The Comparative Effects of Mesenchymal Stem Cell Transplantation Therapy for Spinal Cord Injury in Humans and Animal Models: A Systematic Review and Meta-Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Spinal Cord Injury
1.2. Current Treatments and the Need for New Therapies
1.3. In Vivo Models of Spinal Cord Injury
1.4. Study Objective
2. Materials and Methods
2.1. Literature Searches and Study Selection
2.2. Inclusion and Exclusion Criteria
2.3. Participants
2.4. Outcome Measures
2.5. Statistical Analysis
3. Results
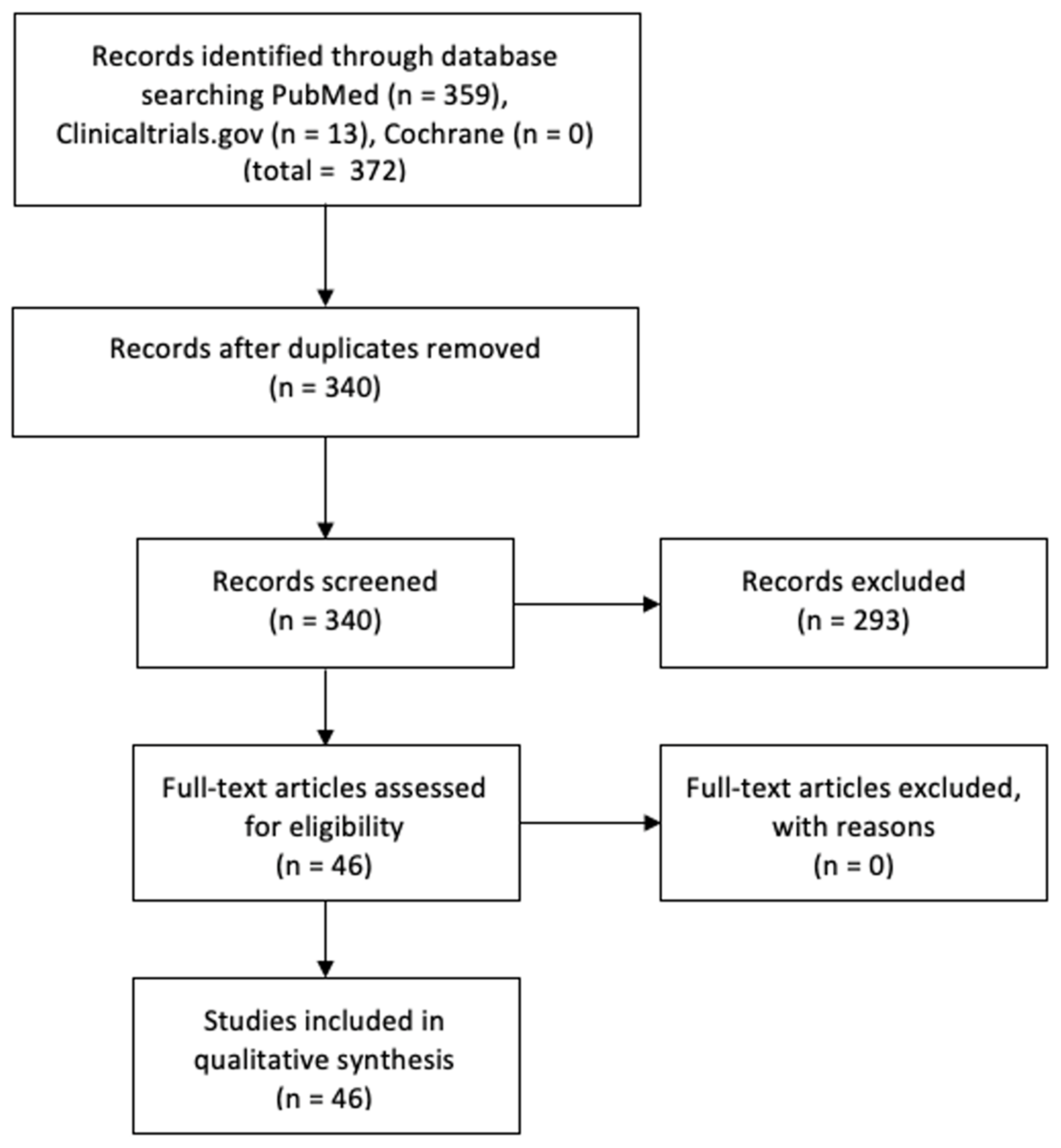
3.1. Study Selection
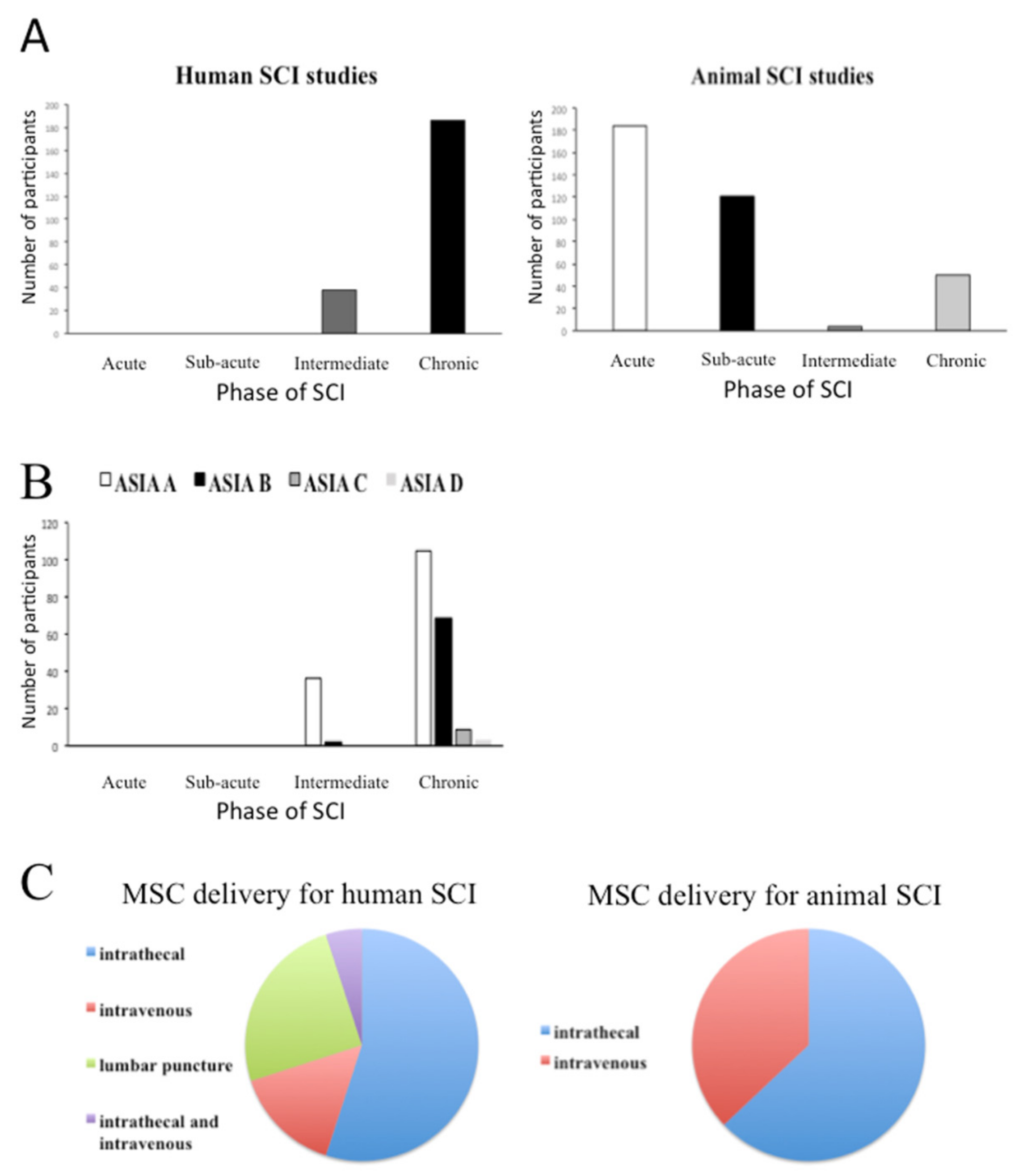
3.2. Summary of Baseline Characteristics of Human and Animal Studies
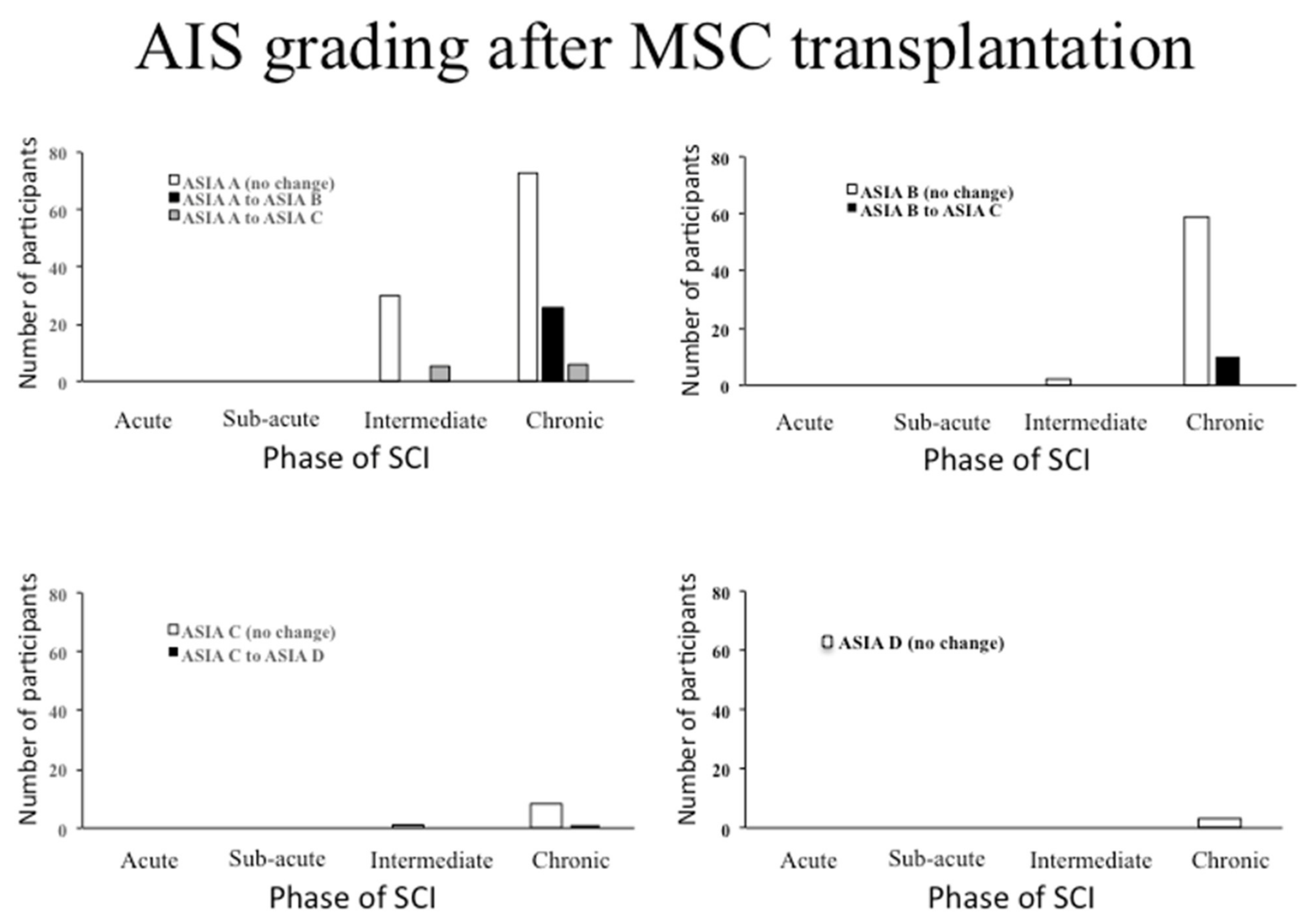
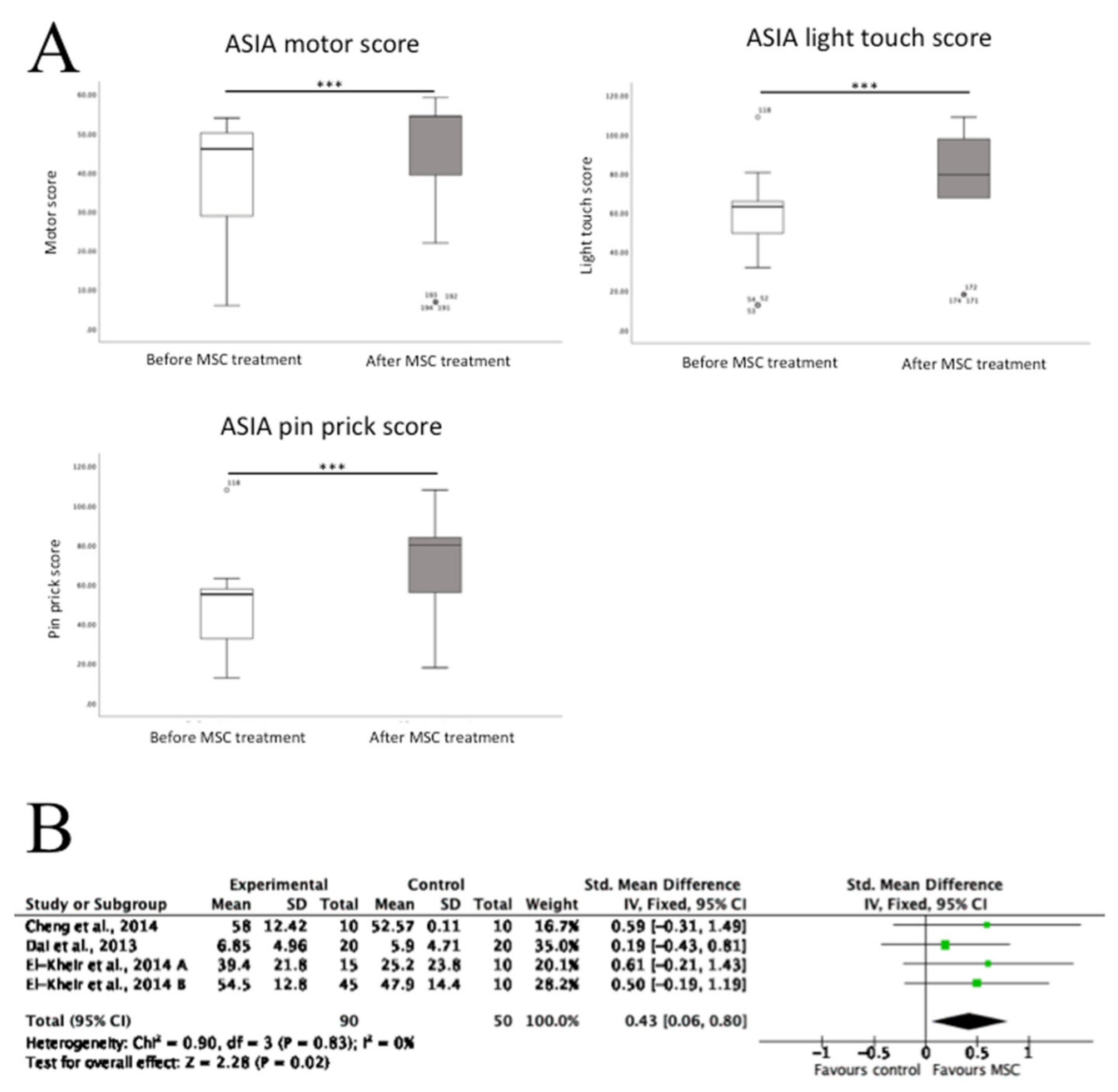
3.3. The Effects of MSC Transplantation on Functional Outcomes in Humans
3.4. The Effects of MSC Transplantation on Functional Outcomes in Animals
4. Discussion
4.1. The Efficacy of MSC Transplantation on Function Outcomes
4.2. The Relevance of SCI Animal Models to Human Studies
4.3. Electrophysiology
4.4. Biomarkers of Functional Recovery
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karsy, M.; Hawryluk, G. Modern Medical Management of Spinal Cord Injury. Curr. Neurol. Neurosci. Rep. 2019, 19, 65. [Google Scholar] [CrossRef]
- WHO. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/spinal-cord-injury (accessed on 30 June 2020).
- Wright, K.T.; El Masri, W.; Osman, A.; Chowdhury, J.; Johnson, W.E.B. Concise Review: Bone Marrow for the Treatment of Spinal Cord Injury: Mechanisms and Clinical Applications. Stem Cells 2011, 29, 169–178. [Google Scholar] [CrossRef] [Green Version]
- Oliveri, R.S.; Bello, S.; Biering-Sørensen, F. Mesenchymal stem cells improve locomotor recovery in traumatic spinal cord injury: Systematic review with meta-analyses of rat models. Neurobiol. Dis. 2014, 62, 338–353. [Google Scholar] [CrossRef] [PubMed]
- Pineau, I.; Lacroix, S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: Multiphasic expression pattern and identification of the cell types involved. J. Comp. Neurol. 2006, 500, 267–285. [Google Scholar] [CrossRef] [PubMed]
- Rowland, J.W.; Hawryluk, G.W.J.; Kwon, B.; Fehlings, M.G. Current status of acute spinal cord injury pathophysiology and emerging therapies: Promise on the horizon. Neurosurg. Focus 2008, 25, E2. [Google Scholar] [CrossRef]
- Yuan, Y.-M.; He, C. The glial scar in spinal cord injury and repair. Neurosci. Bull. 2013, 29, 421–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witiw, C.D.; Fehlings, M.G. Acute Spinal Cord Injury. J. Spinal Disord. Tech. 2015, 28, 202–210. [Google Scholar] [CrossRef]
- Morgenstern, D.A.; Asher, R.A.; Fawcett, J.W. Chapter 22 Chondroitin sulphate proteoglycans in the CNS injury response. Prog. Brain Res. 2002, 137, 313–332. [Google Scholar] [CrossRef]
- McDonald, J.W.; Belegu, V. Demyelination and Remyelination after Spinal Cord Injury. J. Neurotrauma 2006, 23, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Ludwin, S.K. Remyelination in the central nervous system and the peripheral nervous system. Adv. Neurol. 1988, 47, 215–254. [Google Scholar] [PubMed]
- Salewski, R.; Emrani, H.; Fehlings, M.G. Neural Stem/Progenitor Cells for Spinal Cord Regeneration. In Trends in Cell Signaling Pathways in Neuronal Fate Decision; Wislet-Gendebien, S., Ed.; IntechOpen: London, UK, 2013. [Google Scholar]
- Fawcett, J.W.; Curt, A.; Steeves, J.D.; Coleman, W.P.; Tuszynski, M.H.; Lammertse, D.; Bartlett, P.F.; Blight, A.R.; Dietz, V.; Ditunno, J.; et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: Spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord 2006, 45, 190–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karamouzian, S.; Nematollahi-Mahani, S.N.; Nakhaee, N.; Eskandary, H. Clinical safety and primary efficacy of bone marrow mesenchymal cell transplantation in subacute spinal cord injured patients. Clin. Neurol. Neurosurg. 2012, 114, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.W.; Cho, T.-H.; Park, D.-H.; Lee, J.-B.; Park, J.-Y.; Chung, Y.-G. Intrathecal transplantation of autologous adipose-derived mesenchymal stem cells for treating spinal cord injury: A human trial. J. Spinal Cord Med. 2015, 39, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Siddall, P.J.; Middleton, J.W. Spinal cord injury-induced pain: Mechanisms and treatments. Pain Manag. 2015, 5, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Rouanet, C.; Reges, D.; Rocha, E.; Gagliardi, V.; Silva, G.S. Traumatic spinal cord injury: Current concepts and treatment update. Arq. Neuro Psiquiatr. 2017, 75, 387–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evaniew, N.; Belley-Côté, E.P.; Fallah, N.; Noonan, V.K.; Rivers, C.S.; Dvorak, M.F. Methylprednisolone for the Treatment of Patients with Acute Spinal Cord Injuries: A Systematic Review and Meta-Analysis. J. Neurotrauma 2016, 33, 468–481. [Google Scholar] [CrossRef]
- Donovan, J.; Kirshblum, S. Clinical Trials in Traumatic Spinal Cord Injury. Neurotherapeutics 2018, 15, 654–668. [Google Scholar] [CrossRef] [Green Version]
- Qin, C.; Guo, Y.; Yang, D.-G.; Yang, M.-L.; Du, L.-J.; Li, J.-J. Induced Pluripotent Stem Cell Transplantation Improves Locomotor Recovery in Rat Models of Spinal Cord Injury: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Cell. Physiol. Biochem. 2018, 47, 1835–1852. [Google Scholar] [CrossRef]
- Kadoya, K.; Lu, P.; Nguyen, K.; Lee-Kubli, C.; Kumamaru, H.; Yao, L.; Knackert, J.; Poplawski, G.; Dulin, J.N.; Strobl, H.; et al. Spinal cord reconstitution with homologous neural grafts enables robust corticospinal regeneration. Nat. Med. 2016, 22, 479–487. [Google Scholar] [CrossRef] [Green Version]
- Keyvan-Fouladi, N.; Raisman, G.; Li, Y. Functional Repair of the Corticospinal Tract by Delayed Transplantation of Olfactory Ensheathing Cells in Adult Rats. J. Neurosci. 2003, 23, 9428–9434. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, H.; Uchida, K.; Guerrero, A.R.; Watanabe, S.; Sugita, D.; Takeura, N.; Yoshida, A.; Long, G.; Wright, K.T.; Johnson, W.E.; et al. Transplantation of Mesenchymal Stem Cells Promotes an Alternative Pathway of Macrophage Activation and Functional Recovery after Spinal Cord Injury. J. Neurotrauma 2012, 29, 1614–1625. [Google Scholar] [CrossRef] [Green Version]
- Neuhuber, B.; Himes, B.T.; Shumsky, J.S.; Gallo, G.; Fischer, I. Axon growth and recovery of function supported by human bone marrow stromal cells in the injured spinal cord exhibit donor variations. Brain Res. 2005, 1035, 73–85. [Google Scholar] [CrossRef]
- Gnecchi, M.; Danieli, P.; Malpasso, G.; Ciuffreda, M.C. Paracrine Mechanisms of Mesenchymal Stem Cells in Tissue Repair. Methods Mol. Biol. 2016, 1416, 123–146. [Google Scholar] [CrossRef]
- Martins, L.F.; Costa, R.O.; Pedro, J.R.; Aguiar, P.; Serra, S.C.; Teixeira, F.G.; Sousa, N.; Salgado, A.J.; Almeida, R.D. Mesenchymal stem cells secretome-induced axonal outgrowth is mediated by BDNF. Sci. Rep. 2017, 7, 4153. [Google Scholar] [CrossRef] [Green Version]
- Kingham, P.J.; Kolar, M.K.; Novikova, L.N.; Novikov, L.N.; Wiberg, M. Stimulating the Neurotrophic and Angiogenic Properties of Human Adipose-Derived Stem Cells Enhances Nerve Repair. Stem Cells Dev. 2014, 23, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Uchida, K.; Nakajima, H.; Matsuo, H.; Sugita, D.; Yoshida, A.; Honjoh, K.; Johnson, W.E.; Baba, H. Early Transplantation of Mesenchymal Stem Cells After Spinal Cord Injury Relieves Pain Hypersensitivity Through Suppression of Pain-Related Signaling Cascades and Reduced Inflammatory Cell Recruitment. Stem Cells 2015, 33, 1902–1914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, K.T.; El Masri, W.; Osman, A.; Roberts, S.; Chamberlain, G.; Ashton, B.A.; Johnson, W.E. Bone marrow stromal cells stimulate neurite outgrowth over neural proteoglycans (CSPG), myelin associated glycoprotein and Nogo-A. Biochem. Biophys. Res. Commun. 2007, 354, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Hofstetter, C.P.; Schwarz, E.J.; Hess, D.; Widenfalk, J.; El Manira, A.; Prockop, D.J.; Olson, L. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc. Natl. Acad. Sci. USA 2002, 99, 2199–2204. [Google Scholar] [CrossRef] [Green Version]
- Orr, M.B.; Gensel, J.C. Spinal Cord Injury Scarring and Inflammation: Therapies Targeting Glial and Inflammatory Responses. Neurotherapeutics 2018, 15, 541–553. [Google Scholar] [CrossRef] [Green Version]
- DePaul, M.A.; Lin, C.-Y.; Silver, J.; Lee, Y.-S. Combinatory repair strategy to promote axon regeneration and functional recovery after chronic spinal cord injury. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Nori, S.; Khazaei, M.; Ahuja, C.S.; Yokota, K.; Ahlfors, J.-E.; Liu, Y.; Wang, J.; Shibata, S.; Chio, J.; Hettiaratchi, M.H.; et al. Human Oligodendrogenic Neural Progenitor Cells Delivered with Chondroitinase ABC Facilitate Functional Repair of Chronic Spinal Cord Injury. Stem Cell Rep. 2018, 11, 1433–1448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahuja, C.S.; Nori, S.; Tetreault, L.; Wilson, J.; Kwon, B.; Harrop, J.; Choi, D.; Fehlings, M.G. Traumatic Spinal Cord Injury—Repair and Regeneration. Neurosurgery 2017, 80, S9–S22. [Google Scholar] [CrossRef] [PubMed]
- Sarveazad, A.; Babahajian, A.; Bakhtiari, M.; Soleimani, M.; Behnam, B.; Yari, A.; Akbari, A.; Yousefifard, M.; Janzadeh, A.; Amini, N.; et al. The combined application of human adipose derived stem cells and Chondroitinase ABC in treatment of a spinal cord injury model. Neuropeptides 2017, 61, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Huang, X.; Lin, W.; Qiu, Y.; He, Y.; Yu, J.; Xi, Y.; Ye, X. Hypoxic preconditioned bone mesenchymal stem cells ameliorate spinal cord injury in rats via improved survival and migration. Int. J. Mol. Med. 2018, 42, 2538–2550. [Google Scholar] [CrossRef]
- Takahashi, A.; Nakajima, H.; Uchida, K.; Takeura, N.; Honjoh, K.; Watanabe, S.; Kitade, M.; Kokubo, Y.; Johnson, W.E.B.; Matsumine, A. Comparison of Mesenchymal Stromal Cells Isolated from Murine Adipose Tissue and Bone Marrow in the Treatment of Spinal Cord Injury. Cell Transplant. 2018, 27, 1126–1139. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.-P.; Chen, X.-W.; Zhang, L.-Q.; Wu, C.-Y.; Huang, Z.-D.; Lin, J.-H. Effect of Neuroglobin Genetically Modified Bone Marrow Mesenchymal Stem Cells Transplantation on Spinal Cord Injury in Rabbits. PLoS ONE 2013, 8, e63444. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Jo, S.-H.; Kim, W.H.; Kweon, O.-K. Antioxidant and anti-inflammatory effects of intravenously injected adipose derived mesenchymal stem cells in dogs with acute spinal cord injury. Stem Cell Res. Ther. 2015, 6, 229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gossard, J.-P.; Delivet-Mongrain, H.; Martinez, M.; Kundu, A.; Escalona, M.; Rossignol, S. Plastic Changes in Lumbar Locomotor Networks after a Partial Spinal Cord Injury in Cats. J. Neurosci. 2015, 35, 9446–9455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zurita, M.; Aguayo, C.; Bonilla, C.; Otero, L.; Rico, M.; Rodríguez, A.; Vaquero, J. The pig model of chronic paraplegia: A challenge for experimental studies in spinal cord injury. Prog. Neurobiol. 2012, 97, 288–303. [Google Scholar] [CrossRef] [PubMed]
- Capogrosso, M.; Milekovic, T.; Borton, D.; Wagner, F.; Moraud, E.M.; Mignardot, J.-B.; Buse, N.; Gandar, J.; Barraud, Q.; Xing, D.B.D.; et al. A brain–spine interface alleviating gait deficits after spinal cord injury in primates. Nat. Cell Biol. 2016, 539, 284–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harding, J.; Roberts, R.M.; Mirochnitchenko, O. Large animal models for stem cell therapy. Stem Cell Res. Ther. 2013, 4, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Kjell, J.; Olson, L. Rat models of spinal cord injury: From pathology to potential therapies. Dis. Model. Mech. 2016, 9, 1125–1137. [Google Scholar] [CrossRef] [Green Version]
- Inman, D.M.; Steward, O. Ascending sensory, but not other long-tract axons, regenerate into the connective tissue matrix that forms at the site of a spinal cord injury in mice. J. Comp. Neurol. 2003, 462, 431–449. [Google Scholar] [CrossRef]
- Lemon, R.N.; Griffiths, J. Comparing the function of the corticospinal system in different species: Organizational differences for motor specialization? Muscle Nerve 2005, 32, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Courtine, G.; Bunge, M.B.; Fawcett, J.W.; Grossman, R.G.; Kaas, J.H.; Lemon, R.; Maier, I.; Martin, J.; Nudo, R.J.; Ramón-Cueto, A.; et al. Can experiments in nonhuman primates expedite the translation of treatments for spinal cord injury in humans? Nat. Med. 2007, 13, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Granger, N.; Carwardine, D. Acute Spinal Cord Injury. Veter. Clin. N. Am. Small Anim. Pr. 2014, 44, 1131–1156. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.U.; Yoon, Y.; Choi, K.U.; Jo, K.R.; Kim, N.; Lee, E.; Kim, W.H.; Kweon, O.-K. Therapeutic Effects of Intravenous Injection of Fresh and Frozen Thawed HO-1-Overexpressed Ad-MSCs in Dogs with Acute Spinal Cord Injury. Stem Cells Int. 2019, 2019, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Bach, F.S.; Rebelatto, C.L.K.; Fracaro, L.; Senegaglia, A.C.; Fragoso, F.Y.I.; Daga, D.R.; Brofman, P.R.S.; Pimpão, C.T.; Filho, J.R.E.; Montiani-Ferreira, F.; et al. Comparison of the Efficacy of Surgical Decompression Alone and Combined With Canine Adipose Tissue-Derived Stem Cell Transplantation in Dogs With Acute Thoracolumbar Disk Disease and Spinal Cord Injury. Front. Veter. Sci. 2019, 6, 383. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.; Burch, R. The Principles of Humane Experimental Technique. Med. J. Aust. 1960, 1, 500. [Google Scholar] [CrossRef]
- Nardone, R.; Florea, C.; Höller, Y.; Brigo, F.; Versace, V.; Lochner, P.; Golaszewski, S.; Trinka, E. Rodent, large animal and non-human primate models of spinal cord injury. Zoology 2017, 123, 101–114. [Google Scholar] [CrossRef]
- Kirshblum, S.C.; Burns, S.P.; Biering-Sorensen, F.; Donovan, W.; Graves, D.E.; Jha, A.; Johansen, M.; Jones, L.; Krassioukov, A.; Mulcahey, M.; et al. International standards for neurological classification of spinal cord injury (Revised 2011). J. Spinal Cord Med. 2011, 34, 535–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, T.T.; Leonard, G.R.; Cepela, D.J. Classifications in Brief: American Spinal Injury Association (ASIA) Impairment Scale. Clin. Orthop. Relat. Res. 2017, 475, 1499–1504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. A Sensitive and Reliable Locomotor Rating Scale for Open Field Testing in Rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Song, R.B.; Basso, D.M.; Da Costa, R.C.; Fisher, L.C.; Mo, X.; Moore, S.A. Adaptation of the Basso–Beattie–Bresnahan locomotor rating scale for use in a clinical model of spinal cord injury in dogs. J. Neurosci. Methods 2016, 268, 117–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basso, D.M.; Fisher, L.C.; Anderson, A.J.; Jakeman, L.B.; McTigue, D.M.; Popovich, P.G. Basso Mouse Scale for Locomotion Detects Differences in Recovery after Spinal Cord Injury in Five Common Mouse Strains. J. Neurotrauma 2006, 23, 635–659. [Google Scholar] [CrossRef]
- Osaka, M.; Honmou, O.; Murakami, T.; Nonaka, T.; Houkin, K.; Hamada, H.; Kocsis, J.D. Intravenous administration of mesenchymal stem cells derived from bone marrow after contusive spinal cord injury improves functional outcome. Brain Res. 2010, 1343, 226–235. [Google Scholar] [CrossRef]
- Morita, T.; Sasaki, M.; Kataoka-Sasaki, Y.; Nakazaki, M.; Nagahama, H.; Oka, S.; Oshigiri, T.; Takebayashi, T.; Yamashita, T.; Kocsis, J.D.; et al. Intravenous infusion of mesenchymal stem cells promotes functional recovery in a model of chronic spinal cord injury. Neuroscience 2016, 335, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, UK. Cochrane RevMan. 2020. Available online: https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman (accessed on 1 August 2020).
- Mendonça, M.V.P.; LaRocca, T.F.; Souza, B.S.D.F.; Villarreal, C.F.; Silva, L.F.M.; Matos, A.C.; Novaes, M.A.; Bahia, C.M.P.; Martinez, A.C.D.O.M.; Kaneto, C.M.; et al. Safety and neurological assessments after autologous transplantation of bone marrow mesenchymal stem cells in subjects with chronic spinal cord injury. Stem Cell Res. Ther. 2014, 5, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Vaquero, J.; Zurita, M.; Rico, M.A.; Aguayo, C.; Bonilla, C.; Marin, E.; Tapiador, N.; Sevilla, M.; Vazquez, D.; Carballido, J.; et al. Intrathecal administration of autologous mesenchymal stromal cells for spinal cord injury: Safety and efficacy of the 100/3 guideline. Cytotherapy 2018, 20, 806–819. [Google Scholar] [CrossRef]
- Oh, S.K.; Choi, K.H.; Yoo, J.Y.; Kim, D.Y.; Kim, S.J.; Jeon, S.R. A Phase III Clinical Trial Showing Limited Efficacy of Autologous Mesenchymal Stem Cell Therapy for Spinal Cord Injury. Neurosurgery 2016, 78, 436–447. [Google Scholar] [CrossRef] [Green Version]
- Vaquero, J.; Zurita, M.; Rico, M.A.; Bonilla, C.; Aguayo, C.; Fernández, C.; Tapiador, N.; Sevilla, M.; Morejón, C.; Montilla, J.; et al. Repeated subarachnoid administrations of autologous mesenchymal stromal cells supported in autologous plasma improve quality of life in patients suffering incomplete spinal cord injury. Cytotherapy 2017, 19, 349–359. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.; Liu, X.; Hua, R.; Dai, G.; Wang, X.; Gao, J.; An, Y. Clinical observation of umbilical cord mesenchymal stem cell transplantation in treatment for sequelae of thoracolumbar spinal cord injury. J. Transl. Med. 2014, 12, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, G.; Liu, X.; Zhang, Z.; Yang, Z.; Dai, Y.; Xu, R. Transplantation of autologous bone marrow mesenchymal stem cells in the treatment of complete and chronic cervical spinal cord injury. Brain Res. 2013, 1533, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Ra, J.C.; Shin, I.S.; Kim, S.H.; Kang, S.K.; Kang, B.C.; Lee, H.Y.; Kim, Y.J.; Jo, J.Y.; Yoon, E.J.; Choi, H.J.; et al. Safety of Intravenous Infusion of Human Adipose Tissue-Derived Mesenchymal Stem Cells in Animals and Humans. Stem Cells Dev. 2011, 20, 1297–1308. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Venkataramana, N.K.; Bansal, A.; Balaraju, S.; Jan, M.; Chandra, R.; Dixit, A.; Rauthan, A.; Murgod, U.; Totey, S. Ex vivo-expanded autologous bone marrow-derived mesenchymal stromal cells in human spinal cord injury/paraplegia: A pilot clinical study. Cytotherapy 2009, 11, 897–911. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, J.; Zurita, M.; Rico, M.A.; Bonilla, C.; Aguayo, C.; Montilla, J.; Bustamante, S.; Carballido, J.; Marin, E.; Martinez, F.; et al. An approach to personalized cell therapy in chronic complete paraplegia: The Puerta de Hierro phase I/II clinical trial. Cytotherapy 2016, 18, 1025–1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Kheir, W.A.; Gabr, H.; Awad, M.R.; Ghannam, O.; Barakat, Y.; Farghali, H.A.M.A.; El Maadawi, Z.M.; Ewes, I.; Sabaawy, H.E. Autologous Bone Marrow-Derived Cell Therapy Combined with Physical Therapy Induces Functional Improvement in Chronic Spinal Cord Injury Patients. Cell Transpl. 2014, 23, 729–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phedy, P.; Djaja, Y.P.; Gatam, L.; Kusnadi, Y.; Wirawan, R.P.; Tobing, I.M.; Subakir, N.; Mappalilu, A.; Prawira, M.A.; Yauwenas, R.; et al. Motoric Recovery After Transplantation of Bone Marrow Derived Mesenchymal Stem Cells in Chronic Spinal Cord Injury: A Case Report. Am. J. Case Rep. 2019, 20, 1299–1304. [Google Scholar] [CrossRef]
- Bydon, M.; Dietz, A.B.; Goncalves, S.; Moinuddin, F.M.; Alvi, M.A.; Goyal, A.; Yolcu, Y.; Hunt, C.L.; Garlanger, K.L.; Del Fabro, A.S.; et al. CELLTOP Clinical Trial: First Report From a Phase 1 Trial of Autologous Adipose Tissue–Derived Mesenchymal Stem Cells in the Treatment of Paralysis Due to Traumatic Spinal Cord Injury. Mayo Clin. Proc. 2020, 95, 406–414. [Google Scholar] [CrossRef] [Green Version]
- LaRocca, T.F.; Macêdo, C.T.; Souza, B.S.D.F.; Andrade-Souza, Y.M.; Villarreal, C.F.; Matos, A.C.; Silva, D.N.; Da Silva, P.D.; Souza, C.L.E.M.D.; Paixão, D.D.S.; et al. Image-guided percutaneous intralesional administration of mesenchymal stromal cells in subjects with chronic complete spinal cord injury: A pilot study. Cytotherapy 2017, 19, 1189–1196. [Google Scholar] [CrossRef]
- Chotivichit, A.; Ruangchainikom, M.; Chiewvit, P.; Wongkajornsilp, A.; Sujirattanawimol, K. Chronic spinal cord injury treated with transplanted autologous bone marrow-derived mesenchymal stem cells tracked by magnetic resonance imaging: A case report. J. Med. Case Rep. 2015, 9, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hua, R.; Li, P.; Wang, X.; Yang, J.; Zheng, P.; Niu, X.; Li, Y.; An, Y. Evaluation of Somatosensory Evoked Potential and Pain Rating Index in a Patient with Spinal Cord Injury Accepted Cell Therapy. Pain Physician 2016, 19, E659–E666. [Google Scholar] [PubMed]
- Jarocha, D.; Milczarek, O.; Wedrychowicz, A.; Kwiatkowski, S.; Majka, M. Continuous Improvement after Multiple Mesenchymal Stem Cell Transplantations in a Patient with Complete Spinal Cord Injury. Cell Transpl. 2015, 24, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kim, D.Y.; Sung, I.Y.; Choi, G.H.; Jeon, M.H.; Kim, K.K.; Jeon, S.R. Long-term Results of Spinal Cord Injury Therapy Using Mesenchymal Stem Cells Derived From Bone Marrow in Humans. Neurosurgery 2011, 70, 1238–1247. [Google Scholar] [CrossRef]
- Ban, D.-X.; Ning, G.-Z.; Feng, S.-Q.; Wang, Y.; Zhou, X.-H.; Liu, Y.; Chen, J.-T. Combination of activated Schwann cells with bone mesenchymal stem cells: The best cell strategy for repair after spinal cord injury in rats. Regen. Med. 2011, 6, 707–720. [Google Scholar] [CrossRef]
- Chen, L.; Cui, X.; Wu, Z.; Jia, L.; Yu, Y.; Zhou, Q.; Hu, X.; Xu, W.; Luo, D.; Liu, J.; et al. Transplantation of bone marrow mesenchymal stem cells pretreated with valproic acid in rats with an acute spinal cord injury. Biosci. Trends 2014, 8, 111–119. [Google Scholar] [CrossRef] [Green Version]
- Galhom, R.A.; El Raouf, H.H.H.A.; Ali, M.H.M. Role of bone marrow derived mesenchymal stromal cells and Schwann-like cells transplantation on spinal cord injury in adult male albino rats. Biomed. Pharmacother. 2018, 108, 1365–1375. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Sani, M.; Haider, K.H.; Dorvash, M.; Ziaee, S.M.; Karimi, A.; Namavar, M.R. Concomitant use of mesenchymal stem cells and neural stem cells for treatment of spinal cord injury: A combo cell therapy approach. Neurosci. Lett. 2018, 668, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Karaöz, E.; Kabatas, S.; Duruksu, G.; Okcu, A.; Subası, C.; Ay, B.; Musluman, M.; Civelek, E. Reduction of lesion in injured rat spinal cord and partial functional recovery of motility after bone marrow derived mesenchymal stem cell transplantation. Turk. Neurosurg. 2011, 22, 207–217. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-W.; Ha, K.-Y.; Molon, J.N.; Kim, Y.-H. Bone Marrow–Derived Mesenchymal Stem Cell Transplantation for Chronic Spinal Cord Injury in Rats. Spine 2013, 38, E1065–E1074. [Google Scholar] [CrossRef]
- Kim, Y.-C.; Kim, Y.-H.; Kim, J.-W.; Ha, K.-Y. Transplantation of Mesenchymal Stem Cells for Acute Spinal Cord Injury in Rats: Comparative Study between Intralesional Injection and Scaffold Based Transplantation. J. Korean Med. Sci. 2016, 31, 1373–1382. [Google Scholar] [CrossRef] [Green Version]
- Mitsuhara, T.; Takeda, M.; Yamaguchi, S.; Manabe, T.; Matsumoto, M.; Kawahara, Y.; Yuge, L.; Kurisu, K. Simulated microgravity facilitates cell migration and neuroprotection after bone marrow stromal cell transplantation in spinal cord injury. Stem Cell Res. Ther. 2013, 4, 35. [Google Scholar] [CrossRef] [Green Version]
- Quertainmont, R.; Cantinieaux, D.; Botman, O.; Sid, S.; Schoenen, J.; Franzen, R. Mesenchymal Stem Cell Graft Improves Recovery after Spinal Cord Injury in Adult Rats through Neurotrophic and Pro-Angiogenic Actions. PLoS ONE 2012, 7, e39500. [Google Scholar] [CrossRef] [PubMed]
- Rosado, I.; Carvalho, P.; Alves, E.; Tagushi, T.; Carvalho, J.; Silva, J.; Lavor, M.; Oliveira, K.; Serakides, R.; Goes, A.; et al. Immunomodulatory and neuroprotective effect of cryopreserved allogeneic mesenchymal stem cells on spinal cord injury in rats. Genet. Mol. Res. 2017, 16. [Google Scholar] [CrossRef] [PubMed]
- Sandner, B.; Ciatipis, M.; Motsch, M.; Soljanik, I.; Weidner, N.; Blesch, A. Limited Functional Effects of Subacute Syngeneic Bone Marrow Stromal Cell Transplantation after Rat Spinal Cord Contusion Injury. Cell Transpl. 2016, 25, 125–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres-Espín, A.; Redondo-Castro, E.; Hernández, J.; Navarro, X. Bone marrow mesenchymal stromal cells and olfactory ensheathing cells transplantation after spinal cord injury–A morphological and functional comparison in rats. Eur. J. Neurosci. 2014, 39, 1704–1717. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-J.; Zhang, R.-P.; Li, J.-D. Transplantation of neurotrophin-3-expressing bone mesenchymal stem cells improves recovery in a rat model of spinal cord injury. Acta Neurochir. 2014, 156, 1409–1418. [Google Scholar] [CrossRef]
- De Almeida, F.M.; Marques, S.A.; Ramalho, B.D.S.; Massoto, T.B.; Martinez, A.M.B. Chronic spinal cord lesions respond positively to tranplants of mesenchymal stem cells. Restor. Neurol. Neurosci. 2015, 33, 43–55. [Google Scholar] [CrossRef]
- Neirinckx, V.; Agirman, G.; Coste, C.; Marquet, A.; Dion, V.; Rogister, B.; Franzen, R.; Wislet, S. Adult bone marrow mesenchymal and neural crest stem cells are chemoattractive and accelerate motor recovery in a mouse model of spinal cord injury. Stem Cell Res. Ther. 2015, 6, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Kim, Y.; Rhew, D.; Kuk, M.; Kim, M.; Kim, W.H.; Kweon, O.-K. Effect of the combination of mesenchymal stromal cells and chondroitinase ABC on chronic spinal cord injury. Cytotherapy 2015, 17, 1374–1383. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, Y.; Rhew, D.; Kim, A.; Jo, K.R.; Yoon, Y.; Choi, K.U.; Jung, T.; Kim, W.H.; Kweon, O.-K. Effect of canine mesenchymal stromal cells overexpressing heme oxygenase-1 in spinal cord injury. J. Veter. Sci. 2017, 18, 377–386. [Google Scholar] [CrossRef]
- Chalmers, I. The lethal consequences of failing to make use of all relevant evidence about the effects of medical treatments: The need for systematic reviews. In Treating Individuals: From Randomized Trials to Personalised Medicine; Rothwell, P., Ed.; Elsevier: London, UK, 2009; pp. 37–58. [Google Scholar]
- Cofano, F.; Boido, M.; Monticelli, M.; Zenga, F.; Ducati, A.; Vercelli, A.; Garbossa, D. Mesenchymal Stem Cells for Spinal Cord Injury: Current Options, Limitations, and Future of Cell Therapy. Int. J. Mol. Sci. 2019, 20, 2698. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.-L.; Liu, Y.-J.; Yang, S.-G.; Zhao, Q.-J.; Wang, X.; Gong, W.; Han, Z.-B.; Xu, Z.-S.; Lu, Y.-X.; Liu, D.; et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica 2006, 91, 1017–1026. [Google Scholar]
- Jensen, M.P.; Hoffman, A.J.; Cardenas, D.D. Chronic pain in individuals with spinal cord injury: A survey and longitudinal study. Spinal Cord 2005, 43, 704–712. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Johannesen, I.L.; Sindrup, S.; Bach, F.; Jensen, T.S.; Sindrup, S.; Bach, F. Pain and dysesthesia in patients with spinal cord injury: A postal survey. Spinal Cord 2001, 39, 256–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neuhuber, B.; Barshinger, A.L.; Paul, C.; Shumsky, J.S.; Mitsui, T.; Fischer, I. Stem cell delivery by lumbar puncture as a therapeutic alternative to direct injection into injured spinal cord. J. Neurosurg. Spine 2008, 9, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Paul, C.; Samdani, A.F.; Betz, R.R.; Fischer, I.; Neuhuber, B. Grafting of Human Bone Marrow Stromal Cells into Spinal Cord Injury. Spine 2009, 34, 328–334. [Google Scholar] [CrossRef] [Green Version]
- Katoh, S.; El Masry, W.S. Neurological recovery after conservative treatment of cervical cord injuries. J. Bone Jt. Surg. Br. Vol. 1994, 76, 225–228. [Google Scholar] [CrossRef] [Green Version]
- Katoh, S.; El Masry, W.S.; Jaffray, D.; McCall, I.W.; Eisenstein, S.M.; Pringle, R.G.; Pullicino, V.; Ikata, T. Neurologic Outcome in Conservatively Treated Patients With Incomplete Closed Traumatic Cervical Spinal Cord Injuries. Spine 1996, 21, 2345–2351. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.J.; Harrington, G.M.; Hulme, C.H.; Morris, R.; Bennett, A.; Tsang, W.-H.; Osman, A.; Chowdhury, J.; Kumar, N.; Wright, K.T. A Preliminary Cohort Study Assessing Routine Blood Analyte Levels and Neurological Outcome after Spinal Cord Injury. J. Neurotrauma 2020, 37, 466–480. [Google Scholar] [CrossRef] [PubMed]
- Samdani, A.; Chafetz, R.S.; Vogel, L.C.; Betz, R.R.; Gaughan, J.P.; Mulcahey, M.J. The international standards for neurological classification of spinal cord injury: Relationship between S4-5 dermatome testing and anorectal testing. Spinal Cord 2010, 49, 352–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Middendorp, J.J.; EM-SCI Study Group; Hosman, A.J.F.; Pouw, M.H.; Van De Meent, H. Is determination between complete and incomplete traumatic spinal cord injury clinically relevant? Validation of the ASIA sacral sparing criteria in a prospective cohort of 432 patients. Spinal Cord 2009, 47, 809–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cloud, B.A.; Ball, B.G.; Chen, B.K.; Knight, A.M.; Hakim, J.S.; Ortiz, A.M.; Windebank, A.J. Hemisection spinal cord injury in rat: The value of intraoperative somatosensory evoked potential monitoring. J. Neurosci. Methods 2012, 211, 179–184. [Google Scholar] [CrossRef] [Green Version]
- Cruccu, G.; Aminoff, M.; Curio, G.; Guerit, J.; Kakigi, R.; Mauguiere, F.; Rossini, P.; Treede, R.-D.; Garcia-Larrea, L. Recommendations for the clinical use of somatosensory-evoked potentials. Clin. Neurophysiol. 2008, 119, 1705–1719. [Google Scholar] [CrossRef] [PubMed]
- Metz, G.A.; Curt, A.; Van De Meent, H.; Klusman, I.; Schwab, M.E.; Dietz, V. Validation of the Weight-Drop Contusion Model in Rats: A Comparative Study of Human Spinal Cord Injury. J. Neurotrauma 2000, 17, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Tong, B.; Jutzeler, C.R.; Cragg, J.J.; Grassner, L.; Schwab, J.M.; Casha, S.; Geisler, F.; Kramer, J.L.K. Serum Albumin Predicts Long-Term Neurological Outcomes After Acute Spinal Cord Injury. Neurorehabilit. Neural Repair 2017, 32, 7–17. [Google Scholar] [CrossRef]
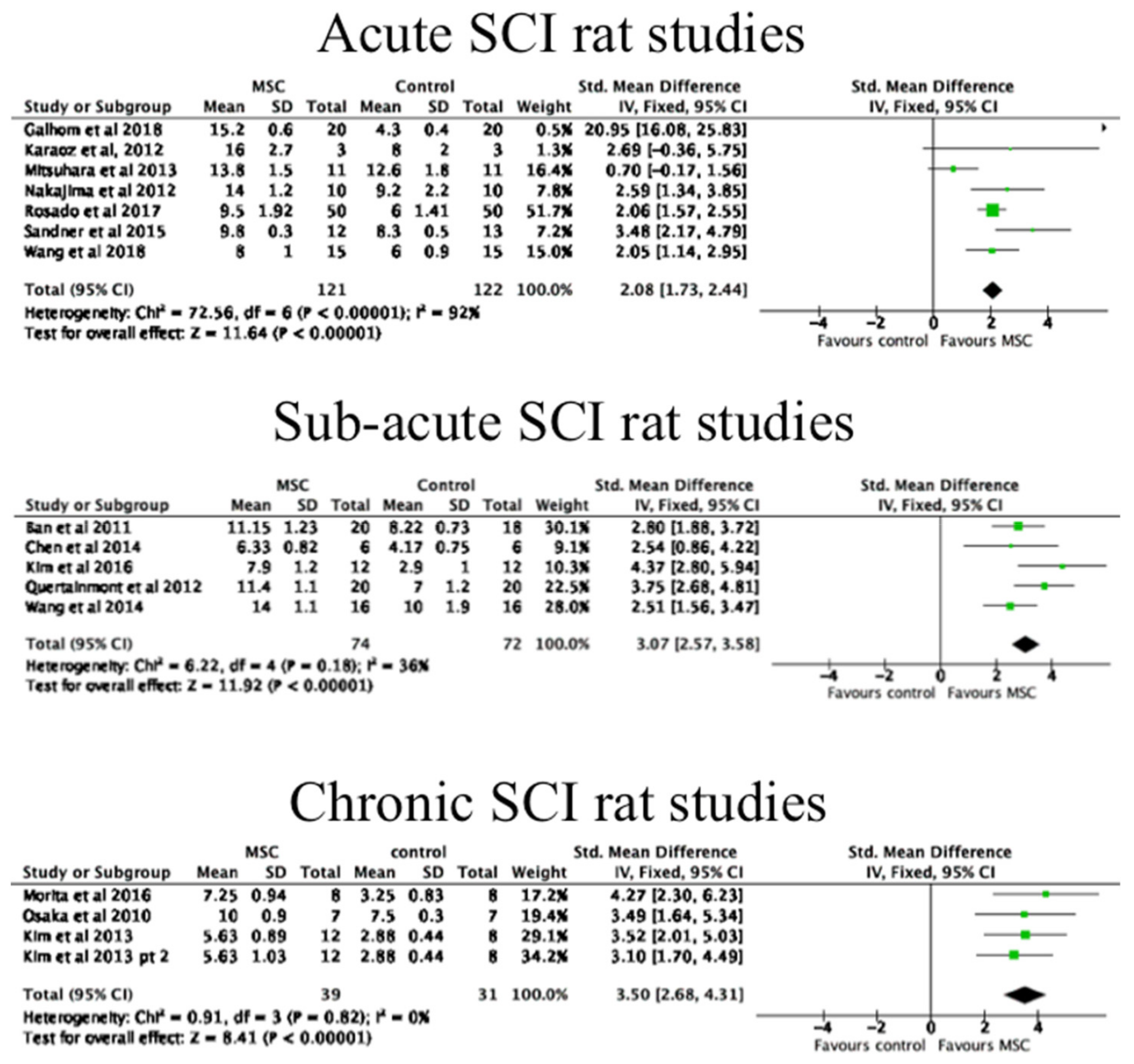
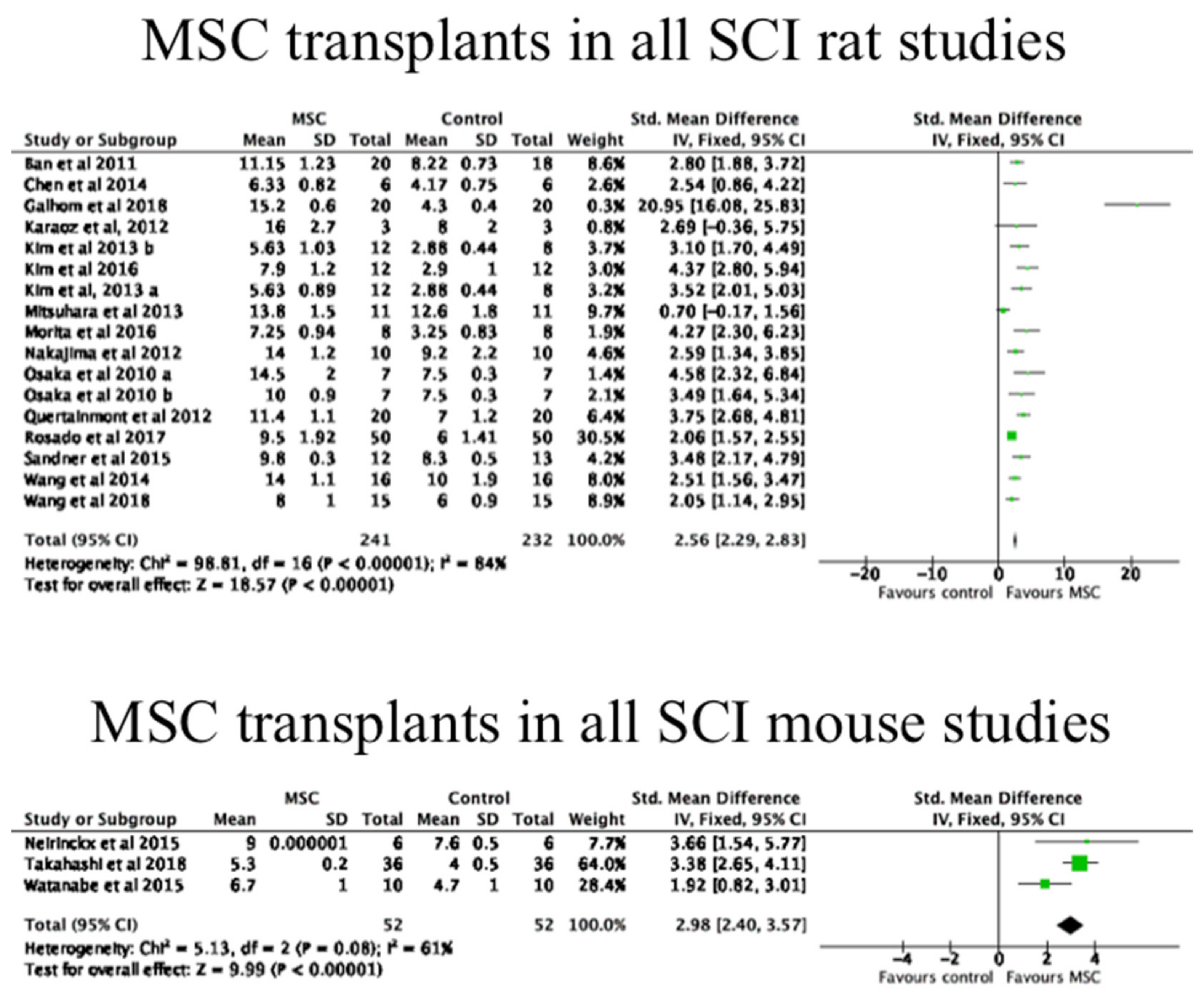
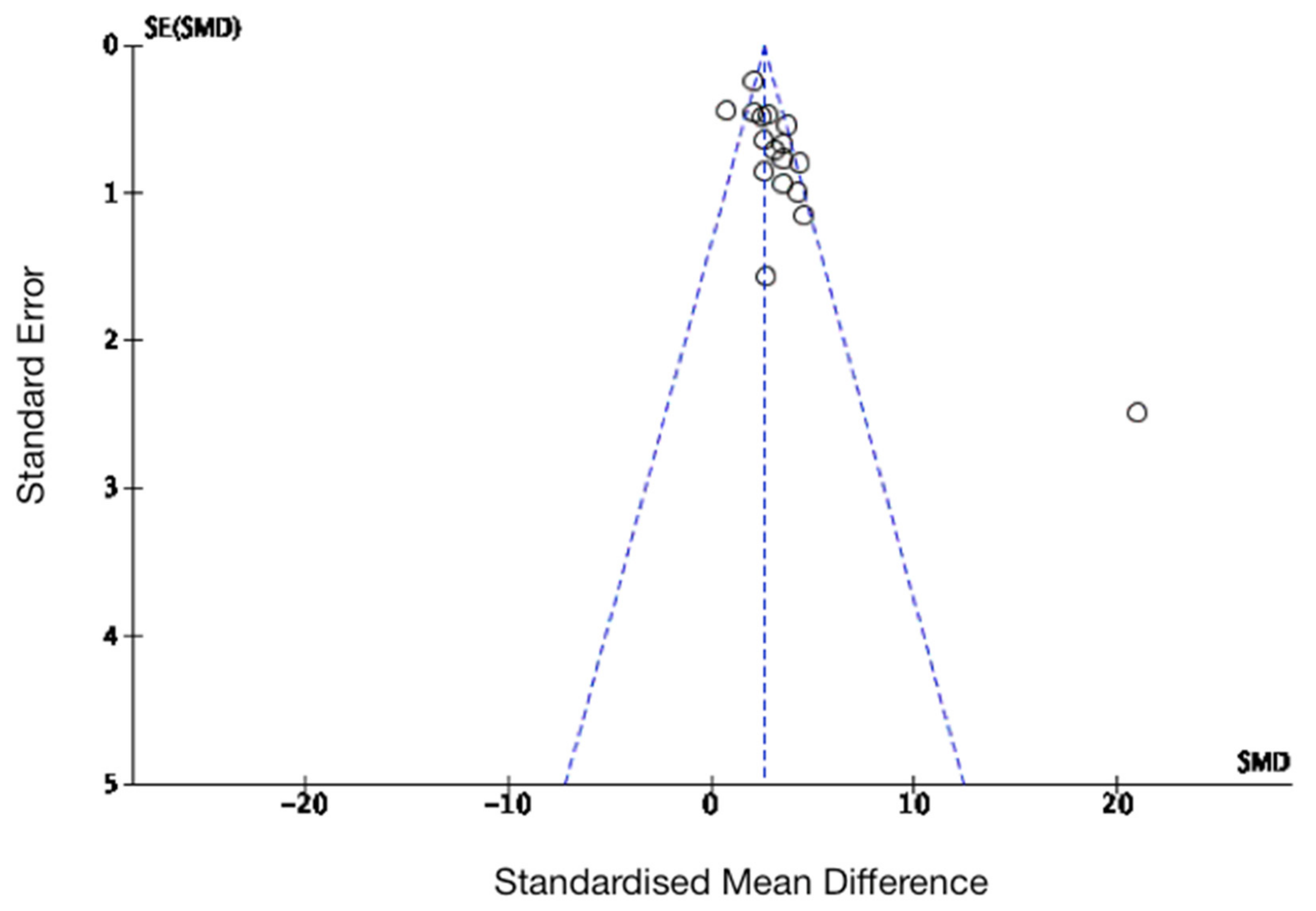
| Author | Study Design | MSC Delivery | Cell Source | MSC Dosage | CP | Participants (n) |
|---|---|---|---|---|---|---|
| Mendonca et al., 2014 [61] | UC, NonRand | IT | Au, BM | 1 × 107 | None | 12 |
| Vaquero et al., 2018 [62] | UC, NonRand | IT | Au, BM | 3 × 108 | 10% DMSO | 11 |
| Oh et al., 2016 [63] | UC, NonRand | IT | Au, BM | 4.8 × 107 | None | 16 |
| Hur et al., 2016 [15] | UC, NonRand | IT | Au, AT | 9 × 107 | None | 14 |
| Vaquero et al., 2017 [64] | UC, NonRand | IT | Au, BM | 1.2 × 108 | NS | 10 |
| Cheng et al., 2014 [65] | C, Rand | IT | Allo, UC | 4 × 107 | None | 10 treated 24 control |
| Dai et al., 2013 [66] | C, Rand | IT | Au, BM | 8 × 105 (MS) | None | 20 treated 20 control |
| Karamouzian et al., 2012 [14] | C, NonRand | LP | Au, BM | 0.7–1.2 × 106 | None | 11 |
| Ra et al., 2011 [67] | UC, NonRand | IV | Au, AT | 4 × 108 | None | 8 |
| Pal et al., 2009 [68] | UC, NonRand | LP | Au, BM | 1 × 106/kg | NS | 30 |
| Vaquero et al., 2016 [69] | UC, NonRand | IT | Au, BM | 1.3–2.6 × 108 | NS | 12 |
| El-Kheir et al., 2014 [70] | C, Rand | LP | Au, BM | 2 × 106/kg | None | 50 treated 20 control |
| Phedy et al., 2019 [71] | Case study | IT + IV | Au, BM | 69.5 × 106 | NS | 1 |
| Bydon et al., 2020 [72] | Case study | LP | Au, AT | 1 × 108 | NS | 1 |
| Larocca et al., 2017 [73] | Pilot, UC, NonRand | IT | Au, BM | 2 × 107 | None | 5 |
| Chotivichit et al., 2015 [74] | Pilot, UC, NonRand | IT | Au, BM | 3 × 107 | None | 1 |
| Hua et al., 2016 [75] | Pilot, UC, NonRand | IT | Au, UC | 4 × 107 | None | 1 |
| Jarocha et al., 2015 [76] | Case study | IT + IV | Au, BM | 1.54 × 108 | None | 1 |
| Park et al., 2012 [77] | UC, NonRand | LP | Au, BM | 1.48 × 108 | 10% DMSO | 10 |
| Author | Species | SCI Phase | MSC Delivery | Cell Source | MSC Dosage | CP | Participants (n/Group) |
|---|---|---|---|---|---|---|---|
| Ban et al., 2011 [78] | Rat | Sub-acute | IT | Allo, BM | 1.2 × 103/kg | None | 20 |
| Chen et al., 2014 [79] | Rat | Sub-acute | IV | Allo, BM | 1 × 106 | None | 6 |
| Galhom et al., 2018 [80] | Rat | Acute | IT | Allo, BM | 1.5 × 106 | None | 20 |
| Hosseini et al., 2018 [81] | Rat | Acute | IV | Allo, BM | 2 × 106/kg | None | 15 |
| Karaoz et al., 2012 [82] | Rat | Acute | IT | Allo, BM | 3 × 105 | None | 3 |
| Kim et al., 2013 [83] | Rat | Chronic | IT | Allo, BM | 1 × 106 | None | 12 |
| Rat | Chronic | IV | Allo, BM | 1 × 106 | None | 12 | |
| Kim et al., 2016 [84] | Rat | Sub-acute | IT | Allo, BM | 1 × 106 | None | 12 |
| Mitsuhara et al., 2013 [85] | Rat | Chronic | IV | Allo, BM | 3 × 105 | None | 11 |
| Morita et al., 2016 [59] | Rat | Acute | IV | Allo, BM | 1 × 106 | None | 8 |
| Nakajima et al., 2012 [23] | Rat | Acute | IT | Allo, BM | 1 × 106 | None | 10 |
| Osaka et al., 2010 [58] | Rat | Acute | IV | Allo, BM | 1 × 106 | None | 7 |
| Rat | Chronic | IV | Allo, BM | 1 × 106 | None | 7 | |
| Quertainmont et al., 2012 [86] | Rat | Sub-acute | IV | Allo, BM | 1 × 106 | None | 20 |
| Rosado et al., 2017 [87] | Rat | Acute | IV | Allo, AT | 1 × 106 | 10% DMSO | 50 |
| Sandner et al., 2015 [88] | Rat | Acute | IT | Allo, BM | 5 × 105 | None | 12 |
| Torres-Espin et al., 2014 [89] | Rat | Sub-acute | IT | Allo, BM | 4.5 × 105 | None | 7 |
| Wang et al., 2014 [90] | Rat | Sub-acute | IT | Allo, BM | 1 × 106 | None | 16 |
| Wang et al., 2018 [36] | Rat | Acute | IT | Allo, BM | 1 × 106 | None | 15 |
| Lin et al., 2013 [38] | Rabbit | Acute | IT | Allo, BM | 5 × 106 | None | 24 |
| de Almeida et al., 2015 [91] | Mouse | Chronic | IT | Allo, BM | 8 × 105 | None | 8 |
| Neirinckx et al., 2015 [92] | Mouse | Acute | IT | Allo, BM | 3 × 104 | None | 6 |
| Takahashi et al., 2018 [37] | Mouse | Acute | IT | Allo, AT | 1 × 105 | None | 10 |
| Watanabe et al., 2015 [28] | Mouse | Sub-acute | IT | Allo, BM | 2 × 105 | None | 36 |
| Khan et al., 2019 [49] | Dog | Acute | IV | Allo, AT | 1 × 107 | 10% DMSO | 4 |
| Lee et al., 2015 [93] | Dog | Sub-acute | IT | Allo, AT | 1 × 107 | None | 4 |
| Lee et al., 2017 [94] | Dog | Intermediate | IT | Allo, AT | 1 × 107 | None | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, L.D.V.; Pickard, M.R.; Johnson, W.E.B. The Comparative Effects of Mesenchymal Stem Cell Transplantation Therapy for Spinal Cord Injury in Humans and Animal Models: A Systematic Review and Meta-Analysis. Biology 2021, 10, 230. https://doi.org/10.3390/biology10030230
Johnson LDV, Pickard MR, Johnson WEB. The Comparative Effects of Mesenchymal Stem Cell Transplantation Therapy for Spinal Cord Injury in Humans and Animal Models: A Systematic Review and Meta-Analysis. Biology. 2021; 10(3):230. https://doi.org/10.3390/biology10030230
Chicago/Turabian StyleJohnson, Louis D. V., Mark R. Pickard, and William E. B. Johnson. 2021. "The Comparative Effects of Mesenchymal Stem Cell Transplantation Therapy for Spinal Cord Injury in Humans and Animal Models: A Systematic Review and Meta-Analysis" Biology 10, no. 3: 230. https://doi.org/10.3390/biology10030230





