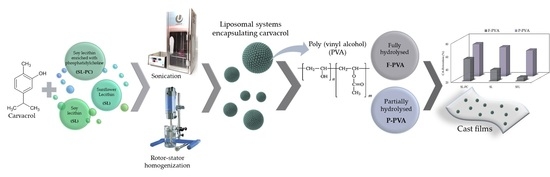
Liposomal Encapsulation of Carvacrol to Obtain Active Poly (Vinyl Alcohol) Films
Abstract
:1. Introduction
2. Results and Discussion
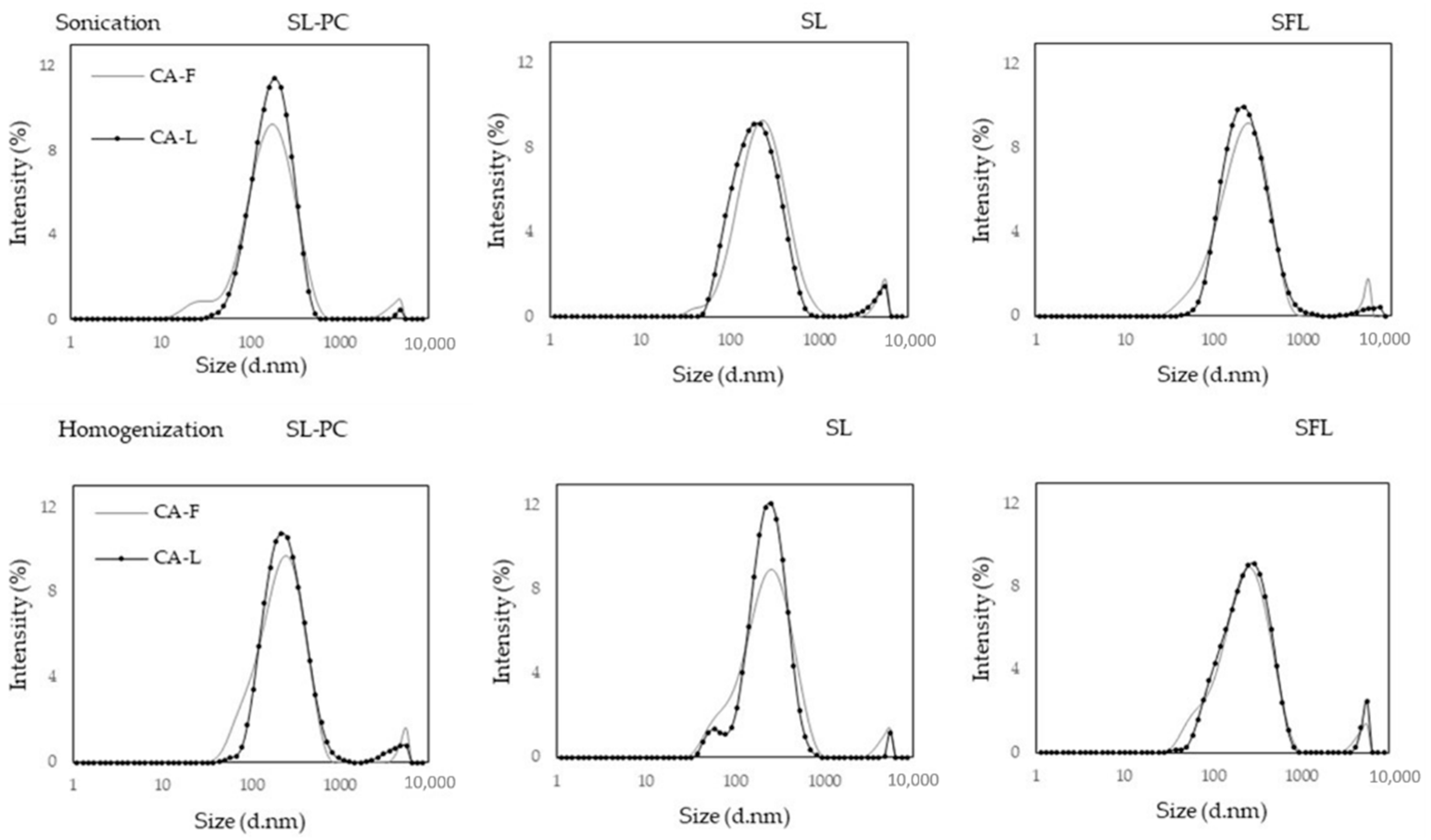
2.1. Liposomal Characteristics
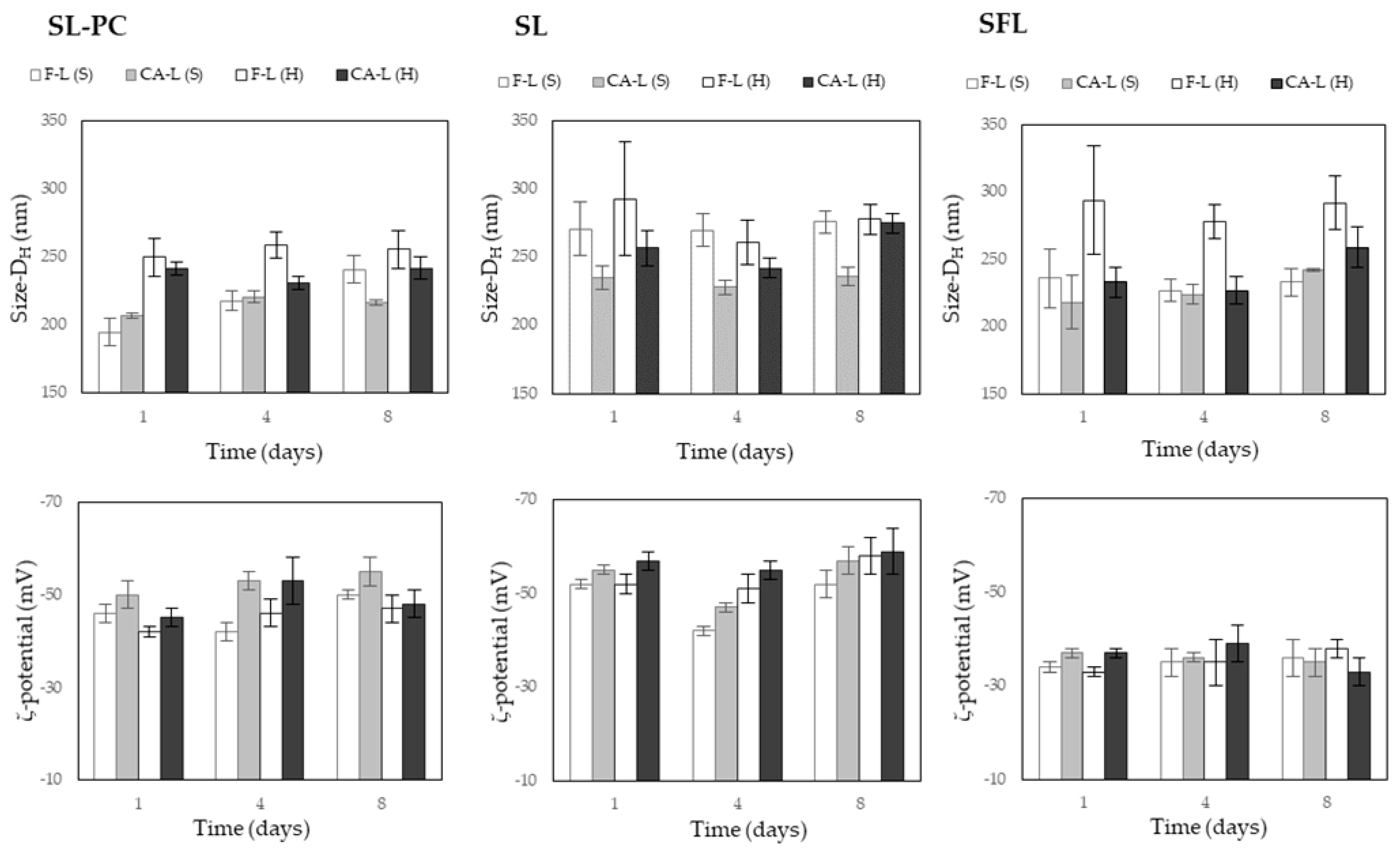
2.2. Liposomal Stability
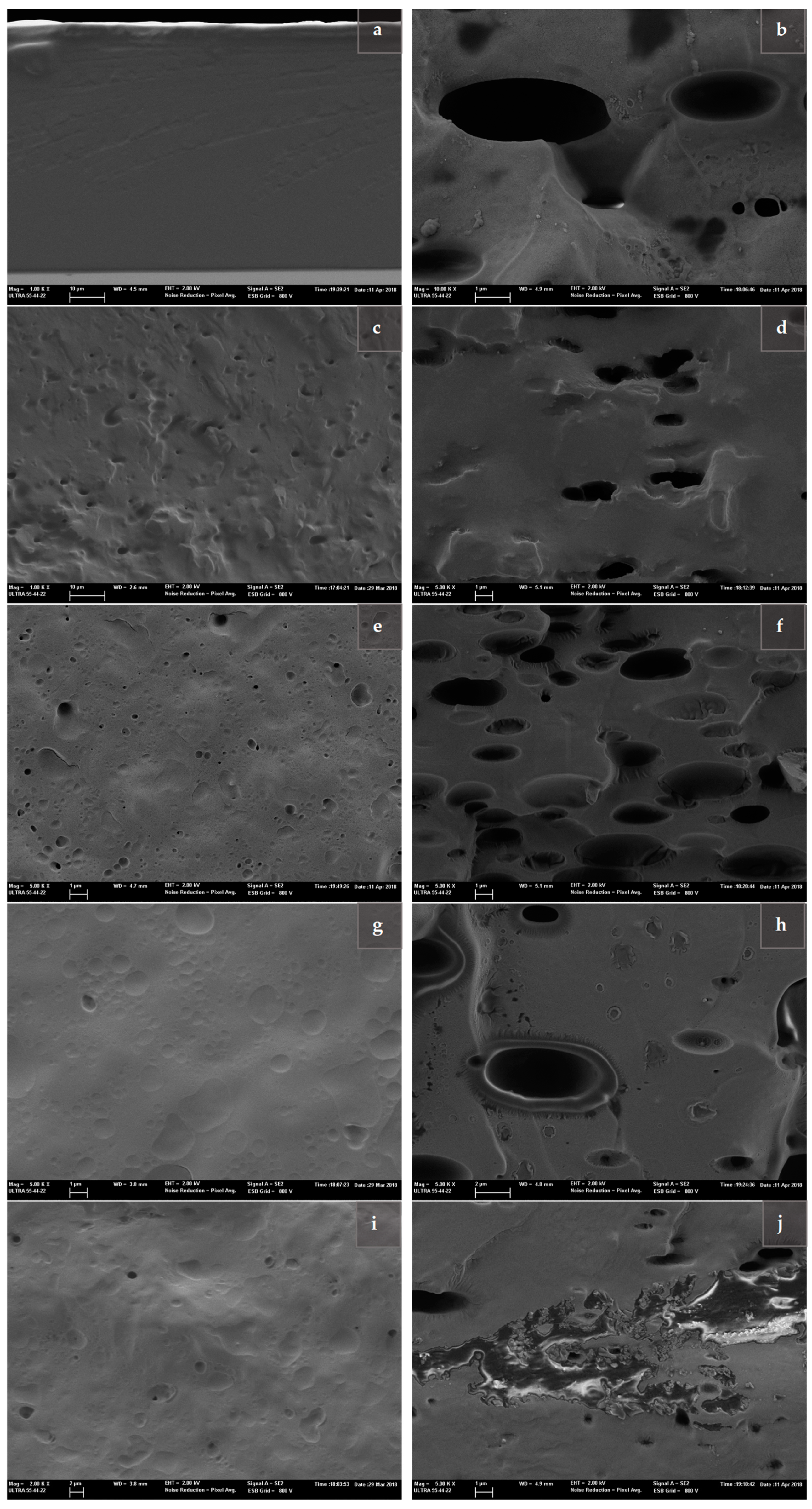
2.3. Carvacrol Content and Film Microstructure of PVA Matrices with Liposomes
3. Materials and Methods
3.1. Materials
3.2. Liposome Production
3.3. Liposome Properties
3.4. Preparation of Films
3.5. Final Carvacrol Content in the Films
3.6. Microstructure of Films
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Altan, A.; Aytac, Z.; Uyar, T. Carvacrol loaded electrospun fibrous films from zein and poly(lactic acid) for active food packaging. Food Hydrocoll. 2018, 81, 48–59. [Google Scholar] [CrossRef] [Green Version]
- Gursul, S.; Karabulut, I.; Durmaz, G. Antioxidant efficacy of thymol and carvacrol in microencapsulated walnut oil triacylglycerols. Food Chem. 2019, 278, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Estaca, J.; López-De-Dicastillo, C.; Hernández-Muñoz, P.; Catalá, R.; Gavara, R. Advances in antioxidant active food packaging. Trends Food Sci. Technol. 2014, 35, 42–51. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential oils as additives in active food packaging. Food Chem. 2020, 343, 128403. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.; González-Martínez, C.; Chiralt, A. Incorporation of carvacrol into poly (vinyl alcohol) films, as affected by the polymer molecular characteristics. Polym. Degrad. Stab. 2020, 12, 497. [Google Scholar] [CrossRef] [Green Version]
- De Vincenzi, M.; Stammati, A.; De Vincenzi, A.; Silano, M. Constituents of aromatic plants: Carvacrol. Fitoterapia 2004, 75, 801–804. [Google Scholar] [CrossRef]
- Veldhuizen, E.J.A.; Tjeerdsma-Van Bokhoven, J.L.M.; Zweijtzer, C.; Burt, S.A.; Haagsman, H.P. Structural Requirements for the Antimicrobial Activity of Carvacrol. J. Agric. Food Chem. 2006, 54, 1874–1879. [Google Scholar] [CrossRef]
- Sapper, M.; Wilcaso, P.; Santamarina, M.P.; Roselló, J.; Chiralt, A. Antifungal and functional properties of starch-gellan films containing thyme (Thymus zygis) essential oil. Food Control 2018, 92, 505–515. [Google Scholar] [CrossRef]
- Requena, R.; Vargas, M.; Chiralt, A. Release kinetics of carvacrol and eugenol from poly(hydroxybutyrate-co-hydroxyvalerate) (PHBV) films for food packaging applications. Eur. Polym. J. 2017, 92, 185–193. [Google Scholar] [CrossRef]
- Coimbra, M.; Isacchi, B.; van Bloois, L.; Torano, J.S.; Ket, A.; Wu, X.; Broere, F.; Metselaar, J.M.; Rijcken, C.J.F.; Storm, G.; et al. Improving solubility and chemical stability of natural compounds for medicinal use by incorporation into liposomes. Int. J. Pharm. 2011, 416, 433–442. [Google Scholar] [CrossRef]
- Sebaaly, C.; Greige-Gerges, H.; Stainmesse, S.; Fessi, H.; Charcosset, C. Effect of composition, hydrogenation of phospholipids and lyophilization on the characteristics of eugenol-loaded liposomes prepared by ethanol injection method. Food Biosci. 2016, 15, 1–10. [Google Scholar] [CrossRef]
- Sebaaly, C.; Charcosset, C.; Stainmesse, S.; Fessi, H.; Greige-Gerges, H. Clove essential oil-in-cyclodextrin-in-liposomes in the aqueous and lyophilized states: From laboratory to large scale using a membrane contactor. Carbohydr. Polym. 2016, 138, 75–85. [Google Scholar] [CrossRef]
- Carvalho, I.T.; Estevinho, B.N.; Santos, L. Application of microencapsulated essential oils in cosmetic and personal healthcare products—A review. Int. J. Cosmet. Sci. 2016, 38, 109–119. [Google Scholar] [CrossRef]
- Hammoud, Z.; Gharib, R.; Fourmentin, S.; Elaissari, A.; Greige-Gerges, H. New findings on the incorporation of essential oil components into liposomes composed of lipoid S100 and cholesterol. Int. J. Pharm. 2019, 561, 161–170. [Google Scholar] [CrossRef]
- Cullis, P.R.; Hope, M.J.; Tilcock, C.P.S. Lipid polymorphism and the roles of lipids in membranes. Chem. Phys. Lipids 1986, 40, 127–144. [Google Scholar] [CrossRef]
- Gruner, S.M.; Cullis, P.R.; Hope, M.J.; Tilcock, C.P.S. Lipid Polymorphism:The Molecular Basis of Nonbilayer Phases. Annu. Rev. Biophys. Biophys. Chem. 1985, 14, 211–238. [Google Scholar] [CrossRef] [PubMed]
- Gillet, A.; Grammenos, A.; Compère, P.; Evrard, B.; Piel, G. Development of a new topical system: Drug-in-cyclodextrin-in-deformable liposome. Int. J. Pharm. 2009, 380, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Taladrid, D.; Marín, D.; Alemán, A.; Álvarez-Acero, I.; Montero, P.; Gómez-Guillén, M.C. Effect of chemical composition and sonication procedure on properties of food-grade soy lecithin liposomes with added glycerol. Food Res. Int. 2017, 100, 541–550. [Google Scholar] [CrossRef] [Green Version]
- Müller, R.H.; Jacobs, C.; Kayser, O. Nanosuspensions as particulate drug formulations in therapy: Rationale for development and what we can expect for the future. Adv. Drug Deliv. Rev. 2001, 47, 3–19. [Google Scholar] [CrossRef]
- Seelig, J.; Macdonald, P.M.; Scherer, P.G. Phospholipid head groups as sensors of electric charge in membranes. Biochemistry 1987, 26, 7535–7541. [Google Scholar] [CrossRef] [PubMed]
- Ramazani, F.; Chen, W.; Van Nostrum, C.F.; Storm, G.; Kiessling, F.; Lammers, T.; Hennink, W.E.; Kok, R.J. Strategies for encapsulation of small hydrophilic and amphiphilic drugs in PLGA microspheres: State-of-the-art and challenges. Int. J. Pharm. 2016, 499, 358–367. [Google Scholar] [CrossRef]
- Talón, E.; Vargas, M.; Chiralt, A.; González-Martínez, C. Antioxidant starch-based films with encapsulated eugenol. Application to sunflower oil preservation. LWT 2019, 113, 108290. [Google Scholar] [CrossRef]
- Valencia-Sullca, C.; Jiménez, M.; Jiménez, A.; Atarés, L.; Vargas, M.; Chiralt, A. Influence of liposome encapsulated essential oils on properties of chitosan films. Polym. Int. 2016, 65, 979–987. [Google Scholar] [CrossRef]
- El Asbahani, A.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.A.; Casabianca, H.; El Mousadik, A.; Hartmann, D.; Jilale, A.; Renaud, F.N.R.; et al. Essential oils: From extraction to encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef]
- Gibis, M.; Thellmann, K.; Thongkaew, C.; Weiss, J. Interaction of polyphenols and multilayered liposomal-encapsulated grape seed extract with native and heat-treated proteins. Food Hydrocoll. 2014, 41, 119–131. [Google Scholar] [CrossRef]
- Silva, R.; Ferreira, H.; Little, C.; Cavaco-Paulo, A. Effect of ultrasound parameters for unilamellar liposome preparation. Ultrason. Sonochem. 2010, 17, 628–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sebaaly, C.; Jraij, A.; Fessi, H.; Charcosset, C.; Greige-Gerges, H. Preparation and characterization of clove essential oil-loaded liposomes. Food Chem. 2015, 178, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Lachataignerais, J.; Pons, R.; Panizza, P.; Courbin, L.; Rouch, J.; Lopez, O. Study and formation of vesicle systems with low polydispersity index by ultrasound method. Chem. Phys. Lipids 2006, 140, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Greenly, J.M.; Tester, J.W. Ultrasonic cavitation for disruption of microalgae. Bioresour. Technol. 2015, 184, 276–279. [Google Scholar] [CrossRef]
- McMahon, H.T.; Gallop, J.L. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nat. Cell Biol. 2005, 438, 590–596. [Google Scholar] [CrossRef]
- Yang, P.L. Metabolomics and Lipidomics: Yet More Ways Your Health is Influenced by Fat, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780128009642. [Google Scholar]
- Maherani, B.; Arab-Tehrany, E.; Kheirolomoom, A.; Geny, D.; Linder, M. Calcein release behavior from liposomal bilayer; influence of physicochemical/mechanical/structural properties of lipids. Biochimie 2013, 95, 2018–2033. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, Z.; Barzegar, M.; Sahari, M.A.; Maherani, B. Nanoliposomal carriers for improvement the bioavailability of high—Valued phenolic compounds of pistachio green hull extract. Food Chem. 2017, 220, 115–122. [Google Scholar] [CrossRef]
- Wink, M. Functions and Biotechnology of Plant Secondary Metabolites, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2010; ISBN 9781405185288. [Google Scholar]
- Olbrich, K.; Rawicz, W.; Needham, D.; Evans, E. Water Permeability and Mechanical Strength of Polyunsaturated Lipid Bilayers. Biophys. J. 2000, 79, 321–327. [Google Scholar] [CrossRef] [Green Version]
- Yi, Z.; Nagao, M.; Bossev, D.P. Bending elasticity of saturated and monounsaturated phospholipid membranes studied by the neutron spin echo technique. J. Phys. Condens. Matter 2009, 21, 155104. [Google Scholar] [CrossRef]
- Wang, G.; Wang, T. Oxidative Stability of Egg and Soy Lecithin as Affected by Transition Metal Ions and pH in Emulsion. J. Agric. Food Chem. 2008, 56, 11424–11431. [Google Scholar] [CrossRef] [Green Version]
- Lu, Q.; Lu, P.-M.; Piao, J.-H.; Xu, X.-L.; Chen, J.; Zhu, L.; Jiang, J.-G. Preparation and physicochemical characteristics of an allicin nanoliposome and its release behavior. LWT Food Sci. Technol. 2014, 57, 686–695. [Google Scholar] [CrossRef]
- Joseph, E.; Singhvi, G. Multifunctional Nanocrystals for Cancer Therapy: A Potential Nanocarrier; Elsevier BV: Amsterdam, The Netherlands, 2019; ISBN 9780128165058. [Google Scholar]
- Li, J.; Chang, C.; Zhani, J.; Yu, H. Ascorbyl palmitate effects on the stability of curcumin-loaded soybeanphosphatidylcholine liposomes. Food Biosci. 2021, 41, 100923. [Google Scholar] [CrossRef]
- Reiner, G.N.; Perillo, M.A.; García, D.A. Effects of propofol and other GABAergic phenols on membrane molecular organization. Colloids Surfaces B Biointerfaces 2013, 101, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Reiner, G.N.; Fraceto, L.F.; de Paula, E.; Perillo, M.A.; García, D.A. Effects of Gabaergic Phenols on Phospholipid Bilayers as Evaluated by 1H-NMR. J. Biomater. Nanobiotechnol. 2013, 4, 28–34. [Google Scholar] [CrossRef]
- Hasan, M.; Belhaj, N.; Benachour, H.; Barberi-Heyob, M.; Kahn, C.J.F.; Jabbari, E.; Linder, M.; Arab-Tehrany, E. Liposome encapsulation of curcumin: Physico-chemical characterizations and effects on MCF7 cancer cell proliferation. Int. J. Pharm. 2014, 461, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Perdones, Á.; Chiralt, A.; Vargas, M. Properties of film-forming dispersions and films based on chitosan containing basil or thyme essential oil. Food Hydrocoll. 2016, 57, 271–279. [Google Scholar] [CrossRef]
- Vélez, M.A.; Perotti, M.C.; Zanel, P.; Hynes, E.R.; Gennaro, A.M. Soy PC liposomes as CLA carriers for food applications: Preparation and physicochemical characterization. J. Food Eng. 2017, 212, 174–180. [Google Scholar] [CrossRef]
- Dos Reis Coimbra, J.S.; Teizeira, J.A. (Eds.) Engineering Aspects of Milk and Dairy Products; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9781787284395. [Google Scholar]
- Andrade, J.; González-Martínez, C.; Chiralt, A. The Incorporation of Carvacrol into Poly (vinyl alcohol) Films Encapsulated in Lecithin Liposomes. Polymers 2020, 12, 497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiśniewska, M.; Bogatyrov, V.; Ostolska, I.; Szewczuk-Karpisz, K.; Terpiłowski, K.; Nosal-Wiercińska, A. Impact of poly(vinyl alcohol) adsorption on the surface characteristics of mixed oxide MnxOy–SiO2. Adsorption 2016, 22, 417–423. [Google Scholar] [CrossRef] [Green Version]
- Tampau, A.; González-Martínez, C.; Chiralt, A. Polyvinyl alcohol-based materials encapsulating carvacrol obtained by solvent casting and electrospinning. React. Funct. Polym. 2020, 153, 104603. [Google Scholar] [CrossRef]
| LEC | CA:LEC | Method | |||||
|---|---|---|---|---|---|---|---|
| Sonication | Homogenisation | ||||||
| DH (nm) * | PDI | ζ (mV) | DH (nm) * | PDI | ζ (mV) | ||
| SL-PC | 0:1 | 195 (10) | 0.28 (0.01) | −46 (2) | 250 (14) | 0.29 (0.03) | −43 (1) |
| 0.5:1 | 211 (1) | 0.24 (0.02) | −47 (2) | 249 (12) | 0.32 (0.01) | −44 (1) | |
| 1:1 | 207 (2) | 0.17 (0.01) | −50 (3) | 242 (5) | 0.19 (0.01) | −45 (2) | |
| SL | 0:1 | 271 (20) | 0.31 (0.03) | −52(1) | 293 (42) | 0.32 (0.04) | −52 (2) |
| 0.5:1 | 219 (8) | 0.32 (0.01) | −53 (1) | 232 (30) | 0.33 (0.01) | −56 (2) | |
| 1:1 | 235 (9) | 0.27 (0.05) | −55 (1) | 257 (13) | 0.31 (0.01) | −57 (2) | |
| SFL | 0:1 | 236 (22) | 0.30(0.02) | −34 (1) | 294 (40) | 0.35 (0.03) | −33 (1) |
| 0.5:1 | 192 (5) | 0.35 (0.02) | −38 (1) | 179 (7) | 0.36 (0.03) | −37 (1) | |
| 1:1 | 218 (20) | 0.25 (0.03) | −37 (1) | 233 (11) | 0.36 (0.01) | −37 (1) | |
| LEC | CA: LEC | CA Retention (%) | |||
|---|---|---|---|---|---|
| F-PVA | P-PVA | ||||
| Sonication | Homogenisation | Sonication | Homogenisation | ||
| SL-PC | 0.5:1 | 54 (2) | 53 (4) | 74 (2) | 71 (5) |
| 1:01 | 57 (3) | 54 (3) | 67 (3) | 65 (3) | |
| SL | 0.5:1 | 47 (1) | 41 (1) | 66 (1) | 64 (1) |
| 1:01 | 45 (4) | 46 (4) | 66 (7) | 62 (5) | |
| SFL | 0.5:1 | 28 (1) | 22 (2) | 61 (7) | 54 (8) |
| 1:01 | 27 (2) | 24 (4) | 63 (3) | 60 (3) | |
| Phospholipid | % (w/w) | ||
|---|---|---|---|
| SL-PC | SL | SFL | |
| PC-phosphatidylcholine | 74 | 14 | 12 |
| PI-Phosphatidylinositol | 12 | 4 | |
| PE-Phosphatidylethanolamine | 11 | 10 | 11 |
| LPC-Lysophosphatidylcholine | 3 | 0 | 3 |
| PA-Phosphatidic acid | 1 | 4 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade, J.; González-Martínez, C.; Chiralt, A. Liposomal Encapsulation of Carvacrol to Obtain Active Poly (Vinyl Alcohol) Films. Molecules 2021, 26, 1589. https://doi.org/10.3390/molecules26061589
Andrade J, González-Martínez C, Chiralt A. Liposomal Encapsulation of Carvacrol to Obtain Active Poly (Vinyl Alcohol) Films. Molecules. 2021; 26(6):1589. https://doi.org/10.3390/molecules26061589
Chicago/Turabian StyleAndrade, Johana, Chelo González-Martínez, and Amparo Chiralt. 2021. "Liposomal Encapsulation of Carvacrol to Obtain Active Poly (Vinyl Alcohol) Films" Molecules 26, no. 6: 1589. https://doi.org/10.3390/molecules26061589
APA StyleAndrade, J., González-Martínez, C., & Chiralt, A. (2021). Liposomal Encapsulation of Carvacrol to Obtain Active Poly (Vinyl Alcohol) Films. Molecules, 26(6), 1589. https://doi.org/10.3390/molecules26061589