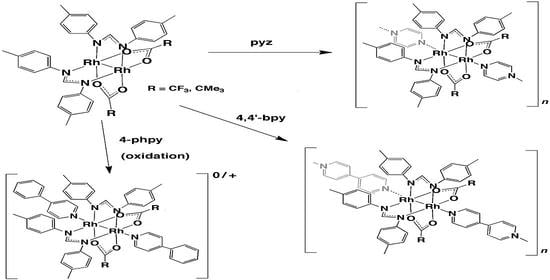
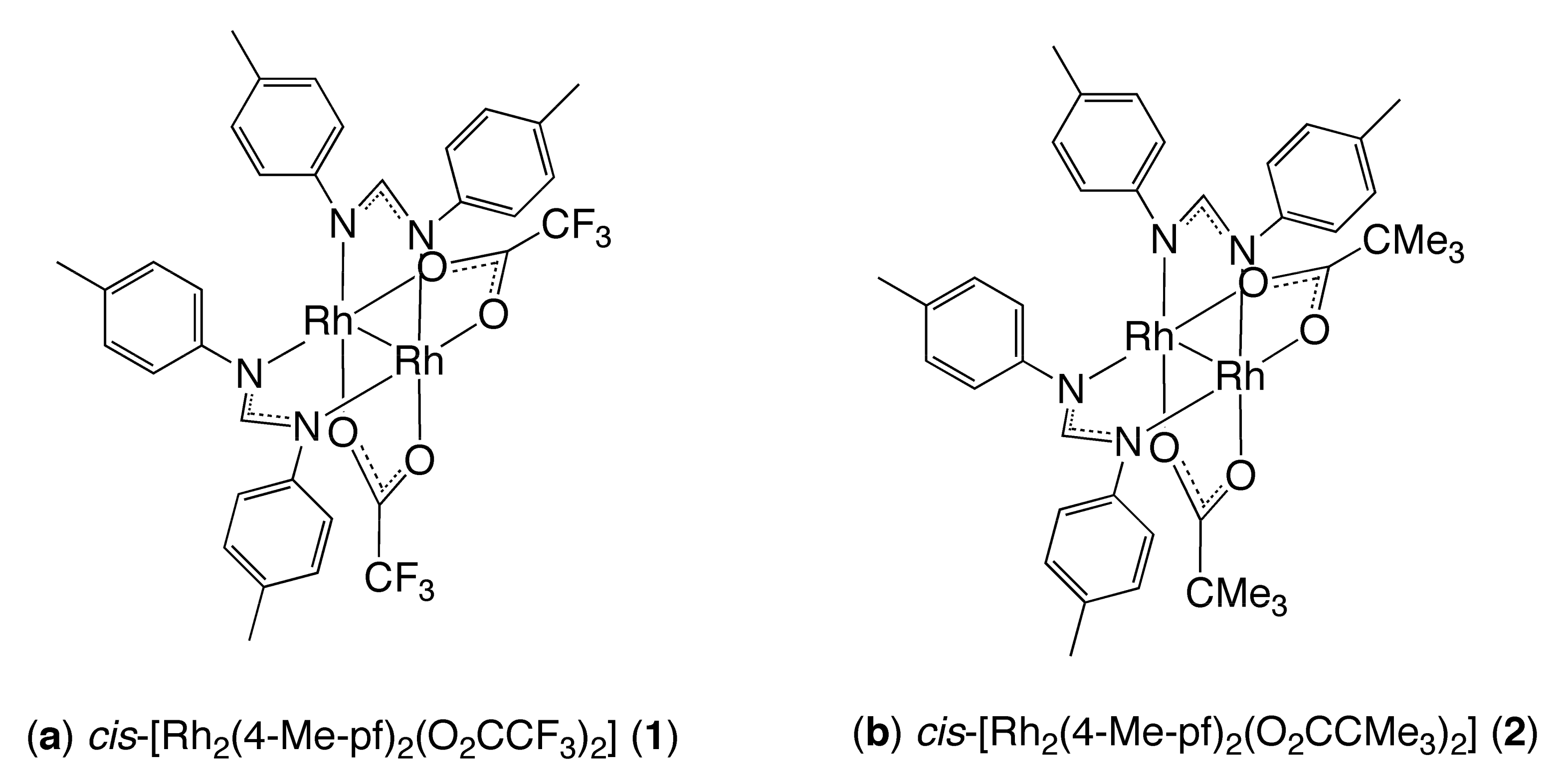
Structures and Properties of 4-phpy, pyz, and 4,4′-bpy Adducts of Lantern-Type Dirhodium Complexes with µ-Formamidinato and µ-Carboxylato Bridges
Abstract
1. Introduction
2. Results and Discussion
2.1. Bis-Adduct Rh2II,II Complexes of 4-phpy
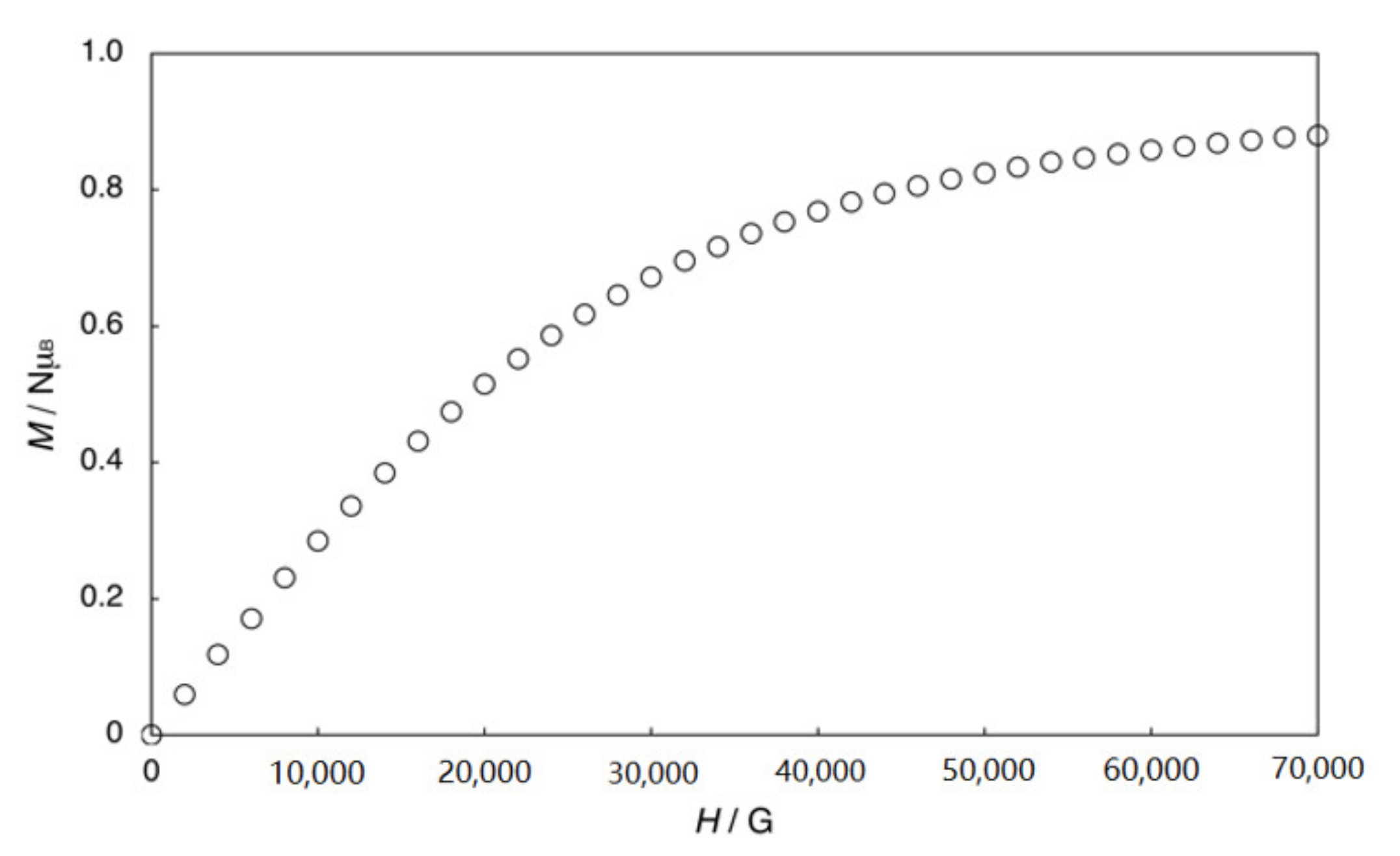
2.2. Bis-Adduct Rh2II,III Complexes of 4-phpy
2.3. Rh2II,II Polymer Complexes of pyz and 4,4′-bpy
3. Materials and Methods
3.1. General Aspects
3.2. Synthesis of Complexes
3.2.1. Synthesis of cis-[Rh2(4-Me-pf)2(O2CCF3)2(4-phpy)2] (3)
3.2.2. Synthesis of cis-[Rh2(4-Me-pf)2(O2CCMe3)2(4-phpy)2] (4)
3.2.3. Synthesis of cis-[Rh2(4-Me-pf)2(O2CCMe3)2(4-phpy)2]BF4 (5)
3.2.4. Synthesis of cis-[Rh2(4-Me-pf)2(O2CCF3)2(pyz)]n (6)
3.2.5. Synthesis of cis-[Rh2(4-Me-pf)2(O2CCMe3)2(pyz)]n (7)
3.2.6. Synthesis of cis-[Rh2(4-Me-pf)2(O2CCF3)2(4,4′-bpy)]n (8)
3.2.7. Synthesis of cis-[Rh2(4-Me-pf)2(O2CCMe3)2(4,4′-bpy)]n (9)
3.3. Crystal Structure Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cotton, F.A.; Murillo, C.A.; Walton, R.A. Multiple Bonds between Metal Atoms, 3rd ed.; Springer: New York, NY, USA, 2005. [Google Scholar]
- Liddle, S.T. Molecular Metal-Metal Bonds, Compounds, Synthesis, Properties; Wiley-VCH: Weinheim, Germany, 2015. [Google Scholar]
- Handa, M.; Kasamatsu, K.; Kasuga, K.; Mikuriya, M.; Fujii, T. Linear Chain Compounds of Molybdenum(II) Acetate Dimer Linked by Linear-Bidentate Ligands, Pyrazine, 4,4′-Bipyridine and 1,4-Diazabicyclo[2.2.2]octane. Chem. Lett. 1990, 19, 1753–1756. [Google Scholar] [CrossRef]
- Handa, M.; Yamada, K.; Nakao, T.; Kasuga, K.; Mikuriya, M.; Kotera, T. Structure and properties of a compound formed by the reaction of molybdenum(II) trifluoroacetate with 4,4′-bipyridine. Chem. Lett. 1993, 22, 1969–1972. [Google Scholar] [CrossRef]
- Handa, M.; Mikuriya, M.; Nukada, R.; Matsumoto, H.; Kasuga, K. Chain compound of molybdenum(II) pivalate bridged by 4,4′-bipyridine. Bull. Chem. Soc. Jpn. 1994, 67, 3125–3127. [Google Scholar] [CrossRef]
- Handa, M.; Mikuriya, M.; Kotera, T.; Yamada, K.; Nakao, T.; Matsumoto, H.; Kasuga, K. Linear Chain Compounds of Molybdenum(II) Acetate Linked by Pyrazine, 4,4′-Bipyridine, and 1,4-Diazabicyclo[2.2.2]octane. Bull. Chem. Soc. Jpn. 1995, 68, 2567–2572. [Google Scholar] [CrossRef]
- Mikuriya, M.; Nukada, R.; Morishita, H.; Handa, M. Chain compounds formed by the reaction of copper(II) carboxylate [Cu2(O2CR)4] (R = C(CH3)3, CCl3) and bridging ligand L (L = pyrazine, 4,4′-bipyridine, and 1,4-diazabicyclo[2.2.2]octane). Chem. Lett. 1995, 24, 617–618. [Google Scholar] [CrossRef]
- Handa, M.; Watanabe, M.; Yoshioka, D.; Kawabata, S.; Nukada, R.; Mikuriya, M.; Azuma, H.; Kasuga, K. Adduct polymers and dimers of rhodium(II) pivalate with pyrazine, 4,4′-bipyridine, 1,4-diazabicyclo[2.2.2]octane, triethylamine, and pyridine. Bull. Chem. Soc. Jpn. 1999, 72, 2681–2686. [Google Scholar] [CrossRef]
- Nukada, R.; Mori, W.; Takamizawa, S.; Mikuriya, M.; Handa, M.; Naono, H. Microporous structure of a chain compound of copper(II) benzoate bridged by pyrazine. Chem. Lett. 1999, 28, 367–368. [Google Scholar] [CrossRef]
- Nukada, R.; Mikuriya, M.; Handa, M.; Naono, H. Hydrophobic micropore in a chain compound of dinuclear copper(II) benzoate with pyrazine—Adsorption properties for N2. CCl4, H2O, CO2, and CH3CN. In Proceedings of the 2nd International Porous and Powder Materials Symposium and Exhibition PPM 2015, Izmir, Turkey, 15–18 September 2015; Ozdemir, S.K., Polat, M., Tanoglu, M., Eds.; The Organizing Committee of The International Porous and Power Materials Symposium and Exhibition: Izmir, Turkey, 2015; pp. 77–81. [Google Scholar]
- Handa, M.; Yoshioka, D.; Mikuriya, M.; Hiromitsu, I.; Kasuga, K. Coordination polymers of ruthenium(II) acetate with pyrazine, 4,4′-bipyridine, and 1,4-diazabicyclo[2.2.2]octane. Mol. Cryst. Liq. Cryst. 2002, 376, 257–262. [Google Scholar] [CrossRef]
- Mikuriya, M.; Higashiguchi, T.; Sakai, T.; Yoshioka, D.; Handa, M. Chain compounds of rhodium(II) benzoate bridged by N,N’-didentate ligands. In Progress in Coordination and Bioinorganic Chemistry; Melnik, N., Sirota, A., Eds.; Slovak University of Technology Press: Bratislava, Slovakia, 2003; pp. 213–218. [Google Scholar]
- Yoshioka, D.; Mikuriya, M.; Handa, M. Synthsis and characterization of polynuclear chain and tetranuclear adducts of mixed-valent ruthenium(II,III) pivalate with N,N’-didentate ligands. Bull. Chem. Soc. Jpn. 2004, 77, 2205–2211. [Google Scholar] [CrossRef]
- Mikuriya, M.; Yamamoto, J.; Yoshioka, D.; Tanaka, H.; Handa, M. Synthesis, crystal structures, and adsorption properties of chain complexes of rhodium(II) pivalate with N,N’-bidentate ligands. In New Trends in Coordination, Bioinorganic and Applied Inorganic Chemistry; Melnik, M., Segla, P., Tatarko, M., Eds.; Slovak University of Technology Press: Bratislava, Slovakia, 2011; pp. 311–319. [Google Scholar]
- Mikuriya, M.; Yamamoto, J.; Ouchi, K.; Yoshioka, D.; Tanaka, H.; Handa, M. Synthesis, Crystal strcuture, and N2-adsorption property of a chain complex of rhodium(II) acetate and 1,4-diazabicyclo[2.2.2]octane. X-ray Struct. Anal. Online 2013, 29, 7–8. [Google Scholar] [CrossRef]
- Mikuriya, M.; Ouchi, K.; Nakanishi, Y.; Yoshioka, D.; Tanaka, H.; Handa, M. Synthesis, crystal structure, and N2-adsorption property of a chain complex of rhodium(II) benzoate and piperazine. X-ray Struct. Anal. Online 2013, 29, 21–22. [Google Scholar] [CrossRef][Green Version]
- Mikuriya, M.; Yamamoto, J.; Ouchi, K.; Takada, S.; Yoshioka, D.; Tanaka, H.; Handa, M. Synthesis and crystal structure of a chain complex of rhodium(II) acetate and 1,2-bis(4-pyridyl)ethane having an N2-adsorption property. X-ray Struct. Anal. Online 2013, 29, 29–30. [Google Scholar] [CrossRef][Green Version]
- Mikuriya, M.; Kaihara, N.; Yamamoto, J.; Takada, S.; Yoshioka, D.; Tanaka, H.; Handa, M. Synthesis and crystal structure of a chain complex of molybdenum(II) acetate and 1,2-bis(4-pyridyl)ethane aiming at N2-adsorption property. X-ray Struct. Anal. Online 2013, 29, 31–32. [Google Scholar] [CrossRef][Green Version]
- Mikuriya, M.; Takada, S.; Yoshioka, D.; Tanaka, H.; Handa, M. Synthesis and crystal structure of a chain complex of molybdenum(II) acetate and trans-1,2-bis(4-pyridyl)ethylene in relation to the N2-adsorption property. X-ray Struct. Anal. Online 2013, 29, 33–34. [Google Scholar] [CrossRef][Green Version]
- Mikuriya, M.; Yamamoto, J.; Ouchi, K.; Yoshioka, D.; Tanaka, H.; Handa, M. Synthesis and crystal structure of a chain complex of rhodium(II) benzoate and 1,4-diazabicyclo[2.2.2]octane having an N2-adsorption property. X-ray Struct. Anal. Online 2013, 29, 45–46. [Google Scholar] [CrossRef][Green Version]
- Mikuriya, M.; Tsukimi, N.; Ono, T.; Tanaka, Y.; Yoshioka, D.; Tanaka, H.; Handa, M. Synthesis and crystal structure of a chain complex of molybdenum(II) benzoate and 4,4′-bipyridyl in relation to the N2-adsorption property. X-ray Struct. Anal. Online 2014, 30, 25–26. [Google Scholar] [CrossRef][Green Version]
- Mikuriya, M.; Kaihara, N.; Ono, T.; Tanaka, Y.; Yoshioka, D.; Tanaka, H.; Handa, M. Synthesis and crystal structure of a chain complex of molybdenum(II) benzoate and 1,2-bis(4-pyridyl)ethane with an N2-adsorption property. X-ray Struct. Anal. Online 2014, 30, 39–40. [Google Scholar] [CrossRef][Green Version]
- Mikuriya, M.; Kaihara, N.; Ono, T.; Tanaka, Y.; Yoshioka, D.; Tanaka, H.; Handa, M. Synthesis and crystal structure of a chain complex of molybdenum(II) benzoate with 1,2-bis(4-pyridyl)ethylene having an N2-adsorption property. X-ray Struct. Anal. Online 2014, 30, 41–42. [Google Scholar] [CrossRef][Green Version]
- Mikuriya, M.; Yano, M.; Takahashi, N.; Yoshioka, D.; Tanaka, H.; Handa, M. Synthesis and crystal structure of a chain complex of copper(II) pivalate and 1,2-bis-(4-pyridyl)ethane in relation to adsorption property for N2. X-ray Struct. Anal. Online 2015, 31, 47–48. [Google Scholar] [CrossRef]
- Takamizawa, S.; Nakata, E.; Yokoyama, H.; Mochizuki, K.; Mori, W. Carbon dioxide inclusion phases of a transformable 1D coordination polymer host [Rh2(O2CPh)4(pyz)]n. Angew. Chem. Int. Ed. 2003, 42, 4331–4334. [Google Scholar] [CrossRef]
- Piraino, P.; Bruno, G.; Tresoldi, G.; Schiavo, S.L.; Zanello, P. Chemical oxidation of binuclear rhodium(I) complexes with silver salts. Synthesis, x-ray crystal structure, and electrochemical properties of the rhodium [Rh24+] mixed-ligand complex Rh2(Form)2(O2CCF3)2(H2O)20.5C6H6 (Form = N,N’-di-p-tolylformamidinate anion). Inorg. Chem. 1987, 26, 91–96. [Google Scholar] [CrossRef]
- Piraino, P.; Bruno, G.; Schiavo, S.L.; Laschi, F.; Zanello, P. Synthesis, X-ray crystal structure, and electrochemical properties of the Rh4+ complex Rh2(form)4 (form = N,N’-di-p-tolylformamidinate anion). Inorg. Chem. 1987, 26, 2205–2211. [Google Scholar] [CrossRef]
- Handa, M.; Yasuda, M.; Muraki, Y.; Yoshioka, D.; Mikuriya, M.; Kasuga, K. Polymer and “trimer-of-dimers” complexes derived from [Rh2(form)4] (form- = N,N’-Di-p-tolylformamidinate anion) and 1,4-diisocyanobenzene. Chem. Lett. 2003, 32, 946–947. [Google Scholar] [CrossRef]
- Handa, M.; Sanaka, A.; Sugikawa, Y.; Ikeue, T.; Tanaka, H.; Yoshioka, D.; Mikuriya, M. Polymer Complex of Rhodium(II) Formamidinate Dimer Linked by 1,4-Diisocyanobenzene with Toluene as Solvent of Crystallization. X-ray Struct. Anal. Online 2011, 27, 73–74. [Google Scholar] [CrossRef][Green Version]
- Bear, J.L.; Han, B.; Wu, Z.; Van Caemelbecke, E.; Kadish, K.M. Synthesis, Electrochemistry, and Spectroscopic Characterization of Bis-dirhodium Complexes Linked by Axial Ligands. Inorg. Chem. 2001, 40, 2275–2281. [Google Scholar] [CrossRef]
- Handa, M.; Ikeue, T.; Sanaka, A.; Nakai, T.; Tanaka, T.; Yoshioka, D.; Mikuriya, M. Assembled complexes of lantern-type rhodium(II) dimers with formamidinato ligands. In New Trends in Coordination, Bioinorganic and Applied Inorganic Chemistry; Melnik, M., Segla, P., Tatarko, M., Eds.; Slovak University of Technology Press: Bratislava, Slovakia, 2011; pp. 133–139. [Google Scholar]
- Handa, M.; Nishiura, S.; Masuda, T.; Yano, N.; Mikuriya, M.; Kataoka, Y. Synthesis, structure, and properties of a lantern-type dinuclear rhodium(II) complex cis-[Rh2(4-Me-pf)2(O2CCMe3)2], 4-Me-pf- = N,N’-di-p-tolylformamidinate anion. Chem. Pap. 2018, 72, 841–851. [Google Scholar] [CrossRef]
- Catalan, K.V.; Hess, J.S.; Maloney, M.M.; Mindiola, D.J.; Ward, D.L.; Dunbar, K.R. Reactions of DNA purines with dirhodium formamidinate compounds that display antitumor behavior. Inorg. Chem. 1999, 38, 3904–3913. [Google Scholar] [CrossRef]
- Drago, R.S.; Long, J.R.; Cosmano, R. Metal synergism in the coordination chemistry of a metal-metal bonded system: Rh2(C3H7COO)4. Inorg. Chem. 1981, 20, 2920–2927. [Google Scholar] [CrossRef]
- Felthouse, T.R. The chemistry, structure, and metal metal-bonding in compounds of rhodium(II). Prog. Inorg. Chem. 1982, 29, 73–166. [Google Scholar] [CrossRef]
- Kruger, H.J.; Holm, R.H. Stabilization of trivalent nickel in tetragonal NiS4N2 and NiN6 environments: Synthesis, structures, redox potentials and observations related to [NiFe]-hydrogenases. J. Am. Chem. Soc. 1990, 112, 2955–2963. [Google Scholar] [CrossRef]
- Ziółkowski, J.J.; Moszner, M.; Glowiak, T. Multiple rhodium(II)–rhodium(III) bond in the dimer [Rh2(O2CMe)4(H2O)2][ClO4]·H2O; X-ray crystal structure. J. Chem. Soc. Chem. Commun. 1977, 760–761. [Google Scholar] [CrossRef]
- Cotton, F.A.; DeBoer, B.G.; LaPrade, M.D.; Pipal, J.R.; Ucko, D.A. The crystal and molecular structures of dichromium tetraacetate dihydrate and dirhodium tetraacetate dihydrate. Acta Crystallogr. Sect. B 1971, 27, 1664–1671. [Google Scholar] [CrossRef]
- Cotton, F.A.; DeBoer, B.G.; LaPrade, M.D.; Pipal, J.R.; Ucko, D.A. Multiple chromium(II)-chromium(II) and rhodium(II)-rhodium(II) bonds. J. Am. Chem. Soc. 1970, 92, 2926–2927. [Google Scholar] [CrossRef]
- Baranovskii, I.B.; Golubnichaya, M.A.; Dikareva, L.M.; Rotov, A.V.; Shchelokov, R.N.; Poraikoshits, M.A. Binuclear acetamide rhodium(II,III) complexes. Russ. J. Inorg. Chem. 1986, 31, 1652–1656. [Google Scholar]
- Ebihara, M.; Fuma, Y. Variation of hydrogen-bonded networks in hexafluorophosphate salts of amidate-bridged dirhodium(II,III) complexes with axial aqua ligands. Acta Crystallogr. Sect. C 2006, 62, m284–m289. [Google Scholar] [CrossRef] [PubMed]
- Fuma, Y.; Ebihara, M. Tetra-μ-acetamidato-κ4N: O;κ4O: N-diaqua-dirhodium(II,III) perrhenate. Acta Crystallogr. Sect. E 2006, 62, m1898–m1900. [Google Scholar] [CrossRef]
- Ahsan, M.Q.; Bernal, I.; John, L. Bear Reaction of tetrakis(acetato)dirhodium with acetamide: Crystal and molecular structure of tetrakis(acetamido)diaquadirhodium trihydrate. Inorg. Chem. 1986, 25, 260–265. [Google Scholar] [CrossRef]
- Bear, J.L.; Yao, C.L.; Lifsey, R.S.; Korp, J.D.; Kadish, K.M. Electrochemical, spectroscopic, and structural characterization of rhodium complexes Rh2(dpf)4, Rh2(dpf)4(CH3CN), and [Rh2(dpf)4(CH3CN)]ClO4, where dpf = N,N’-diphenylformamidinate(1-). Inorg. Chem. 1991, 30, 336–340. [Google Scholar] [CrossRef]
- Kawamura, T.; Maeda, M.; Miyamoto, M.; Usami, H.; Imaeda, K.; Ebihara, M. Geometrical Difference and Electron Configuration of Lantern-Type Rh24+ and Rh25+ Complexes: X-ray Structural and DFT Study. J. Am. Chem. Soc. 1998, 120, 8136–8142. [Google Scholar] [CrossRef]
- Handa, M.; Muraki, Y.; Kawabata, S.; Sugimori, T.; Hiromitsu, I.; Mikuriya, M.; Kasuga, K. Polymer Complexes of Rhodium Acetamidate Dimers Bridged by Pyrazine, 4,4′-Bipyridine, and 1,4-Diazabicyclo[2.2.2]octane. Mol. Cryst. Liq. Cryst. 2002, 379, 327–332. [Google Scholar] [CrossRef]
- Roberts, R.M. The reaction of diarylformamidines with ethyl malonate. J. Org. Chem. 1949, 14, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 229–234. [Google Scholar] [CrossRef]
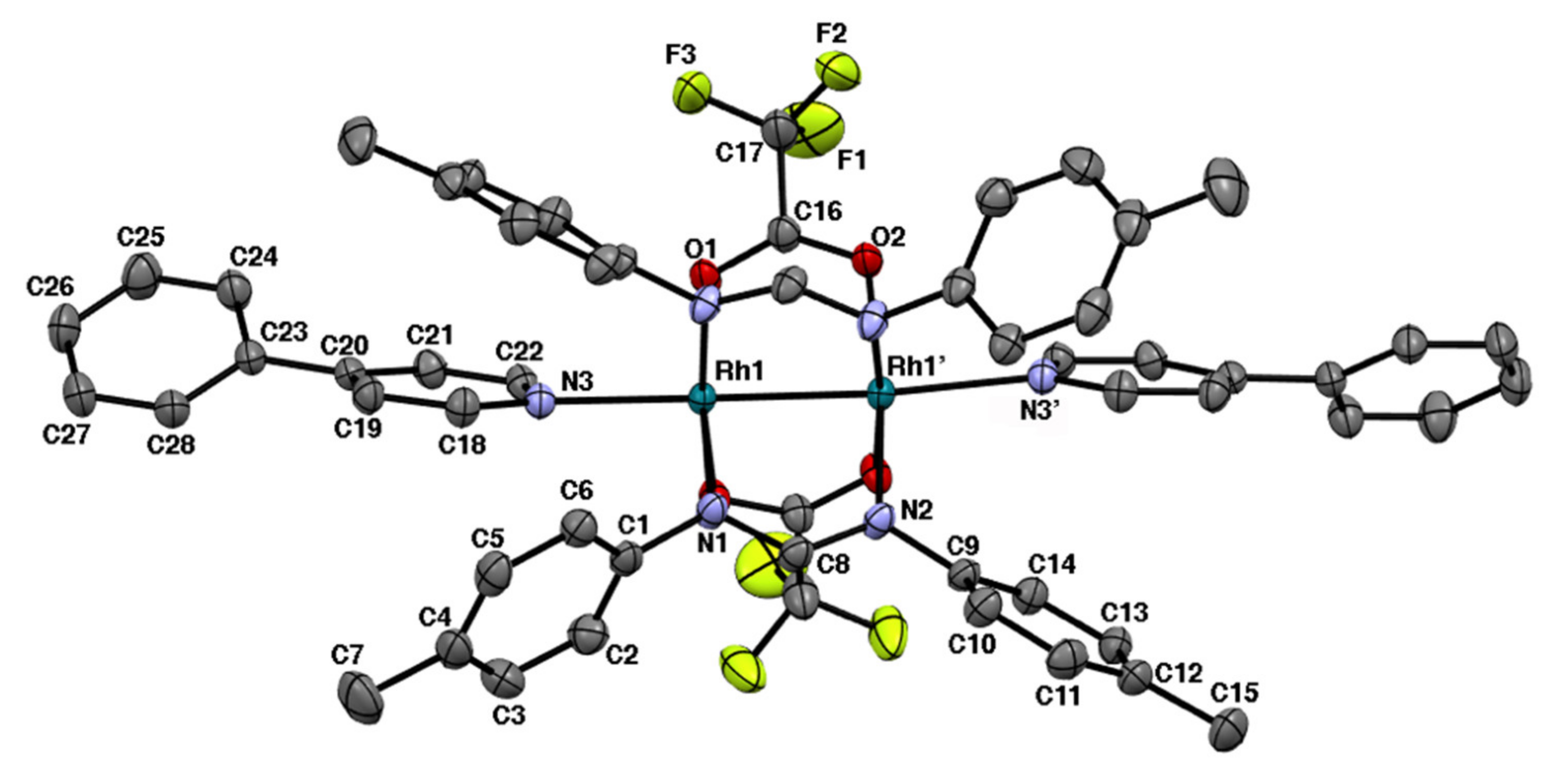
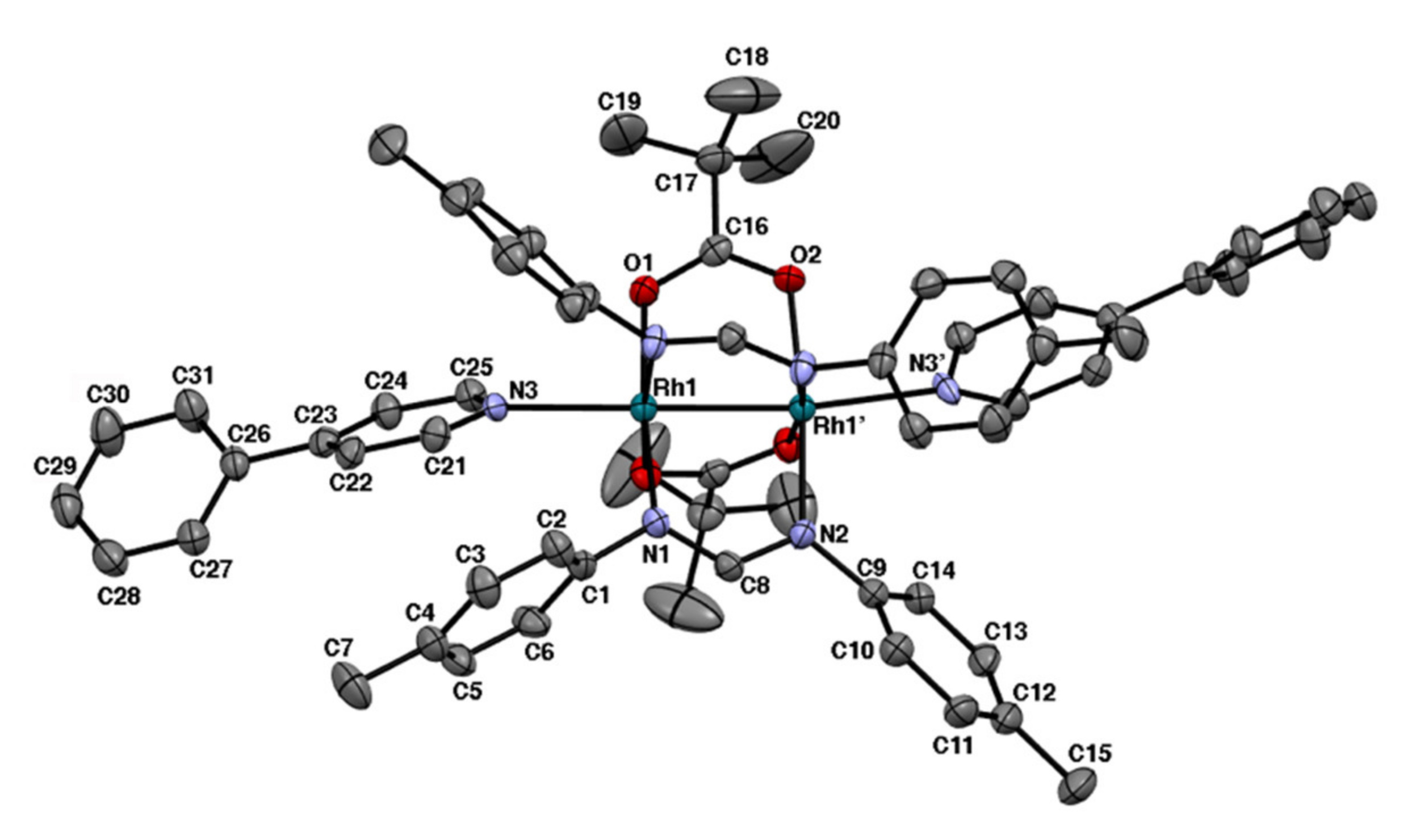

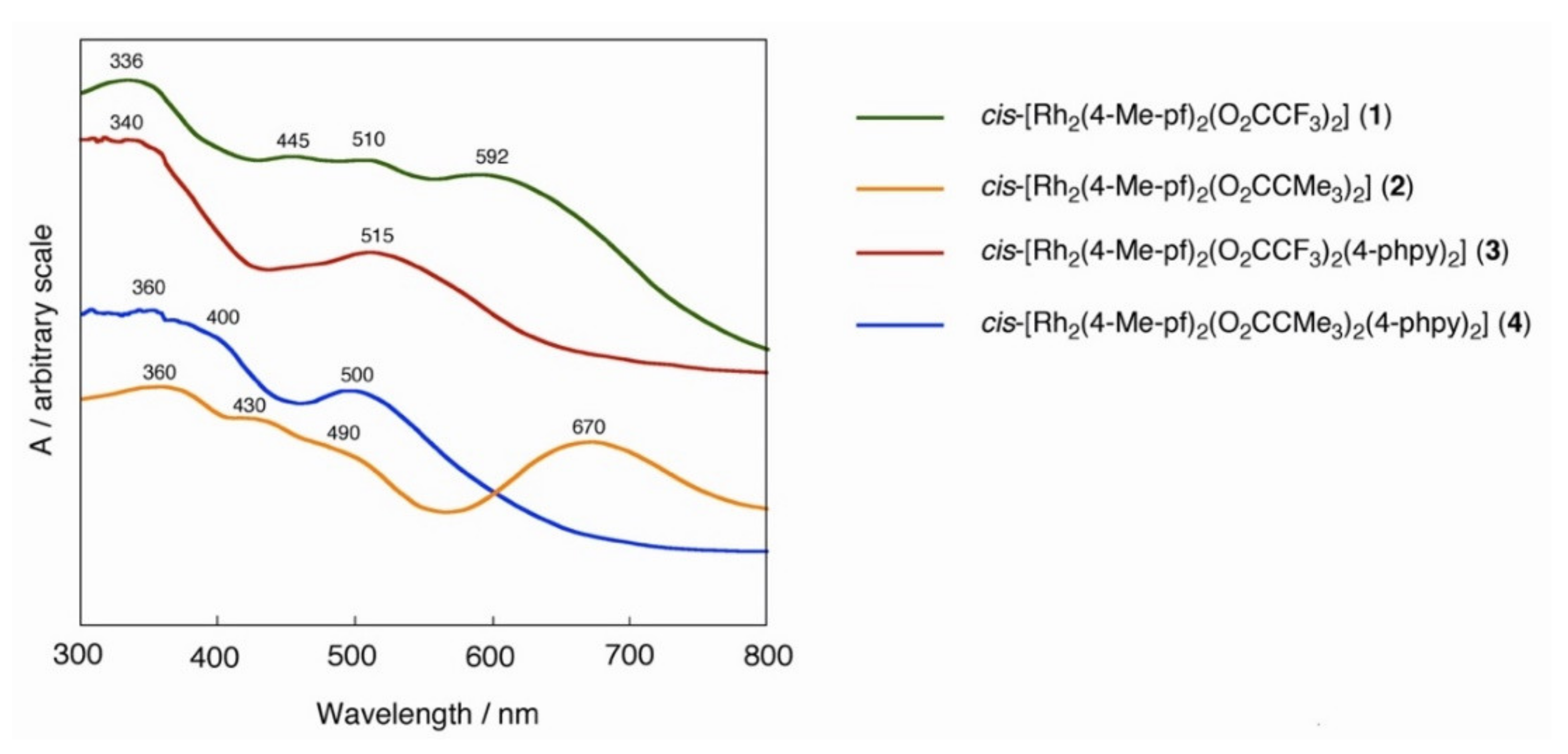
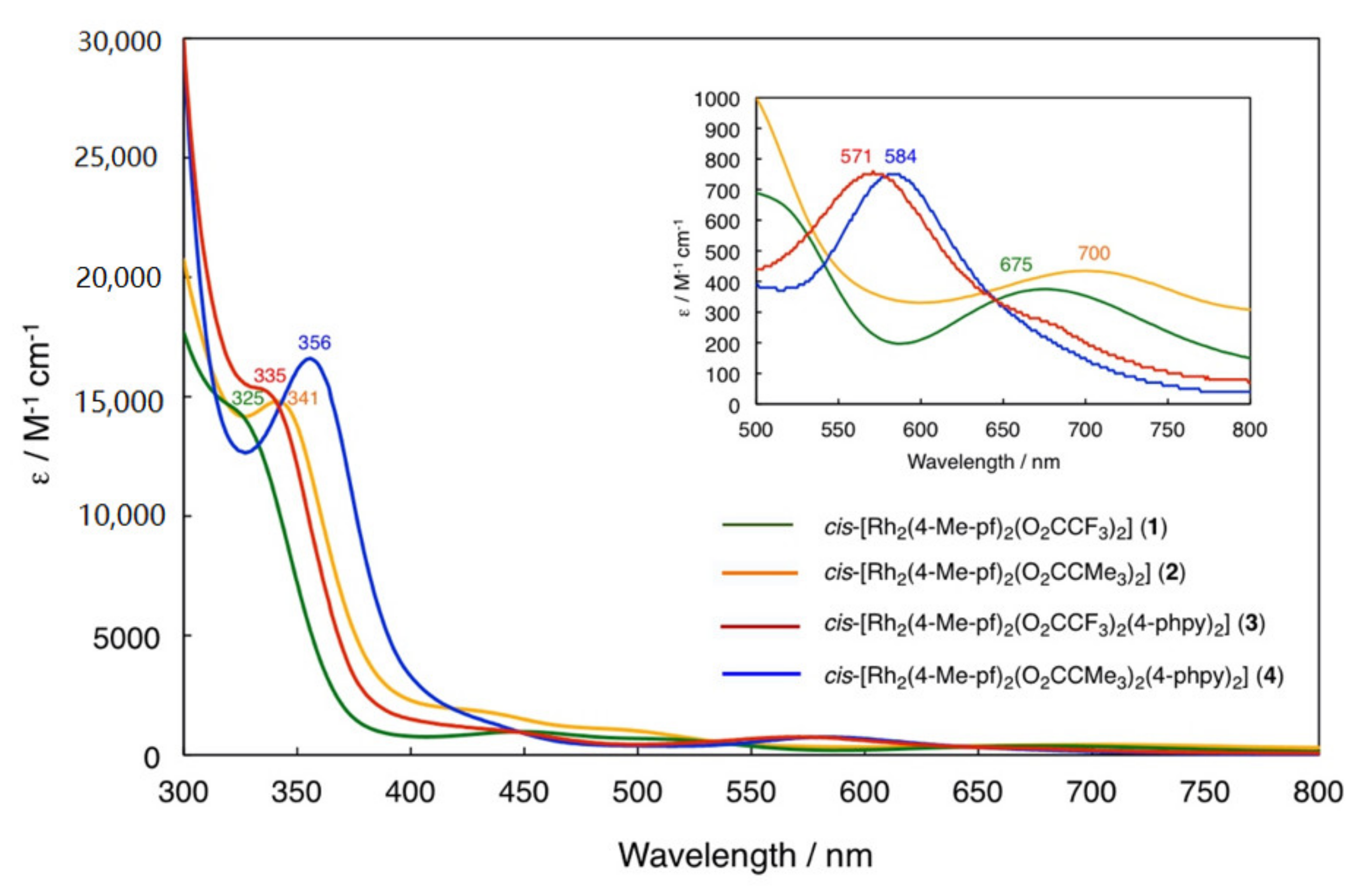

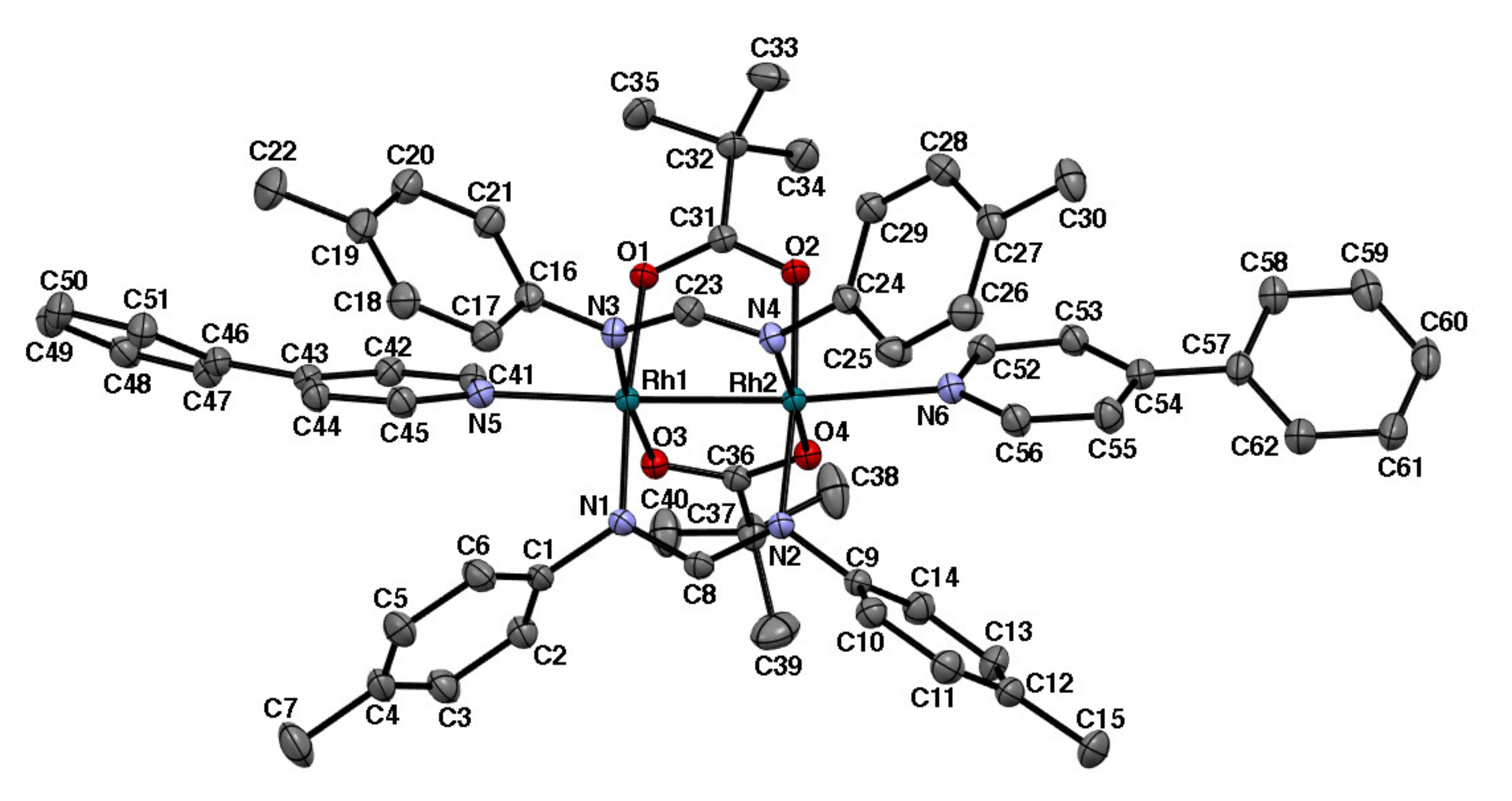
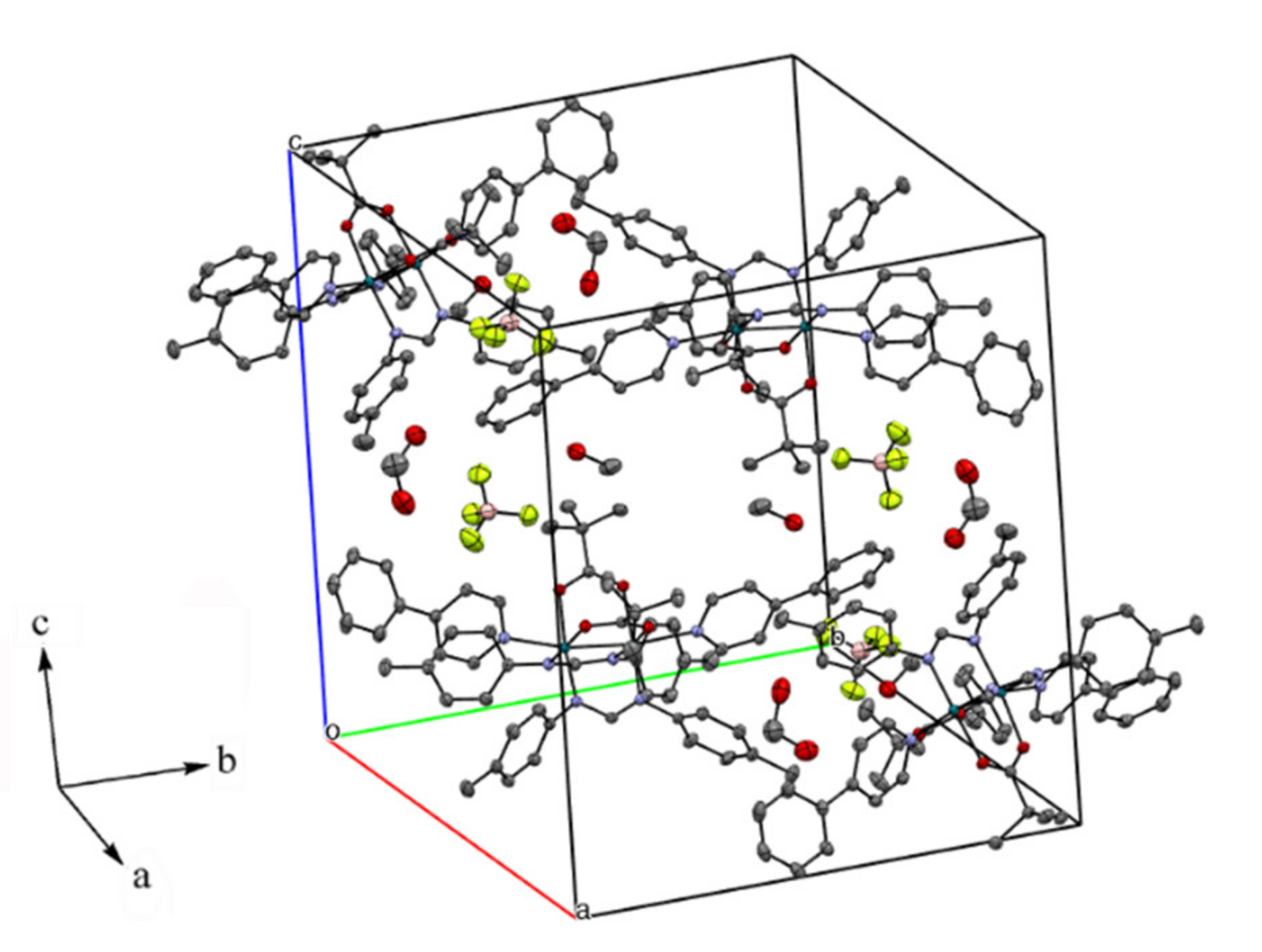
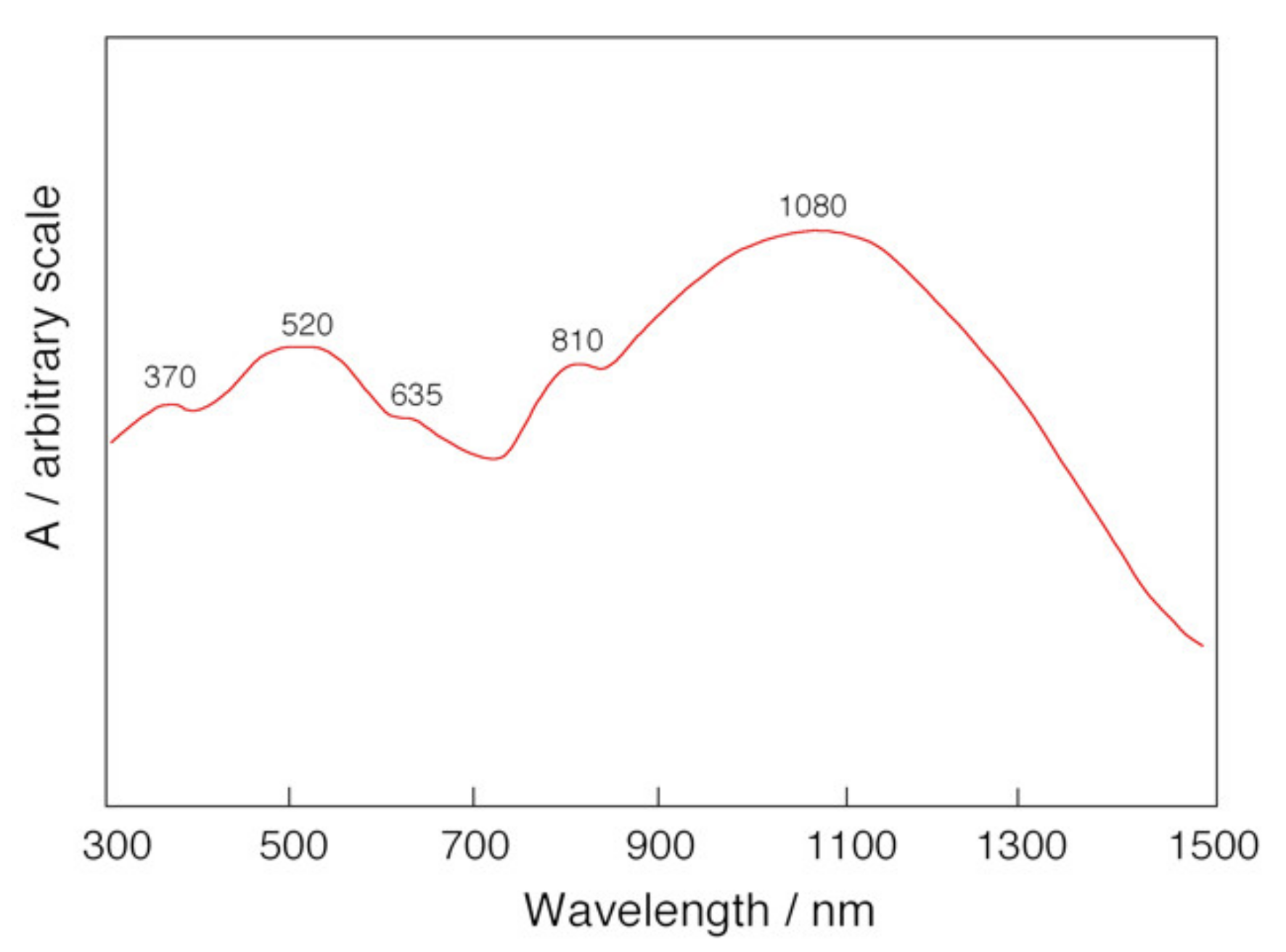
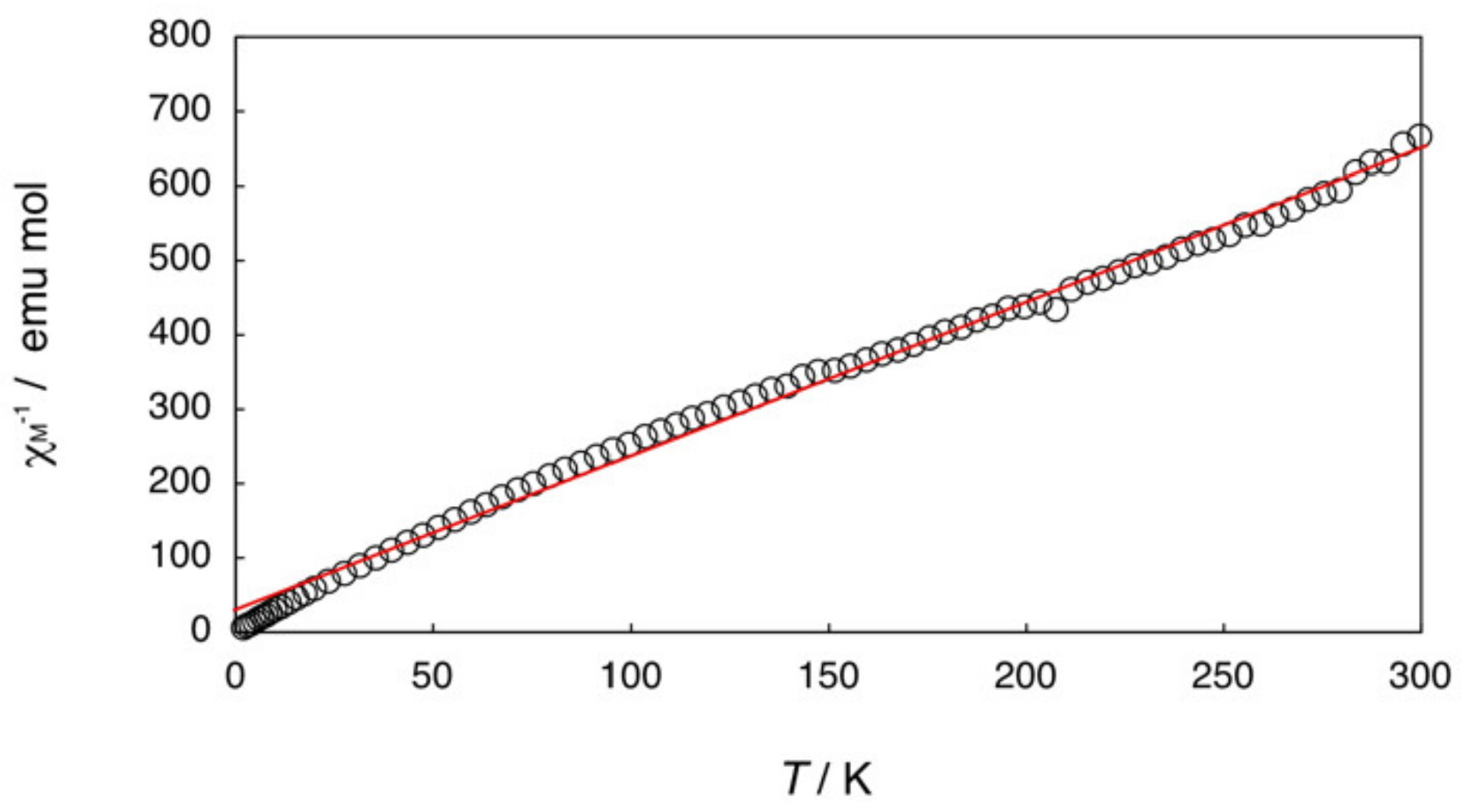
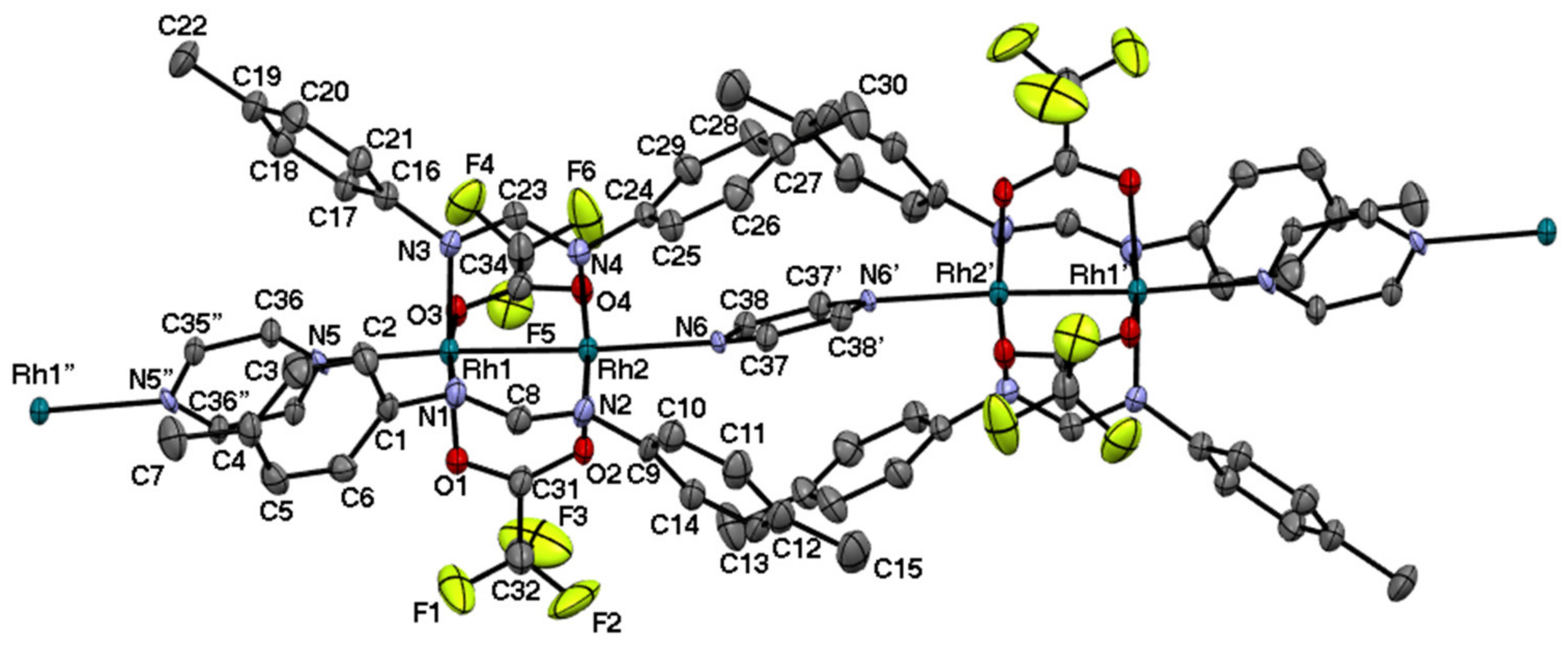
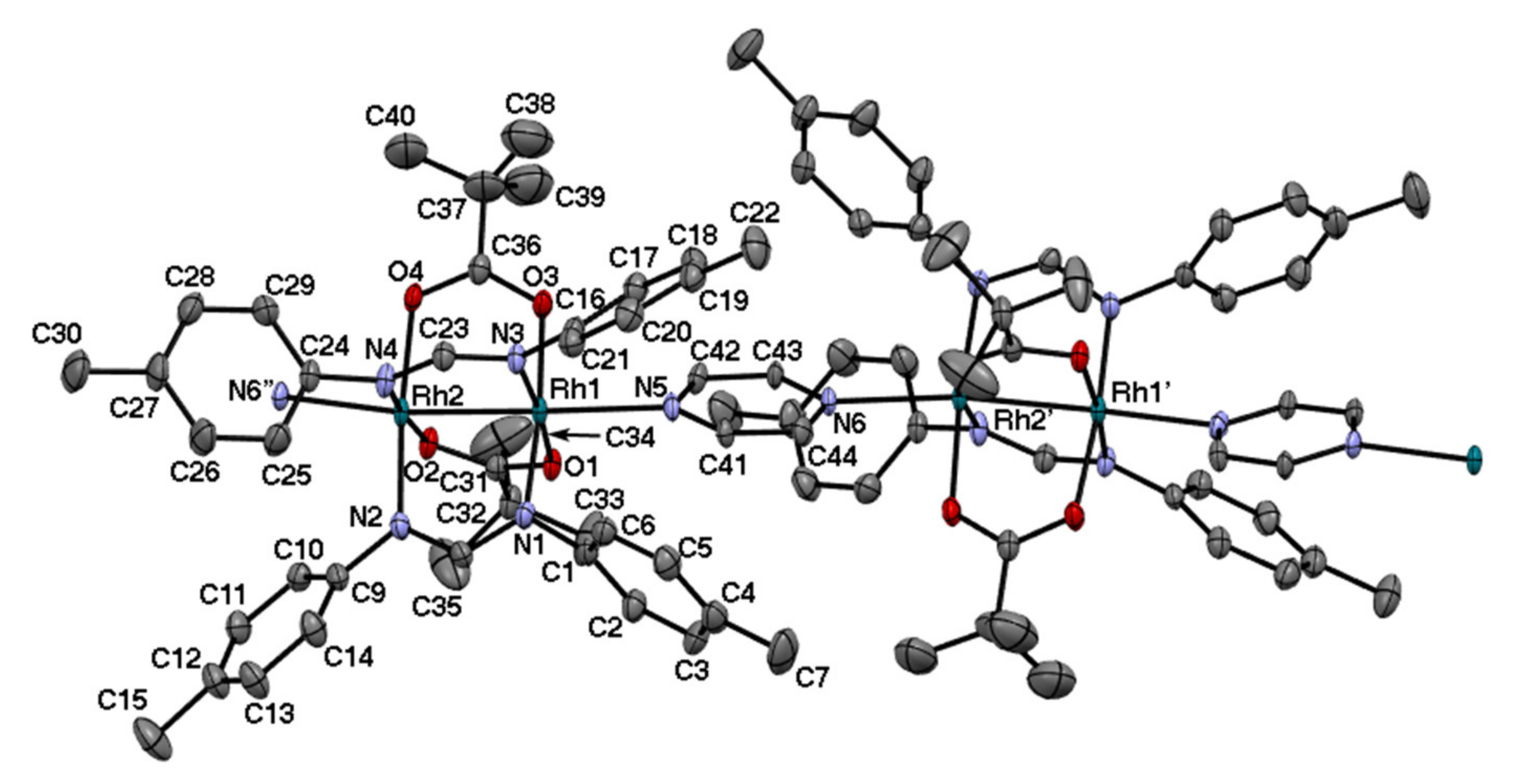
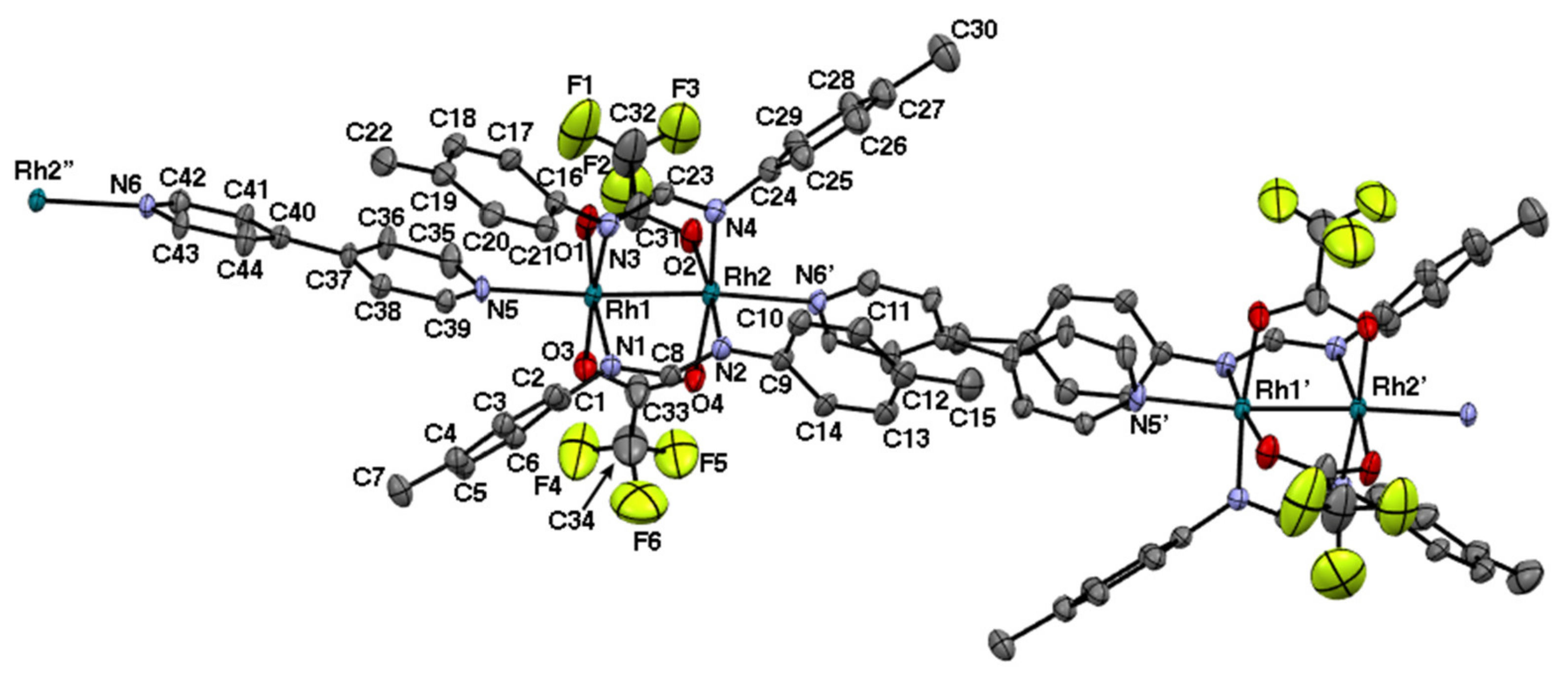
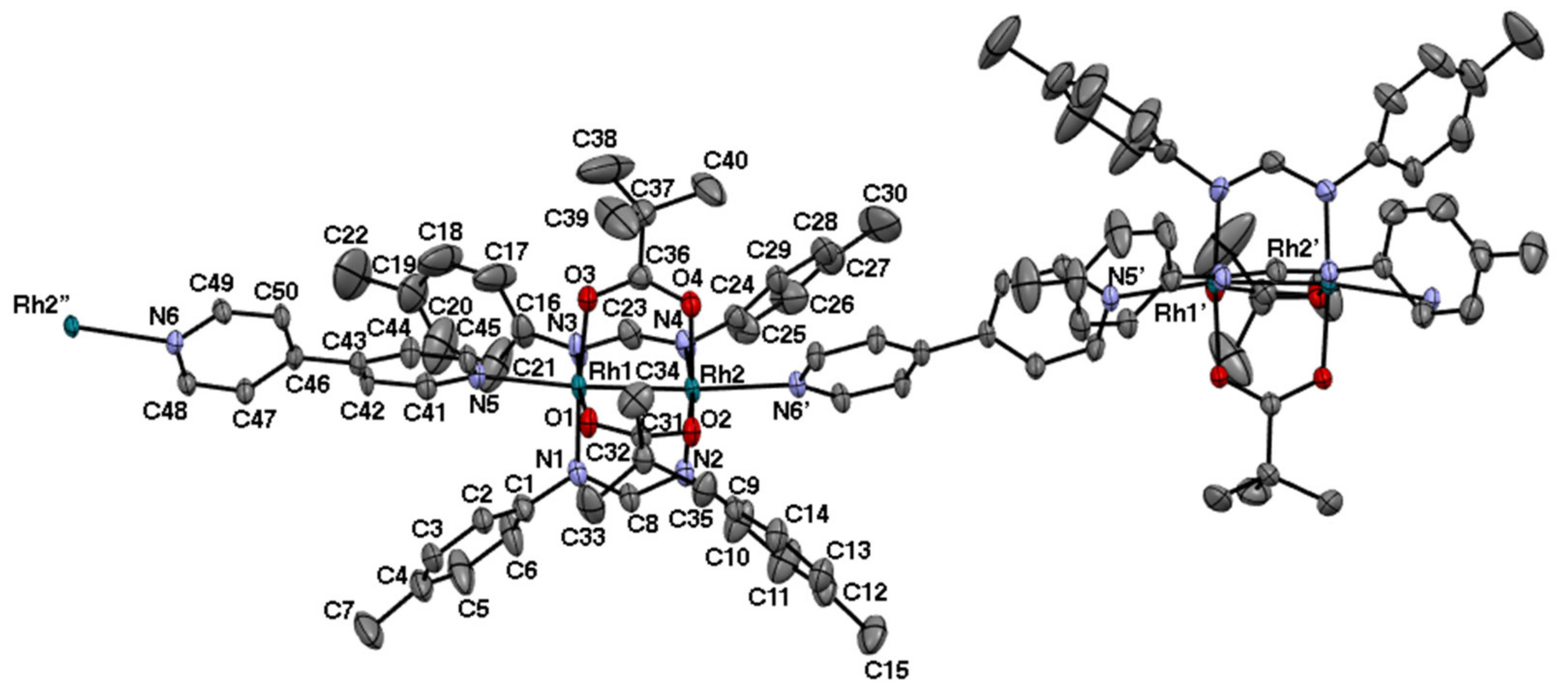
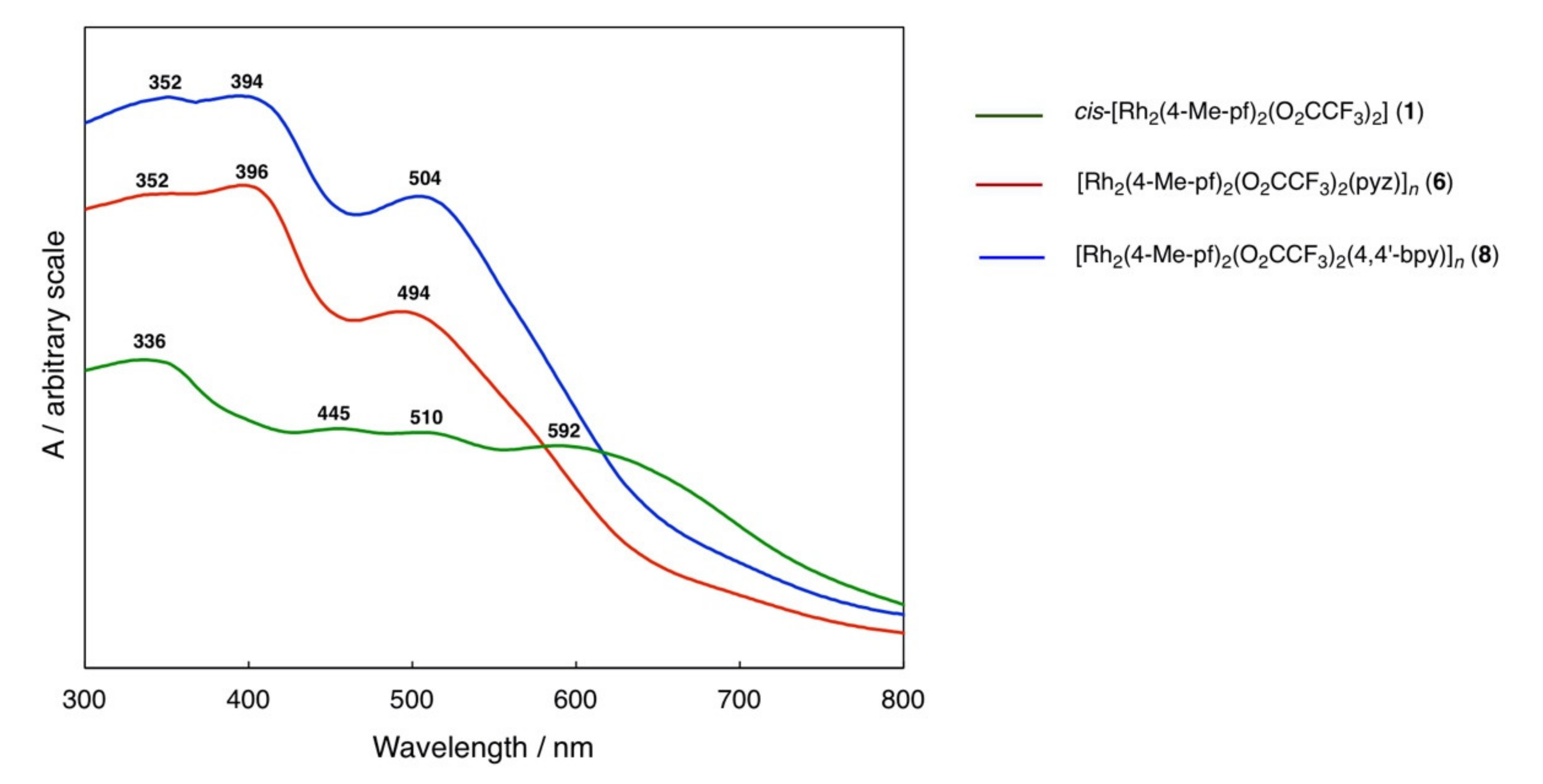

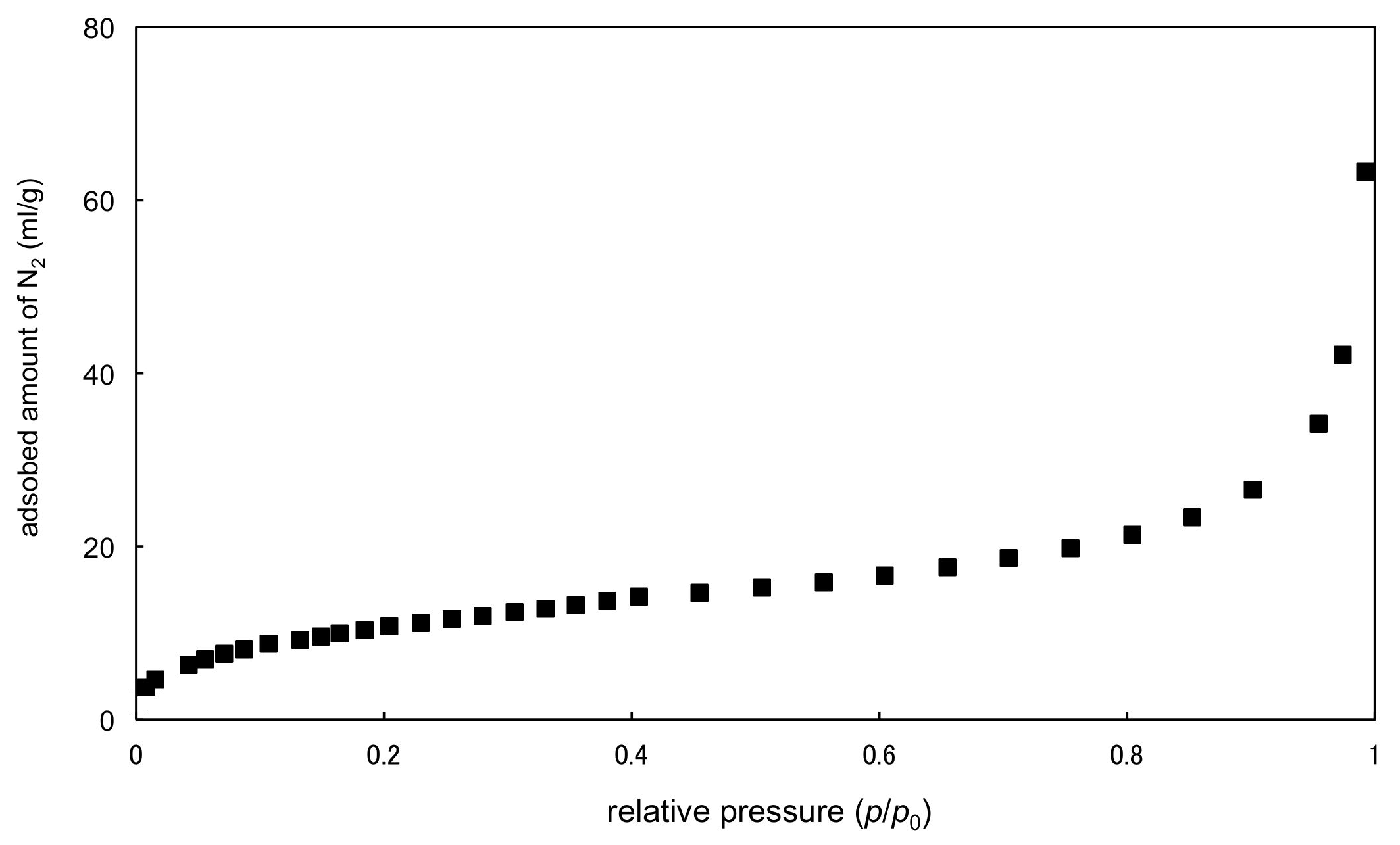
| Complexes | Rh–Rh/Å | Rh-Neq (4-Me-pf−)/Å (a) | Rh-Oeq (RCO2−)/Å (a) | Rh-Nax/Å | T/K (b) |
|---|---|---|---|---|---|
| 3 | 2.4702(2) | 2.0069 | 2.0938 | 2.3045(17) | 150 |
| 4 | 2.4428(4) | 2.025 | 2.070 | 2.289(2) | 150 |
| 5•2MeOH | 2.45320(19) | 1.9747 | 2.0487 | 2.2673(16), 2.2935(16) | 293 |
| 6 | 2.464(3) | 2.019 | 2.100 | 2.301(4), 2.320(4) | 150 |
| 7 | 2.4421(5) | 2.025 | 2.069 | 2.302(4), 2.283(4) | 150 |
| 8 | 2.4678(9) | 2.0193 | 2.102 | 2.300(6), 2.294(6) | 150 |
| 9 | 2.4660(7) | 2.023 | 2.066 | 2.303(3), 2.297(3) | 150 |
| Complexes | 3 | 4 | 5·2MeOH | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|
| Empirical formula | C56H48F6N6O4Rh2 | C62H66N6O4Rh2 | C64H70BF4N6O6Rh2 | C38H34F6N6O4Rh2 | C44H52N6O4Rh2 | C44H38F6N6O4Rh2 | C50H56N6O4Rh2 |
| Formula mass | 1188.82 | 1165.02 | 1311.89 | 958.53 | 934.73 | 1034.62 | 1010.82 |
| Temperature, T (K) | 150 | 150 | 293 | 150 | 150 | 150 | 150 |
| Crystal system | orthorhombic | monoclinic | monoclinic | triclinic | monoclinic | monoclinic | monoclinic |
| Space group | Pccn | C2/c | P21/n | P1 | P21/c | P21/c | I2/a |
| a (Å) | 14.3060(3) | 27.611(2) | 15.0200(2) | 11.517(14) | 10.8066(3) | 9.428(3) | 27.388(6) |
| b (Å) | 17.9846(4) | 10.0319(8) | 20.7383(2) | 11.578(15) | 18.9112(4) | 21.162(6) | 10.390(2) |
| c (Å) | 20.3568(6) | 20.3559(15) | 20.3334(2) | 18.91(2) | 24.2718(8) | 23.223(6) | 38.642(7) |
| α (°) | 90 | 90 | 90 | 95.639(15) | 90 | 90 | 90 |
| β (°) | 90 | 94.966(2) | 107.2500(10) | 105.24(2) | 95.950(3) | 97.122(5) | 101.144(12) |
| γ (°) | 90 | 90 | 90 | 108.23(3) | 90 | 90 | 90 |
| Unit-cell volume, V (Å3) | 5237.6(2) | 5617.2(7) | 6122.12(12) | 2266(5) | 4933.6(2) | 4598(2) | 10789(4) |
| Formula per unit cell, Z | 4 | 4 | 4 | 2 | 4 | 4 | 8 |
| Density, Dcalcd (g cm−3) | 1.508 | 1.378 | 1.423 | 1.405 | 1.258 | 1.495 | 1.245 |
| Crystal size (mm) | 0.602 × 0.399 × 0.103 | 0.350 × 0.200 × 0.170 | 0.20 × 0.10 × 0.050 | 0.100 × 0.100 × 0.010 | 0.220 × 0.020 × 0.020 | 0.130 × 0.100 × 0.030 | 0.220 × 0.200 × 0.190 |
| Absorption coefficient, μ (mm−1) | 0.703 | 0.640 | 0.607 | 0.794 | 0.711 | 0.789 | 0.655 |
| θrange for data collection (°) | 1.819–25.999 | 2.161–24.997 | 1.481–31.594 | 2.500–26.368 | 2.634–24.248 | 3.020–24.247 | 3.003–24.249 |
| Reflections collected/unique | 5160/4473 | 4920/4532 | 16763/19473 | 9091/7035 | 7937/6427 | 7375/5993 | 8648/7419 |
| [R1(I > 2σ(I)); wR2(all data)] | R1 = 0.0275, | R1 = 0.0387, | R1 = 0.0346, | R1 = 0.0507, | R1 = 0.0443, | R1 = 0.0725, | R1 = 0.0442, |
| ωR2 = 0.0777 | ωR2 = 0.0863 | ωR2 = 0.1075 | ωR2 = 0.1495 | ωR2 = 0.1333 | ωR2 = 0.1956 | ωR2 = 0.1265 | |
| Goodness-of-fit on F2 | 1.062 | 1.193 | 1.189 | 1.047 | 1.076 | 1.087 | 1.082 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Handa, M.; Nishiura, S.; Kano, M.; Yano, N.; Akashi, H.; Mikuriya, M.; Tanaka, H.; Kawamoto, T.; Kataoka, Y. Structures and Properties of 4-phpy, pyz, and 4,4′-bpy Adducts of Lantern-Type Dirhodium Complexes with µ-Formamidinato and µ-Carboxylato Bridges. Magnetochemistry 2021, 7, 39. https://doi.org/10.3390/magnetochemistry7030039
Handa M, Nishiura S, Kano M, Yano N, Akashi H, Mikuriya M, Tanaka H, Kawamoto T, Kataoka Y. Structures and Properties of 4-phpy, pyz, and 4,4′-bpy Adducts of Lantern-Type Dirhodium Complexes with µ-Formamidinato and µ-Carboxylato Bridges. Magnetochemistry. 2021; 7(3):39. https://doi.org/10.3390/magnetochemistry7030039
Chicago/Turabian StyleHanda, Makoto, Satoshi Nishiura, Makoto Kano, Natsumi Yano, Haruo Akashi, Masahiro Mikuriya, Hidekazu Tanaka, Tatsuya Kawamoto, and Yusuke Kataoka. 2021. "Structures and Properties of 4-phpy, pyz, and 4,4′-bpy Adducts of Lantern-Type Dirhodium Complexes with µ-Formamidinato and µ-Carboxylato Bridges" Magnetochemistry 7, no. 3: 39. https://doi.org/10.3390/magnetochemistry7030039
APA StyleHanda, M., Nishiura, S., Kano, M., Yano, N., Akashi, H., Mikuriya, M., Tanaka, H., Kawamoto, T., & Kataoka, Y. (2021). Structures and Properties of 4-phpy, pyz, and 4,4′-bpy Adducts of Lantern-Type Dirhodium Complexes with µ-Formamidinato and µ-Carboxylato Bridges. Magnetochemistry, 7(3), 39. https://doi.org/10.3390/magnetochemistry7030039