Fabrication of a Porous Three-Dimensional Scaffold with Interconnected Flow Channels: Co-Cultured Liver Cells and In Vitro Hemocompatibility Assessment
Abstract
1. Introduction
2. Materials and Methods
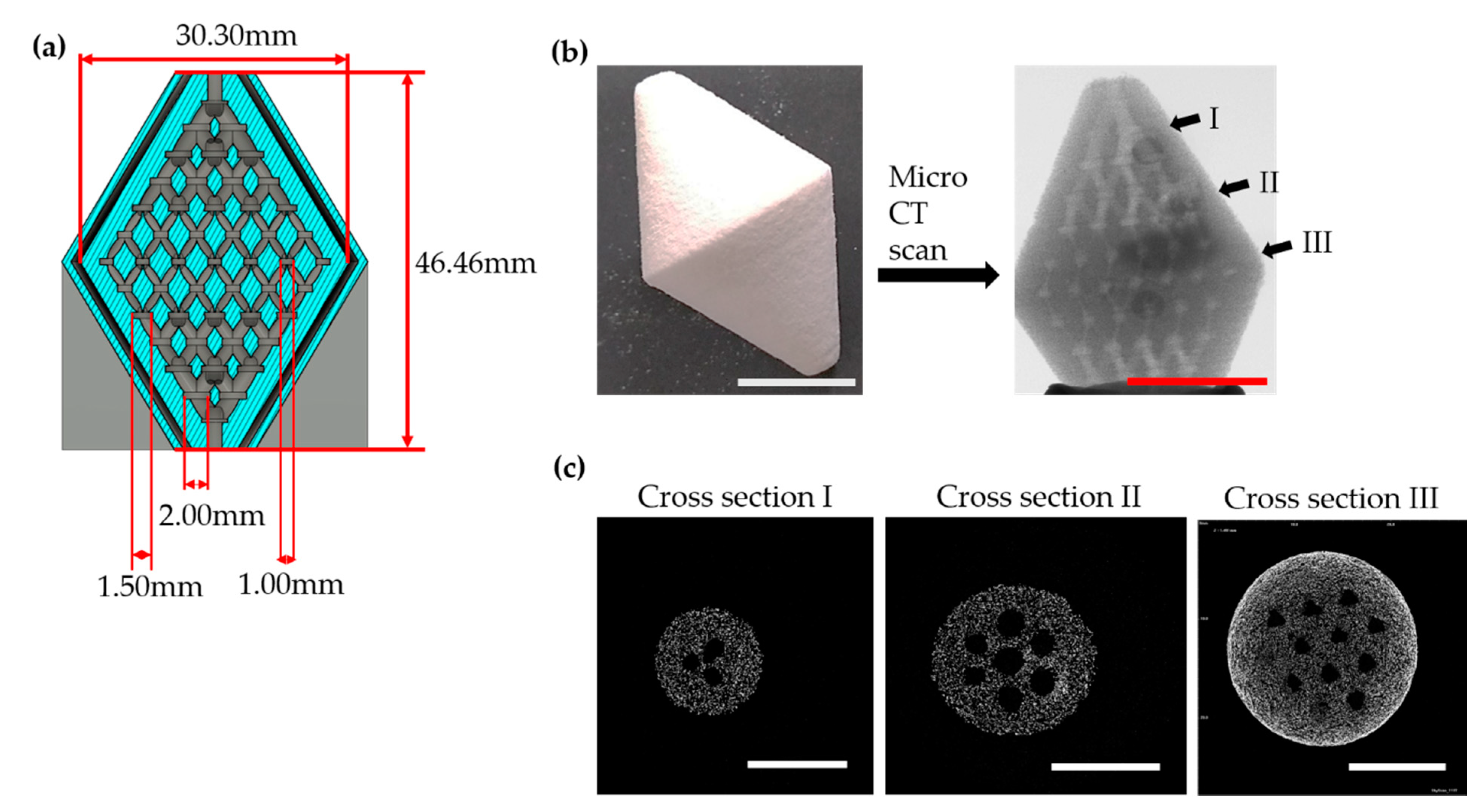
2.1. Design of Scaffold
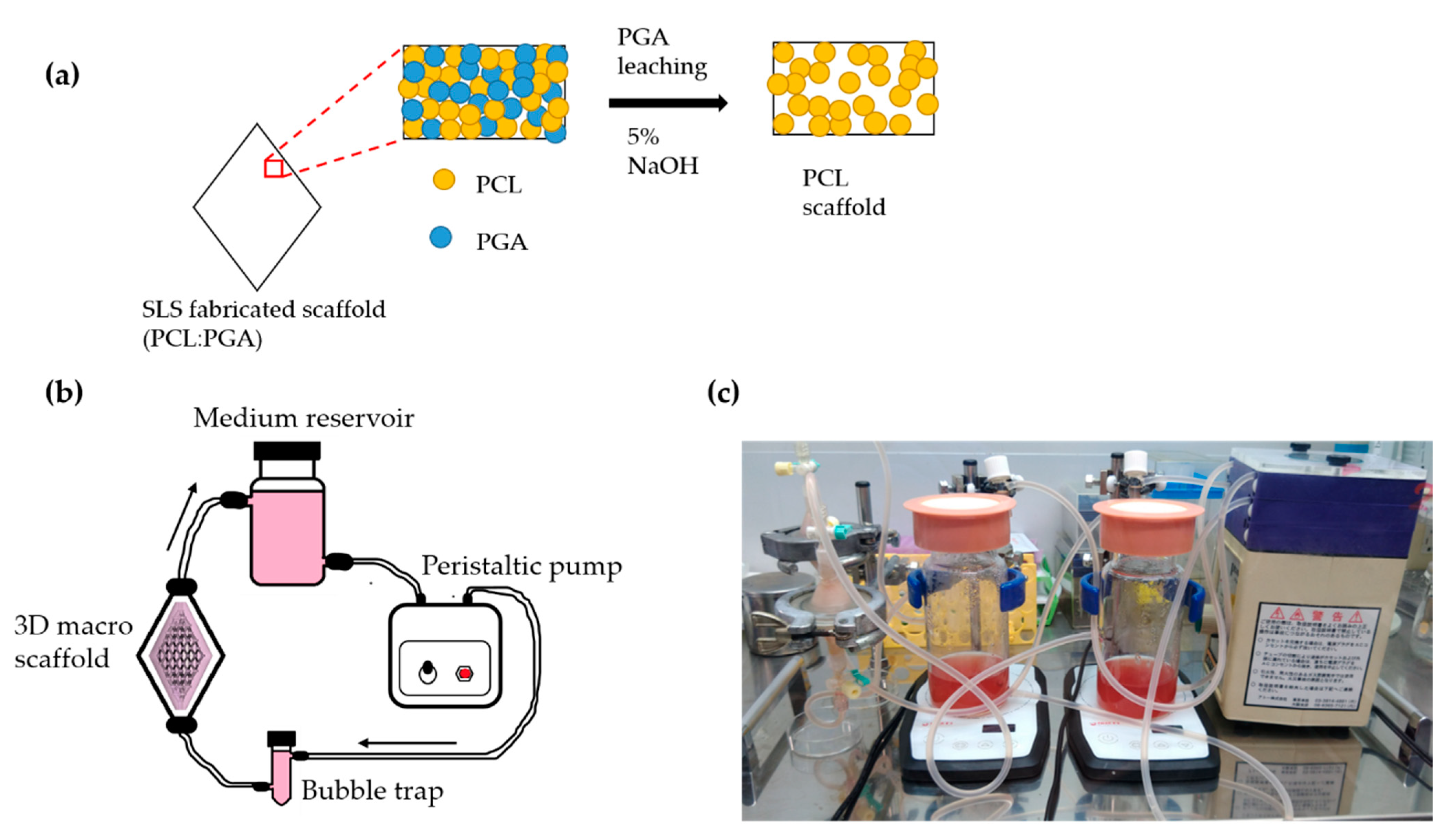
2.2. Fabrication of Scaffold Using Selective Laser Sintering
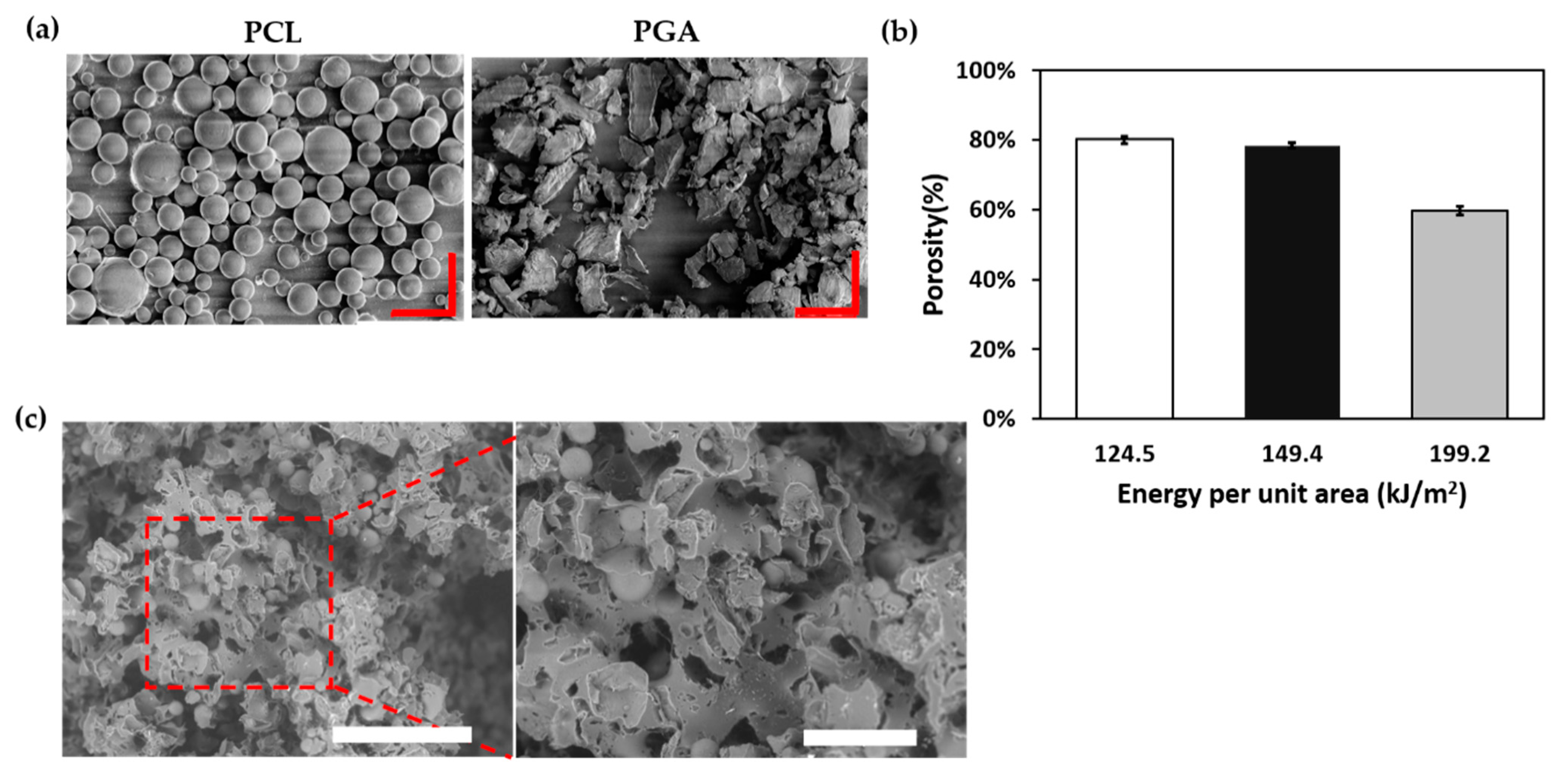
2.3. Porosity Measurement and Morphology of Scaffold
2.4. Perfusion Culture Setup for 3-D Scaffold
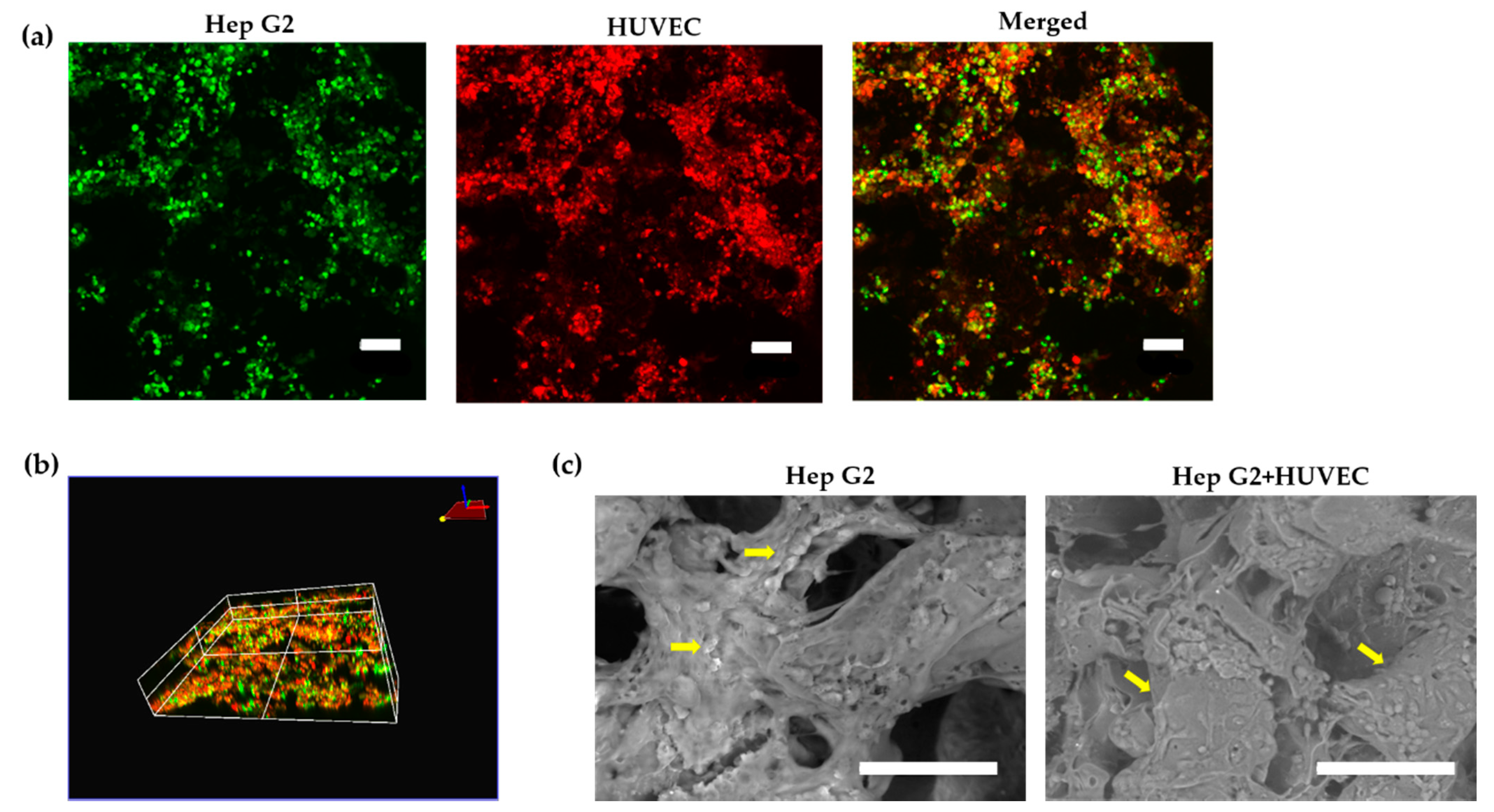
2.5. Cell Localization and Cellular Metabolism
2.6. Platelet Deposition on Scaffold
2.7. Statistical Analysis
3. Results
3.1. Fabrication of Scaffold
3.2. Porosity Measurement and Morphology of Scaffold
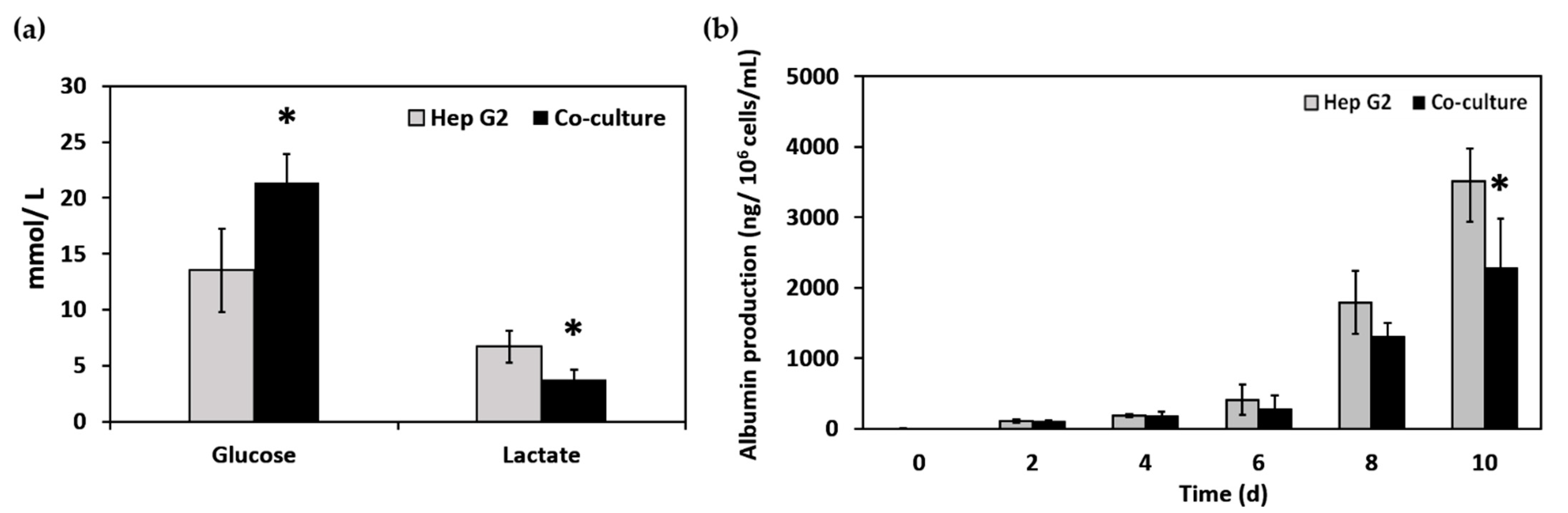
3.3. Cell Localization and Cellular Metabolism
3.4. Platelet Deposition and Adhesion
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Dhariawala, B.; Hunt, E.; Thomas, B. Rapid Prototyping of Tissue-Engineering Constructs, Using Photopolymerizable Hydrogels and Stereolithography. Tissue Eng. 2004, 10, 1316–1322. [Google Scholar] [CrossRef]
- Sakai, Y.; Otsuka, M.; Hanada, S.; Nishiyama, Y.; Konishi, Y.; Yamashita, A. A novel poly-l-lactic acid scaffold that possesses a macroporous structure and a branching/joining three-dimensional flow channel network: Its fabrication and application to perfusion culture of human hepatoma Hep G2 cells. Mater. Sci. Eng. C 2004, 24, 379–386. [Google Scholar] [CrossRef]
- Kaihara, S.; Borenstein, J.; Koka, R.; Lalan, S.; Cunningham, B.; Vacanti, J. 7-Silicon Micromachining to Tissue Engineer Branched Vascular Channels for Liver Fabrication. Tissue Eng. 2000, 6, 105–117. [Google Scholar] [CrossRef]
- Lewis, P.L.; Shah, R.N. 3D Printing for Liver Tissue Engineering: Current Approaches and Future 3D Printing for Liver Tissue Engineering: Current Approaches and Future Challenges. Curr. Transplant. Rep. 2016, 3, 100–108. [Google Scholar] [CrossRef]
- Williams, M.J.; Adewunmi, A.; Schek, R.; Flanagan, C.; Krebsbach, P.; Feinberg, S.; Hollister, S.; Das, S. Bone tissue engineering using polycaorolactone scaffolds fabricated via selective laser sintering. Biomaterials 2005, 26, 4817–4827. [Google Scholar] [CrossRef]
- Chua, C.K.; Leong, K.F.; Wang, Y. Fabrication of customised scaffolds using computer-aided design and rapid prototyping techniques. Rapid Prototyp. J. 2005, 4, 249–259. [Google Scholar] [CrossRef]
- Leong, K.F.; Chua, C.K.; Sudarmadji, N.; Yeong, W.Y. Review article Engineering functionally graded tissue engineering scaffolds. J. Mech. Behav. Biomed. Mater. I 2008, 1, 140–152. [Google Scholar] [CrossRef]
- Cheah, C.M.; Chua, C.K.; Leong, K.F.; Cheong, C.H.; Naing, M.W. Automatic Algorithm for Generating Complex Polyhedral. Tissue Eng. 2004, 10, 595–610. [Google Scholar] [CrossRef] [PubMed]
- Hollister, B.S.J. Scaffold Design and Manufacturing: From Concept to Clinic. Adv. Mater. 2009, 21, 3330–3342. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Cheung, W.L.; Wang, M. Optimized fabrication of Ca – P / PHBV nanocomposite scaffolds via selective laser sintering for bone tissue engineering. Biofabrication 2011, 3, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Wang, S.; Yaszemski, M.J.; Lu, L. Enhanced cell ingrowth and proliferation through three dimensional nanocomposite scaffolds with controlled pore structures. Biomacromolecules 2010, 11, 682–689. [Google Scholar] [CrossRef]
- Huang, H.; Oizumi, S.; Kojima, N.; Niino, T.; Sakai, Y. Avidin-biotin binding-based cell seeding and perfusion culture of liver-derived cells in a porous scaffold with a three-dimensional interconnected flow-channel network. Biomaterials 2007, 28, 3815–3823. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.W.; Vacant, J.P. Toward development of an implantable tissue engineered liver. Biomaterials 1996, 17, 365–372. [Google Scholar] [CrossRef]
- Hammond, J.S.; Beckingham, I.J.; Shakesheff, K.M. Scaffolds for liver tissue engineering. Expert Rev. Med. Devices 2006, 3, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Lohfeld, S.; Tyndyk, M.A.; Cahill, S.; Flaherty, N.; Barron, V.; McHugh, P.E. A method to fabricate small features on scaffolds for tissue engineering via selective laser sintering. J. Biomed. Sci. Eng. 2010, 3, 138–147. [Google Scholar] [CrossRef]
- Kim, S.S.; Utsunomiya, H.; Koski, J.A.; Wu, B.M.; Cima, M.J.; Sohn, J.; Mukai, K.; Griffith, L.G.; Vacanti, J.P. Survival and Function of Hepatocytes on a Novel Three-Dimensional Synthetic Biodegradable Polymer Scaffold With an Intrinsic Network of Channels. Ann. Surg. 1998, 228, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Pang, Y.; Sutoko, S.; Horimoto, Y.; Sun, W.; Niino, T.; Sakai, Y. Design, Fabrication, and Evaluation of Polyglycolic Acid Modules with Canals as Tissue Elements in Cellular—Assembly Technology. Appl. Sci. 2020, 10, 3748. [Google Scholar] [CrossRef]
- Griffith, L.G.; Wells, A.; Stolz, D.B. Engineering liver. Hepatology 2014, 60, 1426–1434. [Google Scholar] [CrossRef] [PubMed]
- Page, C.O.F.; Griffith, L.G.; Naughton, G. Tissue Engineering—Current Challenges and Expanding Opportunities. Science 2002, 295, 1009–1014. [Google Scholar]
- Michael, E.; Schaubel, D.; Cai, S.; Guidinger, M.; Merion, R. Portal vein thrombosis and liver transplant survival benefit. Liver Transpl. 2010, 16, 999–1005. [Google Scholar] [CrossRef]
- Braune, S.; Latour, R.A.; Reinthaler, M.; Landmesser, U.; Lendlein, A.; Jung, F. In Vitro Thrombogenicity Testing of Biomaterials. Adv. Healthc. Mater. 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Reinthaler, M.; Braune, S.; Lendlein, A.; Landmesser, U.; Jung, F. Platelets and coronary artery disease: Interactions with the blood vessel wall and cardiovascular devices. Biointerphases 2016, 11, 029702. [Google Scholar] [CrossRef]
- Braune, S.; Groß, M.; Walter, M.; Zhou, S.; Dietze, S.; Rutschow, S.; Lendlein, A.; Tschöpe, C.; Jung, F. Adhesion and activation of platelets from subjects with coronary artery disease and apparently healthy individuals on biomaterials. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 210–217. [Google Scholar] [CrossRef]
- Denadai, R.; Teixeira, F.V.; Saad-Hossne, R. Management of psoriatic lesions associated with anti-TNF therapy in patients with IBD. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 744. [Google Scholar] [CrossRef]
- Weber, M.; Steinle, H.; Golombek, S.; Hann, L.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Blood-Contacting Biomaterials: In Vitro Evaluation of the Hemocompatibility. Front. Bioeng. Biotechnol. 2018, 6, 99. [Google Scholar] [CrossRef]
- Chen, Y.M.; Tanaka, M.; Gong, J.P.; Yasuda, K.; Yamamoto, S.; Shimomura, M.; Osada, Y. Platelet adhesion to human umbilical vein endothelial cells cultured on anionic hydrogel scaffolds. Biomaterials 2007, 28, 1752–1760. [Google Scholar] [CrossRef]
- Liu, P.; Tian, B.; Yang, L.; Zheng, X.; Zhang, X.; Li, J.; Liu, X. Hemocompatibility improvement of decellularized spleen matrix for constructing transplantable bioartificial liver Hemocompatibility improvement of decellularized spleen matrix for constructing transplantable bioarti fi cial liver. Biomed. Mater. 2019, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Van Hinsbergh, V.W.M. Endothelium—Role in regulation of coagulation and inflammation. Semin. Immunopathol. 2012, 34, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Jesus, C.; Perera, C.; Guadalupe, M.; Baas, C.; Abigail, G.; Lara, A.; Isaai, S.; Borges, R.; Leticia, A.; Guzmán, R.; et al. Characterization and hemocompatibility assessment of porous composite scaffolds with a biomimetic human clavicle macrostructure. Health Technol. 2020, 10, 423–428. [Google Scholar]
- Mcguigan, A.P.; Sefton, M. V Vascularized organoid engineered by modular assembly enables blood perfusion. Proc. Natl. Acad. Sci. USA 2006, 103, 11461–11466. [Google Scholar] [CrossRef] [PubMed]
- Sakai, Y.; Huang, H.; Hanada, S.; Niino, T. Possessing a clinically significant mass. Biochem. Eng. J. 2009, 1–14. [Google Scholar] [CrossRef]

| Parameter | Settings |
|---|---|
| Material | PCL: PGA (1:2) by volume |
| Spot diameter | 170 µm |
| Power | 9 W |
| Pre heat temperature | 40 °C |
| Scan speed | 1.5 m/s |
| Scan line pitch | 40 µm |
| Energy per unit area | 49 kJ/m2 |
| Layer thickness | 200 µm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Khadim, R.R.; Nagayama, M.; Shinohara, M.; Inamura, K.; Danoy, M.; Nishikawa, M.; Furukawa, K.; Sakai, Y.; Niino, T. Fabrication of a Porous Three-Dimensional Scaffold with Interconnected Flow Channels: Co-Cultured Liver Cells and In Vitro Hemocompatibility Assessment. Appl. Sci. 2021, 11, 2473. https://doi.org/10.3390/app11062473
Li M, Khadim RR, Nagayama M, Shinohara M, Inamura K, Danoy M, Nishikawa M, Furukawa K, Sakai Y, Niino T. Fabrication of a Porous Three-Dimensional Scaffold with Interconnected Flow Channels: Co-Cultured Liver Cells and In Vitro Hemocompatibility Assessment. Applied Sciences. 2021; 11(6):2473. https://doi.org/10.3390/app11062473
Chicago/Turabian StyleLi, Muxin, Rubina Rahaman Khadim, Mitsuru Nagayama, Marie Shinohara, Kousuke Inamura, Mathieu Danoy, Masaki Nishikawa, Katsuko Furukawa, Yasuyuki Sakai, and Toshiki Niino. 2021. "Fabrication of a Porous Three-Dimensional Scaffold with Interconnected Flow Channels: Co-Cultured Liver Cells and In Vitro Hemocompatibility Assessment" Applied Sciences 11, no. 6: 2473. https://doi.org/10.3390/app11062473
APA StyleLi, M., Khadim, R. R., Nagayama, M., Shinohara, M., Inamura, K., Danoy, M., Nishikawa, M., Furukawa, K., Sakai, Y., & Niino, T. (2021). Fabrication of a Porous Three-Dimensional Scaffold with Interconnected Flow Channels: Co-Cultured Liver Cells and In Vitro Hemocompatibility Assessment. Applied Sciences, 11(6), 2473. https://doi.org/10.3390/app11062473





