Remote Photoacoustic Sensing Using Single Speckle Analysis by an Ultra-Fast Four Quadrant Photo-Detector
Abstract
:1. Introduction
2. Materials and Methods
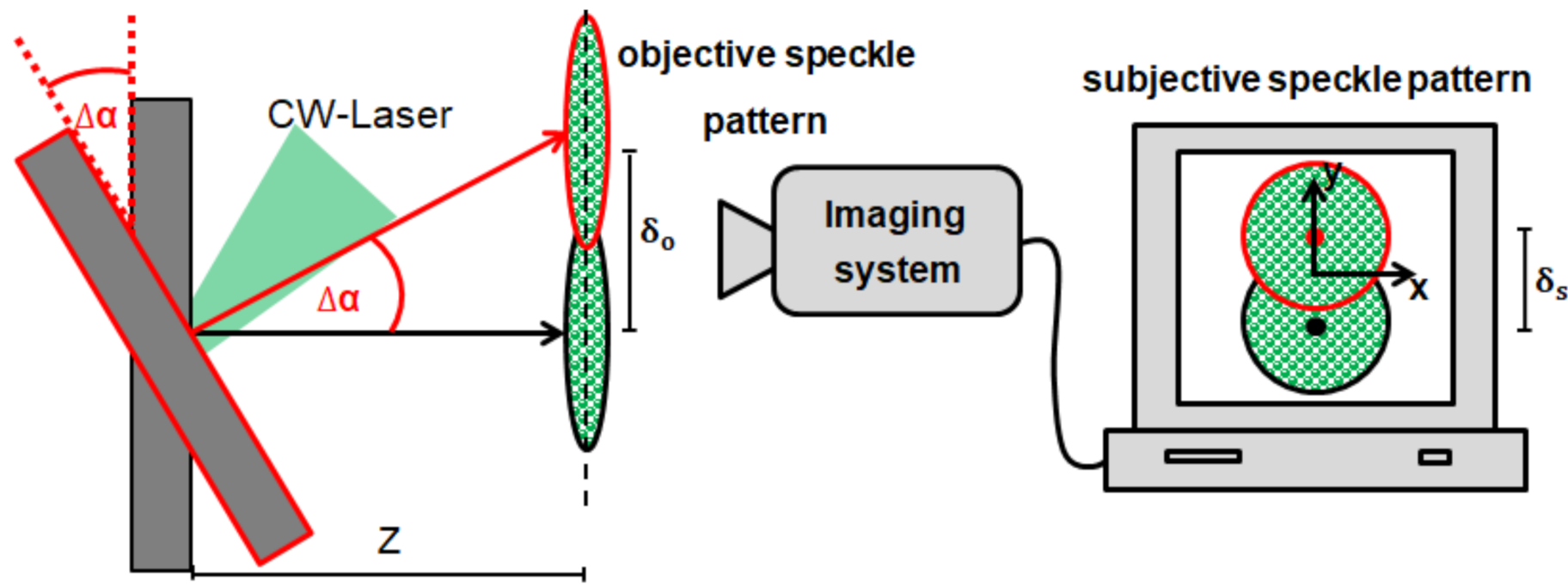
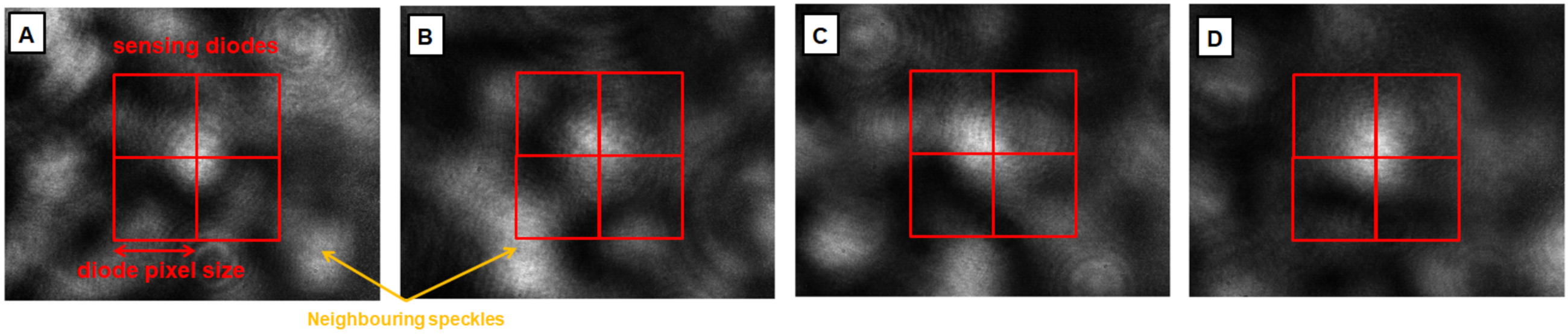
2.1. Speckle Sensing by Multiple Speckles’ Tracking
2.2. Imaging Systems and Setups
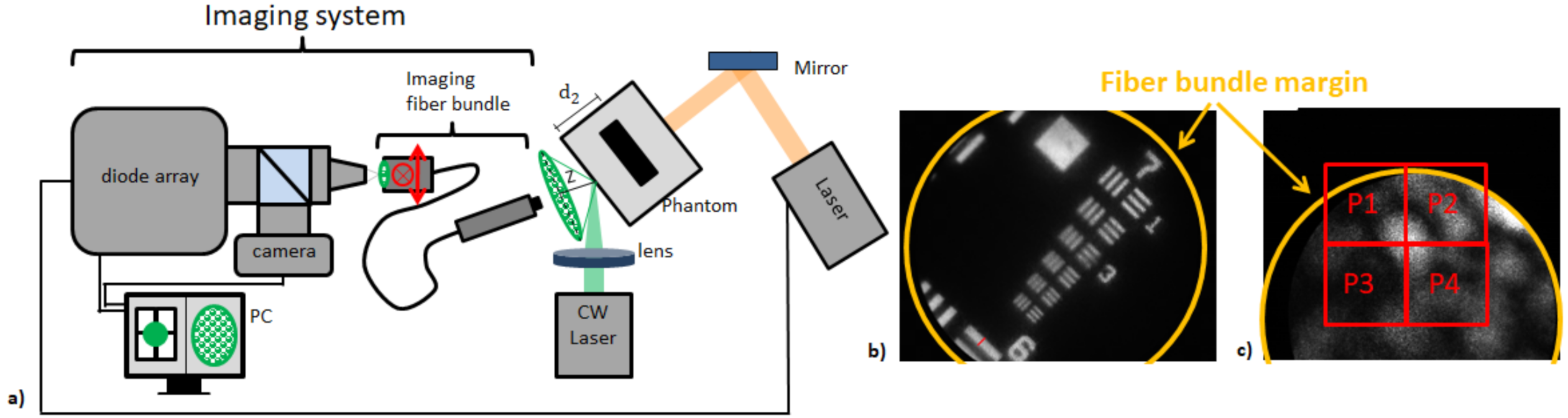
2.2.1. Free-Space Single Speckle Sensing
2.2.2. Fiber-Based Single Speckle Sensing
2.3. Measurement Modes and Samples
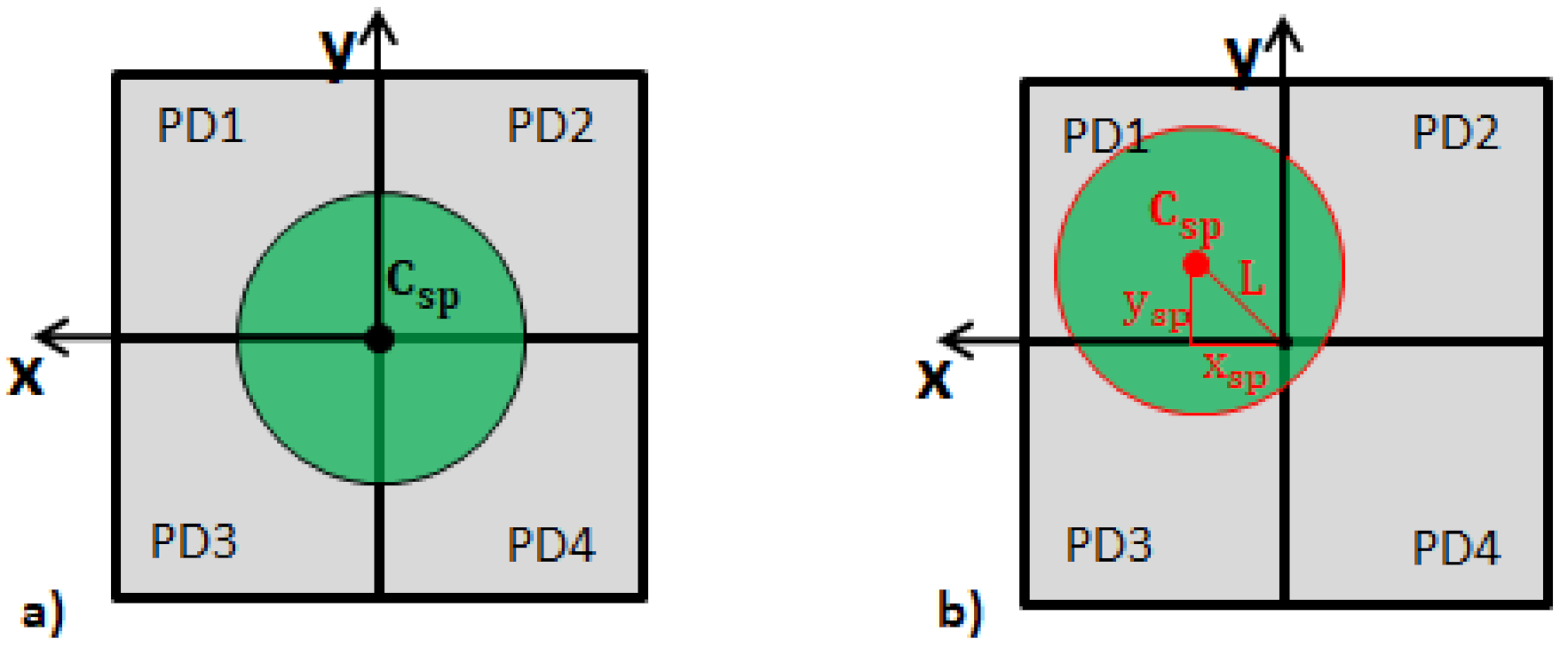
2.4. Data Analysis for Single Speckle Analysis
2.5. Sensing Parameter Evaluation for Single Speckle Analysis
2.5.1. Sensitivity
2.5.2. Sensing Range
2.5.3. Linearity
3. Results and Discussion
3.1. Photoacoustic Measurements
3.2. Sensing Parameters
3.2.1. Sensitivity
3.2.2. Sensing Range
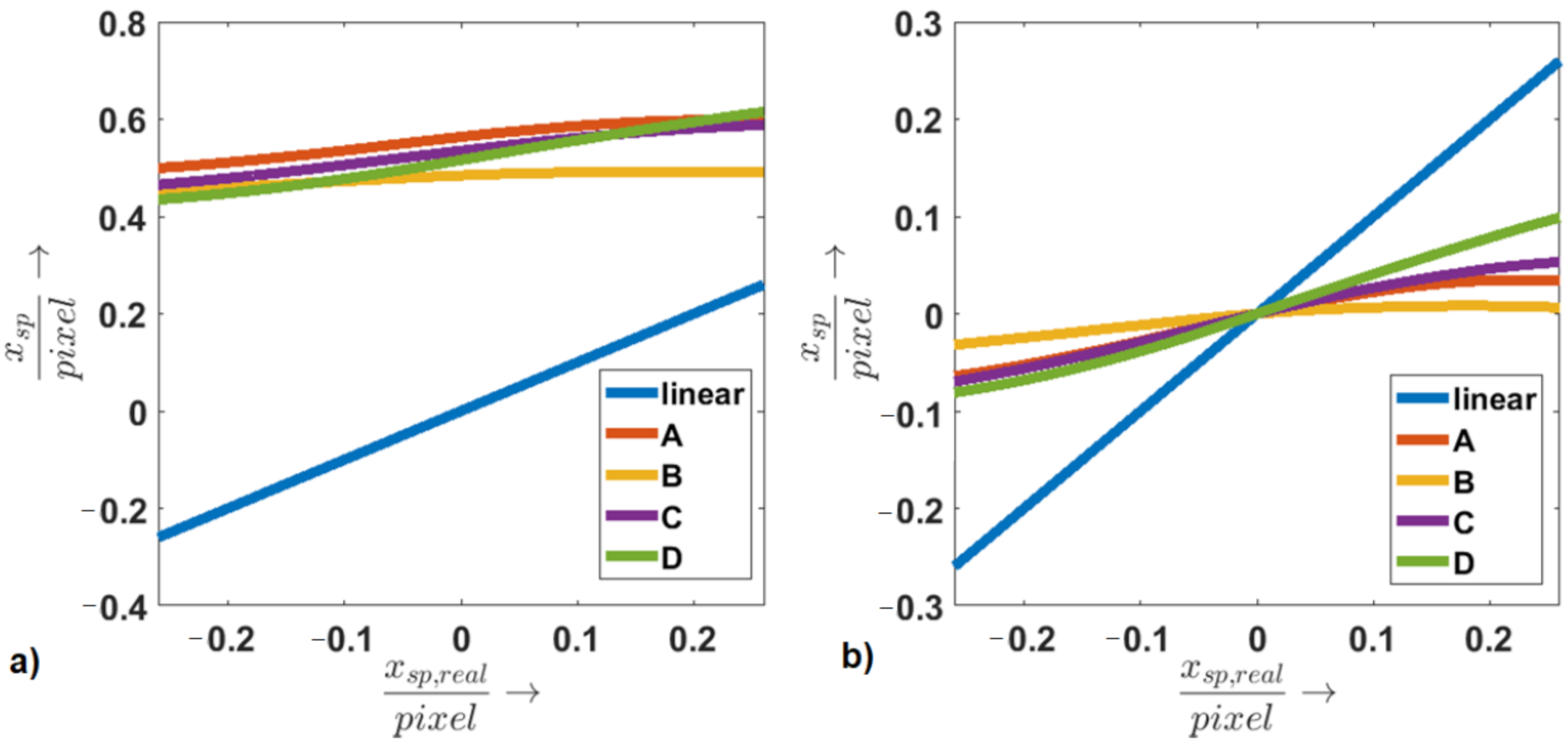
3.2.3. Linearity
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APS | avalanche-photodiode sensor |
| MPE | maximum permitted exposure |
| PA | Photoacoustic |
| PhAT | phantom Setup A transmission mode |
| PhAR | phantom Setup A reflection mode |
| PhBT | phantom Setup B transmission mode |
| PVCP | polyvinyl chloride plastisol |
| skinAT1 | skin sample Setup A transmission mode |
| skinAT2p | skin sample Setup A transmission mode pulsed illumination |
References
- Yao, J.; Xia, J.; Wang, L.V. Multiscale functional and molecular photoacoustic tomography. Ultrason. Imaging 2016, 38, 44–62. [Google Scholar] [CrossRef] [Green Version]
- Jacques, S.L. Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, R37. [Google Scholar] [CrossRef]
- Yao, J.; Wang, L.V. Photoacoustic microscopy. Laser Photonics Rev. 2013, 7, 758–778. [Google Scholar] [CrossRef]
- Kolkman, R.G.; Blomme, E.; Cool, T.; Bilcke, M.; van Leeuwen, T.G.; Steenbergen, W.; Grimbergen, K.A.; den Heeten, G.J. Feasibility of noncontact piezoelectric detection of photoacoustic signals in tissue-mimicking phantoms. J. Biomed. Opt. 2010, 15, 055011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnes, R.A.; Maswadi, S.; Glickman, R.; Shadaram, M. Probe beam deflection technique as acoustic emission directionality sensor with photoacoustic emission source. Appl. Opt. 2014, 53, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L.; van Wijk, K.; Caron, J.N.; Timmerman, M. Gas-coupled laser acoustic detection as a non-contact line detector for photoacoustic and ultrasound imaging. J. Opt. 2016, 18, 024005. [Google Scholar] [CrossRef] [Green Version]
- Carp, S.A.; Guerra, A., III; Duque, S.Q., Jr.; Venugopalan, V. Optoacoustic imaging using interferometric measurement of surface displacement. Appl. Phys. Lett. 2004, 85, 5772–5774. [Google Scholar] [CrossRef]
- Hochreiner, A.; Bauer-Marschallinger, J.; Burgholzer, P.; Jakoby, B.; Berer, T. Non-contact photoacoustic imaging using a fiber based interferometer with optical amplification. Biomed. Opt. Express 2013, 4, 2322–2331. [Google Scholar] [CrossRef] [Green Version]
- Rousseau, G.; Gauthier, B.; Blouin, A.; Monchalin, J.P. Non-contact biomedical photoacoustic and ultrasound imaging. J. Biomed. Opt. 2012, 17, 061217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horstmann, J.; Spahr, H.; Buj, C.; Münter, M.; Brinkmann, R. Full-field speckle interferometry for non-contact photoacoustic tomography. Phys. Med. Biol. 2015, 60, 4045. [Google Scholar] [CrossRef] [Green Version]
- Wissmeyer, G.; Pleitez, M.A.; Rosenthal, A.; Ntziachristos, V. Looking at sound: Optoacoustics with all-optical ultrasound detection. Light. Sci. Appl. 2018, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Clark, M. Optical detection of ultrasound on rough surfaces using speckle correlated spatial filtering. J. Phys. Conf. Ser. IOP Publ. 2011, 278, 012025. [Google Scholar] [CrossRef]
- Sharpies, S.; Light, R.; Achamfuo-Yeboah, S.; Clark, M.; Somekh, M.G. The SKED: Speckle knife edge detector. J. Phys. Conf. Ser. IOP Publ. 2014, 520, 012004. [Google Scholar] [CrossRef] [Green Version]
- Zalevsky, Z.; Beiderman, Y.; Margalit, I.; Gingold, S.; Teicher, M.; Mico, V.; Garcia, J. Simultaneous remote extraction of multiple speech sources and heart beats from secondary speckles pattern. Opt. Express 2009, 17, 21566–21580. [Google Scholar] [CrossRef] [Green Version]
- Hajireza, P.; Shi, W.; Bell, K.; Paproski, R.J.; Zemp, R.J. Non-interferometric photoacoustic remote sensing microscopy. Light. Sci. Appl. 2017, 6, e16278. [Google Scholar] [CrossRef]
- Lengenfelder, B.; Mehari, F.; Hohmann, M.; Heinlein, M.; Chelales, E.; Waldner, M.J.; Klämpfl, F.; Zalevsky, Z.; Schmidt, M. Remote photoacoustic sensing using speckle analysis. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lengenfelder, B.; Mehari, F.; Hohmann, M.; Löhr, C.; Waldner, M.J.; Schmidt, M.; Zalevsky, Z.; Klämpfl, F. Contact-free endoscopic photoacoustic sensing using speckle analysis. J. Biophotonics 2019, 12, e201900130. [Google Scholar] [CrossRef] [Green Version]
- Lengenfelder, B.; Mehari, F.; Hoppe, L.; Klämpfl, F.; Tenner, F.; Zalevsky, Z.; Schmidt, M. Remote photoacoustic tomography using speckle sensing with a high-speed camera. In Optics and the Brain; Optical Society of America: Washington, DC, USA, 2016; p. JM3A–16. [Google Scholar]
- Lengenfelder, B.; Hohmann, M.; Röhm, M.; Schmidt, M.; Zam, A.; Zalevsky, Z.; Klämpfl, F. Image reconstruction for remote photoacoustic tomography using speckle analysis. Tissue Opt. Photonics 2020, 11363, 113631F. [Google Scholar]
- Lengenfelder, B.; Jarkas, H.; Shabairou, N.; Hohmann, M.; Schmidt, M.; Zalevsky, Z.; Klämpfl, F. Remote photoacoustic tomography using diode-array and speckle analysis. Tissue Opt. Photonics 2020, 11363, 1136308. [Google Scholar]
- Horstmann, J. Kontaktlose Photoakustische Tomographie: Realisierung und Evaluation einer Optisch-Holographischen Detektionsmethode; Infinite Science Publishing: Lübeck, Germany, 2016. [Google Scholar]
- Culjat, M.O.; Goldenberg, D.; Tewari, P.; Singh, R.S. A review of tissue substitutes for ultrasound imaging. Ultrasound Med. Biol. 2010, 36, 861–873. [Google Scholar] [CrossRef]
- Wu, J.; Chen, Y.; Gao, S.; Li, Y.; Wu, Z. Improved measurement accuracy of spot position on an InGaAs quadrant detector. Appl. Opt. 2015, 54, 8049–8054. [Google Scholar] [CrossRef]
- Shabairou, N.; Lengenfelder, B.; Hohmann, M.; Klämpfl, F.; Schmidt, M.; Zalevsky, Z. All-optical, an ultra-thin endoscopic photoacoustic sensor using multi mode fiber. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Winkler, A.M.; Maslov, K.I.; Wang, L.V. Noise-equivalent sensitivity of photoacoustics. J. Biomed. Opt. 2013, 18, 097003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Fowlkes, J.B.; Cannata, J.M.; Hu, C.; Carson, P.L. Photoacoustic imaging with a commercial ultrasound system and a custom probe. Ultrasound Med. Biol. 2011, 37, 484–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Position Sensitive Diode, First Sensor. Available online: https://www.first-sensor.com/en/products/optical-sensors/detectors/quadrant-apds-qa/ (accessed on 31 August 2020).
- Huang, C.; Wang, K.; Nie, L.; Wang, L.V.; Anastasio, M.A. Full-wave iterative image reconstruction in photoacoustic tomography with acoustically inhomogeneous media. IEEE Trans. Med. Imaging 2013, 32, 1097–1110. [Google Scholar] [CrossRef] [PubMed]


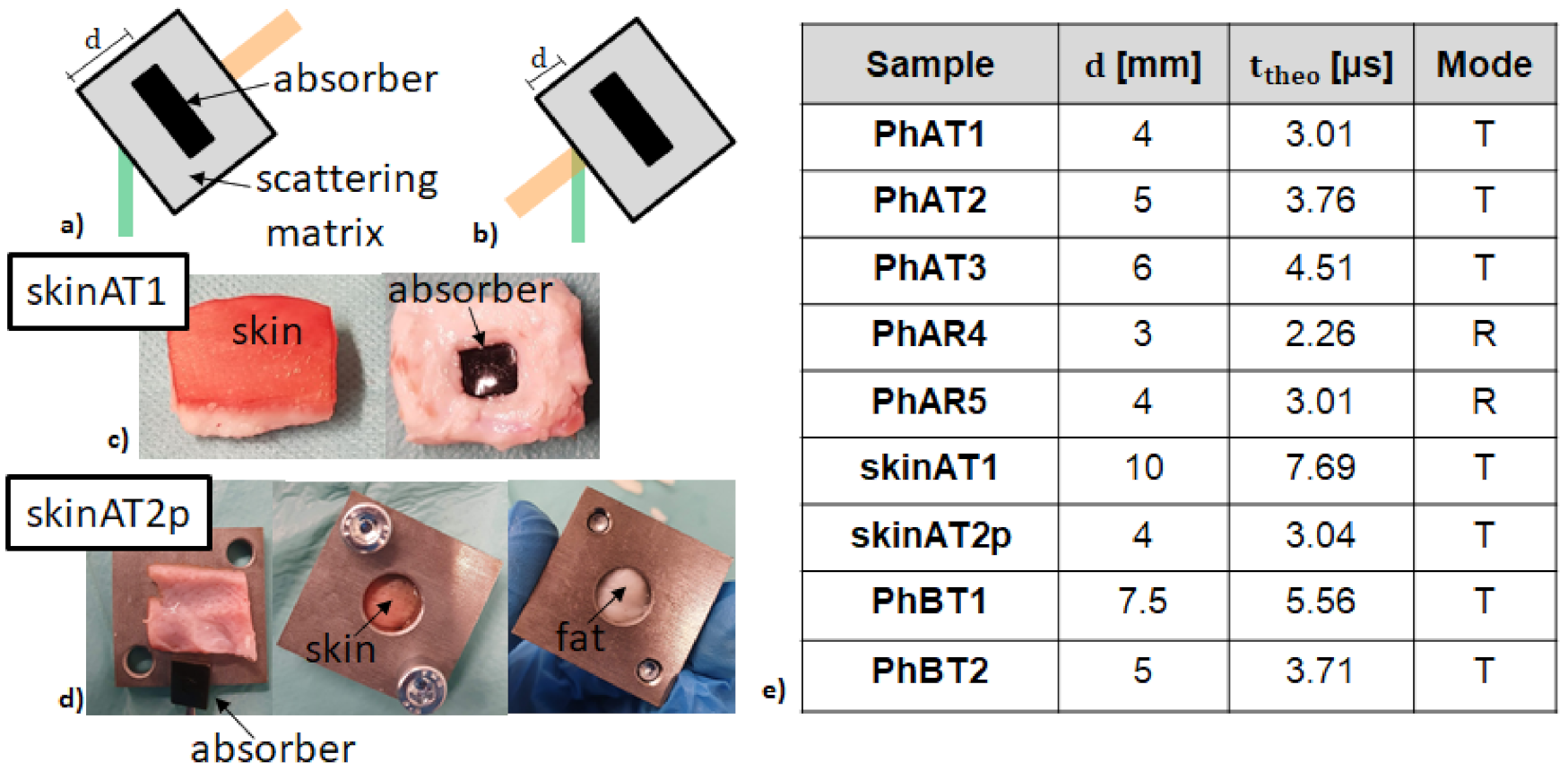
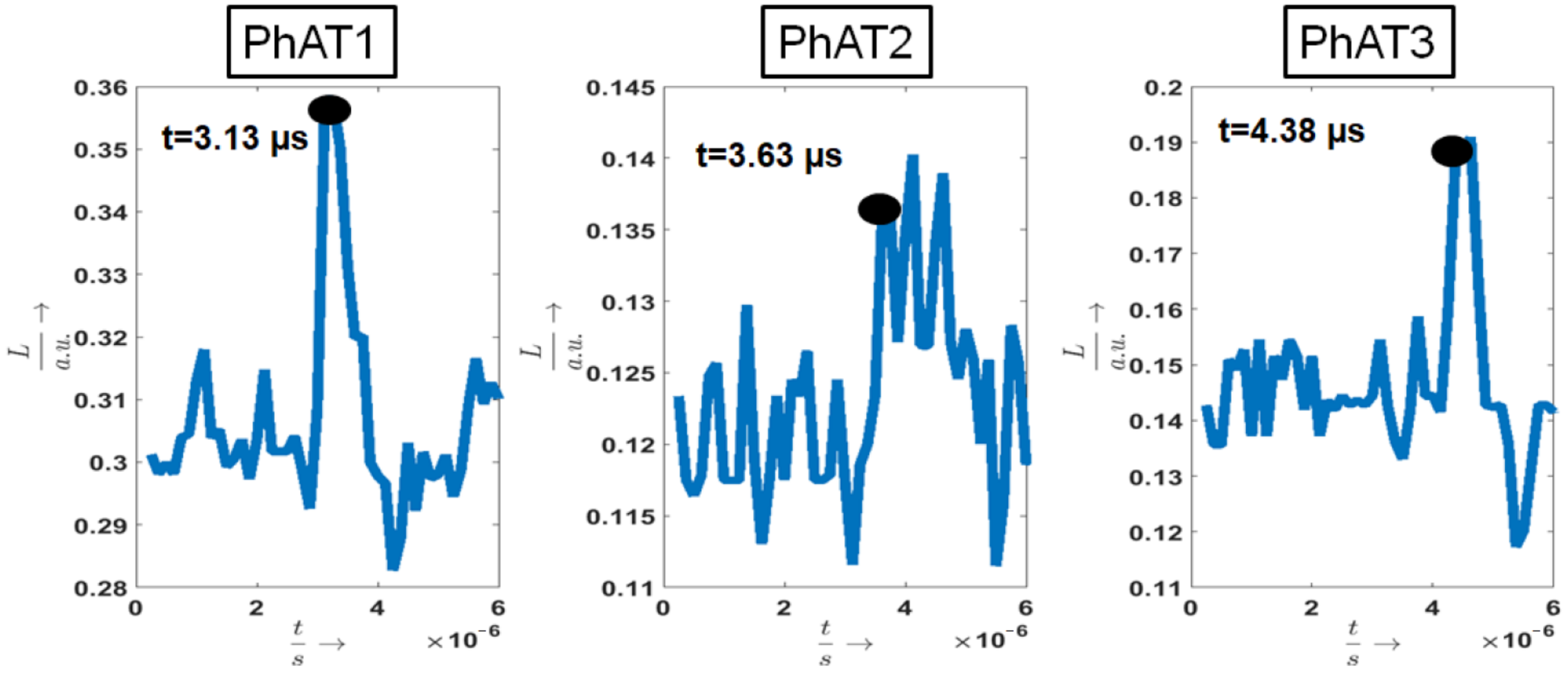
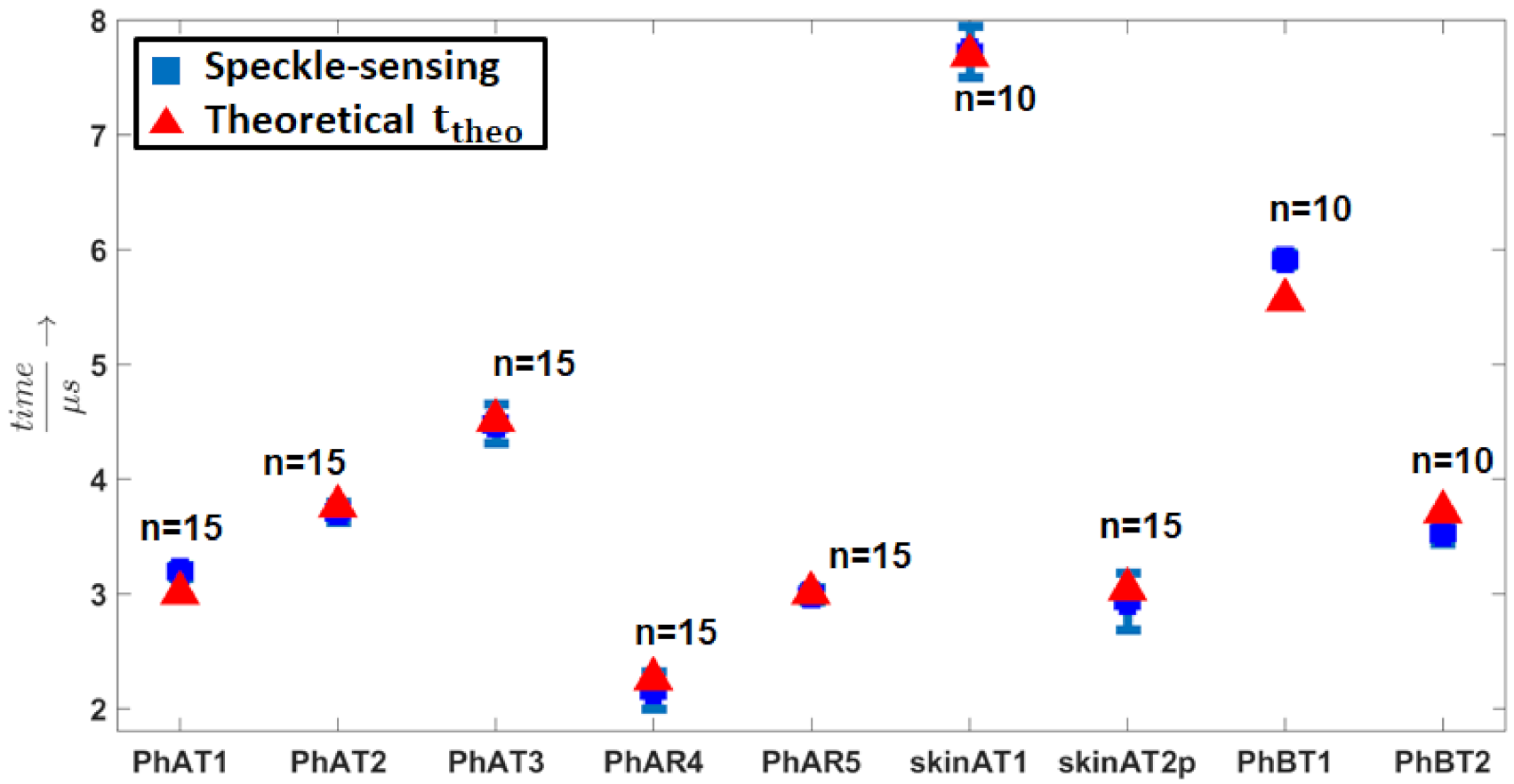
| Sample | (μs) | (μs) | (μs) |
|---|---|---|---|
| PhAT1 | 3.19 | 0.06 | 3.01 |
| PhAT2 | 3.71 | 0.09 | 3.76 |
| PhAT3 | 4.48 | 0.17 | 4.51 |
| PhAR4 | 2.15 | 0.16 | 2.26 |
| PhAR5 | 2.99 | 0.06 | 3.01 |
| skinAT1 | 7.72 | 0.22 | 7.69 |
| skinAT2p | 2.93 | 0.25 | 3.04 |
| PhBT1 | 5.91 | 0.057 | 5.56 |
| PhBT2 | 3.53 | 0.093 | 3.71 |
| Free-Space | Fiber-Guided | ||
|---|---|---|---|
| PVCP | skin | PVCP | |
| 0.006 | 0.007 | 0.0057 | |
| in μm | 1600 | 1600 | 1600 |
| M | 5 | 5 | 50 |
| Z in cm | 20 | 20 | 0.2 |
| in | 55.0 | 64.2 | 522 |
| in mm | 0.75 | 0.75 | 0.05 |
| in nm | 7.2 | 8.4 | 4.56 |
| in | 1.4 | 1.99 | 1.4 |
| in kPa | 31.67 | 52.52 | 20.06 |
| in kPa | 127 | 210 | 80 |
| Single Speckle Analysis | |
|---|---|
| in μm | 1600 |
| in μm | 1120 |
| in | 0.0642 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lengenfelder, B.; Hohmann, M.; Späth, M.; Scherbaum, D.; Weiß, M.; Rupitsch, S.J.; Schmidt, M.; Zalevsky, Z.; Klämpfl, F. Remote Photoacoustic Sensing Using Single Speckle Analysis by an Ultra-Fast Four Quadrant Photo-Detector. Sensors 2021, 21, 2109. https://doi.org/10.3390/s21062109
Lengenfelder B, Hohmann M, Späth M, Scherbaum D, Weiß M, Rupitsch SJ, Schmidt M, Zalevsky Z, Klämpfl F. Remote Photoacoustic Sensing Using Single Speckle Analysis by an Ultra-Fast Four Quadrant Photo-Detector. Sensors. 2021; 21(6):2109. https://doi.org/10.3390/s21062109
Chicago/Turabian StyleLengenfelder, Benjamin, Martin Hohmann, Moritz Späth, Daniel Scherbaum, Manuel Weiß, Stefan J. Rupitsch, Michael Schmidt, Zeev Zalevsky, and Florian Klämpfl. 2021. "Remote Photoacoustic Sensing Using Single Speckle Analysis by an Ultra-Fast Four Quadrant Photo-Detector" Sensors 21, no. 6: 2109. https://doi.org/10.3390/s21062109
APA StyleLengenfelder, B., Hohmann, M., Späth, M., Scherbaum, D., Weiß, M., Rupitsch, S. J., Schmidt, M., Zalevsky, Z., & Klämpfl, F. (2021). Remote Photoacoustic Sensing Using Single Speckle Analysis by an Ultra-Fast Four Quadrant Photo-Detector. Sensors, 21(6), 2109. https://doi.org/10.3390/s21062109








