell Therapy for Neonatal Hypoxic-Ischemic Encephalopathy: A Systematic Review of Preclinical Studies
Abstract
:1. Introduction
2. Therapeutic Hypothermia—The Standard of Care
3. The Potential of Stem Cell Therapy for HIE
3.1. Umbilical Cord Blood Cells
3.2. Mesenchymal Stem/Stromal Cells
3.2.1. Umbilical Cord Tissue-Derived MSCs
3.2.2. Umbilical Cord Blood-Derived MSCs
3.2.3. Placenta-Derived MSCs
3.2.4. Bone Marrow-Derived MSCs
3.3. Endothelial Progenitor Cells
| Cell Type | Animal Model | Delivery Route (Source, Dose/Admin) Time of Treat. | Functional Outcome | Brain Damage/ Apoptosis | SC Engraftment | Other Outcomes/Observations | Ref. |
|---|---|---|---|---|---|---|---|
| BM-MSCs | Rat (RV; 3.5 h hypoxia) | IC (human; 106 cells) 3 dpi | ↑ | = | Yes | MSCs transdifferentiated into astrocytes (astrocytic markers colocalized more with the transplanted MSCs than neuronal or oligodendrocyte markers). | [55] |
| Mouse (RV; 0.75 h hypoxia) | ICV (murine; 105 cells) 3 or 10 dpi | ↑ | ↓ | - | No difference between treatment at 10 and 3 dpi; increase in cell proliferation; no differentiation of MSCs into mature cell types; increase in the number of mature neurons, astrocytes, and oligodendrocytes; decrease in microglial activation. | [57] | |
| Mouse (RV; 0.75 h hypoxia) | IN (murine; 5 × 105 cells) 10 dpi | ↑ | ↓ | Yes | No differentiation of MSCs into mature cell types. | [58] | |
| Mouse (RV; 0.75 h hypoxia) | ICV (murine; 105 cells) 3 and/or 10 dpi | 3 and 10 dpi ↑ | 3 and 10 dpi ↓ | - | Injection at 3 dpi: Increase in mature neurons and oligodendrocytes count. Injection at 10 dpi: Increase in oligodendrocyte maturation, remyelination, and neuronal repair without new cell formation. | [56] | |
| Mouse (RV; 0.75 h hypoxia) | ICV (murine; 105 cells) 3 and 10 dpi | ↑ | ↓ | - | Decrease in the HI-induced contralateral axonal rewiring and HI-induced changes in the white matter; increase in the axonal connectivity in the ipsilateral hemisphere. | [59] | |
| Rat (transient MCAO) | IN (murine; 106 cells) 3 dpi | ↑ | ↓ | - | Increase in cell proliferation. | [61] | |
| Mouse (RV; 0.75 h hypoxia) | IN (murine; 0.25–1 × 106 cells) 3/10/17 dpi | 0.5 × 106 ↑ 3 or 10 dpi ↑ | 0.5 × 106 ↓ 3 or 10 dpi ↓ 17 dpi = | Yes | 0.5 × 106 cells: Lowest dose to produce alterations. | [60] | |
| Sheep Fetuses (0.4 h UCO) | IV (human; 3.5 × 106 cells) 1 h after UCO | - | ↓ | Low | Decrease of the cerebral inflammatory response, T-cell invasion, and electrographic seizure activity; increase in persistent tolerance of T-cells and preOLs count. | [82] | |
| Mouse (RV; 0.75 h hypoxia) | IN (human; 1 or 2 × 106 cells) 10 dpi | ↑ | 1 × 106 = 2 × 106 ↓ | Low | Decrease in astrogliosis and microglial activation. | [62] | |
| Mouse (RV; 0.75 h hypoxia) | IN (murine; 0.5 × 106 cells) 10 dpi | ↑ | ↓ | - | No detection of adverse effects during the animal’s lifespan and no induction of tumors or other lesions in the brain or nasal turbinates. | [63] | |
| Rat (RV; 2.5 h hypoxia) | ICV (murine; 106 cells) 2 dpi | ↑ | ↓ | - | Decrease in TLR2 expression levels. | [64] | |
| Rat (RV; 1.5 h hypoxia) | SC (murine; 0.75–1 × 106 cells or 0.8–1.2 × 105 cells) 7 dpi | ↑ | - | - | Increase in striatal medium spiny projection neurons—restored to uninjured levels with the higher dosage. | [65] | |
| Rat (RV; 2 h hypoxia) | ICV (murine; 2 × 105 cells) 1 dpi | ↑ | - | Yes | Improvement in the neuronal pathological changes induced by the HI insult; increase in autophagy levels. | [66] | |
| Rat (RV; 1 h hypoxia) | IV (murine; 105 cells) 1 dpi | ↑ | ↓ | - | Decrease in microglial activation. | [54] | |
| Rat (RV; 2 h hypoxia) | IV (murine; 106 cells) 3 dpi | ↑ | ↓ | - | Increase in the number of neurons and synapses. | [67] | |
| Rat (RV; 1 h hypoxia) | IV (murine; 105 cells) 4 hpi and 1 dpi | - | ↓ | - | Decrease in microglia activation (M1 phenotype); increase in anti-inflammatory cytokine and growth factor levels. | [73] | |
| Mouse (RV; 1 h hypoxia) | IN + TH (murine; 106 cells) 3 dpi | MSCs/TH ↑ MSCs + TH ↑↑ | MSCs + TH ↑ | - | MSCs or TH: Decrease in growth factor expression levels. MSCs: Decrease in hypomyelination. MSCs + TH (compared to either therapy alone): Increase in the infiltration of endothelial cells and peripheral immune cells; increase in pro-inflammatory cytokines levels; decrease in growth factor expression levels (bellow control levels). | [68] | |
| Rat (RV; 2.5 h hypoxia) | IV (6 × 106 cells) ? | - | - | Yes | Increase in HIF-1α and SDF-1α protein levels in the hippocampus. | [122] | |
| Mouse (RV; 0.75 h hypoxia) | IN (106 cells) 10 dpi | - | ↓ | Yes | Increase in DCX+ cells in the SVZ, number of astrocytes at the lesion site, and the number of neurons; decrease in reactive astrocytes and microglial activation (M2 phenotype). | [80] | |
| Rat (bilateral ligation of cephalic arteries, 1.5 h hypoxia) | IV or ICV (3 × 106 cells) 1 dpi | IV ↑ ICV ↑↑ | IV ↓ ICV ↓↓ | - | Decrease of astrogliosis. | [35] | |
| Rat (RV, 2.5 h hypoxia) | ICV (murine; 2 × 105 cells) 5 dpi | ↑ | - | - | Enhanced long-term potentiation. | [70] | |
| Rat (RV, 2.5 h hypoxia) | ICV (murine; 2 × 105 cells) 5 dpi | ↑ | - | - | Decrease in the number of proliferating astrocytes. | [69] |
4. Therapeutic Hypothermia and Stem Cell Therapy
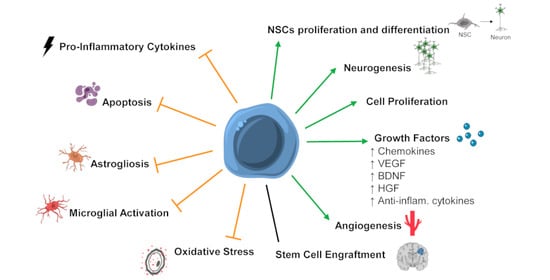
5. Mechanisms of Action of SCT after HI Insult in the Developing Brain
5.1. Stem Cell Engraftment and Differentiation into Mature Neuronal Cells
5.2. Secreted Factors and Paracrine Effects
5.3. Neurogenesis
5.4. Apoptosis
5.5. Astrogliosis and Activated Microglia
5.6. Inflammation
5.7. Angiogenesis
5.8. Oxidative Stress
6. Analysis and Discussion
7. Conclusions and Future Perspectives
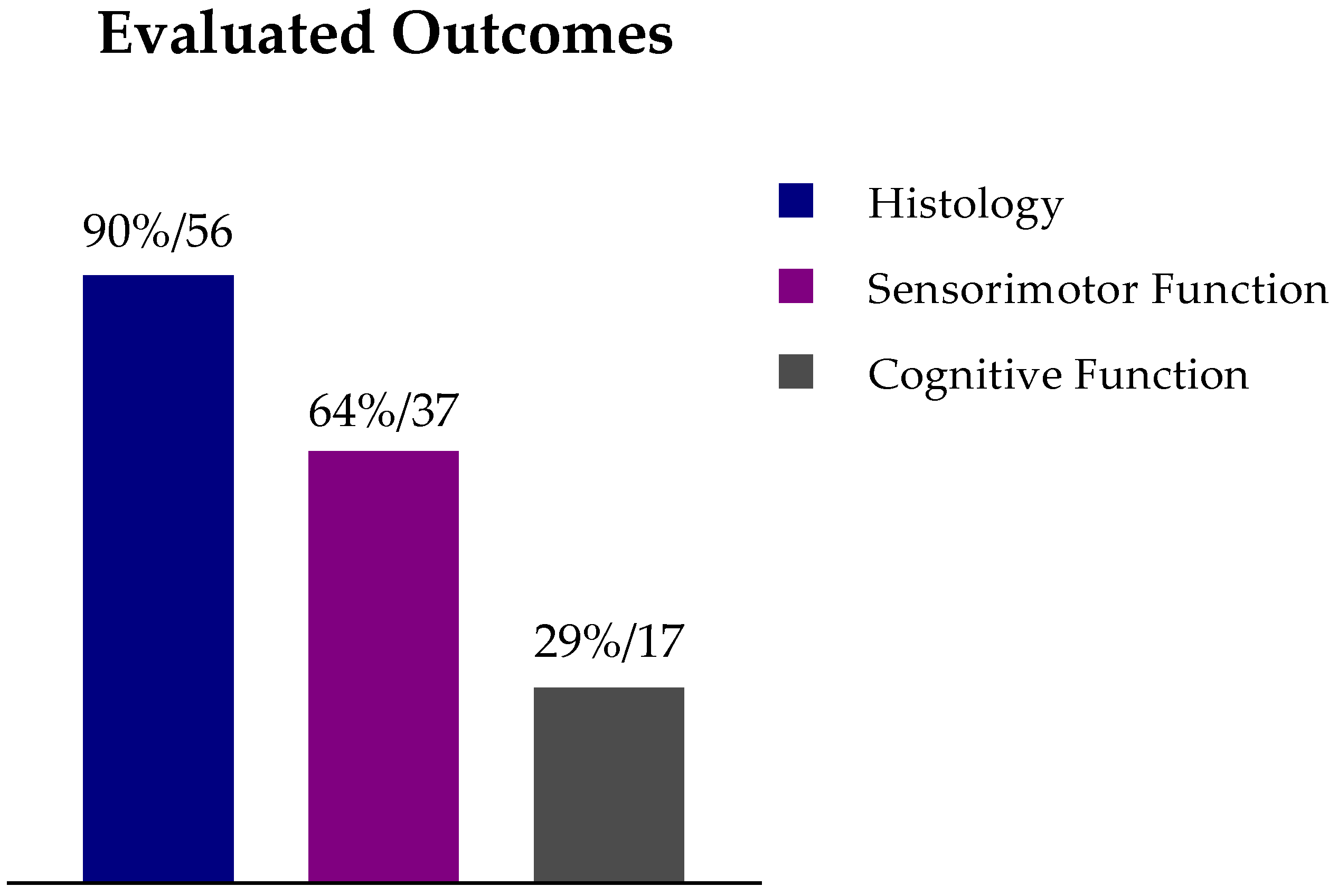
8. Methods
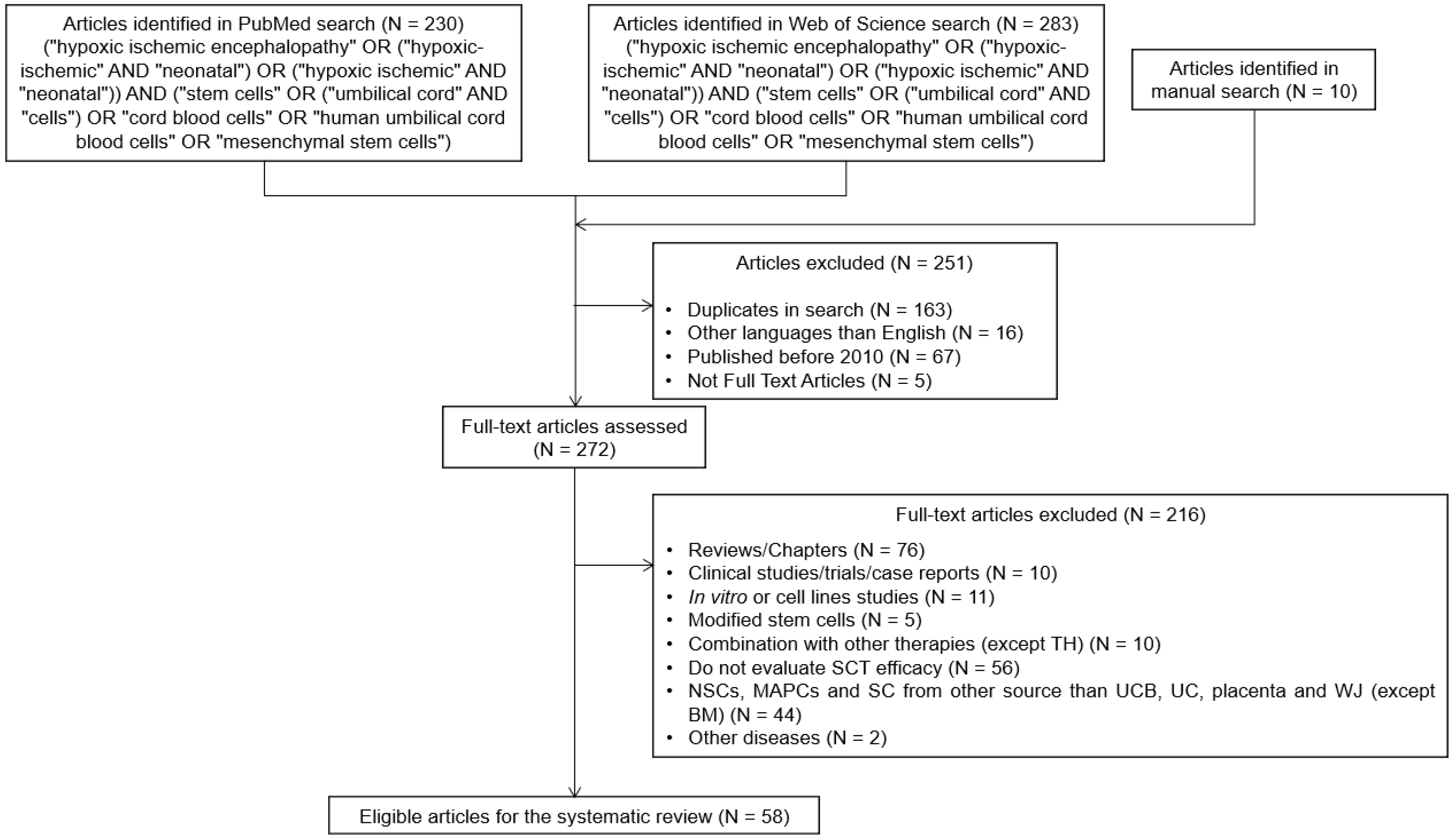
8.1. Literature Search
8.2. Inclusion and Exclusion Criteria
8.3. Data Extraction
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Full form |
| BBB | blood-brain barrier |
| BDNF | brain-derived neurotrophic factor |
| BM | bone marrow |
| BM-MSCs | bone marrow-derived MSCs |
| BW | bodyweight |
| CCA | common carotid artery |
| CNS | central nervous system |
| ECFCs | endothelial colony-forming cells |
| EPCs | endothelial progenitor cells |
| HGF | hepatocyte growth factor |
| HI | hypoxic-ischemic |
| HIE | hypoxic-ischemic encephalopathy |
| hpi/dpi/wpi | hours/days/weeks post insult |
| HSCs | hematopoietic stem cells |
| IA | intra-arterial |
| IC | intracardiac |
| ICV | intraventricular |
| IN | intranasal |
| IP | intraperitoneal |
| IT | intrathecal |
| IV | intravenous |
| IVH | intraventricular hemorrhage |
| MCAO | middle cerebral artery occlusion |
| MNC | UCB mononuclear fraction |
| MSCs | mesenchymal stem/stromal cells |
| NSCs | neuronal stem cells |
| P | postnatal day |
| PD | placenta-derived |
| PD-MSCs | placenta-derived MSCs |
| PWMD | periventricular white matter damage |
| RV | Rice–Vannucci |
| SC | subcutaneous |
| SCT | stem cell therapy |
| SVZ | subventricular zone |
| TH | therapeutic hypothermia |
| Treg | regulatory T-cells |
| UCB | umbilical cord blood |
| UCB cells | human umbilical cord blood cells (mononuclear fraction) |
| UCB-MSCs | umbilical cord blood-derived MSCs |
| UCO | umbilical cord occlusion |
| UCT | umbilical cord tissue |
| UCT-MSCs | umbilical cord tissue-derived MSCs |
| VEGF | vascular endothelial growth factor |
References
- Millar, L.J.; Shi, L.; Hoerder-Suabedissen, A.; Molnar, Z. Neonatal Hypoxia Ischaemia: Mechanisms, Models, and Therapeutic Challenges. Front. Cell Neurosci. 2017, 11, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Li, B.; Zhang, X.; Zhu, C.; Wang, X. Birth Asphyxia Is Associated With Increased Risk of Cerebral Palsy: A Meta-Analysis. Front. Neurol. 2020, 11, 704. [Google Scholar] [CrossRef] [PubMed]
- Honeycutt, A.A.; Grosse, S.D.; Dunlap, L.J.; Schendel, D.E.; Chen, H.; Brann, E.; Homsi, G.a. Economic Costs of Mental Retardation, Cerebral Palsy, Hearing Loss, and Vision Impairment. In Using Survey Data to Study Disability: Results from the National Health Survey on Disability (USA); Emerald Group Publishing Limited: Bingley, UK, 2003; pp. 207–228. [Google Scholar]
- Lawn, J.E.; Cousens, S.; Zupan, J.; Lancet Neonatal Survival Steering Team. 4 million neonatal deaths: When? Where? Why? Lancet 2005, 365, 891–900. [Google Scholar] [CrossRef]
- Kurinczuk, J.J.; White-Koning, M.; Badawi, N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum. Dev. 2010, 86, 329–338. [Google Scholar] [CrossRef]
- Jacobs, S.E.; Berg, M.; Hunt, R.; Tarnow-Mordi, W.O.; Inder, T.E.; Davis, P.G. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst. Rev. 2013, 1, CD003311. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.C.; Kozuki, N.; Blencowe, H.; Vos, T.; Bahalim, A.; Darmstadt, G.L.; Niermeyer, S.; Ellis, M.; Robertson, N.J.; Cousens, S.; et al. Intrapartum-related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatr. Res. 2013, 74, 50–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nabetani, M.; Shintaku, H.; Hamazaki, T. Future perspectives of cell therapy for neonatal hypoxic-ischemic encephalopathy. Pediatr. Res. 2018, 83, 356–363. [Google Scholar] [CrossRef]
- Volpe, J.J.; Kinney, H.C. Volpe’s Neurology of the Newborn, 6th ed.; Elsevier: Philadelphia, PA, USA, 2018; Unit IV, Chapter 20; 1224p. [Google Scholar]
- Ikonomidou, C.; Kaindl, A.M. Neuronal death and oxidative stress in the developing brain. Antioxid. Redox. Signal. 2011, 14, 1535–1550. [Google Scholar] [CrossRef]
- Patel, S.D.; Pierce, L.; Ciardiello, A.J.; Vannucci, S.J. Neonatal encephalopathy: Pre-clinical studies in neuroprotection. Biochem. Soc. Trans. 2014, 42, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.P.; Ferriero, D.M. From selective vulnerability to connectivity: Insights from newborn brain imaging. Trends Neurosci. 2009, 32, 496–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rice, J.E., 3rd; Vannucci, R.C.; Brierley, J.B. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann. Neurol. 1981, 9, 131–141. [Google Scholar] [CrossRef]
- Vannucci, R.C.; Christensen, M.A.; Yager, J.Y. Nature, time-course, and extent of cerebral edema in perinatal hypoxic-ischemic brain damage. Pediatr. Neurol. 1993, 9, 29–34. [Google Scholar] [CrossRef]
- Edwards, A.B.; Feindel, K.W.; Cross, J.L.; Anderton, R.S.; Clark, V.W.; Knuckey, N.W.; Meloni, B.P. Modification to the Rice-Vannucci perinatal hypoxic-ischaemic encephalopathy model in the P7 rat improves the reliability of cerebral infarct development after 48hours. J. Neurosci. Methods 2017, 288, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhao, F.; Qu, Y.; Zhang, L.; Wang, Y.; Mu, D. Animal models of hypoxic-ischemic encephalopathy: Optimal choices for the best outcomes. Rev. Neurosci. 2017, 28, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Davidson, J.O.; Wassink, G.; van den Heuij, L.G.; Bennet, L.; Gunn, A.J. Therapeutic Hypothermia for Neonatal Hypoxic-Ischemic Encephalopathy—Where to from Here? Front. Neurol. 2015, 6, 198. [Google Scholar] [CrossRef] [Green Version]
- Thoresen, M.; Tooley, J.; Liu, X.; Jary, S.; Fleming, P.; Luyt, K.; Jain, A.; Cairns, P.; Harding, D.; Sabir, H. Time is brain: Starting therapeutic hypothermia within three hours after birth improves motor outcome in asphyxiated newborns. Neonatology 2013, 104, 228–233. [Google Scholar] [CrossRef]
- Rao, R.; Trivedi, S.; Vesoulis, Z.; Liao, S.M.; Smyser, C.D.; Mathur, A.M. Safety and Short-Term Outcomes of Therapeutic Hypothermia in Preterm Neonates 34–35 Weeks Gestational Age with Hypoxic-Ischemic Encephalopathy. J. Pediatr. 2017, 183, 37–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yates, N.; Gunn, A.J.; Bennet, L.; Dhillon, S.K.; Davidson, J.O. Preventing Brain Injury in the Preterm Infant-Current Controversies and Potential Therapies. Int. J. Mol. Sci. 2021, 22, 1671. [Google Scholar] [CrossRef]
- Nair, J.; Kumar, V.H.S. Current and Emerging Therapies in the Management of Hypoxic Ischemic Encephalopathy in Neonates. Children 2018, 5, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirzaei, H.; Sahebkar, A.; Sichani, L.S.; Moridikia, A.; Nazari, S.; Sadri Nahand, J.; Salehi, H.; Stenvang, J.; Masoudifar, A.; Mirzaei, H.R.; et al. Therapeutic application of multipotent stem cells. J. Cell. Physiol. 2018, 233, 2815–2823. [Google Scholar] [CrossRef]
- Phuc, P.V.; Ngoc, V.B.; Lam, D.H.; Tam, N.T.; Viet, P.Q.; Ngoc, P.K. Isolation of three important types of stem cells from the same samples of banked umbilical cord blood. Cell Tissue Bank 2012, 13, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.L.; Troyer, D.L. Stem cells in the umbilical cord. Stem. Cell Rev. 2006, 2, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Ballen, K.K.; Barker, J.N.; Stewart, S.K.; Greene, M.F.; Lane, T.A.; American Society of, B.; Marrow, T. Collection and preservation of cord blood for personal use. Biol. Blood Marrow Transpl. 2008, 14, 356–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha, V.; Labopin, M.; Sanz, G.; Arcese, W.; Schwerdtfeger, R.; Bosi, A.; Jacobsen, N.; Ruutu, T.; de Lima, M.; Finke, J.; et al. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N. Engl. J. Med. 2004, 351, 2276–2285. [Google Scholar] [CrossRef]
- Ringden, O.; Okas, M.; Uhlin, M.; Uzunel, M.; Remberger, M.; Mattsson, J. Unrelated cord blood and mismatched unrelated volunteer donor transplants, two alternatives in patients who lack an HLA-identical donor. Bone Marrow Transpl. 2008, 42, 643–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malgieri, A.; Kantzari, E.; Patrizi, M.P.; Gambardella, S. Bone marrow and umbilical cord blood human mesenchymal stem cells: State of the art. Int. J. Clin. Exp. Med. 2010, 3, 248–269. [Google Scholar]
- McDonald, C.A.; Djuliannisaa, Z.; Petraki, M.; Paton, M.C.B.; Penny, T.R.; Sutherland, A.E.; Castillo-Melendez, M.; Novak, I.; Jenkin, G.; Fahey, M.C.; et al. Intranasal Delivery of Mesenchymal Stromal Cells Protects against Neonatal Hypoxic(-)Ischemic Brain Injury. Int. J. Mol. Sci. 2019, 20, 2449. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Gu, J.; Gu, Y.; He, M.; Bi, Y.; Chen, J.; Li, T. Human Umbilical Cord-Derived Mesenchymal Stem Cells Improve Learning and Memory Function in Hypoxic-Ischemic Brain-Damaged Rats via an IL-8-Mediated Secretion Mechanism Rather than Differentiation Pattern Induction. Cell Physiol. Biochem. 2015, 35, 2383–2401. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Feng, Z.; Wang, X.; Xiong, Y.; Wang, L.; Ye, L.; Zhang, H. hUC-MSCs Exert a Neuroprotective Effect via Anti-apoptotic Mechanisms in a Neonatal HIE Rat Model. Cell Transplant. 2019, 28, 1552–1559. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, C.; Chen, J.; Luo, M.; Qu, Y.; Mu, D.; Chen, Q. Umbilical cord mesenchymal stem cells and umbilical cord blood mononuclear cells improve neonatal rat memory after hypoxia-ischemia. Behav. Brain Res. 2019, 362, 56–63. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.; Li, W.; Nie, D.; Chen, W.; Xu, C.; Yi, X.; Shi, J.; Tian, M.; Qin, J.; et al. Therapeutic effect of human umbilical cord mesenchymal stem cells on neonatal rat hypoxic-ischemic encephalopathy. J. Neurosci. Res. 2014, 92, 35–45. [Google Scholar] [CrossRef]
- Mukai, T.; Mori, Y.; Shimazu, T.; Takahashi, A.; Tsunoda, H.; Yamaguchi, S.; Kiryu, S.; Tojo, A.; Nagamura-Inoue, T. Intravenous injection of umbilical cord-derived mesenchymal stromal cells attenuates reactive gliosis and hypomyelination in a neonatal intraventricular hemorrhage model. Neuroscience 2017, 355, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, N.; Ehsan, E.; Abd El Ghany, E.; Sabry, D.; Shamaa, A. Mesenchymal stem cells transplantation attenuates experimentally induced brain injury after neonatal hypoxia by different two routes of administrations. Biocell 2019, 43, 21–28. [Google Scholar] [CrossRef]
- Penny, T.R.; Pham, Y.; Sutherland, A.E.; Mihelakis, J.G.; Lee, J.; Jenkin, G.; Fahey, M.C.; Miller, S.L.; McDonald, C.A. Multiple doses of umbilical cord blood cells improve long-term brain injury in the neonatal rat. Brain Res. 2020, 1746, 147001. [Google Scholar] [CrossRef]
- Pimentel-Coelho, P.M.; Magalhaes, E.S.; Lopes, L.M.; deAzevedo, L.C.; Santiago, M.F.; Mendez-Otero, R. Human cord blood transplantation in a neonatal rat model of hypoxic-ischemic brain damage: Functional outcome related to neuroprotection in the striatum. Stem Cells Dev. 2010, 19, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Geissler, M.; Dinse, H.R.; Neuhoff, S.; Kreikemeier, K.; Meier, C. Human umbilical cord blood cells restore brain damage induced changes in rat somatosensory cortex. PLoS ONE 2011, 6, e20194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Paula, S.; Greggio, S.; Marinowic, D.R.; Machado, D.C.; DaCosta, J.C. The dose-response effect of acute intravenous transplantation of human umbilical cord blood cells on brain damage and spatial memory deficits in neonatal hypoxia-ischemia. Neuroscience 2012, 210, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Wasielewski, B.; Jensen, A.; Roth-Harer, A.; Dermietzel, R.; Meier, C. Neuroglial activation and Cx43 expression are reduced upon transplantation of human umbilical cord blood cells after perinatal hypoxic-ischemic injury. Brain Res. 2012, 1487, 39–53. [Google Scholar] [CrossRef]
- Greggio, S.; de Paula, S.; Azevedo, P.N.; Venturin, G.T.; Dacosta, J.C. Intra-arterial transplantation of human umbilical cord blood mononuclear cells in neonatal hypoxic-ischemic rats. Life Sci. 2014, 96, 33–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grandvuillemin, I.; Garrigue, P.; Ramdani, A.; Boubred, F.; Simeoni, U.; Dignat-George, F.; Sabatier, F.; Guillet, B. Long-Term Recovery After Endothelial Colony-Forming Cells or Human Umbilical Cord Blood Cells Administration in a Rat Model of Neonatal Hypoxic-Ischemic Encephalopathy. Stem Cells Transl. Med. 2017, 6, 1987–1996. [Google Scholar] [CrossRef]
- McDonald, C.A.; Penny, T.R.; Paton, M.C.B.; Sutherland, A.E.; Nekkanti, L.; Yawno, T.; Castillo-Melendez, M.; Fahey, M.C.; Jones, N.M.; Jenkin, G.; et al. Effects of umbilical cord blood cells, and subtypes, to reduce neuroinflammation following perinatal hypoxic-ischemic brain injury. J. Neuroinflamm. 2018, 15, 47. [Google Scholar] [CrossRef] [PubMed]
- Penny, T.R.; Sutherland, A.E.; Mihelakis, J.G.; Paton, M.C.B.; Pham, Y.; Lee, J.; Jones, N.M.; Jenkin, G.; Fahey, M.C.; Miller, S.L.; et al. Human Umbilical Cord Therapy Improves Long-Term Behavioral Outcomes Following Neonatal Hypoxic Ischemic Brain Injury. Front. Physiol. 2019, 10, 283. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yan, Y.; Luo, Z.; Luo, P.; Xiao, N.; Sun, X.; Cheng, L. Effects of human umbilical cord blood CD34(+) cell transplantation in neonatal hypoxic-ischemia rat model. Brain Dev. 2019, 41, 173–181. [Google Scholar] [CrossRef]
- Nakanishi, K.; Sato, Y.; Mizutani, Y.; Ito, M.; Hirakawa, A.; Higashi, Y. Rat umbilical cord blood cells attenuate hypoxic-ischemic brain injury in neonatal rats. Sci. Rep. 2017, 7, 44111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drobyshevsky, A.; Cotten, C.M.; Shi, Z.; Luo, K.; Jiang, R.; Derrick, M.; Tracy, E.T.; Gentry, T.; Goldberg, R.N.; Kurtzberg, J.; et al. Human Umbilical Cord Blood Cells Ameliorate Motor Deficits in Rabbits in a Cerebral Palsy Model. Dev. Neurosci. 2015, 37, 349–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, S.Y.; Chang, Y.S.; Sung, D.K.; Sung, S.I.; Park, W.S. Hypothermia broadens the therapeutic time window of mesenchymal stem cell transplantation for severe neonatal hypoxic ischemic encephalopathy. Sci. Rep. 2018, 8, 7665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, W.S.; Sung, S.I.; Ahn, S.Y.; Yoo, H.S.; Sung, D.K.; Im, G.H.; Choi, S.J.; Chang, Y.S. Hypothermia augments neuroprotective activity of mesenchymal stem cells for neonatal hypoxic-ischemic encephalopathy. PLoS ONE 2015, 10, e0120893. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.S.; Ahn, S.Y.; Im, G.H.; Sung, D.K.; Park, Y.R.; Choi, S.H.; Choi, S.J.; Chang, Y.S.; Oh, W.; Lee, J.H.; et al. Human umbilical cord blood-derived mesenchymal stem cell transplantation attenuates severe brain injury by permanent middle cerebral artery occlusion in newborn rats. Pediatr. Res. 2012, 72, 277–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, G.; Hong, X.; Chen, X.; Lan, F.; Zhang, G.; Liao, L. Intracerebral transplantation of mesenchymal stem cells derived from human umbilical cord blood alleviates hypoxic ischemic brain injury in rat neonates. J. Perinat. Med. 2010, 38, 215–221. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, H.; Ding, H.; Li, D.; Yi, X.; Ma, X.; Li, R.; Huang, M.; Ju, X. Transplantation of placenta-derived mesenchymal stem cells reduces hypoxic-ischemic brain damage in rats by ameliorating the inflammatory response. Cell Mol. Immunol. 2017, 14, 693–701. [Google Scholar] [CrossRef] [Green Version]
- Ding, H.F.; Zhang, H.; Ding, H.F.; Li, D.; Yi, X.H.; Gao, X.Y.; Mou, W.W.; Ju, X.L. Therapeutic effect of placenta-derived mesenchymal stem cells on hypoxic-ischemic brain damage in rats. World J. Pediatr. 2015, 11, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Ueda, K.; Kondo, T.; Hattori, T.; Mikrogeorgiou, A.; Sugiyama, Y.; Suzuki, T.; Yamamoto, M.; Hirata, H.; Hirakawa, A.; et al. Administration of Bone Marrow-Derived Mononuclear Cells Contributed to the Reduction of Hypoxic-Ischemic Brain Injury in Neonatal Rats. Front. Neurol. 2018, 9, 987. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Kim, B.I.; Jo, C.H.; Choi, C.W.; Kim, E.K.; Kim, H.S.; Yoon, K.S.; Choi, J.H. Mesenchymal stem-cell transplantation for hypoxic-ischemic brain injury in neonatal rat model. Pediatr. Res. 2010, 67, 42–46. [Google Scholar] [CrossRef] [Green Version]
- van Velthoven, C.T.; Kavelaars, A.; van Bel, F.; Heijnen, C.J. Repeated mesenchymal stem cell treatment after neonatal hypoxia-ischemia has distinct effects on formation and maturation of new neurons and oligodendrocytes leading to restoration of damage, corticospinal motor tract activity, and sensorimotor function. J. Neurosci. 2010, 30, 9603–9611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Velthoven, C.T.; Kavelaars, A.; van Bel, F.; Heijnen, C.J. Mesenchymal stem cell treatment after neonatal hypoxic-ischemic brain injury improves behavioral outcome and induces neuronal and oligodendrocyte regeneration. Brain Behav. Immun. 2010, 24, 387–393. [Google Scholar] [CrossRef] [PubMed]
- van Velthoven, C.T.; Kavelaars, A.; van Bel, F.; Heijnen, C.J. Nasal administration of stem cells: A promising novel route to treat neonatal ischemic brain damage. Pediatr. Res. 2010, 68, 419–422. [Google Scholar] [CrossRef] [PubMed]
- van Velthoven, C.T.; van de Looij, Y.; Kavelaars, A.; Zijlstra, J.; van Bel, F.; Huppi, P.S.; Sizonenko, S.; Heijnen, C.J. Mesenchymal stem cells restore cortical rewiring after neonatal ischemia in mice. Ann. Neurol. 2012, 71, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Donega, V.; van Velthoven, C.T.; Nijboer, C.H.; van Bel, F.; Kas, M.J.; Kavelaars, A.; Heijnen, C.J. Intranasal mesenchymal stem cell treatment for neonatal brain damage: Long-term cognitive and sensorimotor improvement. PLoS ONE 2013, 8, e51253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Velthoven, C.T.; Sheldon, R.A.; Kavelaars, A.; Derugin, N.; Vexler, Z.S.; Willemen, H.L.; Maas, M.; Heijnen, C.J.; Ferriero, D.M. Mesenchymal stem cell transplantation attenuates brain injury after neonatal stroke. Stroke 2013, 44, 1426–1432. [Google Scholar] [CrossRef] [Green Version]
- Donega, V.; Nijboer, C.H.; Braccioli, L.; Slaper-Cortenbach, I.; Kavelaars, A.; van Bel, F.; Heijnen, C.J. Intranasal administration of human MSC for ischemic brain injury in the mouse: In vitro and in vivo neuroregenerative functions. PLoS ONE 2014, 9, e112339. [Google Scholar] [CrossRef] [Green Version]
- Donega, V.; Nijboer, C.H.; van Velthoven, C.T.; Youssef, S.A.; de Bruin, A.; van Bel, F.; Kavelaars, A.; Heijnen, C.J. Assessment of long-term safety and efficacy of intranasal mesenchymal stem cell treatment for neonatal brain injury in the mouse. Pediatr. Res. 2015, 78, 520–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, Y.; Zhang, Y.; Bi, Y.; Liu, J.; Tan, B.; Gong, M.; Li, T.; Chen, J. Mesenchymal stem cells suppress neuronal apoptosis and decrease IL-10 release via the TLR2/NFkappaB pathway in rats with hypoxic-ischemic brain damage. Mol. Brain. 2015, 8, 65. [Google Scholar] [CrossRef] [Green Version]
- Cameron, S.H.; Alwakeel, A.J.; Goddard, L.; Hobbs, C.E.; Gowing, E.K.; Barnett, E.R.; Kohe, S.E.; Sizemore, R.J.; Oorschot, D.E. Delayed post-treatment with bone marrow-derived mesenchymal stem cells is neurorestorative of striatal medium-spiny projection neurons and improves motor function after neonatal rat hypoxia-ischemia. Mol. Cell Neurosci. 2015, 68, 56–72. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, L.; Qu, Y.; Xiao, G.; Li, S.; Bao, S.; Lu, Q.R.; Mu, D. Mesenchymal Stem Cells Protect Against Hypoxia-Ischemia Brain Damage by Enhancing Autophagy Through Brain Derived Neurotrophic Factor/Mammalin Target of Rapamycin Signaling Pathway. Stem Cells 2018, 36, 1109–1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakai, T.; Sasaki, M.; Kataoka-Sasaki, Y.; Oka, S.; Nakazaki, M.; Fukumura, S.; Kobayashi, M.; Tsutsumi, H.; Kocsis, J.D.; Honmou, O. Functional recovery after the systemic administration of mesenchymal stem cells in a rat model of neonatal hypoxia-ischemia. J. Neurosurg. Pediatr. 2018, 22, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Herz, J.; Koster, C.; Reinboth, B.S.; Dzietko, M.; Hansen, W.; Sabir, H.; van Velthoven, C.; Bendix, I.; Felderhoff-Muser, U. Interaction between hypothermia and delayed mesenchymal stem cell therapy in neonatal hypoxic-ischemic brain injury. Brain Behav. Immun. 2018, 70, 118–130. [Google Scholar] [CrossRef]
- He, M.; Shi, X.; Yang, M.; Yang, T.; Li, T.; Chen, J. Mesenchymal stem cells-derived IL-6 activates AMPK/mTOR signaling to inhibit the proliferation of reactive astrocytes induced by hypoxic-ischemic brain damage. Exp. Neurol. 2019, 311, 15–32. [Google Scholar] [CrossRef]
- Gu, Y.; He, M.; Zhou, X.; Liu, J.; Hou, N.; Bin, T.; Zhang, Y.; Li, T.; Chen, J. Endogenous IL-6 of mesenchymal stem cell improves behavioral outcome of hypoxic-ischemic brain damage neonatal rats by supressing apoptosis in astrocyte. Sci. Rep. 2016, 6, 18587. [Google Scholar] [CrossRef] [Green Version]
- Kidani, Y.; Miki, Y.; Nomimura, N.; Minakawa, S.; Tanaka, N.; Miyoshi, H.; Wakabayashi, K.; Kudo, Y. The therapeutic effect of CD133+ cells derived from human umbilical cord blood on neonatal mouse hypoxic-ischemic encephalopathy model. Life Sci. 2016, 157, 108–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenkranz, K.; Kumbruch, S.; Tenbusch, M.; Marcus, K.; Marschner, K.; Dermietzel, R.; Meier, C. Transplantation of human umbilical cord blood cells mediated beneficial effects on apoptosis, angiogenesis and neuronal survival after hypoxic-ischemic brain injury in rats. Cell Tissue. Res. 2012, 348, 429–438. [Google Scholar] [CrossRef]
- Sugiyama, Y.; Sato, Y.; Kitase, Y.; Suzuki, T.; Kondo, T.; Mikrogeorgiou, A.; Horinouchi, A.; Maruyama, S.; Shimoyama, Y.; Tsuji, M.; et al. Intravenous Administration of Bone Marrow-Derived Mesenchymal Stem Cell, but not Adipose Tissue-Derived Stem Cell, Ameliorated the Neonatal Hypoxic-Ischemic Brain Injury by Changing Cerebral Inflammatory State in Rat. Front. Neurol. 2018, 9, 757. [Google Scholar] [CrossRef]
- Baba, N.; Wang, F.; Iizuka, M.; Shen, Y.; Yamashita, T.; Takaishi, K.; Tsuru, E.; Matsushima, S.; Miyamura, M.; Fujieda, M.; et al. Induction of regional chemokine expression in response to human umbilical cord blood cell infusion in the neonatal mouse ischemia-reperfusion brain injury model. PLoS ONE 2019, 14, e0221111. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.H.; Bai, X.; Zhang, N.; Wang, S.Y.; Li, W.; Jiang, L. Improvement of human umbilical cord mesenchymal stem cell transplantation on glial cell and behavioral function in a neonatal model of periventricular white matter damage. Brain Res. 2014, 1563, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, K.; Liu, H.; Yang, T.; Xiao, D.J.; Wang, Y.S. The neuroprotective effect of mesenchymal stem cells is mediated through inhibition of apoptosis in hypoxic ischemic injury. World J. Pediatr. 2019, 16, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Hattori, T.; Sato, Y.; Kondo, T.; Ichinohashi, Y.; Sugiyama, Y.; Yamamoto, M.; Kotani, T.; Hirata, H.; Hirakawa, A.; Suzuki, S.; et al. Administration of umbilical cord blood cells transiently decreased hypoxic-ischemic brain injury in neonatal rats. Dev. Neurosci. 2015, 37, 95–104. [Google Scholar] [CrossRef]
- Aridas, J.D.; McDonald, C.A.; Paton, M.C.; Yawno, T.; Sutherland, A.E.; Nitsos, I.; Pham, Y.; Ditchfield, M.; Fahey, M.C.; Wong, F.; et al. Cord blood mononuclear cells prevent neuronal apoptosis in response to perinatal asphyxia in the newborn lamb. J. Physiol. 2016, 594, 1421–1435. [Google Scholar] [CrossRef]
- Rosenkranz, K.; Tenbusch, M.; May, C.; Marcus, K.; Meier, C. Changes in Interleukin-1 alpha serum levels after transplantation of umbilical cord blood cells in a model of perinatal hypoxic-ischemic brain damage. Ann. Anat. 2013, 195, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Donega, V.; Nijboer, C.H.; van Tilborg, G.; Dijkhuizen, R.M.; Kavelaars, A.; Heijnen, C.J. Intranasally administered mesenchymal stem cells promote a regenerative niche for repair of neonatal ischemic brain injury. Exp. Neurol. 2014, 261, 53–64. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhao, Y.; Wang, X. Umbilical cord blood cells regulate the differentiation of endogenous neural stem cells in hypoxic ischemic neonatal rats via the hedgehog signaling pathway. Brain Res. 2014, 1560, 18–26. [Google Scholar] [CrossRef]
- Jellema, R.K.; Wolfs, T.G.; Lima Passos, V.; Zwanenburg, A.; Ophelders, D.R.; Kuypers, E.; Hopman, A.H.; Dudink, J.; Steinbusch, H.W.; Andriessen, P.; et al. Mesenchymal stem cells induce T-cell tolerance and protect the preterm brain after global hypoxia-ischemia. PLoS ONE 2013, 8, e73031. [Google Scholar] [CrossRef] [Green Version]
- Cotten, C.M.; Murtha, A.P.; Goldberg, R.N.; Grotegut, C.A.; Smith, P.B.; Goldstein, R.F.; Fisher, K.A.; Gustafson, K.E.; Waters-Pick, B.; Swamy, G.K.; et al. Feasibility of autologous cord blood cells for infants with hypoxic-ischemic encephalopathy. J. Pediatr. 2014, 164, 973–979.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuji, M.; Sawada, M.; Watabe, S.; Sano, H.; Kanai, M.; Tanaka, E.; Ohnishi, S.; Sato, Y.; Sobajima, H.; Hamazaki, T.; et al. Autologous cord blood cell therapy for neonatal hypoxic-ischaemic encephalopathy: A pilot study for feasibility and safety. Sci. Rep. 2020, 10, 4603. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Zhao, Y.S.; Hu, M.Y.; Sun, Y.Q.; Chen, Y.X.; Bi, X.H. Umbilical cord blood cells regulate endogenous neural stem cell proliferation via hedgehog signaling in hypoxic ischemic neonatal rats. Brain Res. 2013, 1518, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, M.; Taguchi, A.; Sato, Y.; Ogawa, Y.; Saito, S.; Yamahara, K.; Ihara, M.; Harada-Shiba, M.; Ikeda, T.; Matsuyama, T.; et al. Evaluations of Intravenous Administration of CD34+ Human Umbilical Cord Blood Cells in a Mouse Model of Neonatal Hypoxic-Ischemic Encephalopathy. Dev. Neurosci. 2016, 38, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.-B. Transplantation of umbilical cord blood mononuclear cells attenuates the expression of IL-1β via the TLR4/NF-κβ pathway in hypoxic-ischemic neonatal rats. J. Neurorestoratol. 2020, 8, 122–130. [Google Scholar] [CrossRef]
- Ali, H.; Bahbahani, H. Umbilical cord blood stem cells—Potential therapeutic tool for neural injuries and disorders. Acta. Neurobiol. Exp. 2010, 70, 316–324. [Google Scholar]
- Aridas, J.D.; Yawno, T.; Sutherland, A.E.; Nitsos, I.; Ditchfield, M.; Wong, F.Y.; Fahey, M.C.; Malhotra, A.; Wallace, E.M.; Jenkin, G.; et al. Detecting brain injury in neonatal hypoxic ischemic encephalopathy: Closing the gap between experimental and clinical research. Exp. Neurol. 2014, 261, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Minguell, J.J.; Erices, A.; Conget, P. Mesenchymal stem cells. Exp. Biol. Med. 2001, 226, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Barlow, S.; Brooke, G.; Chatterjee, K.; Price, G.; Pelekanos, R.; Rossetti, T.; Doody, M.; Venter, D.; Pain, S.; Gilshenan, K.; et al. Comparison of human placenta- and bone marrow-derived multipotent mesenchymal stem cells. Stem Cells Dev. 2008, 17, 1095–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooke, G.; Tong, H.; Levesque, J.P.; Atkinson, K. Molecular trafficking mechanisms of multipotent mesenchymal stem cells derived from human bone marrow and placenta. Stem Cells Dev. 2008, 17, 929–940. [Google Scholar] [CrossRef]
- Wu, L.F.; Wang, N.N.; Liu, Y.S.; Wei, X. Differentiation of Wharton’s jelly primitive stromal cells into insulin-producing cells in comparison with bone marrow mesenchymal stem cells. Tissue Eng. Part A 2009, 15, 2865–2873. [Google Scholar] [CrossRef]
- Chen, M.Y.; Lie, P.C.; Li, Z.L.; Wei, X. Endothelial differentiation of Wharton’s jelly-derived mesenchymal stem cells in comparison with bone marrow-derived mesenchymal stem cells. Exp. Hematol. 2009, 37, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Baksh, D.; Yao, R.; Tuan, R.S. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells 2007, 25, 1384–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Yongda, L.; Jun, M.; Yingyu, S.; Shaoju, Z.; Xinwen, Z.; Mingxue, Z. Culture and neural differentiation of rat bone marrow mesenchymal stem cells in vitro. Cell Biol. Int. 2007, 31, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, F.G.; Carvalho, M.M.; Panchalingam, K.M.; Rodrigues, A.J.; Mendes-Pinheiro, B.; Anjo, S.; Manadas, B.; Behie, L.A.; Sousa, N.; Salgado, A.J. Impact of the Secretome of Human Mesenchymal Stem Cells on Brain Structure and Animal Behavior in a Rat Model of Parkinson’s Disease. Stem Cells Transl. Med. 2017, 6, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Mendes-Pinheiro, B.; Anjo, S.I.; Manadas, B.; Da Silva, J.D.; Marote, A.; Behie, L.A.; Teixeira, F.G.; Salgado, A.J. Bone Marrow Mesenchymal Stem Cells’ Secretome Exerts Neuroprotective Effects in a Parkinson’s Disease Rat Model. Front. Bioeng. Biotechnol. 2019, 7, 294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsuan, Y.C.; Lin, C.H.; Chang, C.P.; Lin, M.T. Mesenchymal stem cell-based treatments for stroke, neural trauma, and heat stroke. Brain Behav. 2016, 6, e00526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, C.; Li, L. Preconditioning influences mesenchymal stem cell properties in vitro and in vivo. J. Cell Mol. Med. 2018, 22, 1428–1442. [Google Scholar] [CrossRef] [Green Version]
- Park, W.S.; Ahn, S.Y.; Sung, S.I.; Ahn, J.Y.; Chang, Y.S. Strategies to enhance paracrine potency of transplanted mesenchymal stem cells in intractable neonatal disorders. Pediatr. Res. 2018, 83, 214–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.; Chen, W.; Wu, L.; Jiang, L.; Qin, H.; Tang, N. Hypoxic preconditioning improves the survival and neural effects of transplanted mesenchymal stem cells via CXCL12/CXCR4 signalling in a rat model of cerebral infarction. Cell Biochem. Funct. 2019, 37, 504–515. [Google Scholar] [CrossRef]
- Ding, D.C.; Chang, Y.H.; Shyu, W.C.; Lin, S.Z. Human umbilical cord mesenchymal stem cells: A new era for stem cell therapy. Cell Transplant. 2015, 24, 339–347. [Google Scholar] [CrossRef]
- Li, X.; Bai, J.; Ji, X.; Li, R.; Xuan, Y.; Wang, Y. Comprehensive characterization of four different populations of human mesenchymal stem cells as regards their immune properties, proliferation and differentiation. Int. J. Mol. Med. 2014, 34, 695–704. [Google Scholar] [CrossRef] [Green Version]
- Wegmeyer, H.; Broske, A.M.; Leddin, M.; Kuentzer, K.; Nisslbeck, A.K.; Hupfeld, J.; Wiechmann, K.; Kuhlen, J.; von Schwerin, C.; Stein, C.; et al. Mesenchymal stromal cell characteristics vary depending on their origin. Stem Cells Dev. 2013, 22, 2606–2618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amable, P.R.; Teixeira, M.V.; Carias, R.B.; Granjeiro, J.M.; Borojevic, R. Mesenchymal stromal cell proliferation, gene expression and protein production in human platelet-rich plasma-supplemented media. PLoS ONE 2014, 9, e104662. [Google Scholar] [CrossRef] [PubMed]
- Kuchroo, P.; Dave, V.; Vijayan, A.; Viswanathan, C.; Ghosh, D. Paracrine factors secreted by umbilical cord-derived mesenchymal stem cells induce angiogenesis in vitro by a VEGF-independent pathway. Stem Cells Dev. 2015, 24, 437–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amable, P.R.; Teixeira, M.V.; Carias, R.B.; Granjeiro, J.M.; Borojevic, R. Protein synthesis and secretion in human mesenchymal cells derived from bone marrow, adipose tissue and Wharton’s jelly. Stem. Cell Res. Ther. 2014, 5, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balasubramanian, S.; Venugopal, P.; Sundarraj, S.; Zakaria, Z.; Majumdar, A.S.; Ta, M. Comparison of chemokine and receptor gene expression between Wharton’s jelly and bone marrow-derived mesenchymal stromal cells. Cytotherapy 2012, 14, 26–33. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Thej, C.; Venugopal, P.; Priya, N.; Zakaria, Z.; Sundarraj, S.; Majumdar, A.S. Higher propensity of Wharton’s jelly derived mesenchymal stromal cells towards neuronal lineage in comparison to those derived from adipose and bone marrow. Cell Biol. Int. 2013, 37, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Prockop, D.J. Concise review: Two negative feedback loops place mesenchymal stem/stromal cells at the center of early regulators of inflammation. Stem Cells 2013, 31, 2042–2046. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hirai, M.; Cantero, S.; Ciubotariu, R.; Dobrila, L.; Hirsh, A.; Igura, K.; Satoh, H.; Yokomi, I.; Nishimura, T.; et al. Isolation and characterization of mesenchymal stem cells from human umbilical cord blood: Reevaluation of critical factors for successful isolation and high ability to proliferate and differentiate to chondrocytes as compared to mesenchymal stem cells from bone marrow and adipose tissue. J. Cell Biochem. 2011, 112, 1206–1218. [Google Scholar] [PubMed]
- Lee, J.K.; Lee, M.K.; Jin, H.J.; Kim, D.S.; Yang, Y.S.; Oh, W.; Yang, S.E.; Park, T.S.; Lee, S.Y.; Kim, B.S.; et al. Efficient intracytoplasmic labeling of human umbilical cord blood mesenchymal stromal cells with ferumoxides. Cell Transpl. 2007, 16, 849–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.E.; Ha, C.W.; Jung, M.; Jin, H.J.; Lee, M.; Song, H.; Choi, S.; Oh, W.; Yang, Y.S. Mesenchymal stem/progenitor cells developed in cultures from UC blood. Cytotherapy 2004, 6, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Erices, A.; Conget, P.; Minguell, J.J. Mesenchymal progenitor cells in human umbilical cord blood. Br. J. Haematol. 2000, 109, 235–242. [Google Scholar] [CrossRef]
- Musina, R.A.; Bekchanova, E.S.; Belyavskii, A.V.; Grinenko, T.S.; Sukhikh, G.T. Umbilical cord blood mesenchymal stem cells. Bull. Exp. Biol. Med. 2007, 143, 127–131. [Google Scholar] [CrossRef]
- Wexler, S.A.; Donaldson, C.; Denning-Kendall, P.; Rice, C.; Bradley, B.; Hows, J.M. Adult bone marrow is a rich source of human mesenchymal ‘stem’ cells but umbilical cord and mobilized adult blood are not. Br. J. Haematol. 2003, 121, 368–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parolini, O.; Alviano, F.; Bagnara, G.P.; Bilic, G.; Buhring, H.J.; Evangelista, M.; Hennerbichler, S.; Liu, B.; Magatti, M.; Mao, N.; et al. Concise review: Isolation and characterization of cells from human term placenta: Outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells 2008, 26, 300–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evangelista, M.; Soncini, M.; Parolini, O. Placenta-derived stem cells: New hope for cell therapy? Cytotechnology 2008, 58, 33–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoder, M.C.; Mead, L.E.; Prater, D.; Krier, T.R.; Mroueh, K.N.; Li, F.; Krasich, R.; Temm, C.J.; Prchal, J.T.; Ingram, D.A. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 2007, 109, 1801–1809. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.; Liu, L.; Lin, J.; Wang, Y.; Xuan, X.; Guo, Y.; Hu, S. SDF-1alpha/CXCR4 Axis Mediates The Migration of Mesenchymal Stem Cells to The Hypoxic-Ischemic Brain Lesion in A Rat Model. Cell J. 2015, 16, 440–447. [Google Scholar] [PubMed]
- Fan, C.G.; Zhang, Q.J.; Tang, F.W.; Han, Z.B.; Wang, G.S.; Han, Z.C. Human umbilical cord blood cells express neurotrophic factors. Neurosci. Lett. 2005, 380, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Newman, M.B.; Willing, A.E.; Manresa, J.J.; Sanberg, C.D.; Sanberg, P.R. Cytokines produced by cultured human umbilical cord blood (HUCB) cells: Implications for brain repair. Exp. Neurol. 2006, 199, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Kupcova Skalnikova, H. Proteomic techniques for characterisation of mesenchymal stem cell secretome. Biochimie 2013, 95, 2196–2211. [Google Scholar] [CrossRef] [PubMed]
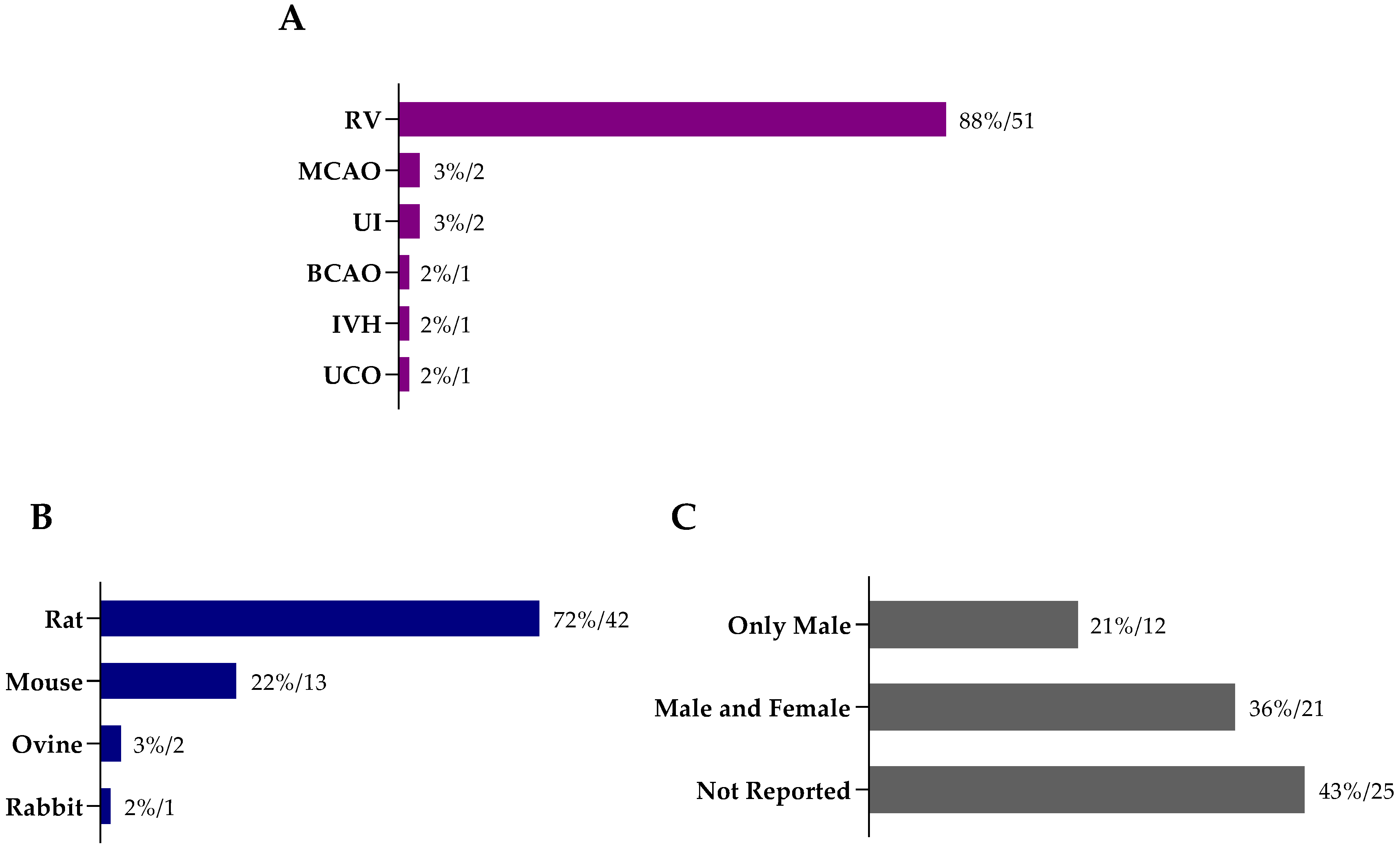
| Cell Type | Animal Model | Delivery Route (Dose/Admin) Time of Treat. | Functional Outcome | Brain Damage/ Apoptosis | SC Engraftment | Other Outcomes/Observations | Ref. |
|---|---|---|---|---|---|---|---|
| UCB cells | Rat (RV; 1.5 h hypoxia) | IP (2 × 106 cells) 3 hpi | ↑ | ↓ | Low | Decrease in microglial activation and number of macrophages. | [37] |
| Rat (RV; 1.3 h hypoxia) | IP (107 cells) 1 dpi | ↑ | = | Yes | Restoration of cortical plasticity. | [38] | |
| Rat (RV; 1.3 h hypoxia) | IP (107 cells) 1 dpi | - | ↓ | - | Increase in mature neuron count, angiogenesis levels, occludin levels, and growth factor expression. | [72] | |
| Rat (RV; 2 h hypoxia) | IV (106/107/108 cells) 1 dpi | 108 ↑ | 107 or 108 ↓ | Yes | [39] | ||
| Rat (RV; 1.3 h hypoxia) | IP or IT (107 cells) 1 dpi | ↑ | - | Yes | Both administration routes led to similar outcomes; decrease in microglial activation, astrogliosis, and invading macrophages. | [40] | |
| Rat (RV; 2 h hypoxia) | ICV (3 × 106 cells) 1 dpi | - | ↓ | - | Increase in NSC proliferation. | [85] | |
| Rat (RV; 1.3 h hypoxia) | IP (107 cells) 1 dpi | - | - | - | Decrease in pro-inflammatory cytokines serum levels, microglial activation, and macrophage infiltration. | [79] | |
| Rat (RV; 2 h hypoxia) | IA (106/107 cells) 1 dpi | 107 ↑ | = | - | [41] | ||
| Rat (RV; 1 h hypoxia) | IP (107 cells) 6 hpi | = | Apoptosis ↓ Brain damage = | - | Decrease in oxidative stress and microglial activation. | [77] | |
| Rat (RV; 1.5 h hypoxia) | IP (107 cells) 2 dpi | ↑ | ↓ | - | Decrease in neuroinflammation; increase in the number of mature neurons; delayed glial scar formation (12 wpi); increase in cerebral capillary density and cerebral blood flow. | [42] | |
| Rat (RV; 3 h hypoxia) | IP (106 cells) 1 dpi | ↑ | Apoptosis = Brain damage ↓ | - | Decrease in infiltrating CD4+ T-cells, number of T-cells with pro-inflammatory phenotype, and microglial activation; no alteration of growth factor expression levels. | [43] | |
| Rat (RV; 3 h hypoxia) | IP (106 cells) 1 dpi | ↑ | = | - | Increase in microglial activation in the somatosensory cortex. | [44] | |
| Rat (RV; 1.5 h hypoxia) | IP/IN (106 cells) 1 dpi or 1, 3, and 10 dpi | 1 + 3 + 10 dpi ↑ | 1 + 3 + 10 dpi ↓ | - | Multiple doses: Decrease in microglial activation. | [36] | |
| Rat (RV; 2.5 h hypoxia) | IV (1.5 × 104 CD34+ cells or 106 MNCs) 7 dpi | ↑ | ↓ | - | Decrease in cerebral atrophy and astrogliosis; increase in DCX and lectin expression. | [45] | |
| Rat (RV; 2 h hypoxia) | IP (murine; 2 × 106 cells) 3 dpi | ↑ | ↓ | Low | Increase in proliferating cells in the hippocampus; decrease in microglial activation and macrophage infiltration. | [46] | |
| Mouse (RV; 0.5 h hypoxia) | IV (105 CD34+ cells) 2 dpi | = | = | - | Increase in CBF in the ischemic penumbra. | [86] | |
| Rabbit (0.67 h UI) | IV (5 × 106 cells or 2.5 × 106 cells) 4 h after birth | 2.5 × 106 ↑ 5 × 106 ↑↑ | - | No | [47] | ||
| Rat (RV; 2 h hypoxia) | ICV (3 × 106 cells) 1 dpi | - | - | - | Increase in the number of neurons and NSC differentiation into neuronal cells; decrease in the number of glial cells and glial differentiation. | [81] | |
| Mouse (RV; 2 h hypoxia) | IV (5 × 106 cells) 3 wpi | - | - | - | Administration of UCB cells induced a shift in chemokine expression profile after HI insult; increase in chemokine levels to the damaged brain tissue. | [74] | |
| Rat (RV, 2 h hypoxia) | ICV (3 × 106 cells) 1 dpi | - | - | - | Increase of neuronal cell count; decrease in TLR4 protein levels and NF-kβ protein levels; decrease in IL-1β level in the ipsilateral cortex. | [87] | |
| Lamb (UCO) | IA (108 cells) 12 hpi | - | ↓ | - | Decrease in astrogliosis, microglial activation, and macrophage infiltration; restoration of normal brain metabolism. | [78] | |
| UCT-MSCs | Rat (PWMD; 4 h hypoxia) | IP (106 cells) 1, 2, and 3 dpi | ↑ | ↓ | Yes | Decrease in astrogliosis and microglial activation. | [75] |
| Rat (RV; 2 h hypoxia) | ICV (2 × 105 cells) 5 dpi | ↑ | ↓ | - | Increase in hippocampal synaptic plasticity, IL-8 protein levels, and angiogenesis in the hippocampus. | [30] | |
| Rat (RV; 2 h hypoxia) | ICV (3 × 105 cells) 3 dpi | - | ↓ | Yes | Decrease in TNF-α and IL-1β expression levels in the damaged nerve cells. | [76] | |
| Rat (RV; 2 h hypoxia) | ICV (5 × 103 cells) 1 hpi | ↑ | ↓ | Yes | [31] | ||
| Rat (RV; 2 h hypoxia) | ICV (106 cells) 1 dpi | ↑ | ↓ | Yes | MSCs did not differentiate into neuronal or glial cells; decrease in astrogliosis. | [32] | |
| Rat (RV; 2 h hypoxia) | IV/IP (IV: 5 × 105 cells IP: 5 × 106 cells) 1 or 3 dpi | ↑ (Best 1 dpi) | - | Yes | IV route: More MSCs detected in the frontal cortex; greatest decrease in astrogliosis. | [33] | |
| Rat (RV; 1.5 h hypoxia) | IN (2 × 105 cells) 1 dpi | ↑ | ↓ | - | Increase in the number of neurons; decrease in astrogliosis and microglial activation; no alteration of BDNF expression levels. | [29] | |
| Mouse (IVH model) | IV (105 cells) 2 dpi | ↑ | ↓ | - | Decrease of reactive gliosis, hypomyelination, and periventricular cell death; increase in BDNF and HGF expression levels in the serum, CBF, and brain tissue. | [34] | |
| UCB-MSCs | Rat (RV; 2.5 h hypoxia) | ICV (105 cells) 3 dpi | ↑ | - | Yes | Some MSCs differentiated into astrocyte-like cells. | [51] |
| Rat (Permanent MCAO) | ICV (105 cells) 6 hpi | ↑ | ↓ | Yes | Decrease in astrogliosis and microglial activation; decrease in mortality; few MSCs differentiated into neuronal or glial cells. | [50] | |
| Rat (RV; 2 h hypoxia) | ICV + TH (105 cells) 6 hpi | MSCs + TH ↑ | MSCs ↓ MSCs + TH ↓↓ | - | MSCs + TH (compared with each treatment alone): Decrease in microglial activation and inflammatory cytokines levels. | [49] | |
| Rat (RV; 2 h hypoxia) | ICV + TH (105 cells) 2 dpi | MSCs + TH ↑ | MSC ↓ MSCs + TH ↓↓ | - | MSCs: Decrease of astrogliosis. MSCs + TH (compared with each treatment alone): Decrease in inflammatory cytokines levels; greatest decrease in astrogliosis and microglial activation. | [48] |
| Cell Type | Animal Model | Delivery Route (Dose/Admin) Time of Treat. | Functional Outcome | Brain Damage/ Apoptosis | SC Engraftment | Other Outcomes/Observations | Ref. |
|---|---|---|---|---|---|---|---|
| PD-MSCs | Rat (RV; 2.5 h hypoxia) | ICV (106 cells) 2 dpi | ↑ | ↓ | No | Improvement of neuronal morphological changes induced by the HI insult; further increase in ROS levels; decrease in lipid peroxidation and free radical generation. | [53] |
| Rat (RV, 2.5 h hypoxia) | ICV (5 × 104 cells) 2 dpi | ↑ | ↓ | - | Decrease in pro-inflammatory cytokines expression levels in the ipsilateral hemisphere and in serum; increase in anti-inflammatory cytokine levels in the serum and the proportion of Treg cells; | [52] | |
| EPCs/ECFCs | Mouse (RV; 0.5 h hypoxia) | IP (105 cells) 1 dpi | ↑ | ↓ | Yes | Decrease in the size of the cystic lesion. | [71] |
| Rat (RV; 1.5 h hypoxia) | IP (5 × 105 cells) 2 dpi | ↑ | ↓ | - | Increase in the number of mature neuronal cells in the ipsilateral hemisphere; decrease in late astrogliosis; increase in cerebral capillary density and cerebral blood flow. | [42] | |
| Rat (RV; 3 h hypoxia) | IP (2 × 105 cells) 1 dpi | ↑ | ↓ | - | Decrease in infiltrating CD4+-T-cells (to uninjured levels), the number of T-cells with pro-inflammatory phenotype, and microglial activation. | [43] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrenho, I.; Rosado, M.; Dinis, A.; M. Cardoso, C.; Grãos, M.; Manadas, B.; Baltazar, G. Stem Cell Therapy for Neonatal Hypoxic-Ischemic Encephalopathy: A Systematic Review of Preclinical Studies. Int. J. Mol. Sci. 2021, 22, 3142. https://doi.org/10.3390/ijms22063142
Serrenho I, Rosado M, Dinis A, M. Cardoso C, Grãos M, Manadas B, Baltazar G. Stem Cell Therapy for Neonatal Hypoxic-Ischemic Encephalopathy: A Systematic Review of Preclinical Studies. International Journal of Molecular Sciences. 2021; 22(6):3142. https://doi.org/10.3390/ijms22063142
Chicago/Turabian StyleSerrenho, Inês, Miguel Rosado, Alexandra Dinis, Carla M. Cardoso, Mário Grãos, Bruno Manadas, and Graça Baltazar. 2021. "Stem Cell Therapy for Neonatal Hypoxic-Ischemic Encephalopathy: A Systematic Review of Preclinical Studies" International Journal of Molecular Sciences 22, no. 6: 3142. https://doi.org/10.3390/ijms22063142